Abstract
Background
It has been hypothesized that cancer treatments cause accelerated aging through a mechanism involving the shortening of telomeres. However, the effect of cancer treatments on telomere length is unclear.
Methods
We systematically reviewed the epidemiological evidence evaluating the associations between cancer treatment and changes in telomere length. Searches were performed in PubMed for the period of January 1966 through November 2016 using the following search strategy: telomere AND (cancer OR tumor OR carcinoma OR neoplasm) AND (survivor OR patient). Data were extracted and the quality of studies was assessed.
Results
A total of 25 studies were included in this review. Ten were solid cancer studies, 11 were hematological malignancy studies, and 4 included a mixed sample of both solid and hematological cancers. Three of the 10 solid tumor studies reported a statistically significant association between cancer treatment and telomere length shortening, and one reported longer telomere length after treatment. Among the hematological cancer studies, three showed statistically significant decreases in telomere length with treatment, and two showed elongation. When these studies were rated using quality criteria, most of the studies were judged to be of moderate quality.
Conclusions
The findings from this review indicate that the effect of cancer treatment on telomere length may differ by cancer type and treatment as well as other factors. Definitive conclusions cannot be made based on the published literature, because sample sizes tended to be small; treatments, cancer types, and biospecimens were heterogenous; and the length of follow-up times differed greatly.
Telomeres are a series of noncoding repeated DNA sequences at the ends of chromosomes that are essential to chromosome stability and integrity (1). Telomeres act as a “mitotic clock” determining the replicative capacity of a cell; when the telomere length of a cell reaches a critically short length, the cell becomes senescent. Telomeres decrease in length with each somatic cell division, and, thus, progressively shorten with age (2,3). Shorter leukocyte telomere length (LTL) has been shown to be associated with earlier mortality (4) and age-related chronic diseases such as cardiovascular disease (5), diabetes (6), and decreased cognitive function (7). Therefore, LTL has been proposed to be a biomarker of aging and is increasingly used in epidemiological studies of age-related diseases.
Both genetic and environmental factors appear to play a role in an individuals’ age-related telomere attrition. For example, cigarette smoking, air pollution, and other chronic stressors have been shown to be associated with shorter LTL or accelerated LTL shortening in the general population (8–10). It has also been hypothesized that chemotherapeutic agents with genotoxic effects adversely affect telomeres in cancer patients and survivors (11); however, findings in the literature, overall, regarding the association between cancer treatment and changes in telomere length have been inconsistent, with some studies reporting shortening of telomeres after treatment (12–14) and others elongation (15) or no association (16,17).
The purpose of this report was to systematically review the epidemiological evidence on the effect of traditional cancer treatments on telomere length.
Methods
Study Search
For this report, we sought all evidence on the associations between traditional cancer treatments (not complementary alternative medicine) and telomere length change reported in epidemiological studies. PubMed was searched for the period of 1966 through November 2016 using the following search strategy: telomere AND (cancer OR tumor OR carcinoma OR neoplasm) AND (survivor OR patient). The search was restricted to human studies and articles written in English. In addition, the study team hand-searched the references cited in the articles chosen for data abstraction as well as relevant review articles identified in the search.
Study Selection
The following exclusion criteria were applied to the abstracts identified in the literature search: 1) no original data (reviews, editorials); 2) studies examining telomere length as a cancer risk or prognostic factor only; 3) telomere length correlated with noncancer treatment factors only; 4) complementary alternative medicine therapy examined in relation to telomere length; 5) telomere length not measured; 6) studies not in cancer survivors or patients; 7) studies not in humans; and 8) case reports. The full-text articles of all references selected after applying these criteria were reviewed using the same exclusion criteria, and the eligibility of each abstract and full-text article was assessed independently in a standardized manner by two reviewers. If separate reports from the same study were published, the report with the most updated data was selected for inclusion.
After full-text review, the study team decided that those articles describing a study investigating the effect of hematopoietic stem cell transplant (HSCT) on telomere length, but not any other type of cancer treatment, would also be excluded, because the mechanism for telomere attrition associated with HSCT is possibly linked to cellular replication stress to achieve engraftment and not necessarily to the toxicity of treatment (18). Further, a number of recent reviews have been published examining the effects of HSCT on telomere length (19,20).
Data Abstraction and Quality Assessment
Data abstraction for the selected articles was performed serially by two reviewers using an electronic abstraction database. Data abstracted included study population, study design, treatment examined, telomere length measurement method, and study results. To assess study quality, criteria published in Longnecker et al. (21) for observational studies were adapted. These criteria included whether the study population was clearly specified and defined, whether a nontreated comparison group was included in the study (if applicable), whether the sample size was more than 50 participants, whether there was a longitudinal component to the study, whether potential key confounding variables (such as age) were measured and adjusted for statistically, whether telomere length was measured at two time points for a least some of the subjects, whether telomere length was measured prior to treatment, and whether quality control information was provided for the telomere length assay used. Each item was coded as “1 = Yes,” “0.5 = To some extent,” and “0 = No/ Unclear/Information not given” according to the information available in the publications. Scores for all quality criteria were added together for an overall quality score; an overall score of 7 or 8 indicated high quality, an overall score of 4 to 6 indicated moderate quality, and an overall score of less than 4 indicated poor quality. Quality criteria scoring disagreements between reviewers were resolved by consensus.
Results
Study Selection
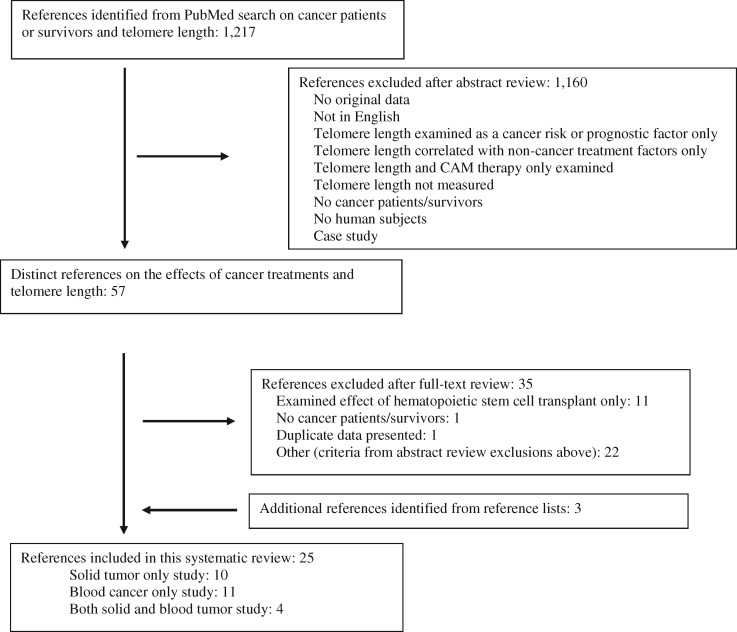
The search yielded 1217 references, of which 1160 were excluded after abstract review. Of the 57 articles obtained for full-text review, 34 studies examined the association between traditional cancer treatments and telomere length. One study was excluded that reported data included in another publication (22), and 11 studies were excluded that investigated the effect of HSCT on telomere length (20,23–32). After full-text review, an additional three eligible references were added based on review of the reference lists (16,33,34). This left 25 studies that met the inclusion criteria (Figure 1) .
Figure 1.
Flowchart of manuscript selection process. CAM = complementary alternative medicine.
Study Characteristics and Findings
Solid Tumor Only Studies.
Ten of the 25 studies included in this review examined the association between a traditional cancer treatment and telomere length among patients diagnosed with a solid tumor (Table 1). Two of these studies were judged to be of overall high quality (14,33); the other 8 were judged to be of moderate quality.
Table 1.
Solid tumor studies in adults examining the effect of cancer treatment on telomere length (N = 10)
| Reference | Cancer type(s) | Study design | Cancer patient sample size | Age range, y | Treatment type | Specimen | Telomere length method | Overall association |
|---|---|---|---|---|---|---|---|---|
| Brouwers et al., 2017 (33) | Breast | Longitudinal | 109 | 70–90 | Chemo: docetaxel+C; endocrine therapy, radiation, G-CSF | PB leukocytes | qPCR | No association |
| Benitez-Buelga et al., 2015 (12) | Breast | Longitudinal, cross-sectional* | 489 | 20–87 | Chemo: AC+T/T+AC or FEC+T/T+FEC (sporadic cancers only) | PB leukocytes | qPCR, high throughput Q-FISH | Shorter telomere length with treatment† |
| Duggan et al., 2014 (16) | Breast | Longitudinal, cross-sectional* | 611 | 18–64 | Varied, included 5-FU, A, C, taxanes; radiation | PB leukocytes | qPCR | No association |
| Sanoff et al., 2014 (35) | Breast | Longitudinal | 33 | 32–69 | Chemo: AC or ACT; FEC (n = 1) | PB leukocytes | TRF analysis | No association |
| Maeda et al., 2013 (36) | Lung, thyroid, prostate, rectal, hepatoma | Longitudinal | 25 | 52–83 | Radiation | PB leukocytes | TRF analysis | No overall association; decrease in proportion of short telomeres with higher dose† |
| Yoon et al., 2007 (37) | Gastric, esophageal, hepatoma, lung, breast, colorectal, ovarian | Longitudinal | 32 | 31–65 | Chemo: varied based on cancer type | PBMC | TRF analysis | Shorter telomere length after treatment† |
| Unryn et al., 2006 (14) | Head and neck | Longitudinal | 20 | 44–75 | Chemo: cisplatin; radiation | PBMC | TRF analysis | Shorter telomere length after treatment† |
| Idei et al., 2002 (38) | Ovarian | Longitudinal, cross-sectional | 42 | NR | Surgery, chemo: cisplatin-based | Free plasma DNA | TRF analysis | Longer telomere length after treatment |
| Schroder et al., 2001 (17) | Breast | Longitudinal | 33 | 29–54 | Radiation, chemo: FEC or FEC+C /thiotepa/carboplatin and autologous PBSCT, G-CSF; tamoxifen | PB leukocytes | TRF analysis | No association |
| Takahashi et al., 2000 (39) | Ovarian | Longitudinal | 21 | 28–78 | Chemo: A, cisplatin, C | Ovarian tumor tissues | TRF analysis | Shorter telomere length after treatment among nonresponders; no association in responders |
No pretreatment sample measured for telomere length. A = doxorubicin; C = cyclophosphamide; FEC = 5-fluorouracil, epirubicin, cyclophosphamide; 5-FU = 5-fluorouracil; G-CSF = granulocyte colony-stimulating factor; PB = peripheral blood; PBMC = peripheral blood mononuclear cells; PBSCT = peripheral blood stem cell transplant; qPCR = quantitative polymerase chain reaction; Q-FISH = quantitative fluorescence in situ hybridization; T = paclitaxel; TRF = terminal restriction fragment.
Result statistically significant.
Of the 10 solid tumor studies, one-half (n = 5) were conducted in a sample of breast cancer patients only (12,16,17,33,35) (Table 1). Only one of these studies showed a statistically significant difference or change (decrease) in telomere length associated with cancer treatment. Specifically, Benitez-Buelga et al. (12), in a cross-sectional analysis, measured LTL of 253 sporadic breast cancer cases (ie, cases that were not suspected to be due to an inherited susceptibility to cancer) at different time points during and post chemotherapy administration [with either a doxorubicin (A), cyclophosphamide (C), and paclitaxel (T) regimen (AC+T/T+AC) or a 5-fluorouracil (F), epirubicin (E), cyclophosphamide (C), and paclitaxel (T) regimen (FEC+T/T+FEC)] and showed a statistically significant negative correlation between number of days on treatment and mean LTL. The correlation was stronger among the breast cancer patients with FEC-based treatment compared with AC-based treatment. However, no LTL measurement was made in pretreatment samples. In a cross-sectional analysis of 236 familial breast cancer cases, Benitez-Buelga et al. (12) also reported that mean LTL was statistically significantly shorter compared with healthy controls during treatment, but not posttreatment (no details on regimen provided). A longitudinal analysis among a subset of seven patients in this study showed elongation of LTL assessed during treatment and again seven years later; the elongation speed was statistically significantly different from the normal shortening of the comparison group. The other four breast cancer studies (16,17,33,35), all of which had a longitudinal component [three with pre- and posttreatment LTL measurements (17,33,35) and one with LTL measurements 6 to 30 months postdiagnosis (16)], showed no statistically significant association between cancer treatment and LTL.
The other five solid tumor studies were of cancer types other than breast cancer (Table 1). Two were conducted among ovarian cancer patients (38,39); one was conducted among head, neck, and nasopharyngeal cancer patients (14); and the remaining two studies were among patients diagnosed with different types of solid tumors (36,37). Of these five, two studies showed overall statistically significant decreases in telomere length associated with cancer treatment (14,37), one reported longer telomere length after treatment (38), and two reported no overall association between cancer treatment and telomere length, although both these studies reported finding shorter telomere length in a subset of patients (36,39).
Both Yoon et al. (37) and Unryn et al. (14) showed decreases in telomere length associated with cancer treatment. In a sample of 32 patients diagnosed with different types of solid tumors (gastric, esophageal, hepatoma, lung, breast, colorectal, or ovarian), Yoon et al. (37) reported that telomere length measured in peripheral blood mononuclear cells (PBMCs) was statistically significantly shorter after four and six cycles of chemotherapy compared with pretreatment. Further, when compared with age-matched healthy controls, mean PBMC telomere length was found to be statistically significantly shorter at all time points (prechemotherapy, after two, four, and six cycles of treatment) for the cancer patients. Similarly, Unryn et al. (14) investigated PBMC telomere length in a sample of 20 head and neck or nasopharyngeal patients and reported a statistically significant decrease in mean telomere length over time, assessing telomere length before, at day 29, and after the completion of radiation and cisplatin chemotherapy. The mean change in PBMC telomere length was greater among older (> 55 years of age) compared with younger patients.
The two ovarian cancer studies showed variable results, although telomere length was measured in different specimen types in these studies (Table 1). Idei et al. (38) reported elongation of telomere length, measured in cell-free plasma DNA as an indicator of tumor burden, after completion of treatment with cisplatin-based multidrug chemotherapy compared to presurgery among late-stage ovarian cancer patients. The authors hypothesized that the elongation of telomere length posttreatment is because shorter tumor telomere restriction fragments are cleared from circulation during treatment and recuperation (38). Takahashi et al. (39) reported no change in telomere length, measured in ovarian cancer tissue, among responders to a chemotherapy regimen of cisplatin, doxorubicin, and cyclophosphamide, but noted decreases in telomere length among nonresponders. No pre- and posttreatment analyses were presented in Takahashi et al. (39) for the entire sample.
Similar to Takahashi et al., Maeda et al. (36) found no overall statistically significant change in mean LTL associated with radiation treatment among 25 patients with lung, thyroid, prostate, rectal, or hepatoma cancer and no difference in the magnitude of LTL change when comparing cancer cases receiving radiation compared with a hospital-based comparison group. However, the data showed a statistically significant decrease in the proportion of short telomeres (defined as those <4.4 kb) with increasing daily radiation dose. The authors suggested two possible explanations: only short telomeres are subjected to the telomere-elongating mechanism or short telomeres disappear from the telomere length distribution with radiation therapy (36).
Hematological Malignancy Only Studies
Eleven of the 25 studies included in this review examined the association between a traditional cancer treatment and telomere length among patients diagnosed with a hematological malignancy (Table 2). Ten of the studies were judged to be of overall moderate quality; one was judged to be of poor quality based on the criteria assessed (48). The types of hematological cancers investigated were chronic myeloid leukemia (CML) in four studies (40,44,46,47), non-Hodgkin lymphoma (NHL) in three studies (41,43,45), and acute promyelocytic leukemia (15), Hodgkin lymphoma (42), acute lymphocytic leukemia (ALL) (48), and lymphoma, not otherwise specified (13), in one study each. Ten of the blood cancer-only studies were conducted on adult patients; Nowak et al. (48) was conducted in a sample of childhood cancer patients. Nine studies examined telomere length in peripheral blood cells (eg, leukocytes, mononuclear cells, or progenitor cells) (15,34,40,42–46,48) and two measured telomere length in bone marrow mononuclear cells (41,47).
Table 2.
Blood cancer studies examining the effect of cancer treatment on telomere length (N = 11)
| Reference | Cancer type(s) | Study design | Cancer patient sample size | Age range, y | Treatment type | Specimen | Telomere length method | Overall association |
|---|---|---|---|---|---|---|---|---|
| Adult | ||||||||
| Lobetti-Bodoni et al., 2012 (40) | Chronic myeloid leukemia | Longitudinal, cross-sectional* | 81 | 23–88 | Varied; cytosine-arabinoside, interferon-α, imatinib, dasatinib | PB PMN; monocyte-depleted PBMC | TRF analysis | No association |
| Guidetti et al., 2011 (41) | Non-Hodgkin lymphoma | Longitudinal* | 53 | 26–76 | High-dose radioimmunotherapy based on 90Y-ibritumomab tiuxetan and autograft | Bone marrow mononuclear cells | TRF analysis | Shorter telomere length after treatment |
| Ghaffari et al., 2008 (15) | Acute promyelocytic leukemia | Longitudinal | 40 | 14–50 | Chemo: arsenic trioxide | PBMC | TRF analysis | Longer telomere length after treatment§ |
| M’kacher et al., 2007 (42) | Hodgkin lymphoma | Longitudinal | 119 | 28–76 | Radiation, chemo (not specified) | PBMC | TRF analysis | No association |
| Ricca et al., 2005 (43) | Non-Hodgkin lymphoma | Longitudinal | 37 | 18–59 | Chemo: high-dose Ara-C (after high dose C and APO); autograft, G-CSF | PB progenitor cells | TRF analysis | Shorter telomere length after treatment§ |
| Drummond et al., 2004 (44) | Chronic myeloid leukemia | Longitudinal | 95 | NR† | Imatinib | PB leukocytes | Flow-FISH | Shorter telomere length in nonresponders to imatinib; longer telomere length in responders |
| Szyper-Kravitz et al., 2003 (13) | Lymphoma | Longitudinal | 10 | 45–80 | Chemo: CHOP; G-CSF | PBMC | Flow-FISH | Shorter telomere length after CHOP without G-CSF§; no change or longer in patients treated with CHOP and G-CSF |
| Lee, et al. 2003 (45) | Non-Hodgkin lymphoma | Longitudinal, cross-sectional | 15 | 19–72 | Varied, included CHOP, radiation, ifosfamide, etoposide, carboplatin | PBMC | TRF analysis | Shorter telomere length after treatment§ |
| Brummendorf et al., 2003 (46) | Chronic myeloid leukemia | Longitudinal, cross-sectional | 206 | 16–81 | Imatinib | PB granulocytes | Flow-FISH | Longer telomere length after treatment§ |
| Iwama et al., 1997 (47) | Chronic myeloid leukemia | Longitudinal | 16 | NR‡ | alpha-interferon | Bone marrow mononuclear cells | TRF analysis | No association (5 of 16 had normalization of telomere length after treatment) |
| Pediatric | ||||||||
| Nowak et al., 2006 (48) | Acute lymphocytic leukemia | Longitudinal | 29 | N/A | Chemo (not specified) | PB lymphocytes | TRF analysis | Only range values before treatment and in remission reported |
No pretreatment sample measured for telomere length. APO = doxorubicin, vincristine, prednisone; Ara-C = cytarabine; C = cyclophosphamide; CHOP = cyclophosphamide, doxorubicin, vincristine and prednisone; FISH = fluorescence in situ hybridization; G-CSF = granulocyte colony-stimulating factor; N/A = not available; NR = not reported; PB = peripheral blood; PBMC = peripheral blood mononuclear cells; PMN = polymorphonucleates; TRF = terminal restriction fragment.
Age range not reported for entire sample; for a subset of the study sample, the age range was 24 to 77 years.
For entire sample of 44 patients reported in the manuscript, the age range was 14 to 72 years; telomere length analyses was limited to a subset of 16 patients.
Result statistically significant.
Among the nine studies examining telomere length in peripheral blood cells, three reported statistically significant shortening of telomere length associated with treatment (13,43,45) (Table 2). In a study of PBMC telomere length change in patients with NHL, Lee et al. (45) showed a statistically significant decline in mean telomere length among five NHL patients with telomere length measurements before and after a CHOP-based (cyclophosphamide, doxorubicin, vincristine, and prednisone) chemotherapy regimen, although there was no correlation between change in telomere length and time since the end of chemotherapy. Mean telomere length both before and after chemotherapy was shorter among the NHL patients (five with pretreatment and 15 with posttreatment measurements) compared with 39 age-matched healthy individuals, and telomere length attrition was suggested to be greater among the cancer patients. However, a statistical comparison examining change over time between the two groups was not presented. Szyper-Kravitz et al. (13) reported that among five lymphoma patients treated with CHOP and not administered granulocyte colony stimulating factor (G-CSF), mean telomere length decreased statistically significantly after two cycles of treatment. Conversely, in the same study, among a separate group of five lymphoma patients treated with CHOP and G-CSF, telomere length was preserved or increased. The investigators suggest these findings were possibly due to upregulation of telomerase activity by G-CSF (13). Finally, Ricca et al. (43) measured telomere length in peripheral blood progenitor cells among 37 NHL patients receiving high-dose cyclophosphamide and high-dose cytarabine (Ara-C) prior to autograft and showed statistically significant shortening in mean telomere length after high-dose Ara-C compared with before Ara-C administration (but after receipt of high-dose cyclophosphamide).
Although P values or other measures of statistical significance were not reported, Drummond et al. (44) reported that mean LTL among CML patients treated with imatinib was shorter in nonresponders (n = 11) but longer in responders (n = 10), eight months and six months after treatment initiation, respectively. Two additional studies showed statistically significant increases in telomere length associated with treatment. Brummendorf et al. (46) found in 119 CML patients with sequential measurements a statistically significant increase in mean peripheral blood granulocyte telomere length when measured after, compared with before, imatinib treatment. Further, mean telomere length was statistically significantly shorter in 197 CML patients early after treatment initiation (<144 days) than in 196 patients with longer treatment duration (>144 days), indicating possible elongation of telomere length with longer imatinib treatment duration (46). Ghaffari et al. (15) reported that, in a sample of 40 patients diagnosed with acute promyelocytic leukemia, telomere length tended to increase as patients responded to standard intensive remission induction and courses of consolidation therapy with arsenic trioxide. Of note, the findings of telomere lengthening associated with treatment in the Brummendorf et al. (46), Ghaffari et al. (15), and Drummond et al. (44) (for responders) studies may be explained by a decrease in the number of malignant cells with shorter telomere lengths over time (ie, a decrease in tumor load) rather than a direct effect of treatment; however, this has not been studied.
The remaining three studies examining peripheral blood cell telomere length either showed no statistically significant change in mean telomere length associated with treatment (42) or did not statistically assess or report change (or before/after treatment differences) in telomere length associated with treatment (40,48). One of the two studies that examined telomere length in bone marrow mononuclear cells reported decreases in mean telomere length associated with specific treatment regimens. Guidetti et al. (41) reported that mean telomere length declined in 54 NHL patients treated with high dose-radioimmunotherapy (90Y-ibritumomab tiuxetan after high-dose cyclophosphamide and/or high-dose Ara-C) when measured at three different time points post, compared with pre-, radioimmunotherapy treatment. No values of statistical significance (eg, P values) were presented, nor were prechemotherapy samples or data from controls collected. In the second study that used bone marrow mononuclear cells to study changes in telomere length associated with cancer treatment, no data were shown or reported for the overall telomere length change among 16 patients administered alpha-interferon, although five of the patients were reported to have longer telomere lengths post- compared with pretreatment (47).
Mixed Solid Tumor and Hematological Malignancy Studies
Four of the 25 studies included in this review examined the association between a traditional cancer treatment and telomere length in a sample of patients diagnosed with either a solid tumor or a hematological cancer (Table 3). Three of these studies were judged to be of moderate quality, and one was judged to be of poor quality (49).
Table 3.
Blood and solid cancer studies examining the effect of cancer treatment on telomere length (N = 4)
| Reference | Cancer type(s) | Study design | Cancer patient sample size | Age Range, y | Treatment type | Specimen | Telomere length method | Overall association |
|---|---|---|---|---|---|---|---|---|
| Adult | ||||||||
| Diker-Cohen et al., 2013 (34) | Non-Hodgkin lymphoma, colon, chronic lymphocytic leukemia, lymphoma | Longitudinal | 42 | 45–74 | Chemo: varied based on cancer type | PBMC | Flow-FISH | Shorter telomere length with treatment† |
| Kronenwett et al., 1996 (49) | Non-Hodgkin lymphoma, multiple myeloma, breast cancer, rhabdomyosarcoma | Cross-sectional* | 54 | 22–60 | Chemo: varied based on cancer type; G-CSF | PB stem cells; mononuclear cells | TRF analysis | No association |
| Pediatric | ||||||||
| Franco et al., 2003 (50) | Acute lymphocytic leukemia, Hodgkin lymphoma, Ewing’s sarcoma, hepatoblastoma, clear cell sarcoma, neuroblastoma, Wilms tumor, brainstem tumor, astrocytoma, severe aplastic anemia | Longitudinal | 24 | 0.7–16 | Chemo: varied based on cancer type, radiation, autologous stem cells | PBMC or bone marrow mononuclear cells and granulocytes | TRF analysis | Shorter PB telomere length with treatment, primarily among solid tumor patients and not hematological cancer patients |
| Engelhardt et al., 1998 (51) | Acute lymphocytic leukemia, acute myeloid leukemia, sarcoma, Wilms tumor, Hodgkin lymphoma, Central nervous system tumors, hepatoblastoma, germ cell | Longitudinal | 25 | 1–15 | Chemo: varied based on cancer type | PBMC and bone marrow mononuclear cells and granulocytes | TRF analysis | Shorter telomere length with treatment |
No pretreatment sample measured for telomere length. FISH = fluorescence in situ hybridization; G-CSF = granulocyte colony-stimulating factor; PB = peripheral blood; PBMC = peripheral blood mononuclear cells; TRF = terminal restriction fragment.
Result statistically significant.
Three of the four mixed solid tumor and hematological cancer studies showed decreases in telomere length in at least one group of patients in the study sample (34,50,51) (Table 3). Diker-Cohen et al. (34) reported that, in 14 adult patients diagnosed with NHL, CHOP treatment resulted, at 6 to 12 months postchemotherapy, in an average PBMC telomere length that was approximately 35% that of a control group of 40 age-matched volunteers (P < .05). At 2 years, telomere length remained shortened to the same degree (35%) of the age-matched control group. Further, in the NHL patients (n = 10) who received more intensive therapy (six cycles plus, after relapse, an additional four cycles of etoposide, cisplatinum, Ara-C and methylprednisolone), more marked telomere shortening was observed 2 months postchemotherapy compared with the standard therapy group and the controls. Average telomere attrition rates over the course of the study were reported as 50 base pairs (bp)/year in the control group, 250 bp/year in the standard chemotherapy group, and 500 bp/year in the intensive chemotherapy group. In the same study, Diker-Cohen et al. (34) reported statistically significant telomere shortening 2 months after either 5-fluorouracil (5-FU) or fludarabine treatment among 10 adult colon cancer patients and 8 adult low-grade lymphoma or chronic lymphocytic leukemia patients, respectively. After 1 year posttreatment, mean telomere length returned to the pretreatment value among the 5-FU group but not the fludarabine group.
Franco et al. (50) examined several subgroups of pediatric cancer patients. Among 10 ALL patients and 9 solid tumor patients treated with various anticancer regimens based on diagnosis, the authors reported pre- to posttreatment shortening in PBMCs and granulocyte telomere length. The magnitude of changes was greater in the solid tumor compared with the ALL patients, although values of statistical significance were not reported. PBMC and granulocyte telomere length losses associated with treatment (based on diagnosis) were also observed among ALL and solid tumor pediatric patients in a study by Engelhardt et al. (51). Additionally, solid tumor patients in this study who received high-dose chemotherapy (n = 2) showed a more pronounced decline in telomere length compared with those who received standard-dose chemotherapy (n = 7). A test of statistical significance was not conducted.
Discussion
For this systematic literature review, the epidemiological evidence pertaining to the effect of traditional cancer treatments on telomere length in cancer patients was identified and critically evaluated to determine whether such a relationship exists. However, an assessment of consistency, and, thus, causality, cannot be made using the current body of evidence, because the studies are not directly comparable. One criterion of the Bradford-Hill set of nine criteria that allow for the assessment of the epidemiological literature as to whether a causal relationship exists between a presumed cause and an observed effect is consistency, or reproducibility, in epidemiological findings across different studies of different patient populations (52). The identified 25 studies did not examine the same treatment regimens over the same period of follow-up time; it is likely that different types of treatments affect telomere length in different ways or may not affect telomere length at all. Further, the studies included in this review collected different specimens for telomere length measurement, adjusted for different (and in some cases a limited set of) potential confounders (eg, age, cigarette smoking), and measured telomere length change over different lengths of time or at different time points on the cancer treatment continuum (eg, pre- to posttreatment or only posttreatment). In addition, different methods were used to assess telomere length across studies, precluding any comparison of telomere length and changes in telomere length between studies and even across different cancer types or treatments. Because of this lack of comparability, a meta-analysis of this literature could not be conducted; thus, results were reported in a descriptive, albeit systematic, manner.
LTL has been hypothesized to be a biomarker of biological aging, with shorter telomeres being indicative of more advanced biological age (53). Numerous studies have shown that various disease states and exposure to chronic stressors, such as cigarette smoking, accelerate LTL shortening (8,9). LTL has also been shown to be a prognostic marker for cancer survival as well as the development of a number of chronic conditions, including cancer and cardiovascular disease (5,54–57). Thus, if known toxic cancer therapies, which cause cycles of cell injury and repair through a variety of different mechanisms to treat cancer, lead to telomere shortening, this may be the biological mechanism by which cancer patients and survivors are at increased risk for accelerated or premature aging.
Indeed, telomere stability has been shown in in vitro studies to be affected by a number of conventional chemotherapies in a manner that depends on the specific drug mechanism of action. For example, both cyclophosphamide, an alkylating agent that is used in regimens to treat breast and other types of cancer, and cisplatin, an alkylating-like agent used to treat testicular, ovarian, and lung cancers in particular, may directly cause telomere damage and shortening by inducing guanine DNA-DNA cross-links (58). Telomeres are specifically susceptible to these cross-links because of their G-rich DNA sequences (58–60). Mitotic inhibitors, such the taxane class of chemotherapy drugs, can cause telomere uncapping, triggering telomere dysfunction and shortening in cancer cells (11). In contrast, G-CSF has been reported to increase LTL through upregulation of telomerase (13,51). Interestingly, four studies included in this review reported G-CSF as part of the treatment regimen and found no association with telomere length change (13,17,33,49). Thus, there is biological plausibility of a telomere shortening effect of certain conventional cancer therapies that may lead to accelerated aging among cancer patients and survivors. It should also be noted that telomere length is also regulated by telomerase activity, and it is possible that cancer treatments have a shortening effect on telomere length through the downregulation of telomerase activity (61). However, there is a paucity of epidemiological evidence on this relationship. A study by Franco et al. (50) included in this review showed no change in telomerase activity associated with cancer treatment among pediatric solid tumor patients and a decrease in the level of telomerase activity among ALL patients associated with induction therapy that continued through maintenance therapy and after the end of treatment.
Although some of the studies included in this review showed telomere shortening associated with cancer treatment, the results of several studies suggest that the shortening may not be permanent and that there may be a recovery period during which telomere length normalizes or lengthens (12,15,38,44,46,47). However, four of these studies were conducted among leukemia patients, and in these studies, pretreatment telomere length measurement is primarily representative of telomere length in cancer cells. In contrast, telomere length measured in specimens during or posttreatment represents telomere length in a mixed population of normal and leukemic cells (15,44,46,47). Of the two solid tumor studies suggesting posttreatment lengthening of telomere length, the results of Benitez-Buelga et al. (12), which observed LTL elongation among a sample of familial breast cancer patients (BRCA1/2 carriers), were based on a small sample size (n = 7) with no treatment details and no pretreatment measurement. The findings of Idei et al. (38) were based on cell-free plasma DNA telomere length measurements. The observed elongation of telomere length in Idei et al. (38) may, like the hematological studies discussed above, reflect a decrease in circulating tumor cells (and DNA) rather than the result of a direct treatment effect on telomere length.
Most of the studies included in this systematic review measured telomere length in leukocytes; this is the most widely used specimen type for telomere length measurement, especially in large population-based or clinical studies due to its accessibility as a DNA source. However, it is possible that the telomere length of the tumor tissues that are most affected by specific cancer treatments (rather than LTL) may best explain the reported findings of cancer treatments and accelerated aging, of which endpoints often include comorbid conditions and functional decline. Leukocytes have a higher replication rate than cells in somatic tissues, suggesting that LTL may decrease at a faster rate than telomere length in other tissues. However, studies comparing LTL with telomere length in other somatic tissues from the same individuals have shown that although absolute differences exist, there are strong correlations (62–64). These findings are not surprising, because telomere length is heritable (65). It should be noted that the relationship between telomere length in leukocytes and diseased tissues (such as solid tumor tissue) is not clear. Furthermore, as discussed above, the studies examining telomere length in leukocytes taken from patients with hematological malignancies may not be an accurate reflection of the true telomere length because the blood samples are a mix of normal and diseased cells, where the proportion would also differ by disease status. Therefore, researchers planning clinical or epidemiological studies must determine the most appropriate biospecimen to collect and telomere length measurement method to use given the type of cancer to be studied and the specific hypothesis to be tested.
An additional challenge of comparing the results across the published studies is that different methods for quantifying telomere length were used, and the utility of the measure depends on the validity and reliability of the techniques. Those used in the studies reviewed here were terminal restriction fragment visualized by Southern blot, quantitative polymerase chain reaction (qPCR), quantitative fluorescence in situ hybridization (qFISH), and flow-FISH. Each of these methods has its strengths and weaknesses; these methods have recently been reviewed by multiple investigators (66–68). In brief, terminal restriction fragment measurement is currently considered the gold standard for telomere length measurement and can be used to measure telomere length in extracted DNA from stored samples, such as those typically available in epidemiological studies. This method provides an actual kilobase size estimate of telomere length, making it feasible to compare results across studies (66). The limitations of terminal restriction fragment measurement are that it often provides an overestimation of true telomere length because of the inclusion of subtelomeric DNA in the measurement, the need for large amounts of DNA, and lower sensitivity to detect very short telomeres (65). To address some of the limitations of terminal restriction fragment measurement, qPCR was developed as a technique to measure telomere length using smaller amounts of DNA in a high throughput manner, making this technique attractive for studies of larger sample sizes. It produces a telomere length result referenced to a standard single copy gene and not an absolute kilobase length estimate; thus, it is difficult to compare telomere length results using this method across studies. Other limitations of the qPCR method are that that reference standards are lacking and there can be variation between and within laboratory batches (66). Both terminal restriction fragment and qPCR telomere length results are limited in that they reflect an average across the population of cells in the sample. In contrast, flow-FISH can more accurately measure LTL, even within specific cell populations; however, viable cells are needed for this method, thus limiting its use in population-based studies (65). It is therefore important when deciding which method to measure telomere length that several factors be evaluated, including the research question, study population, sample size, biospecimen type, timing of analysis, and available resources (66). Further, minimizing technique-related measurement error is imperative in telomere length studies, underscoring the need for rigorous laboratory quality control procedures (65,66).
Advances in cancer treatment have led to better overall survival rates for cancer patients, resulting in a growing number of cancer survivors (69). Despite surviving their cancer, there is increasing evidence that cancer treatments may lead to an accelerated aging phenotype, putting the cancer survivor at risk for premature death from non-cancer aging-related diseases and poor quality of life. A better characterization of the accelerated aging phenotype, the biological mechanisms associated with its occurrence, and which cancer survivors are most at risk is needed. Importantly, LTL may not be the best measure by which accelerated aging may occur with exposure to certain types of cancer treatments; telomere length measured in the tissue most adversely affected by the cancer treatment should be explored as a biomarker of damage related to a specific aging-related outcome. Thus, when planning clinical and epidemiological studies examining cancer treatment and its effect on aging, investigators should determine whether telomere length, measured either in leukocytes or specific tissues, is the mechanism by which cancer treatment leads to accelerated aging and is a valid biomarker to address the research question. If telomere length is used, it is important to select the appropriate epidemiological study design, which includes considering an untreated comparison group, using an adequate sample size, using an accurate telomere length assay, and adjusting for confounders. Further, measuring telomere length longitudinally is crucial; this includes measurement of telomere length prior to and after treatment. The careful design of such studies examining mechanisms of accelerated aging associated with cancer treatments will lead to better quality epidemiological evidence, with the goal of developing interventions to prevent, mitigate, or even reverse deleterious cancer treatment effects.
Funding
This work was supported by the National Institutes of Health (contract no. HHSN261201400011I to Scientific Consulting Group for N.I.S.).
Notes
Affiliations of authors: Epidemiology and Genomics Research Program, Division of Cancer Control and Population Sciences, National Cancer Institute, Rockville, MD (LG, JDM); Clinical Genetics Branch, Division of Cancer Epidemiology and Genetics, National Cancer Institute, Rockville, MD (SMG); Scientific Consulting Group, Inc., Gaithersburg, MD (NIS).
The funder had no role in the design of the study; the collection, analysis, and interpretation of the data; the writing of the manuscript; and the decision to submit the manuscript for publication. The authors have no conflicts of interest to disclose.
References
- 1. Blackburn EH. Structure and function of telomeres. Nature. 1991;3506319:569–573. [DOI] [PubMed] [Google Scholar]
- 2. Allsopp RC, Vaziri H, Patterson C, et al. Telomere length predicts replicative capacity of human fibroblasts. Proc Natl Acad Sci U S A. 1992;8921:10114–10118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Harley CB, Vaziri H, Counter CM, et al. The telomere hypothesis of cellular aging. Exp Gerontol. 1992;274:375–382. [DOI] [PubMed] [Google Scholar]
- 4. Cawthon RM, Smith KR, O’Brien E, et al. Association between telomere length in blood and mortality in people aged 60 years or older. Lancet. 2003;3619355:393–395. [DOI] [PubMed] [Google Scholar]
- 5. Haycock PC, Heydon EE, Kaptoge S, et al. Leucocyte telomere length and risk of cardiovascular disease: systematic review and meta-analysis. BMJ. 2014;349:g4227.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Zee RY, Castonguay AJ, Barton NS, et al. Mean leukocyte telomere length shortening and type 2 diabetes mellitus: a case-control study. Transl Res. 2010;1554:166–169. [DOI] [PubMed] [Google Scholar]
- 7. Yaffe K, Lindquist K, Kluse M, et al. Telomere length and cognitive function in community-dwelling elders: findings from the Health ABC Study. Neurobiol Aging. 2011;3211:2055–2060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Astuti Y, Wardhana A, Watkins J, et al. Cigarette smoking and telomere length: a systematic review of 84 studies and meta-analysis. Environ Res. 2017;158:480–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Oliveira BS, Zunzunegui MV, Quinlan J, et al. Systematic review of the association between chronic social stress and telomere length: a life course perspective. Ageing Res Rev. 2016;26:37–52. [DOI] [PubMed] [Google Scholar]
- 10. Ward-Caviness CK, Nwanaji-Enwerem JC, Wolf K, et al. Long-term exposure to air pollution is associated with biological aging. Oncotarget. 2016;746:74510–74525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lu Y, Leong W, Guerin O, et al. Telomeric impact of conventional chemotherapy. Front Med. 2013;74:411–417. [DOI] [PubMed] [Google Scholar]
- 12. Benitez-Buelga C, Sanchez-Barroso L, Gallardo M, et al. Impact of chemotherapy on telomere length in sporadic and familial breast cancer patients. Breast Cancer Res Treat. 2015;1492:385–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Szyper-Kravitz M, Uziel O, Shapiro H, et al. Granulocyte colony-stimulating factor administration upregulates telomerase activity in CD34+ haematopoietic cells and may prevent telomere attrition after chemotherapy. Br J Haematol. 2003;1202:329–336. [DOI] [PubMed] [Google Scholar]
- 14. Unryn BM, Hao D, Gluck S, et al. Acceleration of telomere loss by chemotherapy is greater in older patients with locally advanced head and neck cancer. Clin Cancer Res. 2006;1221:6345–6350. [DOI] [PubMed] [Google Scholar]
- 15. Ghaffari SH, Shayan-Asl N, Jamialahmadi AH, et al. Telomerase activity and telomere length in patients with acute promyelocytic leukemia: indicative of proliferative activity, disease progression, and overall survival. Ann Oncol. 2008;1911:1927–1934. [DOI] [PubMed] [Google Scholar]
- 16. Duggan C, Risques R, Alfano C, et al. Change in peripheral blood leukocyte telomere length and mortality in breast cancer survivors. J Natl Cancer Inst. 2014;1064:dju035.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Schroder CP, Wisman GB, de Jong S, et al. Telomere length in breast cancer patients before and after chemotherapy with or without stem cell transplantation. Br J Cancer. 2001;8410:1348–1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gadalla SM, Savage SA.. Telomere biology in hematopoiesis and stem cell transplantation. Blood Rev. 2011;256:261–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wang L, Xiao H, Zhang X, et al. The role of telomeres and telomerase in hematologic malignancies and hematopoietic stem cell transplantation. J Hematol Oncol. 2014;7:61–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lee J, Kook H, Chung I, et al. Telomere length changes in patients undergoing hematopoietic stem cell transplantation. Bone Marrow Transplant. 1999;244:411–415. [DOI] [PubMed] [Google Scholar]
- 21. Longnecker MP, Berlin JA, Orza MJ, et al. A meta-analysis of alcohol consumption in relation to risk of breast cancer. JAMA. 1988;2605:652–656. [PubMed] [Google Scholar]
- 22. Brummendorf TH, Ersoz I, Hartmann U, et al. Telomere length in peripheral blood granulocytes reflects response to treatment with imatinib in patients with chronic myeloid leukemia. Blood. 2003;1011:375–376. [DOI] [PubMed] [Google Scholar]
- 23. Ashbridge B, Zehir A, Lubin M, et al. Evaluation of initial telomere length and changes after transplantation in adult double-unit cord blood transplant recipients. Biol Blood Marrow Transplant. 2015;217:1334–1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ruella M, Rocci A, Ricca I, et al. Comparative assessment of telomere length before and after hematopoietic SCT: role of grafted cells in determining post-transplant telomere status. Bone Marrow Transplant. 2010;453:505–512. [DOI] [PubMed] [Google Scholar]
- 25. Chakraborty S, Sun CL, Francisco L, et al. Accelerated telomere shortening precedes development of therapy-related myelodysplasia or acute myelogenous leukemia after autologous transplantation for lymphoma. J Clin Oncol. 2009;275:791–798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Widmann T, Kneer H, Konig J, et al. Sustained telomere erosion due to increased stem cell turnover during triple autologous hematopoietic stem cell transplantation. Exp Hematol. 2008;361:104–110. [DOI] [PubMed] [Google Scholar]
- 27. Rocci A, Ricca I, Dellacasa C, et al. Long-term lymphoma survivors following high-dose chemotherapy and autograft: evidence of permanent telomere shortening in myeloid cells, associated with marked reduction of bone marrow hematopoietic stem cell reservoir. Exp Hematol. 2007;354:673–681. [DOI] [PubMed] [Google Scholar]
- 28. Pipes BL, Tsang T, Peng SX, et al. Telomere length changes after umbilical cord blood transplant. Transfusion. 2006;466:1038–1043. [DOI] [PubMed] [Google Scholar]
- 29. Bhatia R, Van Heijzen K, Palmer A, et al. Longitudinal assessment of hematopoietic abnormalities after autologous hematopoietic cell transplantation for lymphoma. J Clin Oncol. 2005;2327:6699–6711. [DOI] [PubMed] [Google Scholar]
- 30. Lincz LF, Scorgie FE, Sakoff JA, et al. Telomere length predicts neutrophil recovery in the absence of G-CSF after autologous peripheral blood stem cell transplantation. Bone Marrow Transplant. 2004;345:439–445. [DOI] [PubMed] [Google Scholar]
- 31. Robertson JD, Testa NG, Russell NH, et al. Accelerated telomere shortening following allogeneic transplantation is independent of the cell source and occurs within the first year post transplant. Bone Marrow Transplant. 2001;2712:1283–1286. [DOI] [PubMed] [Google Scholar]
- 32. Akiyama M, Asai O, Kuraishi Y, et al. Shortening of telomeres in recipients of both autologous and allogeneic hematopoietic stem cell transplantation. Bone Marrow Transplant. 2000;254:441–447. [DOI] [PubMed] [Google Scholar]
- 33. Brouwers B, Hatse S, Dal Lago L, et al. The impact of adjuvant chemotherapy in older breast cancer patients on clinical and biological aging parameters. Oncotarget. 2016;721:29977–29988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Diker-Cohen T, Uziel O, Szyper-Kravitz M, et al. The effect of chemotherapy on telomere dynamics: clinical results and possible mechanisms. Leuk Lymphoma. 2013;549:2023–2029. [DOI] [PubMed] [Google Scholar]
- 35. Sanoff HK, Deal AM, Krishnamurthy J, et al. Effect of cytotoxic chemotherapy on markers of molecular age in patients with breast cancer. J Natl Cancer Inst. 2014;1064:dju057.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Maeda T, Nakamura K, Atsumi K, et al. Radiation-associated changes in the length of telomeres in peripheral leukocytes from inpatients with cancer. Int J Radiat Biol. 2013;892:106–109. [DOI] [PubMed] [Google Scholar]
- 37. Yoon SY, Sung HJ, Park KH, et al. Telomere length shortening of peripheral blood mononuclear cells in solid-cancer patients undergoing standard-dose chemotherapy might be correlated with good treatment response and neutropenia severity. Acta Haematol. 2007;1181:30–37. [DOI] [PubMed] [Google Scholar]
- 38. Idei T, Sakamoto H, Yamamoto T.. Terminal restriction fragments of telomere are detectable in plasma and their length correlates with clinical status of ovarian cancer patients. J Int Med Res. 2002;303:244–250. [DOI] [PubMed] [Google Scholar]
- 39. Takahashi M, Kigawa J, Oishi T, et al. Alteration of telomerase activity in ovarian cancer after chemotherapy. Gynecol Obstet Invest. 2000;493:204–208. [DOI] [PubMed] [Google Scholar]
- 40. Lobetti-Bodoni C, Ferrero D, Genuardi E, et al. Telomere loss in Philadelphia-negative hematopoiesis after successful treatment of chronic myeloid leukemia: evidence for premature aging of the myeloid compartment. Mech Ageing Dev. 2012;1337:479–488. [DOI] [PubMed] [Google Scholar]
- 41. Guidetti A, Carlo-Stella C, Ruella M, et al. Myeloablative doses of yttrium-90-ibritumomab tiuxetan and the risk of secondary myelodysplasia/acute myelogenous leukemia. Cancer. 2011;11722:5074–5084. [DOI] [PubMed] [Google Scholar]
- 42. M’kacher R, Bennaceur-Griscelli A, Girinsky T, et al. Telomere shortening and associated chromosomal instability in peripheral blood lymphocytes of patients with Hodgkin’s lymphoma prior to any treatment are predictive of second cancers. Int J Radiat Oncol Biol Phys. 2007;682:465–471. [DOI] [PubMed] [Google Scholar]
- 43. Ricca I, Compagno M, Ladetto M, et al. Marked telomere shortening in mobilized peripheral blood progenitor cells (PBPC) following two tightly spaced high-dose chemotherapy courses with G-CSF. Leukemia. 2005;194:644–651. [DOI] [PubMed] [Google Scholar]
- 44. Drummond M, Lennard A, Brummendorf T, et al. Telomere shortening correlates with prognostic score at diagnosis and proceeds rapidly during progression of chronic myeloid leukemia. Leuk Lymphoma. 2004;459:1775–1781. [DOI] [PubMed] [Google Scholar]
- 45. Lee JJ, Nam CE, Cho SH, et al. Telomere length shortening in non-Hodgkin’s lymphoma patients undergoing chemotherapy. Ann Hematol. 2003;828:492–495. [DOI] [PubMed] [Google Scholar]
- 46. Brummendorf TH, Ersoz I, Hartmann U, et al. Normalization of previously shortened telomere length under treatment with imatinib argues against a preexisting telomere length deficit in normal hematopoietic stem cells from patients with chronic myeloid leukemia. Ann N Y Acad Sci. 2003;996:26–38. [DOI] [PubMed] [Google Scholar]
- 47. Iwama H, Ohyashiki K, Ohyashiki JH, et al. The relationship between telomere length and therapy-associated cytogenetic responses in patients with chronic myeloid leukemia. Cancer. 1997;798:1552–1560. [DOI] [PubMed] [Google Scholar]
- 48. Nowak T, Januszkiewicz D, Zawada M, et al. Amplification of hTERT and hTERC genes in leukemic cells with high expression and activity of telomerase. Oncol Rep. 2006;162:301–305. [PubMed] [Google Scholar]
- 49. Kronenwett R, Murea S, Haas R.. Telomere length of blood-derived mononuclear cells from cancer patients during G-CSF-enhanced marrow recovery. Bone Marrow Transplant. 1996;18(suppl 1):S10–S14. [PubMed] [Google Scholar]
- 50. Franco S, Ozkaynak MF, Sandoval C, et al. Telomere dynamics in childhood leukemia and solid tumors: a follow-up study. Leukemia. 2003;172:401–410. [DOI] [PubMed] [Google Scholar]
- 51. Engelhardt M, Ozkaynak MF, Drullinsky P, et al. Telomerase activity and telomere length in pediatric patients with malignancies undergoing chemotherapy. Leukemia. 1998;121:13–24. [DOI] [PubMed] [Google Scholar]
- 52. Hill AB. The environment and disease. Association or causation? Proc R Soc Med. 1965;58:295–300. [PMC free article] [PubMed] [Google Scholar]
- 53. von Zglinicki T, Martin-Ruiz CM.. Telomeres as biomarkers for ageing and age-related diseases. Curr Mol Med. 2005;52:197–203. [DOI] [PubMed] [Google Scholar]
- 54. Ennour-Idrissi K, Maunsell E, Diorio C.. Telomere length and breast cancer prognosis: a systematic review. Cancer Epidemiol Biomarkers Prev. 2017;261:3–10. [DOI] [PubMed] [Google Scholar]
- 55. Xu X, Qu K, Pang Q, et al. Association between telomere length and survival in cancer patients: a meta-analysis and review of literature. Front Med. 2016;102:191–203. [DOI] [PubMed] [Google Scholar]
- 56. Zhang X, Zhao Q, Zhu W, et al. The association of telomere length in peripheral blood cells with cancer risk: a systematic review and meta-analysis of prospective studies. Cancer Epidemiol Biomarkers Prev. 2017;269:1381–1390. [DOI] [PubMed] [Google Scholar]
- 57. D’Mello MJJ, Ross SA, Briel M, et al. Association between shortened leukocyte telomere length and cardiometabolic outcomes: systematic review and meta-analysis. Circ Cardiovasc Genet. 2015;81:82–90. [DOI] [PubMed] [Google Scholar]
- 58. Liu M, Hales BF, Robaire B.. Effects of four chemotherapeutic agents, bleomycin, etoposide, cisplatin, and cyclophosphamide, on DNA damage and telomeres in a mouse spermatogonial cell line. Biol Reprod. 2014;904:72, 1–10. [DOI] [PubMed] [Google Scholar]
- 59. Malayappan B, Johnson L, Nie B, et al. Quantitative high-performance liquid chromatography-electrospray ionization tandem mass spectrometry analysis of bis-N7-guanine DNA-DNA cross-links in white blood cells of cancer patients receiving cyclophosphamide therapy. Anal Chem. 2010;829:3650–3658. [DOI] [PubMed] [Google Scholar]
- 60. Poklar N, Pilch DS, Lippard SJ, et al. Influence of cisplatin intrastrand crosslinking on the conformation, thermal stability, and energetics of a 20-mer DNA duplex. Proc Natl Acad Sci U S A. 1996;9315:7606–7611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Beeharry N, Broccoli D.. Telomere dynamics in response to chemotherapy. Curr Mol Med. 2005;52:187–196. [DOI] [PubMed] [Google Scholar]
- 62. Daniali L, Benetos A, Susser E, et al. Telomeres shorten at equivalent rates in somatic tissues of adults. Nat Commun. 2013;4:1597–1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Friedrich U, Griese E, Schwab M, et al. Telomere length in different tissues of elderly patients. Mech Ageing Dev. 2000;1193:89–99. [DOI] [PubMed] [Google Scholar]
- 64. Takubo K, Izumiyama-Shimomura N, Honma N, et al. Telomere lengths are characteristic in each human individual. Exp Gerontol. 2002;374:523–531. [DOI] [PubMed] [Google Scholar]
- 65. Bodelon C, Savage SA, Gadalla SM.. Telomeres in molecular epidemiology studies. Prog Mol Biol Transl Sci. 2014;125:113–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Montpetit AJ, Alhareeri AA, Montpetit M, et al. Telomere length: a review of methods for measurement. Nurs Res. 2014;634:289–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Aubert G, Hills M, Lansdorp PM.. Telomere length measurement-caveats and a critical assessment of the available technologies and tools. Mutat Res. 2012;730(1-2):59–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Vera E, Blasco MA.. Beyond average: potential for measurement of short telomeres. Aging (Albany NY). 2012;46:379–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Bluethmann SM, Mariotto AB, Rowland JH.. Anticipating the “Silver Tsunami”: prevalence trajectories and comorbidity burden among older cancer survivors in the United States. Cancer Epidemiol Biomarkers Prev. 2016;257:1029–1036. [DOI] [PMC free article] [PubMed] [Google Scholar]