Abstract
Up to 30% of the human population are asymptomatically and permanently colonized with nasal Staphylococcus aureus. To successfully colonize human nares, S. aureus needs to establish solid interactions with human nasal epithelial cells and overcome host defense mechanisms. However, some factors like bacterial interactions in the human nose can influence S. aureus colonization and sometimes prevent colonization. On the other hand, certain host characteristics and environmental factors can predispose to colonization. Nasal colonization can cause opportunistic and sometimes life-threatening infections such as surgical site infections or other infections in non-surgical patients that increase morbidity, mortality as well as healthcare costs.
Keywords: Staphylococcus aureus, nasal colonization, epidemiology, surgical site infections (SSI), nasal microbiota, predisposing factors, nasal carriage
Introduction
Staphylococcus aureus is both a human skin and mucosae commensal but also a frequent cause of serious infections with high morbidity, mortality, and healthcare-associated costs (Schmidt et al., 2015). The most frequent carriage site is the vestibulum nasi (or anterior nares), which serves as reservoir for the spread of the pathogen (Williams, 1963; Sivaraman et al., 2009). This bacteria can establish solid interactions with nasal epithelial cells via various proteins and many cell surface components (Wertheim et al., 2005a; Mulcahy and McLoughlin, 2016), thus transforming into persistent carriage. S. aureus colonizes the anterior nares of 20% to 80% of the human population (Brown et al., 2014). Nasal carriage has been shown to play a key role in the pathogenesis of S. aureus infections (Kluytmans et al., 1997) in patients undergoing surgery (Perl et al., 2002; Bode et al., 2010), dialysis (Kluytmans et al., 1996; Nouwen et al., 2006), and in intensive care unit (ICU) patients (Garrouste-Orgeas et al., 2001), with higher infection risks in persistent carriers (Nouwen et al., 2006).
Previously published reviews on S. aureus carriage have usually focused independently on colonization or infections, or have issued a specific underlying condition or surgery. Here, we will full review recent advances in nasal microbiota composition and interspecies interactions, epidemiology, and risk factors for S. aureus colonization as well as the link between nasal carriage and infections both in community and nosocomial context.
Nasal Microbiota and Interactions Between Bacteria
The adult nasal microbiota differs between individuals, but species belonging to Corynebacterium, Propionibacterium, and Staphylococcus genera are the most abundant bacteria (Frank et al., 2010; Human Microbiome Project Consortium, 2012; Yan et al., 2013; Kaspar et al., 2016). In a study conducted on the nasal microbiota of 178 adults, 88.2% were Corynebacterium carriers, 83.7% Propionibacterium acnes carriers, and 90.4% Staphylococcus epidermidis carriers. Proportional abundance varied considerably between individuals (Liu C.M. et al., 2015).
The health status may influence the nasal microbiota and vice versa. In a study involving healthy and hospitalized individuals, healthy adults harbored nares microbiota dominated by Actinobacteria (mainly Propionibacterium and Corynebacterium spp.) whereas patients microbiota were dominated by S. aureus and S. epidermidis. S. aureus colonization was negatively associated with the presence of other bacteria including S. epidermidis (Frank et al., 2010). Such counterweight effect between bacteria could be the result of interdependent activation-inhibition mechanisms as reviewed by Krismer et al. (2017). In fact, some bacterial species are capable of secreting anti-staphylococcal molecules modulating S. aureus abundance (Figure 1). For instance, in vitro production of H2O2 by Streptococcus pneumoniae can be bactericidal on S. aureus (Regev-Yochay et al., 2006; Selva et al., 2009). Recently, an in vitro and human study demonstrated that lugdunin, a non-ribosomal synthesized bioactive compound produced by Staphylococcus lugdunensis, can prevent S. aureus nasal colonization via a bactericidal effect (Zipperer et al., 2016).
FIGURE 1.
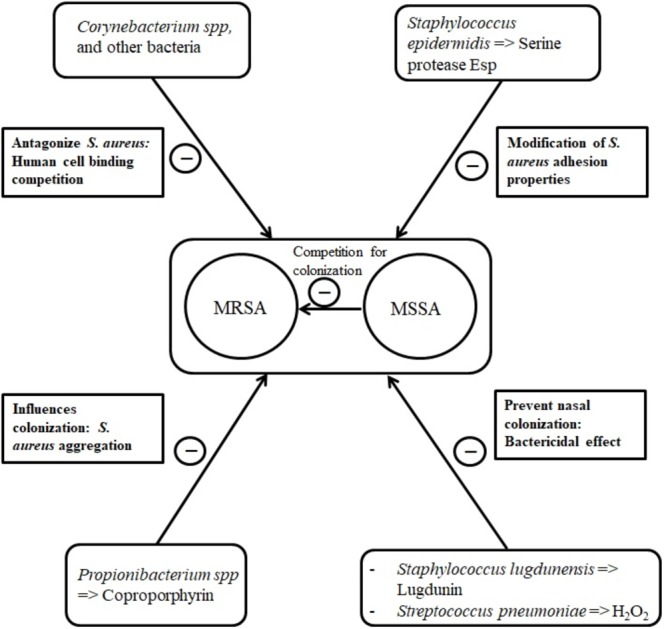
Main bacterial interactions with nasal S. aureus.
In some cases, the bacteria-secreted molecules can modify S. aureus adhesion properties. Some types of S. epidermidis seem to be capable of synthetizing the serine protease Esp that eliminates nasal S. aureus in healthy humans (Iwase et al., 2010), probably by degrading staphylococcal surface proteins and human receptors critical for host–pathogen interaction (Sugimoto et al., 2013). As well, Propionibacterium species produce coproporphyrin III, a porphyrin metabolite that induces S. aureus aggregation which influences nasal colonization (Wollenberg et al., 2014).
Corynebacterium species are suggested to antagonize S. aureus by human cell binding competition mechanisms (Uehara et al., 2000; Lina et al., 2003). In 156 healthy volunteers, Uehara et al. (2000) observed a 71% total eradication rate of nasal S. aureus after performing up to 15 inoculations of a Corynebacterium sp. strain to the nares of S. aureus carriers.
Intra-species competition has also been described. In a cross-sectional clinical study, it was suggested that methicillin-sensitive S. aureus (MSSA) and methicillin-resistant S. aureus (MRSA) compete for colonization, MSSA being protective with regard to MRSA carriage (Dall’Antonia et al., 2005). On the other hand, pre-existing nasal carriage with S. aureus could predispose adult patients to further staphylococcal colonization (Ghasemzadeh-Moghaddam et al., 2015).
Spread and Transmission of S. aureus
Staphylococcus aureus can be found in different body sites like the skin, rectum, vagina, gastrointestinal tract and axilla, the anterior nares appearing as the main reservoir. From a cutaneous commensal site, S. aureus can enter in contact with the nasal mucosa, then interact with epithelial cell ligands such as loricrin and cytokeratin 10 (K10) (Table 1). Once the host’s defenses are overcome, S. aureus can propagate into the anterior nares so that the host becomes an S. aureus nasal carrier (Wertheim et al., 2005a). In human, nasal colonization may begin within the first days of life (Maayan-Metzger et al., 2017). This has been demonstrated in a cohort study evaluating nasal carriage of S. aureus in 100 pairs of infant–mother for a period of 6 months following delivery (Peacock et al., 2003). The carriage rate in the first 8 weeks of life was around 40–50%, thereafter it dropped to 21% at 6 months. In addition, this study found a nasal carriage concordance in 68% of infant–mother pairs attesting the role of environmental factors in S. aureus carriage (Peacock et al., 2003). Another study found identical strains in 80% of infant–mother pairs. In 90% of these newborns, the source of S. aureus was the maternal nasal strain (Leshem et al., 2012; Figure 2).
Table 1.
Major S. aureus-host ligands.
| S. aureus ligand factor | Host ligand | Reference |
|---|---|---|
| ClfB | Loricrin, K10 (cytokeratin 10), K8 (cytokeratin 8), fibrinogen | Perkins et al., 2001; O’Brien et al., 2002; Schaffer et al., 2006; Wertheim et al., 2008; Haim et al., 2010; Mulcahy et al., 2012 |
| IsdA | Fibrinogen, fibronectin | Clarke et al., 2004 |
| SdrC | Unknown | Corrigan et al., 2009 |
| SdrD | Desmoglein 1 | Corrigan et al., 2009; Askarian et al., 2016 |
| SasX | Unknown | Liu Q. et al., 2015 |
| SasG | Unknown | Roche et al., 2003 |
| WTA | Srec-1 | Baur et al., 2014; Weidenmaier et al., 2004 |
FIGURE 2.
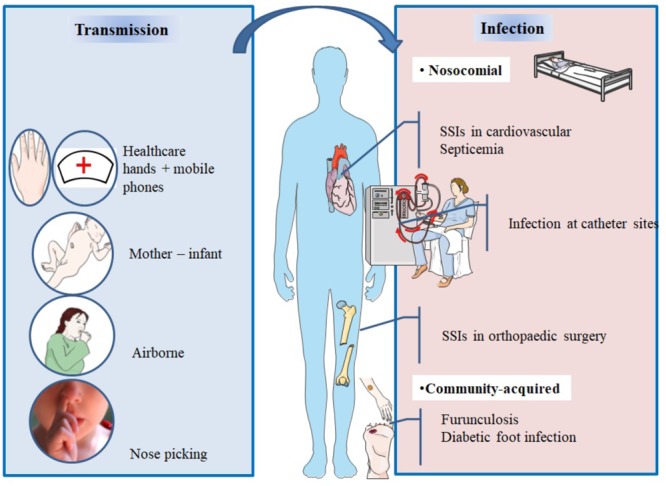
Main spread and transmission mechanisms of S. aureus and impact of nasal carriage on subsequent infections.
After birth, hands are the main vector for S. aureus transmission from surfaces to the nose (Wertheim et al., 2005a). The hypothesis of a link between hand and nose S. aureus carriage is supported by the double blind randomized placebo controlled trial from Reagan et al. (1991), who demonstrated that nasal decolonization with mupirocin applied to health-care workers resulted in a decrease of nose and hand carriage. In a cohort study including outpatients and healthy hospital employees, nasal carriage was evaluated by a single or several swabs. Participants completed a questionnaire about their nose picking behavior, a positive correlation between this habit and nasal carriage of S. aureus was found. However, it is unknown whether nose-picking patients were more frequently colonized at extra nasal sites (Wertheim et al., 2006).
Studies realized in individuals living in the same households have revealed that these people tend to carry genetically similar strains in their nares (Nouwen and Optima Grafische Communicatie, 2004; Muthukrishnan et al., 2013) suggesting horizontal transmission. Multisite MRSA carriage increases the risk for nasal MRSA colonization (Harbarth et al., 2000).
In spite infrequent, airborne transmission is another possible route of S. aureus dissemination (Wertheim et al., 2005a) and may play a role in hospital outbreaks (Sherertz et al., 1996).
During viral upper respiratory infections, the risk of disseminating endogenous S. aureus in the air increases and infection outbreaks may occur. In 1996, an MRSA outbreak involving 8 of 43 patients occurred in a surgical ICU of a university hospital in the United States. Investigations of the cause concluded that a single physician was the source of this outbreak; he was a nasal carrier of MRSA and suffered an upper respiratory infection. To assess airborne dispersal of S. aureus, the authors completed their findings by an experimental clinical test on this physician and showed that transmission of the bacterium increased by 40-fold when he was infected by a rhinovirus infection than when he was not. The use of a mask significantly reduced dispersal (P = 0.015) (Sherertz et al., 1996).
Healthcare workers who are asymptomatic nasal carriers can sometimes be the source of MRSA outbreaks (Wang et al., 2001; Vonberg et al., 2006; Haill et al., 2013; Lamanna et al., 2017). On the other hand, in nonoutbreak situations and in presence of control measures, healthcare workers are infrequently sources of transmission of S. aureus (Price et al., 2017).
Mobile phones of healthcare workers may be a reservoir of S. aureus (Chang et al., 2017). A recent study evaluated incidence of bacterial contamination of mobile phones belonging to medical staff working in the operating room. Seventy two healthcare professionals took bacterial cultures from their phones, anterior nares, and hands. The results revealed that 31 staff had S. aureus isolated from their nares, 8 from their mobile phones, and 4 from their hands. Genotyping confirmed that 7/8 of the mobile phones strains were identical to the ones isolated from the nares (Chang et al., 2017).
Mechanisms of Colonization
The vestibulum nasi or anterior part of the nares is lined by a stratified, keratinized nonciliated squamous epithelium, whereas the rest of the nasal cavity, that is, its inner part is lined with a ciliated columnar epithelium (Peacock et al., 2001; Weidenmaier et al., 2012).
Both epithelia have been described as habitats for S. aureus (Mulcahy et al., 2012; Baur et al., 2014) as it will be developed in this section. Intracellular localization in nasal tissue from healthy volunteers was also described (Hanssen et al., 2017). For a successful colonization, S. aureus expresses adhesive molecules (Burian et al., 2010), fundamental for the establishment of interactions with human cell surface components, as it was demonstrated in vitro and in vivo (Mulcahy et al., 2012; Baur et al., 2014; Figure 3). Major ligands interactions are listed in Table 1.
FIGURE 3.
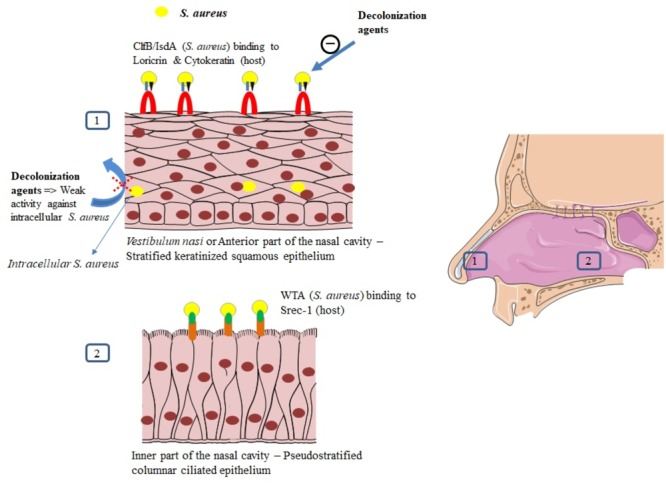
Mechanisms of S. aureus nasal colonization.
Interaction Between S. aureus and the Squamous Epithelium of the Anterior Nares
The vestibulum nasi is lined by skin (Cunningham, 1905; Vuyk and Watts, 2006). The uppermost layer of the epidermis is the stratum corneum or cornified layer (Candi et al., 2005; Eckhart et al., 2013). This layer contains keratinocytes that express proteins, such as loricrin, cytokeratin 10 (K10), involucrin, filaggrin, and other proteins (Eckhart et al., 2013).
Clumping factor B (ClfB) and iron-regulated surface determinant A (IsdA) are staphylococcal surface proteins that can adhere to cornified envelope proteins (Clarke et al., 2004, 2006; Schaffer et al., 2006; Wertheim et al., 2008; Mulcahy et al., 2012) and favor nasal colonization as it will be developed in this section.
Recently, Mulcahy et al. (2012) demonstrated that loricrin, the most abundant protein in the cornified envelope (Steinert and Marekov, 1995), was the major target ligand for ClfB during S. aureus nasal colonization. Nasal colonization by a ClfB+ S. aureus strain was reduced by 80% in loricrin-deficient mice compared to wild-type mice. ClfB has also been shown to interact with cytokeratin 10 (O’Brien et al., 2002; Schaffer et al., 2006; Wertheim et al., 2008), cytokeratin 8 (Haim et al., 2010), and fibrinogen (Perkins et al., 2001; Table 1). The role of ClfB in the adherence of S. aureus to the nasal epithelium has been studied in vitro (O’Brien et al., 2002; Mulcahy et al., 2012), in animal models (Schaffer et al., 2006; Mulcahy et al., 2012), and in human studies (Wertheim et al., 2008).
Schaffer et al. (2006) demonstrated that mutant strains of S. aureus deficient in ClfB resulted in a reduced nasal colonization in both mice and rats, as compared to the wild-type strain (ClfB+). In human, the mutant strain ClfB- of S. aureus has been reported to be cleared faster than the wild-type strain (median 3 ± 1 vs. 7 ± 4 days, p = 0.006), whereas the wild-type strain persisted for 28 days after inoculation (Wertheim et al., 2008).
IsdA also plays a role in the adherence of S. aureus to nasal cells as demonstrated in vitro and in vivo (Clarke et al., 2004). Mutation in IsdA gene reduced the ability of the bacteria to bind human nasal cells in vitro and to colonize the anterior nares in cotton rats (Clarke et al., 2006). This surface protein has also been shown to bind to fibrinogen and fibronectin (Clarke et al., 2004). The role of IsdA in nasal colonization has not been clearly demonstrated in human studies yet.
Other S. aureus surface proteins, such as surface protein G (SasG), SasX, and the serine-aspartate repeat proteins SdrC and SdrD may also serve as ligands to the epithelial cells (Corrigan et al., 2009; Liu Q. et al., 2015). Desmoglein 1 was identified as a host ligand for SdrD (Askarian et al., 2016). Their roles in humans have not been tested yet (Mulcahy et al., 2012).
Interaction in the Inner Nasal Cavity
Apart from the vestibulum nasi, the inner part of the nasal cavity constitutes another ecological niche for S. aureus. The staphylococcal nonprotein adhesin, named cell wall teichoic acid (WTA) is considered as an important factor for the colonization process (Weidenmaier et al., 2004). In an in vivo study, WTA-deficient S. aureus mutants could not adhere to nasal cells and were unable to colonize cotton rat nares compared to wild type control strains (Weidenmaier et al., 2004).
In a study combining in vitro and in vivo assessments, Baur et al. (2014) studied the molecular details of WTA adhesion to nasal cells. They first discovered that SREC-1 (a member of the F-type scavenger receptor) was expressed on epithelial cells in the inner nasal cavity of human and cotton rats. The authors further reported that SREC-1 interacted with WTA and confirmed these findings in infected cotton rats pretreated with antiSREC-1 antibody. A significant decrease in colonization was observed 8 h and 6 days after inoculation in the group receiving antiSREC-1 antibody, as compared to the controls (Baur et al., 2014). In agreement, Weidenmaier et al. (2008) previously demonstrated the role of WTA in the initial stages of S. aureus colonization.
Intracellular Localization of S. aureus
Intracellular localization of S. aureus in the nasal tissue has been described using techniques including immunohistochemical analysis, hematoxylin, and Eosin stains (Hayes et al., 2015; Ou et al., 2016, 2017). Epithelial cells, endothelial cells, and inflammatory cells, especially mast cells (Plouin-Gaudon et al., 2006; Tan et al., 2014; Hayes et al., 2015; Ou et al., 2016) have been described as habitats for intracellular S. aureus (ICSA). Using skin biopsies from the vestibulum nasi of randomly selected healthy participants, Hanssen et al. (2017) detected intracellular S. aureus in the stratum spinosum, one of the layers forming the epidermis. Intracellular localization of S. aureus has been reported both in patients with rhinosinusitis (Clement et al., 2005; Plouin-Gaudon et al., 2006; Ou et al., 2016, 2017) and healthy volunteers (Hanssen et al., 2017). In the control population from the study of Ou et al. (2016), 38% had ICSA. However, in the absence of large scale population study, the true prevalence of ICSA in healthy individuals remains to determine.
Intracellular localization of S. aureus seems to protect the bacteria from host defense mechanisms (Clement et al., 2005) and favor antimicrobial agents’ failure, as it was demonstrated in a recent study evaluating systemic and topical antibiotics. Using a cell model of S. aureus nasal epithelium evasion, Rigaill et al. (2018) found that most of the decolonizing agents, including mupirocin, exhibited weak activity against ICSA. Elimination of ICSA was also studied in a mouse model using vancomycin. In this study, intracellular bacteria were able to establish infection even in the presence of this antibiotic (Lehar et al., 2015), whereas planktonic bacteria were eliminated by vancomycin and did not cause infection. The intracellular residency of S. aureus could explain re-colonization and failure in decolonization observed in some healthy carriers, and the recurrence of infections observed in patients with chronic rhinosinusitis (Clement et al., 2005; Ou et al., 2017; Rigaill et al., 2018).
Immune System and S. aureus Colonization
Our knowledge in terms of host response during S. aureus nasal colonization is still limited as compared to immune response in invasive S. aureus infection (Brown et al., 2014). The association between the host immune system and S. aureus nasal carriage has been recently reviewed by Mulcahy and McLoughlin (2016). The presence of S. aureus in the nares seems to induce both innate and adaptive immune system. S. aureus can otherwise overcome host defense mechanisms. The staphylococcal protein A (SpA) and the exoprotein staphylokinase could play a key role in the bacterial adaptation to the immune system and colonization process (Peschel and Sahl, 2006; Cole et al., 2016).
Innate Immune Response
Human nasal secretions have efficient natural antimicrobial activity (Cole et al., 1999). Indeed, they contain epithelial-cells secreted antimicrobial peptides (AMPs) contributing to the first line defense in the nose. Primary nasal epithelial cells yield AMPs such as hBD3 and RNase-7 after stimulation with cytokines (IFN-g, IL-1b, and TNF-a) (Burgey et al., 2016). Media containing S. aureus induce the expression of these AMPs less efficiently than inflammatory cytokines (Burgey et al., 2016). Differential expression profiles of AMPs could be primary determinants for different S. aureus carriage states (Burgey et al., 2016).
Anti-staphylococcal responses can be induced by the involvement of Toll-like receptors (TLR). Hence, the role of TLR2 has been documented in nasal S. aureus colonization (González-Zorn et al., 2005). Carrier strains of S. aureus have been shown to delay the host’s immune response compared to non-carrier strains due to the delayed TLR2 expression they stimulate in nasal epithelial cells (Quinn and Cole, 2007). Different polymorphisms have been found in S. aureus nasal carriers and non-carriers; they target soluble or membrane-binding molecules such as genes encoding mannose-binding lectin, glucocorticoid receptor, C-reactive protein, beta-defensin 1, TLR-9, and interleukin-4 (van den Akker et al., 2006; Emonts et al., 2008; Ruimy et al., 2010; Mulcahy and McLoughlin, 2016; Nurjadi et al., 2018).
A study evaluating neutrophil depletion was performed in mice to determine the importance of neutrophil influx during carriage. Mice that received an antibody for neutrophil depletion had a significant increase in S. aureus colonization rate as compared to controls. This in vivo study highlights that neutrophil influx plays a role in clearance of S. aureus (Archer et al., 2013).
Adaptive Immune Response
As documented from serum analysis, S. aureus carriage induces an adaptive humoral immune response. Serum levels of immunoglobulin IgG and IgA specific to several staphylococcal proteins have been reported to be higher in persistent carriers than in non-carriers (van Belkum et al., 2009; Colque-Navarro et al., 2010). But evidence that these antibodies are protective against colonization are lacking.
In a murine model of nasal colonization, clearance of S. aureus was found to be B-cell independent but T-cell mediated. The Th17 cells, a functional T-cell lineage distinct from Th1 and Th2 are known to be involved in mucosal barriers and surface pathogen clearance. They typically produce IL-17 cytokine family. Archer et al. (2013, 2016) showed that IL-17A and IL-17F deficient mice failed to clear S. aureus experimentally inoculated through nasal route; IL-17A was required for AMP expression induced by nasal colonization. In a murine model, the IL-10 family cytokine IL-22 was secreted by Th17 cells and facilitated local expression of AMPs. IL-22 also reduced the expression of staphylococcal ligands loricrin and cytokeratin 10 and thus controlled nasal colonization (Mulcahy et al., 2016).
By contrast, in a human study evaluating nasal fluid inflammatory factors and nasal carriage with S. aureus, IL-17 was not detected (Cole et al., 2016). This was surprising and the authors suggested that IL-17 could be insoluble in nasal secretions (Cole et al., 2016). Low Th1 to Th17 cytokines ratio were found to be predictive of S. aureus carriage in volunteers after whole blood stimulation with S. aureus (Nurjadi et al., 2016). Future human studies should be conducted to better understand the role of Th17 cytokines in nasal carriage.
Microbiology-Based Classification of Nasal Carriers
In order to confirm nasal carriage of S. aureus, samples are commonly collected using commercial dry or moistened sterile swabs, with no significant difference found between the two (Warnke et al., 2016). The protocol of sampling is not really standardized but generally consists of rubbing the swab in the anterior nares of each nostril for approximately four rotary movements (Wertheim et al., 2006; Bode et al., 2010). Swabs are then analyzed to check presence of S. aureus. The two commonly used laboratory tests for the identification of S. aureus are the culture on chromogenic solid media – which is the less expensive test – and polymerase chain reaction, which is the gold standard and the most rapid technique for MRSA detection (Pournajaf et al., 2014). It is important to note that chronic carriage of S. aureus may not only result from nasal colonization. Sampling extra-nasal sites like oropharynx, rectum, wounds, axilla increases MRSA detection rate in patients at risk for S. aureus nosocomial infection (Miller et al., 2012; McKinnell et al., 2013).
Epidemiological studies over periods varying from 12 weeks to 3 years have described three nasal carriage patterns for S. aureus among healthy volunteers swabbed several times. Persistent carriers which rates ranged from 10% to 30% (Williams, 1963; Eriksen et al., 1995; VandenBergh et al., 1999; Nouwen et al., 2004; Muthukrishnan et al., 2013), non-carriers which rates ranged from 10% to 47%, and the rest who were considered intermittent carriers (Williams, 1963; Eriksen et al., 1995; VandenBergh et al., 1999; Nouwen et al., 2004). The definitions used for this classification varied from one study to another. In some studies, persistent carriers were the ones who had results of all of their swabs positive for S. aureus (Muthukrishnan et al., 2013). Other studies defined cut-off values for carrier–index (number of positive swabs/number of total swabs for each person) (Eriksen et al., 1995; VandenBergh et al., 1999). However, there is no clear definition on the number of swabs that should be taken and what fraction should be positive before determining carriage state.
Nouwen et al. (2004) proposed a culture rule based on the combination of qualitative and quantitative results of two consecutive nasal culture swabs taken approximately a week apart, to predict S. aureus nasal carrier state among healthy volunteers. When both cultures are positive over 103 colony forming units (cfu) a person is classified as persistent carrier. When only one of the cultures is positive, or when both cultures are positive with a low cfu, the person is considered as an intermittent carrier. van Belkum et al. (2009) performed a study in 51 volunteers with a known carriage state, who were artificially colonized with a mixture of S. aureus strains after a decolonization treatment and followed for 22 weeks. Median nasal bacterial survival was of 4 days in noncarriers, 14 days in intermittent carriers, and more than 154 days in persistent carriers (P = 0.017). The culture swabs of persistent carriers contained more bacterial loads compared to the other groups. Serum levels of anti-staphylococcal antibodies differed between persistent and nonpersistent carriers as previously stated. The authors concluded that intermittent and noncarriers share similar characteristics, and thus suggested a reclassification of S. aureus nasal carriers into persistent carriers and “other” carriers (van Belkum et al., 2009). Persistent carriers have been shown to have higher counts of S. aureus (Nouwen et al., 2004; van Belkum et al., 2009) and a higher risk of infection compared to other carriers (Wertheim et al., 2004; Nouwen et al., 2005). However, bacterial loads in persistent carriers are always variable and it is hard to determine a fixed threshold for diagnosis (Burian et al., 2010; Verhoeven et al., 2012; Nilsson et al., 2015). Persistent carriage is more common in children than in adults, but many people change their carrier state between 10 and 20 years old (Armstrong-Esther, 1976). Conclusions that persistent carriers harbor the same strain for many years, and intermittent carriers appear to have changing strains (Eriksen et al., 1995; VandenBergh et al., 1999) have been recently the subject of controversy (Muthukrishnan et al., 2013).
Cross-sectional studies yield a prevalence of approximately 20–30% of carriers in the general population which is a mix of persistent and intermittent carriers (Gorwitz et al., 2008; den Heijer et al., 2013; Saadatian-Elahi et al., 2013; Chen et al., 2017).
Individual Risk Factors for S. aureus Nasal Colonization
Nasal colonization depends on host factors, such as the underlying condition or diseases (Table 2). Some studies have found that nasal carriage was more frequent in human immunodeficiency virus (HIV)-infected (Raviglione et al., 1990; Weinke et al., 1992; Kotpal et al., 2016) or obese patients (Olsen et al., 2013), compared to healthy individuals. This higher prevalence was also found among diabetic patients undergoing dialysis compared to non-diabetic patients in the same population (Luzar et al., 1990). Other diseases such as granulomatosiss with polyangiitis (formerly known as Wegener’s granulomatosis), rheumatoid arthritis (Laudien et al., 2010), skin and soft tissue infections (Immergluck et al., 2017) atopic dermatitis (Breuer et al., 2002), and recurrent furunculosis (Demos et al., 2012) have been related with an increased carriage rate.
Table 2.
Predisposing factors for nasal carriage.
| Predisposing factors for nasal carriage | Reference |
|---|---|
| HIV-infection | Raviglione et al., 1990; Weinke et al., 1992; Kotpal et al., 2016 |
| Obesity | Olsen et al., 2013 |
| Diabetic patients undergoing dialysis (compared to non-diabetic patients in the same population) | Luzar et al., 1990 |
| Granulomatosis with polyangiitis | Laudien et al., 2010 |
| Rheumatoid arthritis | Laudien et al., 2010 |
| Skin and soft tissue infections | Immergluck et al., 2017 |
| Recurrent furunculosis | Demos et al., 2012 |
| Atopic dermatitis | Breuer et al., 2002 |
| Hemoglobin in nasal secretions | Pynnonen et al., 2011 |
| Histocompatibility antigen phenotype HLA-DR3 | Kinsman et al., 1983 |
| Polymorphisms in genes encoding for the glucocorticoid receptor, interleukin-4, C-reactive proteins, and complement inhibitor proteins | van den Akker et al., 2006; Emonts et al., 2008; Ruimy et al., 2010 |
| Hormonal contraception use | Zanger et al., 2012 |
| Active smokers: controversial | Olsen et al., 2012; Cole et al., 2018 |
| Hospital workers: controversial | Elie-Turenne et al., 2010; Saadatian-Elahi et al., 2013; Chen et al., 2015; Price et al., 2017 |
In healthy subjects, Liu C.M. et al. (2015) found similar carriage rates in men and women, while men had higher bacterial density. Reports of a higher risk of nasal carriage of S. aureus among hospital workers than the rest of the population have not been confirmed (Elie-Turenne et al., 2010; Saadatian-Elahi et al., 2013; Chen et al., 2015; Price et al., 2017). The association between smoking and nasal carriage seems also controversial. In a study by Olsen et al. (2012), active smoking in healthy adults was found to be a protective factor for carriage of S. aureus, with a hypothesized bactericidal activity of cigarette smokes. Conversely, a recent study showed that smokers were more frequently colonized than non-smokers, and cessation from smoking improved clearance of nasal S. aureus in an experimental inoculation study (Cole et al., 2018). Many other host conditions have been punctually studied and reported as additional predisposing factor such as hormonal contraception (Zanger et al., 2012) and presence of hemoglobin in nasal secretions (Pynnonen et al., 2011).
At the genetic level, no correlation was found between genetic factors and S. aureus carriage. No significant heritability for S. aureus nasal colonization was detected in twins and family studies (Roghmann et al., 2011; Andersen et al., 2012). Interestingly, some polymorphisms in host inflammatory response genes have been associated with S. aureus nasal carriage. The presence of the histocompatibility antigen phenotype HLA-DR3 could be a predisposition (Kinsman et al., 1983).
As previously said, at the immune system level, polymorphisms in genes encoding some proteins and differential expression profiles of AMPs could be the determinants of the various carriage states.
In a study involving 93 type 1 diabetes patients, vitamin D receptor polymorphisms were determined in Deoxyribonucleic acid (DNA) extracted from peripheral blood leukocytes. Analysis showed that presence of specific alleles coding for vitamin D receptors were associated with an increased rate of S. aureus colonization (Panierakis et al., 2009).
Nasal Carriage of S. aureus as a Risk Factor for Infections
Staphylococcus aureus nasal colonization has been identified as a major risk factor for the development of patent staphylococcal infections, weather community acquired, or nosocomial (Von Eiff et al., 2001; Wertheim et al., 2004, 2005b) which increases the risk by 2 to 10 times (Perl and Golub, 1998). The risk of infection in nasal carriers has been mainly studied in surgical patients (general, orthopedic, cardiac, and neurosurgeries) (Perl et al., 2002; Bode et al., 2010; Walsh et al., 2017), patients on hemodialysis (Kluytmans et al., 1996; Katneni and Hedayati, 2007), patients on chronic ambulatory peritoneal dialysis (CAPD) (Luzar et al., 1990), HIV-infected patients (Nguyen et al., 1999; Sissolak et al., 2002), and intensive care unit patients (Nardi et al., 2001). It has also been shown to be the primary risk factor for recurrent furunculosis, nasal colonization being present in almost 60% of individuals with furuncles and impetigo (Durupt et al., 2007) (Figure 2 and Table 3).
Table 3.
S. aureus nasal colonization, a risk factor for infections.
| S. aureus nasal colonization, a risk factor for | Reference |
|---|---|
| Surgical site infections after orthopedic surgeries | Kalmeijer et al., 2000; Yano et al., 2000; Weiser and Moucha, 2015 |
| Surgical site infections after cardiac surgeries | Kluytmans et al., 1995; Muñoz et al., 2008 |
| Bacteremia in nonsurgical patients | Wertheim et al., 2004 |
| Catheter-related infections in dialysis patients | Luzar et al., 1990; Katneni and Hedayati, 2007 |
| S. aureus infections in HIV-infected patients | Nguyen et al., 1999; Sissolak et al., 2002 |
| ICU-associated S. aureus infections | Honda et al., 2010 |
| Recurrent furunculosis and impetigo | Durupt et al., 2007; Demos et al., 2012 |
| Diabetic foot ulcer infections | Dunyach-Remy et al., 2017 |
Surgical Site Infections
Developing a post-operative infection is a multifactorial process usually combining preoperative, intraoperative, and post-operative factors (Savage and Anderson, 2013). Nasal colonization can actually be considered as a preoperative risk factor for MRSA and MSSA infections. S. aureus can spread from the anterior nares to other areas on the skin surface and thus contaminate the surgical wound during the operative procedure (NICE Clinical Guidelines, 2008; Savage and Anderson, 2013). It has been shown that around 80% of strains causing a staphylococcal infection at the site of surgery have molecular identity with S. aureus isolates in the nares of concerned patients (Perl et al., 2002).
Surgical site infections (SSI) are one of the most common post-operative complications and represent 20 to 30% of healthcare associated infections (HCAI) (Klevens et al., 2007; Magill et al., 2012; Savage and Anderson, 2013). While enterobacteria and other uro-digestive bacteria are dominant in infections after gastrointestinal, urological and gynecological surgeries (Trautman et al., 2007), S. aureus predominates in orthopedic and cardiac surgery settings (Lepelletier et al., 2005; Trautman et al., 2007; Muñoz et al., 2008). In orthopedic patients, biofilms can form on the implants leading to therapeutic challenges (Chen et al., 2013). According to the French Institute for Public Health Surveillance in 2014, S. aureus composed 19.4% of isolated germs in SSIs after coronary surgeries, 51.9% of organisms causing SSIs in orthopedic surgeries, and 29.3% of organisms causing infections in gynecologic obstetric surgery in 2014 (Le Réseau d’alerte, d’investigation et de surveillance des infections nosocomiales (Raisin), 2015). Similar implications have been also found in other countries (Saadatian-Elahi et al., 2008; Negi et al., 2015).
Several studies including case–control and multivariate analysis have identified nasal carriage of S. aureus an independent risk factor for SSIs (Kluytmans et al., 1995; Kalmeijer et al., 2000; Muñoz et al., 2008). In the case–control study from Kluytmans et al. (1995), cardiac surgery patients were screened for their nasal carriage status the day before surgery and followed for the development of an SSI. S. aureus wound infections occurred in 40 patients as opposed to 120 controls who did not develop infection. Nasal carriage, identified in 52% of cases as compared to 12% of controls, was found to be a significant risk factor for the development of these post-operative infections with an OR = 9.6, 95% CI (3.9–23.7). In a study involving 357 patients undergoing major heart surgery, nasal carriers had a higher incidence of SSI than non-carriers (12.5% vs. 5%, P = 0.01) (Muñoz et al., 2008). Similar conclusions were obtained for orthopedic patients with an incidence increasing from 3- to 11-fold (Kalmeijer et al., 2000; Yano et al., 2000; Weiser and Moucha, 2015).
Surgical site infections have an important impact on the patient and the healthcare system. These infections increase hospital stay (Leaper et al., 2004), mortality and healthcare costs and decrease health-related quality of life (Anderson et al., 2009; Lamarsalle et al., 2013; Savage and Anderson, 2013). A retrospective database analysis in France concluded that staphylococcal infections led to approximately 1.0 and 1.4 additional hospitalizations per patient, 22.1 and 22.4 additional hospital days, and an excess cost of €15,475 and €13,389 after cardiothoracic and orthopedic surgeries respectively (Schmidt et al., 2015). The rate of in-hospital mortality was 2.6 times and six times higher among infected patients than non-infected patients in cardiothoracic and orthopedic procedures (Schmidt et al., 2015).
Methicillin-resistant S. aureus carriage could increase the risk for the development of SSIs. In a systematic review, patients colonized with MRSA were four times more likely to develop invasive infection than patients colonized with MSSA (Safdar and Bradley, 2008). MRSA infections are reported to cause up to 40% of HCAI worldwide with particularly high incidence in the United States and many European countries (McKinnell et al., 2013) and have been shown to have increased morbi-mortality as compared to MSSA (Anderson et al., 2009). In a study carried out by Anderson et al. (2009), patients with SSI due to MRSA had a 2.6 higher risk of dying within 3 months, the duration of hospital stay was 6 days longer, and the related cost was increased of $23,000 compared to patients with SSI due to MSSA. On the other hand, a meta-analysis of 31 cohort studies on S. aureus bacteremia, showed significantly higher mortality with MRSA than with MSSA (Cosgrove et al., 2003). However, the reasons for increased fatality rate with MRSA infections are unclear. Some authors suggest that MRSA and MSSA bacteria are equally virulent but MRSA infections usually develop in patients previously treated with antibiotics. Thus, as previously suggested, the differences in patients’ fatality rate may rather reflect the severity of underlying conditions than a higher bacteria-related increased virulence (Humphreys, 2012; Hraiech et al., 2013).
Infections in Nonsurgical Patients
Nasal carriage of S. aureus has also been found to be a risk factor for subsequent infections in nonsurgical patients. Wertheim et al. (2004) screened 14,008 adults who had nasal swab on admission in a nonsurgical department, 3420 (24%) were positive for S. aureus. The follow-up identified 81 patients who developed S. aureus bacteremia between 2 and 120 days after swabbing, which was three times more frequent in carriers than in non-carriers. Interestingly, the death rate from S. aureus bacteremia was higher in non-carriers than carriers. This could be due to the protective immunity of anti-staphylococcal antibodies (Wertheim et al., 2004; Holtfreter et al., 2006). Approximately, 80% of S. aureus blood isolates causing bacteremia were of endogenous origins and identical to those isolated from the anterior nares of corresponding patients, thus confirming previous report (Von Eiff et al., 2001).
A prospective cohort study evaluated the occurrence of S. aureus infections in 5161 patients who were screened for nasal carriage when admitted to the ICU. ICU-associated S. aureus infections were defined by the development of infection >48 h after their admission to the unit. These infections occurred in 113 patients and nasal colonization was associated with a 2.5 to 4.7-fold increased risk (Honda et al., 2010).
In HIV-positive patients, a prospective cohort study evaluating 231 subjects every 3 months for a minimum of two years, has reported a 6% incidence of S. aureus infections, nasal carriers being more at risk [p = 0.04, OR = 3.6 (0.9–15.4)] (Nguyen et al., 1999).
In hemodialysis and chronic peritoneal dialysis patients, most of infectious complications come from endogenous origin (Luzar et al., 1990; Ena et al., 1994). S. aureus is the most common isolated agent from central venous catheter-related bacteremia (Katneni and Hedayati, 2007), or exit-site infections of peritoneal dialysis catheters (Luzar et al., 1990). Nasal carriers are at increased risk of contracting these infections (Nouwen et al., 2005; Ong et al., 2017).
Staphylococcus aureus is the most frequently isolated pathogen from diabetic foot infections. A study compared the genotypic profiles of S. aureus strains isolated from the nares and diabetic foot ulcer infections of 276 patients. The bacterium was isolated from both sites in 36% of the population, and identical strains were found in 65% of cases. Further investigations should be performed in order to confirm the benefit of screening and treating nasal carriers in this population (Dunyach-Remy et al., 2017).
Nasal carriage of S. aureus constitutes a risk factor for the development of skin and soft tissue infections caused by this germ in non-hospitalized and non-diseased subjects (Chou et al., 2015). It has been shown to be the primary risk factor for recurrent furunculosis (Demos et al., 2012). Nasal carriage is also a risk factor for secondary bacterial pneumonia in patients having influenza A virus infection. The viral infection causes host physiologic changes that generates the dissemination of S. aureus from nasal tissue to the lungs as demonstrated in a mouse model (Reddinger et al., 2016).
Perspectives
Nasal carriage of S. aureus is multifactorial and can predispose carriers to subsequent infections. Nasal decolonization of carriers is therefore recommended in patients undergoing cardiothoracic and orthopedic surgeries (De Jonge et al., 2016).
A full understanding of host-pathogen interactions can help find new decolonization strategies. New fields on colonization mechanisms should be investigated. For example, the role of mycobiota starts to be described in the pathophysiology of chronic respiratory diseases (Brégeon and Rolain, 2015). Interaction of nasal S. aureus with the nasal fungal communities would be an interesting perspective to develop. On the other hand, some reports suggest that S. aureus can regulate host inflammatory gene expression (Modak et al., 2014). Epigenetics mechanisms could be interesting to investigate in order to better understand tolerance mechanisms in S. aureus colonization processes.
Author Contributions
AS wrote the review paper. FB, J-LM, OB, and J-MR corrected the manuscript. All authors approved and revised the final version of the manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Footnotes
Funding. This study was supported by IHU Méditerranée Infection, Marseille, France and by the French Government under the “Investissements d’Avenir” (Investments for the Future) program managed by the “Agence Nationale de la Recherche” (ANR, fr: National Agency for Research) (reference: Méditerranée Infection 10-IAHU-03). This work was supported by Région Provence Alpes Côte d’Azur and European funding FEDER PRIMI. AS was supported by a CIFRE PhD Grant from the “Association Nationale de la Recherche et de la Technologie”.
References
- Andersen P. S., Pedersen J. K., Fode P., Skov R. L., Fowler V. G., Stegger M., et al. (2012). Influence of host genetics and environment on nasal carriage of Staphylococcus aureus in danish middle-aged and elderly twins. J. Infect. Dis. 206 1178–1184. 10.1093/infdis/jis491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson D. J., Kaye K. S., Chen L. F., Schmader K. E., Choi Y., Sloane R., et al. (2009). Clinical and financial outcomes due to methicillin resistant Staphylococcus aureus surgical site infection: a multi-center matched outcomes study. PLoS One 4:e8305. 10.1371/journal.pone.0008305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Archer N. K., Adappa N. D., Palmer J. N., Cohen N. A., Harro J. M., Lee S. K., et al. (2016). Interleukin-17A (IL-17A) and IL-17F are critical for antimicrobial peptide production and clearance of Staphylococcus aureus nasal colonization. Infect. Immun. 84 3575–3583. 10.1128/IAI.00596-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Archer N. K., Harro J. M., Shirtliff M. E. (2013). Clearance of Staphylococcus aureus nasal carriage is T cell dependent and mediated through interleukin-17A expression and neutrophil influx. Infect. Immun. 81 2070–2075. 10.1128/IAI.00084-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong-Esther C. A. (1976). Carriage patterns of Staphylococcus aureus in a healthy non-hospital population of adults and children. Ann. Hum. Biol. 3 221–227. 10.1080/03014467600001381 [DOI] [PubMed] [Google Scholar]
- Askarian F., Ajayi C., Hanssen A.-M., van Sorge N. M., Pettersen I., Diep D. B., et al. (2016). The interaction between Staphylococcus aureus SdrD and desmoglein 1 is important for adhesion to host cells. Sci. Rep. 6:22134. 10.1038/srep22134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baur S., Rautenberg M., Faulstich M., Faulstich M., Grau T., Severin Y., et al. (2014). A nasal epithelial receptor for Staphylococcus aureus WTA governs adhesion to epithelial cells and modulates nasal colonization. PLoS Pathog. 10:e1004089. 10.1371/journal.ppat.1004089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bode L. G. M., Kluytmans J. A. J. W., Wertheim H. F. L., Bogaers D., Vandenbroucke-Grauls C. M. J. E., Roosendaal R., et al. (2010). Preventing surgical-site infections in nasal carriers of Staphylococcus aureus. N. Engl. J. Med. 362 9–17. 10.1056/NEJMoa0808939 [DOI] [PubMed] [Google Scholar]
- Brégeon F., Rolain J.-M. (2015). Le résistome pulmonaire. Méd. Sci. 31 947–950. 10.1051/medsci/20153111003 [DOI] [PubMed] [Google Scholar]
- Breuer K., HAussler S., Kapp A., Werfel T. (2002). Staphylococcus aureus: colonizing features and influence of an antibacterial treatment in adults with atopic dermatitis. Br. J. Dermatol. 147 55–61. 10.1046/j.1365-2133.2002.04872.x [DOI] [PubMed] [Google Scholar]
- Brown A. F., Leech J. M., Rogers T. R., McLoughlin R. M. (2014). Staphylococcus aureus colonization: modulation of host immune response and impact on human vaccine design. Front. Immunol. 4:507. 10.3389/fimmu.2013.00507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgey C., Kern W. V., Römer W., Rieg S. (2016). Differential induction of innate defense antimicrobial peptides in primary nasal epithelial cells upon stimulation with inflammatory cytokines, Th17 cytokines or bacterial conditioned medium from Staphylococcus aureus isolates. Microb. Pathog. 90 69–77. 10.1016/j.micpath.2015.11.023 [DOI] [PubMed] [Google Scholar]
- Burian M., Wolz C., Goerke C. (2010). Regulatory adaptation of Staphylococcus aureus during nasal colonization of humans. PLoS One 5:e10040. 10.1371/journal.pone.0010040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Candi E., Schmidt R., Melino G. (2005). The cornified envelope: a model of cell death in the skin. Nat. Rev. Mol. Cell Biol. 6 328–340. 10.1038/nrm1619 [DOI] [PubMed] [Google Scholar]
- Chang C.-H., Chen S.-Y., Lu J.-J., Chang C.-J., Chang Y., Hsieh P.-H. (2017). Nasal colonization and bacterial contamination of mobile phones carried by medical staff in the operating room. PLoS One 12:e0175811. 10.1371/journal.pone.0175811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen A. F., Heyl A. E., Xu P. Z., Rao N., Klatt B. A. (2013). Preoperative decolonization effective at reducing staphylococcal colonization in total joint arthroplasty patients. J. Arthroplasty 28 18–20. 10.1016/j.arth.2013.03.036 [DOI] [PubMed] [Google Scholar]
- Chen B., Dai X., He B., Pan K., Li H., Liu X., et al. (2015). Differences in Staphylococcus aureus nasal carriage and molecular characteristics among community residents and healthcare workers at Sun Yat-sen University, Guangzhou, Southern China. BMC Infect. Dis. 15:303. 10.1186/s12879-015-1032-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen B. J., Xie X. Y., Ni L. J., Dai X. L., Lu Y., Wu X. Q., et al. (2017). Factors associated with Staphylococcus aureus nasal carriage and molecular characteristics among the general population at a Medical College Campus in Guangzhou, South China. Ann. Clin. Microbiol. Antimicrob. 16:28. 10.1186/s12941-017-0206-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou Y.-H., Lee M.-S., Lin R.-Y., Wu C.-Y. (2015). Risk factors for methicillin-resistant Staphylococcus aureus skin and soft-tissue infections in outpatients in Taiwan. Epidemiol. Infect. 143 749–753. 10.1017/S0950268814001642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke S. R., Brummell K. J., Horsburgh M. J., McDowell P. W., Mohamad S. A. S., Stapleton M. R., et al. (2006). Identification of in vivo-expressed antigens of Staphylococcus aureus and their use in vaccinations for protection against nasal carriage. J. Infect. Dis. 193 1098–1108. 10.1086/501471 [DOI] [PubMed] [Google Scholar]
- Clarke S. R., Wiltshire M. D., Foster S. J. (2004). IsdA of Staphylococcus aureus is a broad spectrum, iron-regulated adhesin. Mol. Microbiol. 51 1509–1519. 10.1111/j.1365-2958.2003.03938.x [DOI] [PubMed] [Google Scholar]
- Clement S., Vaudaux P., Francois P., Schrenzel J., Huggler E., Kampf S., et al. (2005). Evidence of an intracellular reservoir in the nasal mucosa of patients with recurrent Staphylococcus aureus rhinosinusitis. J. Infect. Dis. 192 1023–1028. 10.1086/432735 [DOI] [PubMed] [Google Scholar]
- Cole A. L., Muthukrishnan G., Chong C., Beavis A., Eade C. R., Wood M. P., et al. (2016). Host innate inflammatory factors and staphylococcal protein A influence the duration of human Staphylococcus aureus nasal carriage. Mucosal Immunol. 9 1537–1548. 10.1038/mi.2016.2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole A. L., Schmidt-Owens M., Beavis A. C., Chong C. F., Tarwater P. M., Schaus J., et al. (2018). Cessation from smoking improves innate host defense and clearance of experimentally inoculated nasal S. aureus. Infect. Immun. 86 e912–e917. 10.1128/IAI.00912-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole A. M., Dewan P., Ganz T. (1999). Innate antimicrobial activity of nasal secretions. Infect. Immun. 67 3267–3275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colque-Navarro P., Jacobsson G., Andersson R., Flock J.-I., Möllby R. (2010). Levels of antibody against 11 Staphylococcus aureus antigens in a healthy population. Clin. Vaccine Immunol. 17 1117–1123. 10.1128/CVI.00506-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corrigan R. M., Miajlovic H., Foster T. J., Kluytmans J., van Belkum A., Verbrugh H., et al. (2009). Surface proteins that promote adherence of Staphylococcus aureus to human desquamated nasal epithelial cells. BMC Microbiol. 9:22. 10.1186/1471-2180-9-22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosgrove S. E., Sakoulas G., Perencevich E. N., Schwaber M. J., Karchmer A. W., Carmeli Y. (2003). Comparison of mortality associated with methicillin-resistant and methicillin-susceptible Staphylococcus aureus bacteremia: a meta-analysis. Clin. Infect. Dis. 36 53–59. 10.1086/345476 [DOI] [PubMed] [Google Scholar]
- Cunningham D. J. (1905). Cunningham’s Text-Book of Anatomy. New York, NY?: W. Wood and company. [Google Scholar]
- Dall’Antonia M., Coen P. G., Wilks M., Whiley A., Millar M. (2005). Competition between methicillin-sensitive and -resistant Staphylococcus aureus in the anterior nares. J. Hosp. Infect. 61 62–67. 10.1016/j.jhin.2005.01.008 [DOI] [PubMed] [Google Scholar]
- De Jonge S., Atema J. J., Gans S., Boermeester M. A., Gomes S. M., Solomkin J. S., et al. (2016). Surgical site infections 1 New WHO recommendations on preoperative measures for surgical site infection prevention: an evidence-based global perspective. Lancet Infect. Dis. 16 e276–e287. 10.1016/S1473-3099(16)30398-X [DOI] [PubMed] [Google Scholar]
- Demos M., McLeod M. P., Nouri K. (2012). Recurrent furunculosis: a review of the literature. Br. J. Dermatol. 167 725–732. 10.1111/j.1365-2133.2012.11151.x [DOI] [PubMed] [Google Scholar]
- den Heijer C. D., van Bijnen E. M., Paget W. J., Pringle M., Goossens H., Bruggeman C. A., et al. (2013). Prevalence and resistance of commensal Staphylococcus aureus, including meticillin-resistant S. aureus, in nine European countries: a cross-sectional study. Lancet Infect. Dis. 13 409–415. 10.1016/S1473-3099(13)70036-7 [DOI] [PubMed] [Google Scholar]
- Dunyach-Remy C., Courtais-Coulon C., DeMattei C., Jourdan N., Schuldiner S., Sultan A., et al. (2017). Link between nasal carriage of Staphylococcus aureus and infected diabetic foot ulcers. Diabetes Metab. 43 167–171. 10.1016/j.diabet.2016.09.003 [DOI] [PubMed] [Google Scholar]
- Durupt F., Mayor L., Bes M., Reverdy M.-E., Vandenesch F., Thomas L., et al. (2007). Prevalence of Staphylococcus aureus toxins and nasal carriage in furuncles and impetigo. Br. J. Dermatol. 157 1161–1167. 10.1111/j.1365-2133.2007.08197.x [DOI] [PubMed] [Google Scholar]
- Eckhart L., Lippens S., Tschachler E., Declercq W. (2013). Cell death by cornification. Biochim. Biophys. Acta Mol. Cell Res. 1833 3471–3480. 10.1016/j.bbamcr.2013.06.010 [DOI] [PubMed] [Google Scholar]
- Elie-Turenne M.-C., Fernandes H., Mediavilla J. R., Rosenthal M., Mathema B., Singh A., et al. (2010). Prevalence and characteristics of Staphylococcus aureus colonization among healthcare professionals in an urban teaching hospital. Infect. Control Hosp. Epidemiol. 31 574–580. 10.1086/652525 [DOI] [PubMed] [Google Scholar]
- Emonts M., Uitterlinden A. G., Nouwen J. L., Kardys I., Maat M. P. M., Melles D. C., et al. (2008). Host polymorphisms in interleukin 4 complement factor H, and C-reactive protein associated with nasal carriage of Staphylococcus aureus and occurrence of boils. J. Infect. Dis. 197 1244–1253. 10.1086/533501 [DOI] [PubMed] [Google Scholar]
- Ena J., Boelaert J. R., Boyken L. D., Van Landuyt H. W., Godard C. A., Herwaldt L. A. (1994). Epidemiology of Staphylococcus aureus infections in patients on hemodialysis. Infect. Control Hosp. Epidemiol. 15 78–81. 10.2307/30145535 [DOI] [PubMed] [Google Scholar]
- Eriksen N. H., Espersen F., Rosdahl V. T., Jensen K. (1995). Carriage of Staphylococcus aureus among 104 healthy persons during a 19-month period. Epidemiol. Infect. 115 51–60. 10.1017/S0950268800058118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank D. N., Feazel L. M., Bessesen M. T., Price C. S., Janoff E. N., Pace N. R. (2010). The human nasal microbiota and Staphylococcus aureus carriage. PLoS One 5:e10598. 10.1371/journal.pone.0010598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrouste-Orgeas M., Timsit J. F., Kallel H., Ben Ali A., Dumay M. F., Paoli B., et al. (2001). Colonization with methicillin-resistant Staphylococcus aureus in ICU patients: morbidity, mortality, and glycopeptide use. Infect. Control Hosp. Epidemiol. 22 687–692. 10.1086/501846 [DOI] [PubMed] [Google Scholar]
- Ghasemzadeh-Moghaddam H., Neela V., van Wamel W., Hamat R. A., Shamsudin M. N., Hussin N. S. C., et al. (2015). Nasal carriers are more likely to acquire exogenous Staphylococcus aureus strains than non-carriers. Clin. Microbiol. Infect. 21 998.e1–998.e7. 10.1016/j.cmi.2015.07.006 [DOI] [PubMed] [Google Scholar]
- González-Zorn B., Senna J. P. M., Fiette L., Shorte S., Testard A., Chignard M., et al. (2005). Bacterial and host factors implicated in nasal carriage of methicillin-resistant Staphylococcus aureus in mice. Infect. Immun. 73 1847–1851. 10.1128/IAI.73.3.1847-1851.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorwitz R. J., Kruszon-Moran D., McAllister S. K., McQuillan G., McDougal L. K., Fosheim G. E., et al. (2008). Changes in the prevalence of nasal colonization with Staphylococcus aureus in the united states, 2001–2004. J. Infect. Dis. 197 1226–1234. 10.1086/533494 [DOI] [PubMed] [Google Scholar]
- Haill C., Fletcher S., Archer R., Jones G., Jayarajah M., Frame J., et al. (2013). Prolonged outbreak of meticillin-resistant Staphylococcus aureus in a cardiac surgery unit linked to a single colonized healthcare worker. J. Hosp. Infect. 83 219–225. 10.1016/j.jhin.2012.11.019 [DOI] [PubMed] [Google Scholar]
- Haim M., Trost A., Maier C. J., Achatz G., Feichtner S., Hintner H., et al. (2010). Cytokeratin 8 interacts with clumping factor B: a new possible virulence factor target. Microbiology 156 3710–3721. 10.1099/mic.0.034413-0 [DOI] [PubMed] [Google Scholar]
- Hanssen A.-M., Kindlund B., Stenklev N. C., Furberg A.-S., Fismen S., Olsen R. S., et al. (2017). Localization of Staphylococcus aureus in tissue from the nasal vestibule in healthy carriers. BMC Microbiol. 17:89. 10.1186/s12866-017-0997-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harbarth S., Liassine N., Dharan S., Herrault P., Auckenthaler R., Pittet D. (2000). Risk factors for persistent carriage of methicillin-resistant Staphylococcus aureus. Clin. Infect. Dis. 31 1380–1385. 10.1086/317484 [DOI] [PubMed] [Google Scholar]
- Hayes S. M., Howlin R., Johnston D. A., Webb J. S., Clarke S. C., Stoodley P., et al. (2015). Intracellular residency of Staphylococcus aureus within mast cells in nasal polyps: a novel observation. J. Allergy Clin. Immunol. 135 1648–1651.e5. 10.1016/j.jaci.2014.12.1929 [DOI] [PubMed] [Google Scholar]
- Holtfreter S., Roschack K., Eichler P., Eske K., Holtfreter B., Kohler C., et al. (2006). Staphylococcus aureus carriers neutralize superantigens by antibodies specific for their colonizing strain: a potential explanation for their improved prognosis in severe sepsis. J. Infect. Dis. 193 1275–1278. 10.1086/503048 [DOI] [PubMed] [Google Scholar]
- Honda H., Krauss M. J., Coopersmith C. M., Kollef M. H., Richmond A. M., Fraser V. J., et al. (2010). Staphylococcus aureus nasal colonization and subsequent infection in intensive care unit patients: does methicillin resistance matter? Infect. Control Hosp. Epidemiol. 31 584–591. 10.1086/652530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hraiech S., Roch A., Lepidi H., Atieh T., Audoly G., Rolain J.-M., et al. (2013). Impaired virulence and fitness of a colistin-resistant clinical isolate of Acinetobacter baumannii in a rat model of pneumonia. Antimicrob. Agents Chemother. 57 5120–5121. 10.1128/AAC.00700-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Human Microbiome Project Consortium (2012). Structure, function and diversity of the healthy human microbiome. Nature 486 207–214. 10.1038/nature11234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphreys H. (2012). Staphylococcus aureus: the enduring pathogen in surgery. Surgeon 10 357–360. 10.1016/j.surge.2012.05.003 [DOI] [PubMed] [Google Scholar]
- Immergluck L., Jain S., Ray S., Mayberry R., Satola S., Parker T., et al. (2017). Risk of skin and soft tissue infections among children found to be Staphylococcus aureus MRSA USA300 carriers. West. J. Emerg. Med. 18 201–212. 10.5811/westjem.2016.10.30483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwase T., Uehara Y., Shinji H., Tajima A., Seo H., Takada K., et al. (2010). Staphylococcus epidermidis Esp inhibits Staphylococcus aureus biofilm formation and nasal colonization. Nature 465 346–349. 10.1038/nature09074 [DOI] [PubMed] [Google Scholar]
- Kalmeijer M. D., van Nieuwland-Bollen E., Bogaers-Hofman D., de Baere G. A. (2000). Nasal carriage of Staphylococcus aureus is a major risk factor for surgical-site infections in orthopedic surgery. Infect. Control Hosp. Epidemiol. 21 319–323. 10.1086/501763 [DOI] [PubMed] [Google Scholar]
- Kaspar U., Kriegeskorte A., Schubert T., Peters G., Rudack C., Pieper D. H., et al. (2016). The culturome of the human nose habitats reveals individual bacterial fingerprint patterns. Environ. Microbiol. 18 2130–2142. 10.1111/1462-2920.12891 [DOI] [PubMed] [Google Scholar]
- Katneni R., Hedayati S. S. (2007). Central venous catheter-related bacteremia in chronic hemodialysis patients: epidemiology and evidence-based management. Nat. Clin. Pract. Nephrol. 3 256–266. 10.1038/ncpneph0447 [DOI] [PubMed] [Google Scholar]
- Kinsman O. S., Mckenna R., Noble W. C. (1983). Association between histocompatability antigens (HLA) and nasal carriage of Staphylococcus aureus. J. Med. Microbiol. 16 215–220. 10.1099/00222615-16-2-215 [DOI] [PubMed] [Google Scholar]
- Klevens R. M., Edwards J. R., Richards C. L., Horan T. C., Gaynes R. P., Pollock D. A., et al. (2007). Estimating health care-associated infections and deaths in U.S. hospitals, 2002. Public Health Rep. 122 160–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kluytmans J., van Belkum A., Verbrugh H. (1997). Nasal carriage of Staphylococcus aureus: epidemiology, underlying mechanisms, and associated risks. Clin. Microbiol. Rev. 10 505–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kluytmans J. A., Mouton J. W., Ijzerman E. P., Vandenbroucke-Grauls C. M., Maat A. W., Wagenvoort J. H., et al. (1995). Nasal carriage of Staphylococcus aureus as a major risk factor for wound infections after cardiac surgery. J. Infect. Dis. 171 216–219. 10.1093/infdis/171.1.216 [DOI] [PubMed] [Google Scholar]
- Kluytmans J. A. J. W., Manders M.-J., van Bommel E., Verbrugh H. (1996). Elimination of nasal carriage of Staphylococcus aureus in hemodialysis patients. Infect. Control Hosp. Epidemiol. 17 793–797. 10.2307/30141172 [DOI] [PubMed] [Google Scholar]
- Kotpal R., S K. P., Bhalla P., Dewan R., Kaur R. (2016). Incidence and risk factors of nasal carriage of Staphylococcus aureus in HIV-infected individuals in comparison to HIV-uninfected individuals: a case–control study. J. Int. Assoc. Provid. AIDS Care 15 141–147. 10.1177/2325957414554005 [DOI] [PubMed] [Google Scholar]
- Krismer B., Weidenmaier C., Zipperer A., Peschel A. (2017). The commensal lifestyle of Staphylococcus aureus and its interactions with the nasal microbiota. Nat. Rev. Microbiol. 15 675–687. 10.1038/nrmicro.2017.104 [DOI] [PubMed] [Google Scholar]
- Lamanna O., Bongiorno D., Bertoncello L., Grandesso S., Mazzucato S., Pozzan G. B., et al. (2017). Rapid containment of nosocomial transmission of a rare community-acquired methicillin-resistant Staphylococcus aureus (CA-MRSA) clone, responsible for the Staphylococcal Scalded Skin Syndrome (SSSS). Ital. J. Pediatr. 43:5. 10.1186/s13052-016-0323-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamarsalle L., Hunt B., Schauf M., Szwarcensztein K., Valentine W. J. (2013). Evaluating the clinical and economic burden of healthcare-associated infections during hospitalization for surgery in France. Epidemiol. Infect. 141 2473–2482. 10.1017/S0950268813000253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laudien M., Gadola S. D., Podschun R., Hedderich J., Paulsen J., Reinhold-Keller E., et al. (2010). Nasal carriage of Staphylococcus aureus and endonasal activity in Wegener s granulomatosis as compared to rheumatoid arthritis and chronic Rhinosinusitis with nasal polyps. Clin. Exp. Rheumatol. 28 51–55. [PubMed] [Google Scholar]
- >Le Réseau d’alerte d’investigation et de surveillance des infections nosocomiales (Raisin) (2015). Surveillance des Infections du Site Opératoire, France 2013. France: Institut de veille sanitaire. [Google Scholar]
- Leaper D. J., van Goor H., Reilly J., Petrosillo N., Geiss H. K., Torres A. J., et al. (2004). Surgical site infection - a European perspective of incidence and economic burden. Int. Wound J. 1 247–273. 10.1111/j.1742-4801.2004.00067.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehar S. M., Pillow T., Xu M., Staben L., Kajihara K. K., Vandlen R., et al. (2015). Novel antibody–antibiotic conjugate eliminates intracellular S. aureus. Nature 527 323–328. 10.1038/nature16057 [DOI] [PubMed] [Google Scholar]
- Lepelletier D., Perron S., Bizouarn P., Caillon J., Drugeon H., Michaud J.-L., et al. (2005). Surgical-site infection after cardiac surgery: incidence, microbiology, and risk factors. Infect. Control Hosp. Epidemiol. 26 466–472. 10.1086/502569 [DOI] [PubMed] [Google Scholar]
- Leshem E., Maayan-Metzger A., Rahav G., Dolitzki M., Kuint J., Roytman Y., et al. (2012). Transmission of Staphylococcus aureus from mothers to newborns. Pediatr. Infect. Dis. J. 31 360–363. 10.1097/INF.0b013e318244020e [DOI] [PubMed] [Google Scholar]
- Lina G., Boutite F., Tristan A., Bes M., Etienne J., Vandenesch F. (2003). Bacterial competition for human nasal cavity colonization: role of Staphylococcal agr alleles. Appl. Environ. Microbiol. 69 18–23. 10.1128/aem.69.1.18-23.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C. M., Price L. B., Hungate B. A., Abraham A. G., Larsen L. A., Christensen K., et al. (2015). Staphylococcus aureus and the ecology of the nasal microbiome. Sci. Adv. 1:e1400216. 10.1126/sciadv.1400216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q., Du X., Hong X., Li T., Zheng B., He L., et al. (2015). Targeting surface protein SasX by active and passive vaccination to reduce Staphylococcus aureus colonization and infection. Infect. Immun. 83 2168–2174. 10.1128/IAI.02951-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luzar M. A., Coles G. A., Faller B., Slingeneyer A., Dah G. D., Briat C., et al. (1990). Staphylococcus aureus nasal carriage and infection in patients on continuous ambulatory peritoneal dialysis. N. Engl. J. Med. 322 505–509. 10.1056/NEJM199002223220804 [DOI] [PubMed] [Google Scholar]
- Maayan-Metzger A., Strauss T., Rubin C., Jaber H., Dulitzky M., Reiss-Mandel A., et al. (2017). Clinical evaluation of early acquisition of Staphylococcus aureus carriage by newborns. Int. J. Infect. Dis. 64 9–14. 10.1016/j.ijid.2017.08.013 [DOI] [PubMed] [Google Scholar]
- Magill S. S., Hellinger W., Cohen J., Kay R., Bailey C., Boland B., et al. (2012). Prevalence of healthcare-associated infections in acute care hospitals in Jacksonville, Florida. Infect. Control Hosp. Epidemiol. 33 283–291. 10.1086/664048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKinnell J. A., Huang S. S., Eells S. J., Cui E., Miller L. G. (2013). Quantifying the impact of extranasal testing of body sites for methicillin-resistant Staphylococcus aureus colonization at the time of hospital or intensive care unit admission. Infect. Control Hosp. Epidemiol. 34 161–170. 10.1086/669095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller L. G., Eells S. J., Taylor A. R., David M. Z., Ortiz N., Zychowski D., et al. (2012). Staphylococcus aureus colonization among household contacts of patients with skin infections: risk factors, strain discordance, and complex ecology. Clin. Infect. Dis. 54 1523–1535. 10.1093/cid/cis213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modak R., Das Mitra S., Vasudevan M., Krishnamoorthy P., Kumar M., Bhat A. V, et al. (2014). Epigenetic response in mice mastitis: role of histone H3 acetylation and microRNA(s) in the regulation of host inflammatory gene expression during Staphylococcus aureus infection. Clin. Epigenetics 6:12. 10.1186/1868-7083-6-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulcahy M. E., Geoghegan J. A., Monk I. R., O’Keeffe K. M., Walsh E. J., Foster T. J., et al. (2012). Nasal colonisation by Staphylococcus aureus depends upon clumping factor B binding to the squamous epithelial cell envelope protein loricrin. PLoS Pathog. 8:e1003092. 10.1371/journal.ppat.1003092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulcahy M. E., Leech J. M., Renauld J.-C., Mills K. H., McLoughlin R. M. (2016). Interleukin-22 regulates antimicrobial peptide expression and keratinocyte differentiation to control Staphylococcus aureus colonization of the nasal mucosa. Mucosal Immunol. 9 1429–1441. 10.1038/mi.2016.24 [DOI] [PubMed] [Google Scholar]
- Mulcahy M. E., McLoughlin R. M. (2016). Host–bacterial crosstalk determines Staphylococcus aureus nasal colonization. Trends Microbiol. 24 872–886. 10.1016/j.tim.2016.06.012 [DOI] [PubMed] [Google Scholar]
- Muñoz P., Hortal J., Giannella M., Barrio J. M., Rodríguez-Créixems M., Pérez M. J., et al. (2008). Nasal carriage of S. aureus increases the risk of surgical site infection after major heart surgery. J. Hosp. Infect. 68 25–31. 10.1016/j.jhin.2007.08.010 [DOI] [PubMed] [Google Scholar]
- Muthukrishnan G., Lamers R. P., Ellis A., Paramanandam V., Persaud A. B., Tafur S., et al. (2013). Longitudinal genetic analyses of Staphylococcus aureus nasal carriage dynamics in a diverse population. BMC Infect. Dis. 13:221. 10.1186/1471-2334-13-221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nardi G., Di Silvestre A. D., De Monte A., Massarutti D., Proietti A., Grazia Troncon M., et al. (2001). Reduction in gram-positive pneumonia and antibiotic consumption following the use of a SDD protocol including nasal and oral mupirocin. Eur. J. Emerg. Med. 8 203–214. 10.1097/00063110-200109000-00008 [DOI] [PubMed] [Google Scholar]
- Negi V., Pal S., Juyal D., Sharma M. K., Sharma N. (2015). Bacteriological profile of surgical site infections and their antibiogram: a study from resource constrained rural setting of uttarakhand state, India. J. Clin. Diagn. Res. 9 DC17–DC20. 10.7860/JCDR/2015/15342.6698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen M. H., Kauffman C. A., Goodman R. P., Squier C., Arbeit R. D., Singh N., et al. (1999). Nasal carriage of and infection with Staphylococcus aureus in HIV-infected patients. Ann. Intern. Med. 130 221–225. 10.7326/0003-4819-130-3-199902020-00026 [DOI] [PubMed] [Google Scholar]
- NICE Clinical Guidelines (2008). National Collaborating Centre for Women’s and Children’s Health (UK). Preoperative phase, Surgical Site Infection: Prevention and Treatment of Surgical Site Infection. London: National Institute for Health and Clinical Excellence. [Google Scholar]
- Nilsson A. C., Janson H., Wold H., Fugelli A., Andersson K., Håkangård C., et al. (2015). LTX-109 is a novel agent for nasal decolonization of methicillin-resistant and -sensitive Staphylococcus aureus. Antimicrob. Agents Chemother. 59 145–151. 10.1128/AAC.03513-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nouwen J., Schouten J., Schneebergen P., Snijders S., Maaskant J., Koolen M., et al. (2006). Staphylococcus aureus carriage patterns and the risk of infections associated with continuous peritoneal dialysis. J. Clin. Microbiol. 44 2233–2236. 10.1128/JCM.02083-05 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nouwen J. L., Fieren M. W. J. A., Snijders S., Verbrugh H. A., Van Belkum A. (2005). Persistent (not intermittent) nasal carriage ofStaphylococcus aureus is the determinant of CPD-related infections. Kidney Int. 67 1084–1092. 10.1111/j.1523-1755.2005.00174.x [DOI] [PubMed] [Google Scholar]
- Nouwen J. L. Optima Grafische Communicatie (2004). Determinants, Risks & Dynamics of Staphylococcus aureus Nasal Carriage. Doctoral thesis, Erasmus University Rotterdam; Rotterdam. [Google Scholar]
- Nouwen J. L., Ott A., Kluytmans-Vandenbergh M. F. Q., Boelens H. A. M., Hofman A., van Belkum A., et al. (2004). Predicting the Staphylococcus aureus nasal carrier state: derivation and validation of a “culture rule”. Clin. Infect. Dis. 39 806–811. 10.1086/423376 [DOI] [PubMed] [Google Scholar]
- Nurjadi D., Heeg K., Weber A. N. R., Zanger P. (2018). Toll-like receptor (TLR) -9 promotor polymorphisms and gene expression are associated with persistent Staphylococcus aureus nasal carriage. Clin. Microbiol. Infect. 10.1016/j.cmi.2018.02.014 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- Nurjadi D., Kain M., Marcinek P., Gaile M., Heeg K., Zanger P. (2016). Ratio of T-helper type 1 (Th1) to Th17 cytokines in whole blood is associated with human β-defensin 3 expression in skin and persistent Staphylococcus aureus nasal carriage. J. Infect. Dis. 214 1744–1751. 10.1093/infdis/jiw440 [DOI] [PubMed] [Google Scholar]
- O’Brien L. M., Walsh E. J., Massey R. C., Peacock S. J., Foster T. J. (2002). Staphylococcus aureus clumping factor B (ClfB) promotes adherence to human type I cytokeratin 10: implications for nasal colonization. Cell. Microbiol. 4 759–770. 10.1046/j.1462-5822.2002.00231.x [DOI] [PubMed] [Google Scholar]
- Olsen K., Danielsen K., Wilsgaard T., Sangvik M., Sollid J. U. E., Thune I., et al. (2013). Obesity and Staphylococcus aureus nasal colonization among women and men in a general population. PLoS One 8:e63716. 10.1371/journal.pone.0063716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen K., Falch B. M., Danielsen K., Johannessen M., Ericson Sollid J. U., Thune I., et al. (2012). Staphylococcus aureus nasal carriage is associated with serum 25-hydroxyvitamin D levels, gender and smoking status. The Tromsø Staph and Skin Study. Eur. J. Clin. Microbiol. Infect. Dis. 31 465–473. 10.1007/s10096-011-1331-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ong L. M., Ch’ng C. C., Wee H. C., Supramaniam P., Zainal H., Goh B. L., et al. (2017). Risk of peritoneal dialysis-related peritonitis in a multi-racial asian population. Perit. Dial. Int. 37 35–43. 10.3747/pdi.2015.00141 [DOI] [PubMed] [Google Scholar]
- Ou J., Bassiouni A., Drilling A., Psaltis A. J., Vreugde S., Wormald P. J. (2017). The persistence of intracellular Staphylococcus aureus in the sinuses: a longitudinal study. Rhinol. J. 55 305–311. 10.4193/Rhin16.218 [DOI] [PubMed] [Google Scholar]
- Ou J., Drilling A., Singhal D., Tan N. C.-W., Wallis-Hill D., Vreugde S., et al. (2016). Association of intracellular Staphylococcus aureus with prognosis in chronic rhinosinusitis. Int. Forum Allergy Rhinol. 6 792–799. 10.1002/alr.21758 [DOI] [PubMed] [Google Scholar]
- Panierakis C., Goulielmos G., Mamoulakis D., Maraki S., Papavasiliou E., Galanakis E. (2009). Staphylococcus aureus nasal carriage might be associated with vitamin D receptor polymorphisms in type 1 diabetes. Int. J. Infect. Dis. 13 e437–e443. 10.1016/j.ijid.2009.02.012 [DOI] [PubMed] [Google Scholar]
- Peacock S. J., de Silva I., Lowy F. D. (2001). What determines nasal carriage of Staphylococcus aureus? Trends Microbiol. 9 605–610. [DOI] [PubMed] [Google Scholar]
- Peacock S. J., Justice A., Griffiths D., de Silva G. D. I., Kantzanou M. N., Crook D., et al. (2003). Determinants of acquisition and carriage of Staphylococcus aureus in infancy. J. Clin. Microbiol. 41 5718–5725. 10.1128/JCM.41.12.5718-5725.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins S., Walsh E. J., Deivanayagam C. C., Narayana S. V, Foster T. J., Höök M. (2001). Structural organization of the fibrinogen-binding region of the clumping factor B MSCRAMM of Staphylococcus aureus. J. Biol. Chem. 276 44721–44728. 10.1074/jbc.M106741200 [DOI] [PubMed] [Google Scholar]
- Perl T. M., Cullen J. J., Wenzel R. P., Zimmerman M. B., Pfaller M. A., Sheppard D., et al. (2002). Intranasal mupirocin to prevent postoperative Staphylococcus aureus infections. N. Engl. J. Med. 346 1871–1877. 10.1056/NEJMoa003069 [DOI] [PubMed] [Google Scholar]
- Perl T. M., Golub J. E. (1998). New approaches to reduce Staphylococcus aureus nosocomial infection rates: treating S. aureus nasal carriage. Ann. Pharmacother. 32 S7–S16. [DOI] [PubMed] [Google Scholar]
- Peschel A., Sahl H.-G. (2006). The co-evolution of host cationic antimicrobial peptides and microbial resistance. Nat. Rev. Microbiol. 4 529–536. 10.1038/nrmicro1441 [DOI] [PubMed] [Google Scholar]
- Plouin-Gaudon I., Clement S., Huggler E., Chaponnier C., François P., Lew D., et al. (2006). Intracellular residency is frequently associated with recurrent Staphylococcus aureus rhinosinusitis. Rhinology 44 249–254. [PubMed] [Google Scholar]
- Pournajaf A., Ardebili A., Goudarzi L., Khodabandeh M., Narimani T., Abbaszadeh H. (2014). PCR-based identification of methicillin-resistant Staphylococcus aureus strains and their antibiotic resistance profiles. Asian Pac. J. Trop. Biomed. 4 S293–S297. 10.12980/APJTB.4.2014C423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price J. R., Cole K., Bexley A., Kostiou V., Eyre D. W., Golubchik T., et al. (2017). Transmission of Staphylococcus aureus between health-care workers, the environment, and patients in an intensive care unit: a longitudinal cohort study based on whole-genome sequencing. Lancet Infect. Dis. 17 207–214. 10.1016/S1473-3099(16)30413-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pynnonen M., Stephenson R. E., Schwartz K., Hernandez M., Boles B. R. (2011). Hemoglobin promotes Staphylococcus aureus nasal colonization. PLoS Pathog. 7:e1002104. 10.1371/journal.ppat.1002104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinn G. A., Cole A. M. (2007). Suppression of innate immunity by a nasal carriage strain of Staphylococcus aureus increases its colonization on nasal epithelium. Immunology 122 80–89. 10.1111/j.1365-2567.2007.02615.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raviglione M. C., Mariuz P., Pablos-Mendez A., Battan R., Ottuso P., Taranta A. (1990). High Staphylococcus aureus nasal carriage rate in patients with acquired immunodeficiency syndrome or AIDS-related complex. Am. J. Infect. Control 18 64–69. 10.1016/0196-6553(90)90083-5 [DOI] [PubMed] [Google Scholar]
- Reagan D. R., Doebbeling B. N., Pfaller M. A., Sheetz C. T., Houston A. K., Hollis R. J., et al. (1991). Elimination of coincident Staphylococcus aureus nasal and hand carriage with intranasal application of mupirocin calcium ointment. Ann. Intern. Med. 114 101–106. 10.7326/0003-4819-114-2-101 [DOI] [PubMed] [Google Scholar]
- Reddinger R. M., Luke-Marshall N. R., Hakansson A. P., Campagnari A. A. (2016). Host physiologic changes induced by influenza a virus lead to Staphylococcus aureus biofilm dispersion and transition from asymptomatic colonization to invasive disease. mBio 7:e01235-16. 10.1128/mBio.01235-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regev-Yochay G., Trzcinski K., Thompson C. M., Malley R., Lipsitch M. (2006). Interference between Streptococcus pneumoniae and Staphylococcus aureus: in vitro hydrogen peroxide-mediated killing by Streptococcus pneumoniae. J. Bacteriol. 188 4996–5001. 10.1128/JB.00317-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigaill J., Morgene M. F., Gavid M., Lelonge Y., He Z., Carricajo A., et al. (2018). Intracellular activity of antimicrobial compounds used for Staphylococcus aureus nasal decolonization. J. Antimicrob. Chemother. 10.1093/jac/dky318 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- Roche F. M., Meehan M., Foster T. J. (2003). The Staphylococcus aureus surface protein SasG and its homologues promote bacterial adherence to human desquamated nasal epithelial cells. Microbiology 149 2759–2767. 10.1099/mic.0.26412-0 [DOI] [PubMed] [Google Scholar]
- Roghmann M.-C., Johnson J. K., Stine O. C., Lydecker A. D., Ryan K. A., Mitchell B. D., et al. (2011). Persistent Staphylococcus aureus colonization is not a strongly heritable trait in Amish families. PLoS One 6:e17368. 10.1371/journal.pone.0017368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruimy R., Angebault C., Djossou F., Dupont C., Epelboin L., Jarraud S., et al. (2010). Are host genetics the predominant determinant of persistent nasal Staphylococcus aureus carriage in humans? J. Infect. Dis. 202 924–934. 10.1086/655901 [DOI] [PubMed] [Google Scholar]
- Saadatian-Elahi M., Teyssou R., Vanhems P. (2008). Staphylococcus aureus, the major pathogen in orthopaedic and cardiac surgical site infections: a literature review. Int. J. Surg. 6 238–245. 10.1016/j.ijsu.2007.05.001 [DOI] [PubMed] [Google Scholar]
- Saadatian-Elahi M., Tristan A., Laurent F., Rasigade J.-P., Bouchiat C., Ranc A.-G., et al. (2013). Basic rules of hygiene protect health care and lab workers from nasal colonization by Staphylococcus aureus: an international cross-sectional study. PLoS One 8:e82851. 10.1371/journal.pone.0082851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safdar N., Bradley E. A. (2008). The risk of infection after nasal colonization with Staphylococcus aureus. Am. J. Med. 121 310–315. 10.1016/j.amjmed.2007.07.034 [DOI] [PubMed] [Google Scholar]
- Savage J. W., Anderson P. A. (2013). An update on modifiable factors to reduce the risk of surgical site infections. Spine J. 13 1017–1029. 10.1016/j.spinee.2013.03.051 [DOI] [PubMed] [Google Scholar]
- Schaffer A. C., Solinga R. M., Cocchiaro J., Portoles M., Kiser K. B., Risley A., et al. (2006). Immunization with Staphylococcus aureus clumping factor B, a major determinant in nasal carriage, reduces nasal colonization in a murine model. Infect. Immun. 74 2145–2153. 10.1128/IAI.74.4.2145-2153.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt A., Bénard S., Cyr S. (2015). Hospital cost of staphylococcal infection after cardiothoracic or orthopedic operations in France: a retrospective database analysis. Surg. Infect. 16 428–435. 10.1089/sur.2014.045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selva L., Viana D., Regev-Yochay G., Trzcinski K., Corpa J. M., Lasa I., et al. (2009). Killing niche competitors by remote-control bacteriophage induction. Proc. Natl. Acad. Sci. U.S.A. 106 1234–1238. 10.1073/pnas.0809600106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherertz R. J., Reagan D. R., Hampton K. D., Robertson K. L., Streed S. A., Hoen H. M., et al. (1996). A cloud adult: the Staphylococcus aureus-virus interaction revisited. Ann. Intern. Med. 124 539–547. 10.7326/0003-4819-124-6-199603150-00001 [DOI] [PubMed] [Google Scholar]
- Sissolak D., Geusau A., Heinze G., Witte W., Rotter M. L. (2002). Risk factors for nasal carriage of Staphylococcus aureus in infectious disease patients, including patients infected with HIV, and molecular typing of colonizing strains. Eur. J. Clin. Microbiol. Infect. Dis. 21 88–96. 10.1007/s10096-001-0666-0 [DOI] [PubMed] [Google Scholar]
- Sivaraman K., Venkataraman N., Cole A. M. (2009). Staphylococcus aureus nasal carriage and its contributing factors. Future Microbiol. 4 999–1008. 10.2217/fmb.09.79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinert P. M., Marekov L. N. (1995). The proteins elafin, filaggrin, keratin intermediate filaments, loricrin, and small proline-rich proteins 1 and 2 are isodipeptide cross-linked components of the human epidermal cornified cell envelope. J. Biol. Chem. 270 17702–17711. 10.1074/jbc.270.30.17702 [DOI] [PubMed] [Google Scholar]
- Sugimoto S., Iwamoto T., Takada K., Okuda K.-I., Tajima A., Iwase T., et al. (2013). Staphylococcus epidermidis Esp degrades specific proteins associated with Staphylococcus aureus biofilm formation and host-pathogen interaction. J. Bacteriol. 195 1645–1655. 10.1128/JB.01672-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan N. C.-W., Cooksley C. M., Roscioli E., Drilling A. J., Douglas R., Wormald P.-J., et al. (2014). Small-colony variants and phenotype switching of intracellular Staphylococcus aureus in chronic rhinosinusitis. Allergy 69 1364–1371. 10.1111/all.12457 [DOI] [PubMed] [Google Scholar]
- Trautman M., Stecher J., Luz K., Hemmer W., Hupp T., Grützner P. A. (2007). The use of mupirocin in the eradication of Staphylococcus aureus. Dtsch. Arztebl. 104:A-3259. [Google Scholar]
- Uehara Y., Nakama H., Agematsu K., Uchida M., Kawakami Y., Abdul Fattah A. S. M., et al. (2000). Bacterial interference among nasal inhabitants: eradication of Staphylococcus aureus from nasal cavities by artificial implantation of Corynebacterium sp. J. Hosp. Infect. 44 127–133. 10.1053/jhin.1999.0680 [DOI] [PubMed] [Google Scholar]
- van Belkum A., Verkaik N. J., de Vogel C. P., Boelens H. A., Verveer J., Nouwen J. L., et al. (2009). Reclassification of Staphylococcus aureus nasal carriage types. J. Infect. Dis. 199 1820–1826. 10.1086/599119 [DOI] [PubMed] [Google Scholar]
- van den Akker E. L. T., Nouwen J. L., Melles D. C., van Rossum E. F. C., Koper J. W., Uitterlinden A. G., et al. (2006). Staphylococcus aureus nasal carriage is associated with glucocorticoid receptor gene polymorphisms. J. Infect. Dis. 194 814–818. 10.1086/506367 [DOI] [PubMed] [Google Scholar]
- VandenBergh M. F., Yzerman E. P., van Belkum A., Boelens H. A., Sijmons M., Verbrugh H. A. (1999). Follow-up of Staphylococcus aureus nasal carriage after 8 years: redefining the persistent carrier state. J. Clin. Microbiol. 37 3133–3140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verhoeven P. O., Grattard F., Carricajo A., Lucht F., Cazorla C., Garraud O., et al. (2012). An algorithm based on one or two nasal samples is accurate to identify persistent nasal carriers of Staphylococcus aureus. Clin. Microbiol. Infect. 18 551–557. 10.1111/j.1469-0691.2011.03611.x [DOI] [PubMed] [Google Scholar]
- Von Eiff C., Becker K., Machka K., Stammer H., Peters G. (2001). Nasal carriage as a source of Staphylococcus aureus bacteremia. Study group. N. Engl. J. Med. 344 11–16. 10.1056/NEJM200101043440102 [DOI] [PubMed] [Google Scholar]
- Vonberg R.-P., Stamm-Balderjahn S., Hansen S., Zuschneid I., Rüden H., Behnke M., et al. (2006). How often do asymptomatic healthcare workers cause methicillin-resistant Staphylococcus aureus outbreaks? A systematic evaluation. Infect. Control Hosp. Epidemiol. 27 1123–1127. 10.1086/507922 [DOI] [PubMed] [Google Scholar]
- Vuyk H. D., Watts S. J. (2006). “Nasal reconstruction,” in Facial Plastic and Reconstructive Surgery Vuyk H. D., Lohuis P. J. F. M. (London: Hodder Arnold; ) 455–480. [Google Scholar]
- Walsh T. L., Querry A. M., McCool S., Galdys A. L., Shutt K. A., Saul M. I., et al. (2017). Risk factors for surgical site infections following neurosurgical spinal fusion operations: a case control study. Infect. Control Hosp. Epidemiol. 38 340–347. 10.1017/ice.2016.307 [DOI] [PubMed] [Google Scholar]
- Wang J.-T., Chang S.-C., Ko W.-J., Chang Y.-Y., Chen M.-L., Pan H.-J., et al. (2001). A hospital-acquired outbreak of methicillin-resistant Staphylococcus aureus infection initiated by a surgeon carrier. J. Hosp. Infect. 47 104–109. 10.1053/jhin.2000.0878 [DOI] [PubMed] [Google Scholar]
- Warnke P., Devide A., Weise M., Frickmann H., Schwarz N. G., Schäffler H., et al. (2016). Utilizing moist or dry swabs for the sampling of nasal MRSA carriers? An in vivo and in vitro study. PLoS One 11:e0163073. 10.1371/journal.pone.0163073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weidenmaier C., Goerke C., Wolz C. (2012). Staphylococcus aureus determinants for nasal colonization. Trends Microbiol. 20 243–250. 10.1016/j.tim.2012.03.004 [DOI] [PubMed] [Google Scholar]
- Weidenmaier C., Kokai-Kun J. F., Kristian S. A., Chanturiya T., Kalbacher H., Gross M., et al. (2004). Role of teichoic acids in Staphylococcus aureus nasal colonization, a major risk factor in nosocomial infections. Nat. Med. 10 243–245. 10.1038/nm991 [DOI] [PubMed] [Google Scholar]
- Weidenmaier C., Kokai-Kun J. F., Kulauzovic E., Kohler T., Thumm G., Stoll H., et al. (2008). Differential roles of sortase-anchored surface proteins and wall teichoic acid in Staphylococcus aureus nasal colonization. Int. J. Med. Microbiol. 298 505–513. 10.1016/j.ijmm.2007.11.006 [DOI] [PubMed] [Google Scholar]
- Weinke T., Schiller R., Fehrenbach F. J., Pohle H. D. (1992). Association between Staphylococcus aureus nasopharyngeal colonization and septicemia in patients infected with the human immunodeficiency virus. Eur. J. Clin. Microbiol. Infect. Dis. 11 985–989. 10.1007/BF01967787 [DOI] [PubMed] [Google Scholar]
- Weiser M. C., Moucha C. S. (2015). The current state of screening and decolonization for the prevention of Staphylococcus aureus surgical site infection after total hip and knee arthroplasty. J. Bone Joint Surg. Am. 97 1449–1458. 10.2106/JBJS.N.01114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wertheim H. F. L., Melles D. C., Vos M. C., van Leeuwen W., van Belkum A., Verbrugh H. A., et al. (2005a). The role of nasal carriage in Staphylococcus aureus infections. Lancet Infect. Dis. 5 751–762. 10.1016/S1473-3099(05)70295-4 [DOI] [PubMed] [Google Scholar]
- Wertheim H. F. L., Verveer J., Boelens H. A. M., van Belkum A., Verbrugh H. A., Vos M. C. (2005b). Effect of mupirocin treatment on nasal, pharyngeal, and perineal carriage of Staphylococcus aureus in healthy adults. Antimicrob. Agents Chemother. 49 1465–1467. 10.1128/AAC.49.4.1465-1467.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wertheim H. F. L., van Kleef M., Vos M. C., Ott A., Verbrugh H. A., Fokkens W. (2006). Nose picking and nasal carriage of Staphylococcus aureus. Infect. Control Hosp. Epidemiol. 27 863–867. 10.1086/506401 [DOI] [PubMed] [Google Scholar]
- Wertheim H. F. L., Vos M. C., Ott A., van Belkum A., Voss A., Kluytmans J. A. J. W., et al. (2004). Risk and outcome of nosocomial Staphylococcus aureus bacteraemia in nasal carriers versus non-carriers. Lancet 364 703–705. 10.1016/S0140-6736(04)16897-9 [DOI] [PubMed] [Google Scholar]
- Wertheim H. F. L., Walsh E., Choudhurry R., Melles D. C., Boelens H. A. M., Miajlovic H., et al. (2008). Key role for clumping factor B in Staphylococcus aureus nasal colonization of humans. PLoS Med. 5:e17. 10.1371/journal.pmed.0050017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams R. E. (1963). Healthy carriage of Staphylococcus aureus: its prevalence and importance. Bacteriol. Rev. 27 56–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wollenberg M. S., Claesen J., Escapa I. F., Aldridge K. L., Fischbach M. A., Lemon K. P. (2014). Propionibacterium-produced coproporphyrin III induces Staphylococcus aureus aggregation and biofilm formation. mBio 5:e01286-14. 10.1128/mBio.01286-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan M., Pamp S. J., Fukuyama J., Hwang P. H., Cho D.-Y., Holmes S., et al. (2013). Nasal microenvironments and interspecific interactions influence nasal microbiota complexity and S. aureus carriage. Cell Host Microbe 14 631–640. 10.1016/j.chom.2013.11.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yano M., Doki Y., Inoue M., Tsujinaka T., Shiozaki H., Monden M. (2000). Preoperative intranasal mupirocin ointment significantly reduces postoperative infection with Staphylococcus aureus in patients undergoing upper gastrointestinal surgery. Surg. Today 30 16–21. 10.1007/PL00010040 [DOI] [PubMed] [Google Scholar]
- Zanger P., Nurjadi D., Gaile M., Gabrysch S., Kremsner P. G. (2012). Hormonal contraceptive use and persistent Staphylococcus aureus nasal carriage. Clin. Infect. Dis. 55 1625–1632. 10.1093/cid/cis778 [DOI] [PubMed] [Google Scholar]
- Zipperer A., Konnerth M. C., Laux C., Berscheid A., Janek D., Weidenmaier C., et al. (2016). Human commensals producing a novel antibiotic impair pathogen colonization. Nature 535 511–516. 10.1038/nature18634 [DOI] [PubMed] [Google Scholar]


