Abstract
Objective
To systematically examine the association between alcohol intake and likelihood of having probable rapid eye movement sleep behavior disorder (pRBD) 6 years later.
Methods
The study included 11,905 participants (mean age: 47.7 years) of the Kailuan Study, free of stroke, cancer, Parkinson disease, dementia, and head injury in 2006. We determined pRBD using a validated RBD questionnaire–Hong Kong in 2012. Amounts and types of alcohol intake were collected with questionnaire. Participants were categorized into: nondrinkers, light (women: 0–0.4 servings/day; men: 0–0.9 servings/day), moderate (women: 0.5–1.0 servings/day; men: 1–2 servings/day), and heavy drinkers(women: >1 serving/day; men: >2 servings/day). To examine the alcohol‐pRBD relationship, we used logistic regression to calculate odds ratios (ORs) and 95% confidence intervals (CIs), adjusting for demographic characteristics, smoking, hypertension, diabetes, physical activity, body mass index, and plasma concentrations of lipids and urate.
Results
Compared with nondrinkers, current drinkers had a 23% higher likelihood of having pRBD (adjusted OR 1.23, 95% CI 1.07–1.59). Both moderate (adjusted OR: 1.53, 95% CI 1.01–2.30) and heavy drinkers (adjusted OR: 1.29, 95% CI 1.00–1.66), but not light drinkers (adjusted OR: 1.16, 95% CI 0.94–1.44), had a significantly higher likelihood of having pRBD, relative to nondrinkers. There was a nonsignificant trend between consumption of each individual alcoholic beverages (i.e., beer, wine, or hard liquor) and higher likelihood of having pRBD (adjusted ORs ranged from 1.11 to 1.49).
Conclusions
Alcohol consumption was associated with a higher likelihood of having pRBD. Future prospective studies with clinically confirmed RBD, large sample size for information on types of alcoholic beverage are warranted.
Introduction
Although it has been well documented that rapid eye movement (REM) sleep behavior disorder (RBD) is strongly associated with future risk of developing neurodegenerative diseases,1, 2 etiology and risk factors for RBD remain largely understudied. Understanding these risk factors is important, because besides risk of neurodegeneration, RBD carries the risk of sleep disruption and injury. From recent epidemiological studies of potential risk factors for RBD,3, 4, 5, 6 several identified risk factors are related to neurodegenerative diseases (e.g., head injury and olfactory dysfunction) and cardiovascular diseases (e.g., hyperlipidemia and physical inactivity).3, 4, 5, 6Alcohol consumption was reported in association with higher risk of RBD or probable RBD (pRBD),4, 5, 6 though in some of the studies,4, 5 this association did not reach statistical significance. However, most studies to date were either relatively small or not specifically intended to evaluate the dose and type of alcohol beverage used.4, 5, 6 Alcohol has a strong effect on sleep, and thus may affect risk of sleep disorders. Studying this effect in more detail could also help to understand the potential role of alcohol in the pathogenesis of different health outcomes, which are closely related to RBD. For example, previous studies found that moderate alcohol consumption was associated with a lower risks of cardiovascular disease7, 8 and cognitive impairment (and Alzheimer's disease).9, 10 A large cohort study on Parkinson disease (PD) also reported that different types of alcoholic beverages might have different impacts on PD risk.11 Given that the effect of alcohol on PD might be different from on RBD, which is currently considered as a prodromal symptom of PD, we hypothesized that alcohol may be associated with increased likelihood of having pRBD. We thus conducted a community‐based study to systematically examine the association between different amounts and types of alcohol intake and likelihood of having pRBD.
Methods
Participants
As detailed elsewhere,5, 12 participants of this study included 12,990 Chinese adults (10,725 men and 2265 women), who were free of Parkinson disease and dementia, a subset of an ongoing Chinese cohort, the Kailuan Study.5, 12, 13 In the current analysis, we further excluded 987 participants with a prior history of stroke, cancer, and head injury and 98 participants who did not provide information on alcohol intake or RBD, leaving 11,905 participants (9776 men and 2129 women). The study was approved by the Ethics Committee of the Kailuan General Hospital.
Assessment of pRBD
We used a validated RBD questionnaire–Hong Kong (RBDQ‐HK) to determine probable RBD symptoms in 2012. The RBDQ‐HK was designed as a screening tool for RBD, with 13 Chinese questions regarding dreams and nightmare features, such as frequency of dreams and nightmares, content of dreams, disturbance of sleep of the participant or his/her bed partner, and vocalizations or behaviors during sleep.14 The RBDQ‐HK evaluates lifetime occurrence and recent 1‐year frequency of these symptoms.14 Compared to polysomnography (PSG)‐based diagnosis of RBD, the RBDQ‐HK showed robust sensitivity (82–85%), specificity (81–87%), internal consistency, and test–retest reliability in previous validation studies among general Chinese population and individuals with PD and obstructive sleep apnea (OSA).14, 15 The cut‐off point to estimate RBD for the total scale (score range 0–100) was >18.14
Assessment of alcohol consumption
To reduce the possibility of reverse causality (i.e., pRBD symptoms led to change in drinking status), we used cohort baseline data for alcohol consumption in 2006. Information on alcohol consumption was collected with questionnaire assessing current intake, with questions that included whether consuming any alcoholic beverage in the past 12 months, and if so, the beverage type (beer, wine, and hard liquor) and the amount and frequency of intake (“How many times do you drink in the past 30 days?”), as detailed previously.13 We calculated alcohol consumption in grams per day by multiplying the average times per day by the usual amount of each alcoholic beverage consumption and its average ethanol content (5.0 g for 100 g beer, 12.0 g for wine, and 40.0 g for hard liquor). A standard drink (referred to as “serving” in the report) contains about 14 g of ethanol.13
Based on the standard drinks in the “Dietary Guidelines for Americans 2015–2020,” U.S. Department of Health and Human Services and U.S. Department of Agriculture, we categorized participants into the following groups: nondrinkers, light (women: 0–0.4 servings/day; men: 0–0.9 servings/day), moderate (women: 0.5–1.0 servings/day; men: 1–2 servings/day), and heavy drinkers (women: >1 serving/day; men: >2 servings/day).16 For each type of alcoholic beverage, participants who did not drink or did not drink the indicated type were categorized as negative, otherwise, we grouped them as positive.
To assess the validity of self‐report alcohol consumption, we examined the association between alcohol intake and blood concentration of high‐density lipoprotein cholesterol (HDL‐c) in 71,379 participants of the Kailuan Study.13 As expected, we observed a dose–response relation between alcohol consumption and HDL‐c concentration in a cross‐sectional analysis.13
Assessment of sleep parameters
Information on several sleep parameters including insomnia, daytime sleepiness, sleep duration, snoring, and use of hypnotics was collected in 2012, as detailed below.
Insomnia
Insomnia status in the past 30 days was assessed on the basis of the validated Chinese version of Athens Insomnia Scale (AIS).17, 18 The AIS is a self‐administered questionnaire comprising eight questions on sleep features.17 The cut‐off to determine insomnia for the total scale was ≥6.17
Daytime sleepiness
We determined daytime sleepiness using the Chinese version of Epworth Sleepiness Scale (ESS).19 The higher total score the individual got the higher chance of falling asleep while engaged in specific situations of daily life. The total score ≥10 indicated excessive daytime sleepiness.19 A validation study of the ESS among Chinese individuals has demonstrated good test–retest reliability (ρ = 0.74).20
Snoring status
In the 2014 survey, we included the STOPBANG questionnaire and measured neck circumference to the nearest centimeter using a tape measure by trained field workers to screen individuals at high risk of OSA.21 Questions in STOPBANG comprised eight binary variables, including snoring, daytime tiredness, observed apneas, blood pressure, body mass index (BMI) (>35 kg/m2), age (>50 years), neck circumference (>40 cm), and sex (men). Intermediate or high risk of OSA was defined as three or more of the eight items scored positive.21 Previous validation studies among different populations, including Chinese, showed a high sensitivity (91–94%) to detect OSA.22 In this study, 2280 participants were considered intermediate or high risk of OSA (STOPBANG score ≥ 3).21
Assessment of other potential covariates
Information on education level, occupation, income level, smoking status, and physical activity was collected in 2006 via questionnaires, as detailed elsewhere.5 Weight and height were measured by trained field‐workers. Body mass index (BMI) was defined as body weight (kg) divided by the square of height (m2). We categorized participants into three groups based on their BMI: normal weight (<24 kg/m2), overweight (24–27.9 kg/m2), and obese (≥28 kg/m2). Systolic and diastolic blood pressures were measured twice from the seated position using a mercury sphygmomanometer. We used the average of the two readings for analysis. Hypertension was defined as systolic blood pressure ≥140 mm Hg or diastolic blood pressure ≥90 mm Hg or use of antihypertensive medications in the last 2 weeks regardless of blood pressure status. Prehypertension was grouped as systolic blood pressure between 120 and 139 mm Hg or diastolic blood pressure between 80 and 89 mm Hg. Fasting blood samples were collected, and we determined concentrations of glucose, HDL‐c, low‐density lipoprotein cholesterol (LDL‐c), triglyceride, and urate, using an autoanalyzer (Hitachi 747; Hitachi, Tokyo, Japan) at the central laboratory of the Kailuan General Hospital. Diabetes was defined as a concentration of fasting blood glucose ≥7.0 mmol/L or use of oral hypoglycemic agent or during active treatment with insulin, and prediabetes was classified as a concentration of fasting blood glucose between 5.6 and 6.9 mmol/L. Hyperuricemia was defined as ≥360 μmol/L for women, and ≥400 μmol/L for men.
Statistical analyses
All statistical analyses were conducted using SAS version 9.4 (SAS Institute, Inc, Cary, NC). Formal hypothesis testing was two‐sided with a significant level of 0.05. We used logistic regression to calculate odds ratios (ORs) and 95% confidence intervals (CIs), and to test differences in prevalence of pRBD across drinking status. We fit three multivariate models: model 1 adjusted for age and sex; model 2 further adjusted for education level, occupation, income level, smoking status, hypertension, diabetes, physical activity, BMI, and plasma concentrations of HDL‐c, LDL‐c, triglyceride, and urate. Linear trends were tested for significance by treating categories of drinking status as an ordinal variable.
To examine the robustness of the observed results, we conducted a sensitivity analysis by excluding individuals with hyperuricemia (1462 in men and 175 in women) because urate concentration is strongly associated with alcohol consumption and PD status.23, 24 For the same reason, we conducted another sensitivity analysis using the alternative cut‐off point (range 0–70, >7) for determining pRBD based on seven subgroup behavioral factors of RBDQ‐HK, including sleep talking, shouting, limb movements, and sleep‐related injuries. To minimize the potential effect of RBD mimics on the observed association between alcohol and pRBD, we conducted several sensitivity analyses by excluding participants with other sleep disorders (including excessive daytime sleepiness, and insomnia), or those using hypnotics. We also conducted a sensitivity study by excluding participants with intermediate or high risk of OSA. To introduce prospective component, we examined whether 2006 drinking status predicted RBD symptom onset in the recent 1 year (that is 2011–2012, referred to as “recent‐1‐year pRBD” in the manuscript) based on the RBDQ‐HK. We used PROC GLM to examine the differences in the scores of recent‐1‐year pRBD across 2006 drinking categories.
We examined potential interactions of drinking status with age and sex (as pRBD has been reported to be more common in men and older adults25) in relation to likelihood of pRBD, by including multiplicative terms between sex/age and drinking status in the logistic regression models, with adjustment for other potential confounders in the model 3.
Finally, we examined the relation between each type of alcoholic beverage (beer, wine, and hard liquor), using binary exposure (i.e., positive/negative), and likelihood of having pRBD, with adjustment for other potential confounders in the model 2.
Results
Participants with higher alcohol consumption were more likely to be men, blue collar occupation (compared to white collar occupation), and current smokers, at a lower level of education, income, and physical activity, and to have a higher prevalence of hypertension, diabetes, and higher concentrations of triglyceride, LDL‐c, HDL‐c, and urate (Table 1).
Table 1.
| None | Light | Moderate | Heavy | P value | |
|---|---|---|---|---|---|
| No. | 6564 | 3168 | 408 | 1765 | |
| Age, y | 47.9 (0.14) | 42.7 (0.23) | 48.9 (0.55) | 47.3 (0.29) | <0.001 |
| Women, % | 1968 (30.0) | 152 (4.80) | 1 (0.25) | 8 (0.45) | <0.001 |
| Education, % | |||||
| Primary | 508 (7.7) | 177 (5.6) | 54 (13.2) | 190 (10.8) | <0.001 |
| Middle | 5482 (83.6) | 2469 (78.0) | 330 (80.9) | 1512 (85.7) | |
| College | 570 (8.7) | 520 (16.4) | 24 (5.9) | 62 (3.5) | |
| Occupation, % | |||||
| Blue collar | 6018 (91.8) | 2793 (88.3) | 389 (95.3) | 1693 (96.0) | <0.001 |
| White collar | 536 (8.2) | 370 (11.7) | 19 (4.7) | 71 (4.0) | |
| Income, % | |||||
| <500 RMB/month | 2006 (30.6) | 1149 (36.3) | 127 (31.1) | 735 (41.7) | <0.001 |
| 500–1000 RMB/month | 3343 (51.0) | 1154 (36.4) | 191 (46.8) | 654 (37.1) | |
| >1000 RMB/month | 1210 (18.5) | 865 (27.3) | 90 (22.1) | 375 (21.3) | |
| Smoking status, % | |||||
| Never | 4583 (69.9) | 902 (28.5) | 79 (19.4) | 217 (12.3) | 0.001 |
| Past smoker | 386 (5.9) | 271 (8.6) | 31 (7.6) | 114 (6.5) | |
| Current smoker | 1590 (24.2) | 1993 (63.0) | 298 (73.0) | 1432 (81.2) | |
| Hypertension, % | |||||
| Negative | 1180 (18.1) | 488 (15.5) | 37 (9.2) | 159 (9.0) | <0.001 |
| Prehypertension | 1880 (28.8) | 1116 (35.5) | 114 (28.3) | 447 (25.4) | |
| Hypertension | 3464 (53.1) | 1544 (49.1) | 252 (62.5) | 1152 (65.5) | |
| Diabetes, % | |||||
| Negative | 4207 (64.9) | 2132 (68.0) | 244 (61.3) | 1034 (59.3) | 0.01 |
| Prediabetes | 1458 (22.5) | 717 (22.9) | 101 (25.4) | 471 (27.0) | |
| Diabetes | 814 (12.6) | 285 (9.1) | 53 (13.3) | 238 (13.7) | |
| Physical activity, % | |||||
| Never | 470 (7.2) | 332 (10.5) | 28 (6.9) | 246 (14.0) | <0.001 |
| Every time more than 20 min | |||||
| <4 times/week | 5217 (79.5) | 2394 (75.6) | 310 (76.0) | 1253 (71.1) | |
| ≥4 times/week | 872 (13.3) | 439 (13.9) | 70 (17.2) | 263 (15.0) | |
| Body mass index, kg/m2 | 24.8 (0.05) | 24.9 (0.08) | 24.6 (0.18) | 24.7 (0.09) | 0.29 |
| High‐density lipoprotein cholesterol, mmol/L | 1.58 (0.005) | 1.60 (0.008) | 1.66 (0.02) | 1.74 (0.01) | <0.001 |
| Low‐density lipoprotein cholesterol, mmol/L | 2.11 (0.009) | 2.16 (0.01) | 2.15 (0.04) | 2.20 (0.02) | <0.001 |
| Triglyceride, mmol/L | 1.44 (0.02) | 1.47 (0.03) | 1.41 (0.07) | 1.68 (0.03) | <0.001 |
| Urate, μmol/L | 277 (1.06) | 304 (1.72) | 306 (4.06) | 307 (2.15) | <0.001 |
Values are mean (standard error) adjusted for age and sex, or percentages.
Participants were categorized into: nondrinkers, light (women: 0–0.4 servings/day; men: 0–0.9 servings/day), moderate (women: 0.5–1.0 servings/day; men: 1–2 servings/day), and heavy drinkers (women: >1 serving/day; men: >2 servings/day).
In 2012, a total of 641 participants (5.4%) were determined as pRBD. Compared with noncurrent drinkers, current drinkers had a 23% higher likelihood of having pRBD (OR 1.23, 95% CI 1.07–1.59). Both moderate (adjusted OR 1.53, 95% CI 1.01–2.30) and heavy drinkers (adjusted OR 1.29, 95% CI 1.00–1.66) had a significantly higher likelihood of pRBD, after adjusting for age, sex, education level, occupation, income level, smoking status, hypertension, diabetes, physical activity, BMI, plasma concentrations of lipids and urate (Table 2). We observed similar significant results when using 2006 drinking status to predict scores of recent‐1‐year pRBD (2011–2012) (Table S1), or using 2012 drinking status and pRBD status (Table S2). Results did not change materially in the sensitivity analyses using alternate pRBD definition and excluding participants with hyperuricemia or RBD mimics (Table 3).
Table 2.
The odds ratios (ORs) and 95% confidence intervals (95% CIs) of probable REM sleep behavior disorder (pRBD), according to drinking statusa
| None | Light | Moderate | Heavy | P trend | |
|---|---|---|---|---|---|
| No. (%) | 325 (4.95) | 168 (5.30) | 29 (7.11) | 119 (6.74) | |
| Age and sex adjusted | 1.00 (Ref.) | 1.01 (0.82, 1.23) | 1.34 (0.90, 2.00) | 1.27 (1.01, 1.59) | 0.03 |
| Fully adjustedb | 1.00 (Ref.) | 1.16 (0.94, 1.44) | 1.53 (1.01, 2.30) | 1.29 (1.00, 1.66) | 0.03 |
Participants were categorized into: nondrinkers, light (women: 0–0.4 servings/day; men: 0–0.9 servings/day), moderate (women: 0.5–1.0 servings/day; men: 1–2 servings/day), and heavy drinkers (women: >1 serving/day; men: >2 servings/day).
Adjusted for age, sex, education level (primary, middle, or college and higher), income level (<500 RMB/month, 500–1000 RMB/month, or >1000 RMB/month), occupation (blue collar/white collar), physical activity (never, <4 times/week, or ≥4 times/week), smoking status(never, past, or current smoker), hypertension (negative, prehypertension, or hypertension), diabetes (negative, prediabetes, or diabetes), body mass index (<24 kg/m2, 24–28 kg/m2, or ≥28 kg/m2), plasma concentrations of triglyceride (quartiles), low‐density lipoprotein cholesterol (quartiles), high‐density lipoprotein cholesterol (quartiles), and urate (quartiles).
Table 3.
Sensitivity analyses for odds ratios (ORs) and 95% confidence intervals (95% CIs) of probable REM sleep behavior disorder (pRBD), according to drinking statusa , b
| None | Light | Moderate | Heavy | P trend | |
|---|---|---|---|---|---|
| Excluding 1637 participants with hyperuricemiac | 1.00 (Ref.) | 1.16 (0.93, 1.46) | 1.43 (0.91, 2.23) | 1.34 (1.02, 1.75) | 0.02 |
| Using the alternative definition of pRBDd | 1.00 (Ref.) | 1.18 (0.95, 1.46) | 1.53 (1.01, 2.30) | 1.30 (1.01, 1.67) | 0.03 |
| Excluding 190 participants with excessive daytime sleepinesse | 1.00 (Ref.) | 1.10 (0.88, 1.37) | 1.50 (0.99, 2.28) | 1.27 (0.98, 1.65) | 0.04 |
| Excluding 2121 participants with insomniaf | 1.00 (Ref.) | 1.01 (0.78, 1.31) | 1.44 (0.89, 2.32) | 1.18 (0.87, 1.58) | 0.20 |
| Excluding 1274 participants who used hypnoticsg | 1.00 (Ref.) | 1.16 (0.93, 1.46) | 1.45 (0.94, 2.24) | 1.27 (0.98, 1.65) | 0.05 |
| Excluding 2280 participants with intermediate or high risk of obstructive sleep apneah | 1.00 (Ref.) | 1.17 (0.91, 1.50) | 1.95 (1.25. 3.05) | 1.42 (1.07, 1.90) | 0.006 |
Participants were categorized into: nondrinkers, light (women: 0–0.4 servings/day; men: 0–0.9 servings/day), moderate (women: 0.5–1.0 servings/day; men: 1–2 servings/day), and heavy drinkers (women: >1 serving/day; men: >2 servings/day).
Adjusted for age, sex, education level (primary, middle, or college and higher), income level (<500 RMB/month, 500–1000 RMB/month, or >1000 RMB/month), occupation (blue collar/white collar), physical activity (never, <4 times/week, or ≥4 times/week), smoking status(never, past, or current smoker), hypertension (negative, prehypertension, or hypertension), diabetes (negative, prediabetes, or diabetes), body mass index (<24 kg/m2, 24–28 kg/m2, or ≥ 28 kg/m2), plasma concentrations of triglyceride (quartiles), low‐density lipoprotein cholesterol (quartiles), high‐density lipoprotein cholesterol (quartiles), and urate (quartiles).
1462 men and 175 women; pRBD number in nondrinkers: 302.
Based on seven behavioral factors including sleep talking, shouting, limb movements and sleep‐related injuries (score range 0–70, cut‐off >7); pRBD number in nondrinkers: 369.
The Epworth Sleepiness Scale ≥10; 168 men and 22 women; pRBD number in nondrinkers: 316.
The Athens Insomnia Scale ≥6; 1599 men and 522 women; pRBD number in nondrinkers: 244.
968 men and 306 women; pRBD number in nondrinkers: 284.
The STOPBANG score ≥3; 2256 men and 24 women; pRBD number in nondrinkers: 172.
The interactions between pRBD and sex or age were not significant (P for interaction >0.3 for both) (Table S3). We observed a nonsignificant trend between each alcoholic beverage and higher likelihood of having pRBD (Fig. 1).
Figure 1.
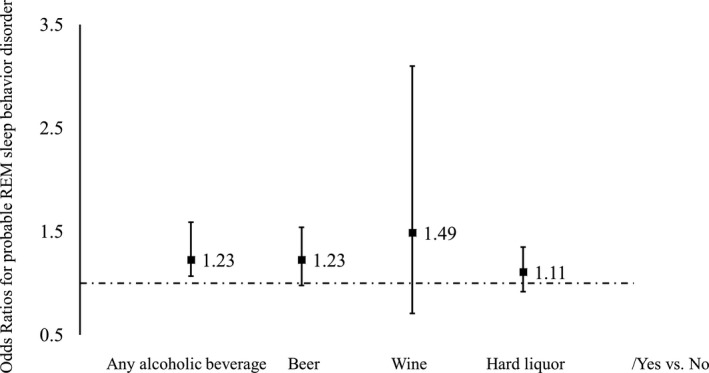
Odds ratios for probable REM sleep behavior disorder by types of alcoholic beverage1,2. 1Adjusted for age, sex, education level (primary, middle, or college and higher), income level (<500 RMB/month, 500–1000 RMB/month, or >1000 RMB/month), occupation (blue collar/white collar), physical activity (never, <4 times/week, or ≥4 times/week), smoking status(never, past, or current smoker), hypertension (no, prehypertension, or hypertension), diabetes (no, prediabetes, or diabetes), body mass index (<24 kg/m2, 24–28 kg/m2, or ≥28 kg/m2), plasma concentrations of triglyceride (quartiles), low‐density lipoprotein cholesterol (quartiles), high‐density lipoprotein cholesterol (quartiles), and urate (quartiles). 2Sample size and percentage of participants with probable REM sleep behavior disorder in any alcoholic beverage group: Negative (325, 4.95%)/Positive (316, 5.92%); beer group: Negative (515, 5.06%)/Positive (111, 6.07%); wine group: Negative (618, 5.38%)/Positive (8, 6.35%); hard liquor group: Negative (389, 5.08%)/Positive (237, 6.00%).
Discussion
In this community‐based study of 11,905 adults, we observed a significant relationship between alcohol consumption and higher likelihood of having pRBD 6 years later. Current drinkers were 23% more likely to have pRBD, independent of potential co‐determinants, comprising age and sex. Excluding individuals with hyperuricemia or RBD mimics did not change the significant association between alcohol consumption and likelihood of pRBD, suggesting that the observed alcohol‐pRBD relationship cannot be totally explained by these two factors.
To our knowledge, limited studies were conducted to investigate risk factors and RBD, and no studies specifically focused on alcohol. A multicenter case‐controlled study of environmental risk factors for idiopathic RBD (iRBD), with 347 cases and 347 controls, found a nonsignificantly higher alcohol intake frequency in the case group, relative to the control group.4 Another community‐based study with 3635 participants showed a significant association between use of alcoholic beverage and pRBD, with an age‐ and sex‐adjusted OR of 2.16 (95% CI: 1.03, 4.50) for current drinkers, versus never drinkers.12
Our study extends prior reports. Alcohol, particularly from beer, had been observed to be associated with lower PD risk in other epidemiological studies.11, 26, 27 In contrast, the positive association between alcohol intake and higher pRBD likelihood was similar across different alcoholic beverages. In this study, moderate drinkers had the largest likelihood of having pRBD. Probably small sample size in this group led to unstable estimations.
Consistently, in a previous study including 3635 Chinese adults, consumption of beer or spirit was both significantly associated with higher odds of having pRBD.12 The opposite direction for the alcohol‐pRBD relationship, particularly for beer, relative to its inverse association with PD, suggests that some RBDs might have different pathogenesis and hence risk factors from PD (i.e., synucleinopathies). Alternately, there is a possibility that alcohol could trigger early presentation or increase severity of synucleine‐mediated RBD. Our observations also suggest that the association could be driven by ethanol, rather than other components (e.g., purine) in alcoholic beverages.
The mechanism by which alcohol may increase RBD risk remains unclear. We could speculate that this may involve changes in the sleep architecture, similar to other REM suppressing substances, such as antidepressants, which are well established as factors that can provoke RBD events. In this study, we also found that moderate and heavy drinkers, but not lighter drinkers, were at increased likelihood of pRBD, probably because the effect on sleep is more significant at these higher doses. Alcohol leads to early sleepiness, and has a REM suppressant effect mainly in the first half of the night. It delays the appearance of the first REM period and reduces the amount of REM in the first half of the night in favor of slow wave sleep.28 It has a short half‐life, and later in the night the number and duration of awakenings tends to increase.29, 30, 31 Patients commonly report nightmares, possibly a reflection of the combination of REM rebound (a consequence of the initial REM suppression) in the latter part of the night and a more fragmented sleep. It may change dream content, favoring more aggressive dreams.32 Dreams reported by patients with RBD are also more likely to be action filled and aggressive, compared to abnormal events from other sleep stages.33, 34
Our study used a questionnaire, and although mostly accurate, may mis‐identify as RBD some events that are of a different nature. These include nightmares with and arousal, behavior related to the alcohol itself, or NREM parasomnias. There can also be arousals secondary to OSA, which can be worsened by alcohol due to its muscle relaxant effect. We have attempted to minimize this effect by eliminating participants who have a large neck circumference and a moderate or high risk of OSA on STOP‐BANG questionnaire. While this does not automatically rule out the presence of OSA, the risk is significantly reduced. Further studies using a PSG for confirmation can provide further information. Based on RBD prevalence, which was shown in a Korean study based on PSG,35 and sensitivity/specificity of the RBDQ‐HK,14 only an expected ~12% of participants who screened positive for pRBD likely had RBD. However, previous studies suggested the individuals with pRBD, which was identified via questionnaire, had higher risk of dementia and PD.36 Given the risk of misclassification of RBD status with this methodology, we used the term pRBD in this report. However, in prior studies with RBD diagnosed by PSG, a similar association between alcohol and RBD was also observed.4 To reduce the effects of potential misclassification of RBD status, we also did sensitivity analyses by excluding participants with RBD mimics. Results did not materially change, suggesting that the effect of these factors on alcohol‐pRBD relation could be small‐to‐modest. Furthermore, although we used exposures from 2006 to predict pRBD status in 2012, our study is still considered as a cross‐sectional study, so the temporal relationship between alcohol consumption and pRBD was unclear. However, using scores of recent‐1‐year pRBD introduced a prospective component, and the analysis with restriction to those with symptom onset in the past year generated consistent results. In addition, high alcohol use might be a sign of mental illness, which has been linked to RBD. We did not have information on mental health or use of antidepressants. However, we still observed a significant association between moderate alcohol intake and high risk of having pRBD. Similarly, we did not collect data on periodic limb movements, which could be another resource of residual confounding, leading to an overestimation regarding the alcohol‐RBD relationship. Furthermore, we did not collect detailed information on OSA until 2014, although we generated similar results after we excluded those who were considered as at intermediate or high risk of OSA.
The advantages of our study include its large sample size, detailed information on alcohol consumption, and rigorous data collection for all potential confounders among the participants. Future studies with clinically confirmed RBD, large sample size for information on types of alcoholic beverage, and prospective study design would be appropriate to further investigate this association.
Conflict of Interest
The authors declare no conflict of interest.
Supporting information
Table S1. The adjusted mean differences and 95% confidence intervals (CIs) of scores of recent‐1‐year probable REM behavior sleep disorder, according to drinking status.
Table S2. The odds ratios (ORs) and 95% confidence intervals (CIs) of probable REM sleep behavior disorder (pRBD), according to drinking status in 2012.
Table S3. The odds ratios (ORs) and 95% confidence intervals (CIs) of probable REM sleep behavior disorder (pRBD), according to drinking status, stratified by age, and sex.
Acknowledgments
This work was supported by the National Institute of Neurological Disorders And Stroke at the National Institutes of Health (NINDS 5R21NS087235‐02 and 1R03NS093245‐01A1).
Funding Information
This work was supported by the National Institute of Neurological Disorders and Stroke at the National Institutes of Health (NINDS 5R21NS087235‐02 and 1R03NS093245‐01A1).
Funding Statement
This work was funded by National Institute of Neurological Disorders and Stroke grants 5R21NS087235‐02 and 1R03NS093245‐01A1; National Institutes of Health grant .
Contributor Information
Shouling Wu, Email: drwusl@163.com.
Xiang Gao, Email: xxg14@psu.edu.
References
- 1. Postuma RB, Gagnon JF, Montplaisir J. Rapid eye movement sleep behavior disorder as a biomarker for neurodegeneration: the past 10 years. Sleep Med 2013;14:763–767. [DOI] [PubMed] [Google Scholar]
- 2. Howell MJ, Schenck CH. Rapid eye movement sleep behavior disorder and neurodegenerative disease. JAMA Neurol 2015;72:707–712. [DOI] [PubMed] [Google Scholar]
- 3. Frauscher B, Jennum P, Ju YE, et al. Comorbidity and medication in REM sleep behavior disorder: a multicenter case‐control study. Neurology 2014;82:1076–1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Postuma RB, Montplaisir JY, Pelletier A, et al. Environmental risk factors for REM sleep behavior disorder: a multicenter case‐control study. Neurology 2012;79:428–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wong JC, Li J, Pavlova M, et al. Risk factors for probable REM sleep behavior disorder: a community‐based study. Neurology 2016;86:1306–1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ma JF, Qiao Y, Gao X, et al. A community‐based study of risk factors for probable rapid eye movement sleep behavior disorder. Sleep Med 2017;30:71–76. [DOI] [PubMed] [Google Scholar]
- 7. Marmot M, Brunner E. Alcohol and cardiovascular disease: the status of the U shaped curve. BMJ 1991;303:565–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Maclure M. Demonstration of deductive meta‐analysis: ethanol intake and risk of myocardial infarction. Epidemiol Rev 1993;15:328–351. [DOI] [PubMed] [Google Scholar]
- 9. Hu N, Yu JT, Tan L, et al. Nutrition and the risk of Alzheimer's disease. Biomed Res Int 2013;2013:524820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wright CB, Elkind MS, Rundek T, et al. Alcohol intake, carotid plaque, and cognition: the Northern Manhattan Study. Stroke 2006;37:1160–1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hernan MA, Chen H, Schwarzschild MA, Ascherio A. Alcohol consumption and the incidence of Parkinson's disease. Ann Neurol 2003;54:170–175. [DOI] [PubMed] [Google Scholar]
- 12. Ma C, Pavlova M, Liu Y, et al. Probable REM sleep behavior disorder and risk of stroke: a prospective study. Neurology 2017;88:1849–1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Huang S, Li J, Shearer GC, et al. Longitudinal study of alcohol consumption and HDL concentrations: a community‐based study. Am J Clin Nutr 2017;105:905–912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Li SX, Wing YK, Lam SP, et al. Validation of a new REM sleep behavior disorder questionnaire (RBDQ‐HK). Sleep Med 2010;11:43–48. [DOI] [PubMed] [Google Scholar]
- 15. Shen SS, Shen Y, Xiong KP, et al. Validation study of REM sleep behavior disorder questionnaire‐Hong Kong (RBDQ‐HK) in east China. Sleep Med 2014;15:952–958. [DOI] [PubMed] [Google Scholar]
- 16. McGuire S. U.S. Department of Agriculture and U.S. Department of Health and Human Services, Dietary Guidelines for Americans, 2010. 7th Edition, Washington, DC: U.S. Government Printing Office, January 2011. Adv Nutr. 2011;2:293–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Chung KF, Kan KK, Yeung WF. Assessing insomnia in adolescents: comparison of Insomnia Severity Index, Athens Insomnia Scale and Sleep Quality Index. Sleep Med 2011;12:463–470. [DOI] [PubMed] [Google Scholar]
- 18. Sun JL, Chiou JF, Lin CC. Validation of the Taiwanese version of the Athens Insomnia Scale and assessment of insomnia in Taiwanese cancer patients. J Pain Symptom Manage 2011;41:904–914. [DOI] [PubMed] [Google Scholar]
- 19. Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep 1991;14:540–545. [DOI] [PubMed] [Google Scholar]
- 20. Chen NH, Johns MW, Li HY, et al. Validation of a Chinese version of the Epworth sleepiness scale. Qual Life Res 2002;11:817–821. [DOI] [PubMed] [Google Scholar]
- 21. Chung F, Yegneswaran B, Liao P, et al. STOP questionnaire: a tool to screen patients for obstructive sleep apnea. Anesthesiology 2008;108:812–821. [DOI] [PubMed] [Google Scholar]
- 22. Nagappa M, Liao P, Wong J, et al. Validation of the STOP‐bang questionnaire as a screening tool for obstructive sleep apnea among different populations: a systematic review and meta‐analysis. PLoS ONE 2015;10:e0143697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Gao X, Chen H, Choi HK, et al. Diet, urate, and Parkinson's disease risk in men. Am J Epidemiol 2008;167:831–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Gao X, O'Reilly EJ, Schwarzschild MA, Ascherio A. Prospective study of plasma urate and risk of Parkinson disease in men and women. Neurology 2016;86:520–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Schenck CH, Bundlie SR, Patterson AL, Mahowald MW. Rapid eye movement sleep behavior disorder. a treatable parasomnia affecting older adults. JAMA 1987;257:1786–1789. [PubMed] [Google Scholar]
- 26. Palacios N, Gao X, O'Reilly E, et al. Alcohol and risk of Parkinson's disease in a large, prospective cohort of men and women. Mov Disord 2012;27:980–987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Noyce AJ, Bestwick JP, Silveira‐Moriyama L, et al. Meta‐analysis of early nonmotor features and risk factors for Parkinson disease. Ann Neurol 2012;72:893–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ebrahim IO, Shapiro CM, Williams AJ, Fenwick PB. Alcohol and sleep I: effects on normal sleep. Alcohol Clin Exp Res 2013;37:539–549. [DOI] [PubMed] [Google Scholar]
- 29. Arnedt JT, Rohsenow DJ, Almeida AB, et al. Sleep following alcohol intoxication in healthy, young adults: effects of sex and family history of alcoholism. Alcohol Clin Exp Res 2011;35:870–878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lands WE. Alcohol, slow wave sleep, and the somatotropic axis. Alcohol 1999;18:109–122. [DOI] [PubMed] [Google Scholar]
- 31. Roehrs T, Roth T. Sleep, sleepiness, sleep disorders and alcohol use and abuse. Sleep Med Rev 2001;5:287–297. [DOI] [PubMed] [Google Scholar]
- 32. Steinig J, Foraita R, Happe S, Heinze M. Perception of sleep and dreams in alcohol‐dependent patients during detoxication and abstinence. Alcohol Alcohol 2011;46:143–147. [DOI] [PubMed] [Google Scholar]
- 33. Fantini ML, Corona A, Clerici S, Ferini‐Strambi L. Aggressive dream content without daytime aggressiveness in REM sleep behavior disorder. Neurology 2005;65:1010–1015. [DOI] [PubMed] [Google Scholar]
- 34. Uguccioni G, Golmard JL, de Fontreaux AN, et al. Fight or flight? Dream content during sleepwalking/sleep terrors vs. rapid eye movement sleep behavior disorder. Sleep Med 2013;14:391–398. [DOI] [PubMed] [Google Scholar]
- 35. Kang SH, Yoon IY, Lee SD, et al. REM sleep behavior disorder in the Korean elderly population: prevalence and clinical characteristics. Sleep 2013;36:1147–1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Boot BP, Boeve BF, Roberts RO, et al. Probable rapid eye movement sleep behavior disorder increases risk for mild cognitive impairment and Parkinson disease: a population‐based study. Ann Neurol 2012;71:49–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. The adjusted mean differences and 95% confidence intervals (CIs) of scores of recent‐1‐year probable REM behavior sleep disorder, according to drinking status.
Table S2. The odds ratios (ORs) and 95% confidence intervals (CIs) of probable REM sleep behavior disorder (pRBD), according to drinking status in 2012.
Table S3. The odds ratios (ORs) and 95% confidence intervals (CIs) of probable REM sleep behavior disorder (pRBD), according to drinking status, stratified by age, and sex.


