Abstract
Background
Mammalian cells express a single functional heparanase, an endoglycosidase that cleaves heparan sulfate and thereby promotes tumor metastasis, angiogenesis, and inflammation. Malignant mesothelioma is highly aggressive and has a poor prognosis because of the lack of markers for early diagnosis and resistance to conventional therapies. The purpose of this study was to elucidate the mode of action and biological significance of heparanase in mesothelioma and test the efficacy of heparanase inhibitors in the treatment of this malignancy.
Methods
The involvement of heparanase in mesothelioma was investigated by applying mouse models of mesothelioma and testing the effect of heparanase gene silencing (n = 18 mice per experiment; two different models) and heparanase inhibitors (ie, PG545, defibrotide; n = 18 per experiment; six different models). Synchronous pleural effusion and plasma samples from patients with mesothelioma (n = 35), other malignancies (12 non–small cell lung cancer, two small cell lung carcinoma, four breast cancer, three gastrointestinal cancers, two lymphomas), and benign effusions (five patients) were collected and analyzed for heparanase content (enzyme-linked immunosorbent assay). Eighty-one mesothelioma biopsies were analyzed by H-Score for the prognostic impact of heparanase using immunohistochemistry. All statistical tests were two-sided.
Results
Mesothelioma tumor growth, measured by bioluminescence or tumor weight at termination, was markedly attenuated by heparanase gene silencing (P = .02) and by heparanase inhibitors (PG545 and defibrotide; P < .001 and P = .01, respectively). A marked increase in survival of the mesothelioma-bearing mice (P < .001) was recorded. Heparanase inhibitors were more potent in vivo than conventional chemotherapy. Clinically, heparanase levels in patients’ pleural effusions could distinguish between malignant and benign effusions, and a heparanase H-score above 90 was associated with reduced patient survival (hazard ratio = 1.89, 95% confidence interval = 1.09 to 3.27, P = .03).
Conclusions
Our results imply that heparanase is clinically relevant in mesothelioma development. Given these preclinical and clinical data, heparanase appears to be an important mediator of mesothelioma, and heparanase inhibitors are worthy of investigation as a new therapeutic modality in mesothelioma clinical trials.
Heparan sulfate (HS) proteoglycans (HSPGs) exert their multiple functional repertoires via several distinct mechanisms that combine structural, biochemical, and regulatory aspects. Through interaction with other macromolecules such as laminin, fibronectin, and collagen, HSPGs dictate the structure, self-assembly, and insolubility of the extracellular matrix (ECM) and basement membrane (1–3). Mammalian cells express a single dominant functional heparanase, an endoglucuronidase that cleaves the HS side chains of HSPG into fragments of 10 to 20 sugar units (4). Cleavage of HS by heparanase leads to disassembly of the ECM, thereby promoting cell dissemination associated with tumor metastasis, angiogenesis, and inflammation (5,6). Heparanase is upregulated in essentially all human tumors examined (5–8). Notably, cancer patients exhibiting high levels of heparanase have a statistically significantly shorter postoperative survival time than patients whose tumors exhibit low levels of heparanase (5,6). A causal role of heparanase in tumor metastasis was demonstrated by the increased lung, liver, and bone colonization of cancer cells following overexpression of heparanase (6) and by a marked decrease in the metastatic potential of cells subjected to heparanase gene silencing (9). Recent studies provide compelling evidence that ties heparanase levels with all steps of tumor formation including tumor initiation, growth, metastasis, and chemoresistance (10–15). These and other results indicate that heparanase is causally involved in cancer progression and hence is a valid target for anticancer drug development. This notion is reinforced by preclinical studies revealing a marked inhibition of tumor growth in mice treated with heparanase inhibitors, now in phase I/Ib clinical trials in cancer patients (16–18). In addition, heparanase appears to facilitate crosstalk between tumors and host cells that control gene expression, ECM degradation, and growth factor/cytokine bioavailability (6,13,19,20).
These aspects are to a large extent relevant to malignant pleural mesothelioma, a highly aggressive tumor characterized by rapid and diffused local growth in the thoracic cavity. The etiology of the disease involves a long latency period that is extended by durable asbestos fibers, the tumor microenvironment, and inflammatory stimuli (21,22). Novel treatments are urgently needed, as current treatment modalities may improve quality of life, but exert modest effects on the overall survival of mesothelioma patients (23,24). The principal hypothesis guiding this research is that heparanase drives mesothelioma aggressiveness, and the goal of the study was to elucidate the biological significance of heparanase as a therapeutic target in mesothelioma.
Methods
Tissues and Clinical Database
Tumor and normal tissue specimens were obtained from the Department of Cardiothoracic Surgery, New York University, Langone Medical Center. All patients signed institutional review board (IRB)–approved informed consent for tissue, blood, and effusion procurement (NYU Lung Cancer Biomarker Center, study number i8896). Surgical specimens (tumor and normal) as well as blood were obtained from patients undergoing extrapleural pneumonectomy or pleurectomy; they were aliquoted, snap-frozen, and stored at –80°C. Tissues and blood from patients without mesothelioma were also collected and similarly processed. Samples were embedded in optimal cutting temperature medium (OCT) for histologic sectioning to estimate tumor cell content of the snap-frozen sample and to provide sections for immunohistochemistry. Slides stained with hematoxylin and eosin were generated from OCT blocks of mesothelioma tissues and reviewed by a pathologist to identify tumor samples with greater than 50% tumor cells among all nucleated cells on the slide. Eighty-one such tumor samples were identified, most with adjacent control normal tissues (lung, pleura, or skeletal muscle) (Table 1). De-identified relevant clinical data were collected and linked to both the specimens and the patient. Synchronous pleural effusion and plasma samples from patients with mesothelioma (n = 35), other malignancies (n = 23) presenting with pleural effusion (12 non–small cell lung cancer, two small cell lung carcinoma, four breast cancer, three gastrointestinal cancers, two lymphomas), and from five patients with benign pleural effusions were collected per National Cancer Institute (NCI) Early Detection Research Network standard operating procedures (https://edrn.nci.nih.gov/resources/standard-operating-procedures) (Table 2). A validation cohort was similarly collected and is described in Table 2. Tissue and blood collection, as well as all analyses, were performed in accordance with and with the approval of the institution’s research review board (IRB protocol 8896).
Table 1.
Demographic and clinical description of 81 mesothelioma patients stained for heparanase
| Characteristic | No. (%) |
|---|---|
| Sex | |
| Male | 60 (74.1) |
| Female | 21 (25.9) |
| Age, mean±SD (range) y | 61.1±11.8 (25–84) |
| Race | |
| White | 76 (93.8) |
| Other | 5 (6.2) |
| Smoking status | |
| Current and former | 46 (56.7) |
| Never | 35 (43.3) |
| Survival status (n = 73) | |
| Alive | 12 (16.4.) |
| Dead | 61 (83.6) |
| Histology | |
| Epithelioid | 58 (71.6) |
| Sarcomatoid | 8 (9.8) |
| Mix | 15 (18.6) |
| T stage (n = 74) | |
| T1 | 4 (5.5) |
| T1a | 3 (4.2) |
| T1b | 4 (5.5) |
| T2 | 20 (25.5) |
| T3 | 24 (32.5) |
| T4 | 19 (25.8) |
| N stage (n = 66) | |
| N0 | 43 (65.2) |
| N1 | 7 (10.6) |
| N2 | 16 (24.2) |
| Stage (n = 81) | |
| IA | 9 (11.1) |
| IB | 25 (30.9) |
| II | 7 (8.6) |
| IIIA | 9 (11.1) |
| IIIB | 14 (17.2) |
| IV | 7 (8.6) |
| NA | 10 (12.5) |
| Heparanase (n = 79) | |
| Negative, staining score = 0 | 29 (36.7) |
| Weak, staining score = 1 | 27 (34.1) |
| Strong, staining score = 2 | 23 (29.2) |
Table 2.
Demographic and clinical characteristics of patients included in the discovery and validation sets, analyzed for heparanase levels in effusions and plasma*
| Characteristic | No. MPM | No. benign | No. other malignancy |
|---|---|---|---|
| Discovery set | |||
| Total No. of patients | 35 | 5 | 23 |
| Age, mean±SD (range), y | 64±10 (39–84) | 73±18 (47–94) | 63±12 (34–86) |
| Histology | |||
| Epithelial | 26 | − | NSCLC (n = 12) |
| Biphasic | 9 | − | SCLC (n = 2) |
| Gastrointestinal cancer (n = 3) | |||
| Breast cancer (n = 4) | |||
| Lymphoma (n = 2) | |||
| Stage | All stage IV | ||
| I | 4 | − | |
| II | 11 | − | |
| III | 12 | − | |
| IV | 8 | − | |
| Validation set | |||
| Total No. of patients | 12 | 8 | 30 |
| Age, mean±SD (range) | 67±9 (56–82) | 78±9 (65–90) | 66±13 (43–88) |
| Histology | |||
| Epithelial | 10 | − | NSCLC (n = 17) |
| Sarcomatoid | 1 | − | SCLC (n = 1) |
| Pleomorphic | 1 | − | Breast cancer (n = 5) |
| Sarcoma (n = 3) | |||
| Lymphoma (n = 1) | |||
| Renal (n = 1) | |||
| Ovarian (n = 1) | |||
| Fibrous tumor pleura (n = 1) | |||
| Stage | − | All stage IV | |
| I | 1 | − | |
| II | 2 | − | |
| III | 4 | − | |
| IV | 4 | − |
Only 21/50 pleural effusions had matching plasma. NSCLC = non–small cell lung cancer; SCLC = small cell lung cancer.
Statistical Analysis
Comparison of continuous variables was performed using a two-sided t test, the Welch variant of the t test when the variances were statistically significantly unequal, and the Mann-Whitney nonparametric test when the distribution of the data was not normal. For the comparison of categorical variables, the chi-square or Fisher exact test was used. Receiver operating characteristic (ROC) curves were used to define differences between plasma and pleural effusion heparanase enzyme-linked immunosorbent assay (ELISA) levels comparing mesothelioma patients with those with benign or nonmesothelioma malignant pleural effusions. Survival analysis was carried out using the Kaplan-Meier method; the Wilcoxon test—which is more powerful in detecting differences early in time, rather than the log-rank test, which is more sensitive in detecting differences late in time—was used to compare survival curves. Five-year survival and median survival time with 95% confidence interval (CI) were calculated according to demographic and clinical studied factors. A multivariable stepwise Cox regression analysis was performed to evaluate independent prognostic factors with and without the influence of stage (age at diagnosis, sex, histology, H-score) and their interactions. All tests were two-tailed and the statistical significance limit was set at a P value of .05 or less. All analyses were realized using STATA version 12.0. Data are presented as mean ± SD.
Additional methods are presented in the Supplementary Methods (available online).
Results
Role of Heparanase in Mesothelioma Cell Invasion, Anchorage-Independent Growth, and Tumor Development
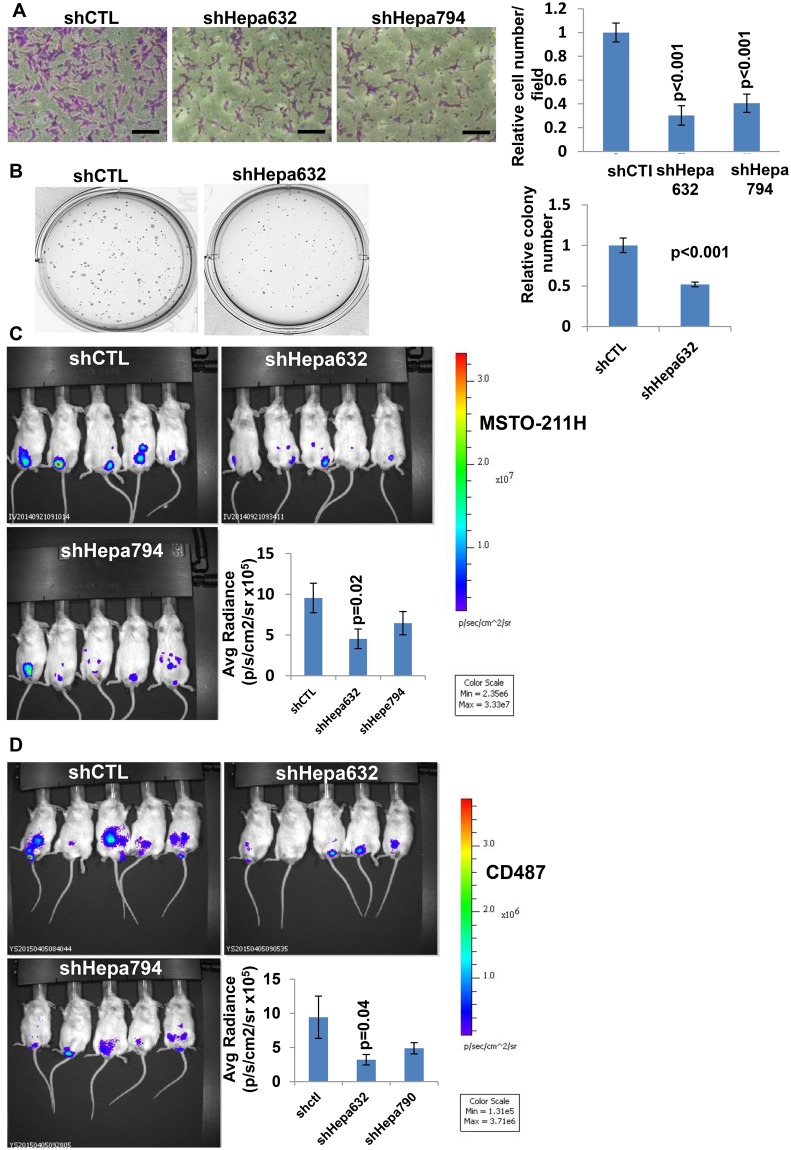
Employing cytology, immunohistochemistry, and polymerase chain reaction analyses, we have previously reported that heparanase is expressed by mesothelioma patients (25,26). Likewise, human (CD484 [not shown], CD487, MSTO-211H, and NCI-H2052) and mouse (AE17, AK7, RN5) pleural mesothelioma cell lines exhibit typical high levels of heparanase activity (Supplementary Figure 1A, available online). To reveal the biological significance of heparanase in mesothelioma, we first utilized heparanase gene silencing methodology and found that heparanase downregulation in MSTO-211H and CD487 cells (Supplementary Figure 1B, available online) results in noticeable inhibition of heparanase activity (Supplementary Figure 1C, available online) and consequently cell invasion (the hallmark of heparanase function) (Figure 1A;Supplementary Figure 2A, available online), as well as modestly attenuated cell proliferation (Supplementary Figure 2B, available online). Moreover, heparanase gene silencing in MSTO-211H cells was associated with reduced number (P < .001) and size of colonies in soft agar (Figure 1B). Most importantly, heparanase silencing in luciferase-labeled MSTO-211H and CD487 mesothelioma cells was associated with a two- to threefold decrease in tumor burden, as evidenced by the in vivo imaging system (IVIS) method (P = .02 and 0.04, respectively) (Figure 1, C and D).
Figure 1.
Gene silencing approach. A) Heparanase gene silencing. shRNA-control (shCTL) and heparanase-silenced (shHepa632/794) MSTO-211H human mesothelioma cells (1 × 105) were plated onto Matrigel-coated 8 μm transwell filters. Invading cells adhering to the lower side of the membrane were visualized (left panels) and counted in 10 random fields (right panel) after 16 hours (mean ±SD; P < .001; two-sided analysis of variance and the Student t test were used for statistical analysis). Scale bars represent 50 µm. B) shCTL and shHepa632 MSTO-211H cells were seeded (2 × 103/35mm dish) in soft agar and grown for two weeks. Shown are representative plates; colony number of shHepa vs control shRNA was quantified in six random fields and is shown graphically in the right panel (mean ±SD; P < .001; two-sided analysis of variance and the Student t test were used for statistical analysis). C) Luciferase-labeled shHepa632/794 and shCTL MSTO-211H cells (2 × 106) were inoculated i.p. into NOD/SCID mice (n = 6/group), and tumor development was inspected by IVIS following administration of luciferin. Quantification of luciferase intensities is shown graphically in the lower right panel. (mean ±SD; P = .02; two-sided analysis of variance and the Student t test were used for statistical analysis). Tumor burden following i.p. administration of control and heparanase gene-silenced CD487 cells in NOD/SCID mice (n = 6) is shown in (D) (mean ±SD; P = .04; two-sided analysis of variance and the Student t test, were used for statistical analysis). Representative images of mice are shown in C and D.
Role of Host Heparanase in Mesothelioma Tumor Development
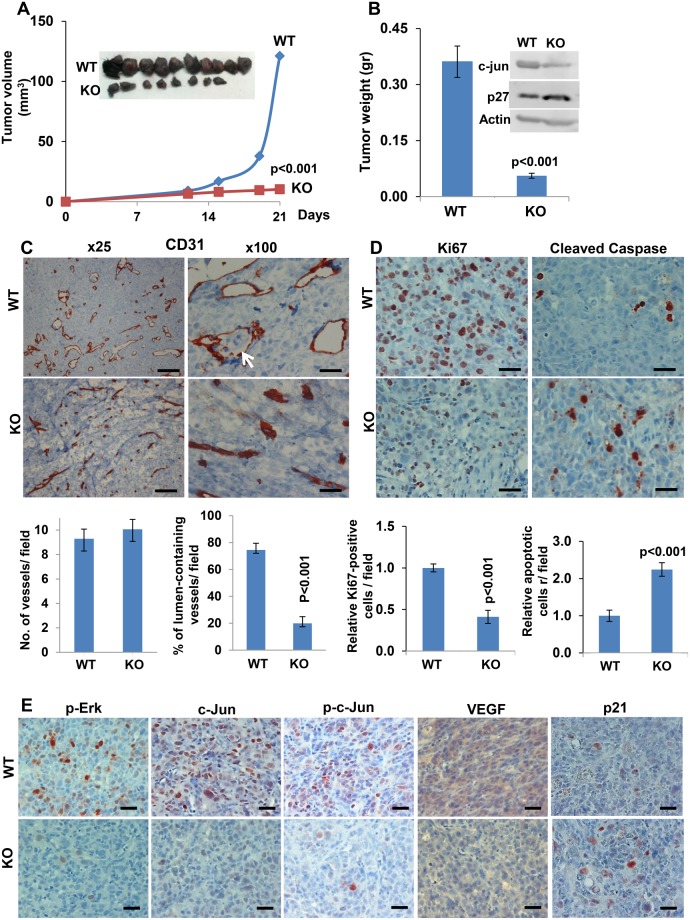
Recent evidence implies that heparanase derived from the tumor microenvironment is also critically important for tumor growth (27). To study this, we implanted mouse AE17 mesothelioma cells into syngeneic wild-type (wt) and heparanase knockout (Hpa-KO) C57BL mice (28). AE17 cells developed smaller tumors (volume and weight) when inoculated into Hpa-KO vs control wt mice (both P < .001) (Figure 2, A and B), associated with impaired angiogenesis evidenced by collapsed blood vessels (P < .001) (Figure 2C, lower panels) along with a decrease in VEGF expression (Figure 2E). Moreover, tumor cells were detected in the vessel lumens of tumors developing in wt but not in Hpa-KO mice (Figure 2C, arrow). Immunostaining further revealed lower proliferation (ie, Ki67; P < .001) and higher apoptosis (ie, cleaved caspase 3; P < .001) rates in tumors that developed in Hpa-KO vs control wt mice (Figure 2D), along with reduced Erk and c-Jun phosphorylation (Figure 2E) and expression (Figure 2B, inset; Figure 2E). Notably, expression of p21 (Figure 2E) and p27 was increased in tumors developed in Hpa-KO mice (Figure 2B, inset), and a similar increase in p21 and p27 expression was observed in cells treated with the heparanase inhibitor PG545 (Supplementary Figure 2C, available online). Decreased tumor growth was seen also in mouse RN5 mesothelioma cells inoculated in Hpa-KO vs wt mice (P = .01) (Supplementary Figure 3A, available online).
Figure 2.
A role for host heparanase in mesothelioma tumor growth. AE17 mouse mesothelioma cells were injected subcutaneously (2 × 106) into heparanase knockout (Hepa-KO; n = 8) vs wild-type (wt) C57BL/6J mice (n = 10). Tumor volume was calculated from external caliper measurements (A). At the end of the experiment on day 21, tumors were resected, photographed (inset), and weighed (B) (mean ±SD; P < .001; two-sided analysis of variance and the Mann-Whitney U test were used for statistical analysis); tumor extracts were prepared and subjected to immunoblotting applying anti-c-Jun, anti-p27, and anti-actin antibodies (B) (inset). Five-micron sections of corresponding tumors were subjected to immunostaining, applying anti-CD31 antibody (C) shown in low (left) and high (right) magnifications. Scale bars represent 200 µm (left) and 50 µm (right). Quantification of blood vessel density (vessels per field in 12 random fields) and the percentage of vessels exhibiting a patent lumen (in 15 random fields) are shown graphically in the lower panels (mean ±SD; P < .001; two-sided analysis of variance and the Mann-Whitney U test were used for statistical analysis). Arrow indicates tumor cells within a blood vessel. Tumor sections were similarly stained with anti-Ki67 (D) (Ki67, left panels) and anti-cleaved caspase 3 (D) (cleaved caspase 3, right panels) antibodies. Quantification of cell proliferation and apoptosis (counted in 10 random fields) are shown graphically in the lower panels (mean ±SD; P < .001; two-sided analysis of variance and the Student t test were used for statistical analysis). Scale bars represent 50 µm. E) Tumor sections were similarly stained with anti phospho-ERK (p-ERK, left panels), anti-c-Jun (second left), anti phospho-c-Jun (p-c-Jun, middle panels), anti-VEGF (VEGF, second right), and anti-p21 (right panels) antibodies. Scale bars represent 50 µm. KO = knockout; WT = wild-type.
Effect of Heparanase Inhibitors on Mesothelioma Xenografts
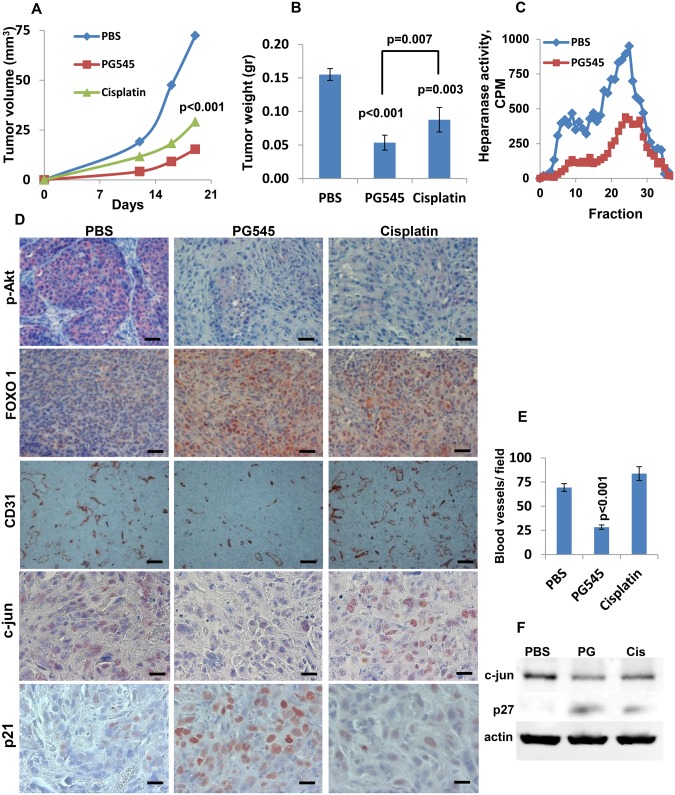
The heparanase inhibitor PG545 (29), a highly sulfated tetrasaccharide, efficiently inhibited heparanase enzymatic activity in mesothelioma cells (Supplementary Figure 3B, available online) and caused a marked decrease in mesothelioma cell invasion (Supplementary Figures 3C and 4A, available online) along with smaller colonies in soft agar (Supplementary Figure 3D, available online). These data were in agreement with the heparanase gene silencing results (Figure 1; Supplementary Figure 2A, available online). Unlike cisplatin, PG545 does appear to be cytotoxic to mesothelioma cells (Supplementary Figure 4B, available online). We next inoculated MSTO-211H cells subcutaneously in NOD/SCID mice, which were then treated with PG545, cisplatin, or control vehicle (PBS). Tumor growth was attenuated by cisplatin, a common chemotherapeutic in mesothelioma, but an even greater threefold inhibition of tumor growth was obtained in mice treated with PG545 (P < .001) (Figure 3, A and B), combined with a marked inhibition of heparanase enzymatic activity within the tumors (Figure 3C). Attenuation of tumor growth by cisplatin or PG545 was accompanied by a prominent decrease in Akt phosphorylation (Figure 3D), an effect also noted in vitro (Supplementary Figure 5A, available online). Reduced Akt phosphorylation by cisplatin and PG545 was accompanied by increased levels of FOXO1 (Figure 3D), as expected (30,31). Notably, tumor angiogenesis and c-Jun expression were inhibited substantially by PG545, but not by cisplatin (Figure 3, D–F). Likewise, expression of p21 and p27 (Figure 3D and F) was induced more prominently by PG545.
Figure 3.
Utilization of a heparanase inhibitor, PG545. A) Tumor growth. MSTO-211H human mesothelioma cells were inoculated subcutaneously (2 × 106) in NOD/SCID mice (n = 7). Mice were treated with PG545 (400 μg/mouse; once a week), cisplatin (3 mg/kg, once every two weeks), or control vehicle (PBS). Tumor volume was calculated from external caliper measurements (A). At the end of the experiment on day 19, tumors were resected and weighed (B) (mean ±SD; P ≤ .007; two-sided analysis of variance and the Mann-Whitney U test were used for statistical analysis), and heparanase enzymatic activity was evaluated in tumor extracts as described in the Methods section (C). D) Immunostaining. Subcutaneous tumors were subjected to immunostaining, applying anti-phospho-Akt (p-Akt, upper panels), anti-FOXO1 (FOXO1, second panels), anti-CD31 (CD31, third panels), anti-c-Jun (fourth panels), and anti-p21 (lower panels) antibodies. Scale bars represent 100 µm (upper and second panels), 200 µm (third panels), and 50 µm (lower panels). Quantification of blood vessel density in 14 random fields is shown graphically in (E) (mean ±SD; P < .001; two-sided analysis of variance and the Student t test were used for statistical analysis). F) Immunoblotting. Extracts of control (PBS), PG545, and cisplatin-treated tumors were subjected to immunoblotting, applying anti-c-Jun (upper panel), anti-p27 (middle panel), and anti-actin (lower panel) antibodies.
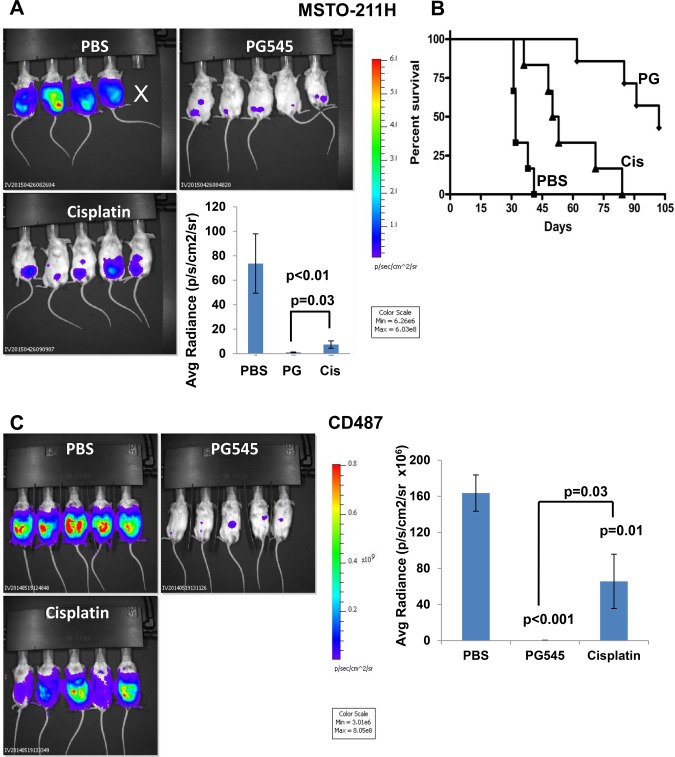
We next employed an intraperitoneal (i.p.) model to further investigate the effect of PG545 and cisplatin on mesothelioma growth. To this end, luciferase-labeled human mesothelioma MSTO-211H cells were inoculated (i.p.) in NOD/SCID mice, and tumor growth was visualized using the IVIS method. Mesothelioma growth in the peritoneum was attenuated markedly by PG545 (P = .006), more so than cisplatin (P = .03 for PG545 vs cisplatin) (Figure 4A). The more potent inhibitory capacity of PG545 was also evident in survival experiments. Strikingly, the median survival of untreated control mice was 32±4.3 days compared with 102±16.9 and 51±17.4 days for PG545 and cisplatin-treated mice, respectively (P < .001, .003, and .001 for PG545 vs PBS, cisplatin vs PBS and PG545 vs cisplatin, respectively) (Figure 4B). Similar superior inhibition of tumor growth (i.p.) by PG545 over cisplatin was observed in luciferase-labeled CD487 mesothelioma cells (P = .03 for PG545 vs cisplatin) (Figure 4C). PG545 was also effective in the inhibition of luciferase-labeled NCI-H2052 (P = .01) (Supplementary Figure 5B, available online).
Figure 4.
Effect of PG545 on orthotopic mesothelioma model. A) Tumor growth. Luciferase-labeled MSTO-211H human mesothelioma cells (2 × 106) were inoculated i.p. into NOD/SCID mice (n = 6/treatment). Mice were treated with PG545 (400 μg/mouse; once a week), cisplatin (once every two weeks; 3 mg/kg), or control vehicle (PBS), and tumor development was inspected by IVIS. Quantification of the luciferase intensities is shown graphically in the lower right panel (mean ±SD; P = .006 for PG545 vs PBS; P = .008 for cisplatin vs PBS, and P = .03 for PG545 vs cisplatin; two-sided analysis of variance and the Mann-Whitney U test were used for statistical analysis). B) Survival. The effect of PG545 and cisplatin on the survival of mice (n = 7) is plotted as Kaplan-Meier curves (P < .001, .003, and .001 for PG545 vs PBS, cisplatin vs PBS, and PG545 vs cisplatin, respectively). The number at risk for the PBS, PG545 and Cisplatin treated mice were: 6, 7 & 6 at baseline; 6, 7 & 5 up to day 41; 0, 7 & 2 from day 42 to 53; and 0, 3 & 0 beyond day 54, respectively. C) Luciferase-labeled CD487 human mesothelioma cells (2 × 106) were inoculated i.p. into 18 NOD/SCID mice. Groups of six mice were treated with PG545 (400 µg/mouse; once a week), cisplatin (3 mg/kg; once every two weeks), or control vehicle (PBS), and tumor development was inspected by IVIS (representative images are shown). Quantification of the luciferase intensities is shown graphically in the right panel (mean ±SD; P ≤ .03; two-sided analysis of variance and the Mann-Whitney U test were used for statistical analysis).
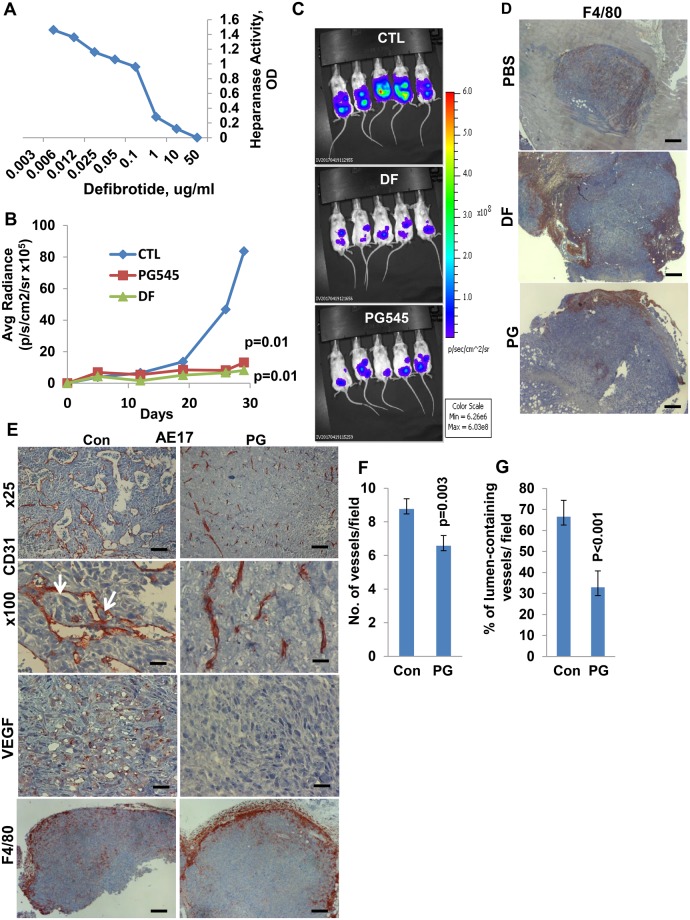
To substantiate these results, we applied another heparanase inhibitor, defibrotide. This compound is a polydisperse oligonucleotide approved for the treatment of severe hepatic veno-occlusive disease (32,33). Applying a colorimetric assay, we first confirmed that defibrotide effectively inhibited heparanase activity (Figure 5A). Next, luciferase-labeled human mesothelioma MSTO-211H cells were inoculated into NOD/SCID mice, and tumor growth was visualized by IVIS following treatment with PG545 or defibrotide. A profound inhibition of tumor growth was exerted by defibrotide (P = .01) (Figure 5, B and C), indistinguishable from PG545, further pointing to a potential critical role of heparanase in mesothelioma pathogenesis.
Figure 5.
Utilization of a heparanase inhibitor, defibrotide. A) Effect of defibrotide in an orthotopic mesothelioma model. Active heparanase (200 ng) was incubated with the indicated concentration of defibrotide, and heparanase activity (OD, colorimetric assay) was examined after 18 hours, as described in the Methods section. B and C) Luciferase-labeled MSTO-211H human mesothelioma cells (2 × 106) were inoculated i.p. into 18 NOD/SCID mice. Groups of six mice were treated with defibrotide (8 mg/mouse; twice a day), PG545 (400 μg/mouse; once a week), or control vehicle (PBS), and tumor development was inspected by IVIS over time (B) (P = .01; two-sided analysis of variance and the Mann-Whitney U test were used for statistical analysis). Representative tumor images at termination are shown in (C). D) Tumors were then collected, and 5-micron sections of formalin-fixed, paraffin-embedded tumor samples were subjected to immunostaining, applying anti-F4/80 antibody (a common marker for mouse macrophages). Scale bars represent 500 µm. E) Mouse AE17 cells. AE17 mouse mesothelioma cells were inoculated (2 × 106) subcutaneously, and mice (n = 6) were treated with PG545 (20 mg/kg, once a week) or PBS (Con) once tumors became palpable. At termination, when tumor growth became evident, tumors were resected, and 5-micron sections were subjected to immunostaining, applying anti-CD31 (upper two panels), anti-VEGF (third panels), and anti-F4/80 (lower panels) antibodies. Scale bars represent 200 µm (upper panels), 50 µm (second and third panels), and 500 µm (lower panels). Quantification of blood vessels per field and the percentage of vessels that show a patent lumen counted in at least 12 high power fields are shown graphically in (F) (mean ±SD) and (G) (mean ±SD), respectively (P ≤ .003; two-sided analysis of variance and the Student t test were used for statistical analysis).
We further examined the heparanase inhibitor PG545 in mouse AE17 mesothelioma cells implanted in syngeneic C57BL/6J mice. Tumor growth was inhibited more than twofold by PG545 (P = .003) (Supplementary Figure 5C, available online), and comparable inhibition of tumor growth by PG545 was noted in mouse mesothelioma AK7 (P = .006) (Supplementary Figure 5D, available online) and RN5 (P < .001) (Supplementary Figure 5E, available online) cells. Notably, PG545 treatment of AE17 cells resulted in impaired vascular density (Figure 5F; P = .003) and a phenotype (Figure 5, E and G) that resembled the collapsed vessels noted in tumors developed in heparanase-KO mice (Figure 2C). Similar vascular phenotypes were noted in AK7 tumors treated with PG545 (Supplementary Figure 6A, available online). Moreover, tumor cells were detected in vessels of control tumors but not following PG545 treatment (Figure 5E, arrows), as was noted in tumors developed in wt vs heparanase-KO mice (Figure 2C). We also analyzed tumor-associated macrophage M1/M2 phenotype, and no differences were observed between control and PG545-treated mice (Supplementary Figure 6B, available online). It was noted, however, that although macrophages populated the entire tumor mass in control mice (Figure 5E), they were noted to be arrested at the tumor periphery following PG545 treatment (Figure 5E). A similar phenotype was found in tumors developed by MSTO-211H cells and treated with PG545 or defibrotide (Figure 5D). In addition, VEGF staining was reduced substantially in tumors treated with PG545, possibly accounting for reduced blood vessel density (Figure 5, E and F).
Quantitating Heparanase Levels in the Plasma And Effusions of Mesothelioma Patients
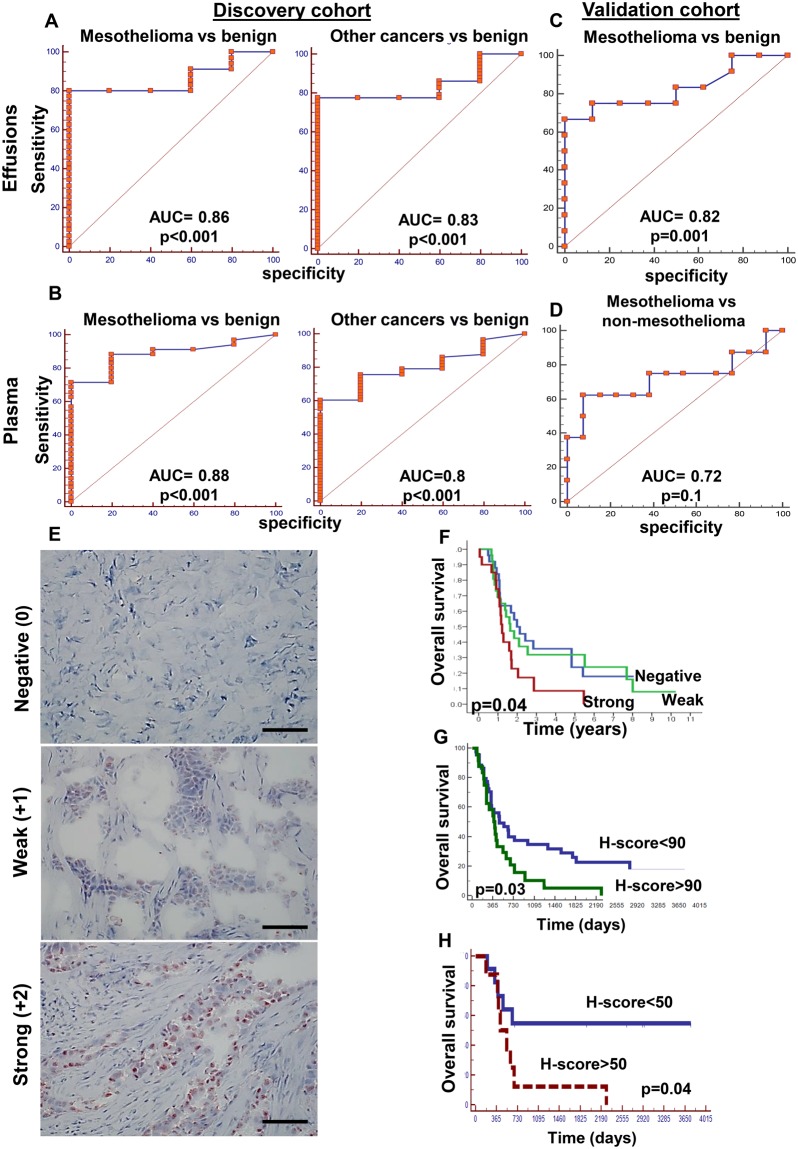
To examine the clinical significance of heparanase in mesothelioma, 63 patients with matched effusion and plasma samples from mesothelioma patients, nonmesothelioma cancer patients, and patients with benign effusions (Table 2) were evaluated by ELISA for their heparanase levels. We found a positive correlation between heparanase levels in the plasma and effusion samples (Supplementary Figure 7A, available online). The ELISA results were further confirmed by heparanase activity in cells collected from the effusions (Supplementary Figure 7B, available online). Thus, high heparanase activity was evident in cells collected from effusions exhibiting high levels of heparanase, whereas no activity was detected in cells collected from effusions with low levels of heparanase (Supplementary Figure 7B, available online). Importantly, heparanase levels in both effusions and plasma (Figure 6, A and B) could discriminate the sample as malignant or benign with high confidence (P < .001), implying that heparanase levels may serve as a marker for malignant effusions. This was further confirmed in a validation cohort of samples collected independently (Figure 6, C and D). Demographic and clinical description of the discovery and validation cohorts is summarized in Table 2.
Figure 6.
Heparanase levels in pleural effusions and plasma. A and B) Discovery cohort (n = 63). Shown are receiver operator characteristic (ROC) curves with calculated areas under the curve (AUC) for heparanase protein levels in pleural effusions (A) and plasma samples (B) from mesothelioma (left panels) and other cancers (right panels) vs patients with benign pleural effusions. AUCs are 0.88 and 0.80 (P < .001) for the plasma samples, and 0.86 and 0.83 (P < .001) for the pleural effusions, respectively. C and D) Validation cohort (n = 50). Shown are ROC curves for heparanase protein levels in pleural effusions from mesothelioma patients vs benign (C) (AUC = 0.82, P = .001) and vs nonmesothelioma cancer (D) (AUC = 0.72, P = .1 of the validation patient cohort). E) Immunostaining. Mesothelioma sections were subjected to immunostaining, applying antiheparanase antibody. Shown are representative images of cases exhibiting no (negative, upper panel) weak (+1, middle), or strong (+2, lower panel) staining intensity. Scale bars represent 100 µm. F) Kaplan-Meier survival analysis of patients according to their heparanase staining intensities. Mesothelioma patients endowed with strong heparanase staining survive less than patients who are found negative for heparanase (P = .04 for strong vs negative). G and H) Patient survival according to heparanase H-score. Heparanase H-score (ie, combining the staining intensity and staining extent) was calculated for 68 mesothelioma patients, and their survival rate (P = .03, Kaplan-Meier) was compared among patients having H-score lower vs higher than 90 (G). A similar analysis was performed for a subgroup of 22 epithelioid patients who did not receive chemotherapy prior to biopsy collection (H) (P = .04). All P values for the ROC areas under the curve were two-sided; Kaplan-Meier curves were used for survival analyses with two-sided P values). AUC = area under the curve.
We next subjected 81 mesothelioma biopsies to immunostaining, applying antiheparanase antibody. Two patients had inadequate tumor tissue for staining. Demographic and clinical characterization of the patients enrolled in this study is summarized in Table 1. The median survival of all the patients was 18 months (Supplementary Figure 7C, available online), very similar to many surgical series as well as previous data (34). Furthermore, we found that patients diagnosed with epithelioid vs other (biphasic/sarcomatoid) histology survived longer (P = .05) (Supplementary Figure 7D, available online), as expected (34). Twenty-nine (36.7%) biopsies were stained negative for heparanase whereas 50 biopsies were positive, exhibiting weak or strong staining (Figure 6E). Notably, patients showing strong staining of heparanase exhibited reduced survival time vs heparanase-negative patients (P = .04) (Figure 6F).
Subsequently, the staining intensity of heparanase was combined with the staining extent (ie, percentage of heparanase-positive tumor cells) to obtain a more accurate H-score in 68 patients (59 epithelial, nine nonepithelial). Strikingly, we found that patients exhibiting heparanase H-scores greater than 90 had reduced survival (P = .03) (Figure 6G). In a group of epithelioid patients who did not receive chemotherapy before being biopsied, the ROC characteristics, as well as the survival data, became even more robust. In this subgroup (n = 22), patients exhibiting heparanase H-scores lower than 50 had a statistically significantly better survival rate (P = .04) (Figure 6H), and 42.2% of the patients survived longer than five years. When multivariable modeling was applied to these 68 patients, together with age, sex, histology, and H-score cut off of 90, H-score (hazard ratio [HR] = 1.89, 95% confidence interval [CI] = 1.09 to 3.27, P < .02 ) and histology (HR = 1.84, CI = 1.02 to 3.32, P = .044) remained independent predictors of death with age and sex excluded from the model. Additionally, when the Eighth International Staging System data were added to the variables, stage (HR = 2.26, 95% CI = 1.65 to 3.08, P < .001), and H-score remained (HR = 1.84, 95% CI = 1.06 to 3.21, P = .031), along with sex (HR = 2.27, 95% CI = 1.14 to 4.49, P = .02) and histology (HR = 2.34, 95% CI = 1.25 to 4.37, P = .008), independent predictors of survival (data not shown). These results strongly suggest that heparanase plays a decisive role in mesothelioma, lending support to the study of PG545 and defibrotide in this disease.
Discussion
These investigations provide compelling evidence that ties heparanase with mesothelioma. Clinically, patients with high heparanase immunostaining or H-score had shorter survival than patients with low levels of heparanase, and an even higher survival rate was noted in a subgroup of patients for whom heparanase H-score was calculated before chemotherapy. This may suggest that chemotherapy induces heparanase expression, as noted in multiple myeloma (35), thus urging the addition of heparanase inhibitors to conventional chemotherapy. In addition, we found that heparanase levels (ELISA) can provide valuable information and categorize the plasma/effusion sample as malignant or benign with high confidence, thus implying its diagnostic potential. The clinical results are supported by the ability of heparanase inhibitors to prominently restrain the growth of mesothelioma tumor xenografts implanted orthotopically, and in vivo, PG545 appeared more effective than cisplatin in prolonging the survival of mesothelioma-bearing mice. Similar potency was also noted for defibrotide, a drug already approved for veno-occlusive disease (VOD).
When AE17 mouse mesothelioma cells were inoculated into heparanase-KO mice, strikingly smaller tumors developed when compared with wt mice. We hypothesize that this may be due to restriction of supportive crosstalk between the tumor cells and the tumor microenvironment, and this concept is supported by the following considerations. Unlike cisplatin, PG545 does not exert a direct cytotoxic effect on mesothelioma cells, suggesting that inhibition of tumor growth could involve the tumor microenvironment. This is best exemplified by impaired vasculature in tumors implanted in heparanase-KO mice or following treatment with heparanase inhibitors. We consistently found that lack of heparanase (KO mice) or its inhibition results in collapsed tumor vasculature devoid of a typically patent lumen. In addition, treatment with PG545 resulted in reduced blood vessel density, in accordance with the anti-angiogenic potency of this drug (29). Moreover, we found that VEGF staining intensity and expression were decreased in tumors that developed in heparanase-KO mice or following treatment with PG545. This is in agreement with reduced VEGF levels in myeloma tumors treated with the heparanase inhibitor Roneparstat (SST0001) (14) and the involvement of heparanase in VEGF gene regulation (36). These results are important because they echo recent data showing that the addition of anti-VEGF monoclonal antibody (bevacizumab; Avastin) to pemetrexed and cisplatin chemotherapy improved survival, probably by targeting the tumor vasculature (24,37), which plays an important role in the progression of mesothelioma (38). Moreover, tumor cells were often detected in blood vessels of control mice but not in PG545-treated tumor vessels or tumors developed in heparanase-KO mice. This agrees with reduced cellular invasion following heparanase gene silencing or inhibition. Notably, vascular invasion has been found to be associated with decreased overall survival of mesothelioma patients (39).
Another cellular constituent of the tumor microenvironment is the macrophage. Interestingly, although neither the total number of macrophages attracted to tumors nor their classification into M1 or M2 type was affected by PG545, their localization was altered. Accordingly, while macrophages were noted to populate the entire tumor mass in control mice, they appeared to accumulate at the tumor periphery in heparanase-KO mice or upon treatment with PG545 or defibrotide. A similar phenotype was found in Lewis lung carcinoma cells implanted in heparanase-KO mice vs control mice (19), suggesting that heparanase is required for macrophages to penetrate tumors. Given the pro-angiogenic properties of macrophages (40), their elimination from the tumor mass may add another explanation for the observed impaired angiogenesis in heparanase-KO mice or following PG545 treatment.
While the above results focus on the tumor microenvironment, heparanase also affects the tumor cells. For example, the expression of p21 and p27, cyclin-dependent kinase inhibitors that function to attenuate the cell cycle (41,42), was induced by PG545, thus further restricting tumor growth. While induction of p21 and attenuation of Akt phosphorylation by PG545 have been reported (13,43,44), a connection between heparanase and c-Jun expression is shown here for the first time. Notably, c-Jun expression and phosphorylation were decreased in mesothelioma tumors developed in Hpa-KO mice and upon treatment with PG545. This is important, given the critical role of c-Jun in asbestos-mediated carcinogenesis and in driving mesothelioma cell proliferation (45), and further ties heparanase with the AP1 transcription machinery (19). Our results demonstrate a profound effect of defibrotide on mesothelioma already as a single agent, supporting further preclinical and clinical studies on the antimesothelioma efficacy of defibrotide, alone and in combination with other treatments, taking advantage of the fact that this drug was already approved by the US Food and Drug Administration for the treatment of VOD.
Collectively, these results strongly imply that heparanase is clinically relevant in mesothelioma development. Taking into account our in vitro, preclinical, and clinical data, heparanase appears to be an important mediator of mesothelioma tumor progression, encouraging further development and testing in clinical trials of existing (ie, PG545, defibrotide) and newly developed (ie, neutralizing antibodies, small molecules) heparanase inhibitors as a new therapeutic modality for this malignancy.
Given the limited number of patients available to the current study, additional research is required to substantiate the importance and clinical significance of heparanase in the pathogenesis of mesothelioma.
Funding
This work was supported by a research grant awarded to HP and IV by the Laura and Isaac Perlmutter Foundation Inc. It was also supported by an Investigator-Initiated Research (IIR) grant awarded to IV and AN by Jazz Pharmaceuticals UK and by research grants awarded to IV by the Israel Science Foundation (grant 601/14); the United States-Israel Binational Science Foundation (BSF); the Israel Cancer Research Fund (ICRF); and by a grant from the National Institutes of Health (CA211752; awarded to Ralph Sanderson, UAB and IV). I. Vlodavsky is a Research Professor of the ICRF.
Notes
Affiliations of authors: Cancer and Vascular Biology Research Center, Rappaport Faculty of Medicine, Technion, Haifa, Israel (UB, SF, NI, IV); Departments of General Thoracic Surgery (ML) and Pathology (YZ) Rambam Health Care Campus, Haifa, Israel; Department of Cardiothoracic Surgery, Langone Medical Center, New York University School of Medicine, New York, NY (CL, AM, CG, HIP); University of Hawaii Cancer Center, Honolulu, HI (HY); Zucero Therapeutics, Darra, Queensland, Australia (EH); Beijing Hospital of Traditional Chinese Medicine, Beijing, China (GZ); Department of Medical Biochemistry and Microbiology, University of Uppsala, Uppsala, Sweden (JPL); Department of Hematology and Bone Marrow Transplantation, Chaim Sheba Medical Center, Tel-Hashomer, Israel (AN).
The funder had no role in the design of the study; the collection, analysis, or interpretation of the data; the writing of the manuscript; or the decision to submit the manuscript for publication.
Edward Hammond is employed by Zucero Therapeutics, Darra, Queensland, Australia. All other authors have no potential conflict of interest to declare.
We thank Dr. Maurizio D'Incalci and Dr. Eugenio Erba (Mario Negri Institute for Pharmacological Research, Italy) for generously providing the CD484 and CD487 human mesothelioma cell lines and for kindly promoting the initiation of this research. AE17 and RN5 mouse mesothelioma cells were kindly provided by Dr. D. Nelson (Curtin University, Australia), and Dr. B. Schwaller (University of Fribourg, Switzerland), respectively.
Supplementary Material
References
- 1. Bernfield M, Gotte M, Park PW, et al. Functions of cell surface heparan sulfate proteoglycans. Annu Rev Biochem. 1999;68:729–777. [DOI] [PubMed] [Google Scholar]
- 2. Timpl R, Brown JC.. Supramolecular assembly of basement membranes. Bioessays. 1996;182:123–132. [DOI] [PubMed] [Google Scholar]
- 3. Udo Hacker KNaNP. Heparan sulphate proteoglycans: The sweet side of development. Nat Rev Mol Cell Biol. 2005;6:530–541. [DOI] [PubMed] [Google Scholar]
- 4. Vlodavsky I, Friedmann Y, Elkin M, et al. Mammalian heparanase: Gene cloning, expression and function in tumor progression and metastasis. Nat Med. 1999;57:793–802. [DOI] [PubMed] [Google Scholar]
- 5. Barash U, Cohen-Kaplan V, Dowek I, et al. Proteoglycans in health and disease: New concepts for heparanase function in tumor progression and metastasis. Febs J. 2010;27719:3890–3903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Vlodavsky I, Beckhove P, Lerner I, et al. Significance of heparanase in cancer and inflammation. Cancer Microenviron. 2012;52:115–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ilan N, Elkin M, Vlodavsky I.. Regulation, function and clinical significance of heparanase in cancer metastasis and angiogenesis. Int J Biochem Cell Biol. 2006;3812:2018–2039. [DOI] [PubMed] [Google Scholar]
- 8. Vreys V, David G.. Mammalian heparanase: What is the message? J Cell Mol Med. 2007;113:427–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Edovitsky E, Elkin M, Zcharia E, et al. Heparanase gene silencing, tumor invasiveness, angiogenesis, and metastasis. J Natl Cancer Inst. 2004;9616:1219–1230. [DOI] [PubMed] [Google Scholar]
- 10. Arvatz G, Shafat I, Levy-Adam F, et al. The heparanase system and tumor metastasis: Is heparanase the seed and soil? Cancer Metastasis Rev. 2011;302:253–268. [DOI] [PubMed] [Google Scholar]
- 11. Barash U, Zohar Y, Wildbaum G, et al. Heparanase enhances myeloma progression via CXCL10 downregulation. Leukemia. 2014;2811:2178–2187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Boyango I, Barash U, Fux L, et al. Targeting heparanase to the mammary epithelium enhances mammary gland development and promotes tumor growth and metastasis. Matrix Biol. 2018;65:91–103. [DOI] [PubMed] [Google Scholar]
- 13. Boyango I, Barash U, Naroditsky I, et al. Heparanase cooperates with Ras to drive breast and skin tumorigenesis. Cancer Res. 2014;74:4504–4514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ramani VC, Zhan F, He J, et al. Targeting heparanase overcomes chemoresistance and diminishes relapse in myeloma. Oncotarget. 2016;7:1598–1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Shteingauz A, Boyango I, Naroditsky I, et al. Heparanase enhances tumor growth and chemoresistance by promoting autophagy. Cancer Res. 2015;7518:3946–3957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ferro V, Hammond E, Fairweather JK.. The development of inhibitors of heparanase, a key enzyme involved in tumour metastasis, angiogenesis and inflammation. Mini Rev Med Chem. 2004;46:693–702. [DOI] [PubMed] [Google Scholar]
- 17. Ritchie JP, Ramani VC, Ren Y, et al. SST0001, a chemically modified heparin, inhibits myeloma growth and angiogenesis via disruption of the heparanase/syndecan-1 axis. Clin Cancer Res. 2011;176:1382–1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Vlodavsky I, Ilan N, Naggi A, et al. Heparanase: Structure, biological functions, and inhibition by heparin-derived mimetics of heparan sulfate. Curr Pharm Des. 2007;1320:2057–2073. [DOI] [PubMed] [Google Scholar]
- 19. Gutter-Kapon L, Alishekevitz D, Shaked Y, et al. Heparanase is required for activation and function of macrophages. Proc Natl Acad Sci U S A. 2016;11348:E7808–E7817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lerner I, Hermano E, Zcharia E, et al. Heparanase powers a chronic inflammatory circuit that promotes colitis-associated tumorigenesis in mice. J Clin Invest. 2011;1215:1709–1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Carbone M, Ly BH, Dodson RF, et al. Malignant mesothelioma: Facts, myths, and hypotheses. J Cell Physiol. 2012;2271:44–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sekido Y. Molecular pathogenesis of malignant mesothelioma. Carcinogenesis. 2013;347:1413–1419. [DOI] [PubMed] [Google Scholar]
- 23. Bononi A, Napolitano A, Pass HI, et al. Latest developments in our understanding of the pathogenesis of mesothelioma and the design of targeted therapies. Exp Rev Respir Med. 2015;95:633–654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Yap TA, Aerts JG, Popat S, et al. Novel insights into mesothelioma biology and implications for therapy. Nat Rev Cancer. 2017;178:475–488. [DOI] [PubMed] [Google Scholar]
- 25. Davidson B, Vintman L, Zcharia E, et al. Heparanase and basic fibroblast growth factor are co-expressed in malignant mesothelioma. Clin Exp Metast. 2004;215:469–476. [DOI] [PubMed] [Google Scholar]
- 26. Doviner V, Maly B, Reinhartz T, et al. Heparanase expression: A potential ancillary diagnostic tool for distinguishing between malignant cells and reactive mesothelium in body cavity effusions. Cytopathology. 2007;181:13–19. [DOI] [PubMed] [Google Scholar]
- 27. Weissmann M, Arvatz G, Horowitz N, et al. Heparanase-neutralizing antibodies attenuate lymphoma tumor growth and metastasis. Proc Natl Acad Sci U S A. 2016;1133:704–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Zcharia E, Jia J, Zhang X, et al. Newly generated heparanase knock-out mice unravel co-regulation of heparanase and matrix metalloproteinases. PLoS One. 2009;44:e5181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Dredge K, Hammond E, Handley P, et al. PG545, a dual heparanase and angiogenesis inhibitor, induces potent anti-tumour and anti-metastatic efficacy in preclinical models. Br J Cancer. 2011;1044:635–642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Aoki M, Jiang H, Vogt PK.. Proteasomal degradation of the FoxO1 transcriptional regulator in cells transformed by the P3k and Akt oncoproteins. Proc Natl Acad Sci U S A. 2004;10137:13613–13617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Arvatz G, Barash U, Nativ O, et al. Post-transcriptional regulation of heparanase gene expression by a 3' AU-rich element. Faseb J. 2011;2412:4969–4976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Richardson P, Linden E, Revta C, et al. Use of defibrotide in the treatment and prevention of veno-occlusive disease. Exp Rev Hematol. 2009;24:365–376. [DOI] [PubMed] [Google Scholar]
- 33. Dalle JH, Giralt SA.. Hepatic veno-occlusive disease after hematopoietic stem cell transplantation: Risk factors and stratification, prophylaxis, and treatment. Biol Blood Marrow Tranplant. 2016;223:400–409. [DOI] [PubMed] [Google Scholar]
- 34. Rusch VW, Chansky K, Kindler HL, et al. The IASLC Mesothelioma Staging Project: Proposals for the M descriptors and for revision of the TNM stage groupings in the forthcoming (eighth) edition of the TNM classification for mesothelioma. J Thorac Oncol. 2016;1112:2112–2119. [DOI] [PubMed] [Google Scholar]
- 35. Ramani VC, Vlodavsky I, Ng M, et al. Chemotherapy induces expression and release of heparanase leading to changes associated with an aggressive tumor phenotype. Matrix Biol. 2016;55:22–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Zetser A, Bashenko Y, Edovitsky E, et al. Heparanase induces vascular endothelial growth factor expression: Correlation with p38 phosphorylation levels and Src activation. Cancer Res. 2006;663:1455–1463. [DOI] [PubMed] [Google Scholar]
- 37. Zalcman G, Mazieres J, Margery J, et al. Bevacizumab for newly diagnosed pleural mesothelioma in the Mesothelioma Avastin Cisplatin Pemetrexed Study (MAPS): A randomised, controlled, open-label, phase 3 trial. Lancet. 2016;38710026:1405–1414. [DOI] [PubMed] [Google Scholar]
- 38. Ohta Y, Shridhar V, Bright RK, et al. VEGF and VEGF type C play an important role in angiogenesis and lymphangiogenesis in human malignant mesothelioma tumours. Br J Cancer. 1999;811:54–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ujiie H, Kadota K, Nitadori JI, et al. The tumoral and stromal immune microenvironment in malignant pleural mesothelioma: A comprehensive analysis reveals prognostic immune markers. Oncoimmunology. 2015;46:e1009285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Lamagna C, Aurrand-Lions M, Imhof BA.. Dual role of macrophages in tumor growth and angiogenesis. J Leukoc Biol. 2006;804:705–713. [DOI] [PubMed] [Google Scholar]
- 41. Abbas T, Dutta A.. p21 in cancer: Intricate networks and multiple activities. Nat Rev Cancer. 2009;96:400-414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Abukhdeir AM, Park BH.. P21 and p27: Roles in carcinogenesis and drug resistance. Exp Rev Mol Med. 2008;10:e19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Singh P, Blatt A, Feld S, et al. The heparanase inhibitor PG545 attenuates colon cancer initiation and growth, associating with increased p21 expression. Neoplasia. 2017;193:175–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Winterhoff B, Freyer L, Hammond E, et al. PG545 enhances anti-cancer activity of chemotherapy in ovarian models and increases surrogate biomarkers such as VEGF in preclinical and clinical plasma samples. Eur J Cancer. 2015;517:879–892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Heintz NH, Janssen-Heininger YM,, Mossman BT.. Asbestos, lung cancers, and mesotheliomas: From molecular approaches to targeting tumor survival pathways. Am J Respir Cell Mol Biol. 2010;422:133–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.