Abstract
Glioblastoma (GBM) is a highly malignant type of primary brain tumor with a high mortality rate. Although the current standard therapy consists of surgery followed by radiation and temozolomide (TMZ), chemotherapy can extend patient’s post-operative survival but most cases eventually demonstrate resistance to TMZ. O6-methylguanine-DNA methyltransferase (MGMT) repairs the main cytotoxic lesion, as O6-methylguanine, generated by TMZ, can be the main mechanism of the drug resistance. In addition, mismatch repair and BER also contribute to TMZ resistance. TMZ treatment can induce self-protective autophagy, a mechanism by which tumor cells resist TMZ treatment. Emerging evidence also demonstrated that a small population of cells expressing stem cell markers, also identified as GBM stem cells (GSCs), contributes to drug resistance and tumor recurrence owing to their ability for self-renewal and invasion into neighboring tissue. Some molecules maintain stem cell properties. Other molecules or signaling pathways regulate stemness and influence MGMT activity, making these GCSs attractive therapeutic targets. Treatments targeting these molecules and pathways result in suppression of GSCs stemness and, in highly resistant cases, a decrease in MGMT activity. Recently, some novel therapeutic strategies, targeted molecules, immunotherapies, and microRNAs have provided new potential treatments for highly resistant GBM cases. In this review, we summarize the current knowledge of different resistance mechanisms, novel strategies for enhancing the effect of TMZ, and emerging therapeutic approaches to eliminate GSCs, all with the aim to produce a successful GBM treatment and discuss future directions for basic and clinical research to achieve this end.
Keywords: glioma, temozolomide, chemosensitivity
Introduction
Glioblastoma (GBM) is one of the most common and aggressive primary malignant brain neoplasms in adults, with a low median survival period of only 12–15 months after the initial diagnosis. Although it’s relatively uncommon, with a low incidence of about 5/100,000 when compared with other malignant tumors,1) GBM still accounts for around 70% of all adult malignant brain tumors.2,3) Owing to its rapid proliferation ability and highly infiltrative growth, complete surgical resection is still difficult to achieve.1,3) The majority of diagnosed GBMs recur, even after an expanded resection of normal brain tissue was performed at the edge of the tumor mass. The benefits of nitrosoureas-based chemotherapy on GBM patient survival were established more than three decades ago.2) Since then, surgical resection, followed by radiation therapy and chemotherapy, has been adopted as conventional therapies for patients with newly diagnosed GBM.
Temozolomide (TMZ) is an oral alkylating agent, for which the antitumor activity was first discovered in 1987,4) and has been widely applied as an effective first-line chemotherapeutic agent for the treatment of GBM patients since FDA approved its efficacy in March, 2005.5,6) When combined with radiation therapy, and employed as an adjuvant therapy, TMZ treatment contributed to a significant increase in median survival period, 2-year survival, progression-free survival, and improved quality of life when compared with radiotherapy alone.6) Since then, hundreds of studies and papers have demonstrated the main cytotoxic mechanism by which TMZ may eliminate GBM tumors. TMZ elicits cytotoxicity by transporting a methyl group that attaches to guanine at the O6 and N7 positions, and adenine at N3 position during DNA replication to form cytotoxic O6-methylguanine (O6-MG), N7-methylguanine (N7-MG), and N3-methyladenine (N3-MA). These cytotoxic groups are composed of mismatched lethal base pairs that result in single and double-strand DNA breaks that induce cell cycle arrest at G2/M, eventually leading to cell apoptosis.1,7–9)
Although subsequent radiation therapy and chemotherapy with TMZ contributed to lengthen survival and improve quality of life,6,9,10) the survival advantages are still palliative.11,12) The increase in TMZ resistance is one of the main reasons for GBM treatment failure.9) O6-methylguanine-DNA methyltransferase (MGMT) is the most important contributor to TMZ chemoresistance that could repair the main cytotoxic lethal base pairs, which are composed of the alkylating agent TMZ. Recently, a series of TMZ resistant cases have been reported, although these cases do not appear to have MGMT activity. So, there should be some other resistance mechanism independent of the MGMT repair system, such as that present in glioma stem cells (GSCs).
Following the first introduction of the concept of cancer stem cells, based on studies of blood formation in hematopoietic stem cells and cancer-initiating cells in leukemia,13) reports gradually proposed that in various human solid tumors, including malignant brain tumors, there is a small population of undifferentiated highly tumorigenic cancer stem cells or cancer-initiating cells, that possess extensive self-renewal capacity and further differentiation ability. This self-renewal and differentiation induce cancer initiation, progression, metastasis, and resistant to conventional therapies.14,15) Recent evidences also demonstrate that GSCs exhibit stronger resistance to conventional therapies than normal neural stem cells (NSCs).16) This increased resistance is probably because of different proliferative ability, expression levels of key proteins and molecules related to cell behavior and survival, or various subsequent markers. In this paper, we will discuss the main mechanism of TMZ cytotoxicity. The TMZ resistance mechanism is caused by a conventional repair system and regulated by GSCs. Strategies to enhance TMZ chemosensitivity against glioma are the latest frontier in current treatments targeting GSCs.
Resistance Mechanism
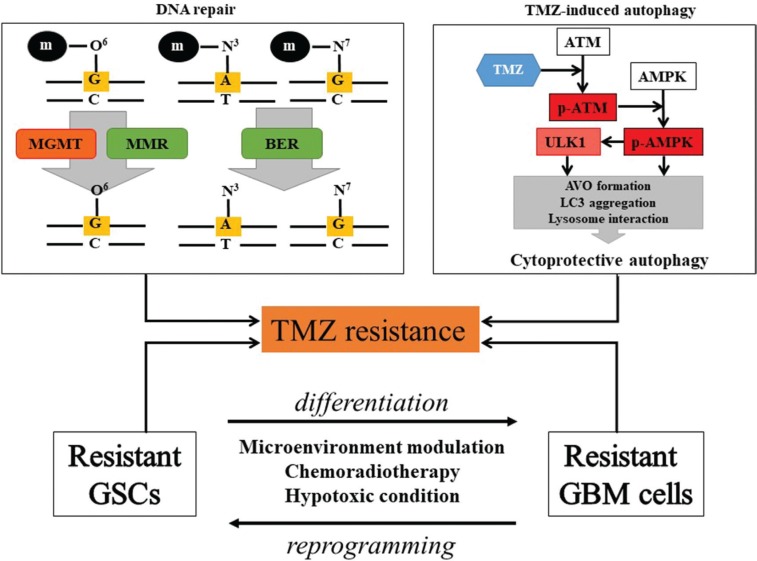
The TMZ response varies in GBM, although TMZ is the standard chemotherapy for GBM treatment.6) In GBM with TMZ resistance, it is difficult to prevent tumor progression or recurrence.17,18) Clarifying the TMZ resistance mechanism is essential to increase drug efficacy, and could provide an important basis for individualized treatment according to different resistance mechanisms. Current researches indicated that TMZ resistance is the result of both the DNA repair system, such as MGMT, mismatch repair (MMR), and base excision repair (BER), as well as other mechanisms like autophagy and GSCs (Fig. 1).1,17,19–28)
Fig. 1.
TMZ resistant mechanism. The causes of TMZ resistance are mainly DNA repair system, autophagy, and GSC. The MGMT and MMR systems remove the O6-guanine methylation, followed by usual DNA replication. Activated BER also contributes to DNA repair through removal of the methylation of N7-guanine and the N3-adenine. TMZ-induced autophagy via the ATM/AMPK pathway can induce AVO formation, LC3 aggregation, which are essential for autophagosome and lysosome interaction, facilitating cytoprotective autophagy and cell survival. GSCs may change their phenotype to TMZ resistant GSCs or differentiate into TMZ resistant GBM cells via tumor microenvironment modulation, chemoradiotherapy, or hypoxic condition. On the other hand, differentiated GBM cells can re-acquire stem cell capacity through reprogramming by the tumor microenvironment modulation. AMPK: AMP-activated protein kinase, ATM: ataxia telangiectasia mutated, AVO: acidic vesicular organelles, BER: base excision repair, GSC: glioblastoma stem cell, LC3: microtubule associated protein light chain 3, MGMT: methylguanine methyltransferase, MMR: mismatch repair, TMZ: Temozolomide, ULK: unc51-like kinase.
DNA repair systems
O6-methylguanine-DNA methyltransferase
The anti-tumor effect of TMZ is reflected in the O6-guanine methylation although TMZ-induced DNA methylation occurs at the N7-guanine (>70%) and the N3-adenine (>9%) to a greater extent than the O6-guanine (5%).17) MGMT is DNA repair enzyme that directly repairs the TMZ-generated cytotoxic lesion by removing the methyl group in the O6-methylguanine, which leads to invalidation of TMZ-induced lethal DNA damage. MGMT expression levels directly correlate with the methylation status of the promoter site in the cysteine-phosphate-guanine (CpG) MGMT gene island. MGMT is diminished by methylation of MGMT promoter and stays active when MGMT promoter remains unmethylated. It was reported that GBM cases, with a methylated MGMT promoter, showed prolonged survival compared to cases with an unmethylated MGMT promoter in a phase II trial evaluating the combination effect of radiotherapy and TMZ for newly diagnosed GBMs.19,21,23,26) Hence, MGMT is the main culprit contributing to TMZ resistance, providing a potentially sensitive target for TMZ therapy.
Mismatch repair
MMR is a system that corrects nucleotide base mismatches generated in the process of DNA synthesis. O6-methylguanine (O6-MeG), induced by TMZ treatment, mispairs with thymine during DNA replication. The MMR system recognizes mispaired O6-MeG/T and excises the newly synthesized strand, leaving the parental strand with O6-MeG intact. These futile cycles repeat, leading to cell cycle arrest and apoptosis. The loss of MMR function does not respond to TMZ-induced mispairing and can be associated with resistance to the cytotoxic effects of TMZ. MMR ability is impaired by mutation of MMR genes, such as melanocyte-stimulating hormone 2 (MSH2), MSH6, mutL homolog 1 (MLH1), and post-meiotic segregation-increased Saccharomyces cerevisiae 2 (PMS2). MSH6 somatic mutations were mainly identified in recurrent GBM, mediated by TMZ compared to primary GBM without TMZ therapy.19,20,25,27) It indicates that mutations in MMR genes are rar in primary GBM, while these genes are vulnerable to TMZ-induced mutations, so that tumor cells will become resistant to TMZ because of a disrupted MMR pathway.19,27)
Base excision repair
The BER system is involved in the repair of DNA damage induced by an oxidizing agent, ionizing radiation or alkylating agents. This system consists of multicatalysis reactions by DNA glycosylase, endonuclease, polymerase, and DNA ligase. The methylation of N7-guanine and the N3-adenine represent more than 90% of the methylation caused by TMZ and are rapidly repaired by BER, so that would promote GBM survival. When one or more components of BER are mutated, it results in the deficiency in the ability of BER to repair DNA damage, furthermore, contributing to TMZ cytotoxicity to GBM. Notably, N3 lesions are lethal if not repaired, unlike N7 lesions. Among the components of BER, poly (ADP-ribose) polymerase-1 (PARP-1) is known as an important enzyme with dual effect. Inhibition of PARP-1 leads to the accumulation of broken DNA in the cells, resulting in cell death. PARP-1 hyper-activation, generated by DNA damage, leads to a depletion of nicotinamide adenine dinucleotide (NAD+) and adenosine triphosphate (ATP) followed by cell death. Thus, the regulation of BER activity will contribute to the treatment of TMZ resistant GBM.17,19–21,27) However, reports indicated that the role of BER in TMZ resistance is less important than MGMT and MMR, although the rate of N7-guanine and the N3-adenine methylation is higher than that of O6-guanine.
Autophagy
Autophagy is the mechanism to maintain homeostasis and survival through the degradation and recycling of cellular proteins, organelles, and other cellular components. Autophagy is commonly regulated by various stimuli including conditions, such as starvation, hypoxia, pathogens, radiation toxic agent, and DNA damage. Autophagy involved in cancer cell behaviors and its implications for cancer therapy have been an appealing direction for cancer research for decades. However, the role of autophagy is still controversial.29,30) It is possible that the effect on autophagy depends on the cellular context and treatment conditions.28) Studies that investigated the relation of autophagy to TMZ treated glioma cells have demonstrated that autophagy, but not apoptosis, is induced in GBM cells treated with TMZ.31,32) Autophagy activity is reduced in cancer cells than in their normal counterparts to maintain the potential to activate self-defensive autophagy.33) Once cancer cells are exposed to radiation or chemotherapeutic agents, a high rate of autophagy is triggered34,35) and cancer cells degrade unnecessary components or molecules for their survival as an adaptation to detrimental conditions caused by cancer therapies, such as TMZ chemotherapy.31) Another study also has reported that very few of GBM cells treated with TMZ underwent apoptosis.36) The role of autophagy is preliminary protective and autophagy induction is considered as a mechanism of chemoresistance.30,37) Based on the findings described above, combination treatments of TMZ with autophagy inhibitors or regulators would be a promising strategy to treat highly resistant GBM cases. However, Investigations showed that additional treatment of TMZ-treated GBM cells with Bafilomycin A1, which inhibits autophagosome and lysosome fusion, resulted in more effective apoptotic cell death as compared with TMZ and/or Bafilomycin A1 treatment alone because of the mechanism by which Bafilomycin A1 blocked TMZ-induced cytoprotective autophagy at the late stage and activated caspase-3 as well as mitochondrial and lysosomal membrane permeabilization.31) In the same study, another autophagy inhibitor, 3-methyladenine (3-MA), which is known to inhibit autophagy at the early stage, has decreased TMZ cytotoxicity when employed in combination therapy, in contrast with Bafilomycin A1.31) Furthermore, it has been reported that inhibition of adenosine monophosphateactivated protein kinase (AMPK), an initiator of autophagosome formation interacting with mammalian autophagyinitiating kinase unc51-like kinase 1 (ULK1),38) augments the cytotoxicity of TMZ in GBM cells in combination treatment,32) These discoveries suggest that autophagy is the main result of TMZ cytotoxicity and inhibition of autophagy significantly influences the TMZ anti-glioma effect. Meanwhile, there should be a focus on how different autophagy inhibitors or regulators play different roles in different steps of the process of TMZ induced autophagy to potentially induce different results.
Glioma stem cells
Since the first description of the cancer stem cell hypothesis was proposed for hematopoietic cancers, GSC was isolated from the bulk of the GBMs. Molecules that have been identified as GSC markers include CD133, CD15, stage-specific embryonic antigen-1 (SSEA1), and nestin. GSCs are capable of self-renewal, tumorigenesis, differentiation, chemo-resistance and radio-resistance.18,24,39,40) GSC chemo-resistance was related to drug efflux transporter (ATP-binding cassette, ABCG2) and GSC diversity.41) GSCs exhibit diversity because the tumor bulk consists of numerous heterogenetic GSC phenotypes based on distinct genomic profiles. The tumor microenvironment can also modulate GSC phenotypic change and may produce different type of GBM cell lines. In contrast, differentiated GBM cells can be reprogrammed by the tumor microenvironment and re-acquire stem cell capacity. Hypoxic condition promotes stemness and enhances MGMT upregulation through hypoxia-inducible factor 1-alpha (HIF-1α) in GSCs.23,42) Chemo-resistance based on GSC theory could be important as a hallmark of recurrent GBM.
Here, it is explained that TMZ resistance is associated with MGMT function, MMR function, BER function, autophagy, and GSC. GBM cell with MGMT promoter unmethylated status and GSC are primarily tolerant of the effect of TMZ. GBM treated with TMZ can acquire TMZ resistant via changes in the MGMT promoter status, mutation of MMR related genes, TMZ induced-autophagy activation, and heterogeneity of GBM cells. It is important that TMZ therapy can be a trigger of resistance.
Strategy for Enhancing TMZ Effect
Strengthen TMZ effect
Modulation of TMZ dosing schedule
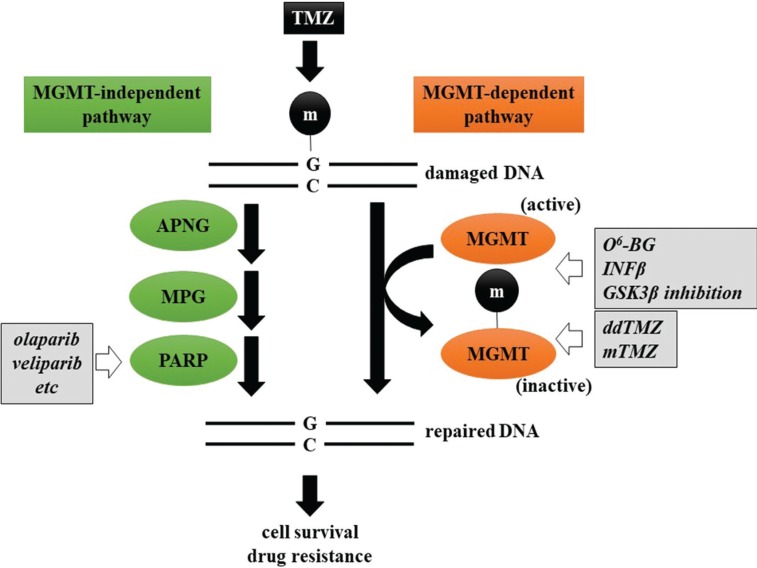
MGMT is one of the mechanisms involved in TMZ resistance. MGMT repairs damaged DNA by transferring a methyl group from the O6 position of guanine, methylated by TMZ, to its cysteine in an active enzymatic domain (Fig. 2). Methylated MGMT loses its enzymatic activity leading to subsequent degradation. Continuous exposure to TMZ, dose-dense TMZ (ddTMZ) or metronomic TMZ (mTMZ) causes accumulation of methylated MGMT and sensitization to TMZ. Biologically, a low dose (20–50 mg/m2) mTMZ regimen might deplete CD4+CD25+Foxp3+ regulatory T cells (Tregs), which play a significant role in hampering antitumor immunity.43)
Fig. 2.
Potential therapeutic approach enhancing the effect of TMZ. TMZ resistance by DNA repair is divided into MGMT independent and dependent mechanisms. Therapeutic approaches to overcome the former include methylation of the MGMT promoter with O6-BG, IFNβ, and GSK3β inhibition and depletion of MGMT by ddTMZ. Currently, PARP inhibition is the only available intervention for the latter mechanism. Circled m represents a methyl group. APNG: alkylpurine-DNA-N-glycosylase, GSK3β: glycogen synthase kinase 3β, INFβ interferon-β, MPG: N-methylpurine DNA glycosylase, O6-BG: O6-benzylguanine, PARP: poly (ADP-ribose) polymerase.
Among several regimens of alternate TMZ dosing, 7 d on/7 d off (7/14 d), 21 d on/7 d off (21/28 d), and continuous or metronomic administration (28/28 d) have been vigorously investigated.44) For newly-diagnosed GBM, a large randomized phase III trial (RTOG0525) that compared the ddTMZ regimen (75–100 mg/m2, 21/28 d) with the standard 5/28 d TMZ regimen showed no survival benefit with ddTMZ.45) It is suggested that newly-diagnosed GBM, which has no history of exposure to TMZ (TMZ-naïve) is susceptible to TMZ. In contrast, a 7/14 d regimen for treatment of recurrent or progressive GBM was superior to the standard regimen with respect to both progression-free survival (PFS) and overall survival (OS), without significantly increasing adverse events.46) Based on these data, ddTMZ should be recommended for the patients with refractory disease formerly exposed to TMZ. However, a phase II trial comparing two ddTMZ regimens (7/14 d vs. 21/28 d) for patients with their first recurrent GBM after standard treatment showed no difference between the two regimens for median overall survival (mOS) 298 d vs. 322 d.47) This study also demonstrated that TMZ rechallenge was suitable for MGMT promoter-methylated GBM after standard therapy.47)
Most ddTMZ regimens also increase the amount of TMZ accumulation over one course (28 d) related to potential toxicity. Based on the available data, no significant increase in toxicity of myelosuppression, which is hallmark of TMZ toxicity, was found. CTCAE grade 3/4 lymphopenia was found 24–53% and 12–68% in 21/28 d and 7/14 d regimen, respectively.44,48) A prophylaxis against opportunistic infection and careful MRI follow-up should be required for all ddTMZ regimens in addition to standard treatment. Dose-intense TMZ could promote invadopodia formation, one of the infiltrating phenotype of tumor cells, via MMP-2 activation.49) Thus, it is also necessary to carefully observe neurological deterioration and tumor progression MRI.
Combination treatment with TMZ enhancer
Concomitant therapy is another approach for enhancing the activity of TMZ. The majority of TMZ-induced methylation site is N7 portion of guanine (>70%), whereas N3 portion of adenine and O6 portion of guanine are methylated at 9% and 5%, respectively. N7-MG and N3-MA are substrates for the BER system consisting of a multistep reaction by several DNA glycosylases (alkylpurine-DNA-N-glycosylase; APNG, etc.), endonucleases (N-methylpurine DNA glycosylase; MPG, etc.), polymerases (poly (ADP-ribose) polymerase; PARP, etc.), and DNA ligases (Fig. 2).17) PARP is one of the most intensively investigated BER enzymes and several PARP inhibitors applied to ovarian, prostate, and breast cancers in the United States, Europe, and Japan.50) Some preclinical studies showed that the combination of TMZ with PARP inhibitor was effective for IDH-mutant glioma.51,52) Currently, several clinical trials have investigated the efficacy of PARP inhibitors (olaparib, veliparib, etc.), with or without TMZ, against WHO grades II–IV gliomas (http://www.clinicaltrials.gov).
It is clear that methylation of the MGMT promoter is a favorable prognostic and a predictive factor for GBM; thus, the agents depleting MGMT and methylation of the MGMT promoter could potentiate TMZ cytotoxicity. O6-benzylguanine (O6-BG), a false substrate of MGMT, could irreversibly inactivate MGMT by antagonizing O6-MeG leading to a reversal of TMZ resistance.17) In phase II clinical trial, O6-BG was shown to restore sensitivity of recurrent high-grade glioma (HGG) to TMZ, but no significant survival benefit was observed.53) Interferon-β (INFβ) enhances chemosensitivity by downregulating MGMT transcription via p53 induction.54) INTEGRA study (JCOG0911), a phase II trial examining the effect of IFNβ on RT + TMZ standard therapy for newly-diagnosed HGG, was conducted in Japan. However, it was disappointing that median PFS (mPFS) and mOS were not significantly different between RT + TMZ and RT + TMZ + IFNβ (mPFS, 10.1 vs. 8.5 months; mOS, 20.3 vs. 24.0 months).55) In addition, we identified several drug candidates enhancing the activity of TMZ (Kitabayashi et al., unpublished data). Among them, glycogen synthase kinase 3β (GSK3β) inhibition has been revealed to enhance the activity of TMZ via conversion of unmethylated guanine to methylguanine at the MGMT promoter.56) A phase I/II clinical study that investigated the efficacy and safety of concomitant GSK3β inhibitors with TMZ against recurrent GBM demonstrated an anti-tumor effect, survival benefit, and enhancement of the TMZ effect without adverse side effects.57)
Treat the GSCs based on TMZ
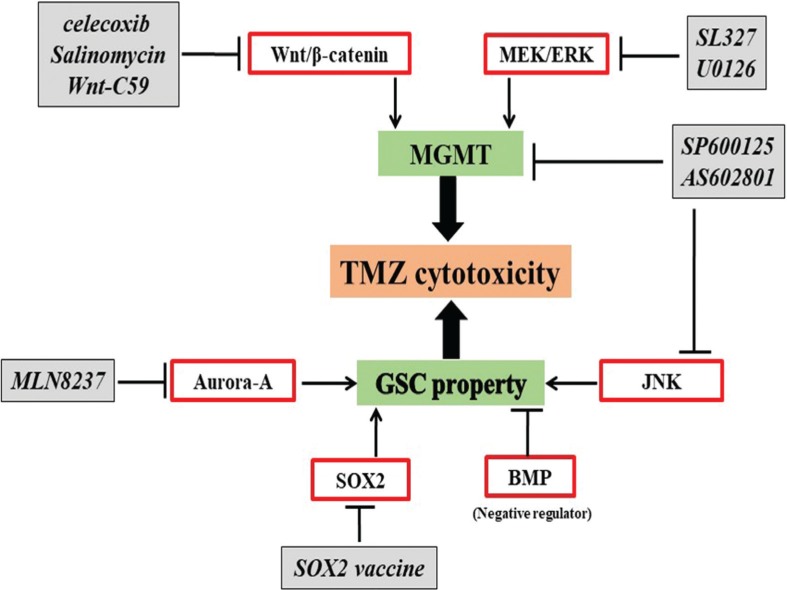
As mentioned, GSCs play an indispensable role in developing resistance to chemoradiotherapy and are the main culprit behind GBM recurrence after initial therapy, owing to their stem-cell-like properties such as self-renewal, capacity of differentiation and tumor initiation. Therefore, novel therapeutic approaches that are effective and successful in eliminating both GSCs and entire tumor bulk are urgently required. Hypothesizes and studies support the idea that molecules involved in maintaining the stem-cell-like properties of GSCs could be novel therapeutic targets to overcome chemoresistance. Recent reports demonstrated that new agents may be effective as single treatments or to synergistically enhance TMZ cytotoxicity against GSCs and eliminate GBM tumor bulks via MGMT promoter methylation, or other MGMT independent pathways (Fig. 3).
Fig. 3.
Treat the GSCs based on TMZ. JNK, MEK/ERK pathways, and Wnt signaling maintain extensive proliferation and self-renewal ability of GSCs in a MGMT dependent manner. Aurora-A kinase, SOX2, and BMP regulate GSC stemness and correlate with tumor aggressiveness and poor prognosis. Targeting these molecules is a promising therapeutic strategy to enhance TMZ. BMP: bone morphogenetic proteins, ERK: extracellular signal regulated kinase, JNK: c-Jun N-terminal kinase, MEK: mitogen-activated protein kinase, SOX2: sex determining region Y-box 2.
MGMT dependent manner
-
JNK inhibition sensitizes TMZ via regulation of MGMT expression.
The c-Jun NH2-terminal kinases (JNKs), also known as stress-activated MAP kinase (SAPK), is a member of the mitogen-activated protein (MAP) kinase family.58) JNK interacts with signals from numerous extracellular stimuli, and is involved in important cellular processes such as proliferation, apoptosis, and differentiation.58,59) JNK is commonly upregulated in a number of human cancers, including GBM. The activation level of JNK in self-renewing cells is obviously superior to that of differentiated cells.59,60) Studies targeting JNK showed that, regardless of whether JNK was inhibited by the JNK specific inhibitor SP600125 or silenced by JNK-shRNA, GSCs after JNK inhibition exhibits weakened stem cell properties with reduced ability to form tumor spheres, lowered expression of stem cell markers, such as Nestin and Sox-2, and elevated expressions of differentiated cell markers such as GFAP and βIII-tubulin..60) Further studies showed that when, GSCs were treated with SP600125, it suppressed the expression of MGMT in both a dose- and time-dependent manner. In combination treatment with TMZ, SP600125 synergistically sensitize TMZ cytotoxicity in GSCs. Knockdown of JNK by shRNA resulted in the same outcome with SP600125. Another novel JNK inhibitor, AS602801, also inhibited GSCs both in vitro and in vivo.59) However, JNK inhibition is ineffective to GSCs without MGMT expression.61) The JNK pathway could be a therapeutic target to overcome GSC related chemo-resistance, and a novel approach to treat GSCs expressing MGMT.
-
MEK inhibitors potentiate TMZ efficacy to GSCs.
MEK-ERK signaling is an important signaling pathway involved in many biological processes like proliferation, differentiation, and apoptosis. Studies demonstrated that MEK/ERK signaling is continuously activated as the consequence of upregulation or abnormalities of upstream molecules of receptor tyrosine kinases, such as EGFR, PDGFR.62,63) Other studies also revealed that ERK phosphorylation is active in glioma cells.64) These findings indicate that therapies targeting MEK/ERK signaling may be potential approaches for treating GSCs. A study discussing the relationship between MEK/ERK signaling and MGMT expression level showed that MEK inhibitors, SL327 and U0126, suppressed MGMT expression in GSCs via activation of p53. MGMT suppression is capable of inhibiting MGMT expression,65) suggesting that MEK activity is required for the maintenance of MGMT expression, regulating TMZ resistance of GSCs. Combination of the MEK inhibitor SL327 with TMZ treatment synergistically sensitized resistant GSCs to TMZ and suppressed the tumorigenic properties of GSCs.66) In vivo experiments also exhibited a significant increase in survival when a combination treatment of SL327 with TMZ was employed, as compared with either SL327 or TMZ treatment alone. These data suggested that the MEK inhibitor plays an effective role as enhancer for TMZ chemotherapy targeting GSCs.
-
Inhibition of Wnt/β-catenin signaling prevents chemo-resistance by down-regulation of MGMT.
Wnt/β-catenin signaling is an ancient and highly conserved system that controls embryonic development and gene homeostasis. According to previous studies, activated Wnt/β-catenin signaling is a key feature of epithelial cancers that regulates self-renewal and differentiation in several adult stem cell niches and interacts with the surrounding microenvironment to influence stemness, cell proliferation and invasive behavior of tumor cells.67,68) Activation of Wnt/β-catenin signaling enhances the motility of GBM cells and contributes to the mesenchymal transition.69) Gene ontology analysis, and pathway-specific gene-expression profiling, also showed that high MGMT expression is significantly related to aberrant Wnt signaling.70) Therefore, Wnt signaling may provide a novel approach to GSC treatment. Wnt signaling inhibitors celecoxib, salinomycin, and porcupine inhibitor Wnt-C59, and genetic inhibition of Wnt signaling by specific shRNA, suppressed MGMT expression.70) The combination of Wnt signaling inhibitors with TMZ restores TMZ sensitivity in vivo.70) The above data illustrates that inhibition of Wnt/β-catenin signaling may offer a potential strategy to treat chemo-resistant GSCs expressing MGMT.
MGMT independent manner
Aurora-A kinase inhibitor suppresses GSC and potentiates TMZ efficacy.
Aurora-A is a serine-threonine kinase essential to centrosome maturation and mitotic entry and exit.71,72) Overexpression of Aurora-A has been reported in several human tumors, including gliomas.73–75) Moreover, Aurora-A kinase activity influences multiple signaling pathways related to cell growth and differentiation and is associated with GBM patient survival.76) Inhibition of Aurora-A by MLN8237, a selective Aurora-A kinase inhibitor, suppressed the ability to form GSC tumor spheres and synergistically potentiated TMZ chemosensitivity in combination treatment.76,77) When treating glioma cell lines with MLN8237 combined with TMZ, it strongly suppressed cell proliferation and significantly inhibited colony formation of GSCs compared to either MLN8237 or TMZ alone.77) Interestingly, MLN8237 at a low concentration renders GSCs sensitivity to radiation therapy.76,77) In summary, Aurora-A kinase may be a new molecular target for GSC treatment.
-
Inhibition of SOX2 decreased GSC activity and TMZ resistance.
Sex-determining region X (SOX) and sex-determining region Y (SRY)—box is a family of transcriptional factors characterized by conserved high mobility group DNA-binding domains that control several important functions and processes involved in the maintenance of stem cell properties in lots of tissues during embryonic development and adulthood, while its genetic inactivation induces stem cell differentiation.78–80) SOX2, a member of the SOX family, is overexpressed in clinical GBM samples and higher SOX2 levels are correlated with tumor aggressiveness and poor prognosis.10,81) Except for promoting the maintenance of GSC stemness, SOX2 also contributes to TMZ resistance.10) These results indicate the potential to target SOX2 as a strategy to eliminate GSCs and potentiate TMZ sensitivity. Several strategies have been proposed to target SOX2, directly or indirectly, to overcome GSCs. Indirect inhibition of SOX2, by inhibition of its upstream molecules such as PDGFR, SHH, and mTOR, suppressed tumor growth significantly through SOX2 downregulation and GSC sensitization. Meanwhile, increased cytotoxicity was observed in GSCs when SOX2 inhibition was combined with TMZ, except for the SHH inhibitor.10,82,83) Immunotherapy, using a peptide vaccination against SOX2, prolonged the survival of GSCs transplanted mice by monotherapy and the vaccination, in combination with TMZ, doubled the survival time.84) Another study showed that direct inhibition of SOX2, using miRNA delivery in GSCs, strongly suppressed tumorigenicity in a mouse xenograft model and increased GSC chemosensitivity to TMZ.85) These data prove that inhibiting SOX2 is a promising and effective strategy targeting GSCs.
-
BMP as new agent to eliminate stemness and chemoresistance.
Bone morphogenetic proteins (BMP) are a member of the TGF superfamily demonstrated to be involved in cell growth, differentiation, and defining stem cell properties. BMP, especially BMP4, acts as a negative regulator of GSC behaviors. Administration of BMP4 prevents tumor growth and motility through BMP receptor-mediated Smad activation, along with induction of anti-proliferative differentiation.14,86) A high-density microarray analysis using high-dose TMZ resistant GSCs demonstrated that BMP7 was the most down-regulated gene, which indicated that internal BMP7 expression correlated with TMZ resistant.87) Administration of BMP7 made highly TMZ resistant GSCs sensitive and suppressed cell proliferation and migration. Furthermore, in a mouse xenograft model, BMP7 treatment synergized to improve TMZ efficacy and extended survival significantly as compared to TMZ alone.87) The main reason for BMP7 potentiation of the TMZ effect may be because BMP activation suppresses the stem cell properties and protective factors involved in chemoresistance, thereby sensitizing GSCs to low-dose TMZ treatment. Overall, these findings indicate that identification of BMPs in GSCs have advantages in individualized treatment, providing an effective strategy for GBM treatment targeting GSCs based on TMZ.
Other therapies that might potentiate the TMZ effect Existing treatments (for GBM or under clinical trial)
-
Anti-angiogenic therapy
An anti-vascular endothelial growth factor (VEGF) antibody bevacizumab with TMZ therapy unfortunately showed no survival benefit for GBM patients in two randomized clinical trials (AVAglio, RTOG0825).88,89) Interestingly, subanalysis of the AVAglio study revealed that the anti-angiogenic therapy could improve clinical symptoms by reducing cerebral edema, resulting in better quality of life. Recently, bevacizumab combined with ddTMZ regimen is expected to have efficacy on progressive or recurrent GBM previously exposed to TMZ.90,91)
-
Tumor treating fields (TTF)
TTF are electric fields of low intensity and intermediate frequency. They demonstrated that cell death is induced by anti-mitotic properties in several cancers. For GBM, its therapeutic potential was first reported in 2007.92) Thereafter, clinical trials were performed for recurrent patients (EF-11 study)93) and then for primary GBM patients (EF-14 study).94) In those studies, TTF with TMZ therapy prolonged both PFS and OS compared with TMZ alone. TTF might delay repair of DNA damage by TMZ,95) suggesting that TTF could potentiate the effect of TMZ. However, cost-effectiveness should be considered.96)
-
Immunotherapy
Cancer immunotherapy is the process by which the body activates its own immune system to fight against existing cancer cells. For several cancers, such as malignant melanoma, prostate cancer, and lung cancer, a few kinds of immunotherapies are incorporated into the standard therapies.97) A variety of immunotherapies, dendritic cell (DC) vaccines, peptide vaccines, such as the EGFRvIII and WT-1, tumor antigen vaccines, adoptive immunotherapy, therapy with NK cells derived from umbilical cord blood, oncolytic virus therapy, and gene transfer therapy, are reported.97–99) For cancer immunotherapy, effective antigens exposed to T cells would be an essential step to generate and maintain immune responses to cancer cells.100) Several GBM antigens have been identified as potential immunotherapeutic targets.101) Some trails assessing the combination of TMZ chemotherapy with immunotherapies also showed enhanced anti-glioma effects in highly TMZ resistant cases.102) Therefore, immunotherapy is also a promising new potential therapeutic strategy for GBM.
Recently, immunotherapy with fusions of DCs and glioma cells (FC therapy) appears promising in GBM patients. In phase I/II trail, treating GBM patients with a combination of standard TMZ chemotherapy and DC-based vaccination significantly prolonged mOS and PFS,102–105) as compared with the standard radio-chemotherapy treatments.6) FC therapy helped the immune system recognize and eliminate the TMZ-induced chemo-resistant peptides, such as WT-1, gp100, and MAGE-A3.102) Aglatimagene besadenovec (AdV-tk) plus valacyclovir (gene-mediated cytotoxic immunotherapy), autologous formalin-fixed tumor vaccine, and dendritic cell vaccination are also promising therapies.106–108) However, tumor-mediated immunosuppression masked the effect when the residual tumor burden is large.107,109) The EGFRvIII vaccine did not demonstrate a benefit for survival.110,111)
-
Immune checkpoint inhibitors
The cytotoxic T-lymphocyte-associated antigen 4 (CTLA-4) and programmed death 1 (PD-1) immune checkpoint pathways are negative regulators of T-cell immune function.112) Flow cytometric analysis on tumor infiltrating lymphocytes showed that, in patients with GBM, the expression of immune checkpoint molecules like PD-1 and CTLA-4 were significantly higher than that in T-cells from peripheral blood mononuclear cells (PBMCs).113) Glioma cells evade immune surveillance by expressing PD-1, CTLA-4, and other immune checkpoint molecules that negatively regulate cytotoxic T-lymphocytes (CTL)114,115) (109, 110) and activate regulatory T-cells (Treg), which suppresses effector T-cells.112) Collectively, PD-1/PD-L1 inhibitors and CTLA-4 inhibitors, called immune checkpoint inhibitors, have been developed for several cancers, including GBMs.114,116,117) Therefore, immune checkpoint inhibitors (nivolumab, pembrolizumab, and ipilimumab) are potential novel therapeutics for GBM.118) Nivolumab has been assessed in a large scaled trial for recurrent GBM treatment (CheckMate-143 trial), but it did not demonstrate a remarkable effect when compared with consistent chemotherapy,119) though the currently randomized trials for newly diagnosed GBM (CheckMate-498, 548) are ongoing. The clinical trials for other inhibitors (NCT 02337686, RTOG 1125, etc.) are currently underway; therefore, the effect of these treatments is yet to be established. The results of these studies, and the analyses of interactions between these drugs and TMZ in GBM, are anticipated.
Potential treatment
-
Existed drugs or techniques
Metformin (MET), the first-line drug for treating diabetes, has been proven to suppresses cell proliferation and selectively kill cancer stem cells.120,121) This common medicine modulated apoptosis by increasing the Bax/Bcl-2 ratio, reduced reactive oxygen species (ROS) production, and inhibited the TGF-β 1-induced epithelial-mesenchymal transition-like process and stem-like properties in GBM cells via the AKT/mTOR/ZEB1 pathway.122,123) It is also been reported that MET sensitized TMZ cytotoxicity to GBMs when employed in combination.124,125) AKT inhibitors or PI3K/mTOR inhibitors also potentiate TMZ.126,127) MET is a potential novel therapeutic directing drug repositioning for GBM. For other drugs, glycogen synthase kinase 3β (GSK3β) inhibitors, cyclin dependent kinase (CDK) inhibitors, and other drugs used for clinical treatment of various diseases have been identified as TMZ enhancers via apoptosis or inhibition of DNA repair56,57,128–134) (Table 1).
-
Targeted molecules
Mutations in ATRX are well known as the most prevalent genetic abnormalities in diffuse astrocytoma with IDH mutation. The presence of ATRX mutations in glioma implies an increased sensitivity to radiotherapy and DNA-damaging agents that primarily induce double-stranded breaks.135,136) Knockdown of ATRX indicates suppression of ATM (ataxia telangiectasia, mutated) dependent DNA damage repair by modulating histone H3 lysine 9 trimethylation (H3K9me3) to enhance TMZ.137) Nuclear factor erythroid 2-related factor 2 (Nrf2) is a redox-sensitive transcription factor reported to regulate the expression of various cytoprotective genes.138,139) Constitutive Nrf2 activation in many cancers enhances cell survival and resistance to anti-cancer drugs,140,141) including TMZ,142) through glutathione (GSH) synthesis, which plays a critical role in neutralizing reactive oxygen species (ROS) induced by chemotherapy.142) Inhibition or genetic silencing of Nrf2 sensitizes highly resistant GBM to TMZ.142,143)
With the identification of oncogenic microRNAs (miRs), and their critical function in tumorigenesis, sequence-specific targeting of growth promoting miRs has emerged a novel and promising therapeutic avenue.144) Many reports have identified miRs are an important key to regulating many cellular behavior, such as cell growth, stemness, apoptosis, and drug resistance of GBM.145,146) As miR-125b has been proven necessary for GSCs fission and for making stem cells insensitive to chemotherapy, inhibition of miR-125b demonstrates increased apoptosis targeting Bak1.147) MiR-128 is upregulated in TMZ-treated glioma cells and considered to be one of the reasons for TMZ-induced apoptotic cytotoxicity via JNK2/c-Jun signaling-mediated mTOR-inhibition.148) MiR-141-3p promotes glioma cell growth and TMZ resistance by directly targeting p53,149) indicating that inhibition of miR-141-3p would provide new therapeutic methods for GBM treatment. Moreover, miR-16 mediates TMZ resistance in glioma cells by modulation of apoptosis via targeting Bcl-2, suggesting that miR-16 and Bcl-2 could be potential therapeutic targets for glioma therapy.150) Introduction of miR-17, miR-21, miR-30a, and miR-101 into chemo-resistant glioma also resulted in an increase in chemosensitivity to TMZ treatment by various regulating pathways and mechanisms.151–154) There are several miRs reported to be overexpressed in GBM compared to normal brain tissues;145) therefore, there is always a potential to provide a broader view in the exploration for new anti-glioma agents. In the near future, more novel breakthroughs in the area of miRs are expected.
Table 1.
Existing TMZ enhancing drugs
| Classification | Common indications | Administration | Mechanism of combination with TMZ | References |
|---|---|---|---|---|
| GSK3β inhibitors | ||||
| Cimetidine (Tagamet) | Gastroduodenal ulcer | IV injection or oral | GSK3β inhibition | 57) |
| Lithium (LIMAS) | Mood disorder | Oral | ||
| Olanzapine (Zyprexa) | Mood disorder | Oral | ||
| Valproate (Depaken) | Seizure | IV injection or oral | ||
| PI3K inhibitors | ||||
| Dactolisib | Breast cancer (trial) | Oral | PI3K/mTOR inhibition | 128, 129) |
| CDK inhibitors | ||||
| Flavopiridol (Aivocidib) | Acute myeloid leukemia | IV injection | Suppresses DNA repair activity in the G2/M transition | 130) |
| Palbociclib (Ibrance) | ER(+)/HER(−) breast cancer | |||
| Abemaciclib (Verzenio) | Advanced or metastatic breast cancer | Oral | Inhibit CDK4/6 to influence cell cycle by inducing G1 arrest and apoptosis | 131) |
| Others | ||||
| Glucophage (metformin) | Type-2 diabetes | Oral | Akt/mTOR inhibition | 120, 121, 124, 125) |
| Levetiracetam (Keppra) | Epilepsy | IV injection or oral | Demethylate the methylated MGMT promoter via suppressing wt-p53 MGMT suppressive function | 133) |
| Bevacizumab (Avastin) | Colorectal cancer, lung cancer, breast cancer, brain cancer eye disease | IV injection | Blocks angiogenetic VEGF-A | 134) |
CDK: cyclin dependent kinase, ER: estrogen receptor, GSK3β: glycogen synthase kinase 3β, HER: human epidermal growth factor, IV: intravenous injection, MGMT: methylguanine methyltransferase, mTOR: mammalian target of rapamycin, PI3K: phosphoinositide 3-kinase, TMZ: temozolomide, VEGF: vascular endothelial growth factor.
Future Prospective
TMZ has been used as standard chemotherapy for malignant glioma since 2005. To date, various kinds of clinical trial have been performed for GBM with novel drugs. However, no drugs exceeded the effect of TMZ. Therefore, understanding the mechanisms of TMZ resistance and identifying the novel modalities of therapy overcoming chemoresistance remains an important focus in the management of GBM patients. While the understanding of these mechanisms underlying intrinsic and acquired chemoresistance in GBM is expanding rapidly, promising therapeutic options will hopefully be discovered in the near future.
Acknowledgments
We thank Otsuka Toshimi Scholarship Foundation and Fujii International Scholarship Foundation for financial support.
Footnotes
Conflicts of Interest Disclosure
The authors declare that they have no conflict of interest. All authors who are the members of The Japan Neurosurgical Society (JNS) have registered online self-reported COI Disclosure Statement Forms through the website for JNS members.
References
- 1).Yan Y, Xu Z, Dai S, Qian L, Sun L, Gong Z: Targeting autophagy to sensitive glioma to temozolomide treatment. J Exp Clin Cancer Res 35: 23, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2).Hirst TC, Vesterinen HM, Sena ES, Egan KJ, Macleod MR, Whittle IR: Systematic review and meta-analysis of temozolomide in animal models of glioma: was clinical efficacy predicted? Br J Cancer 108: 64–71, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3).Wen PY, Kesari S: Malignant gliomas in adults. N Engl J Med 359: 492–507, 2008 [DOI] [PubMed] [Google Scholar]
- 4).Stevens MF, Hickman JA, Langdon SP, et al. : Antitumor activity and pharmacokinetics in mice of 8-carbamoyl-3-methyl-imidazo[5,1-d]-1,2,3,5-tetrazin-4(3H)-one (CCRG 81045; M & B 39831), a novel drug with potential as an alternative to dacarbazine. Cancer Res 47: 5846–5852, 1987 [PubMed] [Google Scholar]
- 5).Nanegrungsunk D, Onchan W, Chattipakorn N, Chattipakorn SC: Current evidence of temozolomide and bevacizumab in treatment of gliomas. Neurol Res 37: 167–183, 2015 [DOI] [PubMed] [Google Scholar]
- 6).Stupp R, Mason WP, van den Bent MJ, et al. European Organisation for Research and Treatment of Cancer Brain Tumor and Radiotherapy Groups. National Cancer Institute of Canada Clinical Trials Group : Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med 352: 987–996, 2005 [DOI] [PubMed] [Google Scholar]
- 7).Kanzawa T, Bedwell J, Kondo Y, Kondo S, Germano IM: Inhibition of DNA repair for sensitizing resistant glioma cells to temozolomide. J Neurosurg 99: 1047–1052, 2003 [DOI] [PubMed] [Google Scholar]
- 8).Lee SY: Temozolomide resistance in glioblastoma multiforme. Genes & Diseases 3: 198–210, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9).Perazzoli G, Prados J, Ortiz R, et al. : Temozolomide resistance in glioblastoma cell lines: implication of MGMT, MMR, P-Glycoprotein and CD133 expression. PLoS ONE 10: e0140131, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10).Garros-Regulez L, Aldaz P, Arrizabalaga O, et al. : mTOR inhibition decreases SOX2-SOX9 mediated glioma stem cell activity and temozolomide resistance. Expert Opin Ther Targets 20: 393–405, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11).Huse JT, Holland EC: Targeting brain cancer: advances in the molecular pathology of malignant glioma and medulloblastoma. Nat Rev Cancer 10: 319–331, 2010 [DOI] [PubMed] [Google Scholar]
- 12).Knisely JP, Baehring JM: A silver lining on the horizon for glioblastoma. Lancet Oncol 10: 434–435, 2009 [DOI] [PubMed] [Google Scholar]
- 13).Lapidot T, Sirard C, Vormoor J, et al. : A cell initiating human acute myeloid leukaemia after transplantation into SCID mice. Nature 367: 645–648, 1994 [DOI] [PubMed] [Google Scholar]
- 14).Cruceru ML, Neagu M, Demoulin JB, Constantinescu SN: Therapy targets in glioblastoma and cancer stem cells: lessons from haematopoietic neoplasms. J Cell Mol Med 17: 1218–1235, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15).Mimeault M, Hauke R, Mehta PP, Batra SK: Recent advances in cancer stem/progenitor cell research: therapeutic implications for overcoming resistance to the most aggressive cancers. J Cell Mol Med 11: 981–1011, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16).Hambardzumyan D, Squatrito M, Carbajal E, Holland EC: Glioma formation, cancer stem cells, and akt signaling. Stem Cell Rev 4: 203–210, 2008 [DOI] [PubMed] [Google Scholar]
- 17).Nakada M, Furuta T, Hayashi Y, Minamoto T, Hamada J: The strategy for enhancing temozolomide against malignant glioma. Front Oncol 2: 98, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18).Van Meir EG, Hadjipanayis CG, Norden AD, Shu HK, Wen PY, Olson JJ: Exciting new advances in neuro-oncology: the avenue to a cure for malignant glioma. CA Cancer J Clin 60: 166–193, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19).Atkins RJ, Ng W, Stylli SS, Hovens CM, Kaye AH: Repair mechanisms help glioblastoma resist treatment. J Clin Neurosci 22: 14–20, 2015 [DOI] [PubMed] [Google Scholar]
- 20).Gil Del Alcazar CR, Todorova PK, Habib AA, Mukherjee B, Burma S: Augmented HR repair mediates acquired temozolomide resistance in glioblastoma. Mol Cancer Res 14: 928–940, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21).Messaoudi K, Clavreul A, Lagarce F: Toward an effective strategy in glioblastoma treatment. Part I: resistance mechanisms and strategies to overcome resistance of glioblastoma to temozolomide. Drug Discov Today 20: 899–905, 2015 [DOI] [PubMed] [Google Scholar]
- 22).Nagel ZD, Kitange GJ, Gupta SK, et al. : DNA repair capacity in multiple pathways predicts chemoresistance in glioblastoma multiforme. Cancer Res 77: 198–206, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23).Hombach-Klonisch S, Mehrpour M, Shojaei S, et al. : Glioblastoma and chemoresistance to alkylating agents: involvement of apoptosis, autophagy, and unfolded protein response. Pharmacol Ther 184: 13–41, 2018 [DOI] [PubMed] [Google Scholar]
- 24).Yamada R, Nakano I: Glioma stem cells: their role in chemoresistance. World Neurosurg 77: 237–240, 2012 [DOI] [PubMed] [Google Scholar]
- 25).Yip S, Miao J, Cahill DP, et al. : MSH6 mutations arise in glioblastomas during temozolomide therapy and mediate temozolomide resistance. Clin Cancer Res 15: 4622–4629, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26).Jung TY, Jung S, Moon KS, et al. : Changes of the O6-methylguanine-DNA methyltransferase promoter methylation and MGMT protein expression after adjuvant treatment in glioblastoma. Oncol Rep 23: 1269–1276, 2010 [DOI] [PubMed] [Google Scholar]
- 27).Erasimus H, Gobin M, Niclou S, Van Dyck E: DNA repair mechanisms and their clinical impact in glioblastoma. Mutat Res Rev Mutat Res 769: 19–35, 2016 [DOI] [PubMed] [Google Scholar]
- 28).Li S, Wang L, Hu Y, Sheng R: Autophagy regulators as potential cancer therapeutic agents: a review. Curr Top Med Chem 15: 720–744, 2015 [DOI] [PubMed] [Google Scholar]
- 29).Rebecca VW, Amaravadi RK: Emerging strategies to effectively target autophagy in cancer. Oncogene 35: 1–11, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30).Thorburn A, Thamm DH, Gustafson DL: Autophagy and cancer therapy. Mol Pharmacol 85: 830–838, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31).Kanzawa T, Germano IM, Komata T, Ito H, Kondo Y, Kondo S: Role of autophagy in temozolomide-induced cytotoxicity for malignant glioma cells. Cell Death Differ 11: 448–457, 2004 [DOI] [PubMed] [Google Scholar]
- 32).Zou Y, Wang Q, Li B, Xie B, Wang W: Temozolomide induces autophagy via ATM AMPK ULK1 pathways in glioma. Mol Med Rep 10: 411–416, 2014 [DOI] [PubMed] [Google Scholar]
- 33).Ogier-Denis E, Codogno P: Autophagy: a barrier or an adaptive response to cancer. Biochim Biophys Acta 1603: 113–128, 2003 [DOI] [PubMed] [Google Scholar]
- 34).Paglin S, Hollister T, Delohery T, et al. : A novel response of cancer cells to radiation involves autophagy and formation of acidic vesicles. Cancer Res 61: 439–444, 2001 [PubMed] [Google Scholar]
- 35).Altan N, Chen Y, Schindler M, Simon SM: Defective acidification in human breast tumor cells and implications for chemotherapy. J Exp Med 187: 1583–1598, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36).Hirose Y, Berger MS, Pieper RO: p53 effects both the duration of G2/M arrest and the fate of temozolomide-treated human glioblastoma cells. Cancer Res 61: 1957–1963, 2001 [PubMed] [Google Scholar]
- 37).Sui X, Chen R, Wang Z, et al. : Autophagy and chemotherapy resistance: a promising therapeutic target for cancer treatment. Cell Death Dis 4: e838, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38).Sanchez AM, Csibi A, Raibon A, et al. : AMPK promotes skeletal muscle autophagy through activation of forkhead FoxO3a and interaction with Ulk1. J Cell Biochem 113: 695–710, 2012 [DOI] [PubMed] [Google Scholar]
- 39).Bao S, Wu Q, McLendon RE, et al. : Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature 444: 756–760, 2006 [DOI] [PubMed] [Google Scholar]
- 40).Mao P, Joshi K, Li J, et al. : Mesenchymal glioma stem cells are maintained by activated glycolytic metabolism involving aldehyde dehydrogenase 1A3. Proc Natl Acad Sci USA 110: 8644–8649, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41).Oberstadt MC, Bien-Möller S, Weitmann K, et al. : Epigenetic modulation of the drug resistance genes MGMT, ABCB1 and ABCG2 in glioblastoma multiforme. BMC Cancer 13: 617, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42).Yuen CA, Asuthkar S, Guda MR, Tsung AJ, Velpula KK: Cancer stem cell molecular reprogramming of the Warburg effect in glioblastomas: a new target gleaned from an old concept. CNS Oncol 5: 101–108, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43).Banissi C, Ghiringhelli F, Chen L, Carpentier AF: Treg depletion with a low-dose metronomic temozolomide regimen in a rat glioma model. Cancer Immunol Immunother 58: 1627–1634, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44).Nagane M: Dose-dense temozolomide: is it still promising? Neurol Med Chir (Tokyo) 55 Suppl 1: 38–49, 2015 [PubMed] [Google Scholar]
- 45).Gilbert MR, Wang M, Aldape KD, et al. : Dose-dense temozolomide for newly diagnosed glioblastoma: a randomized phase III clinical trial. J Clin Oncol 31: 4085–4091, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46).Wei W, Chen X, Ma X, Wang D, Guo Z: The efficacy and safety of various dose-dense regimens of temozolomide for recurrent high-grade glioma: a systematic review with meta-analysis. J Neurooncol 125: 339–349, 2015 [DOI] [PubMed] [Google Scholar]
- 47).Tabatabai G, Wick W, Steinbach JP, et al. : MGMT promoter methylation as a prognostic biomarker for benefit from dose-intensified temozolomide rechallenge in progressive glioblastoma: first results from the randomized phase II DIRECTOR trial. J Clin Oncol 32: Suppl 5 Abst-2015, 2014 [DOI] [PubMed] [Google Scholar]
- 48).Neyns B, Tosoni A, Hwu WJ, Reardon DA: Dose-dense temozolomide regimens: antitumor activity, toxicity, and immunomodulatory effects. Cancer 116: 2868–2877, 2010 [DOI] [PubMed] [Google Scholar]
- 49).Mao L, Whitehead CA, Paradiso L, et al. : Enhancement of invadopodia activity in glioma cells by sublethal doses of irradiation and temozolomide. J Neurosurg 129: 598–610, 2018 [DOI] [PubMed] [Google Scholar]
- 50).Ohmoto A, Yachida S: Current status of poly(ADP-ribose) polymerase inhibitors and future directions. Onco Targets Ther 10: 5195–5208, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51).Lu Y, Kwintkiewicz J, Liu Y, et al. : Chemosensitivity of IDH1-mutated gliomas due to an impairment in PARP1-mediated DNA repair. Cancer Res 77: 1709–1718, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52).Tateishi K, Higuchi F, Miller JJ, et al. : The alkylating chemotherapeutic temozolomide induces metabolic stress in IDH1-mutant cancers and potentiates NAD+ depletion-mediated cytotoxicity. Cancer Res 77: 4102–4115, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53).Quinn JA, Jiang SX, Reardon DA, et al. : Phase II trial of temozolomide plus o6-benzylguanine in adults with recurrent, temozolomide-resistant malignant glioma. J Clin Oncol 27: 1262–1267, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54).Natsume A, Wakabayashi T, Ishii D, et al. : A combination of IFN-beta and temozolomide in human glioma xenograft models: implication of p53-mediated MGMT downregulation. Cancer Chemother Pharmacol 61: 653–659, 2008 [DOI] [PubMed] [Google Scholar]
- 55).Wakabayashi T, Natsume A, Mizusawa J, et al. : At-58JCOG0911 INTEGRA trial: a randomized screening phase II trial of chemoradiotherapy with interferonβ plus temozolomide versus chemoradiotherapy with temozolomide alone for newly-diagnosed glioblastoma. Neuro Oncol 16: v21, 2014 [Google Scholar]
- 56).Pyko IV, Nakada M, Sabit H, et al. : Glycogen synthase kinase 3β inhibition sensitizes human glioblastoma cells to temozolomide by affecting O6-methylguanine DNA methyltransferase promoter methylation via c-Myc signaling. Carcinogenesis 34: 2206–2217, 2013 [DOI] [PubMed] [Google Scholar]
- 57).Furuta T, Sabit H, Dong Y, et al. : Biological basis and clinical study of glycogen synthase kinase- 3β-targeted therapy by drug repositioning for glioblastoma. Oncotarget 8: 22811–22824, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58).Davis RJ: Signal transduction by the JNK group of MAP kinases. Cell 103: 239–252, 2000 [DOI] [PubMed] [Google Scholar]
- 59).Okada M, Kuramoto K, Takeda H, et al. : The novel JNK inhibitor AS602801 inhibits cancer stem cells in vitro and in vivo. Oncotarget 7: 27021–27032, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60).Matsuda K, Sato A, Okada M, et al. : Targeting JNK for therapeutic depletion of stem-like glioblastoma cells. Sci Rep 2: 516, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61).Okada M, Sato A, Shibuya K, et al. : JNK contributes to temozolomide resistance of stem-like glioblastoma cells via regulation of MGMT expression. Int J Oncol 44: 591–599, 2014 [DOI] [PubMed] [Google Scholar]
- 62).Lopez-Gines C, Gil-Benso R, Benito R, et al. : The activation of ERK1/2 MAP kinases in glioblastoma pathobiology and its relationship with EGFR amplification. Neuropathology 28: 507–515, 2008 [DOI] [PubMed] [Google Scholar]
- 63).Mizoguchi M, Betensky RA, Batchelor TT, Bernay DC, Louis DN, Nutt CL: Activation of STAT3, MAPK, and AKT in malignant astrocytic gliomas: correlation with EGFR status, tumor grade, and survival. J Neuropathol Exp Neurol 65: 1181–1188, 2006 [DOI] [PubMed] [Google Scholar]
- 64).Motta C, D’Angeli F, Scalia M, et al. : PJ-34 inhibits PARP-1 expression and ERK phosphorylation in glioma-conditioned brain microvascular endothelial cells. Eur J Pharmacol 761: 55–64, 2015 [DOI] [PubMed] [Google Scholar]
- 65).Harris LC, Remack JS, Houghton PJ, Brent TP: Wild-type p53 suppresses transcription of the human O6-methylguanine-DNA methyltransferase gene. Cancer Res 56: 2029–2032, 1996 [PubMed] [Google Scholar]
- 66).Sato A, Sunayama J, Matsuda K, et al. : MEK-ERK signaling dictates DNA-repair gene MGMT expression and temozolomide resistance of stem-like glioblastoma cells via the MDM2-p53 axis. Stem Cells 29: 1942–1951, 2011 [DOI] [PubMed] [Google Scholar]
- 67).Fodde R, Brabletz T: Wnt/beta-catenin signaling in cancer stemness and malignant behavior. Curr Opin Cell Biol 19: 150–158, 2007 [DOI] [PubMed] [Google Scholar]
- 68).Wend P, Holland JD, Ziebold U, Birchmeier W: Wnt signaling in stem and cancer stem cells. Semin Cell Dev Biol 21: 855–863, 2010 [DOI] [PubMed] [Google Scholar]
- 69).Kahlert UD, Maciaczyk D, Doostkam S, et al. : Activation of canonical WNT/β-catenin signaling enhances in vitro motility of glioblastoma cells by activation of ZEB1 and other activators of epithelial-to-mesenchymal transition. Cancer Lett 325: 42–53, 2012 [DOI] [PubMed] [Google Scholar]
- 70).Wickström M, Dyberg C, Milosevic J, et al. : Wnt/β-catenin pathway regulates MGMT gene expression in cancer and inhibition of Wnt signalling prevents chemoresistance. Nat Commun 6: 8904, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71).Marumoto T, Honda S, Hara T, et al. : Aurora-A kinase maintains the fidelity of early and late mitotic events in HeLa cells. J Biol Chem 278: 51786–51795, 2003 [DOI] [PubMed] [Google Scholar]
- 72).Seki A, Coppinger JA, Jang CY, Yates JR, Fang G: Bora and the kinase Aurora a cooperatively activate the kinase Plk1 and control mitotic entry. Science 320: 1655–1658, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73).Jeng YM, Peng SY, Lin CY, Hsu HC: Overexpression and amplification of Aurora-A in hepatocellular carcinoma. Clin Cancer Res 10: 2065–2071, 2004 [DOI] [PubMed] [Google Scholar]
- 74).Klein A, Reichardt W, Jung V, Zang KD, Meese E, Urbschat S: Overexpression and amplification of STK15 in human gliomas. Int J Oncol 25: 1789–1794, 2004 [PubMed] [Google Scholar]
- 75).Li D, Zhu J, Firozi PF, et al. : Overexpression of oncogenic STK15/BTAK/Aurora A kinase in human pancreatic cancer. Clin Cancer Res 9: 991–997, 2003 [PubMed] [Google Scholar]
- 76).Lehman NL, O’Donnell JP, Whiteley LJ, et al. : Aurora A is differentially expressed in gliomas, is associated with patient survival in glioblastoma and is a potential chemotherapeutic target in gliomas. Cell Cycle 11: 489–502, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77).Hong X, O’Donnell JP, Salazar CR, et al. : The selective Aurora-A kinase inhibitor MLN8237 (alisertib) potently inhibits proliferation of glioblastoma neurosphere tumor stem-like cells and potentiates the effects of temozolomide and ionizing radiation. Cancer Chemother Pharmacol 73: 983–990, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78).Favaro R, Valotta M, Ferri AL, et al. : Hippocampal development and neural stem cell maintenance require Sox2-dependent regulation of Shh. Nat Neurosci 12: 1248–1256, 2009 [DOI] [PubMed] [Google Scholar]
- 79).Kamachi Y, Kondoh H: Sox proteins: regulators of cell fate specification and differentiation. Development 140: 4129–4144, 2013 [DOI] [PubMed] [Google Scholar]
- 80).Sarkar A, Hochedlinger K: The sox family of transcription factors: versatile regulators of stem and progenitor cell fate. Cell Stem Cell 12: 15–30, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81).Ben-Porath I, Thomson MW, Carey VJ, et al. : An embryonic stem cell-like gene expression signature in poorly differentiated aggressive human tumors. Nat Genet 40: 499–507, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82).Garros-Regulez L, Garcia I, Carrasco-Garcia E, et al. : Targeting SOX2 as a therapeutic strategy in glioblastoma. Front Oncol 6: 222, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83).Hägerstrand D, He X, Bradic Lindh M, et al. : Identification of a SOX2-dependent subset of tumor- and sphere-forming glioblastoma cells with a distinct tyrosine kinase inhibitor sensitivity profile. Neuro-oncology 13: 1178–1191, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84).Favaro R, Appolloni I, Pellegatta S, et al. : Sox2 is required to maintain cancer stem cells in a mouse model of high-grade oligodendroglioma. Cancer Res 74: 1833–1844, 2014 [DOI] [PubMed] [Google Scholar]
- 85).Yang YP, Chien Y, Chiou GY, et al. : Inhibition of cancer stem cell-like properties and reduced chemoradioresistance of glioblastoma using microRNA145 with cationic polyurethane-short branch PEI. Biomaterials 33: 1462–1476, 2012 [DOI] [PubMed] [Google Scholar]
- 86).Piccirillo SG, Reynolds BA, Zanetti N, et al. : Bone morphogenetic proteins inhibit the tumorigenic potential of human brain tumour-initiating cells. Nature 444: 761–765, 2006 [DOI] [PubMed] [Google Scholar]
- 87).Tso JL, Yang S, Menjivar JC, et al. : Bone morphogenetic protein 7 sensitizes O6-methylguanine methyltransferase expressing-glioblastoma stem cells to clinically relevant dose of temozolomide. Mol Cancer 14: 189, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88).Gilbert MR, Dignam JJ, Armstrong TS, et al. : A randomized trial of bevacizumab for newly diagnosed glioblastoma. N Engl J Med 370: 699–708, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89).Chinot OL, Wick W, Mason W, et al. : Bevacizumab plus radiotherapy-temozolomide for newly diagnosed glioblastoma. N Engl J Med 370: 709–722, 2014 [DOI] [PubMed] [Google Scholar]
- 90).Gilbert MR, Pugh SL, Aldape K, et al. : NRG oncology RTOG 0625: a randomized phase II trial of bevacizumab with either irinotecan or dose-dense temozolomide in recurrent glioblastoma. J Neurooncol 131: 193–199, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91).Peters KB, Lipp ES, Miller E, et al. : Phase I/II trial of vorinostat, bevacizumab, and daily temozolomide for recurrent malignant gliomas. J Neurooncol 137: 349–356, 2018 [DOI] [PubMed] [Google Scholar]
- 92).Kirson ED, Dbalý V, Tovarys F, et al. : Alternating electric fields arrest cell proliferation in animal tumor models and human brain tumors. Proc Natl Acad Sci USA 104: 10152–10157, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93).Stupp R, Wong ET, Kanner AA, et al. : NovoTTF-100A versus physician’s choice chemotherapy in recurrent glioblastoma: a randomised phase III trial of a novel treatment modality. Eur J Cancer 48: 2192–2202, 2012 [DOI] [PubMed] [Google Scholar]
- 94).Stupp R, Taillibert S, Kanner A, et al. : Effect of tumor-treating fields plus maintenance temozolomide vs maintenance temozolomide alone on survival in patients with glioblastoma: a randomized clinical trial. JAMA 318: 2306–2316, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95).Giladi M, Munster M, Schneiderman RS, et al. : Tumor treating fields (TTFields) delay DNA damage repair following radiation treatment of glioma cells. Radiat Oncol 12: 206, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96).Bernard-Arnoux F, Lamure M, Ducray F, Aulagner G, Honnorat J, Armoiry X: The cost-effectiveness of tumor-treating fields therapy in patients with newly diagnosed glioblastoma. Neuro-oncology 18: 1129–1136, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97).Zhou Q, Wang Y, Ma W: The progress of immunotherapy for glioblastoma. Hum Vaccin Immunother 11: 2654–2658, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98).Desai R, Suryadevara CM, Batich KA, Farber SH, Sanchez-Perez L, Sampson JH: Emerging immunotherapies for glioblastoma. Expert Opin Emerg Drugs 21: 133–145, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99).Boussiotis VA, Charest A: Immunotherapies for malignant glioma. Oncogene 37: 1121–1141, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100).Gunzer M, Jänich S, Varga G, Grabbe S: Dendritic cells and tumor immunity. Semin Immunol 13: 291–302, 2001 [DOI] [PubMed] [Google Scholar]
- 101).Reardon DA, Wucherpfennig KW, Freeman G, et al. : An update on vaccine therapy and other immunotherapeutic approaches for glioblastoma. Expert Rev Vaccines 12: 597–615, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102).Akasaki Y, Kikuchi T, Homma S, et al. : Phase I/II trial of combination of temozolomide chemotherapy and immunotherapy with fusions of dendritic and glioma cells in patients with glioblastoma. Cancer Immunol Immunother 65: 1499–1509, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103).Bregy A, Wong TM, Shah AH, Goldberg JM, Komotar RJ: Active immunotherapy using dendritic cells in the treatment of glioblastoma multiforme. Cancer Treat Rev 39: 891–907, 2013 [DOI] [PubMed] [Google Scholar]
- 104).Kim WJ, Newman WC, Amankulor NM: Phase I/II trial of combination of temozolomide chemotherapy and immunotherapy with fusions of dendritic and glioma cells in patients with glioblastoma. Neurosurgery 81: N11, 2017 [DOI] [PubMed] [Google Scholar]
- 105).Ardon H, Van Gool SW, Verschuere T, et al. : Integration of autologous dendritic cell-based immunotherapy in the standard of care treatment for patients with newly diagnosed glioblastoma: results of the HGG-2006 phase I/II trial. Cancer Immunol Immunother 61: 2033–2044, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106).Inogés S, Tejada S, de Cerio AL, et al. : A phase II trial of autologous dendritic cell vaccination and radiochemotherapy following fluorescence-guided surgery in newly diagnosed glioblastoma patients. J Transl Med 15: 104, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107).Liau LM, Prins RM, Kiertscher SM, et al. : Dendritic cell vaccination in glioblastoma patients induces systemic and intracranial T-cell responses modulated by the local central nervous system tumor microenvironment. Clin Cancer Res 11: 5515–5525, 2005 [DOI] [PubMed] [Google Scholar]
- 108).Ochoa MC, Minute L, Rodriguez I, et al. : Antibody-dependent cell cytotoxicity: immunotherapy strategies enhancing effector NK cells. Immunol Cell Biol 95: 347–355, 2017 [DOI] [PubMed] [Google Scholar]
- 109).Dix AR, Brooks WH, Roszman TL, Morford LA: Immune defects observed in patients with primary malignant brain tumors. J Neuroimmunol 100: 216–232, 1999 [DOI] [PubMed] [Google Scholar]
- 110).Li L, Quang TS, Gracely EJ, et al. : A Phase II study of anti-epidermal growth factor receptor radioimmunotherapy in the treatment of glioblastoma multiforme. J Neurosurg 113: 192–198, 2010 [DOI] [PubMed] [Google Scholar]
- 111).Weller M, Butowski N, Tran DD, et al. ACT IV trial investigators : Rindopepimut with temozolomide for patients with newly diagnosed, EGFRvIII-expressing glioblastoma (ACT IV): a randomised, double-blind, international phase 3 trial. Lancet Oncol 18: 1373–1385, 2017 [DOI] [PubMed] [Google Scholar]
- 112).Buchbinder EI, Desai A: CTLA-4 and PD-1 Pathways: similarities, differences, and implications of their inhibition. Am J Clin Oncol 39: 98–106, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113).Park J, Kwon M, Kim KH, Chang JH, Shin EC: Expression of immune checkpoint molecules on tumor infiltrating lymphocytes in glioblastoma multiforme. J Immunol 198: Suppl 1 196.3, 2017. 27895176 [Google Scholar]
- 114).Xue S, Hu M, Iyer V, Yu J: Blocking the PD-1/PD-L1 pathway in glioma: a potential new treatment strategy. Hematol Oncol 10: 81, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115).Xue S, Hu M, Li P, et al. : Relationship between expression of PD-L1 and tumor angiogenesis, proliferation, and invasion in glioma. Oncotarget 8: 49702–49712, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116).Preusser M, Lim M, Hafler DA, Reardon DA, Sampson JH: Prospects of immune checkpoint modulators in the treatment of glioblastoma. Nat Rev Neurol 11: 504–514, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117).Kim ES, Kim JE, Patel MA, Mangraviti A, Ruzevick J, Lim M: Immune checkpoint modulators: an emerging antiglioma armamentarium. J Immunol Res 2016: 4683607, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118).Heynckes S, Gaebelein A, Haaker G, et al. : Expression differences of programmed death ligand 1 in de-novo and recurrent glioblastoma multiforme. Oncotarget 8: 74170–74177, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119).Filley AC, Henriquez M, Dey M: Recurrent glioma clinical trial, CheckMate-143: the game is not over yet. Oncotarget 8: 91779–91794, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120).Soritau O, Tomuleasa C, Aldea M, et al. : Metformin plus temozolomide-based chemotherapy as adjuvant treatment for WHO grade III and IV malignant gliomas. J BUON 16: 282–289, 2011 [PubMed] [Google Scholar]
- 121).Yang SH, Li S, Lu G, et al. : Metformin treatment reduces temozolomide resistance of glioblastoma cells. Oncotarget 7: 78787–78803, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122).Valtorta S, Dico AL, Raccagni I, et al. : Metformin and temozolomide, a synergic option to overcome resistance in glioblastoma multiforme models. Oncotarget 8: 113090–113104, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123).Song Y, Chen Y, Li Y, et al. : Metformin inhibits TGF-β1-induced epithelial-to-mesenchymal transition-like process and stem-like properties in GBM via AKT/mTOR/ZEB1 pathway. Oncotarget 9: 7023–7035, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124).Yu Z, Zhao G, Xie G, et al. : Metformin and temozolomide act synergistically to inhibit growth of glioma cells and glioma stem cells in vitro and in vivo. Oncotarget 6: 32930–32943, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125).Yu Z, Zhao G, Li P, et al. : Temozolomide in combination with metformin act synergistically to inhibit proliferation and expansion of glioma stem-like cells. Oncol Lett 11: 2792–2800, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126).Narayan RS, Fedrigo CA, Brands E, et al. : The allosteric AKT inhibitor MK2206 shows a synergistic interaction with chemotherapy and radiotherapy in glioblastoma spheroid cultures. BMC Cancer 17: 204, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127).Koul D, Wang S, Wu S, et al. : Preclinical therapeutic efficacy of a novel blood-brain barrier-penetrant dual PI3K/mTOR inhibitor with preferential response in PI3K/PTEN mutant glioma. Oncotarget 8: 21741–21753, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128).Shi F, Zhang J, Liu H, et al. : The dual PI3K/mTOR inhibitor dactolisib elicits anti-tumor activity in vitro and in vivo. Oncotarget 9: 706–717, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129).Yu Z, Xie G, Zhou G, et al. : NVP-BEZ235, a novel dual PI3K-mTOR inhibitor displays anti-glioma activity and reduces chemoresistance to temozolomide in human glioma cells. Cancer Lett 367: 58–68, 2015 [DOI] [PubMed] [Google Scholar]
- 130).Hayashi T, Adachi K, Ohba S, Hirose Y: The Cdk inhibitor flavopiridol enhances temozolomide-induced cytotoxicity in human glioma cells. J Neurooncol 115: 169–178, 2013 [DOI] [PubMed] [Google Scholar]
- 131).Raub TJ, Wishart GN, Kulanthaivel P, et al. : Brain exposure of two selective dual CDK4 and CDK6 inhibitors and the antitumor activity of CDK4 and CDK6 inhibition in combination with temozolomide in an intracranial glioblastoma xenograft. Drug Metab Dispos 43: 1360–1371, 2015 [DOI] [PubMed] [Google Scholar]
- 132).Zhou A, Lin K, Zhang S, et al. : Nuclear GSK3β promotes tumorigenesis by phosphorylating KDM1A and inducing its deubiquitylation by USP22. Nat Cell Biol 18: 954–966, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133).Bobustuc GC, Baker CH, Limaye A, et al. : Levetiracetam enhances p53-mediated MGMT inhibition and sensitizes glioblastoma cells to temozolomide. Neuro-oncology 12: 917–927, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134).Mathieu V, De Nève N, Le Mercier M, et al. : Combining bevacizumab with temozolomide increases the antitumor efficacy of temozolomide in a human glioblastoma orthotopic xenograft model. Neoplasia 10: 1383–1392, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135).Koschmann C, Calinescu AA, Nunez FJ, et al. : ATRX loss promotes tumor growth and impairs nonhomologous end joining DNA repair in glioma. Sci Transl Med 8: 328ra328, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136).Koschmann C, Lowenstein PR, Castro MG: ATRX mutations and glioblastoma: impaired DNA damage repair, alternative lengthening of telomeres, and genetic instability. Mol Cell Oncol 3: e1167158, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137).Han B, Cai J, Gao W, et al. : Loss of ATRX suppresses ATM dependent DNA damage repair by modulating H3K9me3 to enhance temozolomide sensitivity in glioma. Cancer Lett 419: 280–290, 2018 [DOI] [PubMed] [Google Scholar]
- 138).Ding S, Li C, Cheng N, Cui X, Xu X, Zhou G: Redox regulation in cancer stem cells. Oxid Med Cell Longev 2015: 750798, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139).Jang J, Wang Y, Kim HS, Lalli MA, Kosik KS: Nrf2, a regulator of the proteasome, controls self-renewal and pluripotency in human embryonic stem cells. Stem Cells 32: 2616–2625, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140).Kawasaki Y, Ishigami S, Arigami T, et al. : Clinicopathological significance of nuclear factor (erythroid-2)-related factor 2 (Nrf2) expression in gastric cancer. BMC Cancer 15: 5, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141).Singh A, Boldin-Adamsky S, Thimmulappa RK, et al. : RNAi-mediated silencing of nuclear factor erythroid-2-related factor 2 gene expression in non-small cell lung cancer inhibits tumor growth and increases efficacy of chemotherapy. Cancer Res 68: 7975–7984, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142).Rocha CR, Kajitani GS, Quinet A, Fortunato RS, Menck CF: NRF2 and glutathione are key resistance mediators to temozolomide in glioma and melanoma cells. Oncotarget 7: 48081–48092, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143).Zhang L, Wang H: FTY720 inhibits the Nrf2/ARE pathway in human glioblastoma cell lines and sensitizes glioblastoma cells to temozolomide. Pharmacol Rep 69: 1186–1193, 2017 [DOI] [PubMed] [Google Scholar]
- 144).Wong HA, Fatimy RE, Onodera C, et al. : The cancer genome atlas analysis predicts microRNA for rargeting cancer growth and vascularization in glioblastoma. Mol Ther 23: 1234–1247, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145).Shea A, Harish V, Afzal Z, et al. : MicroRNAs in glioblastoma multiforme pathogenesis and therapeutics. Cancer Med 5: 1917–1946, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146).Gao X, Jin W: The emerging role of tumor-suppressive microRNA-218 in targeting glioblastoma stemness. Cancer Lett 353: 25–31, 2014 [DOI] [PubMed] [Google Scholar]
- 147).Chen J, Fu X, Wan Y, Wang Z, Jiang D, Shi L: miR-125b inhibitor enhance the chemosensitivity of glioblastoma stem cells to temozolomide by targeting Bak1. Tumour Biol 35: 6293–6302, 2014 [DOI] [PubMed] [Google Scholar]
- 148).Chen PH, Cheng CH, Shih CM, et al. : The inhibition of microRNA-128 on IGF-1-activating mTOR signaling involves in temozolomide-induced glioma cell apoptotic death. PLoS ONE 11: e0167096, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149).Zhou X, Wu W, Zeng A, et al. : MicroRNA-141-3p promotes glioma cell growth and temozolomide resistance by directly targeting p53. Oncotarget 8: 71080–71094, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150).Han J, Chen Q: MiR-16 modulate temozolomide resistance by regulating BCL-2 in human glioma cells. Int J Clin Exp Pathol 8: 12698–12707, 2015 [PMC free article] [PubMed] [Google Scholar]
- 151).Xu J, Huang H, Peng R, et al. : MicroRNA-30a increases the chemosensitivity of U251 glioblastoma cells to temozolomide by directly targeting beclin 1 and inhibiting autophagy. Exp The Med 15: 4798–4804, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152).Zhang S, Wan Y, Pan T, et al. : MicroRNA-21 inhibitor sensitizes human glioblastoma U251 stem cells to chemotherapeutic drug temozolomide. J Mol Neurosci 47: 346–356, 2012 [DOI] [PubMed] [Google Scholar]
- 153).Comincini S, Allavena G, Palumbo S, et al. : microRNA-17 regulates the expression of ATG7 and modulates the autophagy process, improving the sensitivity to temozolomide and low-dose ionizing radiation treatments in human glioblastoma cells. Cancer Biol Ther 14: 574–586, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154).Tian T, Mingyi M, Qiu X, Qiu Y: MicroRNA-101 reverses temozolomide resistance by inhibition of GSK3β in glioblastoma. Oncotarget 7: 79584–79595, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]