Abstract
Background
Diet-mediated alterations of critical brain nutrient transporters, major facilitator super family domain-containing 2a (Mfsd2a) and glucose transporter 1 (Glut1), have wide reaching implications in brain health and disease.
Objective
The aim of the study was to examine the impact of long-term low- and high-fat diets with lard or fish oil on critical brain nutrient transporters, Mfsd2a and Glut1.
Methods
Eight-week-old male C57BL/6 mice were fed 1 of the following 4 diets for 32 wk: 10% of kcal from lard, 10% of kcal from fish oil, 41% of kcal from lard, or 41% of kcal from fish oil. Body weight and blood chemistries delineated dietary effects. Cortical and subcortical Mfsd2a and Glut1 mRNA and protein expression were evaluated, with other supportive nutrient-sensitive targets also assessed for mRNA expression changes.
Results
Fish-oil diets increased cortical Mfsd2a mRNA expression compared with lard diets. Subcortical Mfsd2a mRNA expression decreased as the percentage of fat in the diet increased. There was an interaction between the type and percentage of fat with cortical and subcortical Mfsd2a and cortical Glut1 protein expression. In the lard diet groups, protein expression of cortical and subcortical Mfsd2a and cortical Glut1 significantly increased as fat percentage increased. As the fat percentage increased in the fish-oil diet groups, protein expression of cortical and subcortical Mfsd2a and cortical Glut1 did not change. When comparing the fish-oil groups with 10% lard, cortical Mfsd2a protein expression was significantly higher in the 10% and 41% fish-oil groups, whereas cortical Glut1 protein expression was significantly higher in only the 10% fish-oil group. A positive correlation between cortical peroxisome proliferator–activated receptor γ mRNA expression and Mfsd2a protein expression was shown.
Conclusion
Corresponding to chronic dietary treatment, an interaction between the type of fat and the percentage of fat exists respective to changes in brain expression of the key nutrient transporters Mfsd2a and Glut1.
Keywords: omega-3 polyunsaturated fatty acid, Western diet, Mfsd2a, Glut1, blood-brain barrier
Introduction
The impact of diet on brain health has received significant attention over the past decade. The Western diet, which is high in SFAs and simple carbohydrates, has been identified as a risk factor for neurodegenerative disease and psychological disorders (1, 2). Western diet models identify increased hippocampal β-amyloid deposition in mouse models of Alzheimer disease (3, 4), increased hippocampal blood-brain barrier (BBB) permeability (3), and increased learning and memory deficits (1, 5) relative to control diets. Conversely, diets containing the long-chain omega-3 PUFAs, such as EPA and DHA found in fish oils, are thought to be protective because they enhanced neural development, cognition, and cerebrovascular function (6). Increasing ω-3 PUFA brain uptake has been proposed as a potential treatment avenue for neurodegenerative disease (7). How lard- or fish-oil–based diets hinder or benefit brain health and function, respectively, may, in part, be dependent on nutrient transport mechanisms. Two vital nutrient transporter proteins for the brain are major facilitator superfamily domain-containing protein 2a (Mfsd2a) and the glucose transporter 1 (Glut1).
Within the brain, Mfsd2a is selectively found at the BBB endothelium (8). Mfsd2a is a sodium-dependent symporter that transports long-chain FAs that are ≥14 carbons in length, including DHA (22:6), esterified to lysophosphatidylcholine (LPC) (8, 9). DHA esterified to LPC at the sn-2 position was preferentially taken up into the brain compared with nonesterified DHA (8, 10). In Mfsd2a knockout mice, brain concentrations of DHA phospholipids were reduced by 58.8% compared with that in wild-type mice (8). Mfsd2a transport of DHA and accretion of DHA in the brain have significant implications related to neuropsychiatric and neurodegenerative disease. Brain concentrations of ω-3 FAs are lower in major depression (11, 12) and in Alzheimer disease (13–15). Moreover, Mfsd2a has been shown to be a key regulator of BBB integrity, regulation, and formation (9). Transcytosis and permeability at the BBB were increased in Mfsd2a knockouts and transgenic mice with a nonfunctional Mfsd2a transporter compared with wild-type mice (8, 16). Recent data also show that increased expression of Mfsd2a mitigated BBB disruption after intracerebral hemorrhage (17).
Glut1 is another member of the major facilitator superfamily, colocalized with Mfsd2a at the BBB (8). Glut1 provides the brain with its main energy source, glucose, and is the primary glucose transporter (18). Glut1 has 2 isoforms: the 55-kDa isoform affiliated with the BBB endothelium and the 45-kDa isoform affiliated with astrocytes (19, 20). The density of Glut1 is closely linked to regional brain glucose utilization (21). Changes in BBB Glut1 function or expression have been implicated in various conditions, including cognitive impairment, Alzheimer disease, and diabetes (22–24). In Glut1 haplo-deficient mice, Glut1 was not only necessary for maintaining glucose transport but also cerebral blood flow and BBB integrity (24). Moreover, ω-3 PUFAs have been identified as regulators of brain metabolism and glucose utilization through regulation of Glut1 protein expression at the BBB (25–27).
Although the importance of Mfsd2a and Glut1 for the functioning of the brain is evident, there remains limited understanding as to how long-term dietary differences affect Mfsd2a and Glut1 in the adult brain. In the present study, we evaluated how the type and amount of dietary fat affect these key nutrient transports of the brain. Herein, C57BL/6J mice were treated over a 32-wk period with 1 of 4 diets: low-fat (10%) or high-fat (41%), with the use of lard and fish oil. Body weights along with blood chemistry measures of total cholesterol, LDL cholesterol, HDL cholesterol, TGs, glucose, and insulin characterized the whole-body impact of the dietary treatments. Protein and mRNA expressions of Mfsd2a and Glut1 were evaluated from both cortical and subcortical brain tissue. The following additional targets affiliated with dietary variables and brain health were assessed for mRNA expression changes: LDL receptor-related protein 1 (Lrp), Ppar-α, Ppar-γ, cluster of differentiation 36/FA translocase (Cd36), ATP-binding cassette transporter 1 (Abca1), insulin receptor (Insr), Apoe, amyloid precursor protein (App), brain-derived neurotrophic factor (Bdnf), superoxide dismutase 1 (Sod1), Sod2, and catalase (Cat).
Methods
Experimental design
All of the procedures were approved by the Institutional Animal Care and Use Committee at Southern Illinois University Edwardsville. Male C57BL/6J mice (Jackson Laboratories) were housed 3 ⋅ cage−1 ⋅ diet−1 at 25°C with a 12-h light-dark cycle. Diets were formulated by Research Diets and are described in Table 1. At age 8 wk, 48 mice (n = 12/group) were randomly assigned to 1 of the following 4 treatment groups ad libitum for 32 wk (α = 0.05): 10% of kcal from lard (D12450H), 10% of kcal from fish (Menhaden) oil (D15071702), 41% of kcal from lard (D15071701), or 41% of kcal from fish oil (D15071703). One mouse (41% lard group) was euthanized for reasons unrelated to diet. The EPA-to-DHA ratio in the fish oil diets was 1.4:1.0 (see Supplemental Table1 for diet FA compositions). The body mass of each mouse was recorded weekly, and energy intake was determined weekly and extrapolated from the number of mice per cage. At the end of the 32-wk treatments, after overnight feed deprivation (12 h) with access to water, mice were killed via sodium pentobarbital. Blood was collected and treated with EDTA, centrifuged at 4°C for 15 min at 1500 × g, and plasma was collected and stored at –80°C. Mice were perfused with 10 mL sterile ice-cold 0.9% NaCl saline. Brains were collected, then washed with saline, and the meninges and choroid plexus were removed before the dissection and separation of the cortical (i.e., cerebral cortex) and subcortical (i.e., hippocampus, caudate putamen, thalamus, globus palladus, basal forebrain, amygdala) portions. Cortical and subcortical samples for real-time qRT-PCR were stored in RNAlater (Thermo Fisher Scientific) at –20°C. For Western blot evaluations, samples were homogenized and stored in a 6-M urea buffer (6 M urea, 0.1% Triton X-100, 1 mM DTT, 5 mM MgCl2, 5 mM EGTA, 150 mM NaCl, and 10 mM Tris, pH 8.0) containing Complete protease inhibitor and PhosSTOP phosphatase inhibitor (Roche); and protein concentrations were determined by bicinchoninic acid protein assay (Thermo Fisher Scientific) before storage. Samples not immediately evaluated were stored at –80°C. Unless otherwise stated, all chemicals were purchased from Sigma-Aldrich.
TABLE 1.
Diet composition
| 10% Lard | 10% Fish oil | 41% Lard | 41% Fish oil | |
|---|---|---|---|---|
| Protein, % of kcal | 20 | 20 | 20 | 20 |
| Carbohydrate, % of kcal | 70 | 70 | 39 | 39 |
| Fat, % of kcal | 10 | 10 | 41 | 41 |
| kcal/g | 3.85 | 3.85 | 4.6 | 4.6 |
| Lard, g | 20 | 0 | 160 | 0 |
| Menhaden oil, g | 0 | 20 | 0 | 160 |
| Soybean oil, g | 25 | 25 | 25 | 25 |
| SFA, % | 23.5 | 21.2 | 31.4 | 27.1 |
| MUFA, % | 12.8 | 10.1 | 61.6 | 40 |
| PUFA, % | 20.2 | 23.2 | 58 | 82.1 |
| Linoleic acid, % | 41.3 | 31.5 | 29.8 | 9.5 |
| Oleic acid, % | 28.6 | 18.1 | 33.5 | 12.6 |
| α-Linolenic acid, % | 4.9 | 5.1 | 2.3 | 2.5 |
| EPA, % | 0 | 6.7 | 0 | 13.6 |
| DHA, % | 0 | 4.9 | 0 | 9.8 |
| Total ω-3 FAs, % | 4.9 | 19.4 | 2.4 | 31.5 |
Glucose-tolerance test
Glucose-tolerance tests (GTTs) were conducted 4 d before harvest. Mice were feed deprived for 4 h with access to drinking water and injected intraperitoneally with 2 mg glucose/g body weight. Blood glucose concentrations (milligrams per deciliter) were measured at 0, 15, 30, 60, and 120 min using a glucose meter (Accu-Chek; Roche). Glucose AUCs were determined with the use of the positive incremental method (28, 29).
Plasma chemistry
Plasma glucose, total cholesterol (TC), and TG concentrations were determined by enzymatic assay following the manufacturer's instructions (Pointe Scientific). Measurement of HDL cholesterol concentration was conducted by the precipitation of apoB-containing lipoproteins using a heparin (14 mM)-MnCl2 (1.58 M) solution and NaHCO3 (1 M) treatment followed by enzymatic measurement of the remaining cholesterol (30). LDL cholesterol concentration was calculated by using the Friedewald equation: LDL cholesterol = TC – HDL cholesterol ÷ (TGs/5) (31).
Plasma insulin was quantified by using an ELISA kit following the manufacturer's instructions (Mercodia). To estimate the degree of insulin resistance, the HOMA- IR was calculated from fasting glucose and insulin concentrations as fasting glucose (mg/dL) × fasting insulin (mU/L) ÷ 405 (32).
Quantitative real-time PCR analysis
Total RNA was extracted from cortical and subcortical samples using Trizol reagent (Life Technologies). Total RNA in each sample was quantified by using the Qubit BR RNA assay kit following the manufacturer's instructions (Invitrogen). DNase I–treated RNA (1 μg) was reverse transcribed into cDNA using the High Capacity Reverse Transcriptase kit (Applied Biosystems) and stored at –20°C. Real-time PCR evaluations were performed with the use of either TaqMan Fast Universal Master Mix (2×; Thermo Fisher Scientific) or Kapa SYBR Green Fast Rox Low Master Mix (2×; Kapa Biosystems). Evaluations were performed in duplicate with 10 μL Master Mix, 200–500 nM primer, and 10 ng cDNA. Melt curves were performed following all SYBR green assays to verify the absence of primer-dimer formation. Relative mRNA expression for each target was calculated by using the 2(–ΔΔCt) method (33), ΔΔCt represents change relative to 10% lard group, referenced to Gadph. Reactions were carried out with the use of a 7500 Fast PCR system (Applied Biosystems). SYBR green assay primers (Integrated DNA Technologies) included Ppar-γ (Mm.PT.58.31161924), and TaqMan assay primers (Applied Biosystems) included Mfsd2a (Mm01192208_m1), Glut1 (Mm00441480_m1), Insr (Mm01211875_m1), Lrp1 (Mm00464608_m1), Abca1 (Mm00442646_m1), Cd36 (Mm00432403_m1), Ppar-α (Mm00440939_m1), Apoe (Mm01307193_g1), App (Mm01344172_m1), Bndf (Mm04230607_s1), Sod1 (Mm01344233_g1), Sod2 (Mm01313000_m1), and Cat (Mm00437992_m1). Gadph (Mm99999915_g1) served as the reference gene.
Western blot and deglycosylation
Samples were heated at 95°C for 5 min in 2× SDS and 20× reducing agent (Bio-Rad) and loaded into 10% Bis/Tris Criterion XT gels (Bio-Rad). The electrophoretic field was set at 200 V for 35 min followed by transfer to nitrocellulose membranes at 240 mA for 45 min and blocked in 5% (wt:vol) nonfat milk in Tris-buffered saline for 4 h before incubation overnight with primary antibodies. Membranes were independently blotted for Mfsd2a (1:1000; PA5-21049) and Glut1 (1:1000; PA1-46152) (Thermo Fisher Scientific). Blots were developed with the use of Amersham ECL Western Blotting Detection Reagent (GE Healthcare Biosciences) and visualized on a ChemiDoc XRS+ (Bio-Rad), and analyses were performed using Image Lab software 3.0 (Bio-Rad). Membranes were stripped (Re-blot Plus; Thermo Fisher Scientific) and reprobed with β-actin antibody (1:2000; AC-40) (Sigma-Aldrich). Samples sets were run in duplicate, and the respective protein expressions were normalized to the average of the 10% lard group normalized to β-actin.
Peptide:N-Glycosidase F (PNGase F) assay (New England BioLabs, Inc.) catalyzes the cleavage of an internal glycoside bond from N-linked glycoproteins. Samples were mixed with glycoprotein denaturing buffer (10×) and deionized H2O, heated at 100°C for 10 min, then centrifuged at 4°C for 10 s at 2000 × g, followed by addition of 2 μL GlycoBuffer-2 (10×) and 2 μL 10% NP-40, and mixed on a vortex for 10 s. Then, 3 μL PNGaseF or 3 μL dH2O (control) was added and incubated in a dry heater for 24 h followed by Western blot analysis as described above.
Statistical analyses
A 2-level linear mixed model was used to evaluate diet on individual weekly body weights over time. Two-factor ANOVA was used to identify differences in means of variables with the type of fat in the diet, the percentage of fat in the diet, and their interaction at the conclusion of the treatment period, with the significance level set at P < 0.05. If significance was found for the type of fat, percentage of fat, or their interaction, a Tukey-Kramer post hoc test was conducted. Analyses were performed using SAS version 9.4 (SAS Institute). Data are presented as means ± SEMs.
Results
Diet and body weight
Body weights and blood chemistry measures were used to ascertain the whole-body impact of the dietary treatments. The effect of diet on weekly body weight with time was evaluated with the use of a linear mixed model (Figure 1). A significant interaction between diet and time (F = 60.83, df = 3, P < 0.0001) was found for body weight [model estimates—intercept estimate: 23.6275, t = 31.39, P < 0.0001; time in weeks estimate: 0.4201 g/wk, t = 43.06, P < 0.0001; diet estimates relative to 10% lard: 41% fish-oil estimate: 1.7054, t = 1.60, P = 0.1165; 41% lard estimate: 0.8935, t = 0.82, P = 0.4162; 10% fish-oil estimate: 0.8483, t = 0.80, P = 0.4299; 10% fish oil × time estimate: –0.1026 g/wk, t = –7.43, P < 0.0001; 41% fish oil × time estimate: –0.0370 g/wk, t = –2.68, P = 0.0074; 41% lard × time estimate: 0.0842 g/wk, t = 5.97, P < 0.0001; reference group: 10% lard (Figure 1)]. The negative estimates obtained for the interaction between time and diet for the 10% and 41% fish-oil groups indicated that, with each week, mice in these groups gained less weight per week relative to the 10% lard group. The positive estimate obtained for the interaction between diet and time for the 41% lard group indicated that the 41% lard group gained more weight with each week than did the 10% lard group. Post hoc analyses showed that starting at week 15, the 41% lard group had gained significantly more weight compared with the 10% fish-oil group (P < 0.05); at week 23, the 41% lard group had gained significantly more weight compared with the 10% lard group (P < 0.05); at week 29, the 41% fish-oil group had gained more weight compared with the 10% fish-oil group (P < 0.05); and at week 30, the 41% fish-oil group had gained more weight compared with the 41% lard group (P < 0.05).
FIGURE 1.
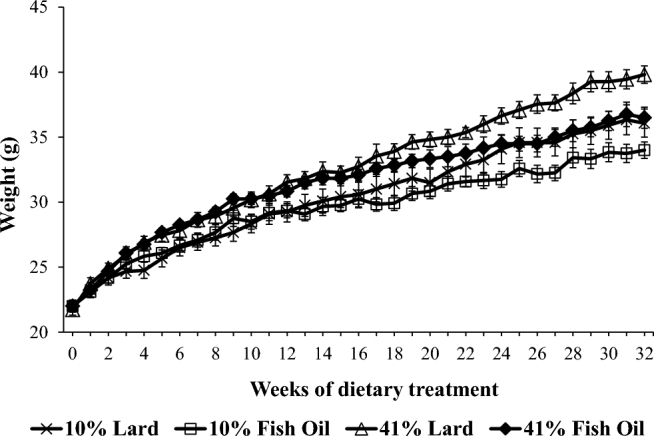
Body-weight change in C57/Bl6 mice respective to dietary treatment group over 32 wk of diet treatment. Data were modeled by using a 2-level linear mixed model. Type III tests for fixed effects: time in weeks (df = 1, F = 6780.70, P < 0.0001), diet (df = 3, F = 0.86, P = 0.4633), interaction of diet with time (df = 3, F = 60.93, P < 0.0001). Values are means ± SEMs.
When looking at how the percentage of fat, type of fat, and their interaction affected the total weight gain across the study, only the type of fat and percentage of fat significantly affected total weight gain (Table 2). Mice fed fish-oil diets gained less total weight than those fed the lard diets (mean weight gain—fish oil: 13.25 g; lard: 16.00 g; P = 0.0133). Mice fed the 41% fat diet gained more weight per week than those fed the 10% fat diets (41% fat: 16.22 g; 10% fat: 13.04 g; P = 0.005).
TABLE 2.
Weight, energy intake, and plasma chemistry evaluations at 32 wk of dietary treatment1
| P | |||||||
|---|---|---|---|---|---|---|---|
| 10% Lard | 10% Fish oil | 41% Lard | 41% Fish oil | Type of fat | Percentage of fat | Interaction | |
| Final weight, g | 36.1 ± 1.1 [12] | 34.0 ± 0.6 [12] | 39.8 ± 2.0 [11] | 36.5 ± 0.8 [12] | 0.0314¥ | 0.0138¥ | 0.6136 |
| Weight gain,2 g | 14.1 ± 0.9 [12] | 12.0 ± 0.5 [12] | 18.1 ± 1.9 [11] | 14.5 ± 0.8 [12] | 0.0133¥ | 0.005¥ | 0.4963 |
| Energy intake,3 kcal ⋅ body weight−1 ⋅ wk−1 | 70.0 ± 1.9 [8] | 70.8 ± 2.1 [8] | 76.1 ± 2.2 [8] | 78.2 ± 3.4 [8] | 0.5491 | 0.0109¥ | 0.7866 |
| GTT AUC, mg/dL | 6928 ± 730 [12] | 5555 ± 729 [12] | 9277 ± 1058 [11] | 5985 ± 921 [11] | 0.0098¥ | 0.1146 | 0.2721 |
| Total cholesterol, mg/dL | 107 ± 3.3 [12]†¤‡ | 74.6 ± 3.3 [12]*¤ | 152 ± 16.0 [11]*†‡ | 70.3 ± 3.9 [12]*¤ | <0.0001¥ | 0.0167¥ | 0.0042¥ |
| TGs, mg/dL | 48.4 ± 2.8 [12] | 47.8 ± 4.6 [12] | 41.8 ± 2.5 [11] | 47.5 ± 6.6 [12] | 0.5777 | 0.4524 | 0.4831 |
| HDL cholesterol, mg/dL | 54.8 ± 5.1 [12] | 43.8 ± 3.9 [12] | 72.5 ± 9.6 [11] | 40.3 ± 3.3 [12] | 0.0006¥ | 0.2309 | 0.0748 |
| LDL cholesterol, mg/dL | 42.5 ± 5.4 [12]¤ | 26.6 ± 3.2 [9] ¤ | 77.0 ± 11.0 [10]*†‡ | 23.3 ± 3.4 [10]¤ | <0.0001¥ | 0.0239¥ | 0.0071¥ |
| HDL cholesterol:TC | 0.51 ± 0.04 [12] | 0.59 ± 0.04 [12] | 0.49 ± 0.51 [11] | 0.58 ± 0.04 [12] | 0.0692 | 0.7098 | 0.8347 |
| HDL cholesterol:LDL cholesterol | 1.6 ± 0.3 [12] | 1.7 ± 0.3 [9] | 1.1 ± 0.2 [10] | 1.9 ± 0.3 [10] | 0.0855 | 0.5269 | 0.1292 |
| Glucose, mg/dL | 135 ± 7.7 [12]¤ | 138 ± 10.2 [12]¤ | 208 ± 7.4 [11]*†‡ | 151 ± 18.0 [12]¤ | 0.0259¥ | 0.0008¥ | 0.0161¥ |
| Insulin, mU/L | 14.7 ± 1.8 [12] | 13.0 ± 1.5 [12] | 40.3 ± 10.8 [11] | 16.8 ± 3.9 [12] | 0.0283¥ | 0.0116¥ | 0.0571 |
| HOMA-IR | 4.9 ± 0.6 [12]¤ | 4.3 ± 0.5 [12]¤ | 21.0 ± 6.0 [11]*†‡ | 6.2 ± 1.3 [12]¤ | 0.0120¥ | 0.0035¥ | 0.0188¥ |
Values are means ± SEMs; n in brackets. Two-factor ANOVA was used to identify the difference between type, percentage, and interaction (¥P < 0.05), with Tukey-Kramer post hoc test if significant. *Different from 10% lard, P < 0.05; †different from 10% fish oil, P < 0.05; ¤different from 41% lard, P < 0.05; ‡different from 41% fish oil, P < 0.05.
Weight gain determined from initial diet start-day and final harvest-day weights.
Energy intake extrapolated from number of mice/cage.
When examining how the percentage of fat, type of fat, and their interaction affected mean weekly caloric intake, only the percentage of fat affected mean weekly energy intake (Table 2). Mice fed 41% fat had a higher weekly energy intake compared with those fed 10% fat (41% fat: 77.16 g, 10% fat: 70.43 g; P = 0.0109).
Plasma chemistry
Significant interactions between the type and percentage of fat were found on mean TC and plasma LDL-cholesterol concentrations (Table 2). As the percentage of fat increased in the fish-oil diets, the mean TC and LDL cholesterol did not change. As the percentage of fat increased in lard-based diets, the mean TC as well as the mean LDL cholesterol increased, with TC and LDL-cholesterol concentrations being significantly higher in the 41% lard group compared with the 10% lard group (P = 0.0021 and 0.0027, respectively). No significant differences were found in mean TGs with the type of fat, the percentage of fat, or their interaction (Table 2).
Mean total plasma HDL-cholesterol concentrations were found to differ only with the type of fat (Table 2). Mice fed lard diets had higher HDL-cholesterol concentrations than those fed fish oil (fish oil: 42.06 mg/dL; lard: 63.26 mg/dL; P = 0.0006).
When looking at how the percentage of fat, type of fat, and their interaction affected the AUCs respective to the GTT, only the type of fat affected this measurement (Table 2). Mice fed lard-based diets had a higher AUC than those fed fish oil (fish oil: 5769.81 mg/dL; lard: 8051.36 mg/dL; P = 0.0098).
Significant interactions were found between the type of fat and percentage of fat on mean feed-deprived plasma glucose concentrations and HOMA-IR. As the percentage of fat increased in mice fed fish-oil diets, mean glucose concentrations and HOMA-IR were similar. As the percentage of fat increased in mice fed lard diets, mean glucose concentrations and HOMA-IR increased and their mean values were significantly higher for those fed 41% lard than for those fed 10% lard (P = 0.0006 and 0.0020, respectively).
Mean fasting plasma insulin concentrations differed only with the type and percentage of fat (Table 2). With respect to type of fat, mice fed lard had higher concentrations of insulin than did those fed fish oil (fish oil: 14.89 mU/L; lard: 26.95 mU/L; P = 0.0283). Mice fed 41% fat had higher insulin concentrations than did those fed 10% fat (10% fat: 13.87 mU/L; 41% fat: 28.02 mU/L; P = 0.0116).
mRNA expression
Mean cortical Mfsd2a mRNA expression only differed with the type of fat (Table 3). A lower mean ΔΔCt for Mfsd2a was found for mice fed fish oil compared with those fed lard (fish oil: –0.22; lard: 0.39; P = 0.0384), translating to a higher fold Mfsd2a mRNA expression in those fed fish oil compared with those fed lard [2(–ΔΔCt) fish oil: 1.16; lard: 0.76].
TABLE 3.
Cortical brain tissue mRNA expression at the end of the 32-wk diet treatment1
| P | |||||||
|---|---|---|---|---|---|---|---|
| Gene | 10% Lard | 10% Fish oil | 41% Lard | 41% Fish oil | Type of fat | Percentage of fat | Interaction |
| Mfsd2a | |||||||
| ΔΔCt | 0.000 ± 0.215 | –0.352 ± 0.282 | 0.790 ± 0.265 | –0.086 ± 0.352 | 0.0384¥ | 0.0729 | 0.3671 |
| 2(–ΔΔCt) | 1.000 (0.718, 1.393) | 1.276 (0.830, 1.962) | 0.579 (0.384, 0.871) | 1.061 (0.620, 1.816) | |||
| Glut1 | |||||||
| ΔΔCt | 0.000 ± 0.172 | 0.159 ± 0.193 | 0.150 ± 0.171 | –0.103 ± 0.249 | 0.8166 | 0.7829 | 0.3136 |
| 2(–ΔΔCt) | 1.000 (0.766, 1.305) | 0.896 (0.667, 1.203) | 0.901 (0.692, 1.174) | 1.074 (0.735, 1.570) | |||
| Ppar-α | |||||||
| ΔΔCt | 0.000 ± 0.095 | 0.058 ± 0.190 | 0.295 ± 0.201 | –0.208 ± 0.291 | 0.2777 | 0.9433 | 0.1730 |
| 2(–ΔΔCt) | 1.000 (0.865, 1.156) | 0.961 (0.718, 1.285) | 0.815 (0.597, 1.112) | 1.150 (0.737, 1.502) | |||
| Ppar-γ | |||||||
| ΔΔCt | 0.000 ± 0.113 | –0.325 ± 0.047 | –0.323 ± 0.079 | –0.334 ± 0.054 | 0.0388¥ | 0.0419¥ | 0.0533 |
| 2(–ΔΔCt) | 1.000 (0.842, 1.188) | 1.253 (1.164, 1.348) | 1.250 (1.106, 1.414) | 1.260 (1.161, 1.368) | |||
| Insr | |||||||
| ΔΔCt | 0.000 ± 0.120 | 0.221 ± 0.153 | 0.088 ± 0.169 | –0.268 ± 0.193 | 0.6753 | 0.2202 | 0.0804 |
| 2(–ΔΔCt) | 1.000 (0.831, 1.204) | 0.858 (0.694, 1.084) | 0.941 (0.724, 1.222) | 1.205 (0.893, 1.624) | |||
| Lrp1 | |||||||
| ΔΔCt | 0.000 ± 0.151 | 0.149 ± 0.157 | 0.087 ± 0.198 | –0.218 ± 0.215 | 0.6737 | 0.4471 | 0.2215 |
| 2(–ΔΔCt) | 1.000 (0.793, 1.262) | 0.902 (0.709, 1.146) | 0.942 (0.694, 1.278) | 1.163 (0.838, 1.614) | |||
| Abca1 | |||||||
| ΔΔCt | 0.000 ± 0.114 | 0.219 ± 0.155 | 0.375 ± 0.173 | –0.032 ± 0.220 | 0.5818 | 0.7163 | 0.0717 |
| 2(–ΔΔCt) | 1.000 (0.839, 1.192) | 0.859 (0.678, 1.089) | 0.771 (0.590, 1.008) | 1.022 (0.728, 1.436) | |||
| Cd36 | |||||||
| ΔΔCt | 0.000 ± 0.138 | 0.139 ± 0.220 | 0.181 ± 0.255 | –0.122 ± 0.226 | 0.7065 | 0.8527 | 0.3104 |
| 2(–ΔΔCt) | 1.000 (0.808, 1.238) | 0.908 (0.649, 1.270) | 0.882 (0.595, 1.308) | 1.088 (0.771, 1.535) | |||
| Apoe | |||||||
| ΔΔCt | 0.000 ± 0.129 | 0.254 ± 0.147 | 0.382 ± 0.177 | 0.107 ± 0.199 | P = 0.9510 | P = 0.4845 | P = 0.1193 |
| 2(–ΔΔCt) | 1.000 (0.819, 1.221) | 0.838 (0.670, 1.049) | 0.767 (0.584, 1.008) | 0.928 (0.685, 1.259) | |||
| App | |||||||
| ΔΔCt | 0.000 ± 0.160 | 0.385 ± 0.141 | 0.392 ± 0.197 | –0.065 ± 0.145 | 0.8253 | 0.8564 | 0.0127¥ |
| 2(–ΔΔCt) | 1.000 (0.783, 1.277) | 0.766 (0.617, 0.950) | 0.762 (0.562, 1.034) | 1.046 (0.836, 1.310) | |||
| Bdnf | |||||||
| ΔΔCt | 0.000 ± 0.303 | 0.095 ± 0.348 | –0.158 ± 0.254 | –0.451 ± 0.229 | 0.7354 | 0.2333 | 0.5095 |
| 2(–ΔΔCt) | 1.000 (0.630, 1.587) | 0.936 (0.551, 1.592) | 1.116 (0.755, 1.651) | 1.367 (0.959, 1.949) | |||
| Sod1 | |||||||
| ΔΔCt | 0.000 ± 0.138 | 0.240 ± 0.162 | 0.178 ± 0.196 | –0.019 ± 0.209 | 0.9058 | 0.8229 | 0.2315 |
| 2(–ΔΔCt) | 1.000 (0.808, 1.238) | 0.847 (0.662, 1.084) | 0.884 (0.653, 1.197) | 1.013 (0.737, 1.394) | |||
| Sod2 | |||||||
| ΔΔCt | 0.000 ± 0.076 | 0.094 ± 0.064 | 0.070 ± 0.062 | 0.008 ± 0.076 | 0.8215 | 0.9115 | 0.2780 |
| 2(–ΔΔCt) | 1.000 (0.900, 1.124) | 0.937 (0.849, 1.034) | 0.953 (0.866, 1.048) | 0.994 (0.885, 1.117) | |||
| Cat | |||||||
| ΔΔCt | 0.000 ± 0.074 | 0.098 ± 0.076 | 0.117 ± 0.072 | –0.007 ± 0.095 | 0.8683 | 0.9387 | 0.1761 |
| 2(–ΔΔCt) | 1.000 (0.891, 1.122) | 0.937 (0.831, 1.052) | 0.922 (0.825, 1.030) | 1.005 (0.870, 1.161) | |||
Values are means ± SEMs or fold differences relative to 10% lard (95% CIs). Two-factor ANOVA was used to identify the differences between type, percentage, and interaction (¥P < 0.05), with Tukey-Kramer post hoc conducted if significant. Abca1, ATP-binding cassette transporter 1; Apoe, apolipoprotein E; App, amyloid precursor protein; Bdnf, brain-derived neurotrophic factor; Cat, catalase; Cd36, cluster of differentiation 36/FA translocase; Insr, insulin receptor; Glut1, glucose transporter 1; Lrp1, LDL receptor–related protein 1; Mfsd2a, major facilitator super family domain-containing 2a; Sod1, superoxide dismutase 1; Sod2, superoxide dismutase 2; ΔΔCt, change relative to 10% Lard, with Gadph as reference gene.
Mean subcortical Mfsd2a mRNA expression levels only differed with percentage of fat (Table 4). Mice fed the 41% fat diet had a higher mean ΔΔCt for Mfsd2a compared with those fed the 10% fat diets (41% fat: 0.47; 10% fat: 0.02; P = 0.0454), translating to a lower fold Mfsd2a mRNA expression in the 41% fat group compared with the 10% fat group [2(–ΔΔCt) 41% fat: 0.72; 10% fat: 0.99].
TABLE 4.
Subcortical brain tissue mRNA expression at the end of the 32-wk diet treatment1
| P | |||||||
|---|---|---|---|---|---|---|---|
| Gene | 10% Lard | 10% Fish oil | 41% Lard | 41% Fish oil | Type of fat | Percentage of fat | Interaction |
| Mfsd2a | |||||||
| ΔΔCt | 0.000 ± 0.235 | 0.030 ± 0.205 | 0.792 ± 0.284 | 0.167 ± 0.172 | 0.1936 | 0.0454¥ | 0.1535 |
| 2(–ΔΔCt) | 1.000 (0.695, 1.439) | 0.896 (0.716, 1.340) | 0.578 (0.323, 0.901) | 0.891 (0.685, 1.159) | |||
| Glut1 | |||||||
| ΔΔCt | 0.000 ± 0.179 | –0.307 ± 0.145 | –0.040 ± 0.167 | –0.063 ± 0.147 | 0.3095 | 0.5280 | 0.3802 |
| 2(–ΔΔCt) | 1.000 (0.761, 1.314) | 1.238 (0.992, 1.544) | 1.028 (0.791, 1.336) | 1.045 (0.835, 1.308) | |||
| Pparα | |||||||
| ΔΔCt | 0.000 ± 0.194 | –0.564 ± 0.206 | –0.089 ± 0.259 | –0.261 ± 0.113 | 0.0687 | 0.5906 | 0.3271 |
| 2(–ΔΔCt) | 1.000 (0.744, 1.344) | 1.478 (1.079, 2.024) | 1.063 (0.713, 1.587) | 1.199 (1.009, 1.423) | |||
| Pparγ | |||||||
| ΔΔCt | 0.000 ± 0.080 | –0.004 ± 0.061 | –0.184 ± 0.077 | –0.074 ± 0.071 | 0.4668 | 0.0875 | 0.4372 |
| 2(–ΔΔCt) | 1.000 (0.880, 1.137) | 1.003 (0.914, 1.100) | 1.136 (1.008, 1.280) | 1.052 (0.944, 1.173) | |||
| Insr | |||||||
| ΔΔCt | 0.000 ± 0.133 | –0.206 ± 0.128 | –0.228 ± 0.170 | –0.148 ± 0.125 | 0.6557 | 0.5493 | 0.3129 |
| 2(–ΔΔCt) | 1.000 (0.811, 1.232) | 1.154 (0.948, 1.404) | 1.171 (0.901, 1.523) | 1.108 (0.915, 1.341) | |||
| Lrp1 | |||||||
| ΔΔCt | 0.000 ± 0.163 | –0.080 ± 0.127 | –0.177 ± 0.185 | –0.039 ± 0.149 | 0.8508 | 0.6654 | 0.4891 |
| 2(–ΔΔCt) | 1.000 (0.774, 1.292) | 1.057 (0.870, 1.283) | 1.131 (0.850, 1.505) | 1.027 (0.818, 1.290) | |||
| Abca1 | |||||||
| ΔΔCt | 0.000 ± 0.135 | –0.132 ± 0.085 | 0.143 ± 0.091 | 0.150 ± 0.119 | 0.5678 | 0.0560 | 0.5201 |
| 2(–ΔΔCt) | 1.000 (0.809, 1.237) | 1.096 (0.963, 1.248) | 0.906 (0.787, 1.042) | 0.901 (0.752, 1.080) | |||
| Cd36 | |||||||
| ΔΔCt | 0.000 ± 0.205 | –0.025 ± 0.128 | 0.009 ± 0.166 | 0.086 ± 0.172 | 0.8766 | 0.7234 | 0.7614 |
| 2(–ΔΔCt) | 1.000 (0.729, 1.372) | 1.018 (0.837, 1.237) | 0.994 (0.770, 1.284) | 0.942 (0.724, 1.225) | |||
| Apoe | |||||||
| ΔΔCt | 0.000 ± 0.106 | –0.039 ± 0.079 | –0.081 ± 0.102 | 0.154 ± 0.103 | 0.3232 | 0.5702 | 0.1687 |
| 2(–ΔΔCt) | 1.000 (0.847, 1.181) | 1.028 (0.911, 1.159) | 1.058 (0.903, 1.239) | 0.899 (0.769, 1.052) | |||
| App | |||||||
| ΔΔCt | 0.000 ± 0.088 | 0.142 ± 0.055 | 0.059 ± 0.094 | 0.193 ± 0.069 | 0.0807 | 0.4773 | 0.9568 |
| 2(–ΔΔCt) | 1.000 (0.873, 1.146) | 0.906 (0.833, 0.986) | 0.960 (0.830, 1.109) | 0.875 (0.788, 0.971) | |||
| Bdnf | |||||||
| ΔΔCt | 0.000 ± 0.216 | –0.362 ± 0.186 | –0.220 ± 0.274 | 0.144 ± 0.206 | 0.9974 | 0.5199 | 0.1080 |
| 2(–ΔΔCt) | 1.000 (0.716, 1.397) | 1.286 (0.969, 1.707) | 1.165 (0.762, 1.779) | 0.905 (0.661, 1.239) | |||
| Sod1 | |||||||
| ΔΔCt | 0.000 ± 0.166 | 0.021 ± 0.100 | –0.003 ± 0.174 | 0.161 ± 0.123 | 0.5189 | 0.6297 | 0.6163 |
| 2(–ΔΔCt) | 1.000 (0.773, 1.293) | 0.986 (0.846, 1.148) | 1.002 (0.765, 1.312) | 0.894 (0.741, 1.079) | |||
| Sod2 | |||||||
| ΔΔCt | 0.000 ± 0.048 | 0.060 ± 0.068 | –0.037 ± 0.054 | –0.048 ± 0.077 | 0.7103 | 0.2692 | 0.5878 |
| 2(–ΔΔCt) | 1.000 (0.927, 1.079) | 0.959 (0.865, 1.064) | 1.026 (0.944, 1.116) | 1.034 (0.919, 1.164) | |||
| Cat | |||||||
| ΔΔCt | 0.000 ± 0.051 | 0.047 ± 0.039 | –0.020 ± 0.048 | –0.038 ± 0.055 | 0.7733 | 0.2870 | 0.5078 |
| 2(–ΔΔCt) | 1.000 (0.891, 1.084) | 0.968 (0.912, 1.028) | 1.014 (0.941, 1.093) | 1.027 (0.942, 1.119) | |||
Values are means ± SEMs or fold differences relative to 10% lard (95% CIs). Two-factor ANOVA was used to identify the differences between type, percentage, and interaction (¥P < 0.05), with Tukey-Kramer post hoc conducted if significant. Abca1, ATP-binding cassette transporter 1; Apoe, apolipoprotein E; App, amyloid precursor protein; Bdnf, brain-derived neurotrophic factor; Cat, catalase; Cd36, cluster of differentiation 36/FA translocase; Insr, insulin receptor; Lrp1, LDL receptor–related protein 1; Mfsd2a, major facilitator super family domain-containing 2a; Pparα, peroxisome proliferator-activated receptor α; Pparγ, peroxisome proliferator-activated receptor γ; Glut1, glucose transporter 1; Sod1, superoxide dismutase 1; Sod2, superoxide dismutase 2; ΔΔCt, change relative to 10% Lard, with Gadph as reference gene.
Mean cortical Ppar-γ mRNA levels differed with type and percentage of fat (Table 3). Mice fed fish oil had a lower mean ΔΔCt for Ppar-γ than did those fed lard (fish oil: –0.33, lard: –0.15; P = 0.0388), translating to a higher fold Ppar-γ mRNA expression in those fed fish oil compared with those fed lard [2(–ΔΔCt) fish oil: 1.26; lard: 1.11]. Mice fed 41% fat had a lower ΔΔCt for Ppar-γ compared with those fed 10% fat (41% fat: –0.33; 10% fat: –0.16; P = 0.0419), translating to higher-fold Ppar-γ mRNA expression in the 41% fat compared with the 10% fat group [2(–ΔΔCt) 41% fat: 1.26; 10% fat: 1.11]. An interaction between percentage and type of fat was found with the cortical App mRNA levels (P = 0.0127). As the percentage of fat increased in mice fed fish oil, there was a trend toward a lower ΔΔCt and higher fold expression of App. In mice fed lard-based diets, as the percentage of fat increased, there was a trend toward a higher ΔΔCt, and lower fold expression of App. However, post hoc tests did not identify any significant differences between groups. No other significant differences were found in mRNA expression of other genes in either the cortical or subcortical tissues.
Protein expression
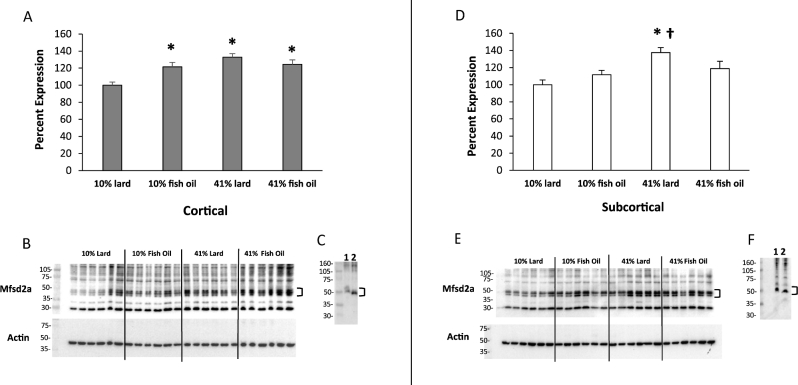
Significant interactions between the type and percentage of fat were found for both cortical (Figure 2A, B) and subcortical (Figure 2D, E) Mfsd2a protein expression (P = 0.0024 and 0.0185, respectively). As the percentage of fat increased in the fish-oil diets, cortical and subcortical Mfsd2a expression levels were similar. As the percentage of fat increased in the lard diets, cortical and subcortical Mfsd2a expression increased and was higher in the 41% lard compared with the 10% lard group (P < 0.0001 and P = 0.0007, respectively). Cortical Mfsd2a expression was significantly higher in both the 10% fish-oil (P = 0.0091) and 41% fish-oil (P = 0.0023) groups compared with the 10% lard group. There was also a significant positive correlation between cortical Ppar-γ mRNA expression and Mfsd2a protein expression (r = 0.39, P = 0.0076). Subcortical Mfsd2a expression was also significantly higher in the 41% lard group compared with the 10% fish-oil group (P = 0.0293). A downward band shift with the deglycosylation assay identified that Mfsd2a was N-glycosylated (Figure 2C, F).
FIGURE 2.
Western blot analysis bar charts show differences in Mfsd2a (∼50 kDa) protein expression in cortical (A) and subcortical (D) brain tissue at the end of the 32-wk diet treatment. Representative blots of cortical (B) and subcortical (E) brain tissue with actin reprobes are shown. Deglycosylation evaluation blots of cortical (C) and subcortical (F) brain tissue show nondeglycosylated control (1) and deglycosylated (2) samples (41% lard). Values are means ± SEMs, n = 11–12/group. Two-factor ANOVA with Tukey-Kramer post hoc test was conducted. *Different from 10% lard, P < 0.05; †Different from 10% fish oil, P < 0.05. Mfsd2a, major facilitator super family domain containing 2a.
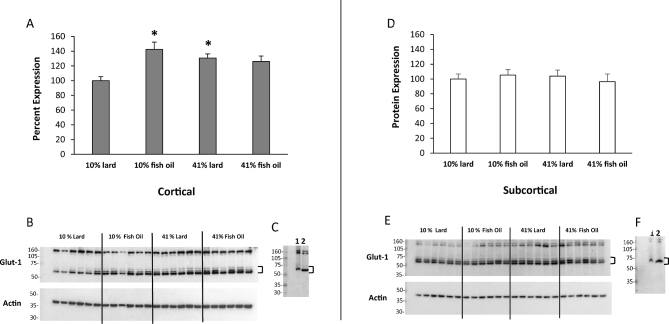
For mean cortical Glut1 protein expression, a significant interaction was found between the percentage and type of fat (P = 0.0025; Figure 3A, B). As the percentage of fat increased in the fish-oil diets, cortical Glut-1 expression levels were similar. As the percentage of fat increased in the lard diets, cortical Glut1 expression increased and was higher in the 41% lard group than in the 10% lard group (P = 0.0272). When compared with 10% lard, cortical Glut1 expression was significantly higher in the 10% fish-oil group (P = 0.0009) and trended lower in the 41% fish-oil group (P = 0.0690). Subcortical Glut1 protein expression did not significantly differ with type or percentage of fat, or their interaction (Figure 3D, E). A downward band shift with the deglycosylation assay suggested Glut1 was N-glycosylated (Figure 3C, F).
FIGURE 3.
Western blot analysis bar charts show differences in Glut1 (∼55 kDa) protein expression in cortical (A) and subcortical (D) brain tissue at the end of the 32-wk diet treatment. Representative blots of cortical (B) and subcortical (E) brain tissue with actin reprobes are shown. Deglycosylation evaluation blots of cortical (C) and subcortical (F) brain tissue show nondeglycosylated control (1) and deglycosylated (2) samples (41% lard). Values are means ± SEMs, n = 11–12/group. Two-factor ANOVA with Tukey-Kramer post hoc test was conducted when interactions were significant. *Different from 10% lard, P < 0.05. Glut-1, glucose-1 transporter Slc2a1.
Discussion
In this study, the impacts of long-term low- and high-fat diets with lard or fish oil were examined. In response to the diets, body weight and plasma chemistries were altered in a manner generally consistent with rodent and human studies (34). Mice fed fish-oil diets showed less weight gain with time and had less total weight gain than did those fed the lard diets, even as the fat percentage increased in the fish-oil diet. When looking at the impact of diet on blood plasma lipids, the 10% and 41% fish-oil diets were protective against increases in TC and LDL cholesterol. Furthermore, mice fed fish-oil diets had lower TC compared with those fed lard diets. In contrast, TC and LDL cholesterol increased as the percentage of lard increased. Lard diets also significantly increased HDL cholesterol compared with the fish-oil diets. Despite the changes in HDL and LDL cholesterol with the type and percentage of fat, the HDL-to-LDL ratios were similar, indicating that they offset each other.
TG concentrations were not altered by the type or percentage of fat, albeit none of the groups showed signs of hypertriglyceridemia at 32 wk of treatment. Although ω-3 PUFA supplementation is well established in reducing TGs in humans, such studies heavily focused on the treatment of highly dyslipidemic populations (35, 36).
Blood glucose and HOMA-IR increased in mice fed lard as the percentage of fat increased, while remaining similar in those fed fish oil. A similar trend was observed for GTT AUCs and insulin measurements. Taken together, these findings support the protective role of fish-oil–based diets on blood glucose control, TC, and LDL cholesterol. This identifies that the type of fat over the percentage of fat is a primary mitigating factor in the protective action on blood chemistries within the range of fat assessed in this investigation.
The impacts of the type and percentage of fat on cortical and subcortical mRNA and protein expression of Mfsd2a and Glut1 were examined. The fish-oil diets increased cortical Mfsd2a mRNA expression compared with the lard diets. In contrast, subcortical Mfsd2a mRNA expression was unaffected by the type of fat, but was only affected by the percentage of fat. Subcortical Mfsd2a mRNA expression decreased as the percentage of fat in the diet increased. Respective to protein expression, an interaction between the type and percentage of fat was found for Mfsd2a in cortical and subcortical tissue. Both fish-oil and the high-fat lard diets increased cortical Mfsd2a protein expression compared with the low-fat lard group. The high-fat lard diet lacking EPA and DHA may have increased cortical Mfsd2a protein expression due to higher concentrations of other FAs. Although Mfsd2a is best known as a transporter of DHA, Mfsd2a transports other phospholipids attached to LPC, including oleate and palmitate (5), which were proportionally higher in the high-fat lard diet. The increased subcortical Mfsd2a protein expression in the high-fat lard diet group further supports this assertion. Thus, Mfsd2a protein expression appears to be regulated as a function of use, regardless of the specific molecule transported, in a positive feedback manner.
Last, the data also suggest that the increased Mfsd2a protein expression is a result of post-translational modification. Mfsd2a has been noted to be N-glycosylated (37), with glycosylation associated with increased transport activity. Differences between the cortical and subcortical regions may be accounted for by the different cellular composition and nutrient transport requirements. Cortical tissue is uniformly gray matter, whereas the subcortical tissue has a high white-matter composition. Although gray matter is primarily neurons and ∼40% lipids, white matter includes myelin and comprises 50–70% lipids (38). Critically, membrane-rich neurons, associated with gray matter, have increased affinity for DHA (39). This also corresponds to energy usage, because cerebral DHA concentrations are highest in high-energy glucose–utilizing brain tissue (40).
No mRNA changes in cortical or subcortical Glut1 mRNA were found with the type or percentage of fat. Unlike Mfsd2a, Glut1 mRNA expression cannot be characterized solely by the endothelial component of the brain. This may explain differences between mRNA and protein expression experiments. An interaction between the type and percentage of fat was found with cortical protein expression of the endothelium-affiliated 55-kDa isoform of Glut1 (19, 20). Although cortical Glut1 protein expression increased with the percentage of fat in mice fed lard, it remained similar as the percentage of fat increased in mice fed fish oil. However, protein expression in the 10% fish-oil group was significantly higher compared with that in the 10% lard group. Similar to Mfsd2a protein expression, the 41% lard group showed increased cortical Glut1 protein expression compared with the 10% lard group, identifying a further association between Glut1 and Mfsd2a with respect to the regulation at the BBB. The lack of observable impact on subcortical Glut1 protein expression may again be attributed to the compositional differences in gray and white matter.
Other studies have also identified FA modulation of Glut1 protein density and glucose transport at the BBB endothelium. Rats fed an ω-3 PUFA–deficient diet showed decreased cortical glucose utilization and expression of the 55-kDa isoform of Glut1 compared with those fed an ω-3 PUFA–adequate diet (25, 27). In rats fed a high ω-3 PUFA diet, expression of the 55-kDa isoform of Glut1 increased by 35% compared with rats fed the ω-3 PUFA–adequate diet (25). Correspondingly, when DHA was added to the culture medium in rat brain endothelial cells initially depleted of DHA, glucose transport activity and Glut1 density increased (25, 26). The previous research also showed no change in the Glut1 mRNA expression. The authors hypothesized the increased Glut1 protein expression was through post-translational events downstream of Ppar-γ activation (27). Our evaluations would support this hypothesis, with increased cortical Glut1 protein expression paralleling the increased Ppar-γ mRNA expression.
Last, Glut1 likewise utilizes N-glycosylation to regulate transporter activity (41), and our evaluations support Glut1 posttranslational modifications through N-glycosylation. Given that Glut1 and Mfsd2a are colocalized at the BBB and members of the same major facilitator superfamily (8), both may be regulated in a similar cascade of events corresponding to Ppar-γ activation with subsequent post-translational changes via N-glycosylation. Interestingly, PPAR-α has been proposed to regulate MFSD2A protein expression in the peripheral tissues (42); and although the Berger et al. (42) evaluation did not evaluate PPAR-γ, there is significant crossover in their downstream activity within the PPAR family (43). Although no changes in cortical or subcortical mRNA Ppar-α expression were found in our evaluation, changes in cortical Ppar-γ mRNA expression also paralleled increases in cortical Mfsd2a protein expression. Together, this supports the premise that ω-3 PUFA activation of Ppar-γ may regulate Mfsd2a protein expression. However, although Mfsd2a is isolated to the BBB, Ppar-γ is present throughout the brain (37), and thus further evaluation of the relation between Ppar-γ and Mfsd2a in response to ω-3 PUFA treatment in isolated brain microvascular endothelial cells would be required to support this assertion.
This study showed an interaction between the type and percentage of fat on cortical expression of Mfsd2a and Glut1, with protein expression increases in the cortical tissue that correspond to an increase in Ppar-γ mRNA expression. We hypothesize that PUFA-rich diets activate Ppar-γ, resulting in an increased posttranslational N-glycosylation of Mfsd2a and Glut1. Several qualifiers and considerations exist with our study. Mfsd2a and Glut1 (55-kDa band) protein expression data are associated with the BBB endothelial component (9, 20) and most likely reflect BBB expression. Ppar-γ mRNA expression changes are affiliated with whole tissue, and thus further evaluation in isolated BBB endothelium or in vitro models are required to fully clarify the interaction. Likewise, precise brain sections were not delineated (e.g., hippocampus), such that regionally specific changes in mRNA expression were not evaluated. In addition, changes in the function, activity, or both of the transporters could also result from variation in the physical properties and membrane structure of the endothelium subsequent to the incorporation of FAs in membrane phospholipids (26, 44). No significant changes in mRNA expression of targets affiliated with lipid protein regulation (Apoe, Lrp1, Abca1, Cd36), oxidative stress (Sod1, Sod2, Cat), or Bdnf were shown in cortical or subcortical tissue, despite other studies showing diet-dependent effects (1, 2, 6, 7, 45). Further regional delineation may provide further understanding. It should also be noted that our dietary treatment did not result in overt pathology. Manifestations of dietary benefit or detriment may likely become more pronounced under pathological inflammatory conditions. Essentially, the effect of diet on the “resilience capacity” of BBB nutrient transporter regulation, as well as general integrity, may be best determined in conjunction with an acute pathological event. Acute inflammation induction subsequent to chronic dietary changes could provide a greater understanding of nutrient transport regulation. Alternatively, nutrient transporter alterations associated with diet respective to neurodegenerative disease would require assessment upon disease manifestation over an extended time course.
Supplementary Material
Acknowledgments
The authors’ responsibilities were as follows—KES, KAW and JSW: designed the research and had primary responsibility for the final content; KES, KAW, JSW, MPH, MLS, and DSU: conducted the research; KES, KAW, JSW, and DSU: analyzed the data; KES and KAW: wrote the manuscript; and all authors: read and approved the final manuscript.
Notes
Supported by the NIH, National Institute of Neurological Disorders and Stroke (grant R21NS090282).
Author disclosures: KES, JSW, MPH, MLS, DSU, and KAW, no conflicts of interest.
Supplemental Table 1 is available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/cdn/.
Abbreviations used:
- Abca1
ATP-binding cassette transporter 1
- App
amyloid precursor protein
- BBB
blood-brain barrier
- Cat
catalase
- Cd36
cluster of differentiation 36/FA translocase
- Glut1
glucose transporter 1
- GTT
glucose-tolerance test
- LPC
lysophosphatidylcholine
- Mfsd2a
major facilitator super family domain-containing 2a
- Sod
superoxide dismutase
- TC
total cholesterol
References
- 1. Hsu TM, Kanoski SE. Blood-brain barrier disruption: mechanistic links between Western diet consumption and dementia. Front Aging Neurosci 2014;6:88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Sellbom KS, Gunstad J. Cognitive function and decline in obesity. J Alzheimers Dis 2012;30(Suppl 2):S89–95. [DOI] [PubMed] [Google Scholar]
- 3. Lin B, Hasegawa Y, Takane K, Koibuchi N, Cao C, Kim-Mitsuyama S. High-fat-diet intake enhances cerebral amyloid angiopathy and cognitive impairment in a mouse model of Alzheimer's disease, independently of metabolic disorders. J Am Heart Assoc 2016;5(6):1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hooijmans CR, Rutters F, Dederen PJ, Gambarota G, Veltien A, van Groen T, Broersen LM, Lutjohann D, Heerschap A, Tanila H, et al. Changes in cerebral blood volume and amyloid pathology in aged Alzheimer APP/PS1 mice on a docosahexaenoic acid (DHA) diet or cholesterol enriched typical Western diet (TWD). Neurobiol Dis 2007;28(1):16–29. [DOI] [PubMed] [Google Scholar]
- 5. Hargrave SL, Davidson TL, Zheng W, Kinzig KP. Western diets induce blood-brain barrier leakage and alter spatial strategies in rats. Behav Neurosci 2016;130(1):123–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Janssen CI, Kiliaan AJ. Long-chain polyunsaturated fatty acids (LCPUFA) from genesis to senescence: the influence of LCPUFA on neural development, aging, and neurodegeneration. Prog Lipid Res 2014;53:1–17. [DOI] [PubMed] [Google Scholar]
- 7. Lo Van A, Sakayori N, Hachem M, Belkouch M, Picq M, Lagarde M, Osumi N, Bernoud-Hubac N. Mechanisms of DHA transport to the brain and potential therapy to neurodegenerative diseases. Biochimie 2016;130:163–7. [DOI] [PubMed] [Google Scholar]
- 8. Nguyen LN, Ma D, Shui G, Wong P, Cazenave-Gassiot A, Zhang X, Wenk MR, Goh EL, Silver DL. Mfsd2a is a transporter for the essential omega-3 fatty acid docosahexaenoic acid. Nature 2014;509(7501):503–6. [DOI] [PubMed] [Google Scholar]
- 9. Ben-Zvi A, Lacoste B, Kur E, Andreone BJ, Mayshar Y, Yan H, Gu C. Mfsd2a is critical for the formation and function of the blood-brain barrier. Nature 2014;509(7501):507–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Thies F, Pillon C, Moliere P, Lagarde M, Lecerf J. Preferential incorporation of sn-2 lysoPC DHA over unesterified DHA in the young rat brain. Am J Physiol 1994;267(5 Part 2):R1273–9. [DOI] [PubMed] [Google Scholar]
- 11. Lin PY, Huang SY, Su KP. A meta-analytic review of polyunsaturated fatty acid compositions in patients with depression. Biol Psychiatry 2010;68(2):140–7. [DOI] [PubMed] [Google Scholar]
- 12. Liu JJ, Green P, John Mann J, Rapoport SI, Sublette ME. Pathways of polyunsaturated fatty acid utilization: implications for brain function in neuropsychiatric health and disease. Brain Res 2015;1597:220–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cunnane SC, Schneider JA, Tangney C, Tremblay-Mercier J, Fortier M, Bennett DA, Morris MC. Plasma and brain fatty acid profiles in mild cognitive impairment and Alzheimer's disease. J Alzheimers Dis 2012;29(3):691–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Soderberg M, Edlund C, Kristensson K, Dallner G. Fatty acid composition of brain phospholipids in aging and in Alzheimer's disease. Lipids 1991;26(6):421–5. [DOI] [PubMed] [Google Scholar]
- 15. Pan Y, Khalil H, Nicolazzo JA. The impact of docosahexaenoic acid on Alzheimer's disease: is there a role of the blood-brain barrier? Curr Clin Pharmacol 2015;10(3):222–41. [DOI] [PubMed] [Google Scholar]
- 16. Andreone BJ, Chow BW, Tata A, Lacoste B, Ben-Zvi A, Bullock K, Deik AA, Ginty DD, Clish CB, Gu C. Blood-brain barrier permeability is regulated by lipid transport-dependent suppression of caveolae-mediated transcytosis. Neuron 2017;94(3):581–94, e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Yang YR, Xiong XY, Liu J, Wu LR, Zhong Q, Zhou K, Meng ZY, Liu L, Wang FX, Gong QW, et al. Mfsd2a (major facilitator superfamily domain containing 2a) attenuates intracerebral hemorrhage-induced blood-brain barrier disruption by inhibiting vesicular transcytosis. J Am Heart Assoc 2017;6(7):e005811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Mergenthaler P, Lindauer U, Dienel GA, Meisel A. Sugar for the brain: the role of glucose in physiological and pathological brain function. Trends Neurosci 2013;36(10):587–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Maher F, Vannucci SJ, Simpson IA. Glucose transporter isoforms in brain: absence of GLUT3 from the blood-brain barrier. J Cereb Blood Flow Metab 1993;13(2):342–5. [DOI] [PubMed] [Google Scholar]
- 20. Pardridge WM, Boado RJ, Farrell CR. Brain-type glucose transporter (GLUT-1) is selectively localized to the blood-brain barrier: studies with quantitative Western blotting and in situ hybridization. J Biol Chem 1990;265(29):18035–40. [PubMed] [Google Scholar]
- 21. Duelli R, Kuschinsky W. Brain glucose transporters: relationship to local energy demand. News Physiol Sci 2001;16:71–6. [DOI] [PubMed] [Google Scholar]
- 22. Pearson TS, Akman C, Hinton VJ, Engelstad K, De Vivo DC. Phenotypic spectrum of glucose transporter type 1 deficiency syndrome (Glut1 DS). Curr Neurol Neurosci Rep 2013;13(4):342. [DOI] [PubMed] [Google Scholar]
- 23. Shah K, Desilva S, Abbruscato T. The role of glucose transporters in brain disease: diabetes and Alzheimer's Disease. Int J Mol Sci 2012;13(10):12629–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Winkler EA, Nishida Y, Sagare AP, Rege SV, Bell RD, Perlmutter D, Sengillo JD, Hillman S, Kong P, Nelson AR, et al. GLUT1 reductions exacerbate Alzheimer's disease vasculo-neuronal dysfunction and degeneration. Nat Neurosci 2015;18(4):521–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Pifferi F, Jouin M, Alessandri JM, Haedke U, Roux F, Perriere N, Denis I, Lavialle M, Guesnet P. n-3 Fatty acids modulate brain glucose transport in endothelial cells of the blood-brain barrier. Prostaglandins Leukot Essent Fatty Acids 2007;77(5–6):279–86. [DOI] [PubMed] [Google Scholar]
- 26. Pifferi F, Jouin M, Alessandri JM, Roux F, Perriere N, Langelier B, Lavialle M, Cunnane S, Guesnet P. n-3 Long-chain fatty acids and regulation of glucose transport in two models of rat brain endothelial cells. Neurochem Int 2010;56(5):703–10. [DOI] [PubMed] [Google Scholar]
- 27. Pifferi F, Roux F, Langelier B, Alessandri JM, Vancassel S, Jouin M, Lavialle M, Guesnet P. (n-3) Polyunsaturated fatty acid deficiency reduces the expression of both isoforms of the brain glucose transporter GLUT1 in rats. J Nutr 2005;135(9):2241–6. [DOI] [PubMed] [Google Scholar]
- 28. Potteiger JA, Jacobsen DJ, Donnelly JE. A comparison of methods for analyzing glucose and insulin areas under the curve following nine months of exercise in overweight adults. Int J Obes Relat Metab Disord 2002;26(1):87–9. [DOI] [PubMed] [Google Scholar]
- 29. Wolever TM, Jenkins DJ. The use of the glycemic index in predicting the blood glucose response to mixed meals. Am J Clin Nutr 1986;43(1):167–72. [DOI] [PubMed] [Google Scholar]
- 30. Warnick GR, Albers JJ. A comprehensive evaluation of the heparin-manganese precipitation procedure for estimating high density lipoprotein cholesterol. J Lipid Res 1978;19(1):65–76. [PubMed] [Google Scholar]
- 31. Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem 1972;18(6):499–502. [PubMed] [Google Scholar]
- 32. Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985;28(7):412–9. [DOI] [PubMed] [Google Scholar]
- 33. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001;25(4):402–8. [DOI] [PubMed] [Google Scholar]
- 34. Martinez-Fernandez L, Laiglesia LM, Huerta AE, Martinez JA, Moreno-Aliaga MJ. Omega-3 fatty acids and adipose tissue function in obesity and metabolic syndrome. Prostaglandins Other Lipid Mediat 2015;121(Part A):24–41. [DOI] [PubMed] [Google Scholar]
- 35. Huang CW, Chien YS, Chen YJ, Ajuwon KM, Mersmann HM, Ding ST. Role of n-3 polyunsaturated fatty acids in ameliorating the obesity-induced metabolic syndrome in animal models and humans. Int J Mol Sci 2016;17(10):1–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Leslie MA, Cohen DJ, Liddle DM, Robinson LE, Ma DW. A review of the effect of omega-3 polyunsaturated fatty acids on blood triacylglycerol levels in normolipidemic and borderline hyperlipidemic individuals. Lipids Health Dis 2015;14:53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Villapol S. Roles of peroxisome proliferator-activated receptor gamma on brain and peripheral inflammation. Cell Mol Neurobiol 2018;38(1):121–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Bradbury J. Docosahexaenoic acid (DHA): an ancient nutrient for the modern human brain. Nutrients 2011;3(5):529–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Crawford MA, Bloom M, Broadhurst CL, Schmidt WF, Cunnane SC, Galli C, Gehbremeskel K, Linseisen F, Lloyd-Smith J, Parkington J. Evidence for the unique function of docosahexaenoic acid during the evolution of the modern hominid brain. Lipids 1999;34(Suppl):S39–47. [DOI] [PubMed] [Google Scholar]
- 40. Brenna JT, Diau GY. The influence of dietary docosahexaenoic acid and arachidonic acid on central nervous system polyunsaturated fatty acid composition. Prostaglandins Leukot Essent Fatty Acids 2007;77(5–6):247–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Asano T, Katagiri H, Takata K, Lin JL, Ishihara H, Inukai K, Tsukuda K, Kikuchi M, Hirano H, Yazaki Y et al. The role of N-glycosylation of GLUT1 for glucose transport activity. J Biol Chem 1991;266(36):24632–6. [PubMed] [Google Scholar]
- 42. Berger JH, Charron MJ, Silver DL. Major facilitator superfamily domain-containing protein 2a (MFSD2A) has roles in body growth, motor function, and lipid metabolism. PLoS One 2012;7(11):e50629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Zolezzi JM, Santos MJ, Bastias-Candia S, Pinto C, Godoy JA, Inestrosa NC. PPARs in the central nervous system: roles in neurodegeneration and neuroinflammation. Biol Rev Camb Philos Soc 2017;92(4):2046–69. [DOI] [PubMed] [Google Scholar]
- 44. Hashimoto M, Hossain S, Yamasaki H, Yazawa K, Masumura S. Effects of eicosapentaenoic acid and docosahexaenoic acid on plasma membrane fluidity of aortic endothelial cells. Lipids 1999;34(12):1297–304. [DOI] [PubMed] [Google Scholar]
- 45. Busquets O, Ettcheto M, Pallas M, Beas-Zarate C, Verdaguer E, Auladell C, Folch J, Camins A. Long-term exposition to a high fat diet favors the appearance of beta-amyloid depositions in the brain of C57BL/6J mice: a potential model of sporadic Alzheimer's disease. Mech Ageing Dev 2017;162:38–45. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.