Abstract
Introduction
Glucocorticoid-induced leucine zipper is a regulatory protein that sequesters activated nuclear factor-kappa B p65. Previously, we showed that rationally designed analogs of the p65-binding domain of glucocorticoid-induced leucine zipper, referred to as glucocorticoid-induced leucine zipper analogs (GAs), inhibited amyloid β–induced metabolic activity and inflammatory cytokines in mixed brain cell cultures. Here, we investigate the therapeutic efficacy of GA in an Alzheimer's disease model.
Methods
GA and control peptides were synthesized covalently as peptide amides with the cell-penetrating agent. C57Bl/6J mice induced with lipopolysaccharide-mediated neuroinflammation (250 mg/kg i.p/day for six days) were treated on alternate days with GA-1, GA-2, or control peptides (25 mg/kg i.v). Brain tissues were assessed for gliosis, cytokines, and antiapoptotic factors.
Results
The brain tissues of GA-1– and GA-2–treated mice exhibited significantly reduced gliosis, suppressed inflammatory cytokines, and elevated antiapoptotic factors.
Discussion
The antineuroinflammatory effects of GA suggest potential therapeutic application for Alzheimer's disease.
Keywords: Neuroinflammation, NF-κB p65 blockade, Neurodegeneration, Alzheimer's disease, Peptide therapeutics
1. Background
Alzheimer's disease (AD) is a degenerative brain disease and the most common form of dementia [1]. Pathologically, AD brains exhibit accumulations of amyloid β (Aβ) plaques and neurofibrillary tangles as well as elevated inflammation-related proteins such as innate immune receptors and proinflammatory cytokines. Elucidations of pathogenesis suggests that both local and systemic inflammations aggravate neurotoxicity and accelerate neurodegeneration in individuals with increased risk for AD [2], [3]. Nuclear factor-kappa B is a critical transcription factor that regulates inflammatory responses, Aβ generation, and tau deposition [4], [5]. Hence, NF-κB has long been recognized as a critical therapeutic target for AD.
The nuclear factor-kappa B family consists of five members (p50, c-rel, p65, RelB, and p52) that diversely combine to form transcriptionally active dimers. In the central nervous system (CNS), positive and negative regulatory mechanisms maintain a homeostatic balance between the neuroprotective c-rel-containing dimers and the predominantly neurotoxic p65:p50 dimers [6], [7]. In AD, age-related changes such as elevated oxidative stress induce excessive activation of p65:p50 dimers. This in turn mediates increased transcription of inflammatory mediators and of endogenous β secretase-1, both of which enhance Aβ production [8], [9]. Observation of increased proportion of neurons with nuclear p65 in the hippocampus and cortex around Aβ plaques in postmortem AD tissues supports a critical role for activated p65 in the process of cholinergic degeneration [10], [11], [12]. Taken together, selective inhibition of activated NF-κB p65 represents an attractive therapeutic strategy for AD [9], [13], [14], [15].
Glucocorticoid-induced leucine zipper (GILZ) is a transcriptional regulatory protein that is constitutively expressed in many tissues including the brain [16], [17]. Functionally, GILZ sequesters the activated p65 in the cytoplasm, preventing its nuclear translocation and thereby transactivation of target genes [18], [19]. Structurally, GILZ binds the transactivation domain (TAD) of p65 via a proline glutamic acid–rich region [20], [21], [22]. The key prolines of GILZ at interface with p65 TAD adopt an extended polyproline type II (PPII) helical conformation [23] and potentially interact with the side chain of phenylalanine residues (F534/F542) of p65 critical for its transactivation ability [24], [25]. Highly represented in transient intermolecular interfaces in human proteome, PPII helical motifs are considered excellent epitope/s for drug development [26], [27]. We developed rationally designed peptide mimics of the p65-binding motif of GILZ, which form stable PPII helix in the context of p65-TAD [23] and showed that select glucocorticoid-induced leucine zipper analogs (GAs) inhibited Aβ-induced metabolic activity and inflammatory cytokines in human mixed brain cell cultures.
A key limitation of drug discovery in AD is that the currently available animal models of AD do not recapitulate all features of the disease precluding true and meaningful extrapolation of findings from models to humans [28], [29]. Although transgenic animal models emphasize the genetic component of the disease, exogenous agent–induced neuroinflammation models highlight the nongenetic processes that accelerate the degeneration in AD [28], [29], [30]. Yet, recognizing and accommodating for these limitations, models of neuroinflammation have been used to test the efficacy of potential therapeutic agents that attempt to slow the disease progression [31]. For example, the validity of nutraceuticals such as resveratrol, glycyrrhizin, and flavonoids for AD was assessed in the lipopolysaccharide (LPS)-induced model of neuroinflammation [32], [33], [34]. The LPS binds the co-receptor CD14 on microglial cells, and the complex then interacts with the toll-like receptor-4. Subsequent signal transduction increases NF-κB p65-mediated transactivation of pathological mediators, leading to neuroinflammation and neurodegeneration [3], [31]. Here, we assessed the effects of GA in mice following systemic LPS administration and show that treatment with select GA suppressed neuroinflammation as indicated by reduced gliosis, decreased inflammatory cytokines, and lessened proapoptotic transcription.
2. Materials and methods
2.1. Peptides
The experimental PPII helical peptides GA-1 and GA-2 or control peptides (sequence [CP-1] or conformation [CP-2] or unstructured [CP-3]). All peptides were co-synthesized with the cell-penetrating agent transactivator of transcription of human immunodeficiency virus (GRKKRRQRRRPQ) for intracellular delivery as peptide amides with amino terminal acetylation (GenScript, Piscataway, NJ) [23], [35]. All peptides were purified by semipreparative reverse-phase high performance liquid chromatography, and the identity of the purified peptide was confirmed by mass spectrometry.
2.2. Animals and LPS-induced neuroinflammation
LPS from Escherichia coli 026:B6, = 10,000 EU/mg, was purchased from Sigma (Sigma Aldrich, MO, USA). Adult C57/6J mice were randomly divided into seven groups of five mice/group: LPS, LPS + GA-1 or GA-2 or CP-1 or CP-2 or CP-3 or transactivator of transcription of human immunodeficiency virus. The LPS was administered intraperitoneally at 250 mg/kg in 100 μL of sterile saline followed by corn oil in the morning daily for 6 days [31], [36]. The GA or CP was administered intravenously at 25 mg/kg in 100 μL of sterile saline on alternate days. Additional control group included mice receiving saline alone. All animal experiments were approved by the Institutional Animal Care and Use Committee of the Indiana University Purdue University at Indianapolis.
2.3. Histology and immunohistochemistry
The mice were sacrificed on day 7 by intracardial perfusion of 25 mL of 0.9% saline. The brain from each mouse was removed, one half was immediately frozen, and the other half fixed in 4% paraformaldehyde for paraffin embedding. Hematoxylin- and eosin-stained sections were assessed for inflammation.
Serial 5-μ-thick coronal sections of each brain specimen were immunostained for markers of microglial cells, astrocytes, and NF-κB p65. Briefly, after deparaffinization and hydration, sections were subjected to antigen retrieval by microwave incubation in 10 mM sodium citrate buffer (pH 6.0) followed by sequential incubation in 30% hydrogen peroxide and blocking buffer (Enzo biosciences, Farmingdale, NY) to reduce nonspecific binding. The sections were then stained with the following primary monoclonal antibodies from EMD Millipore Corporation, Temecula, CA, USA: anti-Iba-1 (AIF1 clone: Cat. # MABN92) or anti-glial fibrillary acidic protein (GFAP) clone GA5 (Cat. # MAB3402, Millipore) or anti-CD11b (Cat. #. MABF513) clone M1/70 or anti-NF-κB p65 subunit clone 12H11 (Cat. #. MAB3026). For monostaining of Iba-1 and GFAP, the detection was performed with the use of immunohistochemistry select immunoperoxidase secondary detection system (Millipore). The peroxidase-conjugated streptavidin-biotin method was used, and brown staining was considered positive. For dual staining of CD11b and NF-κB p65, detection was achieved using the MULTIVIEW (mouse-HRP/rabbit-AP) IHC kit (Catalog #: ADI-950-100, ENZO Life Sciences). For each marker, to confirm the specificity of the antibodies, a separate set of sections from each group were incubated with secondary antibodies alone.
2.4. Quantitative analysis of staining intensity
Images of the IHC-stained sections were captured using the NIKON Multiphoton microscope with attached DS Ri2 camera. Five areas encompassing the hippocampus of each brain section were analyzed using the ImageJ Software [37]. The number and the relative optical density of Iba1+ and GFAP + glial cells were determined using the multipoint tool and the IHC-tool box. Training for brown/red color was performed before measurement to define the threshold for positive selection. Regions of interest encompassing the hippocampus were identified in each section, and the corresponding images were quantified using integrated optical density, normalized to the background optical density. Measurement of pixel intensity was restricted to the 3,3′-diaminobenzidine- or alkaline phosphatase-stained pixels of cells within defined circularity. Five consecutive coronal sections were assessed for each mouse.
2.5. Enzyme-linked immunosorbent assay for cytokines
Frozen brain tissue was homogenized in RIPA lysis buffer (50 mmol/LTris–HCl, pH 6.8, 150 mmol/L NaCl, 5 mmol/L EDTA, 0.5% sodium deoxycholate, 0.5% NP-40) supplemented with a cocktail containing protease and phosphatase inhibitors (Chemicon, Millipore) on ice and then centrifuged at 16,000 × g for 30 min [14], [15], [36]. The supernatant was stored at −80°C until further analysis. For each sample, 10 μL of extracted protein was used for measuring the cytokines interleukin (IL)-6, tumor necrosis factor-alpha, interferon-gamma, IL-10, and transformation growth factor-β using specific OptEIA kits (BD Biosciences, CA).
2.5.1. NF-κB assay
Brain homogenates processed as mentioned previously were assessed by the Cell Signaling Technology PathScan Phospho-NF-κB p65 (Ser536) Sandwich ELISA kit following manufacturer's protocol (Cell Signaling Technology, Boston, MA). Briefly, 40 μL of the extracted protein mixed with 60 μl of the sample diluent was added to phosphor p65 (Ser536) mouse mAb–coated microwells and incubated overnight at 4°C. After washing, the captured phospho-NF-κB p65 protein was detected by incubating with horseradish peroxidase–conjugated NF-κB p65 rabbit mAb followed by color development with 3,3′,5,5′-tetramethylbenzidine substrate. Absorbance at 450 nM was measured using a microplate reader (Model 680; Bio-Rad Laboratories, CA, USA). Nuclear extracts of Raji cells served as the positive control.
2.6. Real-time polymerase chain reaction
The total cellular RNA was isolated from each brain tissue using Qiagen kit (Invitrogen, Carlsbad, CA) and reverse transcribed using iScript cDNA synthesis kit (Bio-Rad, CA). Equal amount of cDNA measured by the NanoDrop (Thermo Fisher Scientific, Waltham, USA) was used for amplification of IL-1β, IL-12, CD14, B-cell lymphoma-extra large (Bcl-xL), and B-cell lymphoma 2 (Bcl-2). Real-time polymerase chain reaction was performed using SYBR green/ROX quantitative polymerase chain reaction master mix (SA Biosciences, Frederick, MD) on the ABI Prism 7000 sequence detection system (Applied Biosystems, Foster City, California, USA) as described [23]. The primers used were as follows: F-βActin: 5′TCATGAAGTGTGACGTTGACATCCGTA3′; R-βActin: 5′CCTAGAAGCATT TGCGCTGCACGAT GG3′ (102 bp); F-IL-1β:5′AGCTGATGGCCCTAAACAGA3′; R-IL-1β:5′GGTCGGAGAT TC GTAGCTGG3′ (89 bp); F-CD14:5′GAGCTAGACGAGGAAAGTTGT3′; R-CD14:5′ACCGTAAGCCGCTT TAA GGACAGA3′ (206 bp); F-Bcl-xL:5′TGGAGTAAACTGGGGTCGCATC-3′; R-Bcl-xL:5′AGCCACAGTCAT GCCC GTCAGG3′ (84 bp) and F-Bcl-2:5′CTCGTCGCTACCGTCGTCACTTCG3′; R-Bcl-2:5′GTGGCCCAGGT ATG ACCCAG3′ (96 bp). The gene-specific threshold cycle (Ct) was corrected by subtracting the Ct for the housekeeping gene β-actin. The ΔCt for experimental samples was subtracted by the ΔCt for the control samples (ΔΔCt). The difference in each gene-specific threshold between the sample from vehicle treated and GA- or CP-treated cells was determined to obtain the relative change in the specific mRNA. The magnitude of change in each gene was determined by the 2-ΔΔCt method. Each measurement of a sample was performed in duplicates.
2.6.1. Statistical analysis
Statistical significance of the difference in the staining intensity, cytokine concentration, and the transcript quantitation between the GA-treated and the untreated or control-peptide–treated groups was determined by one way analysis of variance and Tuckey's post hoc test. A value of P < .05 was considered significant.
3. Results
3.1. Identification of druggable site: The interface of p65:GILZ interaction
Structurally, p65 has dimerizing amino terminal rel homology domain, a nuclear localization sequence masked by the IκB inhibitory complex and carboxy terminal TAD. The transactivation activity of p65 is mediated by interactions of the TAD with co-regulators and the basal transcription machinery. The p65-TAD is commonly divided into two distinct regions, TAD-1521-551 consisting of 36 amino acids and TAD-2428-520 with 92 residues [24]. While TAD1 accounts for nearly 95% of the transactivation potential of full-length p65, TAD-2 alone is less potent mediating about 30% activation. In particular, the conserved aromatic residues (F534, F542), acidic residues (D531, D533), and phosphorylation sites (Ser529, Ser536) in p65-TAD1 have been identified as critical for transactivation [38], [39].
GILZ is a p65-TAD–binding protein that sequesters activated p65 and inhibits transactivation of inflammatory and apoptotic factors [20]. Mutational, binding and structural studies suggested that the p65-binding region of GILZ adopts a PPII helical conformation in the context of critical phenylalanine in p65-TAD [7], [19]. PPII helices highly represented at interfaces of transient protein interactions in human proteome and often behave as adaptable gloves in obtaining the correct binding orientation. The specificity of PPII interaction is determined by residues of the interacting partner. PPII interface mimics constitute excellent drug templates [26], [27], [40]. GAs are rationally designed peptides with residue substitutions in the p65-binding motif of GILZ with increased propensity to form stability of PPII helical conformation in the context of p65-TAD (Fig. 1). Screening 40 GAs, we selected two analogs referred to as GA-1 and GA-2 that exhibit good PPII potential, near-native docking with p65-TAD, acceptable binding strength for transient interactions, and inhibitory potential in cellular assays for evaluating the in-vivo therapeutic efficacy. In silico predictions suggest that the predominant proteolytic products of GA-1 or GA-2 are to the right of the cell-penetrating carrier, suggesting that the GA is potentially delivered intracellularly as intact cargo to bind the activated p65 in the cytosol.
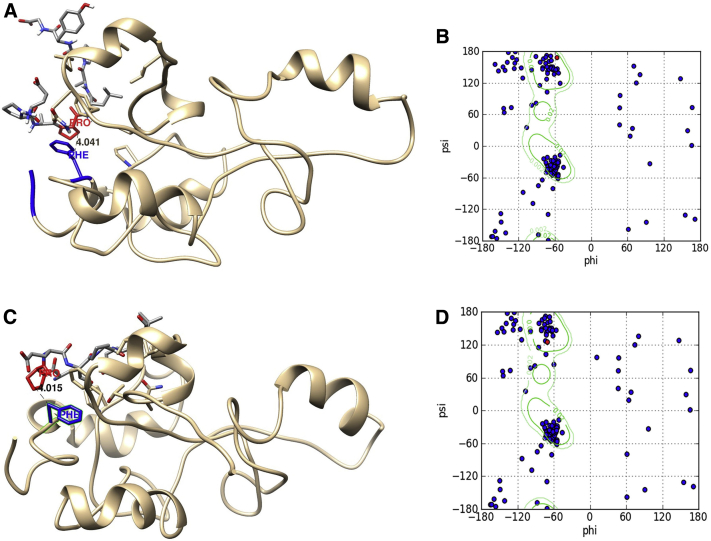
Fig. 1.
The docked complex of GA-1 with human p65-TAD. Representative molecular model of p65-TAD docked with GA-1 (A) or GA-2 (B). The critical proline in each GA and the critical phenylalanine are highlighted in red and blue, respectively. The distance between the Cβ atoms of the two residues in the interface is shown. (C and D) show Ramachandran plot of GA-1 (C) or GA-2 (D) with the phi and psi angles of the critical proline (in red) suggestive of PPII helical conformation. Abbreviations: TAD, transactivation domain; GA, glucocorticoid induced leucine zipper analog; PPII, polyproline type II.
3.2. GA prevented LPS-induced microglial and astrocyte activation in the hippocampus
It has been well documented that peripherally injected LPS induce a variety of central effects. Multiple injections of LPS increase the number of F4/80+, CD11b+, or Iba1-+ cells and induce morphological changes characteristic of activated microglia in the CNS [31], [36], [41], [42]. We observed that the mice induced neuroinflammation and treated with GA-1 or GA-2 exhibited reduced Iba1+ microglial cells and GFAP+ astroglial cells in the hippocampus as compared with that in the hippocampus of mice treated with CP or left untreated (Figs. 2 and 3). Furthermore, we also observed that the number of CD11b+ p65+ glial cells was significantly reduced in number, and intensity in the hippocampus of GA-1– or GA-2–treated mice as compared with that in untreated or CP-treated mice (Fig. 4).
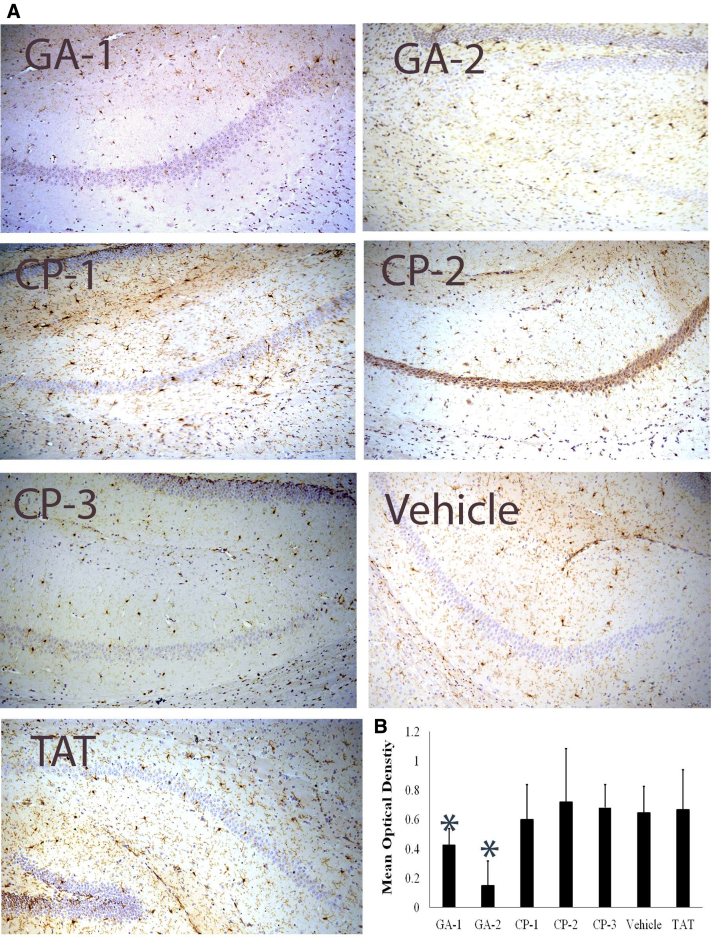
Fig. 2.
Representative images of degrees of microgliosis in mouse hippocampus. (A) shows representative IHC section stained for Iba + microglia in hippocampus of representative mouse treated with the indicated compound (B) shows the mean optical density of the 3,3′-DAB-positive cells depicting microglia in groups of vehicle or indicated compound-treated mice. Abbreviations: CP, control peptide; GA, glucocorticoid induced leucine zipper analog; DAB, diaminobenzidine; IHC, immunohistochemistry. *P < .05.
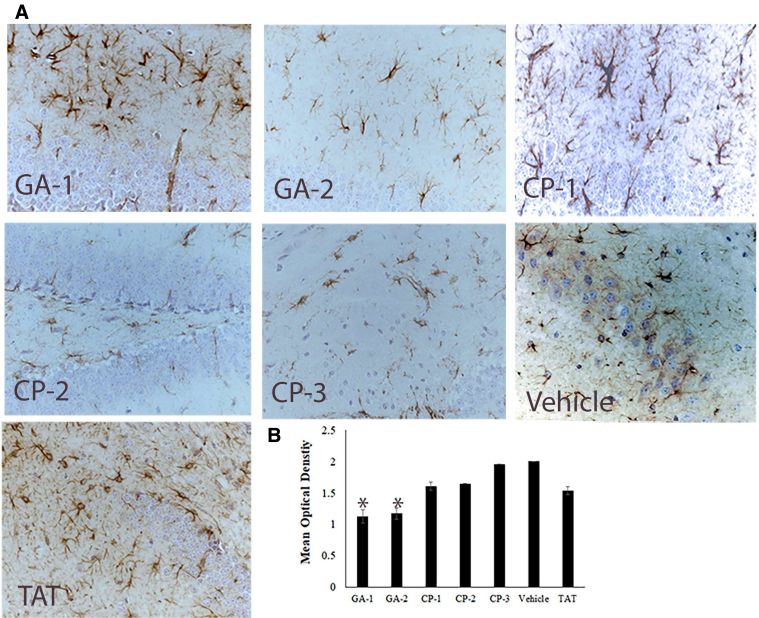
Fig. 3.
Representative images of degrees of astrogliosis in mouse hippocampus. (A) shows representative IHC section stained for GFAP+ microglia in hippocampus of representative mouse treated with the indicated compound (B) shows the mean optical density of the 3,3′-diaminobenzidine-positive cells depicting astrocytes in groups of vehicle or indicated compound-treated mice. Abbreviations: CP, control peptide; GA, glucocorticoid induced leucine zipper analog; GFAP, glial fibrillary acidic protein; DAB, diaminobenzidine; IHC, immunohistochemistry. *P < .05.
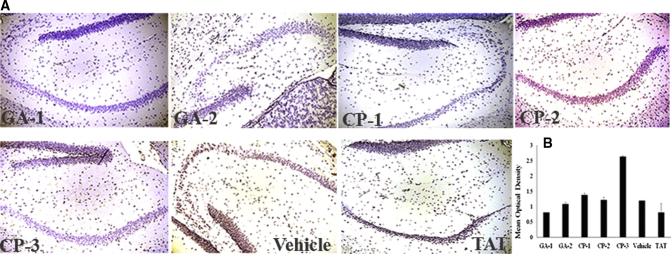
Fig. 4.
Representative images of NF-kB p65+ microglia in mouse hippocampus. (A) Shows representative IHC section stained for CD11b+ (red) and p65+ (brown) microglia (B) shows the mean optical density of the 3,3′-DAB-positive cells depicting p65 microglia in groups of vehicle or indicated compound-treated mice. Abbreviations: NF-kB, nuclear factor-kappa B; CP, control peptide; GA, glucocorticoid induced leucine zipper analogs; DAB, diaminobenzidine; IHC, immunohistochemistry.
3.3. GA suppresses LPS-induced accumulation of inflammatory proteins in the hippocampus
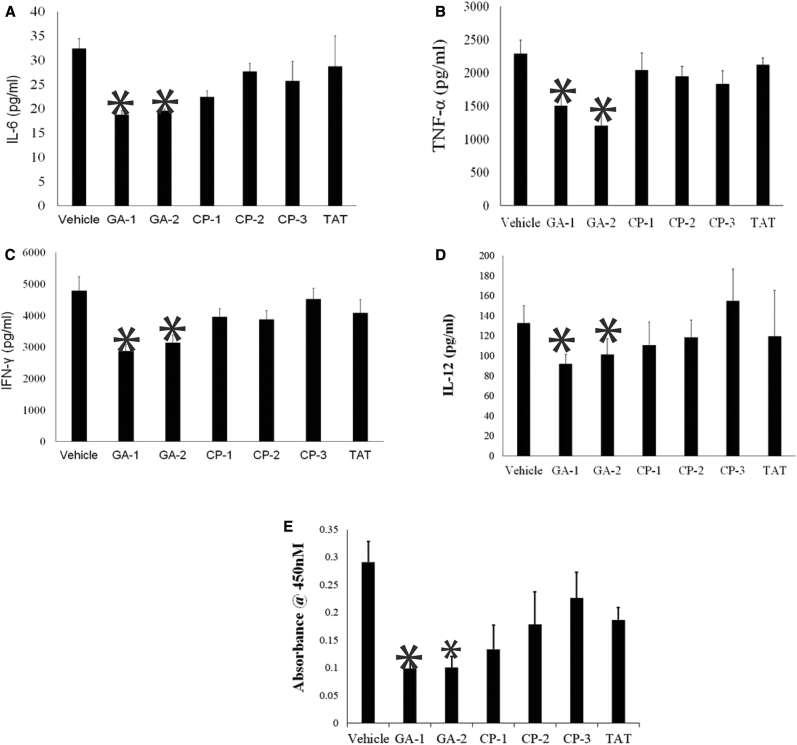
We next investigated the brain tissues for cytokines known to be critical for microglial activation and gliosis in LPS-induced neuroinflammation [31], [36], [42]. Brain tissue homogenates of GA-1– or GA-2–treated mice exhibited reduced IL-6 (18.7 ± 2 pg/mL and 19.6 ± 0.84 pg/mL, respectively) as compared with the homogenates of untreated (32.4 ± 4.7 pg/mL) or CP-treated mice (Fig. 5A). Similarly, brain homogenates of GA-1– or GA-2–treated mice exhibited reduced tumor necrosis factor-alpha (1500.1 ± 476 pg/mL and 1199.2 ± 356.4 pg/mL respectively) as compared with the untreated (2287.7 ± 473 pg/mL) or the CP-treated mice (Fig. 5B). The concentration of interferon-gamma was also significantly lower in the brain tissue of GA-1 (2863.2 ± 697.5 pg/mL) or GA-2 (3125.1 ± 815 pg/mL) treated mice as compared with the untreated (4788.2 ± 2069.1 pg/mL) mice (Fig. 5C). The concentration of IL-12 was also significantly lower in brain homogenates of GA-1– or GA-2–treated mice (92.0 ± 9.3 pg/mL, 101.3 ± 15.9 pg/mL) than that in the brain tissues of untreated mice (132.9 ± 17 pg/mL) or CP-treated mice (Fig. 5D).
Fig. 5.
Effect of GA treatment on cytokines in brain tissues in LPS-induced neuroinflammation: Adult C57Bl/6 mice were subjected to LPS-induced neuroinflammation and treated with the indicated GA or control peptides as described in materials and methods. Protein extracts of brain tissues harvested at the end of experiment was assessed for indicated proinflammatory cytokines (A) IL-6, (B) TNF-α, (C) IFN-γ, (D) IL-12 and (E) activated p65 NF-kB subunit. Values are average/-S.D. * P < .05 as compared with vehicle-treated mice, @ P < .05 as compared with CP-1 or CP-2 or CP-3–treated mice. Abbreviations: NF-kB, nuclear factor-kappa B; GILZ, glucocorticoid-induced leucine zipper; LPS, lipopolysaccharide; GA, glucocorticoid induced leucine zipper analog; CP, control peptide; IL, interleukin; TNF-α, tumor necrosis factor-alpha; IFN-γ, Interferon-gamma.
3.4. GA treatment suppressed activated NF-κB p65 in LPS-induced neuroinflammation
Because LPS is a potent stimulator of NF-κB and GA are designed to sequester activated p65, we measured the activated p65 in the brain homogenates using specific ELISA kits. We observed that the absorbance suggestive of activated p65 was significantly lower in the brain protein extract from mice treated with GA-1 (0.098 ± 0.03) or GA-2 (0.12 ± 0.07) than that of extract from mice left untreated (0.29 ± 0.08) or treated with CP-3 (0.23 ± 0.09) (Fig. 5E).
3.5. GA treatment inhibited LPS-induced suppression of antiapoptotic factors
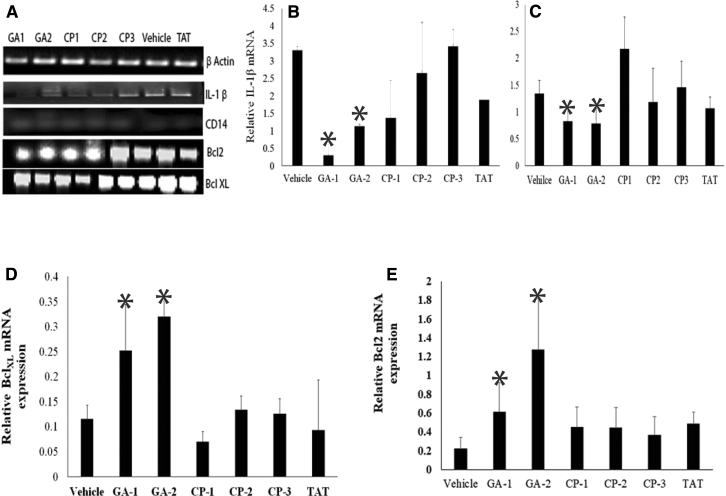
To investigate the effects of GA on neurotoxicity, we determined the expressions of antiapoptotic factors Bcl-xL and Bcl-2 by real-time polymerase chain reaction. In addition, inhibition of LPS-induced cytokine secretion correlated with the decreased levels of steady-state mRNA for IL-1β in the brain tissues of in GA-1– or GA-2–treated mice (Fig. 6A and B). We observed that the relative expression of CD14 mRNA was significantly lower in brain tissues of GA-1 (0.48 ± 0.1) or GA-2 (0.64 ± 0.4) treated mice than that in the brain tissues of untreated (4.0 ± 1.7) or CP (CP-1:1 ± 0.3; CP-2:2.4 ± 2; CP-3:1.2 ± 0.1) treated mice (Fig. 6A and C). The relative expression of Bcl-xL and Bcl-2 was significantly higher in brain tissues of GA-1– (0.32 ± 0.15 and 1.25 ± 0.5, respectively) or GA-2– (0.25 ± 0.4 and 1.9 ± 0.9, respectively) treated mice than that in brain tissues of untreated (0.11 ± 0.1 and 0.22 ± 0.5, respectively) or CP (CP-1:0.06 ± 0.08 and 0.36 ± 0.8, CP-2: 0.13 ± 0.12 and 0.45 ± 0.9; CP-3: 0.13 ± 0.1 and 0.44 ± 0.9, respectively) treated mice (Fig. 6A, D and E).
Fig. 6.
Effect of GA treatment on apoptosis-related molecules in brain tissues of LPS-induced neuroinflammation: Groups of adult C57Bl/6 mice were subjected to LPS-induced neuroinflammation and treated with the indicated GA or control peptides as described in materials and methods. Equal quantity of cDNA isolated from brain tissues of mice treated with GA or CP were amplified for IL-1β, CD14, Bcl-xL, and Bcl-2 mRNA by quantitative PCR. The mRNA expression in each sample was finally determined after correction with GAPDH expression. (A) Gel electrophoresis of the PCR products GAPDH (111 bp), IL-1β (89 bp), CD14 (206 bp), Bcl-xL (84 bp), and Bcl-2 (96 bp). (B) Relative mRNA quantitation of the indicated product with respect to that of housekeeping gene GAPDH is shown. Data are average ± SD. * P < .05 with respect to vehicle- and CP-treated group. Abbreviations: GILZ, glucocorticoid induced leucine zipper; GA, glucocorticoid induced leucine zipper analog; LPS, lipopolysaccharide; CP, control peptide; Bcl-xL, B-cell lymphoma-extra large; Bcl-2, B-cell lymphoma 2; PCR, polymerase chain reaction; IL, interleukin.
4. Discussion
Disproportionate increase in activated NF-κB p65:p50 dimers is implicated in neuroinflammatory and neurotoxic responses. Meta-analysis of differentially expressed genes showed that the increased p65 is associated with 101 of the 168 pathways perturbed in late onset AD [43]. Systemic inflammation and increased infiltration of mononuclear cells with high nuclear p65 are observed in sporadic AD. Furthermore, recent elucidations suggest that the activated NF-κB increases the transcription of proinflammatory microRNA, which in turn downregulate many phagocytic-, synaptogenic-, and neurotrophism-relevant genes. Elevated NF-κB–mediated mi-RNA 125b has been observed in the affected tissues and in the cerebrospinal fluid in AD [44]. Decoy nucleotides that inhibit NF-κb–mediated transcriptional activity have been shown to inhibit Aβ-triggered release of cytochrome c, rescue expression of Bcl-xL, and interfere with intracellular accumulation and extracellular deposition of Aβ [9], [45]. Here, we report the efficacy of a novel peptide agent that selectively inhibits activated p65 to suppress neuroinflammation.
Interfaces of protein-protein interactions are considered excellent targets for developing peptide inhibitors [46]. Rapid proteolytic degradation and poor stability are considered significant shortcomings of peptides as drugs. Strategies to overcome these inherent drawbacks and increase in-vivo half-life include blockade of end groups as incorporated in GA. Indeed, the stability of end group–blocked Aβ peptide has been shown to be several folds higher than that of free peptide with a half-life of over 6 h [47]. In addition, amino-acetylation of GA could increase the lipophilicity and improve membrane permeability, thereby enhancing delivery across the blood-brain barrier [48].
Traditionally, neuroinflammation in AD is considered a reactive phenomenon secondary to damaged neurons that provoke glial activation. However, accumulating evidence suggest that under physiological conditions, a delicate balance between the transient inflammatory responses by glial cells to stress/insults and the endogenous anti-inflammatory and neuroprotective responses in the brain maintain the healthy status. Any perturbation of this balance that leads to more severe and persistent neuroinflammatory cycle could promote neurodegeneration culminating in clinical disease. Central or systemic LPS administration has been shown to increase the number of glial cells, upregulate proinflammatory cytokines in the brain, and eventually precipitate dopaminergic neurotoxicity [13], [22], [41], [49]. Culture medium of LPS-stimulated microglia cells enhanced NF-κB p65 signaling and downregulated Bcl-2 expression in neuronal cells. In contrast, activation of NF-κB c-rel dimers has been shown to enhance Bcl-xL expression and protect against neuronal loss [50], [51], [52]. Our data show that in mice subjected to LPS-induced neuroinflammation, treatment with GA-suppressed gliosis and inflammatory cytokines as well as prevented downregulation of Bcl-2 and Bcl-xL expression largely by inhibiting activated p65 as indicated by the significant reduction in phosphor-p65 in the CNS.
Although the model used here does not replicate the dementia in human AD, the ability of GA to inhibit activated NF-κB p65 and suppress gliosis and inflammatory cytokines suggests potential therapeutic benefits for AD (Fig. 7). Interestingly, GILZ shares significant sequence and structural homology with delta sleep-inducing immunoreactive peptide originally identified by antibodies to a CNS-penetrating hydrophilic nanopeptide [23], [53]. The cross-reaction is attributed to the sequence homology between the nanopeptide and the carboxy terminal of delta sleep-inducing immunoreactive peptide shared by GILZ [53], [54], [55]. This suggests that the GA designed from the carboxy terminal of GILZ will have potential ability to reach the CNS independently. Future studies will evaluate the efficacy of end groups blocked GA independent of transactivator of transcription of human immunodeficiency virus in models of AD. Given the limitations of the Aβ-centric therapies, the potential for alternative strategies such as neuroinflammation targeting disease-modifying therapies for AD is increasing recognized as suggested by the rapid development of receptor for advanced glycation end products antagonists and p38 kinase inhibitors [56], [57]. GA represents a class of agents that can be added to this growing list of disease-modifiers for AD.
Research in Context.
-
1.
Systematic review: The authors searched the literature (using PubMed) for research articles and reviews describing studies on therapies targeting neuroinflammation and nuclear factor-kappa B for Alzheimer's disease. This search pointed to the increased interest for disease-modifying therapies that slow disease progression. There is considerable excitement for development of novel compounds that target mechanistic links between neuroinflammation, amyloid deposition, and neurodegeneration.
-
2.
Interpretation: We identified a unique strategy to selectively suppress activated nuclear factor-kappa B p65, the master regulator of inflammatory responses that also is critical for Aβ production. Our data show that rationally designed analogs of the p65-binding motif of a nuclear factor-kappa B interacting protein, glucocorticoid induced leucine zipper suppressed gliosis and inflammatory responses in the lipopolysaccharide induced neuroinflammation model of Alzheimer's disease.
-
3.
Future directions: These results are of significant translational value and position GA as a potential disease-modifying agent for the treatment of Alzheimer’s disease.
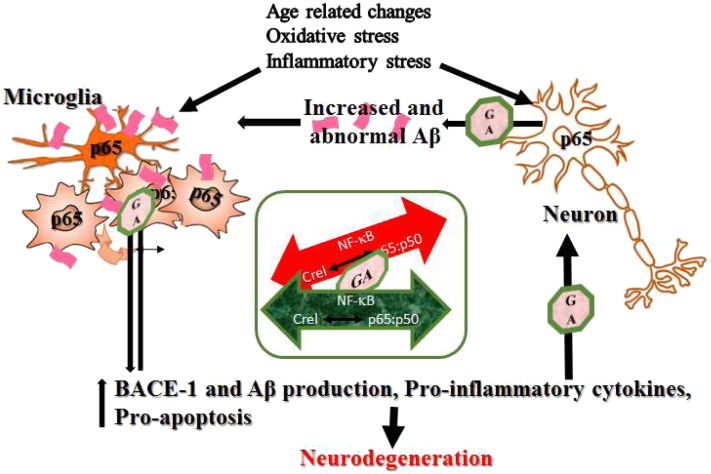
Fig. 7.
Schematic representation of the role of NF-κB in AD pathology and the sites of action of GA: In health, a homeostatic balance between activated c-rel containing dimers and the p65:p50 dimers plays a role in maintaining synaptic activity, neuronal plasticity, and health. In susceptible individuals, age-related noxious stimuli activate NF-κB p65 in neuronal cells, increase transactivation of BACE-1 and consequently Aβ generation. Stimulation and NF-κB p65 activation in glial cells lead to increased secretion of proinflammatory genes (such as IL-12, IL-17), proapoptotic genes and neurotoxic factors (glutamate, iNOS). A feed forward loop ensues culminating in neurodegeneration. By selectively targeting activated p65, GA edges the disrupted balance toward the homeostatic level. The insight shows the rationale for the efficacy of GA: an attempt to re-establish the NF-κB homeostasis and suppress excessive glial activation. Abbreviations: NF-κB, nuclear factor-kappa B; GA, glucocorticoid induced leucine zipper analog; Aβ, amyloid β; AD, Alzheimer’s disease; BACE-1, β secretase-1; iNOS, induced nitric oxide synthase; IL, interleukin.
Acknowledgments
The authors sincerely appreciate the grant support from the NIH (1R41 AG053117) to M.S., from Alzheimer's Association (IIRG-11-206418) and the National Institute on Aging, NIH (5R21AG042804 and 1R21AG047) to D.K.L. The authors sincerely appreciate the grant supports from the Indiana CTSI to M.S. and D.K.L.
Contributor Information
Mythily Srinivasan, Email: mysriniv@iupui.edu.
Debomoy K. Lahiri, Email: dlahiri@iupui.edu.
References
- 1.Association As 2018 Alzheimer's disease facts and figures. Alzheimers Dement. 2018;14:367–429. [Google Scholar]
- 2.Bales K.R., Du Y., Holtzman D., Cordell B., Paul S.M. Neuroinflammation and Alzheimer's disease: critical roles for cytokine/Abeta-induced glial activation, NF-kappaB, and apolipoprotein E. Neurobiology Aging. 2000;21:427–432. doi: 10.1016/s0197-4580(00)00143-3. 451–423. [DOI] [PubMed] [Google Scholar]
- 3.Lopez-Valdes H.E., Martinez-Coria H. The role of neuroinflammation in age-related dementias. Rev Invest Clin. 2016;68:40–48. [PubMed] [Google Scholar]
- 4.Buggia-Prevot V., Sevalle J., Rossner S., Checler F. NFkappaB-dependent control of BACE1 promoter transactivation by Abeta42. J Biol Chem. 2008;283:10037–10047. doi: 10.1074/jbc.M706579200. [DOI] [PubMed] [Google Scholar]
- 5.Snow W.M., Albensi B.C. Neuronal gene targets of NF-kappaB and their dysregulation in Alzheimer's disease. Front Mol Neurosci. 2016;9:118. doi: 10.3389/fnmol.2016.00118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Malek R., Borowicz K.K., Jargiello M., Czuczwar S.J. Role of nuclear factor kappaB in the central nervous system. Pharmacol Rep. 2007;59:25–33. [PubMed] [Google Scholar]
- 7.Srinivasan M., Lahiri D.K. Significance of NF-kappaB as a pivotal therapeutic target in the neurodegenerative pathologies of Alzheimer's disease and multiple sclerosis. Expert Opin Ther Targets. 2015;19:471–487. doi: 10.1517/14728222.2014.989834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chami L., Buggia-Prevot V., Duplan E., Delprete D., Chami M., Peyron J. Nuclear factor-kappaB regulates betaAPP and beta- and gamma-secretases differently at physiological and supraphysiological Abeta concentrations. J Biol Chem. 2012;287:24573–24584. doi: 10.1074/jbc.M111.333054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Valerio A., Boroni F., Benarese M., Sarnico I., Ghisi V., Bresciani L.G. NF-kappaB pathway: a target for preventing beta-amyloid (Abeta)-induced neuronal damage and Abeta42 production. Eur J Neurosci. 2006;23:1711–1720. doi: 10.1111/j.1460-9568.2006.04722.x. [DOI] [PubMed] [Google Scholar]
- 10.Boissiere F., Hunot S., Faucheux B., Duyckaerts C., Hauw J.J., Agid Y. Nuclear translocation of NF-kappaB in cholinergic neurons of patients with Alzheimer's disease. Neuroreport. 1997;8:2849–2852. doi: 10.1097/00001756-199709080-00009. [DOI] [PubMed] [Google Scholar]
- 11.Coulson D.T., Beyer N., Quinn J.G., Brockbank S., Hellemans J., Irvine G.B. BACE1 mRNA expression in Alzheimer's disease postmortem brain tissue. J Alzheimers Dis. 2010;22:1111–1122. doi: 10.3233/JAD-2010-101254. [DOI] [PubMed] [Google Scholar]
- 12.Terai K., Matsuo A., McGeer P.L. Enhancement of immunoreactivity for NF-kappa B in the hippocampal formation and cerebral cortex of Alzheimer's disease. Brain Res. 1996;735:159–168. doi: 10.1016/0006-8993(96)00310-1. [DOI] [PubMed] [Google Scholar]
- 13.Chen C.H., Zhou W., Liu S., Deng Y., Cai F., Tone M. Increased NF-kappaB signalling up-regulates BACE1 expression and its therapeutic potential in Alzheimer's disease. Int J Neuropsychopharmacol. 2012;15:77–90. doi: 10.1017/S1461145711000149. [DOI] [PubMed] [Google Scholar]
- 14.Chen J., Zhou Y., Mueller-Steiner S., Chen L.F., Kwon H., Yi S. SIRT1 protects against microglia-dependent amyloid-beta toxicity through inhibiting NF-kappaB signaling. J Biol Chem. 2005;280:40364–40374. doi: 10.1074/jbc.M509329200. [DOI] [PubMed] [Google Scholar]
- 15.Rangasamy S.B., Corbett G.T., Roy A., Modi K.K., Bennett D.A., Mufson E.J. Intranasal delivery of NEMO-binding domain peptide prevents memory loss in a mouse model of Alzheimer's disease. J Alzheimers Dis. 2015;47:385–402. doi: 10.3233/JAD-150040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ayyar V.S., Almon R.R., Jusko W.J., DuBois D.C. Quantitative tissue-specific dynamics of in vivo GILZ mRNA expression and regulation by endogenous and exogenous glucocorticoids. Physiol Rep. 2015;3 doi: 10.14814/phy2.12382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cannarile L., Zollo O., D'Adamio F., Ayroldi E., Marchetti C., Tabilio A. Cloning, chromosomal assignment and tissue distribution of human GILZ, a glucocorticoid hormone-induced gene. Cell Death Differ. 2001;8:201–203. doi: 10.1038/sj.cdd.4400798. [DOI] [PubMed] [Google Scholar]
- 18.D'Adamio F., Zollo O., Moraca R., Ayroldi E., Bruscoli S., Bartoli A. A new dexamethasone-induced gene of the leucine zipper family protects T lymphocytes from TCR/CD3-activated cell death. Immunity. 1997;7:803–812. doi: 10.1016/s1074-7613(00)80398-2. [DOI] [PubMed] [Google Scholar]
- 19.Di Marco B., Massetti M., Bruscoli S., Macchiarulo A., Di Virgilio R., Velardi E. Glucocorticoid-induced leucine zipper (GILZ)/NF-kappaB interaction: role of GILZ homo-dimerization and C-terminal domain. Nucleic Acids Res. 2007;35:517–528. doi: 10.1093/nar/gkl1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ayroldi E., Riccardi C. Glucocorticoid-induced leucine zipper (GILZ): a new important mediator of glucocorticoid action. FASEB J. 2009;23:3649–3658. doi: 10.1096/fj.09-134684. [DOI] [PubMed] [Google Scholar]
- 21.Di L. Strategic approaches to optimizing peptide ADME properties. AAPS J. 2015;17:134–143. doi: 10.1208/s12248-014-9687-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Riccardi C. [GILZ (glucocorticoid-induced leucine zipper), a mediator of the anti-inflammatory and immunosuppressive activity of glucocorticoids] Ann Ig. 2010;22:53–59. [PubMed] [Google Scholar]
- 23.Srinivasan M., Janardhanam S. Novel p65 binding glucocorticoid-induced leucine zipper peptide suppresses experimental autoimmune encephalomyelitis. J Biol Chem. 2011;286:44799–44810. doi: 10.1074/jbc.M111.279257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.O'Shea J.M., Perkins N.D. Regulation of the RelA (p65) transactivation domain. Biochem Soc Trans. 2008;36:603–608. doi: 10.1042/BST0360603. [DOI] [PubMed] [Google Scholar]
- 25.Schmitz M.L., Stelzer G., Altmann H., Meisterernst M., Baeuerle P.A. Interaction of the COOH-terminal transactivation domain of p65 NF-kappa B with TATA-binding protein, transcription factor IIB, and coactivators. J Biol Chem. 1995;270:7219–7226. doi: 10.1074/jbc.270.13.7219. [DOI] [PubMed] [Google Scholar]
- 26.Cubellis M.V., Caillez F., Blundell T.L., Lovell S.C. Properties of polyproline II, a secondary structure element implicated in protein-protein interactions. Proteins. 2005;58:880–892. doi: 10.1002/prot.20327. [DOI] [PubMed] [Google Scholar]
- 27.Srinivasan M., Dunker A.K. Proline rich motifs as drug targets in immune mediated disorders. Int J Pept. 2012;2012:634769. doi: 10.1155/2012/634769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nazem A., Sankowski R., Bacher M., Al-Abed Y. Rodent models of neuroinflammation for Alzheimer's disease. J Neuroinflammation. 2015;12:74. doi: 10.1186/s12974-015-0291-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Puzzo D., Gulisano W., Palmeri A., Arancio O. Rodent models for Alzheimer's disease drug discovery. Expert Opin Drug Discov. 2015;10:703–711. doi: 10.1517/17460441.2015.1041913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hauss-Wegrzyniak B., Dobrzanski P., Stoehr J.D., Wenk G.L. Chronic neuroinflammation in rats reproduces components of the neurobiology of Alzheimer's disease. Brain Res. 1998;780:294–303. doi: 10.1016/s0006-8993(97)01215-8. [DOI] [PubMed] [Google Scholar]
- 31.Catorce M.N., Gevorkian G. LPS-induced murine neuroinflammation model: main features and suitability for pre-clinical assessment of nutraceuticals. Curr Neuropharmacol. 2016;14:155–164. doi: 10.2174/1570159X14666151204122017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.El-Sayed N.S., Bayan Y. Possible role of resveratrol targeting estradiol and neprilysin pathways in lipopolysaccharide model of Alzheimer disease. Adv Exp Med Biol. 2015;822:107–118. doi: 10.1007/978-3-319-08927-0_12. [DOI] [PubMed] [Google Scholar]
- 33.Lee Y.J., Choi D.Y., Yun Y.P., Han S.B., Oh K.W., Hong J.T. Epigallocatechin-3-gallate prevents systemic inflammation-induced memory deficiency and amyloidogenesis via its anti-neuroinflammatory properties. J Nutr Biochem. 2013;24:298–310. doi: 10.1016/j.jnutbio.2012.06.011. [DOI] [PubMed] [Google Scholar]
- 34.Song J.H., Lee J.W., Shim B., Lee C.Y., Choi S., Kang C. Glycyrrhizin alleviates neuroinflammation and memory deficit induced by systemic lipopolysaccharide treatment in mice. Molecules. 2013;18:15788–15803. doi: 10.3390/molecules181215788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Morris M.C., Deshayes S., Heitz F., Divita G. Cell-penetrating peptides: from molecular mechanisms to therapeutics. Biol Cell. 2008;100:201–217. doi: 10.1042/BC20070116. [DOI] [PubMed] [Google Scholar]
- 36.Ifuku M., Katafuchi T., Mawatari S., Noda M., Miake K., Sugiyama M. Anti-inflammatory/anti-amyloidogenic effects of plasmalogens in lipopolysaccharide-induced neuroinflammation in adult mice. J Neuroinflammation. 2012;9:197. doi: 10.1186/1742-2094-9-197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yamamoto M., Horiba M., Buescher J.L., Huang D., Gendelman H.E., Ransohoff R.M. Overexpression of monocyte chemotactic protein-1/CCL2 in beta-amyloid precursor protein transgenic mice show accelerated diffuse beta-amyloid deposition. Am J Pathol. 2005;166:1475–1485. doi: 10.1016/s0002-9440(10)62364-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Blair W.S., Bogerd H.P., Madore S.J., Cullen B.R. Mutational analysis of the transcription activation domain of RelA: identification of a highly synergistic minimal acidic activation module. Mol Cell Biol. 1994;14:7226–7234. doi: 10.1128/mcb.14.11.7226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schmitz M.L., dos Santos Silva M.A., Altmann H., Czisch M., Holak T.A., Baeuerle P.A. Structural and functional analysis of the NF-kappa B p65 C terminus. An acidic and modular transactivation domain with the potential to adopt an alpha-helical conformation. J Biol Chem. 1994;269:25613–25620. [PubMed] [Google Scholar]
- 40.Ball L.J., Kuhne R., Schneider-Mergener J., Oschkinat H. Recognition of proline-rich motifs by protein-protein-interaction domains. Angew Chem Int Ed Engl. 2005;44:2852–2869. doi: 10.1002/anie.200400618. [DOI] [PubMed] [Google Scholar]
- 41.Wu L.Y., Bao X.Q., Pang H.Y., Sun H., Zhang D. FLZ attenuates learning and memory deficits via suppressing neuroinflammation induced by LPS in mice. J Asian Nat Prod Res. 2015;17:306–317. doi: 10.1080/10286020.2014.1003183. [DOI] [PubMed] [Google Scholar]
- 42.Qin L., Wu X., Block M.L., Liu Y., Breese G.R., Hong J.S. Systemic LPS causes chronic neuroinflammation and progressive neurodegeneration. Glia. 2007;55:453–462. doi: 10.1002/glia.20467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Li X., Long J., He T., Belshaw R., Scott J. Integrated genomic approaches identify major pathways and upstream regulators in late onset Alzheimer's disease. Sci Rep. 2015;5:12393. doi: 10.1038/srep12393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lukiw W.J. NF-kappaB-regulated, proinflammatory miRNAs in Alzheimer's disease. Alzheimers Res Ther. 2012;4:47. doi: 10.1186/alzrt150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Du Y., Chen X., Wei X., Bales K.R., Berg D.T., Paul S.M. NF-(kappa)B mediates amyloid beta peptide-stimulated activity of the human apolipoprotein E gene promoter in human astroglial cells. Brain Res Mol Brain Res. 2005;136:177–188. doi: 10.1016/j.molbrainres.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 46.Srinivasan M. Interface peptide mimetics: rationale and applications as therapeutic agents. Med Chem (Los Angeles) 2016;6:189–194. [Google Scholar]
- 47.Doig A.J. Peptide inhibitors of beta-amyloid aggregation. Curr Opin Drug Discov Devel. 2007;10:533–539. [PubMed] [Google Scholar]
- 48.Adessi C., Soto C. Strategies to improve stability and bioavailability of peptide drugs. Front Med Chem. 2004;1:513–528. doi: 10.2174/0929867024606731. [DOI] [PubMed] [Google Scholar]
- 49.Zhao W.X., Zhang J.H., Cao J.B., Wang W., Wang D.X., Zhang X.Y. Acetaminophen attenuates lipopolysaccharide-induced cognitive impairment through antioxidant activity. J neuroinflammation. 2017;14:17. doi: 10.1186/s12974-016-0781-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pizzi M., Goffi F., Boroni F., Benarese M., Perkins S.E., Liou H.C. Opposing roles for NF-kappa B/Rel factors p65 and c-Rel in the modulation of neuron survival elicited by glutamate and interleukin-1beta. J Biol Chem. 2002;277:20717–20723. doi: 10.1074/jbc.M201014200. [DOI] [PubMed] [Google Scholar]
- 51.Pizzi M., Sarnico I., Boroni F., Benarese M., Steimberg N., Mazzoleni G. NF-kappaB factor c-Rel mediates neuroprotection elicited by mGlu5 receptor agonists against amyloid beta-peptide toxicity. Cell Death Differ. 2005;12:761–772. doi: 10.1038/sj.cdd.4401598. [DOI] [PubMed] [Google Scholar]
- 52.Qin Z.H., Tao L.Y., Chen X. Dual roles of NF-kappaB in cell survival and implications of NF-kappaB inhibitors in neuroprotective therapy. Acta Pharmacol Sin. 2007;28:1859–1872. doi: 10.1111/j.1745-7254.2007.00741.x. [DOI] [PubMed] [Google Scholar]
- 53.Sillard R., Schulz-Knappe P., Vogel P., Raida M., Bensc K.W., Forssmann W.G. A novel 77-residue peptide from porcine brain contains a leucine-zipper motif and is recognized by an antiserum to delta-sleep-inducing peptide. Eur J Biochem. 1993;216:429–436. doi: 10.1111/j.1432-1033.1993.tb18160.x. [DOI] [PubMed] [Google Scholar]
- 54.Bjartell A., Ekman R., Hedenbro J., Sjolund K., Sundler F. Delta sleep-inducing peptide (DSIP)-like immunoreactivity in gut: coexistence with known peptide hormones. Peptides. 1989;10:163–170. doi: 10.1016/0196-9781(89)90093-4. [DOI] [PubMed] [Google Scholar]
- 55.Monnier M., Dudler L., Gachter R., Maier P.F., Tobler H.J., Schoenenberger G.A. The delta sleep inducing peptide (DSIP). Comparative properties of the original and synthetic nonapeptide. Experientia. 1977;33:548–552. doi: 10.1007/BF01922266. [DOI] [PubMed] [Google Scholar]
- 56.Cummings J., Aisen P.S., DuBois B., Frölich L., Jack C.R., Jr, Jones R.W. Drug development in Alzheimer's disease: the path to 2025. Alzheimers Res Ther. 2016;8:39. doi: 10.1186/s13195-016-0207-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cummings J., Lee G., Mortsdorf T., Ritter A., Zhong K. Alzheimer's disease drug development pipeline. Alzheimers Dement (NY) 2017;2017:367–384. doi: 10.1016/j.trci.2017.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]