Abstract
Objective
The objective of this study was to evaluate prespecified and post hoc analyses in RENEW subgroups to identify participants more likely to benefit from opicinumab.
Methods
RENEW assessed the efficacy/safety of opicinumab versus placebo in participants with a first unilateral acute optic neuritis (AON) episode. Difference in visual evoked potential (VEP) latency of the affected eye at 24 weeks versus the fellow eye at baseline was the primary endpoint. Interactions between the primary endpoint and prespecified baseline variables (including age, timing of treatment initiation, and visual impairment) using the median as cut‐off were evaluated in the per protocol population using analysis of covariance (ANCOVA); subgroups based on preexisting brain T2 lesion volume were also analyzed. Interactions between the primary endpoint and retinal ganglion cell layer/inner plexiform layer (RGCL/IPL) and retinal nerve fiber layer (RNFL) thickness were assessed post hoc as was weight gain by treatment.
Results
Treatment benefit of opicinumab (n = 33) over placebo (n = 36) on the primary endpoint was greatest in participants older than the median age at baseline (≥33 years); the difference versus placebo for baseline age ≥33 years was −14.17 msec [P = 0.01] versus −0.89 msec for baseline age <33 years, [P = 0.87]). Post hoc analysis showed that VEP latency recovery was significantly associated with less RGCL/IPL thinning (P = 0.0164), occurring early on.
Interpretation
Age was the strongest prespecified baseline characteristic associated with a treatment effect of opicinumab. A strong association between VEP latency recovery at week 24 and early RGCL/IPL preservation was observed.
Introduction
Acute optic neuritis (AON), frequently the first manifestation of multiple sclerosis (MS), is characterized by optic nerve inflammatory demyelination and axonal injury. While some spontaneous remyelination occurs, most patients have residual structural and clinical deficits.1, 2, 3, 4 A physiological hallmark of AON is prolonged visual evoked potential (VEP) latency, resulting from persistent demyelination of the affected optic nerve.2, 5
Therapies enhancing remyelination and thus supporting axonal integrity and function remain an unmet need for demyelinating diseases such as AON and MS.6 Opicinumab (anti‐LINGO‐1, BIIB033) is a human monoclonal antibody against leucine‐rich repeat and immunoglobulin‐like domain‐containing protein 1 (LINGO‐1; an oligodendrocyte differentiation and myelination suppressor) that was genetically engineered to reduce immunoglobulin effector function. In preclinical models, opicinumab has no apparent effects on the immune system; remyelination/neuroprotection effects in the central nervous system (CNS) as well as acceptable tolerability were observed.7, 8, 9, 10 Therefore, opicinumab is being further tested in Phase II proof‐of‐biology/concept studies.
In RENEW, previously healthy participants with a first unilateral AON episode were randomized to opicinumab or placebo intravenous (IV) infusions every 4 weeks. The primary endpoint was the difference in full‐field VEP latency of the affected eye at 24 weeks versus baseline of the unaffected fellow eye in the intent‐to‐treat (ITT) population. Efficacy for opicinumab versus placebo was also examined at the end of study (week 32) in the ITT population, and at 24 and 32 weeks in the per protocol (PP) population. Although the ITT analysis did not demonstrate a statistically significant treatment effect, the PP analysis showed improvement in mean VEP latency favoring the opicinumab‐treated cohort, with treatment effect most evident at week 32 versus week 24. The overall incidence and severity of adverse events were comparable between treatment groups, except for two treatment‐emergent adverse events, hypersensitivity reactions and mild‐moderate weight gain that were more frequent in the opicinumab group.11
Designed as a proof‐of‐concept study, RENEW was not powered for statistical significance; it aimed to investigate baseline demographic and disease characteristics associated with treatment response to LINGO‐1 blockade for remyelination and neuroprotection with opicinumab. Here we present results of the prespecified efficacy analyses aimed to identify participant subgroups more likely to benefit from opicinumab, as well as a post hoc analysis of baseline characteristics associated with weight gain and relationships between structural and functional endpoints.
Methods
Study design and participants
RENEW (NCT01721161) was a previously reported randomized, double‐masked, placebo‐controlled, multicenter study.11 Eligible participants were previously healthy adults with no history of MS who were experiencing a first unilateral AON episode, with normal VEP in the fellow eye. All participants were treated with high‐dose methylprednisolone (1 g IV/day for 3–5 days), then randomized 1:1 within 28 days of first symptom onset to placebo or 100 mg/kg opicinumab IV every 4 weeks (total of 6 infusions) and followed to end of treatment (week 24) and end of study (week 32).11
The primary outcome, difference in VEP latency for the affected eye from the baseline of the unaffected fellow eye, was recorded at weeks 24 and 32 using P100 latency (msec).11 The average normal P100 latency is ~100 msec.12 Latency prolongation was also measured as the interocular difference by comparing the affected eye at all time points with the baseline value for the unaffected fellow eye. The fellow eye was selected as the reference control because baseline latency in the affected eye is not measurable in the acute setting due to conduction block and residual inflammation post high‐dose IV steroids. Neuroaxonal retinal thinning, a secondary endpoint assessing potential neuroprotective treatment effects of opicinumab on retinal ganglion cells (RGC) and the retinal nerve fiber layer (RNFL), was measured using the Duke Optical Coherence Tomography Retinal Analysis Program (DOCTRAP) software on two different spectral‐domain optical coherence tomography (SD‐OCT) systems – either the Cirrus HD‐OCT (Carl Zeiss Meditec, Inc., Dublin, California) or Spectralis (Heidelberg Engineering, Inc., Franklin, Massachusetts) devices.13, 14 Assessments were performed according to prespecified standard protocols and interpreted by a central reader with strict quality control.11 Due to reading differences between the Cirrus and Spectralis devices and analysis software, RNFL thinning was reported as a percentage. On the other hand, a value in microns could be used for retinal ganglion cell layer/inner plexiform layer (RGCL/IPL) thinning, because using fellow eye baseline measurements in this study, DOCTRAP results were generally comparable between Cirrus and Spectralis scans (unpublished). VEP amplitude, high‐contrast visual acuity (HCVA), and low‐contrast letter acuity (LCLA; 1.25% and 2.5% Sloan charts) also were assessed.11
Participants who completed the study, missed ≤1 dose, and did not receive MS disease‐modifying therapy were included in the prespecified PP population. The safety and ITT populations comprised all participants who received ≥1 study dose.11 For the subgroup analyses reported in this manuscript, we focused on the PP population but also reported findings in the ITT population as supplementary material.
Subgroup analyses for the PP population
A subgroup analysis for the PP population was performed because this cohort most closely adhered to the treatment protocol, was free of imputations, and had stronger treatment effects than the ITT study population. The primary efficacy endpoint was assessed in prespecified subgroups of participants classified by the following baseline demographic and disease characteristics: age, days between onset of AON and initiation of study treatment, days between completing steroid therapy and study treatment initiation, LCLA score in affected eye, HCVA score in affected eye, and brain T2 lesion volume. With the exception of brain T2 lesion volume, the median value was used as the cutoff to classify the subgroups for each of the baseline characteristics; 0 and >0 were used for baseline brain T2 lesion volume.
VEP latency recovery (to normal) was prespecified as affected eye VEP latency ≤10% worse than the baseline fellow eye.11 Post hoc analyses were performed to determine the interaction between VEP latency recovery (over 24 weeks) and RGCL/IPL thickness, irrespective of treatment group. We also compared participants with and without VEP latency recovery for corresponding changes over 24 weeks in VEP amplitude, RNFL thickness, LCLA score, and HCVA score.
Subgroup analyses for the safety population
The prespecified safety analyses showed that the overall incidence and severity of adverse events in RENEW were comparable between the opicinumab and placebo groups. However, weight gain during the study was found to be greater in opicinumab‐ than placebo‐treated participants.11 Hence, post hoc analyses of participants who had weight gain >7% during the study were undertaken to determine whether any baseline characteristics were associated with this outcome. A 7% change in weight from baseline is a frequently used cutoff to assess weight gain or loss in clinical studies.
Statistical analyses
For participants classified by prespecified baseline characteristics, the adjusted mean change for each treatment group at week 24, difference compared with placebo, 95% confidence interval (CI), and P‐value (compared with placebo and subgroup‐by‐treatment interaction) were evaluated by analysis of covariance (ANCOVA). The adjusted mean change in efficacy endpoints also was determined for the groups of participants with and without latency recovery at week 24, and the difference between these groups (with 95% CI and P‐value) was calculated using ANCOVA. Post hoc sensitivity analyses were performed to determine if 10% was an appropriate cut‐off to define latency recovery.11 Means and standard deviations for baseline characteristics in participants with and without post‐baseline weight increase >7% were calculated.
Results
Randomized participants
Eighty‐two participants were randomized to placebo or opicinumab. The baseline clinical and demographic characteristics were similar in both groups of the ITT and PP populations, with the exception of more AON severe cases randomized to opicinumab than placebo.11 Baseline VEP latency in the affected eye was not measurable in a number of participants in the acute setting due to conduction failure (affected eye conduction failure at baseline: ITT, n = 15; PP, n = 11). Sixty‐nine participants comprised the PP population, 36 randomized to placebo and 33 to opicinumab; 82 participants were included in the ITT and safety population, 41 in each treatment group.11
Efficacy subgroup analyses in the PP population
The primary endpoint analysis in subgroups classified by prespecified baseline characteristics showed that the treatment benefit for opicinumab versus placebo at week 24 was largest in the older subgroup of participants (≥33 years of age; −14.17 msec (−24.83, −3.52) versus the younger subgroup (<33 years of age; −0.89 msec [−11.43, 9.65]), reaching statistical significance (P = 0.01); there was only a trend in the subgroup‐by‐treatment interaction. Moreover, trends for increased benefit were observed in participants who received the first dose sooner (<25 days from the onset of AON) and in participants with more severe pretreatment visual acuity impairment (HCVA score <49; Tables 1 and 2; see Table S1 for results in the ITT population). Although none of the other subgroups based on baseline characteristics reached statistical significance on the primary endpoint, there was a consistent treatment difference favoring opicinumab across all prespecified treatment subgroups examined (Tables 1 and 2).
Table 1.
Differences in VEP latencies at week 24 in the affected eye compared with the unaffected fellow eye and treatment difference for opicinumab versus placebo for PP population subgroups classified by prespecified demographic and time to treatment baseline characteristics
| Adjusted mean change in VEP at week 24 by baseline characteristic | Placebo | Opicinumab | Treatment difference (95% CI); P‐value |
|---|---|---|---|
| Age | |||
| <33 years | 17.83 (n = 17) | 16.93 (n = 17) |
−0.89 (−11.43, 9.65) P = 0.87 |
| ≥33 years | 26.32 (n = 19) | 12.15 (n = 16) |
−14.17 (−24.83, −3.52) P = 0.01 |
| Subgroup‐by‐treatment interaction P‐value | P = 0.08 | ||
| Treatment window | |||
| Received treatment <25 days from onset of AON | 20.20 (n = 16) | 11.19 (n = 14) |
−9.01 (−20.44, 2.42) P = 0.12 |
| Received treatment ≥25 days from onset of AON | 23.91 (n = 20) | 17.23 (n = 19) |
−6.68 (−16.75, 3.39) P = 0.19 |
| Subgroup‐by‐treatment interaction P‐value | P = 0.76 | ||
| Timing of steroid administration | |||
| Received treatment <15 days after completing steroid therapy | 22.01 (n = 20) | 13.80 (n = 14) |
−8.21 (−19.20, 2.78) P = 0.14 |
| Received treatment ≥15 days after completing steroid therapy | 22.52 (n = 16) | 15.36 (n = 19) |
−7.16 (−18.09, 3.77) P = 0.20 |
| Subgroup‐by‐treatment interaction P‐value | P = 0.89 | ||
AON, acute optic neuritis; CI, confidence interval; PP, per‐protocol; VEP, visual evoked potential.
Table 2.
Difference in VEP latencies at week 24 in the affected eye compared to the baseline of the unaffected fellow eye and treatment difference for opicinumab versus placebo for PP population subgroups classified by prespecified disease baseline characteristics
| Adjusted mean change in VEP at week 24 by baseline characteristic | Placebo | Opicinumab | Treatment difference (95% CI); P‐value |
|---|---|---|---|
| LCLA impairment | |||
| LCLA score = 0 (2.5% chart) | 25.68 (n = 21) | 19.22 (n = 11) |
−6.46 (−18.01, 5.10) P = 0.27 |
| LCLA score >0 (2.5% chart) | 17.40 (n = 15) | 13.61 (n = 20) |
−3.79 (−14.40, 6.82) P = 0.48 |
| Subgroup‐by‐treatment interaction P‐value | P = 0.73 | ||
| HCVA impairment | |||
| HCVA score <49 | 25.21 (n = 16) | 14.29 (n = 12) |
−10.92 (−23.01, 1.18) P = 0.08 |
| HCVA score ≥49 | 19.80 (n = 20) | 15.66 (n = 20) |
−4.14 (−14.14, 5.86) P = 0.41 |
| Subgroup‐by‐treatment interaction P‐value | P = 0.39 | ||
| MRI burden of disease | |||
| Brain T2 lesion volume = 0 | 17.88 (n = 5) | 7.40 (n = 8) |
−10.48 (−28.35, 7.38) P = 0.25 |
| Brain T2 lesion volume >0 | 22.21 (n = 29) | 17.08 (n = 25) |
−5.13 (−13.66, 3.40) P = 0.23 |
| Subgroup‐by‐treatment interaction P‐value | P = 0.59 | ||
CI, confidence interval; HCVA, high‐contrast visual acuity; LCLA, low‐contrast letter acuity; MRI, magnetic resonance imaging; PP, per‐protocol; VEP, visual evoked potential.
For interactions between functional (VEP) and structural (SD‐OCT) outcomes in the visual pathway analyzed post hoc, significantly less RGCL/IPL thinning was observed in the subgroup of participants with VEP latency recovery than in participants without latency recovery (Fig. 1, left panel). After 24 weeks, there was minimal, if any, additional RGCL/IPL thinning (Fig. 1, right panel). Similar results were observed in the ITT population (Fig. S1).
Figure 1.
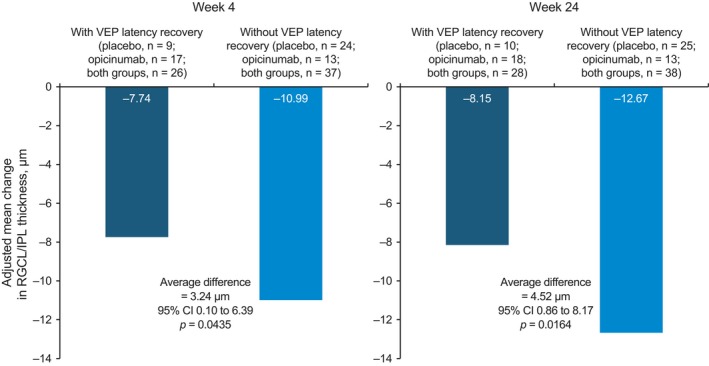
Retinal ganglion cell layer/inner plexiform layer (RGCL/IPL) thinning (spectral‐domain optical coherence tomography) at weeks 4 and 24 in participants from the per‐protocol population with and without visual evoked potential (VEP) latency recovery. CI, confidence interval.
Additional analyses of differences at week 24 between participants classified according to VEP latency recovery showed that absence of VEP latency recovery corresponded to worse average outcomes on VEP amplitude, 1.25% LCLA, and 2.5% LCLA than those with latency recovery (Table 3). None of these observations reached statistical significance (see Table S2 for data in the ITT population).
Table 3.
Post hoc analyses of efficacy endpoints in participants from the PP population without versus with VEP latency recovery at week 24
| Endpoint | Without VEP latency recovery at week 24 n = 38 | With VEP latency recovery at week 24 n = 28 | Difference versus without latency recovery (95% CI); P‐value |
|---|---|---|---|
| Adjusted mean change in VEP amplitude, μVa | −3.43 | −2.57 |
0.85 (−1.28, 2.99) P = 0.43 |
| Adjusted mean change in RGCL/IPL thickness (SD‐OCT), μma | −12.67 | −8.15 |
4.52 (0.86, 8.17) P = 0.02 |
| Adjusted mean percentage change in RNFL thickness (SD‐OCT)a | −16.22 | −10.73 |
5.49 (−0.72, 11.70) P = 0.08 |
| Adjusted mean change in LCLA,1.25% Sloan chartb | 5.64 | 8.87 |
3.23 (−2.52, 8.97) P = 0.27 |
| Adjusted mean change in LCLA, 2.5% Sloan chartb | 11.08 | 12.69 |
1.61 (−4.56, 7.78) P = 0.60 |
| Adjusted mean change in HCVAb | 11.16 | 9.74 |
−1.42 (−6.47, 3.63) P = 0.58 |
CI, confidence interval; HCVA, high‐contrast visual acuity; LCLA, low‐contrast letter acuity; PP, per‐protocol; RGCL/IPL, retinal ganglion cell layer/inner plexiform layer; RNFL, retinal nerve fiber layer; SD‐OCT, spectral‐domain optical coherence tomography; VEP, visual evoked potential.
Adjusted mean versus baseline of the fellow eye.
Adjusted mean versus baseline of the affected eye.
Safety subgroup analyses
Seventeen participants in the RENEW study had weight gain >7% from baseline, 4 (10%) in the placebo group and 13 (32%) in the opicinumab group.11 Post hoc subgroup analyses showed that participants in the opicinumab arm with weight gain >7% during the study had worse baseline AON disease, indicated by worse HCVA impairment, greater VEP latency prolongation, and a higher frequency of conduction failure (absence of VEP P100 amplitude wave) at baseline. However, this was not consistent for the placebo group because VEP latency delay and worse HCVA impairment were more common among patients without weight gain >7%.
Discussion
The RENEW study was the first to investigate, in humans, the potential efficacy of LINGO‐1 blockade with opicinumab for enhancing remyelination using the difference in VEP latency of the affected eye at 24 weeks versus the fellow eye at baseline as the primary outcome measure. VEP latency is a sensitive indicator of demyelination and subsequent remyelination in models of optic neuritis.15, 16 RENEW used the latency of the unaffected eye at baseline to measure the extent of recovery in the affected eye across 32 weeks. Due to the likely influence of baseline demographic and disease severity on the potential efficacy of opicinumab in AON, the study explored efficacy across prespecified demographic and baseline disease characteristics: age range 18–55 years, disease duration between 1 and 28 days, time to steroid administration, and variable baseline disease severity (mild–severe).11
Of all the parameters analyzed, only age had a statistically significant treatment effect with a strong trend in the subgroup‐by‐treatment interaction analysis. We expected trends rather than significant P values due to the small sample size of the RENEW study. In fact, the primary endpoint of RENEW was not itself powered for statistical significance.11 Among all subgroups analyzed, the greatest VEP latency recovery was observed in the older half of participants treated with opicinumab in the PP population (baseline age ≥33 years), while the worst latency delay was observed in the older participants treated with placebo. The younger half (<33 years of age) experienced similar and intermediate degrees of recovery in the two treatment arms.
The finding that older placebo‐treated participants experienced worse VEP latency recovery is consistent with the biological concept that spontaneous remyelination is negatively affected by aging.17, 18, 19, 20, 21 That the strongest opicinumab treatment effect was observed in this subgroup, suggests that LINGO‐1 blockade may be more effective in individuals whose initial clinical episode of CNS demyelination occurs at an older age. Results from a Phase 2 trial showing a modest reduction in VEP latency in patients (mean age = 40.1 years) with relapsing MS with preexisting optic neuritis and good preservation of the RNFL treated with clemastine fumarate are consistent with this finding.22
The hypothesis that older individuals with AON may be more responsive to LINGO‐1 blockade with opicinumab could be explained by one or more of the following reasons. First, younger participants may have greater inherent recovery potential and spontaneous remyelination, which may dampen any therapeutic effect of opicinumab; conversely, intrinsic remyelination may be weaker in older participants, with a greater margin for therapeutic enhancement in this subgroup.21 Second, younger participants may be less responsive to opicinumab because increased LINGO‐1 expression may not play a role in the lack of spontaneous remyelination. Third, younger participants are more likely to have active disease activity (even asymptomatic MS) compared with older patients, confounding any beneficial treatment effect of reparative candidate treatments such as opicinumab. Fourth, the initial demyelination may be more severe in the older participants making it unlikely for spontaneous remyelination to be clinically meaningful. In this context, conduction block at baseline was more frequent in older participants (8/35 vs. 3/34 for younger participants). Fifth, the findings may be spurious, possibly attributable to chance. Additional efficacy studies with opicinumab are needed to shed light on the effect of baseline age on response to therapeutic remyelination.
The lack of statistically significant interaction between the primary endpoint and treatment window or timing of steroid administration at week 24 may be attributed to the small sample size, as the RENEW study was powered only for an 80% treatment effect with one‐tail alpha of 0.1 for the primary endpoint. Notwithstanding, there appears to be a consistent numerical trend suggesting greater improvement in patients treated sooner (<25 days from onset of AON) with opicinumab (P = 0.12, vs. placebo) and in patients randomized to opicinumab and treated sooner with high‐dose methylprednisolone (<15 days from onset of AON; P = 0.14, vs. placebo). The axonal protective potential of opicinumab, if given soon after onset of CNS inflammatory demyelinating injury, should be evaluated in additional studies aiming to initiate treatment sooner that the 28‐day window in this study. Results from a single‐center academic study with phenytoin that enrolled within 14 days of AON onset suggest that treatment with candidate RGCL protective agents could be initiated earlier after onset of AON symptoms.23
The apparent lack of influence of visual impairment and brain MRI data in stratifying patients according to VEP latency prolongation at week 24 is noteworthy. However, the subgroup analyses showed a trend for a treatment benefit in participants with more impaired pretreatment HCVA (P = 0.08). This could indicate that opicinumab‐mediated repair via remyelination may be more effective and relevant in participants with greater pre‐treatment HCVA impairment, barring severe injury to the optic nerve including the ganglion cell neurons in the retina. In this study, the interaction between VEP latency and brain T2 lesion volume is difficult to interpret due to the small sample size and the focus on patients with first episode of AON.
Based on data from the post hoc analyses comparing measures of structure and function, improvement on the primary endpoint was associated with less reduction in RGCL/IPL and RNFL thickness (P = 0.02 and P = 0.08, respectively), which occurred early on. Corresponding findings in the ITT population were also statistically significant for both (Table S2). Our data suggest that an adjusted mean loss in RGCL/IPL of approximately 4–5 μm (Table 3) may be used as a predictor of poor VEP latency recovery following AON. This is of similar magnitude to the average loss on RNFL thickness in MS as a result of AON.24
The initial safety analyses highlighted that the group randomized to opicinumab appeared to have a higher frequency of weight gain than the placebo group.11 Weight gain is unlikely to have been related to intravenous steroid pretreatment because of short‐term single use (3–5 days), although individual differential effects of steroids are possible. It is unclear if the weight gain may be related to more severe disease because neither restricted mobility nor decreased physical activity was assessed. There does not appear to be evidence in the literature that acute visual impairment is associated with weight gain. In addition, no evidence of weight gain was seen in previous preclinical studies of opicinumab in toxicology studies (Biogen, data on file) or in clinical (Phase I) studies in healthy volunteers or individuals with MS.8 Analysis of results from SYNERGY, which assessed opicinumab in disabled participants with relapsing‐remitting and secondary progressive MS treated for ≤18 months, may further elucidate whether weight gain may be treatment‐related.25
The main limitation of these RENEW subgroup analyses is the small sample size. RENEW was designed to assess efficacy trends early in clinical development of opicinumab on both the primary endpoint and subgroup analysis. This likely explains why none of the prespecified baseline characteristics included in the subgroup‐by‐treatment interaction analyses reached statistical significance. Although the majority of the subgroup analyses were prespecified in the statistical analysis plan, the observations require further investigation. A systematic analysis of all baseline characteristics was not performed due to the small sample size. All reported results are based on a univariate subgroup analysis approach of prespecified baseline characteristics that may not account for all confounding issues; data mining results and conclusions from such analyses may need further study with larger sample size for validation.
These RENEW subgroup analyses could have important implications for the design of future clinical trials of CNS remyelinating therapies in the context of AON and beyond. Importantly, these analyses support further investigation of opicinumab as a potential treatment for MS and other CNS demyelinating diseases. Future subgroup analyses in a larger population, including participants enrolled in the recently completed SYNERGY trial25 and the ongoing AFFINITY study (NCT03222973) will help to confirm whether age and other baseline demographic or disease characteristics can identify subgroups of patients more likely to benefit from therapeutic remyelination with opicinumab.
Author Contribution
DC, LB, SG, YC, and LX designed the study with input from the other authors and were responsible for the conduct of the study. OA, TZ, LJV, LL, MSF, GTP, JLP, FZ, and LM collected study data and gave input on analysis. YC and LX analyzed the data, and all authors were involved in data interpretation.
Conflict of Interest
This study was supported by Biogen (Cambridge, MA, USA). DC: former employee of and may hold stock/stock options in Biogen; current employee of Fulcrum Therapeutics; patent WO 2016112270 A1 assigned to Biogen: Lingo‐1 antagonists and uses for treatment of demyelinating disorders. LB: grant and consulting/advisor fees from Biogen. SG: consulting fees from Biogen. OA: advisor fees or honoraria from Almirall, Bayer HealthCare, Biogen, MedImmune, Merck, Novartis, and Teva; research support from Bayer HealthCare, Biogen, Novartis, Roche, and Teva. TZ: consulting fees from Almirall, Bayer HealthCare, Biogen, Genzyme, GlaxoSmithKline, Merck Serono, MSD, Novartis, Sanofi‐Aventis, Synthon, and Teva; research support from Bayer HealthCare, Biogen, Merck Serono, Novartis, Sanofi‐Aventis, and Teva. LJV: consulting/advisor fees or honoraria from Biogen, Merck, Novartis, Roche, and Sanofi Genzyme. LL: honoraria for consulting and/or speaking activities from Biogen, Merck, Roche and Teva; research support from Biogen, Merck, and Novartis; travel support from Almirall, Biogen, Merck, Novartis, and Roche. MSF: honoraria for consulting or speaking activities from Actelion, Bayer HealthCare, Biogen, Chugai, Clene Nanomedicine, EMD Inc., Genzyme, Roche, Sanofi, and Teva; research support from Genzyme; study support from Biogen. GTP: consulting fees and/or research support from Biogen. JLP: consulting fees and/or research support from Biogen, Merck, Novartis, Roche, Sanofi‐Genzyme, and Teva. FZ: consulting fees from Alimera, Allergan, Bayer HealthCare, Boehringer‐Ingelheim, Novartis, Optos, MSD, and Roche; research support from Novartis. LM: advisor honoraria from Biogen, Mylan, Roche, and Sanofi‐Genzyme; research support from Biogen, Merck Serono, and Sanofi‐Genzyme; scientific meeting support from Merck Serono, Novartis, and Teva. YC and LX: former employees of and may hold stock/stock options in Biogen.
Supporting information
Appendix S1. RENEW study investigators.
Figure S1. Retinal ganglion cell layer/inner plexiform layer (RGCL/IPL) thinning (spectral‐domain optical coherence tomography) at weeks 4 and 24 in participants from the intent‐to‐treat population with and without visual evoked potential (VEP) latency recovery. CI, confidence interval.
Table S1. Differences in VEP latencies at week 24 in the affected eye compared with the unaffected fellow eye and treatment difference for opicinumab versus placebo for ITT population subgroups classified by prespecified baseline characteristics.
Table S2. Post hoc analyses of efficacy endpoints in participants from the ITT population without versus with VEP latency recovery at week 24.
Acknowledgments
The authors thank all the participants and investigators in the RENEW study. Thanks also are extended to the following for their assistance with this study: the study's contract research organization, Pharmaceutical Product Development, LLC (Wilmington, North Carolina); the staff at NeuroRx Research (Montreal, Quebec, Canada), who read and interpreted all magnetic resonance imaging results; and the staff from Duke University (Durham, North Carolina), the central reading center for the SD‐OCT and VEP data. Biogen provided funding for medical writing support in the development of this paper; Rebecca J. Gardner from Excel Scientific Solutions wrote the first draft of the manuscript based on input from authors, and Kristen DeYoung from Excel Scientific Solutions copyedited and styled the manuscript per journal requirements. Biogen reviewed and provided feedback on the paper to the authors. The authors had full editorial control of the paper and provided their final approval of all content.
Funding Information
Biogen provided funding for medical writing support in the development of this paper; Rebecca J. Gardner from Excel Scientific Solutions wrote the first draft of the manuscript based on input from authors, and Kristen DeYoung from Excel Scientific Solutions copyedited and styled the manuscript per journal requirements.
A full list of study investigators is provided in the Appendix S1.
Funding Statement
This work was funded by Biogen grant .
References
- 1. Cole SR, Beck RW, Moke PS, et al. The National Eye Institute Visual Function Questionnaire: experience of the ONTT. Invest Ophthalmol Vis Sci 2000;41:1017–1021. [PubMed] [Google Scholar]
- 2. Galetta SL, Villoslada P, Levin N, et al. Acute optic neuritis: unmet clinical needs and model for new therapies. Neurol Neuroimmunol Neuroinflamm 2015;2:e135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Trobe JD, Beck RW, Moke PS, Cleary PA. Contrast sensitivity and other vision tests in the optic neuritis treatment trial. Am J Ophthalmol 1996;121:547–553. [DOI] [PubMed] [Google Scholar]
- 4. Moro SI, Rodriguez‐Carmona ML, Frost EC, et al. Recovery of vision and pupil responses in optic neuritis and multiple sclerosis. Ophthalmic Physiol Opt 2007;27:451–460. [DOI] [PubMed] [Google Scholar]
- 5. Klistorner A, Arvind H, Garrick R, et al. Remyelination of optic nerve lesions: spatial and temporal factors. Mult Scler 2010;16:786–795. [DOI] [PubMed] [Google Scholar]
- 6. Wingerchuk DM, Carter JL. Multiple sclerosis: current and emerging disease‐modifying therapies and treatment strategies. Mayo Clin Proc 2014;89:225–240. [DOI] [PubMed] [Google Scholar]
- 7. Mi S, Pepinsky RB, Cadavid D. Blocking LINGO‐1 as a therapy to promote CNS repair: from concept to the clinic. CNS Drugs 2013;27:493–503. [DOI] [PubMed] [Google Scholar]
- 8. Tran JQ, Rana J, Barkhof F, et al. Randomized phase I trials of the safety/tolerability of anti‐LINGO‐1 monoclonal antibody BIIB033. Neurol Neuroimmunol Neuroinflamm 2014;1:e18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mi S, Hu B, Hahm K, et al. LINGO‐1 antagonist promotes spinal cord remyelination and axonal integrity in MOG‐induced experimental autoimmune encephalomyelitis. Nat Med 2007;13:1228–1233. [DOI] [PubMed] [Google Scholar]
- 10. Ranger ARS, Szak S, Dearth A, et al. Blocking LINGO‐1 does not affect inflammatory markers in the cerebrospinal fluid of individuals with multiple sclerosis. Neurology 2014;82(suppl 10):P1.186. [Google Scholar]
- 11. Cadavid D, Balcer L, Galetta S, et al. Safety and efficacy of opicinumab in acute optic neuritis (RENEW): a randomised, placebo‐controlled, phase 2 trial. Lancet Neurol 2017;16:189–199. [DOI] [PubMed] [Google Scholar]
- 12. Halliday AM. Evoked potentials in clinical testing. Edinburgh: Churchill Livingstone, 1993. [Google Scholar]
- 13. Chiu SJ, Li XT, Nicholas P, et al. Automatic segmentation of seven retinal layers in SDOCT images congruent with expert manual segmentation. Opt Express 2010;18:19413–19428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lee JY, Chiu SJ, Srinivasan PP, et al. Fully automatic software for retinal thickness in eyes with diabetic macular edema from images acquired by cirrus and spectralis systems. Invest Ophthalmol Vis Sci 2013;54:7595–7602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. You Y, Klistorner A, Thie J, Graham SL. Latency delay of visual evoked potential is a real measurement of demyelination in a rat model of optic neuritis. Invest Ophthalmol Vis Sci 2011;52:6911–6918. [DOI] [PubMed] [Google Scholar]
- 16. Mozafari S, Sherafat MA, Javan M, et al. Visual evoked potentials and MBP gene expression imply endogenous myelin repair in adult rat optic nerve and chiasm following local lysolecithin induced demyelination. Brain Res 2010;1351:50–56. [DOI] [PubMed] [Google Scholar]
- 17. Franklin RJ, Goldman SA. Glia disease and repair–remyelination. Cold Spring Harb Perspect Biol 2015;7:a020594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Shen S, Sandoval J, Swiss VA, et al. Age‐dependent epigenetic control of differentiation inhibitors is critical for remyelination efficiency. Nat Neurosci 2008;11:1024–1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Jones SJ. Visual evoked potentials after optic neuritis. Effect of time interval, age and disease dissemination. J Neurol 1993;240:489–494. [DOI] [PubMed] [Google Scholar]
- 20. Kriss A, Francis DA, Cuendet F, et al. Recovery after optic neuritis in childhood. J Neurol Neurosurg Psychiatry 1988;51:1253–1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ruckh JM, Zhao JW, Shadrach JL, et al. Rejuvenation of regeneration in the aging central nervous system. Cell Stem Cell 2012;10:96–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Green AJ, Gelfand JM, Cree BA, et al. Clemastine fumarate as a remyelinating therapy for multiple sclerosis (ReBUILD): a randomised, controlled, double‐blind, crossover trial. Lancet 2017;390:2481–2489. [DOI] [PubMed] [Google Scholar]
- 23. Raftopoulos R, Hickman SJ, Toosy A, et al. Phenytoin for neuroprotection in patients with acute optic neuritis: a randomised, placebo‐controlled, phase 2 trial. Lancet Neurol 2016;15:259–269. [DOI] [PubMed] [Google Scholar]
- 24. Nolan RC, Galetta SL, Frohman TC, et al. Optimal intereye difference thresholds in retinal nerve fiber layer thickness for predicting a unilateral optic nerve lesion in multiple sclerosis. J Neuroophthalmol 2018; 10.1097/WNO.0000000000000629. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Cadavid D, Phillips G, Dong‐Si T, et al. Efficacy and safety of anti LINGO‐1 for the treatment of relapsing forms of multiple sclerosis: design of the phase 2 SYNERGY trial. Neurology 2014;82(suppl 10):P3.154. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. RENEW study investigators.
Figure S1. Retinal ganglion cell layer/inner plexiform layer (RGCL/IPL) thinning (spectral‐domain optical coherence tomography) at weeks 4 and 24 in participants from the intent‐to‐treat population with and without visual evoked potential (VEP) latency recovery. CI, confidence interval.
Table S1. Differences in VEP latencies at week 24 in the affected eye compared with the unaffected fellow eye and treatment difference for opicinumab versus placebo for ITT population subgroups classified by prespecified baseline characteristics.
Table S2. Post hoc analyses of efficacy endpoints in participants from the ITT population without versus with VEP latency recovery at week 24.


