Abstract
Introduction
The INPULSIS-ON trial demonstrated that nintedanib reduced decline in forced vital capacity (FVC) and low pulmonary function (%FVC < 50%) of patients with idiopathic pulmonary fibrosis (IPF). However, there is no sufficient evidence in real world.
Objectives
Reveal the utility and adverse events of nintedanib for severe IPF patients.
Methods
This was a single-center retrospective study. Patients who met the eligibility criteria of the INPULSIS trial (%FVC ≥ 50%; %DLCO [diffusing capacity of the lung carbon monoxide % predicted] ≥ 30%) were classified as Mild to Moderate Group (n = 34); patients who did not meet the criteria were classified as Severe Group (n=17).
Results
The body mass index (24.7 ± 3.4 vs 22.4 ± 3.6 kg/m2; P = 0.021) were significantly low in Severe Group. Main adverse events (diarrhea, nausea, liver disorder, and acute exacerbation) tended to be more in Severe Group than in Mild to Moderate Group; however, the difference was not significant (P = 0.76, 0.14, 0.18, and 0.67, respectively). The continuation rates over 12 months tended to be higher in Mild to Moderate Group than in Severe Group (77% vs 44%; P = 0.027). Log-rank test revealed that the prognosis was significantly better in Mild to Moderate Group than in Severe Group (P = 0.014). In the Severe Group, patients who were able to continue nintedanib for more than 3 months had significantly better prognosis compared to those who could not (P = 0.007).
Conclusion
The benefit from nintedanib was reduced in patients in Severe Group when compared to those in Mild to Moderate Group; however, the prognosis is expected to improve with control of side effects and long-term administration. It is more important to control the side effects in Severe Group.
Keywords: idiopathic pulmonary fibrosis, nintedanib, triple tyrosine kinase inhibitor, INPULSIS trials, forced vital capacity
Introduction
IPF is a chronic progressive interstitial pneumonia.1 It has been reported that an average survival time of IPF is 2–3 years after diagnosis, and the prognosis of IPF is as poor as many types of cancers.2–4 The pulmonary function test is important for evaluation of the severity of IPF. The FVC and DLCO have also been reported to be important prognostic factors of IPF patients.2,5–7
Nintedanib is a tyrosine-kinase inhibitor and targets vascular endothelial growth factor receptors, fibroblast growth factor receptors, and platelet-derived growth factor receptors.8,9 In the most recent international clinical practice guidelines, nintedanib received a conditional recommendation for the treatment of IPF.10 The TOMORROW trial (nintedanib Phase II clinical trial)11 and INPULSIS-1/2 trial (Phase III clinical trial)12 revealed that nintedanib was associated with a reduction in the decline in lung function and with fewer acute exacerbations. However, the eligibility criteria of these three clinical trials were an FVC ≥ 50% of the predicted value and a DLCO of 30%–79% of the predicted value. Therefore, it is unknown whether nintedanib can be expected to affect progressing (or more severe) IPF patients with declining pulmonary function. The INPULSIS-ON trial (NCT 01619085), the open-label extension of the INPULSIS trial, included IPF patients who had progressed, and their FVC decreased to < 50% of the predicted value during the INPULSIS examination period. This trial demonstrated that the nintedanib inhibited FVC reduction in both advanced and non-advanced cases. Additionally, adverse events were also considered equivalent in both cases.13 Tzouvelekis et al reported that even in patients with %FVC < 50%, the efficacy of nintedanib was obtained to the same extent as those with %FVC > 50%.14 In the current clinical setting, since this extensive trial is the only report that investigated the efficacy or safety of nintedanib for advanced IPF, further real world-based evidence is needed.
Thus, this study aimed to verify the effect and safety of nintedanib on patients with severe IPF under clinical conditions.
Patients and methods
This single-center, retrospective study was performed in accordance with the amended Declaration of Helsinki. The research protocol was approved by the Human Ethics Committee of Chiba University Hospital (Approval no 2083). All patients provided written informed consent for participation in the study.
Patients
We diagnosed IPF based on the ATS/ERS/JRS/ALAT IPF guidelines of 2011.1 Patients who met one of the eligibility criteria of the INPULSIS trial (%FVC ≥ 50% and %DLCO ≥ 30%) were classified as Mild to Moderate Group, and patients who did not meet the criteria were classified as Severe Group.12
Nintedanib administration
The criteria of nintedanib administration in our hospital was %FVC decline > 10% and/or radiological worsening. We also administered nintedanib to patients in whom administration of PFD, an other antifibrotic drug, was difficult due to side effects. The initial dose was 150 mg twice daily in all patients. Patients were followed up with liver function, renal function, complete blood count, KL – 6, etc. every 1–3 months. When an adverse event was observed, the dose was reduced or stopped at the discretion of his/her attending physician.
Evaluated parameters
We compared the baseline data, change in FVC, frequency of adverse events, and survival rate of the two groups before one year (±3 months), at the initiation, and after one year (±3 months) of nintedanib administration.
Statistical analysis
The clinical data are expressed as mean ± SD. All statistical analyses were performed using the EZR software package (Saitama Medical Center, Saitama, Japan),15 a graphical user interface for R (R version 3.2.0, The R Foundation for Statistical Computing, Vienna, Austria). A P-value < 0.05 was considered significant.
Results
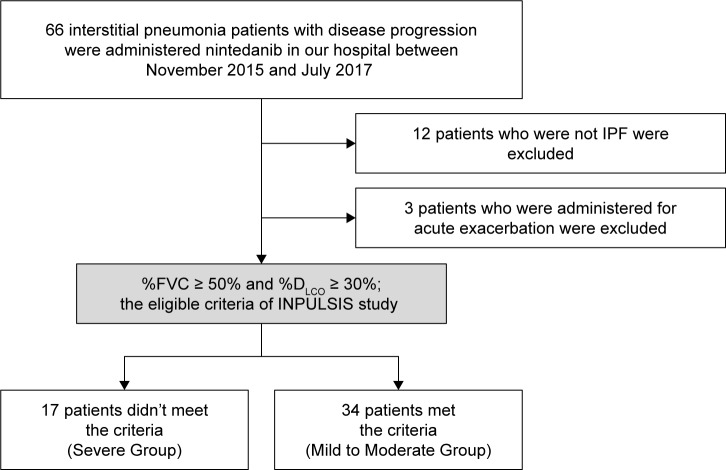
A total of 66 patients received nintedanib at Chiba University Hospital between November 2015 and January 2017. Twelve patients who were not diagnosed as IPF were excluded from the study, as were three patients who received nintedanib in the acute exacerbation stage. Finally, a total of 51 IPF patients were enrolled (Figure 1). The baseline characteristics of the included patients (34 Mild to Moderate Group; 17 Severe Group) are shown in Table 1. There was no significant difference between the two groups in terms of age, sex, or height. Body weight (64.3 ± 11.8 vs 55.8 ± 10.3 kg; P = 0.021), BMI (24.7 ± 3.4 vs 22.4 ± 3.6 kg/m2; P = 0.016), FVC (2,273 ± 672 vs 1,289 ± 310 mL; P < 0.001), %FVC (72.6 ± 15.5 vs 46.4% ± 7.0%; P < 0.001), and %DLCO (57.5 ± 19.0 vs 32.2% ± 13.5%; P < 0.001) were significantly lower in Severe Group. In Mild to Moderate Group, NAC inhalation (n = 6), and PFD (n = 5) were administered before nintedanib; 23 patients did not receive the pre-treatment. In the Severe Group, NAC (n = 3), PFD (n = 8), and NAC + PFD (n = 2) were administered before nintedanib; four patients did not receive the pre-treatment. There were significantly more patients in Mild to Moderate Group who did not receive the pre-treatment (P = 0.002).
Figure 1.
Study flow chart.
Notes: Between November 2015 and July 2017, 66 patients received nintedanib at Chiba University Hospital. Of these 66 patients, 11 who did not have idiopathic pulmonary fibrosis (IPF) and three who received nintedanib for acute exacerbation were excluded. Patients who met one of the eligible criteria of the INPULSIS trial (% forced vital capacity [FVC] ≥ 50%; % diffusion capacity of the lung for carbon monoxide [DLCO] ≥ 30%) were classified as Mild to Moderate Group, and those who did not meet the criteria were classified as Severe Group.
Table 1.
Baseline characteristics of total 51 patients
| Total (n = 51) | Mild to Moderate Group (n = 34) | Severe Group (n = 17) | P-value | |
|---|---|---|---|---|
|
| ||||
| Age (years) | 70.5 ± 6.8 | 70.6 ± 6.0 | 70.1 ± 8.3 | 0.71 |
| Male/female | 31/20 | 23/11 | 8/9 | 0.22 |
| Height (cm) | 159.8 ± 9.7 | 160.0 ± 9.5 | 157.5 ± 9.9 | 0.20 |
| Body weight (kg) | 61.5 ± 11.9 | 64.3 ± 11.8 | 55.8 ± 10.3 | 0.021 |
| BMI (kg/m2) | 24.0 ± 3.6 | 24.7 ± 3.4 | 22.4 ± 3.6 | 0.016 |
| Severity (I/II/III/IV) | 11/2/12/26 | 11/2/9/12 | 0/0/3/14 | 0.0040 |
| GAP stage (I/II/III) | 12/26/13 | 12/20/2 | 0/6/11 | <0.001 |
| Pre-treatment | 9/13/2/27 | 6/5/0/23 | 3/8/2/4 | 0.0031 |
| (NAC/PFD/NAC + PFD/none) | ||||
| Home oxygen therapy | 19 | 8 | 11 | <0.001 |
| KL-6 (U/mL) | 1,691 ± 1284 | 1,581 ± 1,321 | 1,918 ± 1,211 | 0.25 |
| BNP (pg/mL) | 33.3 ± 35.6 | 35.0 ± 40.9 | 30.0 ± 23.0 | 0.69 |
| FVC (mL) | 1,945 ± 740 | 2,273 ± 672 | 1,289 ± 310 | <0.001 |
| %FVC (%) | 63.9 ± 18.2 | 72.6 ± 15.5 | 46.4 ± 7.0 | <0.001 |
| %DLCO (%) | 49.8 ± 21.0 | 57.5 ± 19.0 | 32.2 ± 13.5 | <0.001 |
Notes: Data are expressed as mean ± SD. Fisher’s exact test: male/female, severity, pre-treatment, Mann–Whitney U test: other parameters.
Abbreviations: BMI, body mass index; BNP, brain natriuretic peptide; DLCO, diffusion capacity of the lung for carbon monoxide; FVC, force vital capacity; GAP, Gender-Age-Physiology Index; KL-6, Krebs von den Lungen-6; NAC, N-acetylcysteine inhalation; PFD, pirfenidone.
Among 34 patients in Mild to Moderate Group, six were unavailable for a follow-up pulmonary function test 1 year later (four died, one was transferred to another hospital, and one had disease progression). Among 17 patients of Severe Group, six were unavailable for follow-up 1 year later (two died, three were transferred to another hospital, and one had disease progression) (Figure 2, Table 2).
Figure 2.
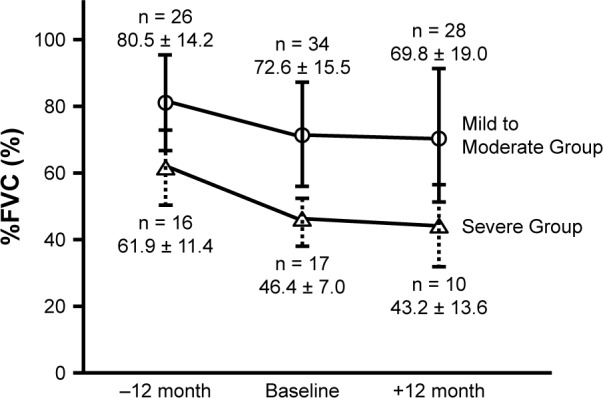
Average forced vital capacity of patients with idiopathic pulmonary fibrosis.
Notes: The mean %FVC 1 year before nintedanib administration was 80.5% ± 14.2% and 61.9% ± 11.4% in Mild to Moderate Group and Severe Group, respectively. The %FVC at the start of nintedanib administration was 72.6% ± 15.5% and 46.4% ± 7.0%, respectively; and the %FVC after 1 year of nintedanib administration was 69.8% ± 19.0% and 43.2% ± 13.6%. In both groups, nintedanib significantly suppressed the deterioration of the FVC.
Table 2.
Side effects and tolerability within 12 months
| Total (n = 51) | Mild to Moderate Group (n = 34) | Severe Group (n = 17) | P-value | |
|---|---|---|---|---|
|
| ||||
| All adverse events (CTCAE grade I+II/III+IV+V) | 38 (27/11) | 24 (19/5) | 14 (8/6) | 0.50 |
| Diarrhea | 24 (21/3) | 17 (15/2) | 7 (6/1) | 0.76 |
| Nausea | 11 (9/2) | 5 (5/0) | 6 (4/2) | 0.14 |
| Liver disorder | 14 (14/0) | 7 (7/0) | 7 (7/0) | 0.18 |
| Headache | 1 (1/0) | – | 1 (1/0) | 0.33 |
| Dizziness | 1 (0/1) | 1 (0/1) | – | 1.0 |
| Pulmonary artery hypertension | 2 (1/1) | – | 2 (1/1) | 0.10 |
| Acute exacerbation | 7 (0/7) | 4 (0/4) | 3 (0/3) | 0.67 |
| Death | 7 (0/7) | 4 (0/4) | 3 (0/3) | 0.67 |
| Continuable patients for >3 months | 44 | 31 | 13 | 0.20 |
| Continuable patients for >12 months | 35 | 27 | 8 | 0.027 |
Notes: CTCAE: Common Terminology Criteria for Adverse Events v4.0, Fisher’s exact test: all parameters.
The mean %FVC at 1 year before nintedanib administration was 80.5 ± 14.2 and 61.9% ± 11.4% in Mild to Moderate Group and Severe Group, respectively. The %FVC at the start of nintedanib administration was 72.6 ± 15.5 and 46.4% ± 7.0%, respectively; and the %FVC after 1 year of nintedanib administration was 69.8 ± 19.0 and 43.2% ± 13.6% (Figure 2). In Severe group, ∆FVC significantly different between before and after 1 year from nintedanib administration (−459 ± 347, −143 ± 276 mL; Wilcoxon’s signed rank test; P = 0.029) (Figure 3).
Figure 3.
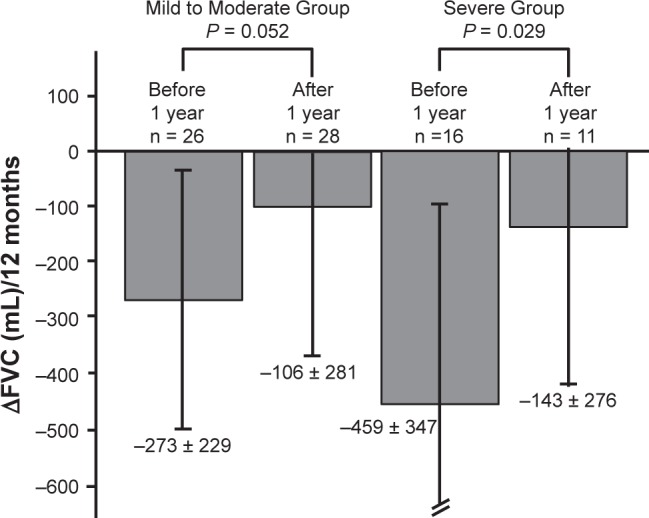
Decrease in forced vital capacity over 12 months.
Notes: Changes in the forced vital capacity (FVC) before and after 12 months. In Severe group, there were significant differences between before and after 1 year from nintedanib administration (−459 ± 347 mL, −143 ± 276 mL; P = 0.029). Statistical analysis was performed with Wilcoxon’s signed rank.
Any adverse events were observed in 24 patients of Mild to Moderate Group and 14 patients of Severe Group (P = 0.50); Grade III/IV of any adverse events occurred in five patients of Mild to Moderate Group and six of Severe Group (Table 2). The primary adverse events were diarrhea (Mild to Moderate Group, 17; Severe Group, 7), nausea (Mild to Moderate Group, 5; Severe Group, 6), and liver dysfunction (Mild to Moderate Group, 7; Severe Group, 7). Acute exacerbations were observed in four patients of Mild to Moderate Group and three of Severe Group (P = 0.67). Four of Mild to Moderate Group and three of Severe Group died within 12 months (P = 0.67). All seven deaths were caused by respiratory failure due to progression of IPF. Seven (20%) patients (four death, two disease progression, one side effect) of Mild to Moderate Group and nine (52%) patients (three death, three disease progression, three side effect) of Severe Group were not able to continue nintedanib for 12 months; the continuation rate was significantly higher in Mild to Moderate Group (P = 0.027). The log-rank test revealed there was significant difference in long-term continuation rate between two groups (P = 0.012) (Figure 4); in long-term mortality between two groups (P = 0.014) (Figure 5). In Severe Group, patients who were able to continue nintedanib for more than 3 months (n = 4) exhibited significantly better prognosis compared to those who could not (n = 13) (P = 0.007) (Figure 6).
Figure 4.
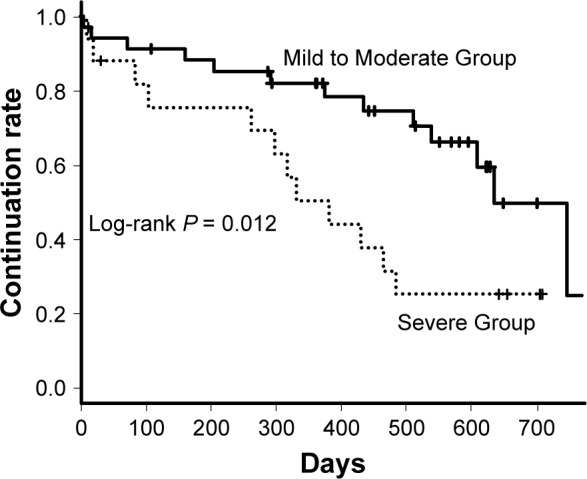
Nintedanib continuable curves of Mild to Moderate and Severe patients.
Note: A log-rank test revealed that there was significant difference in the continuation rate between two groups (P = 0.012).
Figure 5.
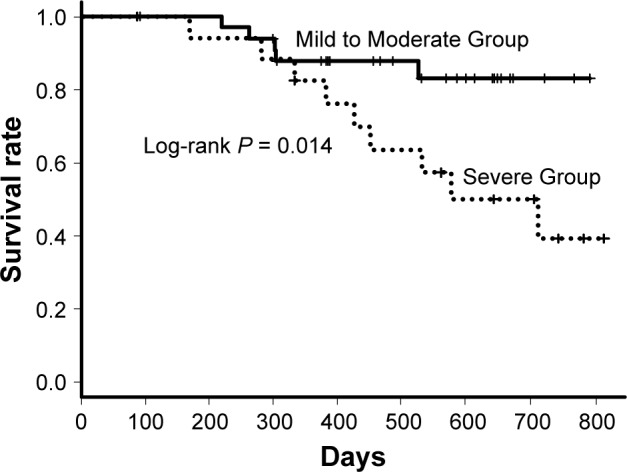
Survival curves of Mild to Moderate and Severe patients.
Note: A log-rank test revealed that there was significant difference in the survival rate between two groups (P = 0.014).
Figure 6.
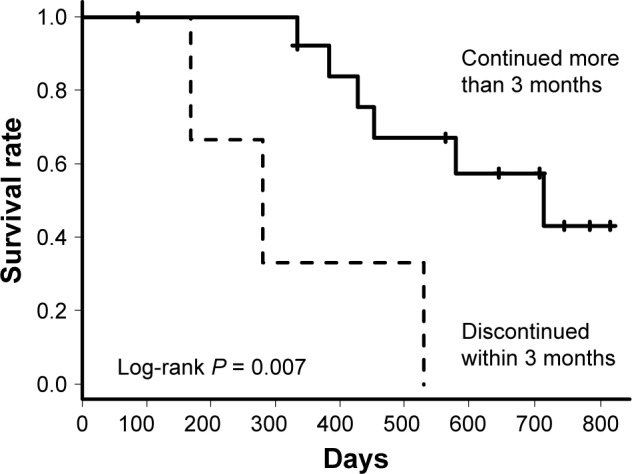
Survival curves of patients in Severe Group depending on whether nintedanib was continued for more than 3 months or not.
Note: A log-rank test revealed that patients who could continue nintedanib for more than 3 months had significantly better prognosis (P = 0.007).
Discussion
Even in Severe Group, the reduction of %FVC was suppressed by nintedanib administration like Mild to Moderate Group. However, the frequency of side effects tended to be more and prognosis was significantly worse in Severe Group than in Mild to Moderate Group. Despite this, patients in Severe Group who were able to continue nintedanib for more than 3 months had significantly better prognosis compared with those who could not (P = 0.007), as shown in Figure 6.
In the present study, the evaluation included 33% of advanced IPF patients (FVC < 50% or DLCO < 30%), while the major two clinical trials of nintedanib (TOMORROW, INPULSIS) included %FVC ≥ 50% and DLCO 30%–79%. The majority of patients included in these large trials were Mild–Moderate disease severity. So, it is unclear whether the efficacy of nintedanib can be expected in progressing IPF patients with declining respiratory function. The INPULSIS-ON trial (NCT 0161908; open-label extension trial of the INPULSIS trial) included patients who had progressed and had an FVC < 50% of the predicted value in the INPULSIS examination period.13 During the INPULSIS trial, 41 of 731 patients with a %FVC < 50% were included, and it was reported that nintedanib was also effective in these advanced IPF patients. Tzouvelekis et al reported that even in patients with %FVC < 50%, the efficacy of nintedanib was obtained to the same extent as the patients of %FVC > 50% from their observational study in Greece.14 In our study, eight patients of Severe Group had already been treated with other anti-fibrotic compounds such as PFD. The usefulness of PFD has been demonstrated in several clinical trials.16–18 However, there is no evidence indicating which of the two anti-fibrotic compounds is superior. In addition, when the effect of one drug is insufficient, there is no evidence to indicate whether it is better to continue the drug or switch to another drug. According to our report, even if the effect of PFD is insufficient, it is possible that switching to nintedanib may be effective.
Regarding adverse events of nintedanib, Severe Group tended to experience more side effects (eg, diarrhea 41%, nausea 35%), although there was no significant difference compared to Mild to Moderate Group (eg, diarrhea 50%, nausea 14%). The number of patients who could continue to receive the treatment for 12 months was lower in Severe Group than in Mild to Moderate Group (27 [79%] and 8 [47%], P = 0.027) (Table 2). However, long-term continuation rate revealed by log-rank test was significantly lower in Severe Group (P = 0.012) (Figure 4) because of death and transferring to another hospital. In Severe Group, as compared with Mild to Moderate Group, there were a few cases who died or transferred to another hospital, so this result is apparently. In Severe Group, patients who were able to continue nintedanib for more than 3 months had significantly better prognosis compared with those who could not (P = 0.007) (Figure 6). This indicates that controlling side effects is more important in Severe Group. In the INPULSIS-ON trial, Wuyts et al reported that although the frequency of side effects did not increase even in advanced patients (advanced patients 100% vs not advanced patients 94%), adverse events leading to treatment discontinuation tended to be more frequent (advanced patients 41.5% vs not advanced patients 22.5%).13 Unlike that report, Severe Group in our study had significantly lower body weights (55.8 vs 64.3 kg, P = 0.021) and BMIs at the start of administration compared to Mild to Moderate Group. In patients with IPF, it was reported that the decrease in BMI became a prognostic factor.19 The patients’ condition at baseline usually has a major impact on the prognosis. Side effects may have increased due to the higher blood concentration of the drug. Ikeda et al reported an association between liver dysfunction due to nintedanib and BMI and concluded that it is appropriate to adjust the dosage of nintedanib for patients with low body weights.20 The standard dosage of nintedanib is 150 mg twice daily, which is the amount used in clinical trials, but the report suggested that 100 mg twice daily might be an option for patients with low body weights.
This study had some limitations. First, the study design was a retrospective single-center study, and the sample size was small. So, we did not mention their pre-treatment medicine. Second, in Severe Group, 6 of 17 patients were not available for a 1-year follow-up pulmonary function test due to death or disease progression. However, delayed progression due to administration of nintedanib was also expected in Severe Group.
Conclusion
Survival benefit from nintedanib is reduced in patients with severe IPF when compared to those with Mild to Moderate IPF; however, an improvement in prognosis may be expected when side effects are controlled and the drug is administered over long-term. Hence, controlling side effects in Severe Group is very important.
Acknowledgments
We would like to thank Editage (www.editage.jp) for English language editing.
Abbreviations
- ALAT
Latin American Thoracic Association
- ATS
American Thoracic Society
- BMI
body mass index
- BNP
brain natriuretic peptide
- CT
computed tomography
- CTCAE
Common Terminology Criteria for Adverse Events
- DLCO
diffusing capacity of lung carbon monoxide
- ERS
European Respiratory Society Thoracic Association
- FVC
forced vital capacity
- HRCT
high-resolution computed tomography
- IPF
idiopathic pulmonary fibrosis
- JRS
Japanese Respiratory Society
- KL-6
Krebs von den Lungen-6
- NAC
N-acetylcysteine
- PFD
pirfenidone
- SD
standard deviation
- TKI
tyrosine kinase inhibitor
Footnotes
Author contributions
MA, KTs, KS, MS, JI, JT, and KTa contributed to the study concept and design. MA, KTs, KS, and MS examined the enrolled patients in the hospital. MA collected data. MA and KTs analyzed data. MA wrote the paper. All authors contributed to data analysis, drafting and revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
KTs has received lecture fee from Boehringer Ingelheim. The other authors report no conflicts of interest in this work.
References
- 1.Raghu G, Collard HR, Egan JJ, et al. An official ATS/ERS/JRS/ALAT statement: idiopathic pulmonary fibrosis: evidence-based guidelines for diagnosis and management. Am J Respir Crit Care Med. 2011;183(6):788–824. doi: 10.1164/rccm.2009-040GL. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vancheri C, Failla M, Crimi N, Raghu G. Idiopathic pulmonary fibrosis: a disease with similarities and links to cancer biology. Eur Respir J. 2010;35(3):496–504. doi: 10.1183/09031936.00077309. [DOI] [PubMed] [Google Scholar]
- 3.Olson AL, Swigris JJ, Lezotte DC, Norris JM, Wilson CG, Brown KK. Mortality from pulmonary fibrosis increased in the United States from 1992 to 2003. Am J Respir Crit Care Med. 2007;176(3):277–284. doi: 10.1164/rccm.200701-044OC. [DOI] [PubMed] [Google Scholar]
- 4.Natsuizaka M, Chiba H, Kuronuma K, et al. Epidemiologic survey of Japanese patients with idiopathic pulmonary fibrosis and investigation of ethnic differences. Am J Respir Crit Care Med. 2014;190(7):773–779. doi: 10.1164/rccm.201403-0566OC. [DOI] [PubMed] [Google Scholar]
- 5.du Bois RM, Nathan SD, Richeldi L, Schwarz MI, Noble PW. Idiopathic pulmonary fibrosis: lung function is a clinically meaningful endpoint for phase III trials. Am J Respir Crit Care Med. 2012;186(8):712–715. doi: 10.1164/rccm.201206-1010PP. [DOI] [PubMed] [Google Scholar]
- 6.Karimi-Shah BA, Chowdhury BA. Forced vital capacity in idiopathic pulmonary fibrosis – FDA review of pirfenidone and nintedanib. N Engl J Med. 2015;372(13):1189–1191. doi: 10.1056/NEJMp1500526. [DOI] [PubMed] [Google Scholar]
- 7.Ley B, Collard HR, King TE. Clinical course and prediction of survival in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2011;183(4):431–440. doi: 10.1164/rccm.201006-0894CI. [DOI] [PubMed] [Google Scholar]
- 8.Hilberg F, Roth GJ, Krssak M, et al. BIBF 1120: triple angiokinase inhibitor with sustained receptor blockade and good antitumor efficacy. Cancer Res. 2008;68(12):4774–4782. doi: 10.1158/0008-5472.CAN-07-6307. [DOI] [PubMed] [Google Scholar]
- 9.Wollin L, Wex E, Pautsch A, et al. Mode of action of nintedanib in the treatment of idiopathic pulmonary fibrosis. Eur Respir J. 2015;45(5):1434–1445. doi: 10.1183/09031936.00174914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Raghu G, Rochwerg B, Zhang Y, et al. An Official ATS/ERS/JRS/ALAT Clinical Practice Guideline: treatment of idiopathic pulmonary fibrosis. An update of the 2011 Clinical Practice Guideline. Am J Respir Crit Care Med. 2015;192(2):e3–e19. doi: 10.1164/rccm.201506-1063ST. [DOI] [PubMed] [Google Scholar]
- 11.Richeldi L, Costabel U, Selman M, et al. Efficacy of a tyrosine kinase inhibitor in idiopathic pulmonary fibrosis. N Engl J Med. 2011;365(12):1079–1087. doi: 10.1056/NEJMoa1103690. [DOI] [PubMed] [Google Scholar]
- 12.Richeldi L, du Bois RM, Raghu G, et al. Efficacy and safety of nintedanib in idiopathic pulmonary fibrosis. N Engl J Med. 2014;370(22):2071–2082. doi: 10.1056/NEJMoa1402584. [DOI] [PubMed] [Google Scholar]
- 13.Wuyts WA, Kolb M, Stowasser S, Stansen W, Huggins JT, Raghu G. First data on efficacy and safety of nintedanib in patients with idiopathic pulmonary fibrosis and forced vital capacity of ≤ 50% of predicted value. Lung. 2016;194(5):739–743. doi: 10.1007/s00408-016-9912-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tzouvelekis A, Karampitsakos T, Kontou M, et al. Safety and efficacy of nintedanib in idiopathic pulmonary fibrosis: a real-life observational study in Greece. Pulm Pharmacol Ther. 2018;49:61–66. doi: 10.1016/j.pupt.2018.01.006. [DOI] [PubMed] [Google Scholar]
- 15.Kanda Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant. 2013;48(3):452–458. doi: 10.1038/bmt.2012.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kishaba T, Nagano H, Nei Y, Yamashiro S. Body mass index-percent forced vital capacity-respiratory hospitalization: new staging for idiopathic pulmonary fibrosis patients. J Thorac Dis. 2016;8(12):3596–3604. doi: 10.21037/jtd.2016.12.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Taniguchi H, Ebina M, Kondoh Y, et al. Pirfenidone in idiopathic pulmonary fibrosis. Eur Respir J. 2010;35(4):821–829. doi: 10.1183/09031936.00005209. [DOI] [PubMed] [Google Scholar]
- 18.Noble PW, Albera C, Bradford WZ, et al. Pirfenidone in patients with idiopathic pulmonary fibrosis (CAPACITY): two randomised trials. Lancet. 2011;377(9779):1760–1769. doi: 10.1016/S0140-6736(11)60405-4. [DOI] [PubMed] [Google Scholar]
- 19.King TE, Bradford WZ, Castro-Bernardini S, et al. A phase 3 trial of pirfenidone in patients with idiopathic pulmonary fibrosis. N Engl J Med. 2014;370(22):2083–2092. doi: 10.1056/NEJMoa1402582. [DOI] [PubMed] [Google Scholar]
- 20.Ikeda S, Sekine A, Baba T, et al. Hepatotoxicity of nintedanib in patients with idiopathic pulmonary fibrosis: a single-center experience. Respir Investig. 2017;55(1):51–54. doi: 10.1016/j.resinv.2016.08.003. [DOI] [PubMed] [Google Scholar]