Abstract
The purpose of this study was to assess the prognostic value of programmed death ligand-1 (PD-L1) positivity in a non-clear cell renal cell carcinoma (non-ccRCC) cohort. PD-L1 expression was evaluated by immunohistochemistry (IHC) using formalin-fixed paraffin-embedded (FFPE) specimens from 45 non-ccRCC patients with available tissue. PD-L1 positivity was defined as ≥1% of staining. Histopathological characteristics and oncological outcomes were correlated to PD-L1 expression. Cancer-specific survival (CSS) and recurrence-free survival (RFS) stratified by PD-L1 status were estimated using the Kaplan–Meier method. Median age was 58 years and median follow-up was 40 months. Non-ccRCC subtypes included sarcomatoid (n = 9), rhabdoid (n = 6), medullary (n = 2), Xp11.2 translocation (n = 2), collecting duct (n = 1), papillary type I (n = 11), and papillary type II (n = 14). PD-L1 positivity was noted in nine (20%) patients. PD-L1 positivity was significantly associated with higher Fuhrman nuclear grade (P = 0.048) and perineural invasion (P = 0.043). Five-year CSS was 73.2 and 83% for PD-L1 positive and negative tumors, respectively (P = 0.47). Five-year RFS was 55.6 and 61.5% for PD-L1 positive and negative tumors, respectively (P = 0.58). PD-L1 was expressed in a fifth of non-ccRCC cases and was associated with adverse histopathologic features. Expression of biomarkers such PD-L1 may help better risk-stratify non-ccRCC patients to guide treatment decisions and follow-up strategies.
Keywords: non-clear cell renal cell carcinoma, prognosis, programmed death ligand-1, renal cell cancer, tumor marker
Introduction
Renal cell carcinoma (RCC) is a lethal urologic malignancy accounting for 62,700 new cases and 14,240 deaths per year in the Unites States (1). Although diagnosis in earlier stages has become more frequent due to increased use of abdominal imaging, mortality rates have not decreased (2, 3). In cases of clinically localized disease, surgical resection remains the mainstay of curative treatment although recurrences can occur in 20–40% of cases (4).
RCC is considered to be immuno-responsive with cases of complete regression reported with high-dose immunotherapy (5). Newer treatments have mainly relied on the vascular endothelial growth factor (VEGF) or mammalian target of rapamycin (mTOR) pathways, although no durable responses have been observed to date (6, 7). Recent advances in the understanding of molecular contributors of RCC have led to the emergence of novel therapeutic agents such as immune checkpoint inhibitors (8). In 2015, Nivolumab, an anti-programmed cell death-1 (PD-1) inhibitor, was approved for patients with advanced clear cell RCC (ccRCC) with failed prior anti-angiogenic therapy due to significantly higher objective response rates and an acceptable toxicity profile (9, 10).
Despite exciting breakthroughs and increased knowledge of the immune response in RCC carcinogenesis, a significant proportion of patients with non-clear cell RCC (non-ccRCC) subtypes were excluded from pivotal clinical trials. This, in turn, limits the available data needed for the development of evidence-based recommendations. Thus far, the prognostic value of programmed death ligand-1 (PD-L1) positivity in non-ccRCC remains unclear. In this study, we sought to examine the clinical significance of PD-L1 expression in a contemporary cohort of patients treated for non-ccRCC at a single institution.
Materials and Methods
Patient selection
A cohort of non-ccRCC patients treated between 2005 and 2015 was retrospectively identified from our RCC database. Tissue was available for 45 patients with non-clear cell subtypes. The study was approved by the institutional review board.
PD-L1 Immunohistochemistry (IHC)
Tissue microarray (TMA) blocks were prepared from 45 non-ccRCC formalin-fixed paraffin-embedded (FFPE) specimens. A hematoxylin and eosin (H&E) slide of each archival specimen was evaluated, and a block representing the overall tumor was chosen for TMA preparation. Three cores of 1-mm diameter per case were selected and sections were cut into 4-μm thicknesses and placed on positively charged slides for immunostaining. For cases with sarcomatoid or rhabdoid features, only the non-clear cell component was evaluated. Slides were subjected to immunostaining using the Ventana Discovery XT automated system (Ventana Medical Systems, Tucson, AZ) using commercially available antibodies against PD-L1 (E1L3N; Cell Signaling Technology, Beverly, MA). PD-L1 expression was defined in tumor cells if membranous alone or membranous and cytoplasmic staining was present. The positivity was further subclassified as ≥1, ≥5, ≥10, ≥25, and ≥50%. PD-L1 positivity of tumor cells was defined as ≥1% of staining. This cutoff level was based on published data showing successful anti-PD-1 therapy in patients with low PD-L1 expression (11). An expert in genitourinary pathology (J.D.) completed this work and decided on IHC positivity.
There is no definitive cutoff for PD-L1 positivity, with more than one threshold being reported for most antibodies in the literature. Published data for PD-L1 tests are mainly focused on immunohistochemistry tests for lung cancer. The variability in test cutoffs and standards for PD-L1 testing suggests that there is presently no standardized approach. Data on renal cell cancers are scarce. Hence, in this study we followed the approach that has been followed by many published studies in the literature based on lung cancer where the cutoff value of ≥1% for PDL-1 has been considered positive. According to Festino et al., there is no definitive threshold result that can be universally applied to predict clinical response to PD-L1–targeted precision treatments (12).
Statistical analysis
The association between PD-L1 expression status and histopathologic characteristics was evaluated using the Mann–Whitney or chi-square test and Fisher’s exact test for numeric and categorical variables, respectively. Data were expressed as median and interquartile range (IQR) for continuous variables, and binary variables were reported as counts and percentages (%). Survival endpoints evaluated were cancer-specific survival (CSS) and recurrence-free survival (RFS) and were defined as the time from surgery to death from disease and recurrence of disease, respectively. CSS and RFS stratified by PD-L1 status were estimated using the Kaplan–Meier method. Statistical significance was defined as P < 0.05. All statistical analyses were performed using Statistical Package for the Social Sciences (SPSS), version 24.0 (IMB Corp, Armonk, NY).
Results
Clinicopathologic characteristics
Between 2005 and 2015, we identified 45 non-ccRCC patients with available tissue. Clinicopathologic characteristics are provided in Table 1. Median age was 58 years (IQR: 53–68.5). Of the patients, 13 (29%) underwent partial nephrectomy and 32 (71%) underwent radical nephrectomy. Regional lymph node dissection was performed in seven (16%) patients, while inferior vena cava thrombectomy was completed in three (6.7%) patients. Histology subtypes included sarcomatoid RCC (n = 9), rhabdoid RCC (n = 6), medullary RCC (n = 2), Xp11.2 translocation RCC (n = 2), collecting duct RCC (n = 1), papillary type I RCC (n = 11), and papillary type II RCC (n = 14). Median tumor size was 7 cm (IQR: 4.5–12.3). Approximately 51% of tumors were pT3–4 and 75.5% were Fuhrman nuclear grades 3–4.
Table 1.
Patients and tumor characteristics.
| Patients characteristics (n = 45) | |
| Median age, years (IQR) | 58 (53–68.5) |
| Gender (%) | |
| Male | 34 (75.6) |
| Female | 11 (24.4) |
| Race (%) | |
| White | 27 (60) |
| Non-white | 18 (40) |
| Karnofsky Performance Scale (%) | |
| <80% | 8 (17.8) |
| ≥80% | 37 (82.2) |
| Surgical procedure (%) | |
| Partial nephrectomy | 13 (28.9) |
| Radical nephrectomy | 32 (71.1) |
| Regional lymph node dissection | 7 (15.6) |
| IVC thrombectomy | 3 (6.7) |
| Tumor characteristics (n = 45) | |
| Non-clear cell subtypes (%) | |
| Sarcomatoid RCC | 9 (20) |
| Rhabdoid RCC | 6 (13.3) |
| Medullary RCC | 2 (4.4) |
| Xp11.2 translocation RCC | 2 (4.4) |
| Collecting duct RCC | 1 (2.2) |
| Papillary type 1 RCC | 14 (31.1) |
| Papillary type 2 RCC | 11 (24.4) |
| AJCC pathologic T stage (%) | |
| pT1 | 16 (35.6) |
| pT2 | 6 (13.3) |
| pT3 | 21 (46.7) |
| pT4 | 2 (4.4) |
| AJCC clinical M stage (%) | |
| cM0 | 39 (86.7) |
| cM1 | 6 (13.3) |
| Median tumor size, cm (IQR) | 7 (4.5–12.3) |
| Fuhrman nuclear grade (%) | |
| I | 3 (6.7) |
| II | 8 (17.8) |
| III | 19 (42.2) |
| IV | 15 (33.3) |
| Adverse features (%) | |
| Lymphovascular invasion | 12 (26.7) |
| Tumor necrosis | 23 (51.1) |
| Perineural invasion | 1 (2.2) |
| Tumor thrombus | 8 (17.8) |
| Surgical margin status | |
| Negative | 40 (88.9) |
| Positive | 4 (8.9) |
| Unknown | 1 (2.2) |
| PD-L1 expression status | |
| <1% (negative) | 36 (80) |
| ≥1% (positive) | 9 (20) |
PD-L1 expression and histopathologic characteristics in non-ccRCC
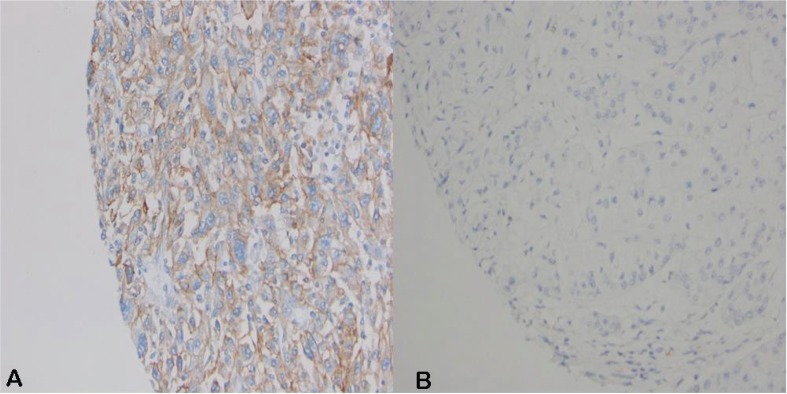
PD-L1 positivity on tumor cells was noted in nine (20%) patients (Figure 1), including two papillary type 2 (18%), three sarcomatoid (33%), three rhabdoid (50%), and one Xp11.2 translocation tumors (50%). PD-L1 positivity was significantly associated with higher Fuhrman nuclear grade (P = 0.048) and perineural invasion (P = 0.043) (Table 2). Although not statistically significant, patients with PD-L1-positive tumors had higher pT stage, more cM1 disease, greater tumor size, more lymphovascular invasion, and more tumor necrosis.
Figure 1.
FFPE non-ccRCC specimens stained with anti-PD-L1 antibody (E1L3N). (A) Positive PD-L1 staining; ≥50% positivity (20X). (B) Negative PD-L1 staining; <1% positivity (20X).
Table 2.
Association of PD-L1 expression and histopathologic characteristics in non-ccRCC.
| Characteristic | PD-L1(-) n = 36 | PD-L1(+) n = 9 | P value |
|---|---|---|---|
| AJCC pathologic (pT) (%) | |||
| pT1/2 | 19 (52.8) | 3 (33.3) | 0.252 |
| pT3/4 | 17 (47.2) | 6 (66.7) | |
| AJCC clinical (cM) (%) | |||
| cM0 | 32 (88.9) | 7 (77.8) | 0.344 |
| cM1 | 4 (11.1) | 2 (22.2) | |
| Median tumor size, cm (IQR) | 7 (4.5–12.6) | 8.5 (3.8–12.2) | 0.865 |
| Fuhrman nuclear grade (%) | |||
| I/II | 9 (25) | 2 (22.2) | 0.048 |
| III/IV | 27 (75) | 7 (77.8) | |
| Lymphovascular invasion (%) | |||
| Negative | 28 (77.8) | 5 (55.6) | 0.225 |
| Positive | 8 (22.2) | 4 (44.4) | |
| Perineural invasion (%) | |||
| Negative | 36 (100) | 8 (88.9) | 0.043 |
| Positive | 0 (0) | 1 (11.1) | |
| Tumor necrosis (%) | |||
| Negative | 18 (50) | 3 (33.3) | 0.303 |
| Positive | 18 (50) | 6 (66.7) | |
| No. Non-clear cell subtypes (%) | |||
| Sarcomatoid RCC | 6 (66.6) | 3 (33.3) | 0.135 |
| Rhabdoid RCC | 3 (50) | 3 (50) | |
| Medullary RCC | 2 (100) | 0 (0) | |
| Xp11.2 translocation RCC | 1 (50) | 1 (50) | |
| Collecting duct RCC | 1 (100) | 0 (0) | |
| Papillary type 1 RCC | 14 (100) | 0 (0) | |
| Papillary type 2 RCC | 9 (81.8) | 2 (18.2) |
Bold values indicate p<0.05
PD-L1 expression and oncological outcomes in non-ccRCC
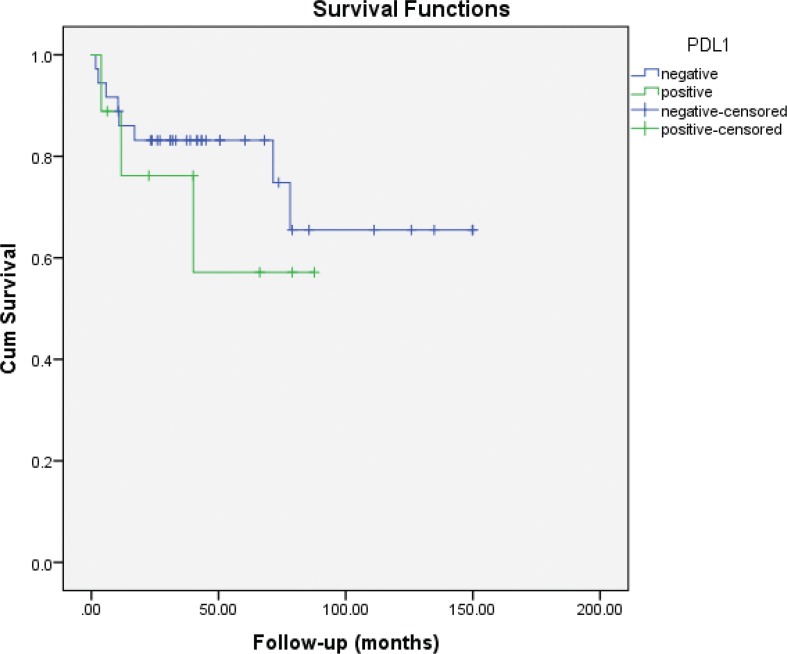
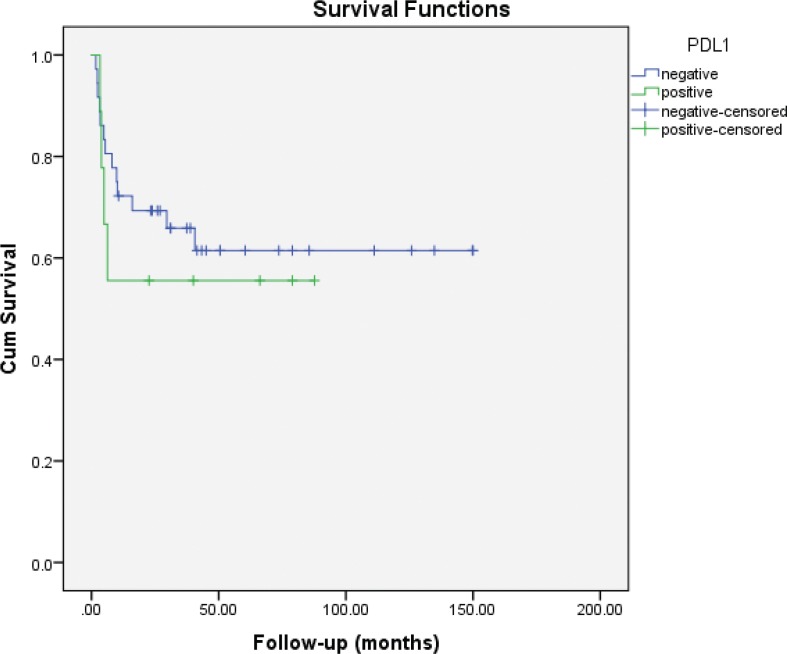
Median follow-up was 40 months (IQR: 22.9–72.5). Eleven (24.4%) patients had died of disease at the time of the analysis. Five-year CSS was 83 and 73% for PD-L1 negative and positive cases, respectively (log-rank P = 0.47) (Figure 2). Five-year RFS was 61.5 and 55.6% for PD-L1 negative and positive cases, respectively (log-rank P = 0.58) (Figure 3).
Figure 2.
Kaplan–Meier plots of cancer-specific survival (CSS) according to PD-L1 expression status in non-ccRCC patients.
Figure 3.
Kaplan–Meier plots of recurrence-free survival (RFS) according to PD-L1 expression status in non-ccRCC patients.
Discussion
Immunomodulatory agents targeting the PD-1/PD-L1 axis have revolutionized treatment of several malignancies, including advanced RCC (10). Unfortunately, much of this progress has been limited to patients with ccRCC, partly due to the relative rarity of non-clear cell variants and the paucity of knowledge about the molecular and biologic drivers of disease. In this study, PD-L1 was expressed in a fifth of non-ccRCC cohort while being associated with adverse histopathologic features.
PD-1 is a member of the B7–CD28 family and serves as a cell surface inhibitory receptor on T cells (13, 14). Mechanisms of action of immune checkpoint inhibitors are nonspecific but generally lead to immune system activation (6, 15). Expression of PD-1 by tumor-infiltrating lymphocyte mononuclear cells (TIMC) and PD-L1 on tumor cells has been associated with poor outcomes in several tumor types (15–19). Thompson et al. were one of the first groups to demonstrate PD-L1 expression as a significant predictor of cancer progression and mortality in ccRCC (20, 21).
To date, only two studies evaluating PD-L1 expression in non-ccRCC have been published. Choueiri et al. assessed PD-L1 expression in 101 non-ccRCC patients including chromophobe (n = 36), papillary (n = 50), collecting duct (n = 5), and Xp.11.2 translocation (n = 10) variants (22). PD-L1 positivity on tumor cells (11 cases, 10.9%) was significantly associated with higher stage and Fuhrman grade, and a worse overall survival. Similarly, our study revealed that PD-L1 positivity was associated with higher Fuhrman grade and perineural invasion, with a trend towards worse oncological outcomes. Conversely, Abbas et al. found no significant correlation between PD-1/PD-L1 expression and oncological outcomes in 63 cases of papillary, chromophobe, and sarcomatoid RCC variants (23).
Because of the lack of available treatments, studies combining non-clear and clear cell carcinomas have focused on angiogenesis inhibitors (anti-VEGF) and other targeted therapies (mTOR inhibitors). A recent meta-analysis of 20 studies including 1244 non-ccRCC and 6300 ccRCC patients revealed that the objective response rate to targeted therapy was significantly lower in those with non-clear cell subtypes (9.2% vs 14.8%) (24). Progression-free survival and overall survival were also shorter in non-ccRCC (7.5 and 13.2 months) versus ccRCC patients (10.5 and 15.7 months). Further studies with non-ccRCC cohorts are needed to assess the immune checkpoint therapy for expanding therapeutic options for these patients.
We acknowledge the several limitations of this study, including inherent biases associated with its retrospective design, small sample size, and population heterogeneity. Although we correlated PD-L1 expression with oncologic outcomes, causality cannot be established. The limited number of cases and events underpowered our analysis. Nevertheless, this is a common problem due to the rarity of non-ccRCC and a main cause of poor accrual in clinical trials. Moreover, this study validates the findings of the only previous report showing a correlation between PD-L1 positivity and adverse histopathologic features in non-ccRCC.
Conclusion
PD-L1 is expressed in non-ccRCC and is associated with a more aggressive tumor phenotype. There was also a trend toward worse oncological outcomes in patients with PD-L1-positive tumors. Expression of biomarkers such PD-L1 may help better risk-stratify non-ccRCC patients to guide treatment decisions and follow-up strategies. Further investigation of anti-PD-1 and anti-PD-L1 immune checkpoint inhibition in patients with non-ccRCC is warranted.
Conflict of interest
The authors declare no potential conflicts of interest with respect to research, authorship and/or publication of this article.
Footnotes
J.C. and M.A. contributed equally to this work.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66(1):7–30. 10.3322/caac.21332 [DOI] [PubMed] [Google Scholar]
- 2.Shah BK, Ghimire KB. Survival trends among patients with advanced renal cell carcinoma in the United States. Urol Int. 2015;94(2):133–6. 10.1159/000364951 [DOI] [PubMed] [Google Scholar]
- 3.Lam JS, Belldegrun AS, Pantuck AJ. Long-term outcomes of the surgical management of renal cell carcinoma. World J Urol. 2006;24(3):255–66. 10.1007/s00345-006-0055-5 [DOI] [PubMed] [Google Scholar]
- 4.Chin AI, Lam JS, Figlin RA, Belldegrun AS. Surveillance strategies for renal cell carcinoma patients following nephrectomy. Rev Urol. 2006;8(1):1–7. [PMC free article] [PubMed] [Google Scholar]
- 5.Rosenberg SA. Interleukin 2 for patients with renal cancer. Nat Clin Pract Oncol. 2007;4(9):497 10.1038/ncponc0926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mann SA, Lopez-Beltran A, Massari F, Pili R, Fiorentino M, Koch MO, et al. . Targeting the programmed Cell Death-1 pathway in genitourinary tumors: Current progress and future perspectives. Curr Drug Metab. 2017;18(8):700–11. 10.2174/1389200218666170518162500 [DOI] [PubMed] [Google Scholar]
- 7.Motzer RJ, Bacik J, Schwartz LH, Reuter V, Russo P, Marion S, et al. . Prognostic factors for survival in previously treated patients with metastatic renal cell carcinoma. J Clin Oncol. 2004;22(3):454–63. 10.1200/JCO.2004.06.132 [DOI] [PubMed] [Google Scholar]
- 8.Ball MW, Allaf ME, Drake CG. Recent advances in immunotherapy for kidney cancer. Discov Med. 2016;21(116):305–13. [PubMed] [Google Scholar]
- 9.McDermott DF, Drake CG, Sznol M, Choueiri TK, Powderly JD, Smith DC, et al. . Survival, durable response, and long-term safety in patients with previously treated advanced renal cell carcinoma receiving nivolumab. J Clin Oncol. 2015;33(18):2013–20. 10.1200/JCO.2014.58.1041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Motzer RJ, Escudier B, McDermott DF, George S, Hammers HJ, Srinivas S, et al. . Nivolumab versus everolimus in advanced renal-cell carcinoma. N Engl J Med. 2015;373(19):1803–13. 10.1056/NEJMoa1510665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carbognin L, Pilotto S, Milella M, Vaccaro V, Brunelli M, Calio A, et al. . Differential activity of Nivolumab, Pembrolizumab and MPDL3280A according to the tumor expression of programmed death-Ligand-1 (PD-L1): Sensitivity analysis of trials in melanoma, lung and genitourinary cancers. PLoS One. 2015;10(6):e0130142 10.1371/journal.pone.0130142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Festino L, Botti G, Lorigan P, Masucci GV, Hipp JD, Horak CE, et al. . Cancer treatment with anti-PD-1/PD-L1 Agents: Is PD-L1 expression a biomarker for patient selection? Drugs. 2016;76(9):925–45. 10.1007/s40265-016-0588-x [DOI] [PubMed] [Google Scholar]
- 13.Ishida Y, Agata Y, Shibahara K, Honjo T. Induced expression of PD-1, a novel member of the immunoglobulin gene superfamily, upon programmed cell death. EMBO J. 1992;11(11):3887–95. 10.1002/j.1460-2075.1992.tb05481.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Keir ME, Butte MJ, Freeman GJ, Sharpe AH. PD-1 and its ligands in tolerance and immunity. Annu Rev Immunol. 2008;26:677–704. 10.1146/annurev.immunol.26.021607.090331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McDermott DF, Atkins MB. PD-1 as a potential target in cancer therapy. Cancer Med. 2013;2(5):662–73. 10.1002/cam4.106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thompson RH, Dong H, Lohse CM, Leibovich BC, Blute ML, Cheville JC, et al. . PD-1 is expressed by tumor-infiltrating immune cells and is associated with poor outcome for patients with renal cell carcinoma. Clin Cancer Res. 2007;13(6):1757–61. 10.1158/1078-0432.CCR-06-2599 [DOI] [PubMed] [Google Scholar]
- 17.Mu CY, Huang JA, Chen Y, Chen C, Zhang XG. High expression of PD-L1 in lung cancer may contribute to poor prognosis and tumor cells immune escape through suppressing tumor infiltrating dendritic cells maturation. Med Oncol. 2011;28(3):682–8. 10.1007/s12032-010-9515-2 [DOI] [PubMed] [Google Scholar]
- 18.Kammerer-Jacquet SF, Crouzet L, Brunot A, Dagher J, Pladys A, Edeline J, et al. . Independent association of PD-L1 expression with noninactivated VHL clear cell renal cell carcinoma-A finding with therapeutic potential. Int J Cancer. 2017;140(1):142–8. 10.1002/ijc.30429 [DOI] [PubMed] [Google Scholar]
- 19.Hino R, Kabashima K, Kato Y, Yagi H, Nakamura M, Honjo T, et al. . Tumor cell expression of programmed cell death-1 ligand 1 is a prognostic factor for malignant melanoma. Cancer. 2010;116(7):1757–66. 10.1002/cncr.24899 [DOI] [PubMed] [Google Scholar]
- 20.Thompson RH, Kuntz SM, Leibovich BC, Dong H, Lohse CM, Webster WS, et al. . Tumor B7-H1 is associated with poor prognosis in renal cell carcinoma patients with long-term follow-up. Cancer Res. 2006;66(7):3381–5. 10.1158/0008-5472.CAN-05-4303 [DOI] [PubMed] [Google Scholar]
- 21.Thompson RH, Gillett MD, Cheville JC, Lohse CM, Dong H, Webster WS, et al. . Costimulatory molecule B7-H1 in primary and metastatic clear cell renal cell carcinoma. Cancer. 2005;104(10):2084–91. 10.1002/cncr.21470 [DOI] [PubMed] [Google Scholar]
- 22.Choueiri TK, Fay AP, Gray KP, Callea M, Ho TH, Albiges L, et al. . PD-L1 expression in nonclear-cell renal cell carcinoma. Ann Oncol. 2014;25(11):2178–84. 10.1093/annonc/mdu445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Abbas M, Steffens S, Bellut M, Becker JU, Grosshennig A, Eggers H, et al. . Do programmed death 1 (PD-1) and its ligand (PD-L1) play a role in patients with non-clear cell renal cell carcinoma? Med Oncol. 2016;33(6):59 10.1007/s12032-016-0770-8 [DOI] [PubMed] [Google Scholar]
- 24.Vera-Badillo FE, Templeton AJ, Duran I, Ocana A, de Gouveia P, Aneja P, et al. . Systemic therapy for non-clear cell renal cell carcinomas: A systematic review and meta-analysis. Eur Urol. 2015;67(4):740–9. 10.1016/j.eururo.2014.05.010 [DOI] [PubMed] [Google Scholar]