Coronary artery disease is a common form of heart disease affecting large numbers of people across the world. Coronary artery disease is caused by atherosclerosis that narrow the coronary artery lumen and limit myocardial blood supply. A thrombotic complication of coronary artery disease [e.g., acute myocardial infarction (MI) and acute coronary syndrome (ACS)] is the major leading cause of death. Identification of the patient’s risk for adverse outcomes is important in determining treatment strategy. The ultimate goal is identifying high-risk patients for adverse outcomes who may benefit from revascularizations such as percutaneous coronary intervention (PCI) and coronary artery bypass graft. Similarly, the identification of low-risk patients may avoid unnecessary revascularizations, which potentially could be harmful.
In the past two decades or so, several imaging techniques (i.e., anatomical assessments) have attempted to establish predictors of adverse outcomes in patients with coronary artery disease. Coronary angiography is the gold standard for assessing coronary anatomy and the presence of luminal obstruction of coronary arteries. The CASS registry showed that the survival rate during 12 years with medical therapy was 91% in patients with normal coronary arteries, 86% in patients with mild stenosis, 79% in patients with intermediate stenosis, 74% in patients with one-vessel disease, 59% in patients with two-vessel disease, and 40% in patients with three-vessel disease (1). Also, a large registry of coronary angiography showed that the annual mortality was 1.4% in patients with one-vessel disease whereas it increased to 8.2% in patients with three-vessel disease with proximal left anterior descending coronary artery (LAD) stenosis >95% (2). Intravascular ultrasound (IVUS) allows us to identify atherosclerotic plaque and determine its size, morphology and tissue composition. The PROSPECT study showed that after complete revascularization with PCI, the patients with non-culprit lesions characterized by a small lumen area (minimal lumen area ≤4.0 mm2), large plaque burden (percent plaque area ≥70%), and thin-capped fibroatheroma on the basis of ultrasound radiofrequency spectrum analysis had a higher risk of major adverse cardiovascular events (MACE: defined as a composite of cardiac death, MI, or unstable angina) compared with patients without those lesions during a 3-year follow-up (18.0% vs. 1.9%, P<0.001) (3). Angioscopy is the only technique that enables direct visualization of the plaque surface in color. In angioscopy, atherosclerotic plaques are observed as white indicating a fibrotic composition or yellow indicating a lipid deposition. A prospective angioscopy study showed that the patients with non-culprit yellow plaques ≥2 in coronary arteries had a 2-fold higher risk of MACE (defined as a new-onset ACS) compared with those with non-culprit yellow plaques ≤1 during a 5-year follow-up (4). Optical coherence tomography (OCT) is a light-based intravascular imaging technique, which provides high-resolution (10–20 µm) images of coronary atherosclerosis. In OCT, lipid-rich plaques are characterized by low-signal regions (i.e., lipid cores) with overlying high-signal layers (i.e., fibrous caps). A large scale international OCT registry showed that OCT-derived lipid-rich plaque was associated with an approximately 2-fold increase in MACE (defined as a composite of cardiac death, MI, and ischemia-driven revascularization) during a 2-year follow-up (7.2% vs. 2.6%, P=0.033) (5).
Physiologic assessment of coronary artery disease is also useful for predicting patient prognosis. Fractional flow reserve (FFR) is an index for physiologic severity of coronary stenosis. FFR is the ratio of coronary blood flow in the artery with stenosis relative to coronary blood flow in the same artery if the stenosis was absent. For example, an FFR value of 0.75 means that coronary blood flow is reduced to 75% of normal blood flow due to the stenosis. During diagnostic coronary angiography, FFR is measured as the pressure at distal to a stenosis (obtained with a pressure guidewire) divided by the pressure at coronary ostium (obtained with a guiding catheter) during maximal coronary blood flow (called maximal hyperemia) induced by intracoronary/intravenous administration of adenosine or other vasodilators. An FFR ≤0.75 identifies reversible myocardial ischemia with a sensitivity of 100% and a specificity of 88% (6).
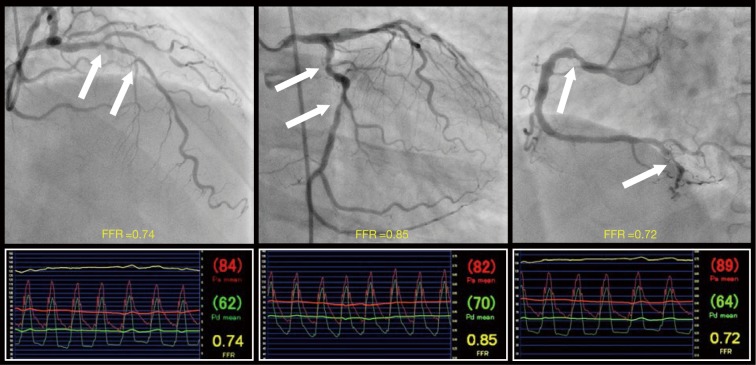
Recently, Lee et al. reported the results of the 3V FFR-FRIENDS study in the March 2018 issue of the European Heart Journal (7). This study was a prospective, multinational (Korea, China, and Japan), and multicenter (12 centres) study investigating 2-year clinical outcomes according to the value of 3V-FFR defined as a sum of FFR values of three major epicardial coronary arteries (range of values, 0.00–3.00) (Figure 1). Patients who had stenosis presenting angiographic diameter stenosis (DS) >30% in major epicardial coronary arteries were enrolled in this study. The FFR measurements were performed after diagnostic angiography in three major epicardial coronary arteries. In cases of PCI, the FFR values at post-PCI were used for the calculation of 3V-FFR. The primary endpoint was MACE (defined as a composite of cardiac death, MI or ischaemia-driven revascularization) during a 2-year follow-up. A total of 1,136 patients (3,298 coronary arteries) were analyzed. Median values of angiographic DS and FFR were 36% [interquartile range (IQR): 25–48%] and 0.91 (IQR: 0.85–0.96), respectively. The median value of 3V-FFR was 2.72 (IQR: 2.57–2.79). According to this median value of 3V-FFR, patients were classified into the high 3V-FFR group or the low 3V-FFR group. The low 3V-FFR group had a 2-fold higher risk of MACE compared with the high 3V-FFR group (7.1% vs. 3.8%, P=0.011). The higher rate of MACE in the low 3V-FFR group was mainly due to the higher rate of ischaemia-driven revascularization (6.2% vs. 2.7%, P=0.008). In the multivariate analysis, the low 3V-FFR value was an independent predictor of MACE (hazard ratio: 2.0, 95% confidence interval: 1.1–3.8, P=0.029). Those results suggest that 3V-FFR is a surrogate marker exhibiting physiologic severity of atherosclerosis in the entire coronary arterial tree and a prognostic indicator in patients with coronary artery disease.
Figure 1.
Measurement of 3V-FFR. The value of 3V-FFR (defined as a sum of FFR values of three major epicardial coronary arteries) was 2.31. Coronary angiography demonstrated three-vessel disease. However, FFR revealed double-vessel disease because FFR in LAD and RCA had ≤0.80 (positive for ischemia) but FFR in LCX showed >0.80 (negative for ischemia). After incorporating FFR into the SYNTAX score to calculate functional SYNTAX score, the score decreased from 29 (classified into an intermediate-risk group) to 20 (classified into a low-risk group). Arrow indicates a lesion with DS >50%. FFR, fractional flow reserve; LCX, left circumflex coronary artery; V, vessel.
The results of the 3V FFR-FRIENDS study are supported by previous researches regarding the prognostic value of single vessel FFR measurement in patient with deferral of PCI in favor of medical therapy. The DEFER study showed that the patients with intermediate stenosis presenting FFR ≥0.75 had a favorable prognosis: the rates of a composite of cardiac death or MI at 2-, 5-, and 15-year follow-up were as low as 2.2%, 3.3%, and 6.6%, respectively (8-10). The FAME study showed that in patients with intermediate stenosis presenting FFR >0.80, the rates of a composite of cardiac death or MI and revascularization during a 2-year follow-up were only 0.2% and 3.2%, respectively (11). Muller et al. reported that even if the patient had a stenosis presenting FFR >0.80 in proximal LAD, the rate of death during a 3-year follow-up was 1.6%, which was similar to a control group without known coronary artery disease (12). The FAME 2 study showed that the patients with FFR <0.80 had an extremely high rate (12.7%) of MACE (defined as a composite of death, MI, or urgent revascularization) during 1 year with medical therapy (13). Shiono et al. reported that the medically treated patients with FFR 0.75–0.80 were at higher risk of MACE (defined as a composite of cardiac death, MI, or ischemia-driven target vessel revascularization) during a 3-year follow-up than those with FFR >0.80 (16% vs. 3%, P=0.015) (14). Adjedj et al. reported that a progressive increase in rate of MACE (defined as a composite of death, MI, or any revascularization) was observed from the highest FFR stratum of 0.81–0.85 (8.5%) to the intermediate FFR stratum of 0.76–0.80 (13.9%) to the lowest FFR stratum of 0.70–0.75 (22.6%) during a 2-year follow-up (15). The CVIT-DEFER registry in Japan showed that the lesions presenting FFR ≤0.8 was associated with a higher rate of MACE (defined as a composite of death, stroke, MI, or any revascularization) compared with lesions presenting FFR >0.80 during a 1-year follow-up (3.1% vs. 4.9%, P=0.010) (16). A meta-analysis of FFR studies demonstrated that the rate of MACE (defined as a composite of death, MI, or any revascularization) increased as FFR decreased, and revascularization had a greater benefit for the patients with lower FFR values at baseline (cutoff value of FFR ≈ 0.75–0.80) (17). The IRIS-FFR registry in Korea demonstrated that the optimal cutoff value of FFR to predict benefit from revascularization over medical therapy was 0.75 for MACE (a composite of cardiac death, MI, or repeat revascularization) and 0.67 for a composite of death or MI (18).
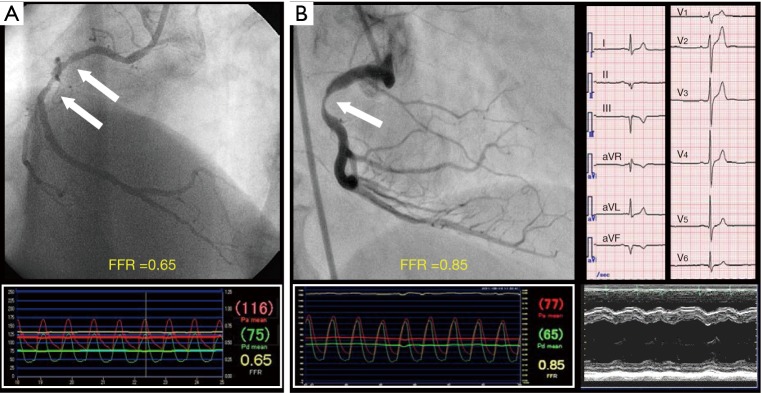
A mismatch between anatomy (e.g., angiography) and physiology (e.g., FFR) is not rare in coronary arteries with atherosclerosis (Figure 2). The rate of the mismatch between angiographic DS and FFR is reported to be 33–40% in previous studies (19-21). The underling mechanisms of mismatch between angiographic DS and FFR are considered to be as follows: (I) the quantitative coronary angiography may be inaccurate due to insufficient border detection, foreshortening of the stenotic segment, or superimposition of side branches. Also, the coronary pressure measurements may be inaccurate due to pressure signal drift, inappropriate pressure wire positioning (i.e., not far enough to the distal coronary artery), coronary flow disturbance due to deep seating of the guiding catheter into the coronary ostium, and insufficient hyperemia; (II) angiographic DS does not take into account viable myocardial mass and coronary supply area that are associated with the stenosis. Stated another way, a stenosis in the proximal segment of large coronary artery is more likely to be physiologically significant, even when its angiographic DS is mild; (III) angiography cannot provide any information about the vasodilatory capabilities of the micro-vasculature in the downstream territory. Therefore, for a similar degree of angiographic DS, FFR is higher in patients with coronary microcirculatory disorders (e.g., elderly patients and patients with diabetes mellitus) (20,22); (IV) angiography has a limited capability for identifying morphological lesion characteristics such as surface roughness, plaque rupture and thrombus which influence lesion hemodynamics (20). The FAME 2 substudy compared the accuracy of both approaches in predicting the natural history of coronary artery disease (21). The coronary stenoses were divided into 4 groups according to values of FFR and angiographic DS: positive concordance (FFR ≤0.80 and DS ≥50%), negative concordance (FFR >0.80 and DS <50%), positive mismatch (FFR ≤0.80 and DS <50%), and negative mismatch (FFR >0.80 and DS ≥50%). The rate of MACE (defined as a composite of cardiac death, target vessel-related MI, target vessel-related revascularization) during a 2-year follow-up was highest in the positive concordance group (39.4%) and lowest in the negative concordance group (7.9%). The rate of MACE was higher in the positive mismatch group (32.7%) than in the negative mismatch group (14.2%) (P<0.001). There was no significant difference in the rate of MACE between the positive concordance group and the positive mismatch group (P=0.149) and no significant difference in the rate of MACE between the negative mismatch group and the negative concordance group (P=0.067). Those results suggest that FFR is a more important determinant of the natural history of coronary stenosis than angiographic DS in patients with coronary artery disease.
Figure 2.
Mismatch between angiographic DS and FFR. (A) Positive mismatch (FFR ≤0.80 and DS <50%); (B) negative mismatch (FFR >0.80 and DS ≥50%) due to small amount of viable myocardium. Electrocardiogram shows QS pattern in II, III, aVF leads. Echocardiogram shows akinesis in left ventricular inferior wall. Arrow indicates a stenosis. DS, diameter stenosis; FFR, fractional flow reserve.
The FFR-guided SYNTAX score, termed “functional SYNTAX score”, has been proposed as a predictor of clinical outcome in patients with multi-vessel coronary artery disease. Functional SYNTAX score is calculated by adding the individual scores of lesions with FFR ≤0.80 and ignoring lesions with FFR >0.80. Nam et al. showed that after incorporating FFR into the SYNTAX score to calculate functional SYNTAX score, one third of the patients moved from the higher-risk group to the lower-risk group (23). In addition, the functional SYNTAX score had a better predictive accuracy for MACE (defined as a composite of death, MI, or any repeat revascularization) during a 1-year follow-up compared with the conventional SYNTAX score in patients undergoing PCI (23).
The major limitation of 3V-FFR is invasive and complex procedure for measurements. To estimate the value of 3V-FFR, the FFR measurement should be performed even in normal or mildly stenotic coronary arteries. The FFR measurements in three major epicardial coronary arteries require additional time for coronary catheterization and increase risk of procedure-related complications such as coronary artery dissection. Recently, computed tomography-derived FFR (FFR-CT) and coronary angiography-based index for estimating FFR [quantitative flow ratio (QFR)] have emerged as less-invasive methods for physiologic assessment of coronary artery disease (24,25). These novel, less-invasive methods rather than invasive FFR may be appropriate for three-vessel physiologic assessment.
In conclusion, 3V-FFR is an important prognostic marker in patients with coronary artery disease. The clinical indication of FFR measurement might be expanded beyond the identification of coronary stenosis responsible for myocardial ischemia.
Acknowledgements
None.
Provenance: This is an invited Editorial commissioned by the Section Editor Xiaoyan Wang (Department of Cardiology, Zhongshan Hospital, Fudan University, Shanghai, China).
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Emond M, Mock MB, Davis KB, et al. Long-term survival of medically treated patients in the Coronary Artery Surgery Study (CASS) Registry. Circulation 1994;90:2645-57. 10.1161/01.CIR.90.6.2645 [DOI] [PubMed] [Google Scholar]
- 2.Mark DB, Nelson CL, Califf RM, et al. Continuing evolution of therapy for coronary artery disease. Initial results from the era of coronary angioplasty. Circulation 1994;89:2015-25. 10.1161/01.CIR.89.5.2015 [DOI] [PubMed] [Google Scholar]
- 3.Stone GW, Maehara A, Lansky AJ, et al. A prospective natural-history study of coronary atherosclerosis. N Engl J Med 2011;364:226-35. Erratum in: N Engl J Med 2011;365:2040. 10.1056/NEJMoa1002358 [DOI] [PubMed] [Google Scholar]
- 4.Ohtani T, Ueda Y, Mizote I, et al. Number of yellow plaques detected in a coronary artery is associated with future risk of acute coronary syndrome: detection of vulnerable patients by angioscopy. J Am Coll Cardiol 2006;47:2194-200. 10.1016/j.jacc.2006.01.064 [DOI] [PubMed] [Google Scholar]
- 5.Xing L, Higuma T, Wang Z, et al. Clinical Significance of Lipid-Rich Plaque Detected by Optical Coherence Tomography: A 4-Year Follow-Up Study. J Am Coll Cardiol 2017;69:2502-13. 10.1016/j.jacc.2017.03.556 [DOI] [PubMed] [Google Scholar]
- 6.Pijls NH, De Bruyne B, Peels K, et al. Measurement of fractional flow reserve to assess the functional severity of coronary-artery stenoses. N Engl J Med 1996;334:1703-8. 10.1056/NEJM199606273342604 [DOI] [PubMed] [Google Scholar]
- 7.Lee JM, Koo BK, Shin ES, et al. Clinical implications of three-vessel fractional flow reserve measurement in patients with coronary artery disease. Eur Heart J 2018;39:945-51. 10.1093/eurheartj/ehx458 [DOI] [PubMed] [Google Scholar]
- 8.Bech GJ, De Bruyne B, Pijls NH, et al. Fractional flow reserve to determine the appropriateness of angioplasty in moderate coronary stenosis: a randomized trial. Circulation 2001;103:2928-34. 10.1161/01.CIR.103.24.2928 [DOI] [PubMed] [Google Scholar]
- 9.Pijls NH, van Schaardenburgh P, Manoharan G, et al. Percutaneous coronary intervention of functionally nonsignificant stenosis: 5-year follow-up of the DEFER Study. J Am Coll Cardiol 2007;49:2105-11. 10.1016/j.jacc.2007.01.087 [DOI] [PubMed] [Google Scholar]
- 10.Zimmermann FM, Ferrara A, Johnson NP, et al. Deferral vs. performance of percutaneous coronary intervention of functionally non-significant coronary stenosis: 15-year follow-up of the DEFER trial. Eur Heart J 2015;36:3182-8. 10.1093/eurheartj/ehv452 [DOI] [PubMed] [Google Scholar]
- 11.Tonino PA, De Bruyne B, Pijls NH, et al. Fractional flow reserve versus angiography for guiding percutaneous coronary intervention. N Engl J Med 2009;360:213-24. 10.1056/NEJMoa0807611 [DOI] [PubMed] [Google Scholar]
- 12.Muller O, Mangiacapra F, Ntalianis A, et al. Long-term follow-up after fractional flow reserve-guided treatment strategy in patients with an isolated proximal left anterior descending coronary artery stenosis. JACC Cardiovasc Interv 2011;4:1175-82. 10.1016/j.jcin.2011.09.007 [DOI] [PubMed] [Google Scholar]
- 13.De Bruyne B, Pijls NH, Kalesan B, et al. Fractional flow reserve-guided PCI versus medical therapy in stable coronary disease. N Engl J Med 2012;367:991-1001. Erratum in: N Engl J Med 2012;367:1768. 10.1056/NEJMoa1205361 [DOI] [PubMed] [Google Scholar]
- 14.Shiono Y, Kubo T, Tanaka A, et al. Long-term outcome after deferral of revascularization in patients with intermediate coronary stenosis and gray-zone fractional flow reserve. Circ J 2015;79:91-5. 10.1253/circj.CJ-14-0671 [DOI] [PubMed] [Google Scholar]
- 15.Adjedj J, De Bruyne B, Floré V, et al. Significance of Intermediate Values of Fractional Flow Reserve in Patients With Coronary Artery Disease. Circulation 2016;133:502-8. 10.1161/CIRCULATIONAHA.115.018747 [DOI] [PubMed] [Google Scholar]
- 16.Tanaka N, Nakamura M, Akasaka T, et al. One-Year Outcome of Fractional Flow Reserve-Based Coronary Intervention in Japanese Daily Practice- CVIT-DEFER Registry. Circ J 2017;81:1301-6. 10.1253/circj.CJ-16-1213 [DOI] [PubMed] [Google Scholar]
- 17.Johnson NP, Tóth GG, Lai D, et al. Prognostic value of fractional flow reserve: linking physiologic severity to clinical outcomes. J Am Coll Cardiol 2014;64:1641-54. 10.1016/j.jacc.2014.07.973 [DOI] [PubMed] [Google Scholar]
- 18.Ahn JM, Park DW, Shin ES, et al. Fractional Flow Reserve and Cardiac Events in Coronary Artery Disease: Data From a Prospective IRIS-FFR Registry (Interventional Cardiology Research Incooperation Society Fractional Flow Reserve). Circulation 2017;135:2241-51. 10.1161/CIRCULATIONAHA.116.024433 [DOI] [PubMed] [Google Scholar]
- 19.Toth G, Hamilos M, Pyxaras S, et al. Evolving concepts of angiogram: fractional flow reserve discordances in 4000 coronary stenoses. Eur Heart J 2014;35:2831-8. 10.1093/eurheartj/ehu094 [DOI] [PubMed] [Google Scholar]
- 20.Park SJ, Kang SJ, Ahn JM, et al. Visual-functional mismatch between coronary angiography and fractional flow reserve. JACC Cardiovasc Interv 2012;5:1029-36. 10.1016/j.jcin.2012.07.007 [DOI] [PubMed] [Google Scholar]
- 21.Ciccarelli G, Barbato E, Toth GG, et al. Angiography versus hemodynamics to predict the natural history of coronary stenoses: fractional flow reserve versus angiography in multivessel evaluation 2 substudy. Circulation 2018;137:1475-85. 10.1161/CIRCULATIONAHA.117.028782 [DOI] [PubMed] [Google Scholar]
- 22.Jin X, Lim HS, Tahk SJ, et al. Impact of Age on the Functional Significance of Intermediate Epicardial Artery Disease. Circ J 2016;80:1583-9. 10.1253/circj.CJ-15-1402 [DOI] [PubMed] [Google Scholar]
- 23.Nam CW, Mangiacapra F, Entjes R, et al. Functional SYNTAX score for risk assessment in multivessel coronary artery disease. J Am Coll Cardiol 2011;58:1211-8. 10.1016/j.jacc.2011.06.020 [DOI] [PubMed] [Google Scholar]
- 24.Kitabata H, Leipsic J, Patel MR, et al. Incidence and predictors of lesion-specific ischemia by FFR(CT): Learnings from the international ADVANCE registry. J Cardiovasc Comput Tomogr 2018;12:95-100. 10.1016/j.jcct.2018.01.008 [DOI] [PubMed] [Google Scholar]
- 25.Emori H, Kubo T, Kameyama T, et al. Diagnostic Accuracy of Quantitative Flow Ratio for Assessing Myocardial Ischemia in Prior Myocardial Infarction. Circ J 2018;82:807-14. 10.1253/circj.CJ-17-0949 [DOI] [PubMed] [Google Scholar]