Depression and anxiety are associated with delayed presentation to HIV care but not late testing in South Africa. Interventions aimed at improving engagement in HIV care should consider screening for depression and anxiety when patients first present for HIV testing.
Keywords: HIV/AIDS, delayed presenters, late testers, depression, anxiety
Abstract
Background
Facility- and community-based efforts to improve human immunodeficiency virus (HIV) testing in sub-Saharan Africa may benefit from understanding how mental health influences HIV care-seeking behavior.
Methods
We conducted a study among adults presenting for HIV testing in the Umlazi township of South Africa. Prior to testing, we measured depression using the 9-item Patient Health Questionnaire and anxiety using the 7-item Generalized Anxiety Disorder scale. We categorized patients as delayed presenters (presenting to clinic >3 months after first HIV-positive test), late testers (presenting within 3 months of diagnosis with a CD4 count ≤200 cells per µL), or neither. We used multinomial logistic regression adjusting for sociodemographic and behavioral characteristics to determine the effects of depression and anxiety on HIV care-seeking behavior.
Results
Among 1482 HIV-infected adults, 59% were female and mean age was 33 years. The prevalence of depression in the cohort was 33% and anxiety was 9%. In adjusted models, mild to moderate depression was not associated with delayed presentation or late testing. HIV-infected adults with severe depression had 3.6 greater odds (95% confidence interval [CI], 1.2–10.2) of delayed presentation and 2.2 greater odds (95% CI, 1.01–4.8) of late testing compared with those without depression. Individuals with generalized anxiety had 2.3 greater odds (95% CI, 1.3–4.2) of delayed presentation compared with those without anxiety.
Conclusions
Severe depression was associated with delayed presentation and late testing, while anxiety was associated only with delayed presentation. Screening for mental health services may improve antiretroviral therapy initiation and linkage to care following HIV testing.
Half of the estimated 36 million people living with human immunodeficiency virus (PLWH) worldwide have not initiated antiretroviral therapy (ART) [1]. The “90-90-90” Joint United Nations Programme on HIV/AIDS treatment program targets diagnosis of 90% of PLWH, ART treatment for 90% of diagnosed PLWH, and viral suppression for 90% of people receiving treatment. As of 2016 in South Africa, 86% of PLWH were aware of their status, 65% were on therapy, and 81% had achieved viral suppression [2]. Although this progress is remarkable, it has been challenging to bridge the gap between human immunodeficiency virus (HIV) testing and ART initiation.
Early diagnosis and treatment initiation are critical for improving outcomes and reducing transmission. Worldwide, 22%–49% of newly diagnosed HIV-infected individuals present at clinics with CD4 <200 cells/µL, and those diagnosed in late stages of infection have advanced immunosuppression, with a significantly greater risk of morbidity and mortality [3, 4]. In 2015, the World Health Organization (WHO) recommended that all HIV-infected persons initiate ART irrespective of CD4 count, and South Africa implemented this universal test-and-treat rule in September 2016. Two randomized controlled trials conducted in sub-Saharan Africa found that although the universal test-and-treat approach resulted in increased testing in this population, linkage to care and treatment adherence were still a problem [5, 6]. A better understanding of the risk factors of delay in presentation to HIV care and ART initiation may be crucial for ensuring success of HIV programs.
Definitions of different stages at presentation to HIV care have been recently defined in an effort to standardize data collection and reporting in various settings [7, 8]. “Late testing” is commonly expressed in terms of an AIDS-defining illness and/or CD4 T-cell counts ranging from 50 cells/µL to 350 cells/µL at the time of testing, with 200 cells/µL being the most frequently used criterion [9, 10]. “Delayed presentation” is a concept that measures the time between first HIV diagnosis and presentation to clinical care, without regard to disease stage and severity [7]. Most studies of presentation to HIV care have not differentiated between people who present to the clinic for the first time with a low CD4 count (late testers) and people who present for testing or treatment having had a prior diagnosis of HIV (delayed presenters).
Because depression and anxiety disorders may be common among PLWH [11, 12], we hypothesized that delayed treatment-seeking behaviors of HIV-infected adults who know their HIV status may be associated with depression and anxiety. While an association between depression and low CD4 cell counts and treatment adherence has been described before [13, 14], there is limited understanding of the relationship between depression/anxiety and delayed care-seeking behavior among HIV-infected individuals. Therefore, we sought to determine whether depression and anxiety are risk factors for delayed presentation to HIV care among HIV-infected individuals in an HIV clinic in Umlazi, South Africa.
METHODS
Site and Participants
We conducted a prospective cohort study of HIV-infected adults in an urban township in KwaZulu-Natal, South Africa. We recruited patients presenting for HIV testing at the outpatient department of iThembalabantu Clinic in the Umlazi township from September 2013 to July 2016. Eligible participants were ≥18 years of age, presenting for voluntary HIV testing, and ART naive at the time of enrollment. We excluded females known to be pregnant. All participants provided written informed consent in either English or Zulu. The study was approved by the institutional review board (number 49563) of the University of Washington in Seattle and the Medical Research Ethics Committee of the University of KwaZulu-Natal in Durban (protocol number BF052/13).
Data Collection
Upon enrollment and before HIV testing, a research assistant collected information on sociodemographic and behavioral factors, HIV testing history, and knowledge of partner’s HIV status using a standardized questionnaire. Additional questions assessed several known risk factors for late testing, including distance to the clinic and travel time. Questions about experienced/ anticipated stigma and stigmatizing attitudes were adapted from an HIV stigma scale validated in Uganda [15]. All responses to the questionnaire were self-reported. This was followed by HIV testing conducted by nonresearch clinic staff as per standard of care. For those who tested HIV positive, a research nurse performed routine clinical assessment for signs/symptoms of disease, a clinical examination, and baseline laboratory testing, including CD4 T-cell count. Collected blood samples were tested for CD4 count at the National Health Laboratory Service at Prince Mshiyeni Memorial Hospital.
Measurement of Depression and Anxiety
The main exposures of interest were depression and anxiety. We used the 9-item Patient Health Questionnaire (PHQ-9) scale to assess depression, which has been used before among HIV-infected populations in South Africa [16, 17], Kenya [18], and the United States [19]. Each response is scored on a 4-point Likert scale (0 for “not at all” to 4 for “nearly every day”), with all responses being summed into a severity score for each participant. We used the 7-item Generalized Anxiety Disorder scale (GAD-7) to measure anxiety. The anxiety severity score was calculated in a manner similar to the PHQ-9 scale. The depression and anxiety scores were analyzed using severity score cutoffs described for the PHQ-9 and GAD-7 scales, respectively [20, 21]. Depression was categorized as no depression (PHQ-9 score <5), mild to moderate depression (PHQ-9 score 5–14), or severe depression (PHQ-9 score >14). Anxiety was categorized as no anxiety (GAD-7 score <10) or generalized anxiety disorder (GAD-7 score ≥10). We administered questions related to depression, anxiety, and stigma before HIV testing to eliminate any bias that may arise from knowledge of the HIV test result.
Outcome Definitions
We differentiate between delayed presenters and late testers in this study. We calculated delay in care-seeking as date of enrollment in the study minus the date of first self-reported positive HIV test. A gap of >90 days between the first HIV-positive test and study enrollment was considered delayed presentation to care for the purpose of this study. HIV-infected participants were categorized as delayed presenters or late testers based only on their status at the time of enrollment.
We categorized HIV-care seeking behavior as follows:
Delayed presenters: Participants who first tested positive for HIV >90 days prior to study enrollment, regardless of their CD4 count at enrollment.
Late testers: Participants who tested positive at the time of study enrollment or ≤90 days prior to enrollment and had baseline CD4 count <200 cells/µL.
Neither: Participants who tested positive for HIV at the time of study enrollment or ≤90 days prior to enrollment and had a baseline CD4 count >200 cells/µL.
Statistical Analysis
Univariate multinomial logistic regression models were used to identify independent risk factors associated with HIV care-seeking behavior. We measured the correlation between depression and anxiety using Pearson correlation coefficient. Separate logistic regression models were fit for depression and anxiety due to high correlation between them. Variables that were significantly associated with the outcome in univariate models were included in the final multivariable models. Age, sex, and stigma were hypothesized to be confounders in the association of both depression and anxiety with HIV care-seeking behavior and were adjusted for in the multivariable models regardless of statistical significance in univariate models. We categorized age as ≤25 years, 26–35 years, 36–45 years, and ≥46 years. We broadly categorized stigma as “experienced stigma” and “stigmatizing attitudes.” A positive response to at least 1 question indicative of either of these 2 categories classified a person as exposed to HIV-related stigma. All reported P values were 2-tailed, and a P value <.05 was considered statistically significant. We conducted analyses using SAS software version 9.4 (SAS Institute, Cary, North Carolina).
RESULTS
Cohort Characteristics
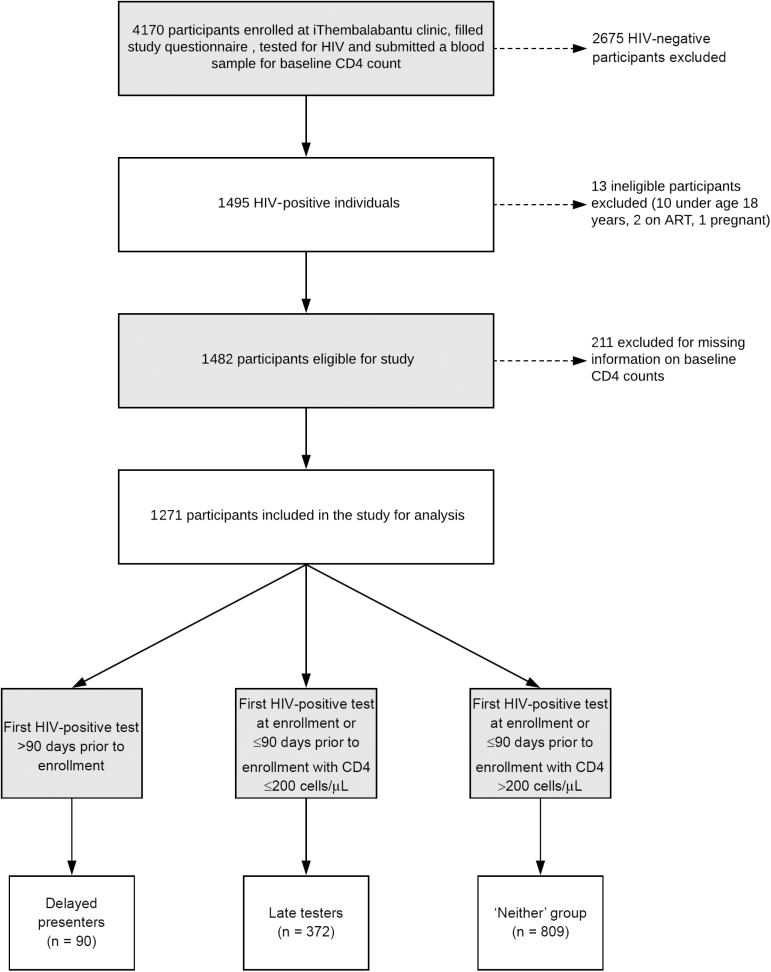
Of the 4170 participants enrolled, 1482 (36%) were HIV infected and met the inclusion criteria. Of these, 211 (14%) participants were missing information on baseline CD4 counts and were excluded. Among the 1271 participants that were included, 90 (7%) were delayed presenters, 372 (29%) were late testers, and 809 (63%) were neither (Figure 1).
Figure 1.
Flowchart of participants enrolled at the iThembalabantu clinic. Abbreviations: ART, antiretroviral therapy; HIV, human immunodeficiency virus.
Within the study cohort, about half (47%) were 26–35 years old, 756 (59%) were female, 796 (63%) had not completed high school, a majority (82%) had at least 1 child, and 96 (7%) were married. About half of the cohort was unemployed (56%), and 82% of the cohort earned <2000 South African rand (162 US dollars) per month. A majority of the participants (86%) lived within 5 km of the clinic and about 21% had to use public transit to travel to the clinic. Eight hundred thirty-seven participants (66%) had tested at least once for HIV before, and 113 (9%) reported having a positive HIV test before presenting for testing at the iThembalabantu clinic. The mean CD4 count was 294 cells/µL for delayed presenters, 101 cells/µL for late testers, and 447 cells/µL for the control group. Smoking and alcohol consumption was low in this cohort (24% ever smoked, 35% ever consumed alcohol). About a third of the cohort had HIV-infected partners (29%), but more than half of the cohort (57%) did not know their partner’s HIV status (Table 1). The proportion of delayed presenters declined significantly over time from 27% (46/172) in 2013 to 0% (0/172) in 2016, whereas the proportion of late testers increased moderately from 24% (42/172) in 2013 to 34% (58/172) in 2016.
Table 1.
Cohort Characteristics of Human Immunodeficiency Virus-Infected Adults, by Outcome Status
| Characteristic | Total | Delayed Presenters | Late Testers | Control | P Valueb |
|---|---|---|---|---|---|
| Total | 1271 | 90 | 372 | 809 | |
| Sociodemographic factors | |||||
| Year enrolled | |||||
| 2013 | 172 (13.5) | 46 (51.1) | 42 (11.3) | 84 (10.4) | <.001 |
| 2014 | 478 (37.6) | 34 (37.8) | 140 (37.6) | 304 (37.5) | |
| 2015 | 449 (35.4) | 10 (11.1) | 132 (35.5) | 307 (38.0) | |
| 2016 | 172 (13.5) | 0 (0.0) | 58 (15.6) | 114 (14.1) | |
| Age, y | |||||
| ≤25 | 214 (16.8) | 12 (13.3) | 43 (11.6) | 159 (19.6) | .013 |
| 26–35 | 600 (47.2) | 43 (47.8) | 170 (45.7) | 387 (47.8) | |
| 36–45 | 311 (24.4) | 23 (25.6) | 104 (28.0) | 184 (22.7) | |
| ≥46 | 146 (11.4) | 12 (13.3) | 55 (14.8) | 79 (8.6) | |
| Sex | |||||
| Female | 756 (59.4) | 66 (73.3) | 176 (47.3) | 514 (63.5) | <.001 |
| Education | |||||
| Less than high school | 796 (62.6) | 54 (60.0) | 245 (65.9) | 497 (61.4) | .0003 |
| Employment status | |||||
| Unemployed | 708 (55.6) | 58 (64.4) | 202 (54.3) | 448 (55.3) | .3 |
| Have children | |||||
| Yes | 1044 (82.3) | 79 (87.8) | 297 (79.8) | 668 (82.8) | .6 |
| Income, ZAR/moc | |||||
| <2000 | 1031 (82.5) | 63 (70.0) | 305 (82.0) | 663 (83.4) | .06 |
| Marital status | |||||
| Unmarried, widowed, or divorced | 1175 (92.4) | 84 (93.3) | 347 (93.3) | 744 (92.0) | .5 |
| Travel | |||||
| Use public transit or other | 266 (20.9) | 22 (24.4) | 79 (21.2) | 165 (20.5) | .3 |
| Distance traveled to clinic | |||||
| <5 km | 1095 (86.2) | 66 (73.3) | 328 (88.2) | 701 (86.7) | <.0001 |
| HIV testing history | |||||
| Had a prior HIV test | 837 (65.8) | 90 (100.0) | 216 (58.1) | 531 (65.6) | <.001 |
| Had a prior HIV-positive test | 113 (8.8) | 90 (100.0) | 7 (0.8) | 16 (2.0) | <.001 |
| Average No. of times tested for HIV prior to enrollmenta | 3.5 (2.3) | 2.8 (1.9) | 3.04 (1.8) | 3.7 (2.4) | <.001 |
| Mean CD4 count at enrollmenta | 335 (239) | 294 (214) | 101 (58) | 447 (212) | <.001 |
| Behavioral factors | |||||
| Smoking | |||||
| Ever smoker | 307 (24.2) | 24 (26.7) | 98 (26.3) | 185 (23.0) | .6 |
| Alcohol | |||||
| Ever consumed alcohol | 441 (34.8) | 33 (36.7) | 139 (37.4) | 269 (33.4) | .0015 |
| Partner’s HIV status | |||||
| No/unknown | 729 (57.5) | 42 (46.7) | 228 (61.3) | 459 (56.8) | .013 |
| Yes negative | 170 (13.3) | 17 (18.9) | 41 (11.1) | 112 (13.8) | |
| Yes positive | 369 (29.0) | 31 (34.4) | 101 (27.1) | 237 (29.3) | |
| Depression | |||||
| Mild to moderate | 382 (30.0) | 51 (56.7) | 116 (31.2) | 215 (26.5) | <.001 |
| Severe | 36 (2.8) | 7 (7.8) | 14 (3.8) | 15 (1.9) | |
| Anxiety | |||||
| Yes | 117 (9.2) | 20 (22.2) | 37 (9.9) | 60 (7.4) | <.001 |
| Stigma | |||||
| Stigmatizing attitudes | 232 (18.2) | 25 (27.7) | 69 (18.5) | 138 (17.0) | .04 |
| Experienced stigma | 228 (17.9) | 32 (35.5) | 62 (16.7) | 134 (16.6) | <.001 |
Data are presented as No. (%) unless otherwise indicated. Statistically significant P-values are in bold.
Abbreviations: HIV, human immunodeficiency virus; ZAR, South African rand.
aContinuous variables are summarized as mean (standard deviation) of the variable. The P values are for analysis of variance (ANOVA) test comparing means for ≥2 levels.
bFor categorical variables, P values are for χ2 test comparing proportions in each group. For continuous variables, P values are for ANOVA tests comparing group means.
c1 ZAR = 0.08 US dollars.
Prevalence of Depression and Anxiety
The prevalence of mild to moderate depression in the study sample was 33% (n = 382) and that of severe depression was 3% (n = 36). The prevalence of generalized anxiety disorder was 9% (n = 117). Depression and anxiety were highly and positively correlated (Pearson correlation coefficient, ρ = 0.75) in this cohort.
Correlates of Delayed Presentation
In the univariate analysis comparing delayed presenters to the comparison (“neither”) group, depression, anxiety, stigma, high income, and distance from clinic were significantly associated with delayed presentation (Table 2). Women had 40% greater odds of being delayed presenters, but this association was only moderately significant. Compared to HIV-infected individuals with no depression, the odds of delayed presentation were 1.6 times greater (95% confidence interval [CI], .9–2.8) for those with mild to moderate depression, and 3.6 times greater (95% CI, 1.2–10.2) for those with severe depression, after adjusting for calendar year, age, sex, stigma, distance from clinic, and income (Table 3). Participants with anxiety had odds of delayed presentation 2.3 times higher (95% CI, 1.3–4.2) compared to those with no anxiety (Table 3). Thus, severe depression and generalized anxiety were significantly associated with delayed presentation in our study. Calendar year, sex, stigma, and distance from clinic were still significantly associated with delayed presentation in multivariable models for both depression and anxiety.
Table 2.
Univariate Correlates of Delayed Presenters and Late Testersa (N = 1271)
| Characteristic | Delayed Presenters | Late Testers | ||
|---|---|---|---|---|
| OR (95% CI) | P Value | OR (95% CI) | P Value | |
| Enrollment year | ||||
| 2013 | 1 | … | 1 | … |
| 2014 | 0.2 (.1–0.3) | <.001 | 0.9 (.6–1.4) | .7 |
| 2015 | 0.06 (.03–0.1) | <.001 | 0.9 (.5–1.3) | .5 |
| 2016 | … | … | 1.02 (.6–1.6) | .9 |
| Age, y | ||||
| ≤25 | 1 | … | 1 | … |
| 26–35 | 1.4 (.7–2.8) | .2 | 1.6 (1.1–2.3) | .01 |
| 36–45 | 1.6 (.8–3.4) | .1 | 2.1 (1.3–3.1) | <.001 |
| ≥46 | 2.0 (.8–4.6) | .1 | 2.6 (1.6–4.1) | <.001 |
| Sex | ||||
| Female | 1 | … | 1 | … |
| Male | 0.6 (.4–1.0) | .06 | 1.9 (1.5–2.5) | <.001 |
| Education | ||||
| Less than high school | 1 | … | 1 | … |
| High school or greater | 1.1 (.7–1.7) | .8 | 0.8 (.6–1.1) | .13 |
| Income, ZAR/mob | ||||
| <2000 | 1 | … | 1 | … |
| ≥2000 | 2.0 (1.2–3.3) | .006 | 1.0 (.7–1.4) | .9 |
| Children | ||||
| No | 1 | … | 1 | … |
| Yes | 1.5 (.7–2.8) | .2 | 0.8 (.6–1.1) | .2 |
| Employment | ||||
| No | 1 | … | 1 | … |
| Yes | 0.7 (.4–1.1) | .09 | 1.0 (.8–1.3) | .7 |
| Marital status | ||||
| No | 1 | … | 1 | … |
| Yes | 0.8 (.3–1.9) | .6 | 0.8 (.5–1.3) | .4 |
| Travel | ||||
| Walk | 1 | … | 1 | … |
| Use public transit or other | 0.8 (.5–1.3) | .4 | 0.9 (.7–1.3) | .8 |
| Distance | 1 | … | 1 | … |
| <5 km from clinic | ||||
| ≥5 km from clinic | 2.4 (1.4–3.9) | <.001 | 0.8 (.6–1.3) | .5 |
| Smoking | ||||
| Never smoker | 1 | … | 1 | … |
| Ever smoker | 1.2 (0.7–1.9) | .4 | 1.2 (.9–1.6) | .2 |
| Alcohol | ||||
| Ever consumed alcohol | 1 | … | 1 | … |
| Never consumed alcohol | 1.1 (.7–1.8) | .5 | 1.2 (.9–1.5) | .2 |
| Partners’ HIV status | ||||
| No/unknown | 1 | … | 1 | … |
| Yes/HIV negative | 1.6 (.9–3.0) | .09 | 0.7 (.5–1.1) | .1 |
| Yes/HIV infected | 1.4 (.9–2.3) | .1 | 0.8 (.6–1.1) | .3 |
| Depression | ||||
| No depression | 1 | … | 1 | … |
| Mild to moderatec | 4.3 (2.7–6.9) | <.001 | 1.3 (.9–1.7) | .06 |
| Severed | 8.4 (3.2–22.2) | <.001 | 2.2 (1.1–4.7) | .03 |
| Anxietye | ||||
| No | 1 | … | 1 | … |
| Yes | 3.6 (2.0–6.2) | <.001 | 1.4 (.9–2.1) | .1 |
| Stigmaf | ||||
| No | 1 | … | 1 | … |
| Yes | 3.4 (2.2–5.3) | <.001 | 1.1 (.8–1.5) | .5 |
Statistically significant P-value are in bold.
Abbreviations: HIV, human immunodeficiency virus; ZAR, South African rand.
aComparison group consists of individuals who presented to the clinic within 3 months of a positive HIV test with a CD4 count >200 cells/µL.
b1 ZAR = 0.08 US dollars.
cMild to moderate depression: 9-item Patient Health Questionnaire (PHQ-9) score 5–13.
dSevere depression: PHQ-9 score ≥14.
eAnxiety: 7-item Generalized Anxiety Disorder scale score ≥10.
fStigma was recategorized as a binary yes/no variable and defined as participants who experienced stigma and/or stigmatizing attitudes.
Table 3.
Multivariable Analyses for Association Between Depression/Anxiety and Delayed Care-Seeking Behaviora
| Delayed Presenters | Late Testers | |||
|---|---|---|---|---|
| Characteristic | aORb (95% CI) | P Value | aORb (95% CI) | P Value |
| Depression | ||||
| No depression | Ref | … | Ref | … |
| Mild to moderate depressionc | 1.6 (.9–2.8) | .08 | 1.3 (.9–1.8) | .08 |
| Severe depressiond | 3.6 (1.2–10.2) | .01 | 2.2 (1.01–4.8) | .04 |
| Anxietye | ||||
| No anxiety | Ref | … | Ref | … |
| Anxiety | 2.3 (1.3–4.2) | .006 | 1.3 (.8–2.0) | .3 |
Statistically significant P-values are in bold.
Abbreviations: aOR, adjusted odds ratio; CI, confidence interval.
aTwo separate logistic models for depression and anxiety due to high correlation between them.
bModels are adjusted for calendar year, age, sex, stigma, income, and distance from clinic.
cMild to moderate depression: 9-item Patient Health Questionnaire (PHQ-9) score 5–14.
dSevere depression: PHQ-9 score ≥14.
eAnxiety: 7-item Generalized Anxiety Disorder scale score ≥10.
Correlates of Late Testing
In the univariate analysis comparing late testers to the comparison group, only age, sex, and severe depression were significantly associated with late testing (Table 2). Compared to HIV-positive individuals with no depression, the odds of late testing were 1.3 times greater (95% CI, .9–1.8) for those with mild to moderate depression, and 2.2 times greater (95% CI, 1.01–4.8) for those with severe depression, after adjusting for calendar year, age, sex, stigma, distance from clinic, and income (Table 3). Odds of late testing were not significantly different (odds ratio, 1.3 [95% CI, .8–2.0]) among those with anxiety compared to those without anxiety (Table 3). Thus, in the multivariable models, severe depression was moderately significantly associated with late testing, but anxiety was not.
DISCUSSION
In this HIV-infected cohort in South Africa, people who were classified as delayed presenters for care were significantly more likely to have severe depression and generalized anxiety than patients who presented earlier, regardless of CD4 count. Late testing was not significantly associated with mild to moderate depression and anxiety and was weakly associated with severe depression. Other risk factors for delayed presentation included female sex, high income, and living far from the clinic. Brief mental health screening at presentation in these high-risk groups may improve enrollment and linkage in HIV treatment programs. This is in accordance with the new WHO recommendations for prevention and management of mental health conditions in HIV populations [22].
Few studies have explored the psychosocial barriers associated with HIV care-seeking behavior. Fear of HIV-related stigma and low perceived risk of infection have been shown to be associated with lower rates of testing [23–26]. A recent study in South Africa found that poor social support and mental health affected HIV testing behavior [27]. In other studies, depression and anxiety have been linked with lower CD4 cell counts and poor treatment adherence [28, 29], but not with testing behavior. To our knowledge, our study was the first to explore the association of depression and anxiety with HIV care-seeking behavior, which we studied in a large cohort of South Africans presenting for HIV testing in a province with high HIV prevalence. Prior studies addressing psychosocial barriers also did not distinguish between delayed presenters and late testers. This study underscores the importance of distinguishing between types of delayed care-seeking behaviors, as different risk factors might be associated with them. Our findings indicate that programs may benefit from conducting a brief depression and anxiety screening assessment to identify those at risk of delayed presentation and may want to have more frequent contact with high-risk groups or offer same-day ART initiation to them.
We hypothesized that delayed presenters with a prior HIV-positive test may experience increased psychological distress or respond with denial, which might cause them to delay initiating treatment by several months, and our study results confirm this. The fear and stigma associated with disclosing their HIV status to their family or the community might also demotivate individuals from seeking treatment in a timely manner [4]. Domestic violence is another important risk factor for HIV among women in this region that may prevent timely treatment [30] as well as cause traumatic stress, which can lead to depression [31]. We found weak evidence of association between late testing and severe depression in our study and no association between late testing and anxiety. This might be because only 7 (2%) of late testers had a prior positive HIV diagnosis. The fear and stigma related to a positive diagnosis could, thus, be lower in this group, which could have resulted in low prevalence of depression and anxiety. While some studies have shown that depression may inhibit HIV testing, the exact nature of the relationship between testing-related fear and distress and depression is unclear [4, 32]. More research explicitly defining the mechanism through which depression/anxiety affects late testing among undiagnosed individuals is warranted.
In this cohort, the prevalence of depression (33%) and anxiety (9%) were comparable to those reported in other studies in Africa [33–35]. Studies that have examined demographic and social factors associated with a general category of late presentation for HIV care have found older age, foreign origin [25], low socioeconomic status, unemployment [36], doubting partners’ faithfulness [25, 36], counseling at time of first positive test [8], and being male [36, 37] as predictive of care-seeking behavior. Association between more perceived stigma and late presentation has also been described [38]. We too found older age, female sex, and HIV-related stigma to be associated with delayed presentation. Older age and male sex were associated with late testing, which confirms the findings of other studies.
A total of 113 (9%) participants presented at the iThembalabantu clinic for a voluntary HIV test despite having a prior positive HIV diagnosis, and 80% of them (90/113) were delayed presenters. While there is little research around repeat testing behavior of HIV-infected individuals, some reasons could be doubts about their HIV diagnosis at first test, lack of access to ART at first facility, stigma-related factors, or transportation issues [39].
While late testing remained unchanged during the period of enrollment, South Africa implemented the universal test-and-treat strategy in September 2016, which was after study enrollment ended. This may lead to a decrease in late testing behavior [40].
Our study had several strengths and limitations. We reduced the potential for bias of our outcomes by administering the depression and anxiety questionnaires before testing for HIV. However, the cross-sectional study design precludes ascertainment of temporality and assertions of causality. About 14% of participants were excluded from the study because they did not have a laboratory result for baseline CD4 count. We assumed that these data are missing completely at random and performed a complete case analysis. Participants self-reported details of previous HIV diagnosis that allowed us to classify them as delayed presenters. Recall bias or social desirability bias may result in an underestimation of the number of delayed presenters. We were unable to assess and control for previously reported risk factors either because the data were not collected in our questionnaire (foreign origin, perception of partner’s faithfulness, domestic violence) or were only collected on a subset of participants (perception of HIV acquisition), or the prevalence of the risk factor in our cohort was negligible (drug use) [4, 8, 24]. In our cohort, there is little variation in drug use and country of origin across exposure groups, so they are unlikely to be important confounders. Domestic violence is a potential confounder of the association between depression and HIV care-seeking behavior that may have biased our findings. Finally, while we did measure stigma, it has been challenging to obtain a standardized and validated measure of HIV-related stigma in this population, despite recent efforts [15].
In conclusion, these findings suggest that interventions aimed at reducing delays in engagement in HIV care should acknowledge that persons with depression and anxiety are at higher risk of delayed presentation for care. HIV testing programs could do brief anxiety and depression screening, engage in closer follow-up, and encourage same-day HIV treatment initiation to reduce delays in HIV care linkage.
Notes
Acknowledgments. We are thankful to Dr Dumezweni Ntshangase and Meighan Krows for assisting with the planning, coordination, and execution of the study.
Author contributions. M. S. R., T. H., and P. K. D. designed the study. P. K. D., H. T., and S. G. acquired study data. M. S. R., T. H., and P. K. D. analyzed and interpreted the data. M. S. R., T. H., P. K. D., C. C., and M. Y. M. drafted and revised the manuscript.
Financial support. This work was supported by the Institute for Allergy and Infectious Diseases of the National Institutes of Health (NIH) (grant number K23 AI108293).
Potential conflicts of interest. T. H. reports receiving a grant from the NIH. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Joint United Nations Programme on HIV/AIDS. Regional statistics—2015. Geneva, Switzerland: UNAIDS, 2016:18–25. [Google Scholar]
- 2. Joint United Nations Programme on HIV/AIDS. Ending AIDS progress towards the 90-90-90 targets. Glob Aids Updat 2017:198. [Google Scholar]
- 3. Diaz RS, Inocêncio LA, Sucupira MCA, et al. The virological and immunological characteristics of the HIV-1-infected population in Brazil: from initial diagnosis to impact of antiretroviral use. PLoS One 2015; 10:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Mukolo A, Villegas R, Aliyu M, Wallston KA. Predictors of late presentation for HIV diagnosis: a literature review and suggested way forward. AIDS Behav 2013; 17:5–30. [DOI] [PubMed] [Google Scholar]
- 5. Hayes R, Floyd S, Schaap A, et al. A universal testing and treatment intervention to improve HIV control: one-year results from intervention communities in Zambia in the HPTN 071 (PopART) cluster-randomised trial. PLoS Med 2017; 14:1–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Iwuji CC, Orne-Gliemann J, Larmarange J, et al. Universal test and treat and the HIV epidemic in rural South Africa: a phase 4, open-label, community cluster randomised trial. Lancet HIV 2017; 2018:1–10. [DOI] [PubMed] [Google Scholar]
- 7. Kozak M, Zinski A, Leeper C, Willig JH, Mugavero MJ. Late diagnosis, delayed presentation and late presentation in HIV: proposed definitions, methodological considerations and health implications. Antivir Ther 2013; 18:17–23. [DOI] [PubMed] [Google Scholar]
- 8. Girardi E, Aloisi MS, Arici C, et al. Delayed presentation and late testing for HIV: demographic and behavioral risk factors in a multicenter study in Italy. J Acquir Immune Defic Syndr 2004; 36:951–9. [DOI] [PubMed] [Google Scholar]
- 9. Antinori A, Coenen T, Costagiola D, et al. ; European Late Presenter Consensus Working Group. Late presentation of HIV infection: a consensus definition. HIV Med 2011; 12:61–4. [DOI] [PubMed] [Google Scholar]
- 10. Johnson M, Sabin C, Girardi E. Definition and epidemiology of late presentation in Europe. Antivir Ther 2010; 15(Suppl 1):3–8. [DOI] [PubMed] [Google Scholar]
- 11. Pence BW, Miller WC, Whetten K, Eron JJ, Gaynes BN. Prevalence of DSM-IV-defined mood, anxiety, and substance use disorders in an HIV clinic in the southeastern United States. J Acquir Immune Defic Syndr 2006; 42:298–306. [DOI] [PubMed] [Google Scholar]
- 12. Ciesla JA, Roberts JE. Meta-analysis of the relationship between HIV infection and risk for depressive disorders. Am J Psychiatry 2001; 158:725–30. [DOI] [PubMed] [Google Scholar]
- 13. Memiah P, Shumba C, Etienne-Mesubi M, et al. The effect of depressive symptoms and CD4 count on adherence to highly active antiretroviral therapy in sub-Saharan Africa. J Int Assoc Provid AIDS Care 2013; 13:346–52. [PubMed] [Google Scholar]
- 14. Kaharuza FM, Bunnell R, Moss S, et al. Depression and CD4 cell count among persons with HIV infection in Uganda. AIDS Behav 2006; 10:105–11. [DOI] [PubMed] [Google Scholar]
- 15. Kipp AM, Audet CM, Earnshaw VA, Owens J, McGowan CC, Wallston KA. Re-validation of the Van Rie HIV/AIDS-related stigma scale for use with people living with HIV in the United States. PLoS One 2015; 10:1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Chibanda D, Cowan F, Gibson L, Weiss HA, Lund C. Prevalence and correlates of probable common mental disorders in a population with high prevalence of HIV in Zimbabwe. BMC Psychiatry 2016; 16:55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Tuthill EL, Pellowski JA, Young SL, Butler LM. Perinatal depression among HIV-infected women in KwaZulu-Natal South Africa: prenatal depression predicts lower rates of exclusive breastfeeding. AIDS Behav 2017; 21:1691–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Monahan PO, Shacham E, Reece M, et al. Validity/reliability of PHQ-9 and PHQ-2 depression scales among adults living with HIV/AIDS in western Kenya. J Gen Intern Med 2009; 24:189–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Crane PK, Gibbons LE, Willig JH, et al. Measuring depression levels in HIV-infected patients as part of routine clinical care using the nine-item Patient Health Questionnaire (PHQ-9). AIDS Care 2010; 22:874–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med 2001; 16:606–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Spitzer RL, Kroenke K, Williams JB, Löwe B. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch Intern Med 2006; 166:1092–7. [DOI] [PubMed] [Google Scholar]
- 22. World Health Organization. HIV prevention, diagnosis, treatment and care for key populations (2016 update). Geneva, Switzerland: WHO, 2016:155. [PubMed] [Google Scholar]
- 23. Carrizosa CM, Blumberg EJ, Hovell MF, et al. Determinants and prevalence of late HIV testing in Tijuana, Mexico. AIDS Patient Care STDS 2010; 24:333–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Mayben JK, Kramer JR, Kallen MA, Franzini L, Lairson DR, Giordano TP. Predictors of delayed HIV diagnosis in a recently diagnosed cohort. AIDS Patient Care STDS 2007; 21:195–204. [DOI] [PubMed] [Google Scholar]
- 25. Bonjour MA, Montagne M, Zambrano M, et al. Determinants of late disease-stage presentation at diagnosis of HIV infection in Venezuela: a case-case comparison. AIDS Res Ther 2008; 5:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Abaynew Y, Deribew A, Deribe K. Factors associated with late presentation to HIV/AIDS care in South Wollo ZoneEthiopia: a case-control study. AIDS Res Ther 2011; 8:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Drain PK, Losina E, Coleman SM, et al. Social support and mental health among adults prior to HIV counseling and testing in Durban, South Africa. AIDS Care 2015; 27:1231–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Tao J, Wang L, Kipp AM, et al. Relationship of stigma and depression among newly HIV-diagnosed Chinese men who have sex with men. AIDS Behav 2016; 21:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Fonsah JY, Njamnshi AK, Kouanfack C, et al. Adherence to antiretroviral therapy (ART) in Yaoundé-Cameroon: association with opportunistic infections, depression, ART regimen and side effects. PLoS One 2017;12:1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Adams J, Hansen N, Fox A, et al. Correlates of HIV testing among abused women in South Africa. Violence Against Women 2011; 8:1014–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Devries KM, Mak JY, Bacchus LJ, et al. Intimate partner violence and incident depressive symptoms and suicide attempts: a systematic review of longitudinal studies. PLoS Med 2013; 10: e1001439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Mayston R, Lazarus A, Patel V, et al. Pathways to HIV testing and care in Goa, India: exploring psychosocial barriers and facilitators using mixed methods. BMC Public Health 2016; 16:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Pappin M, Wouters E, Booysen FL. Anxiety and depression amongst patients enrolled in a public sector antiretroviral treatment programme in South Africa: a cross-sectional study. BMC Public Health 2012; 12:244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Marwick KF, Kaaya SF. Prevalence of depression and anxiety disorders in HIV-positive outpatients in rural Tanzania. AIDS Care 2010; 22:415–9. [DOI] [PubMed] [Google Scholar]
- 35. Olagunju AT, Adeyemi JD, Ogbolu RE, Campbell EA. A study on epidemiological profile of anxiety disorders among people living with HIV/AIDS in a sub-Saharan Africa HIV clinic. AIDS Behav 2012; 16:2192–7. [DOI] [PubMed] [Google Scholar]
- 36. Chadborn TR, Delpech VC, Sabin CA, Sinka K, Evans BG. The late diagnosis and consequent short-term mortality of HIV-infected heterosexuals (England and Wales, 2000–2004). AIDS 2006; 20:2371–9. [DOI] [PubMed] [Google Scholar]
- 37. Castilla J, Sobrino P, del Amo J; EPI-VIH Study Group HIV infection among people of foreign origin voluntarily tested in Spain. A comparison with national subjects. Sex Transm Infect 2002; 78:250–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Gesesew HA, Gebremedhin AT, Demissie TD, Kerie MW, Sudhakar M, Mwanri L. Significant association between perceived HIV related stigma and late presentation for HIV/AIDS care in low and middle-income countries : a systematic review and meta—analysis. 2017; 12:1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kulkarni S, Tymejczyk O, Gadisa T, et al. ‘Testing, testing’: multiple HIV-positive tests among patients initiating antiretroviral therapy in Ethiopia. J Int Assoc Provid AIDS Care 2017; 16:546–54. [DOI] [PubMed] [Google Scholar]
- 40. Meyer-Rath G, Johnson LF, Pillay Y, et al. Changing the South African national antiretroviral therapy guidelines: the role of cost modelling. PLoS One 2017; 12:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]