Abstract
Although inflammation is known to influence bone turnover and bone mineral density (BMD), less is known about role of soluble tumor necrosis factor alpha receptor 1 (sTNFα-R1) in changes in bone turnover and BMD in the year after hip fracture. We studied 245 persons (117 men and 128 women) from the Baltimore Hip Studies. Bone turnover markers of resorption (carboxy-terminal type I collagen cross-links [CTX-I]) and formation (amino-terminal propeptide type I collagen [P1NP]), BMD of the contralateral hip, and sTNFα-R1 were measured within 15 days of hospitalization and 2, 6, and 12 months later. Latent class growth modeling was used to determine sTNFα-R1 trajectories. Weighted generalized estimating equations were used to examine the association of sTNFα-R1 trajectories with serum levels of CTX-I and P1NP and BMD; standardized beta coefficients () are reported. Higher baseline sTNFα-R1 was significantly associated with a greater rate of CTX-I change ( = 0.26, p = 0.004). Four distinct sTNFα-R1 trajectories were identified. The two groups with higher sTNFα-R1 levels during the year following fracture had faster increasing levels of CTX-I compared to the group with lowest sTNFα-R1 levels (men: group 3: = 0.76, p = 0.02; group 4: = 1.4, p < 0.001; women: group 3; = 0.67, p = 0.02; group 4: = 1.3, p = 0.004). Men in the highest sTNFα-R1 group had a greater decline in BMD compared to the lowest sTNFα-R1 group (2-month = −0.01, p = 0.01; 6-month: = −0.09, p = 0.001; 12-months: = −0.1, p < 0.001). An increasing rate of CTX-I was associated with a steeper decline in total hip BMD in those within higher sTNFα-R1 trajectory groups (p < 0.001). CTX-I was significantly increased with sTNFα-R1 in both sexes. CTX-I and the highest sTNFα-R1 trajectory were significantly associated with declines in total hip BMD in men. Interventions that reduce systemic inflammation should be explored to reduce bone resorption and prevent a decline in BMD after hip fracture.
Keywords: HIP FRACTURE, AGING, BONE TURNOVER, BONE MINERAL DENSITY, CYTOKINES, TUMOR NECROSIS FACTOR ALPHA RECEPTOR-1, SEX DIFFERENCES
Introduction
Hip fracture is an acute event often followed by a debilitating state in older adults with an increased risk of new fractures.(1–3) About one-third of women and one-fifth of men with a previous history of osteoporotic hip fracture experience a second fracture within the next 5 years.(4)
Bone loss can be induced by a negative balance between osteoblastic and osteoclastic activities, responsible for bone formation and resorption, respectively. Osteoporosis or decline in bone mineral density (BMD) is mediated by the loss of homeostasis between bone formation and bone resorption. Typically, this is an increase in bone resorption without a sufficient corresponding increase in bone formation and associated with an increased risk of fracture in older adults. Of note, osteoporosis is reported to occur in the presence of chronic inflammatory states, such as rheumatoid arthritis or aging.(5–8) Therefore, inflammation has been a center of focus in recent years in the study of mechanisms underlying age-related chronic diseases.(9–11) In an earlier Baltimore Hip Studies cohort, investigators reported that elevated inflammatory biomarkers during the postfracture year were adversely associated with recovery of lower extremity function after hip fracture.(12) However, associations of bone resorption and formation with acute and subacute inflammatory states following fracture have not been well described.
Tumor necrosis factor alpha (TNFα) is an inflammatory biomarker that rises in response to an acute injury and is required for initial injury healing.(13–15) There is a body of evidence suggests that TNFα affects bone metabolism through stimulation of its receptors on osteoclasts and osteoblasts and induces their differentiation from their progenitor cells. Additionally, through tumor necrosis factor alpha receptor 1 (TNFα-R1), TNFα induces monocyte lineage stem cells to differentiate into osteoclast precursors.(16, 17) For clinical purposes, soluble TNFα-R1 (sTNFα-R1) with its long-term bioavailability in stored serum is an optimal proxy of activated TNFα and a biomarker of systemic inflammation. Moreover, clinical data using TNF inhibitors in patients with rheumatoid arthritis (RA) or axial spondyloarthritis (AS) have shown improvements in bone metabolite profiles and decreases in bone loss.(18, 19) Thus, we chose sTNFα-R1 as a biomarker of inflammation for this study.
Following the acute rise in inflammation at the time of fracture, complete resolution of inflammation may not occur(12); by the same token, an increased baseline sTNFα-R1 may remain high fora longer time. Although the initial activation of the TNFα signaling pathway plays a pivotal role in injury healing,(14, 15) and TNFα is particularly important as a regulator of bone metabolism, less is known about a role that long-term activation of TNFα following fractures may play in relation to the changes in bone turnover biomarkers and BMD. Therefore, we aim to identify patterns of sTNFα-R1 trajectories following hip fracture and its association with more immediate bone-related outcomes, including measures of bone turnover, carboxy-terminal type I collagen cross-links (CTX-I), and amino-terminal propeptide of type I collagen (P1NP), biomarkers of bone resorption (osteoclast activity) and formation (osteoblast activity), respectively,(20) and change in BMD over a year following hip fracture.
In previous studies, descriptions of changes in total hip BMD, bone turnover, and inflammation following hip fracture have largely been restricted to women,(12, 21–23) with few studies conducted in men.(24) During the past year, we reported a decrement of BMD following hip fractures, with a steeper decline in men than women.(25) However, the reason for this discrepancy is not well understood.
Taken together, in this study, we hypothesize that there are distinct trajectories of sTNFα-R1 after hip fracture and that those with higher levels will have increased bone resorption, which is an early surrogate of osteoporosis,(26) and subsequent decreased BMD. We will also consider whether the trajectories of sTNFα-R1, at least in part, explain the discrepancy of BMD in men and women following the hip fracture.
Subjects and Methods
Study design and participants
Baltimore Hip Studies (BHS) is a program of research comprising longitudinal studies of individuals who were hospitalized for hip fracture and followed prospectively. The seventh cohort, BHS-7, included patients aged 65 years and older who were admitted to one of eight Baltimore-area hospitals. Recruitment of male and female hip fracture patients was frequency matched (1:1) on calendar time of fracture and hospital. Patients were excluded if they were non–community dwelling at the time of fracture, resided greater than 70 miles from the study hospital, were non-English speaking, had a pathologic fracture, weighed >300 pounds, were bedridden for 6 months before the fracture, did not have surgical repair of the fracture, or had hardware in the contralateral hip. Participants or their proxies provided written informed consent within 15 days of admission. The study was approved by the institutional review boards (IRBs) of the University of Maryland–Baltimore, and each of the eight study hospitals.
A total of 362 participants were enrolled; 23 participants were withdrawn (five participants failed to provide data at baseline, and another 18 participants were removed from the analytic sample as a result of an IRB-requested postprocedure audit), leaving a final sample of 339 (168 men, 171 women). Of 253 patients with measured BMD, 245 patients also had both total hip BMD and serum sTNFα-R1 available, resulting in an analytical sample of 128 women and 117 men.
Measures
Demographic variables and comorbidity
Participant characteristics at baseline included age, sex, body mass index (BMI) (kg/m2), years of education, marital status, race, and smoking habits. Medication history was ascertained as (i) any use of bone suppressing medications such as glucocorticoids (prednisone, cortisone, hydrocortisone, dexamethasone, or any other steroids) or thyroid medications such as levothyroxine; (ii) use of any bone-active medications such as bisphosphonates and teriparatide; (iii) hormone therapy (any form of estrogen in women and testosterone in men); (iv) calcium supplements in any form; and (v) hydrochlorothiazide. The number of comorbid conditions abstracted from medical charts was determined using the modified Charlson comorbidity index,(27) in which mild liverdisease was eliminated, a history of diabetes mellitus (DM, “yes” versus “no”), and estimated glomerular filtration rate (eGFR) using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) formula as an indicator of renal function were additionally considered.(28) We also measured serum parathyroid hormone (PTH) to adjust for plausible secondary hyperparathyroidism as a results of renal function decline.(29)
Follow-up measurements
Follow-up visits were conducted at 2, 6, and 12 months post-hospital admission. Fasting blood specimens at all visits were collected between the hours of 7:00 a.m. and 10:00 a.m. and processed no later than 1:00 p.m., so that samples were processed within 6 hours of collection and serum was stored at −80°C until assayed. Blood collected at each time point was used to measure biomarkers of interest. The assay measurements were performed at the Clinical Research Core Laboratory, Johns Hopkins Institute for Clinical & Translational Research.
Soluble tumor necrosis factor alpha receptor1
Soluble tumor necrosis factor alpha receptor1 (sTNFα-R1) was measured in duplicate, using the Quantikine Human sTNF enzyme-linked immunosorbent assay (ELISA) (R&D Systems, Minneapolis, MN, USA) and V-Plex MSD (Meso Scale Diagnostics, Gaithersburg, MD, USA), with a sensitivity of 0.77 pg/mL and interassay and intraassay coefficients of variation (CV%) of 10.31 and 8.95, respectively. Because osteoprotegerin (OPG) and receptor activator of nuclear factor-κB (NF-κB) ligand (RANKL) also belong to the TNF superfamily that play roles in modification of bone turnover pathways(30, 31) we evaluated whether cross-reactivity occurs between anti-TNFα-R1 antibody and these two proteins, performing the test in the standard solution as a control and also in our study participants’ serum samples. The details of these tests are explained in the Supporting Information.
Bone turnover biomarkers
Serum levels of carboxy-terminal type I collagen cross-links (CTX-I: sensitivity = 0.020 ng/mL, intraassay CV% = 8.15, interassay CV% = 10.31) and amino-terminal propeptide of type I collagen (P1NP: sensitivity 2.0 μg/L, intraassay CV% = 4.14, interassay CV% = 2.74) indicated bone resorption and bone formation, respectively. CTX-I was determined using ELISA and P1NP using radio-immunoassay (RIA).
BMD
Total hip BMD was assessed at baseline and 2-month, 6-month, and 12-month follow-up visits using dual-energy X-ray absorptiometry (DXA) at the contralateral hip (g/cm2) in one of seven DXA facilities where four sites used Lunar Prodigy machines (Madison, WI, USA) and three sites used Hologic machines (Waltham, MA, USA). Men and women were matched to the clinical sites and, therefore, two types of DXA machines in equal ratios, and the types of machines remained the same during follow-up measurements. To ensure the reproducibility of results and avoid systematic bias, the DXA operators were certified and trained equally to use the same procedures. We excluded images with poor quality or low anatomical coverage. To account for any inter-site and machine differences, statistical models include a time-varying indicator to capture the different DXA sites and machines, an approach that has been used previously in the study of BMD changes in patients with osteoporosis.(32) Additionally, during the analyses, we adjusted for type of machine used.
Statistical analyses
We compared baseline characteristics by sex. We computed mean ± standard deviation (SD), or median and interquartile range (IQR) for continuous variables and percentages for categorical variables. All biomarkers were assessed for their degree of skewness and were natural log-transformed in regression analyses. Analyses were initially conducted on all participants, then repeated, stratified by sex.
sTNFα-R1
To examine sex differences in sTNFα-R1 over time, we included sex by time interaction terms in the multivariate models and examined sex-based differences in sTNFα-R1 levels over time. We examined the association of baseline sTNFα-R1 with covariates. To investigate population diversity in the trajectory of sTNFα-R1 over time, we evaluated the multinomial heterogeneity in sTNFα-R1 concentrations using latent class growth modeling (LCGM) to identify distinct groups of individuals who have a similarity in the pattern of changes. More details of this method are reported in the supplement.(33) We also compared baseline sTNFα-R1 levels using a one-way ANOVA test and performed the Tukey-Kramer post hoc test for comparisons between each pair of groups.
Bone outcomes and associations with sTNFα-R1
First, we examined sex differences in standardized serum levels of CTX-I and P1NP; then we fit a multivariate regression model and included a sex-by-time interaction term to examine sex differences in CTX-I and P1NP over time. We evaluated whether baseline sTNFα-R1 was associated with the rate of change in the bone turnover biomarkers over time, adjusting for demographic variables, fasting state at the time of blood draw, BMI, baseline CTX-I, and comorbidity. We evaluated whether a change in total hip BMD at each visit differed between distinct trajectory groups of sTNFα-R1 in men and women after adjustment for baseline CTX-I. Additionally, we examined the association between the rate of change in BMD over a year following the hip fracture and rate of change in the bone resorption biomarker in each distinct trajectory group of sTNFα-R1 after adjustment for the covariates. To address missing data and truncation by death, we used inverse probability weighted generalized estimating equations (W-GEE).(34) Covariates were entered in the backward stepwise multivariate W-GEE models. All continuous variables were standardized to a mean of 0 and SD of 1 to permit comparison of their incremental predictive associations; standardized betas are reported (). All statistical analyses were performed using STATA 14.1 (StataCorp, College Station, TX, USA)
Results
Baseline characteristics
Of 245 participants at baseline, 147, 123, and 113 participants at 2, 6, and 12 months, respectively, were analyzed (Supporting Fig. 1). Participants’ baseline characteristics are summarized in Table 1. The mean age was 80.9 ± 7.9 years with an equal sex distribution. About one-half of men and one-quarter of women in the cohort were married. Of note, men had a higher Charlson comorbidity index than women (p < 0.001). More women than men used bone-active drugs. Importantly, men had a higher baseline sTNFα-R1 level than women (p = 0.005). Although women had lower BMD than men at baseline (p < 0.05), men had higher postfracture bone resorption as measured by CTX-I levels (p < 0.001) (Table 1).
Table 1.
Demographic Characteristics, Medical History, and Inflammatory and Bone Biomarkers at Baseline in Total Participants and Stratified by Sex
| Total (n = 245) |
Women (n = 128) |
Men (n = 117) |
pa | |
|---|---|---|---|---|
| Demographic variables | ||||
| Age (years), mean ± SD | 80.7 ± 7.5 | 81.2 ± 7.7 | 80.2 ± 7.3 | 0.2 |
| Gender (male), % | 48.6 | – | – | – |
| Marital status, % | 0.001 | |||
| Married | 40.3 | 30 | 51.2 | |
| Unmarried | 59.7 | 70 | 48.8 | |
| Education (years), mean ± SD | 13.1 ± 3.4 | 13.0 ± 3.0 | 13.2 ± 3.8 | 0.5 |
| Current smoking (yes), n (%) | 17 (7.0) | 6.8 | 5.8 | 0.7 |
| Alcohol abuse (yes), n (%) | 9 (3.6) | 0.77 | 6.5 | 0.01 |
| BMI (kg/m2), mean ± SD | 25.3 ± 5.1 | 25.0 ± 5.6 | 25.5 ± 4.6 | 0.3 |
| Medical history | ||||
| Charlson comorbidity index, median (range) | 2 (0–8) | 1 (0–6) | 2 (0–8) | <0.001 |
| Renal disease, n (%) | 11 (4.3) | 4 (3.1) | 7 (5.7) | 0.3 |
| DM, n (%) | 57 (22.5) | 26 (21.1) | 31 (23.9) | 0.6 |
| eGFR, mean ± SD | 68.9 ± 19.7 | 68.9 ± 18.0 | 68.8 ± 21.5 | 0.5 |
| PTH (pg/mL), mean ± SD | 59.4 ± 36.3 | 59.2 ± 40.7 | 59.6 ± 31.4 | 0.9 |
| Breast cancer, n (%) | – | 24 (14.8) | – | – |
| Prostate cancer, n (%) | – | – | 32 (20.1) | – |
| Bone-activating drugs, n (%) | 76 (23.5) | 62 (81.6) | 14 (18.4) | 0.001 |
| Hydrochlorothiazide, n (%) | 61 (19.2) | 55.7 | 44.3 | 0.36 |
| Use of testosterone, n (%) | 5 (3.0) | – | 5 (3.0) | – |
| Use of estrogen, n (%) | 58 (35.4) | 58 (35.4) | – | – |
| Baseline inflammation and bone biomarkers, median (IQR) | ||||
| sTNFα-R1 (pg/mL) | 3003 (2424–3884) | 2766 (2319–3517) | 3228 (2587–4217) | 0.005 |
| CTX-I (ng/mL) | 0.80 (0.6–1.1) | 0.7 (0.48–1.0) | 0.8 (0.65–1.2) | <0.001 |
| P1NP (μg/L) | 100.6 (66.9–146.8) | 92.1 (66.7–133.2) | 109.6 (71.0–157.9) | 0.17 |
| Baseline BMD (g/cm2), mean ± SD (range) | ||||
| Total hip BMD | ||||
| Lunar machine (n = 198) | 0.76 ± 0.14 (0.4–1.2) | 0.7 ± 0.13 (0.4–1.2) | 0.8 ± 0.14 (0.5–1.1) | 0.001 |
| Hologic machine (n = 55) | 0.74 ± 0.13 (0.4–1.1) | 0.69 ± 0.11 (0.4–0.9) | 0.78 ± 0.14 (0.5–1.1) | 0.02 |
DM = diabetes mellitus; eGFR = estimated glomerular filtration rate using CKD-EPI formula (mL/min/1.73 m2); PTH = parathyroid hormones; IQR = interquartile range; sTNFα-R1 = soluble tumor necrosis factor alpha receptor-1; CTX-I = C- terminal telopeptide; P1NP = N-terminal propeptide of type 1 procollagen; BMD = bone mineral density.
p value: men compared to women.
Baseline and longitudinal sTNFαR-1
Overall, after accounting for other covariates, older age, a greater number of comorbidities, and baseline renal function were significantly associated with higher baseline serum sTNFα-R1 levels (Supporting Table 1). In a sex-stratified analysis, age, Charlson comorbidity index, and baseline renal function were significantly associated with baseline sTNFα-R1 levels in both men and women. Additionally, in women but not men, higher BMI and DM were significantly associated with baseline serum sTNFα-R1 levels (Supporting Table 1).
We identified four distinct latent groups of sTNFα-R1 trajectories over 12 months with significant pairwise differences in mean baseline sTNFα-R1 levels between these four trajectory groups (p < 0.001; Supporting Table 2). Group 1 had the lowest sTNFα-R1 levels at baseline and over 12 months and accounted for 28.7% of the participants. Groups 2 and 3 accounted for 41.1%, and 21.1%, respectively, falling in the intermediate levels of sTNFαR-1, with group 3 higher than group 2. Group 4, with the highest levels and an increasing trend in sTNFα-R1 to 12 months, accounted for 9.2% of total participants. Members of each group had a posterior probability greater than 70% of group membership. Women were more likely to be in groups 1 and 2 than men, and men were more likely to be in groups 3 and 4 than women (p = 0.03; Supporting Table 3). There were significant differences of intercepts and slopes between each pair of trajectory groups (p < 0.001), except for groups 1 and 2 (Fig. 1; Supporting Table 4).
Fig. 1.
Trajectories of sTNFα-R1 over 1-year follow-up using LCGM. Dark lines: four classes of trajectories, symbols: observed group means at each visit. The gray dashed lines are 95% pointwise confidence on the estimated sTNFα-R1 trajectories. G = sTNFα-R1 trajectory groups; LCGM = latent class growth modeling.
In the cross-reactivity and replication tests, the range of sTNFα-R1 levels was 1346.9 to 7101.0 pg/mL, or 1.3 ng/mL to 7.1 ng/mL. Spiking with 50ng/mL RANKL or OPG (50 ng/mL exceeds maximum serum levels of sTNFα-R1 by about sevenfold) reduces captured TNFα-R1 by 5%, which falls within the interassay CV range. Because the RANKL and OPG in the serum are at levels of more than 50 ng/mL, there is unlikely to be an effect on sTNFα-R1 detection with this sandwich-based TNFα-R1 ELISA kit (R&D Systems) (Supporting Figs. 2 and 3). The mean values of antibody detection adding OPG and RANKL was 0.00 pg/mL.
Bone turnover biomarkers (CTX-I, P1NP) and BMD
Serum CTX-I levels in men were higher than those in women over 1-year follow-up (p < 0.001). A 1-SD increment in baseline sTNFα-R1 was associated with a 0.26-SD faster rate of increase of CTX-I in men (p = 0.004). Although higher baseline sTNFα-R1 was associated with a greater rate of increase of CTX-I in women, this association was not statistically significant ( = 0.14, p = 0.26; Table 2).
Table 2.
Association of Baseline sTNFα-R1 and Slope (Rate of Change Over 12 Months) of Bone Turnover Biomarkers, Adjusted for Other Covariates
| Slope CTX | Slope P1NP | |
|---|---|---|
| Baseline sTNFα-R1 | ( ± SE, p) | ( ± SE, p) |
| Total | 0.22 ± 0.07, 0.002 | 0.04 ± 0.03, 0.6 |
| Men | 0.26 ± 0.09, 0.004 | −0.01 ± 0.009, 0.9 |
| Women | 0.14 ± 0.1, 0.26 | 0.15 ± 0.1, 0.2 |
Multivariate weighted-generalized estimated equation was used. Current smoking status, use of bone-suppressing drugs, calcium or vitamin D supplements, breast, or prostate cancer, DM, and BMI not significantly associated with the bone outcome; adjustment for baseline bone mineral density and bone-activate medications were made.
sTNFα-R1 = soluble tumor necrosis factor alpha receptor-1; CTX-I = carboxy-terminal type I collagen cross-links (bone resorption biomarker); P1NP = N-terminal propeptide of type 1 procollagen; = standardized beta.
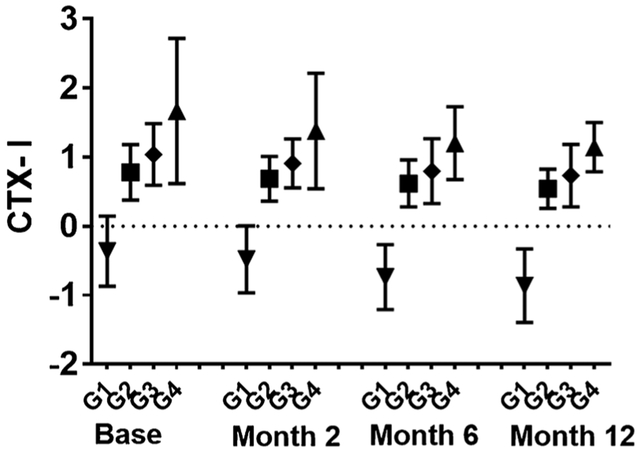
In an unadjusted analysis of the total sample, we also found that CTX-I was higher at baseline and during the entire 1-year postfracture period for inflammatory trajectory groups with the highest sTNFα-R1 levels (p < 0.001; Fig. 2). The two trajectory groups (groups 3 and 4) having the highest levels of sTNFα-R1 were significantly associated with the rate of change of CTX-I, independent of bone-active medications, calcium supplement use, renal function, and other covariates (group 3 versus 1: = 0.5, p = 0.01; group 4 versus 1: = 1.4, p = 0.001), and the results remained significant when stratified by sex: (group 3 versus 1 in men = 0.76, p = 0.02; group 4 versus 1 in men: = 1.4, p < 0.001; group 3 versus 1 in women = 0.67, p = 0.02; group 4 versus 1 in women: = 1.3, p = 0.004; Table 3). Men had a significantly greater rate of change in CTX-I than women over the 12-month follow-up ( = 0.3, p = 0.008). Baseline sTNFα-R1 or the sTNFα-R1 trajectory groups did not significantly relate to change in P1NP over time (Tables 2 and 3). In men, those in the sTNFα-R1 trajectory group 4 had a BMD that significantly declined at 2, 6, and 12 months from baseline more than those in sTNFα-R1 trajectory group 1 (2-month = −0.01, p = 0.01; 6-month: = −0.09, p = 0.001; 12-month: = −0.1, p < 0.001) (Table 4), and the magnitude of the decrement become larger over time. In women, comparing the same trajectory groups, BMD declined more in group 4 compared to group 1. However, it was not statistically significant. In men, a higher CTX-I level was associated with a greater decline in BMD within the three highest sTNFα-R1 trajectory groups (groups 2, 3, and 4) (group 2: = −0.15, p < 0.001; group 3: = −0.11, p < 0.0061; group 4: = −0.21, p < 0.001; Supporting Table 5). The results are after adjustment for PTH level and other covariates such as renal comorbidities and medication use history.
Fig. 2.
Standardized CTX-I levels (ng/mL) at each visit by sTNFα-R1 trajectory groups over 1 year after the hip fracture. G = sTNFα-R1 trajectory groups.
Table 3.
Association of sTNFα-R1 Trajectory Groups With Slope (Rate of Change Over 12 Months) of Bone Turnover Biomarkers Adjusted for Other Covariates
| Slope CTX-I ( ± SE; p) |
Slope P1NP ( ± SE; p) |
|||||
|---|---|---|---|---|---|---|
| sTNFα-R1 trajectory groups | Total | Men | Women | Total | Men | Women |
| Group 1 (Ref) | – | – | – | – | – | – |
| Group 2 | 0.09 ± 0.1; 0.5 | 0.23 ± 0.2; 0.3 | −0.09 ± 0.7; 0.7 | 0.09 ± 0.1; 0.5 | 0.15 ± 0.3; 0.6 | 0.15 ± 0.1; 0.6 |
| Group 3 | 0.5 ± 0.2; 0.01 | 0.76 ± 0.3; 0.02 | 0.67 ± 0.3; 0.02 | 0.29 ± 0.2; 0.1 | 0.14 ± 0.3; 0.6 | 0.4 ± 0.3; 0.3 |
| Group 4 | 1.4 ± 0.2; 0.001 | 1.4 ± 0.4; <0.001 | 1.3 ± 0.4; 0.004 | 0.27 ± 0.2; 0.3 | 0.45 ± 0.3; 0.3 | 0.6 ± 0.4; 0.2 |
Multivariate weighted-generalized estimated equation was used. Current smoking status, use of bone-activating drugs, calcium or vitamin D supplements, breast or prostate cancer, DM, and BMI were not significant.
sTNFα-R1 = soluble tumor necrosis factor alpha receptor-1; CTX-I = carboxy-terminal type I collagen cross-links (bone resorption biomarker); P1NP = N-terminal propeptide of type 1 procollagen; = standardized beta; DM = diabetes mellitus.
Table 4.
Association of sTNFα-R1 Trajectories and Change in Total Hip BMD at 2, 6, and 12 Months From Baseline, Adjusted for Other Covariates
| Δ BMD Baseline to 2-month ( ± SE; p) |
Δ BMD Baseline to 6-month ( ± SE; p) |
Δ BMD Baseline to 12-month ( ± SE; p) |
||||
|---|---|---|---|---|---|---|
| sTNFα-R1 trajectory groups |
Men | Women | Men | Women | Men | Women |
| Group 1 (Ref) | – | – | – | – | – | – |
| Group 2 | 0.003 ± 0.001; 0.7 | 0.002 ± 0.001; 0.8 | −0.01 ± 0.01; 0.3 | −0.002 ± 0.007; 0.8 | −0.007 ± 0.06; 0.6 | 0.01 ± 0.01; 0.3 |
| Group 3 | 0.02 ± 0.01; 0.3 | 0.005 ± 0.02; 0.6 | −0.007 ± 0.06; 0.6 | −0.006 ± 0.06; 0.6 | 0.01 ± 0.01; 0.4 | 0.01 ± 0.01; 0.3 |
| Group 4 | −0.03 ± 0.01; 0.01 | 0.006 ± 0.004; 0.6 | −0.09 ± 0.02; 0.001 | −0.01 ± 0.01; 0.4 | −0.1 ± 0.02; <0.001 | −0.01 ± 0.01; 0.8 |
Multivariate weighted-generalized estimated equation was used. Current smoking status, use of bone-activating drugs, calcium or vitamin D supplements, breast or prostate cancer, DM, and BMI were not significant.
sTNFα-R1 = soluble tumor necrosis factor alpha receptor-1; BMD = bone mineral density; = standardized beta; DM = diabetes mellitus.
Discussion
Most studies of hip fracture have focused on BMD decrements and bone metabolism in women. Little is known about bone turnover and BMD in men, and even less is known about the role that postfracture inflammation may play in bone turnover changes or BMD in either sex. Moreover, a study of white women showed that an increase in soluble TNFα receptor was associated with bone loss following hip fracture.(35)
In this study, we focused on sTNFα-R1 as a proxy of TNFα activation as the key inflammatory cytokine because it is an inflammatory biomarker that rises in response to an acute injury. In this study, we sought to evaluate the association of sTNFα-R1 and bone turnover biomarkers and BMD within the year following hip fracture in both men and women. Notably, men had higher levels of sTNFα-R1 at baseline that remained higher during the 1-year follow-up.
We identified four distinct groups of sTNFα-R1 trajectories following hip fracture with significant differences at baseline levels and slopes of sTNFα-R1. The first group had relatively low sTNFα-R1 levels at baseline and over the year of follow-up; the second and third groups had intermediate levels of sTNFα-R1, with the third group higher than the second; and the fourth group had the highest levels of sTNFα-R1. Our group previously reported a greater decline in BMD in men than women after 12 months.(36) Consistent with this, we found that men had higher levels of bone resorption than women over 1 year after adjustment for baseline BMD. In the current study, we found that the two distinct trajectories of sTNFα-R1 with the highest levels also had an accelerated rate of bone resorption and bone loss, after adjustment for baseline BMD, baseline CTX-I, and other covariates. Accelerated bone resorption was associated with the accelerated bone loss in men with higher sTNFα-R1 trajectories, which may explain, at least partially, why men experience greater BMD decline than women. Although women with higher sTNFα-R1 trajectories showed an association with BMD decline, the effect size, which is not influenced by sample size, was small and nonsignificant. One possible explanation is that men had higher levels of BMD at baseline and therefore, had more potential for manifesting significant BMD loss over the year following the hip fracture. It is also plausible that in older women who usually have lower BMD than men, a larger sample size is required to detect a significant BMD change following hip fracture.
The median P1NP in premenopausal women is about 36.0 ng/mL whereas beta CTX-I is about 0.29 ng/mL.(37) However, both the bone formation (P1NP) and bone resorption (CTX-I) biomarkers may increase in older populations with osteoporosis. Similarly, in our study, both baseline turnover biomarkers in both men and women were threefold higher than premenopausal women. However, only the bone resorption marker was significantly associated with the change in BMD.
sTNFα-R1 is a systemic inflammatory cytokine; thus, its role in inflammatory pathways plausibly explains its association with bone turnover postfracture. Indeed, studies have shown that in individuals with chronic inflammatory diseases, elevated levels of pro-inflammatory cytokines have been associated with bone resorption.(5–8) TNFα through TNFα-R1 induces receptor activator of nuclear factor κ B (RANK) to increase responsiveness to its ligand (RANKL). The RANKL, the TNFR-related RANK receptor, and osteoprotegerin (OPG), regulate osteoclastogenesis through TNF receptor associated factor (TRAF)-6 and I kappa B kinase (IKK), which activates the nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) pathway. TNFα stimulates expression of the RANK receptor and RANKL, an action that leads to further stimulation of TNFα in a positive feedback fashion. Moreover, TNFα binds to TNFα-R1, which in turn through TRAF2 activates IKK, and thus the NF-κB pathway. This complex interaction could serve to enhance the osteoclastogenic response.(38–41) Additionally, use of anti-TNFα medication in patients with chronic inflammatory states, such as RA or AS, has been shown to decrease bone resorption, increase bone formation, prevent erosion formation in RA patients (which is due to osteoclast-mediated bone destruction), and decrease bone loss.(18, 19)
Although acute activation of NF-κB following stress or injury is critical for healing(42) a sustained activation of TNFα and sTNFα-R1 level can potentially disturb the balance between osteoblast (cells involved in bone formation) and osteoclast (cells involved in bone resorption) activity through increased production RANKL, favoring bone resorption(43) Cauley and colleagues(44) revealed that an elevated serum level of sTNFα-R1 was associated with increased incidence of fracture in men. Studies have also shown that increased TNFα or its soluble receptors were associated with increased risk of fracture in older women.(35,45)
Although TNFα is just one of several cytokines participating in the pathophysiology of osteoporosis, there is evidence that activation of TNFα induces IL-1 and IL-6 production that further enhances osteoclastogenesis (42,46)
A long-term imbalance in bone resorption and formation biomarkers has been shown to result in greater bone loss, bone fragility, and therefore increased risk of recurrent fractures(23,47) We found a significant decrement in BMD only in the group with highest levels of sTNFα-R1. Although BMD is a valid measure for diagnosis of long-term osteoporosis, it may be less sensitive for capturing short-term changes in bone remodeling after a hip fracture. For example, we did not detect a sex-differences in BMD values earlier than 12 months, although there were significant sex differences in bone resorption biomarkers after adjustment for baseline BMD.(36) Cauley and colleagues44 also did not detect an association between baseline sTNFα-R1 and BMD in women over time, although they did detect an increased incidence of fractures. As an alternative, studies have suggested the use of bone turnover biomarkers as independent tools for an immediate evaluation of risk for fracture or as a measure of treatment effects independent of BMD. The clinical use of bone turnover biomarkers for an early diagnosis of increased risk of bone loss, and therefore early intervention, at least in part, may yield a better long-term bone-related outcome following a hip fracture.
In our study, neither baseline nor rate of sTNFα-R1 change over time was associated with the bone formation biomarker P1NP in either men or women. Some have suggested that a bone resorption biomarker is a stronger indicator of bone loss compared to a bone formation biomarker.(48,49) The median P1NP in premenopausal women is about 36.0 μg/mL, whereas beta CTX-I is about 0.29 ng/mL.(37) Both the bone formation (P1NP) and bone resorption (CTX-I) biomarkers may increase in an older population with osteoporosis. Similarly, in our study, both baseline CTX-I and PINP were threefold higher than in premenopausal women. However, only the bone resorption biomarker was significantly associated with change in BMD.
The results of our study are novel for multiple reasons. First, we identified distinct trajectories of sTNFα-R1 following hip fracture. Second, we found that these trajectories were related to accelerated bone resorption and bone loss in men. In our study we assessed cross-reactivity between anti-TNFα-R1 antibody, and RANKL and OPG recombinant proteins. Our data showed no cross-reactivity because adding about sevenfold recombinant proteins to the serum did not increase the level of captured anti-TNFα-R1. In our analysis, we also adjusted for the use of bisphosphonate-family medications that target RANKL-OPG pathways. The significant associations of sTNFα-R1 with bone outcomes were independent of these medications. These results suggest that sTNFα-R1 may exert its effect on bone loss independent of RANKL and OPG pathways. Third, we compared the associations of this inflammatory biomarker and bone turnover markers after adjustment for renal function and parathyroid hormone levels in men and women after fracture.
An important strength of the study, in addition to inclusion of both men and women after fracture, is that we had comprehensive measures of medical and medication history and BMD, for which we could adjust the analyses. However, our results are limited to a selected group of hip fracture patients entering one of eight hospitals in Baltimore, MD, and therefore, the results may not necessarily be applied to all hip fracture patients. Moreover, the potential for assay cross-reactivity is always a concern in biomarker studies. In this particular project, according to the assay’s manufacturer, the regions of homology of sTNFα-R1 and RANKL-OPG are remarkably low.
In conclusion, we found that men had increased levels of both CTX-I and sTNFα-R1. Higher levels of baseline sTNFα-R1 and the patterns of change in sTNFα-R1 during the year after hip fracture were associated with faster increases in CTX-I, indicating accelerated bone resorption in men. sTNFα-R1 in acute, subacute, and chronic states may exert effects through distinct pathways. Therefore, what is warranted next is a more comprehensive evaluation of inflammatory biomarkers and related metabolite pathways to shed more light on the mechanisms by which inflammation plays a role in determining bone outcomes. Results also suggest that modalities that modify the sTNFα-R1 pathway in the subacute stage following hip fracture, as a way of reducing bone loss, may maintain bone density and therefore, reduce possible risks of secondary fractures.
Supplementary Material
Acknowledgments
This work was supported by grants from the NIH National Institute on Aging (R37 AG09901, R01 AG029315, T32 AG00262, and P30 AG028747). We thank all hospital personnel for their support of the research and the participants. The University of Maryland Claude D. Pepper Older Americans Independence Center (UM-OAIC) and their families for their generous commitment to this project.
Authors’ roles: Current study design: SS, JMG, MH, and JM. BHS-7 study design: JM, MH, DO, AG-B, and RM. Study management: JM, DO, MH, and JMG. Data analysis: SS, MDS, and NF. Interpretation of results: SS, JMG, JM, MDS, MH, and NF. Drafting manuscript: SS, JM, and JMG. Critical review: SS, JM, MDS, JMG, AG-B, DO, and RM.
Disclosures
SS: Supported by NIH training grant T32 AG00262. MDS: None. RM: Full-time employee of the Novartis Institutes for Biomedical Research and stock owner in Novartis and GlaxoSmithKline. AGB: None. DO: None. NF: None. MCH: Consultant to the following entities: Bristol Myers Squibb, EMD Serono, Flexion Therapeutics Inc., Galapagos, Genentech/Roche, IBSA Biotechniq SA, Innovative Sciences, Novartis Pharma AG, Pfizer Inc., Plexxikon, Samumed LLC, Theralogix LLC, TissueGene Inc., TLC Biopharmaceuticals, Inc., and Zynerba. JMG: Consultant for Ammonett, Novartis, Pluristem and Boehringer Ingelheim Pharma. JM: Consultant and advisory boards for: American Orthopaedic Association, Ammonett, Novartis, Pluristem, and Viking. There are no potential conflicts of interest for the authors.
Footnotes
Additional Supporting Information may be found in the online version of this article.
References
- 1.Wehren LE, Magaziner J. Hip fracture: risk factors and outcomes. Curr Osteoporos Rep. 2003;1(2):78–85. [DOI] [PubMed] [Google Scholar]
- 2.Orwig DL, Chan J, Magaziner J. Hip fracture and its consequences: differences between men and women. Orthop Clin North Am. 2006;37(4):611–22. [DOI] [PubMed] [Google Scholar]
- 3.Dyer SM, Crotty M, Fairhall N, et al. A critical review of the long-term disability outcomes following hip fracture. BMC Geriatr. 2016;16:158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bliuc D, Nguyen ND, Milch VE, Nguyen TV, Eisman JA, Center JR. Mortality risk associated with low-trauma osteoporotic fracture and subsequent fracture in men and women. JAMA. 2009;301(5):513–21. [DOI] [PubMed] [Google Scholar]
- 5.Pietschmann P, Mechtcheriakova D, Meshcheryakova A, Foger-Samwald U, Ellinger I. Immunology of osteoporosis: a mini-review. Gerontology. 2016;62(2):128–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rodriguez-Bores L, Barahona-Garrido J,Yamamoto-Furusho JK. Basic and clinical aspects of osteoporosis in inflammatory bowel disease. World J Gastroenterol. 2007;13(46):6156–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Toussirot E, Wendling D. Antiinflammatory treatment with bisphosphonates in ankylosing spondylitis. Curr Opin Rheumatol. 2007;19(4):340–5. [DOI] [PubMed] [Google Scholar]
- 8.McInnes IB, Schett G. The pathogenesis of rheumatoid arthritis. N Engl J Med. 2011;365(23):2205–19. [DOI] [PubMed] [Google Scholar]
- 9.Bandeen-Roche K, Walston JD, Huang Y, Semba RD, Ferrucci L. Measuring systemic inflammatory regulation in older adults: evidence and utility. Rejuvenation Res. 2009;12(6):403–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fabbri E, An Y, Zoli M, et al. Aging and the burden of multimorbidity: associations with inflammatory and anabolic hormonal biomarkers. J Gerontol A Biol Sci Med Sci. 2015;70(1):63–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fougère B, Boulanger E, Nourhashémi F, Guyonnet S, Cesari M. Chronic inflammation: accelerator of biological aging. J Gerontol A Biol Sci Med Sci. 2017. September 1;72(9):1218–25. [DOI] [PubMed] [Google Scholar]
- 12.Miller RR, Cappola AR, Shardell MD, et al. Persistent changes in interleukin-6 and lower extremity function following hip fracture. J Gerontol A Biol Sci Med Sci. 2006;61(10):1053–8. [DOI] [PubMed] [Google Scholar]
- 13.Glass GE, Chan JK, Freidin A, Feldmann M, Horwood NJ, Nanchahal J. TNF-alpha promotes fracture repair by augmenting the recruitment and differentiation of muscle-derived stromal cells. Proc Natl Acad Sci U S A. 2011;108(4):1585–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gerstenfeld LC, Cho TJ, Kon T, et al. Impaired fracture healing in the absence of TNF-alpha signaling: the role of TNF-alpha in endochondral cartilage resorption. J Bone Miner Res. 2003;18(9):1584–92. [DOI] [PubMed] [Google Scholar]
- 15.Marsell R, Einhorn TA. The biology of fracture healing. Injury. 2011; 42(6):551−5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nanes MS. Tumor necrosis factor-alpha: molecular and cellular mechanisms in skeletal pathology. Gene. 2003;321:1–15. [DOI] [PubMed] [Google Scholar]
- 17.Li P, Schwarz EM, O’Keefe RJ, et al. Systemic tumor necrosis factor alpha mediates an increase in peripheral CD11bhigh osteoclast precursors in tumor necrosis factor alpha-transgenic mice. Arthritis Rheum. 2004;50(1):265–76. [DOI] [PubMed] [Google Scholar]
- 18.Arends S, Spoorenberg A, Brouwer E, van der Veer E. Clinical studies on bone-related outcome and the effect of TNF-alpha blocking therapy in ankylosing spondylitis. Curr Opin Rheumatol. 2014;26(3): 259–68. [DOI] [PubMed] [Google Scholar]
- 19.Zerbini CAF, Clark P, Mendez-Sanchez L, et al. Biologic therapies and bone loss in rheumatoid arthritis. Osteoporos Int. 2017;28(2):429–46. [DOI] [PubMed] [Google Scholar]
- 20.Vasikaran S, Eastell R, Bruyere O, et al. Markers of bone turnover for the prediction of fracture risk and monitoring of osteoporosis treatment: a need for international reference standards. Osteoporos Int. 2011;22(2):391−420. [DOI] [PubMed] [Google Scholar]
- 21.Yu-Yahiro JA, Michael RH, Dubin NH, et al. Serum and urine markers of bone metabolism during the year after hip fracture. J Am Geriatr Soc. 2001;49(7):877–83. [DOI] [PubMed] [Google Scholar]
- 22.Magaziner J, Wehren L, Hawkes WG, et al. Women with hip fracture have a greater rate of decline in bone mineral density than expected: another significant consequence of a common geriatric problem. Osteoporos Int. 2006;17(7):971–7. [DOI] [PubMed] [Google Scholar]
- 23.Riggs BL, Melton LJ 3rd, O’Fallon WM. Drug therapy for vertebral fractures in osteoporosis: evidence that decreases in bone turnover and increases in bone mass both determine antifracture efficacy. Bone. 1996;18(3 Suppl):197S–201S. [DOI] [PubMed] [Google Scholar]
- 24.Lambert JK, Zaidi M, Mechanick JI. Male osteoporosis: epidemiology and the pathogenesis of aging bones. Curr Osteoporos Rep. 2011;9(4):229–36. [DOI] [PubMed] [Google Scholar]
- 25.Rathbun AM, Shardell M, Orwig D, et al. Difference in the trajectory of change in bone geometry as measured by hip structural analysis in the narrow neck, intertrochanteric region, and femoral shaft between men and women following hip fracture. Bone. 2016;92: 124–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Seibel MJ. Biochemical markers of bone turnover part II: clinical applications in the management of osteoporosis. Clin Biochem Rev. 2006;27(3):123–38. [PMC free article] [PubMed] [Google Scholar]
- 27.Charlson M, Szatrowski TP, Peterson J, Gold J. Validation of a combined comorbidity index. J Clin Epidemiol. 1994;47(11):1245–51. [DOI] [PubMed] [Google Scholar]
- 28.Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nikodimopoulou M, Liakos S. Secondary hyperparathyroidism and target organs in chronic kidney disease. Hippokratia. 2011;15(Suppl 1):33–8. [PMC free article] [PubMed] [Google Scholar]
- 30.Lacey DL, Timms E, Tan HL, et al. Osteoprotegerin ligand is a cytokine that regulates osteoclast differentiation and activation. Cell. 1998;93(2):165–76. [DOI] [PubMed] [Google Scholar]
- 31.Wong BR, Josien R, Choi Y. TRANCE is a TNF family member that regulates dendritic cell and osteoclast function. J Leukoc Biol. 1999;65(6):715–24. [DOI] [PubMed] [Google Scholar]
- 32.Cawthon PM, Ewing SK, McCulloch CE, et al. Loss of hip BMD in older men: the osteoporotic fractures in men (MrOS) study. J Bone Miner Res. 2009;24(10):1728–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rabe-Hesketh S, Skrondal A. Multilevel and longitudinal modeling using Stata: volume I: continuous responses. Stat Methods Med Res. 2016;25(6):3069. [Google Scholar]
- 34.Shardell M, Hicks GE, Ferrucci L. Doubly robust estimation and causal inference in longitudinal studies with dropout and truncation by death. Biostatistics. 2015;16(1):155–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Barbour KE, Lui LY, Ensrud KE, et al. Inflammatory markers and risk of hip fracture in older white women: the study of osteoporotic fractures. J Bone Miner Res. 2014;29(9):2057–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rathbun AM, Shardell M, Orwig D, et al. Differences in the trajectory of bone mineral density change measured at the total hip and femoral neck between men and women following hip fracture. Arch Osteoporos. 2016;11:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Glover SJ, Gall M, Schoenborn-Kellenberger O, et al. Establishing a reference interval for bone turnover markers in 637 healthy, young, premenopausal women from the United Kingdom, France, Belgium, and the United States. J Bone Miner Res. 2009;24(3):389–97. [DOI] [PubMed] [Google Scholar]
- 38.Mundy GR. Osteoporosis and inflammation. Nutr Rev. 2007; 65(12 Pt 2):S147–51. [DOI] [PubMed] [Google Scholar]
- 39.Ginaldi L, Di Benedetto MC, De Martinis M. Osteoporosis, inflammation and ageing. Immun Ageing. 2005;2:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pfeilschifter J, Chenu C, Bird A, Mundy GR, Roodman GD. Interleukin-1 and tumor necrosis factor stimulate the formation of human osteoclastlike cells in vitro. J Bone Miner Res. 1989;4(1):113–8. [DOI] [PubMed] [Google Scholar]
- 41.Kobayashi K, Takahashi N, Jimi E, et al. Tumor necrosis factor alpha stimulates osteoclast differentiation by a mechanism independent of the ODF/RANKL-RANK interaction. J Exp Med. 2000;191(2): 275–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pahl HL. Activators and target genes of Rel/NF-kappaB transcription factors. Oncogene. 1999;18(49):6853–66. [DOI] [PubMed] [Google Scholar]
- 43.Zupan J, Jeras M, Marc J. Osteoimmunology and the influence of pro-inflammatory cytokines on osteoclasts. Biochem Med (Zagreb). 2013;23(1):43–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cauley JA, Barbour KE, Harrison SL, et al. Inflammatory markers and the risk of hip and vertebral fractures in men: the Osteoporotic Fractures in Men (MrOS). J Bone Miner Res. 2016;31(12):2129–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Moffett SP, Zmuda JM, Oakley JI, et al. Tumor necrosis factor-alpha polymorphism, bone strength phenotypes, and the risk of fracture in older women. J Clin Endocrinol Metab. 2005;90(6):3491−7. [DOI] [PubMed] [Google Scholar]
- 46.Hashizume M, Hayakawa N, Mihara M. IL-6 trans-signalling directly induces RANKL on fibroblast-like synovial cells and is involved in RANKL induction by TNF-alpha and IL-17. Rheumatology (Oxford). 2008;47(11):1635–40. [DOI] [PubMed] [Google Scholar]
- 47.Akesson K, Ljunghall S, Jonsson B, et al. Assessment of biochemical markers of bone metabolism in relation to the occurrence of fracture: a retrospective and prospective population-based study of women. J Bone Miner Res. 1995;10(11):1823–9. [DOI] [PubMed] [Google Scholar]
- 48.Cosman F, Nieves J, Wilkinson C, Schnering D, Shen V, Lindsay R. Bone density change and biochemical indices of skeletal turnover. Calcif Tissue Int. 1996;58(4):236–43. [DOI] [PubMed] [Google Scholar]
- 49.Ross PD, Knowlton W. Rapid bone loss is associated with increased levels of biochemical markers. J Bone Miner Res. 1998;13(2): 297–302. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.