Abstract
Objective
To determine if altered tryptophan (Trp) metabolism is associated with MS risk or disease severity in children.
Methods
Participants with pediatric‐onset MS and clinically isolated syndrome (CIS) within 4 years of disease onset and healthy controls underwent collection of serum. Longitudinal disability and processing speed measures and relapse data were collected in cases. Global metabolomics were conducted in 69/67 cases/controls. Targeted Trp measurement was performed in a discovery group (82 cases, 50 controls) and a validation group (92 cases, 50 controls), while functional gut microbiome analysis was done in 17 cases. Adjusted logistic, linear and negative binomial regression and Cox‐proportional hazard models were used.
Results
Using global metabolomics data, higher relative abundances of Trp and indole lactate, a known gut microbiota‐derived Trp metabolite, were associated with lower risk of MS. In cases, higher relative abundances of gut microbiota‐derived Trp metabolites were associated with lower disability and higher processing speed scores and higher relative abundance of kynurenine was associated with higher relapse rate. Using targeted tryptophan measures, in the discovery and validation groups, each 1 mcg/mL increase in serum Trp level was associated with 20% (95% CI: 4–34%) and 32% (95% CI: 16–44%) decrease in adjusted odds of having MS, respectively. A lower relative abundance of gut microbial genes involved in Trp catabolism was associated with higher relapse risk.
Interpretation
Trp metabolism by the gut microbiota and the kynurenine pathway may be relevant to the risk of MS in children as well as MS activity and severity.
Introduction
Multiple sclerosis (MS) is an inflammatory and degenerative disease of the central nervous system (CNS). While the causes of MS remain unclear and even less is known as to what might trigger MS relapses,1, 2, 3, 4, 5 both genetic and environmental exposures are thought to contribute.6 Diet may influence the risk of both disease onset and activity, although findings have been mixed.6 For example, dietary salt intake has been studied as a possible contributing factor to disease onset and subsequent disease activity with conflicting results.7, 8, 9, 10 In addition, lower iron intake (but not other dietary factors) were associated with increased risk of pediatric MS, while higher saturated fat and vegetable intake were associated with increased and decreased relapse risk, respectively.11, 12 Although prevention of relapses is an important treatment outcome, preservation of cognitive and physical functioning in patients with MS are also important goals. However, our understanding of environmental and dietary factors that affect cognitive and physical disability outcomes is even more limited.
Tryptophan (Trp), an essential amino acid, and its metabolites have recently been reported to affect the immune response in MS and in an animal model of MS, experimental autoimmune encephalomyelitis (EAE).13 Several Trp metabolites downregulate CNS immune responses through activation of the aryl hydrocarbon receptor (AHR) on glial cells.13, 14 Interestingly, these Trp metabolites are produced by intestinal bacteria and this may affect the risk of MS.15 On the other hand, some endogenous Trp metabolites (for example those produced in the kynurenine pathway) have pro‐inflammatory and neurotoxic properties and may be associated with worse neurodegeneration in MS.16
Pediatric MS, while relatively rare, represents a unique opportunity to investigate risk factors for disease onset and course, by leveraging a possibly higher genetic and environmental exposure burden for susceptibility and a higher relapse rate compared to adults with the disease.17 The goal of this study was to determine if alterations in Trp metabolic pathways are associated with the risk of MS onset and disease course and severity in pediatric MS.
Methods
Study participants
An overview of the study design is depicted in Figure 1. We first performed non‐targeted metabolomics, assessing tryptophan and its endogenous and exogenous metabolites in a group of cases and controls (metabolomics set). Those analyses guided further studies using targeted Trp measurement in two larger discovery (TRP1) and replication (TRP2) cohorts. Non‐targeted metabolomics is the comprehensive analysis of all metabolites in a sample (including the unknown chemicals) and provides the opportunity for discovering new targets and generating hypotheses. Targeted metabolomics, as its name implies, is the measurement of predefined and chemically characterized metabolites.18
Figure 1.
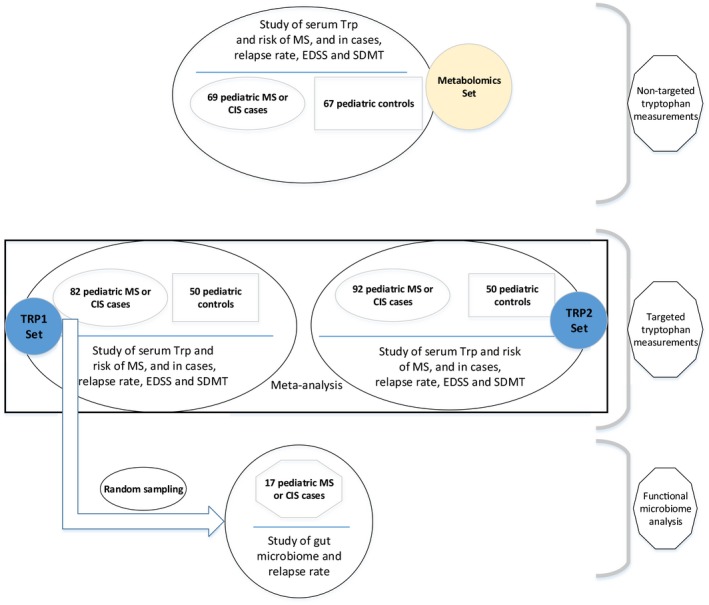
Overview of the study design.
All MS cases were recruited at the University of California, San Francisco (UCSF) Pediatric MS Center. Cases in the global metabolomics cohort (metabolomics set) were randomly chosen from one of these cohorts: (1) pediatric MS or clinically isolated syndrome (CIS) patients who were evaluated at UCSF Pediatric MS clinic from January 2006 to November 2011 and were enrolled in a prospective cohort study (with average follow‐up time of 2.8 years) or (2) pediatric MS or CIS patients who were part of a multicenter case‐control study of genetic and environmental risk factors in pediatric MS whose demographic and clinical data, including prospective relapse information were collected from November 2011 up until February 2016 (with average follow‐up time of 3.6 years) (R01NS071463, PI Waubant). For both groups, patients with relapsing‐remitting MS or CIS suggestive of MS (at least 2 silent T2‐bright foci on MRI) occurring before age 18 years and seen within 4 years of symptom onset19, 20 were eligible for inclusion. Case status was confirmed by a pediatric MS specialist panel. Patients provided blood samples at the time of recruitment in the study; a subset also provided a stool sample. For targeted Trp analyses (TRP1 and TRP2 sets) all cases enrolled at UCSF in both the cohort and multicenter case‐control studies for whom serum was available were included.
For both the global metabolomics (metabolomics set) and targeted tryptophan discovery and replication sets (TRP1 and TRP2), control subjects were chosen randomly, without replacement, from a larger pool of controls enrolled at UCSF as part of the multicenter case‐control study of genetic and environmental risk factors in pediatric MS (R01NS071463, PI Waubant). Control subjects were recruited at general or specialty pediatric clinics and fulfilled the following eligibility criteria: age less than 22 years, absence of autoimmune disorders except eczema or asthma, no previous history of severe health conditions or treatment with immunosuppressive medications, and biological parents and siblings without MS. In the metabolomics set, the cases and controls were similar with regard to age (±1 year), sex and race/ethnicity.
The Institutional Review Board at UCSF approved the study. Participants and their parents provided assent and informed consent.
Demographic and clinical information
For participating cases and controls, demographic data were collected at the time of enrollment, with race and ethnicity self‐reported according to the USA's National Institute of Health criteria.21 We then categorized the race/ethnicities into four groups: (1) White, non‐Hispanic, (2) White, Hispanic, (3) Black, (4) others.
Clinical data (cases only) included number of relapses, follow‐up time and use of disease‐modifying therapies (DMTs), as well as the Expanded Disability Status Scale (EDSS) and, from 2013 onwards, the Symbol Digit Modalities Test (SDMT). The EDSS is the most widely used disability measure in MS studies; a higher score denotes more severe disability.22 The SDMT is a measure of processing speed, one of the most‐commonly affected cognitive domains in MS; lower scores indicate more impaired processing speed.23 The EDSS and SDMT recorded at a clinic visit at the same time or after blood collection were used for analyses. Relapses were defined as new or recurrent neurological symptoms localizing to the CNS that lasted for a minimum of 24 h after a remission of at least 30 days since the previous attack in the absence of fever or infection. Only the prospectively collected relapses (i.e., those occurring after study enrollment) were included in the analyses.
Follow‐up duration was calculated from the time of enrollment to the end of available follow‐up. Dates of initiation and cessation of disease‐modifying therapy (DMT) were recorded for all cases at enrollment and during follow‐up. DMT use was reported as ‘exposed versus. unexposed,’ with ‘exposed’ defined as >50% of time on DMT during follow‐up. For sensitivity analyses, we used the proportion of follow‐up time on a DMT as a continuous variable in the models.
Non‐targeted metabolomics analysis
Serum samples were frozen within 3 h of collection at −80°. Global untargeted metabolomics utilizing liquid/gas chromatography followed by tandem mass spectrometry was performed as previously described (Metabolon Inc., Morrisville, NC).24, 25 Briefly, after proteins were precipitated, samples were divided in five fractions for analyses by different mass spectrometry techniques. Metabolites were identified by automated comparison of ion features in the study samples to a reference library. After quantification of peaks using area under the curve, raw values for each metabolite were normalized. Standard quality control measures were applied.
Trp metabolites identified through this method included tryptophan, kynurenine, kynurenate, N‐formyl anthranilic acid, xanthurenate, picolinate, serotonin, N‐formyl anthranilic acid, indole lactate, indole acetate, indole propionate and 3‐indoxyl sulfate (Fig. 2).
Figure 2.
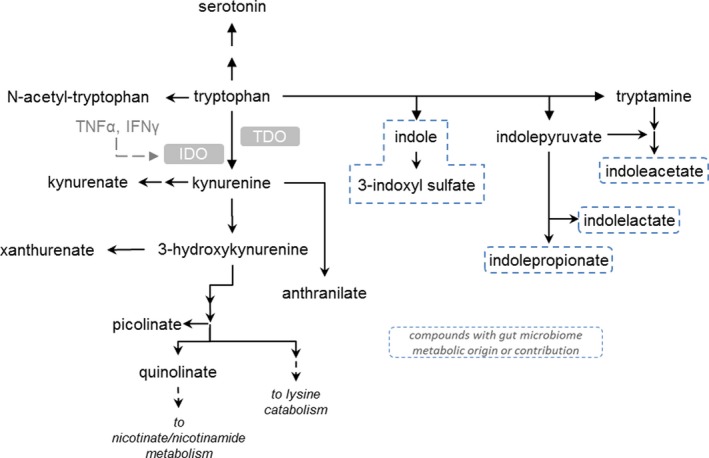
Tryptophan metabolism pathway. TNF, tumor necrosis factor; IFNg, Interferon gamma; IDO, indoleamine 2,3‐dioxygenase; TDO, tryptophan 2,3‐dioxygenase
Targeted serum Trp measurements
Because difference in Trp levels between cases and controls was the strongest finding, targeted Trp analysis was performed in a larger group of cases and controls (TRP1 set) and was replicated in a second group of cases and controls (TRP2 set). Trp levels were measured by liquid chromatography‐mass spectrometry (Metabolon Inc., Morrisville, NC). The range of quantitation was 2.00–100 μg/mL based on the analysis of 0.05 mL of serum. Calibration samples were prepared at eight different concentration levels by spiking phosphate buffered saline solution with corresponding calibration spiking solutions. Calibration samples, study samples and quality control samples were spiked with internal standard solution (Trp‐D3) and were subjected to protein precipitation with an organic solvent. Following centrifugation, an aliquot of the supernatant was injected onto an Agilent 1290/AB Sciex QTrap 5500 liquid chromatography‐mass spectrometry/mass spectrometry system equipped with a C18 reversed phase ultra‐high‐performance liquid chromatography (UHPLC) column. The mass spectrometer was operated in positive mode using electrospray ionization. The peak area of the m/z 205.1 → 146.1 product ion of Trp was measured against the peak area of the Trp‐D3 product ion of m/z 208.1 → 146.1. Quantitation was performed using a weighted (1/x) linear least squares regression analysis generated from fortified calibration standards prepared immediately prior to each run.
Serum 25(OH)D measurements
Serum 25(OH) vitamin D (25OHD) levels were measured using chemiluminescence assay as previously described (Heartland Assays, Ames, IA).26 25OHD levels were used to adjust analyses because they are associated with risk of MS onset and MS outcomes, such as relapse and possibly disability.26, 27
Gut microbiome analysis
Stool samples were available from 17 of the 82 cases in the discovery set.28 We extracted and amplified DNA from stool samples and performed 16S rRNA sequencing. We then applied a validated algorithm to enable metagenomic predictions from this sequencing data, and draw inferences as to the potential functional capacity of the gut microbiota (PiCRUSt; Phylogenetic investigation of Communities by Reconstruction of Unobserved States).29
Statistical analysis
For untargeted metabolomics data, we normalized (log transformation) and standardized the reported metabolite abundance and then performed logistic and only in cases, negative binomial regression to analyze the association with disease odds and relapse risk, respectively. We used a linear regression model to assess the cross‐sectional association between Trp metabolites and EDSS and SDMT. We adjusted all models for age, sex, race/ethnicity and serum 25OHD, and the negative binomial model for DMT use. Regression coefficients, odds ratio (OR), incident rate ratio (IRR) and corresponding P‐values were reported, as appropriate. P‐values <0.05 were considered statistically significant. Because of the exploratory nature of the study, correction for multiple comparisons was not performed. As we used the normalized values of relative abundance of metabolites in our statistical models, for non‐targeted analyses, the point estimates should only be interpreted in relative terms.
For targeted Trp analysis, serum Trp level was included in the models as a continuous variable. The association between serum Trp level and disease risk was assessed by logistic regression models. The results in TRP1 and TRP2 datasets were pooled using fixed‐effects inverse‐variance meta‐analyses. Negative binomial regression models were used to assess the association between serum Trp levels and relapse rate. Regression coefficient, OR, IRR, corresponding 95% confidence intervals (CIs) and P‐values were reported, as appropriate.
For the PiCRUSt analysis, we specifically selected the Trp pathway and assessed its association with subsequent relapse risk using Kaplan‐Meier curves, followed by a Cox model adjusted for DMT use at the time of the stool sample (exposed vs. unexposed). Analyses were performed using Stata 13.1 (StataCorp, TX) and the Statistical Package for the Social Sciences (SPSS) (IBM Corp. Version 24).
Results
Characteristics of cases and controls
Global untargeted metabolomics analysis was performed on 69 MS/CIS cases and 67 age, sex and race/ethnicity similar controls in the metabolomics set. Targeted measurements of serum Trp were done in 82 MS/CIS cases and 50 controls in the TRP1 set, and 92 MS/CIS cases and 50 controls in the TRP2 set (see Fig. 1). In targeted Trp datasets, the controls were significantly different to the cases on age (older), proportion female (TRP2), and race/ethnicity. Clinical and demographic characteristics of cases and controls in the non‐targeted and targeted Trp analyses are summarized in Tables 1 and 2, respectively.
Table 1.
Baseline characteristics (at enrollment) of the pediatric‐onset MS cases and controls included in the Trp metabolites analysis (non‐targeted assays)
| MS Cases | Control | P‐value1 | |
|---|---|---|---|
| N | 69 | 67 | |
| Age in years, mean (SD) | 14.3 (2.8) | 14.5 (2.6) | 0.63 |
| Female (%) | 32 (46%) | 32 (48%) | 0.87 |
| Race/ethnicity (%) | |||
| White, non‐Hispanic | 36 (52%) | 36 (54%) | 0.92 |
| White, Hispanic | 22 (32%) | 22 (33%) | |
| Black | 5 (7%) | 3 (4%) | |
| Others | 6 (9%) | 6 (9%) | |
| 25(OH) vitamin D in ng/mL, mean (SD) | 24.5 (9.8) | 23.5 (8.8) | 0.55 |
| DMT exposed (%) | 50 (74) | N/A | – |
| Follow‐up duration in years, mean (SD) | 3.2 (1.8) | N/A | – |
| Median EDSS (IQR) | 3 (2–4) | N/A | – |
| Mean SDMT (SD) | 53.2 (15.1) [32 out of 69 patients had scores available] | N/A | – |
DMT, disease‐modifying therapy.
1Student T‐test for continuous variables and chi‐square test for categorical variables.
Table 2.
Baseline characteristics (at enrollment) of the pediatric MS cases and controls and subsequent follow‐up for cases (targeted tryptophan analysis)
| TRP1 set (Discovery) | TRP2 set (Replication) | |||||
|---|---|---|---|---|---|---|
| Cases | Control | P‐value1 | Cases | Controls | P‐value1 | |
| N | 82 | 50 | 92 | 50 | ||
| Age, mean (SD) | 13.4 (3.5) | 15.1 (2.8) | 0.004 | 12.6 (4.5) | 14.5 (2.9) | 0.01 |
| Female (%) | 56 (68%) | 27 (54%) | 0.10 | 62 (67%) | 17 (34%) | <0.001 |
| Race/ethnicity (%) | ||||||
| White, non‐Hispanic | 24 (29%) | 26 (52%) | 24 (26%) | 29 (58%) | ||
| White, Hispanic | 34 (41%) | 16 (32%) | 0.035 | 42 (46%) | 13 (26%) | 0.002 |
| Black | 6 (7%) | 4 (8%) | 7 (8%) | 1 (2%) | ||
| Others | 18 (22%) | 4 (8%) | 19 (21%) | 7 (14%) | ||
| Trp in mcg/mL, mean (SD) | 11.0 (2.3) | 12.3 (2.4) | 0.003 | 10.3 (2.6) | 12.1 (2.2) | <0.001 |
| 25(OH) vitamin D, ng/mL, mean (SD) | 24.6 (9.8) | 23.7 (7.3) | 0.57 | 24.2 (10.8) | 22.8 (10.2) | 0.43 |
| DMT exposed (%) | 66 (80%) | N/A | – | 69 (76%) | N/A | – |
| Follow‐up duration (from baseline to study end), years, mean (SD) | 3.5 (1.7) | N/A | – | 3.5 (2.3) | N/A | – |
| Median EDSS (IQR) | 3 (2–4) | N/A | – | 3 (2–4) | N/A | – |
| Mean SDMT (SD) | 51.3 (13.2) [62 out 82 patients had scores available] | N/A | – | 45.7 (13.2) [13 out of 92 patients had scores available] | ||
Trp, tryptophan; DMT, disease‐modifying therapy.
1Student T‐test for continuous variables and chi‐square test for categorical variables.
Serum levels of Trp metabolites and risk of pediatric‐onset MS
In the non‐targeted metabolomics sample, a higher relative abundance of Trp and indole lactate in the serum were associated with lower risk of developing pediatric MS (For Trp: adjusted OR: <0.01, P‐value of <0.0001; for indole lactate: adjusted OR: 0.11, P < 0.001). There were no significant associations between kynurenine, kynurenate, N‐formyl anthranilic acid, xanthurenate, picolinate, serotonin, N‐formyl anthranilic acid, indole acetate, indole propionate and 3‐indoxyl sulfate and pediatric MS risk (Table 3).
Table 3.
Association between the levels of serum tryptophan metabolites (non‐targeted analysis) and the risk of MS, relapse rate, EDSS and SDMT
| MS risk* (case‐control) | Relapse rate** | EDSS** | SDMT** | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Odds ratio*** | P‐value | IRR*** | P‐value | Regression coefficient*** | P‐value | Regression coefficient*** | P‐value | ||
| Tryptophan | 0.002 | <0.001 | 4.2 | 0.08 | −1.4 | 0.19 | −0.68 | 0.97 | |
| Serotonin | 0.97 | 0.90 | 0.95 | 0.80 | 0.19 | 0.47 | 4.7 | 0.33 | |
| Gut microbiota‐derived metabolites | Indole lactate | 0.11 | <0.001 | 1.21 | 0.70 | −0.66 | 0.30 | 4.34 | 0.69 |
| Indole acetate | 0.94 | 0.78 | 1.16 | 0.38 | −0.20 | 0.36 | 7.8 | 0.021 | |
| Indole propionate | 1.04 | 0.81 | 1.16 | 0.17 | −0.40 | 0.004 | 4.7 | 0.016 | |
| 3‐indoxyl sulfate | 0.63 | 0.20 | 1.16 | 0.61 | −0.06 | 0.88 | 1.56 | 0.79 | |
| Kynurenine pathway metabolites | Kynurenine | 0.33 | 0.15 | 5.8 | 0.003 | −0.37 | 0.63 | −11.6 | 0.30 |
| Kynurenate | 0.61 | 0.25 | 1.45 | 0.23 | −0.51 | 0.19 | −6.1 | 0.34 | |
| Xanthurenate | 0.66 | 0.14 | 1.25 | 0.18 | −0.21 | 0.42 | −7.5 | 0.10 | |
| picolinate | 0.41 | 0.16 | 1.66 | 0.15 | −0.22 | 0.63 | −9.9 | 0.23 | |
| N‐formyl anthranilic acid | 0.96 | 0.76 | 1.03 | 0.69 | 0.15 | 0.17 | −2.0 | 0.17 | |
IRR, incidence rate ratio; EDSS, expanded disability status scale; SDMT, symbol digit modalities test.
Statistically significant results are highlighted.
*Adjusted for age sex, race‐ethnicity and serum 25‐OH vitamin D level
**Adjusted for age of onset of the disease, sex, race‐ethnicity, serum 25‐OH vitamin D level and use of DMT
***The relative abundance of metabolites was scaled and log transformed. The odds ratios, IRRs and regression coefficients should not be interpreted as the change for each one‐unit difference in the serum level of the metabolite.
Because Trp had the strongest association with the risk of MS, we performed targeted measurements in two larger groups of cases and controls. In the TRP1 set, the mean (standard deviation) serum Trp level was 11.0 (2.3) mcg/mL for cases and 12.3 (2.4) mcg/mL for controls; after adjusting for age, sex, race/ethnicity and serum 25OHD levels, each additional 1 mcg/mL in serum Trp level was associated with decreased odds of MS by 20% (95% CI: 4–34%, P‐value: 0.017) (Table 4). In the TRP2 set, the mean (standard deviation) serum Trp level was 10.3 (2.6) mcg/mL in cases and 12.1 (2.4) mcg/mL in controls. After adjusting for age, sex, race/ethnicity and serum 25OHD levels, each additional 1 mcg/mL in serum Trp level was associated with decreased odds of MS by 32% (95% CI: 16–44%, P‐value < 0.001) (Table 4).
Table 4.
Association between baseline serum tryptophan levels and risk of having pediatric MS (targeted tryptophan analysis)
| TRP1 set (Discovery) | TRP2 set (Replication) | |||||
|---|---|---|---|---|---|---|
| OR | 95% CI | P‐value | OR | 95% CI | P‐value | |
| Serum Trp (per 1 mcg/mL) (unadjusted) | 0.79 | 0.68–0.93 | 0.004 | 0.75 | 0.65–0.88 | <0.001 |
| Serum Trp (per 1 mcg/mL) (adjusted) | 0.80 | 0.67–0.96 | 0.017 | 0.68 | 0.56–0.84 | <0.001 |
| Age (1‐year increment) | 0.80 | 0.68–0.94 | 0.006 | 0.79 | 0.69–0.91 | 0.001 |
| Sex (female [reference]) | 1.72 | 0.70–4.23 | 0.237 | 4.44 | 1.78–11.07 | 0.001 |
| Race/ethnicity (vs. White non‐Hispanic [reference]) | ||||||
| White Hispanic | 2.93 | 1.11–7.73 | 0.070 | 7.39 | 2.57–21.26 | 0.001 |
| Black | 1.77 | 0.41–7.77 | 13.32 | 1.20–147.35 | ||
| Other | 4.43 | 1.14–17.16 | 4.50 | 1.23–16.46 | ||
| Serum 25(OH) vitamin D (per 1 ng/mL) | 1.01 | 0.96–1.06 | 0.669 | 1.03 | 0.98–1.08 | 0.201 |
Key: Trp, tryptophan.
Statistically significant results are highlighted.
Meta‐analysis of TRP1 and TRP2 datasets yielded a 30% decrease in the odds of having MS (95% CI: 16–43%, P‐value < 0.001) for each additional mcg/mL of serum Trp.
Serum Trp metabolite levels and relapse rate
In the metabolomics set, a total of 110 relapses occurred in 219.7 patient‐year follow‐up. Relative abundance of kynurenine was positively associated with the relapse rate (IRR = 5.8, P‐value = 0.003) (Table 3). There was no statistically significant association between other tryptophan metabolites and the relapse rate in the metabolomics set.
In the TRP1 set, a total of 106 relapses occurred in 284.6 patient‐years of follow‐up. In the TRP2 set, a total of 214 relapses occurred in 314.7 patient‐years of follow‐up. We did not find any significant associations between serum Trp levels and risk of relapse in the TRP1 or TRP2 sets (Table 5).
Table 5.
Association between baseline serum tryptophan level and subsequent relapse risk (targeted tryptophan analysis)
| TRP1 (Discovery) | TRP2 (Replication) | |||||
|---|---|---|---|---|---|---|
| IRR | 95% CI | P‐value | IRR | 95% CI | P‐value | |
| Serum Trp (per 1 mcg/mL) (univariable) | 0.99 | 0.91–1.08 | 0.908 | 1.00 | 0.92–1.09 | 0.965 |
| Serum Trp (per 1 mcg/mL) (multivariable) | 1.01 | 0.93–1.09 | 0.860 | 1.02 | 0.94–1.12 | 0.588 |
| Age at disease onset (1‐year increment) | 1.03 | 0.97–1.10 | 0.308 | 1.02 | 0.98–1.07 | 0.328 |
| Sex (female) | 1.35 | 0.86–2.11 | 0.187 | 1.07 | 0.68–1.68 | 0.785 |
| Race/ethnicity (vs. White non‐Hispanic) | 0.381 | 0.959 | ||||
| White Hispanic | 1.56 | 0.90–2.70 | 0.92 | 0.56–1.53 | ||
| Black | 1.06 | 0.46–2.47 | 0.85 | 0.36–2.00 | ||
| Other | 1.22 | 0.66–2.26 | 1.03 | 0.56–1.90 | ||
| Serum 25(OH) vitamin D (per 1 ng/mL) | 1.00 | 0.98–1.02 | 0.941 | 0.98 | 0.97–1.00 | 0.103 |
| DMT exposed [no = reference value] | 0.84 | 0.47–1.49 | 0.543 | 1.50 | 0.81–2.78 | 0.191 |
Trp, tryptophan; DMT, disease‐modifying therapy; IRR, incidence rate ratio.
In a sensitivity analysis, using the proportion of follow‐up time on a DMT as a continuous variable in the models did not change the results (data not shown).
Serum Trp metabolite levels, EDSS and SDMT
In the metabolomics set, the median EDSS score was 1.5 (IQR = 1–2). In multivariable models adjusted for age, sex, race/ethnicity and serum 25OHD, relative abundance of serum indole propionate was inversely associated with the EDSS score (regression coefficient = −0.40, P‐value = 004). In the metabolomics set, 32 patients (out of 69 patients) had SDMT measured in a clinic visit at or after serum sample collection. In multivariable models adjusted for age, sex, race/ethnicity and serum 25OHD, relative abundance of serum indole propionate and indole acetate were positively associated with SDMT score (for indole propionate: regression coefficient: 4.7, P‐value = 0.016; for indole acetate: regression coefficient: 7.8, P‐value = 0.021). There was no association between serum levels of Trp or other metabolites and EDSS or SDMT scores in the metabolomics, TRP1 and TRP2 datasets (Data from the metabolomics set are shown in Table 3; data from TRP1 and TRP2 sets not shown).
Relative abundance of microbial genes involved in Trp catabolism and relapse risk in pediatric MS
Microbiome data were available only in 17 cases (from the TRP1 set). Cases with a lower relative abundance of microbial genes involved in Trp catabolism had a shorter time to relapse during follow‐up than those with higher abundance (≤vs. > median relative abundance, log‐rank test P = 0.011, Fig. 3). Similar findings were observed with the Cox model, with a lower abundance associated with a higher hazard of relapse (unadjusted hazard ratio (HR) = 3.1, 95% CI: 1.1–9.2, P‐value = 0.036). The direction of effect remained the same when adjusting for baseline DMT exposure, although failed to reach significance in this small sample (adjusted HR = 2.9; 95% CI: 0.99–8.8, P‐value = 0.053).
Figure 3.
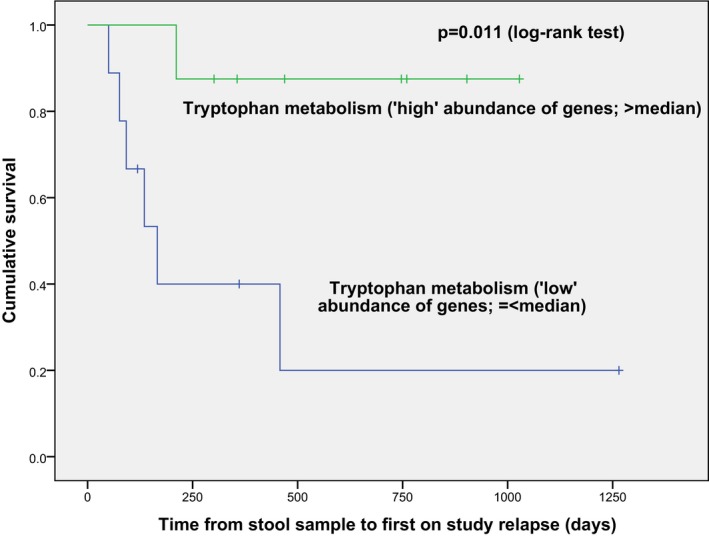
A low abundance (vs. high abundance) of gut microbial genes encoding tryptophan metabolism (as predicted from 16S rRNA sequencing and Phylogenetic Investigation of Communities by Reconstruction of Unobserved States (PiCRUSt)) was associated with a shorter time to relapse (Kaplan‐Meier curves, P = 0.011, log‐rank test).
Discussion
Our investigation of the tryptophan pathway in pediatric MS suggests that various metabolites may contribute to distinct aspects of the disease, that is. disease onset, risk of relapse and accumulation of disability. While higher serum levels of Trp and relative abundance of indole lactate, a Trp metabolite (produced by gut microbiota and an AHR ligand) were associated with a lower risk of pediatric MS, they were not associated with MS phenotype (relapse, EDSS and SDMT). In contrast, higher abundance of other Trp metabolites (indole propionate and indole acetate) was associated with lower disability and better processing speed, but not with the risk of developing the disease. Finally, higher kynurenine levels were associated with higher relapse rate. These results are generally in line with our understanding of the effects of different tryptophan metabolites on the risk and severity of MS.13, 14, 16 While metabolomics analyses have identified alterations in multiple metabolic processes in MS, these techniques have not been previously applied to pediatric MS and have not studied the tryptophan pathway as a whole.30 Reduced plasma levels of Trp and some of its metabolites have been reported in adult MS patients compared to healthy subjects and dynamic modulation of some of these metabolites in the course of the disease has been described,13, 31 but the study of Trp metabolism in the risk of MS and disease activity and severity is novel.
Reduced serum Trp and indole lactate in patients with MS compared to controls were the strongest and the most consistent findings in our study. Two processes can lead to reduced serum Trp: reduced dietary intake or intestinal absorption of tryptophan, and increased catabolism either by the gut microbiota or endogenously (such as increased utilization of tryptophan by lymphocytes). In a mouse model of uncontrolled inflammation, activated T‐cells uptake and accumulate tryptophan and several other amino acids. This process leads to a systemic decrease in the serum amino acid levels and eventually, cerebral deficit in several key neurotransmitters. These metabolic alterations are associated with behavioral changes in the animals.32 Alternatively, decreased serum Trp could be caused by alterations in human endogenous Trp metabolism (kynurenine metabolic pathway) or altered gut microbial Trp metabolism. These pathways may not only affect the risk of MS development, but also, as demonstrated in this study, may be associated with disease course.
Tryptophan metabolism through the kynurenine pathway has been associated with autoimmune and neurodegenerative diseases.33, 34 Pro‐inflammatory cytokines stimulate indoleamine 2,3‐dioxygenase, the first enzyme in the kynurenine pathway. Kynurenine metabolites have pro‐inflammatory, as well as, neurotoxic and neuroprotective effects, and the kynurenine pathway metabolic signature may discriminate clinical disease subtypes.16 Here, we showed that higher relative abundance of kynurenine in the serum is associated with higher relapse rate in pediatric patients with MS. Unlike the previous report,16 we did not find a correlation between other kynurenine pathway metabolites and the risk of developing MS or physical or cognitive disability scores in our cohorts. This discrepancy might be due to the difference in the age of participants (pediatric vs. adult) or statistical modeling (e.g. adjusting the models for potential confounders).
Our most intriguing results point to the potential role of Trp metabolism by gut microbiota in neuro‐inflammation.13 Some of the indole metabolites (such as indole lactate, indole propionate, indole‐3‐aldehyde and indole) produced by the action of various gut commensals on Trp are ligands or precursors of ligands of the AHR. Several of these metabolites are predominantly or exclusively produced by the gut microbiota and circulating levels are determined by the composition of the gut microbiota.35 Activation of AHR on astrocytes and microglia controls microglial activation, modulates the transcriptional program of astrocytes14 and dampens neuroinflammation. A Trp depleted diet worsened EAE which was reversed by Trp supplementation in a wild‐type mice, while mice lacking AHR on their astrocytes did not respond to Trp supplementation.13 Precursors of AHR agonists are produced by ampicillin‐sensitive, vancomycin‐resistant gut bacteria and treating late‐stage EAE mice with ampicillin impairs their neurological recovery.13 With reduced availability of dietary Trp to the gut microbiota that produce anti‐inflammatory metabolites, individuals might be at higher risk of developing MS. The reduction in these anti‐inflammatory Trp metabolites is not only associated with an increased risk of developing MS (indole lactate), but also with worse physical disability (indole propionate) and slower processing speed (indole propionate and indole acetate) in patients with pediatric MS. In addition, our gut microbiome functional analysis in a subgroup of patients suggested an association between shorter time to relapse and lower relative abundance of microbial genes associated with Trp catabolism (hence likely lower production of AHR agonists). These preliminary results raise the possibility that a complex interplay among Trp, gut microbiota and microbiota‐derived Trp metabolites affect neuroinflammation.
Depressed levels of serum Trp may also affect metabolic pathways for serotonin, melatonin and other proteins in the brain. Melatonin has been shown to have immunomodulatory properties and was recently implicated in the development of MS.36 While melatonin was not studied with the metabolomics platform used in our study, serotonin levels were not associated with MS risk or disease severity.
The study of young patients close to MS onset is a strength as these children have potentially fewer irrelevant environmental exposures, thereby facilitating the identification of important risk factors. Additional study strengths include stringent case ascertainment, short disease duration at the time of enrollment, prospective capture of relapses after serum and stool collection, use of both targeted and untargeted approaches to measure Trp and confirmation of findings in independent cohorts. Analyses were also adjusted for 25OHD levels and use of DMTs, both of which are associated with relapse risk, and a portion of our subjects also contributed gut metagenome data pertaining to the Trp pathway.
We acknowledge limitations of the study. Serum Trp was obtained after the onset of MS and there is a possibility that levels were affected by the disease itself, such as patients changing their diet after diagnosis. However, as patients were enrolled shortly after disease and there is no consensus about specific dietary recommendations for MS,11 this appears unlikely. We did not have MRI data to assess the association of serum Trp with radiological disease activity. However, by measuring relapses, we assessed a clinically relevant outcome as relapses are used as the primary outcomes in trials testing promising DMTs for MS. Because of the hypothesis‐based and exploratory nature of our study, we did not correct the results for multiple hypotheses testing. For the microbiome and global untargeted metabolomics studies, the number of samples analyzed was constrained by the available funding and a sample size calculation was not performed. As such, we cannot rule out that null results might be due to an inadequate sample size. The dietary intake of tryptophan was not measured in this study. Finally, the role of unmeasured or unknown confounders cannot be excluded.
Overall, our data support a role for Trp and some of its metabolites in MS risk and disease course; specifically, those that are produced by the kynurenine pathway enzymes and gut microbiota. Our findings do not support a Trp‐rich diet or Trp supplementation for prevention or treatment of MS. This cannot be overemphasized considering the possible association between Trp supplements and the development of eosinophilia‐myalgia syndrome.37 Future studies with measurements of dietary Trp intake and prospective and longitudinal assessment of serum Trp metabolites measured by targeted approaches, are first needed to allow a deeper evaluation of the role of the Trp pathways in the MS course.
Conflict of Interest
The authors report no disclosure relevant to the manuscript.
Acknowledgments
This work was supported by several grants (R01NS071463, PI Waubant; Race to Erase MS, PI Waubant; NMSS diet: RG‐1701‐26635, PI Waubant), Career Transition Award from the National MS Society to PB and John F Kurtzke Clinician Scientist Development award from the AAN to PB. We are thankful for patients and their families to have participated in this research.
Funding Information
This work was supported by several grants (R01NS071463, PI Waubant; Race to Erase MS, PI Waubant; NMSS: RG‐1701‐26635, PI Waubant).
Bardia Nourbakhsh conducted the statistical analysis.
Funding Statement
This work was funded by grant R01NS071463; Race to Erase MS grant ; NMSS grant RG‐1701‐26635.
References
- 1. Lucas RM, Hughes AM, Lay M‐LJ, et al. Epstein‐Barr virus and multiple sclerosis. J Neurol Neurosurg Psychiatr 2011;82:1142–1148. [DOI] [PubMed] [Google Scholar]
- 2. Weston M, Constantinescu CS. What role does tobacco smoking play in multiple sclerosis disability and mortality? A review of the evidence. Neurodegener Dis Manag 2015;5:19–25. [DOI] [PubMed] [Google Scholar]
- 3. Correale J, Gaitán MI. Multiple sclerosis and environmental factors: the role of vitamin D, parasites, and Epstein‐Barr virus infection. Acta Neurol Scand 2015;132:46–55. [DOI] [PubMed] [Google Scholar]
- 4. Mokry LE, Ross S, Timpson NJ, et al. Obesity and multiple sclerosis: a mendelian randomization study. PLoS Med 2016;13:e1002053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Goodin DS. The epidemiology of multiple sclerosis: insights to a causal cascade. Handb Clin Neurol 2016;138:173–206. [DOI] [PubMed] [Google Scholar]
- 6. McKay KA, Jahanfar S, Duggan T, et al. Factors associated with onset, relapses or progression in multiple sclerosis: a systematic review. Neurotoxicology 2017;61:189–212. [DOI] [PubMed] [Google Scholar]
- 7. Farez MF, Fiol MP, Gaitán MI, et al. Sodium intake is associated with increased disease activity in multiple sclerosis. J Neurol Neurosurg Psychiatr 2015;86:26–31. [DOI] [PubMed] [Google Scholar]
- 8. Nourbakhsh B, Graves J, Casper TC, et al. Dietary salt intake and time to relapse in paediatric multiple sclerosis. J Neurol Neurosurg Psychiatr 2016;87(12):1350–1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. McDonald J, Graves J, Waldman A, et al. A case‐control study of dietary salt intake in pediatric‐onset multiple sclerosis. Mult Scler Relat Disord 2016;6:87–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Fitzgerald KC, Munger KL, Hartung HP, et al. Sodium intake and multiple sclerosis activity and progression in BENEFIT. Ann Neurol 2017;82:20–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Pakpoor J, Seminatore B, Graves JS, et al. Dietary factors and pediatric multiple sclerosis: a case‐control study. Mult Scler 2018;24(8):1067–1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Azary S, Schreiner T, Graves J, et al. Contribution of dietary intake to relapse rate in early paediatric multiple sclerosis. J Neurol Neurosurg Psychiatry 2018;89:28–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Rothhammer V, Mascanfroni ID, Bunse L, et al. Type I interferons and microbial metabolites of tryptophan modulate astrocyte activity and central nervous system inflammation via the aryl hydrocarbon receptor. Nat Med 2016;22:586–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Rothhammer V, Borucki DM, Tjon EC, et al. Microglial control of astrocytes in response to microbial metabolites. Nature 2018;557:724–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Tremlett H, Waubant E. The gut microbiota and pediatric multiple sclerosis: recent findings. Neurotherapeutics 2018;15(1):102–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lim CK, Bilgin A, Lovejoy DB, et al. Kynurenine pathway metabolomics predicts and provides mechanistic insight into multiple sclerosis progression. Sci Rep 2017;7:41473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gorman MP, Healy BC, Polgar‐Turcsanyi M, Chitnis T. Increased relapse rate in pediatric‐onset compared with adult‐onset multiple sclerosis. Arch Neurol 2009;66:54–59. [DOI] [PubMed] [Google Scholar]
- 18. Roberts LD, Souza AL, Gerszten RE, Clish CB. Targeted metabolomics. Curr Protoc Mol Biol 2012. Apr; CHAPTER:Unit30.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Polman CH, Reingold SC, Banwell B, et al. Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann Neurol 2011;69:292–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Krupp LB, Tardieu M, Amato MP, et al. International Pediatric Multiple Sclerosis Study Group criteria for pediatric multiple sclerosis and immune‐mediated central nervous system demyelinating disorders: revisions to the 2007 definitions. Mult Scler 2013;19:1261–1267. [DOI] [PubMed] [Google Scholar]
- 21. NOT‐OD‐15‐089: Racial and Ethnic Categories and Definitions for NIH Diversity Programs and for Other Reporting Purposes [Internet]. [date unknown];[cited 2018 Jul 28] Available from: https://grants.nih.gov/grants/guide/notice-files/NOT-OD-15-089.html
- 22. Kurtzke JF. Rating neurologic impairment in multiple sclerosis: an expanded disability status scale (EDSS). Neurology 1983;33:1444–1452. [DOI] [PubMed] [Google Scholar]
- 23. Chiaravalloti ND, DeLuca J. Cognitive impairment in multiple sclerosis. Lancet Neurol 2008;7:1139–1151. [DOI] [PubMed] [Google Scholar]
- 24. Sha W, da Costa K‐A, Fischer LM, et al. Metabolomic profiling can predict which humans will develop liver dysfunction when deprived of dietary choline. FASEB J 2010;24:2962–2975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Evans AM, DeHaven CD, Barrett T, et al. Integrated, nontargeted ultrahigh performance liquid chromatography/electrospray ionization tandem mass spectrometry platform for the identification and relative quantification of the small‐molecule complement of biological systems. Anal Chem 2009;81:6656–6667. [DOI] [PubMed] [Google Scholar]
- 26. Mowry EM, Krupp LB, Milazzo M, et al. Vitamin D status is associated with relapse rate in pediatric‐onset multiple sclerosis. Ann Neurol 2010;67:618–624. [DOI] [PubMed] [Google Scholar]
- 27. Gianfrancesco MA, Stridh P, Rhead B, et al. Evidence for a causal relationship between low vitamin D, high BMI, and pediatric‐onset MS. Neurology 2017;88(17):1623–1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Tremlett H, Fadrosh DW, Faruqi AA, et al. Gut microbiota composition and relapse risk in pediatric MS: a pilot study. J Neurol Sci 2016;363:153–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Langille MGI, Zaneveld J, Caporaso JG, et al. Predictive functional profiling of microbial communities using 16S rRNA marker gene sequences. Nat Biotechnol 2013;31:814–821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Bhargava P, Fitzgerald KC, Calabresi PA, Mowry EM. Metabolic alterations in multiple sclerosis and the impact of vitamin D supplementation. JCI Insight 2017;2(19):e95302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Rothhammer V, Borucki DM, Garcia Sanchez MI, et al. Dynamic regulation of serum aryl hydrocarbon receptor agonists in MS. Neurol Neuroimmunol Neuroinflamm 2017;4:e359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Miyajima M, Zhang B, Sugiura Y, et al. Metabolic shift induced by systemic activation of T cells in PD‐1‐deficient mice perturbs brain monoamines and emotional behavior. Nat Immunol 2017;18:1342–1352. [DOI] [PubMed] [Google Scholar]
- 33. Åkesson K, Pettersson S, Ståhl S, et al. Kynurenine pathway is altered in patients with SLE and associated with severe fatigue. Lupus Sci Med 2018;5:e000254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Maddison DC, Giorgini F. The kynurenine pathway and neurodegenerative disease. Semin Cell Dev Biol 2015;40:134–141. [DOI] [PubMed] [Google Scholar]
- 35. Wikoff WR, Anfora AT, Liu J, et al. Metabolomics analysis reveals large effects of gut microflora on mammalian blood metabolites. Proc Natl Acad Sci USA 2009;106:3698–3703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Farez MF, Mascanfroni ID, Méndez‐Huergo SP, et al. Melatonin contributes to the seasonality of multiple sclerosis relapses. Cell 2015;162:1338–1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Blackburn WD. Eosinophilia myalgia syndrome. Semin Arthritis Rheum 1997;26:788–793. [DOI] [PubMed] [Google Scholar]


