Summary
Information is encoded in neural networks through changes in synaptic weights. Synaptic learning rules involve a combination of rapid Hebbian plasticity and slower homeostatic synaptic plasticity that regulates neuronal activity through global synaptic scaling. Hebbian and homeostatic plasticity have been extensively investigated, whereas much less is known about their interaction. Here we investigated structural and functional consequences of homeostatic plasticity at dendritic spines of mouse hippocampal neurons. We found that prolonged activity blockade induced spine growth, paralleling synaptic strength increases. Following activity blockade, glutamate uncaging-mediated stimulation at single spines led to size-dependent structural potentiation: smaller spines underwent robust growth, whereas larger spines remained unchanged. Moreover, spines near the stimulated spine exhibited volume changes following homeostatic plasticity, indicating that there was a breakdown of input specificity following homeostatic plasticity. Overall, these findings demonstrate that Hebbian and homeostatic plasticity interact to shape neural connectivity through non-uniform structural plasticity at inputs.
Subject Areas: Optical Imaging, Neuroscience, Cellular Neuroscience
Graphical Abstract
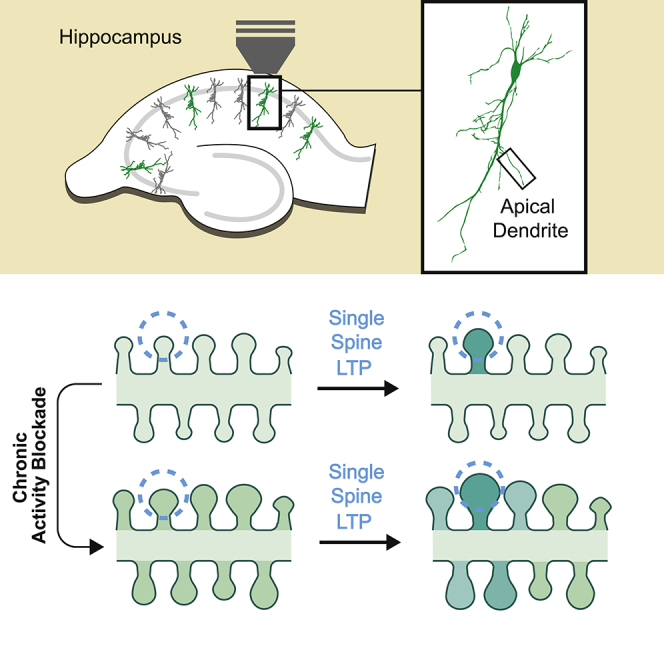
Highlights
-
•
Chronic activity blockade leads to enlarged hippocampal spines and structural scaling
-
•
Homeostatic plasticity affects subsequent Hebbian plasticity according to size of spines
-
•
Neighbors also grow after potentiation of single spines, compromising input specificity
Optical Imaging; Neuroscience; Cellular Neuroscience
Introduction
Hebbian synaptic plasticity, widely regarded as the leading biological mechanism for information storage, involves activity-dependent changes in synaptic connectivity (Malenka and Bear, 2004). Notably, these changes have a physical component and excitatory postsynaptic current is highly correlated with dendritic spine volume. However, left unchecked activity would lead to a positive feedback loop in which changes in synaptic weight are further reinforced by future events. This has been proposed to result in loss of functionality of the system, by either driving synapses toward saturation, or through silencing the population. How neurons maintain stability in the face of destabilizing cellular and circuit events has been a long-standing question. One method neurons employ to resolve this is homeostatic synaptic plasticity (HSP), a feedback mechanism through which a population of synapses can be maintained to function within optimal bounds. During HSP, a decrease in global activity drives a counteracting scalar increase in synaptic strengths to bring the network into an optimal functioning range, and conversely, network activity increases will promote synaptic weakening (Turrigiano, 2011). This scaling is instantiated in part by AMPA receptor trafficking to and from the postsynaptic density, as well as through presynaptic changes that modify neurotransmitter release, leading to concomitant changes in synaptic strength (Murthy et al., 2001, O'Brien et al., 1998). HSP has been observed both in vitro and in vivo (Desai et al., 2002, Turrigiano et al., 1998), supporting a fundamental role for this mechanism in the proper functioning of the neural system.
Increasing evidence points to structural correlates of homeostatic plasticity (Barnes et al., 2017, Keck et al., 2013, Kirov and Harris, 1999). Although it is well established that HSP modulates synaptic function, it is currently unknown how this process dynamically regulates spine structural changes and how such alterations affect the encoding of subsequent activity. Specifically, how HSP affects the induction and longevity of subsequent forms of Hebbian plasticity at individual inputs is yet to be determined. In this study, we examine how the induction of HSP affects the structure and function of hippocampal pyramidal neuron synapses and what are the consequences of these changes for future Hebbian plasticity. We find that the induction of HSP, due to chronic activity blockade, leads to an initial supralinear increase in the size of spines that over time becomes linear, thus producing structural scaling that matches the functional scaling of synapses. Through precise 2-photon-mediated glutamate uncaging, we further investigate how these homeostatic functional and structural plasticity changes affect the ability of individual inputs to undergo subsequent plasticity. We demonstrate that after HSP, spines express increased longevity of long-term potentiation (LTP), an increased growth rate after stimulation, and a reduced plasticity threshold. We find that HSP enhances the magnitude of synaptic potentiation through the preferential modulation of small spines and by promoting structural plasticity at clustered inputs following Hebbian activity at single synapses. Together, these changes provide a mechanism by which homeostatic plasticity can modulate synaptic efficacy and enhance future learning without compromising previously stored information.
Results
Structural Correlates of Homeostatic Plasticity in Hippocampal Neurons
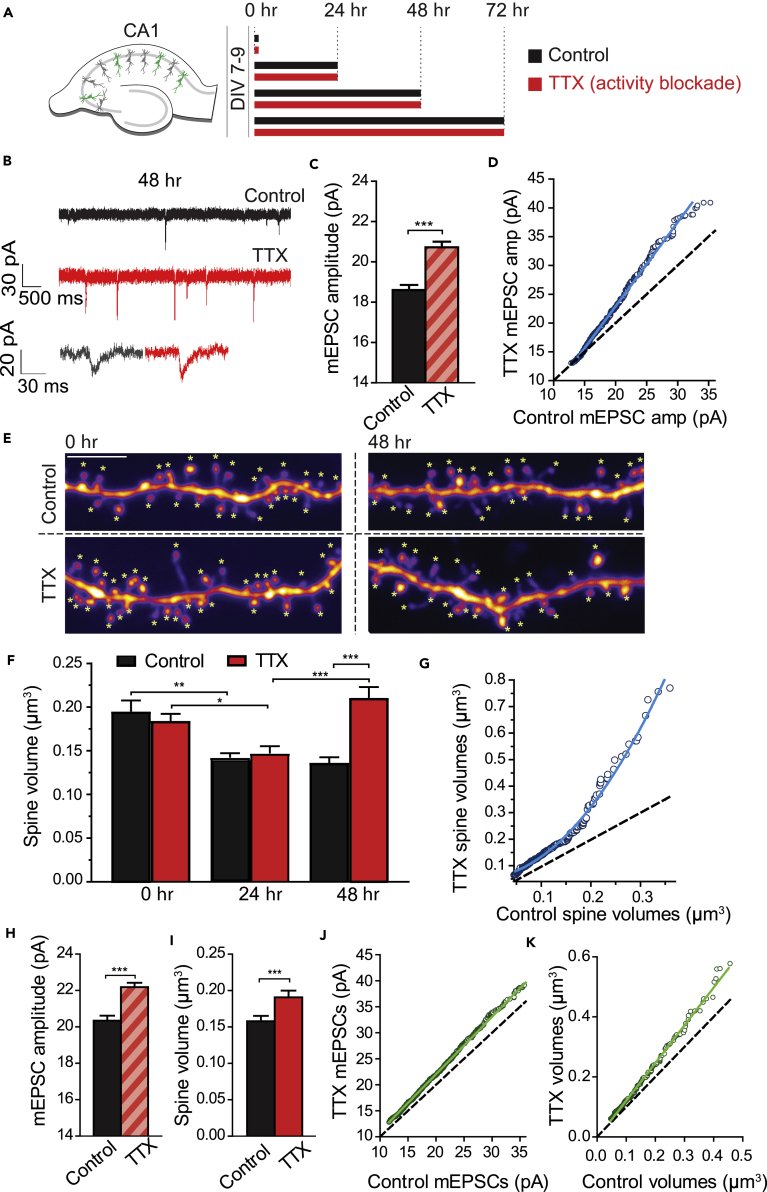
Hebbian plasticity at an individual input is linearly correlated with volume changes in the corresponding spine (Arellano et al., 2007, Govindarajan et al., 2011, Harvey and Svoboda, 2007, Matsuzaki et al., 2004), and thus changes in the volume of a spine can be used as a reliable proxy for functional changes at the corresponding synapse. Since HSP is known to change the functional properties of the spine, we reasoned that homeostatic modifications of efficacy should be accompanied by structural plasticity of inputs. Supporting this idea, a partial blockade of activity in the visual cortex leads to the growth of distal spines in vivo (Keck et al., 2013). Thus, applying a chronic activity blockade ex vivo in the hippocampus would allow us to model the structural consequences of homeostatic plasticity and to test how such changes affect further plasticity at single spines. We therefore induced HSP using tetrodotoxin (TTX) inhibition of sodium channels, for 0, 24, 48, or 72 hr (Figure 1A, see Transparent Methods), as this has been shown to induce scaling of synaptic strengths in a variety of systems (Karmarkar and Buonomano, 2006, Turrigiano et al., 1998). We chose to conduct our experiments in mouse hippocampal organotypic slice cultures, which maintain a physiologically relevant tissue architecture and in which the relationship between synaptic structure and function can be probed at the level of single spines. To verify that functional synaptic scaling occurred, we recorded spontaneous miniature excitatory postsynaptic currents (mEPSCs) from both TTX-treated and control CA1 hippocampal pyramidal neurons at 48 hr after the beginning of the HSP induction period (Figures 1B–1D). All recordings were performed in the presence of acute TTX to block action potentials. As expected, we found a significant increase in mEPSC amplitude following 48 hr of activity blockade (Figures 1B and 1C; control = 18.6 ± 0.25 pA, TTX = 20.72 ± 0.28 pA, p = 1.55 × 10−8, Mann-Whitney). The distribution of the TTX mEPSCs scaled linearly to overlay with the control distribution (Figure 1D), in accordance with the synaptic scaling theory. We next investigated whether homeostatic plasticity leads to structural modification of synapses by live, 2-photon imaging of GFP-labeled CA1 dendrites (Figure 1E). The volumes of all visible spines within the image were measured using SpineS, a custom in-house-developed MATLAB toolbox (Erdil et al., 2012, Ghani et al., 2017). We found that spines chronically treated with TTX were significantly bigger than those from control cells beginning at 48 hr (Figures 1E and 1F; control = 0.136 ± 0.0062 μm³, TTX = 0.211 ± 0.0123 μm³, p = 1.9e-10). As both control and TTX-treated spines initially reduced in volume (Figure 1F, 24 hr), it is unlikely that the spine enlargement we observe reflects an activity-induced blockade of spine shrinkage by TTX (Figure 1F; mean ± SEM: control0h = 0.195 ± 0.0125, control24h = 0.142 ± 0.005, p = 0.003 [two-way ANOVA]; TTX0h = 0.184 ± 0.008, TTX24h = 0.147 ± 0.008, p = 0.03 [two-way ANOVA]). However, by 48 hr TTX spines were significantly bigger than the 24-hr TTX spines, and than the 48-hr control spines (Figures 1E and 1F; control = 0.136 ± 0.0062 μm³, TTX = 0.211 ± 0.0123 μm³, p = 1.9e-10; Figure S2A). Surprisingly, as opposed to the linear functional scaling of mEPSCs seen in response to HSP, we found that structural scaling of spine volumes at 48 hr are best fit to controls with a second-order equation (Figure 1G). This superlinear scaling resulted from a preponderance of large spines after TTX treatment, for which correspondingly large mEPSCs were not observed.
Figure 1.
Spines Undergo Structural Scaling following Activity Blockade
(A) Experimental protocol to induce HSP in hippocampal organotypic slices.
(B) Example mEPSC recording traces from control and TTX-treated conditions at the 48 hr time point. Bottom: representative individual mEPSCs.
(C) Quantification of mEPSC amplitudes after 48 hr of activity blockade, represented as mean ± SEM. ***p = 1.553 × 10−8, Mann-Whitney. For all data presented, total number of spines or mEPSCs are represented by the first number (n) and followed by the number of independent neurons (N, in parentheses) from which they were collected. Number of mEPSCs (n) from independent neurons (N): control = 552 (8), TTX = 742 (8).
(D) Ranked control mEPSC amplitudes plotted against TTX condition at 48 hr. Best fit line to the data: TTX = control × 1.45–6.06.
(E) Representative images of dendrites at either 0 or 48 hr after TTX treatment or in control media. Asterisks indicate all the spines that were analyzed. Scale bar, 5 μm.
(F) Quantification of spine volumes for the indicated conditions, represented as mean ± SEM. **p0 control –24 control = 0.003; *p0 TTX–24 TTX = 0.03; ***pTTX 24–TTX 48 = <0.0001; ***p48 control–48 TTX = 1.9e-10. All statistics: two-way ANOVA, post-hoc Bonferroni. Number of spines (n) from independent neurons (N) for the 0, 24, and 48 hr time points: control = 103(6), 245(8), 285 (7). TTX = 172 (7), 232 (5), 208 (6).
(G) Ranked control spine volumes plotted against TTX condition at 48 hr. Best fit line to the data: TTX = control2 × 5.44 + control × 0.25 + 0.55.
(H) Quantification of mEPSC amplitudes after 72 hr in TTX, represented as mean ± SEM. ***p = 1.883 × 10−15, Mann-Whitney. Number of mEPSCs (n) from independent neurons (N): control = 1,164 (10), TTX = 1,302 (12).
(I) Quantification of spine volume after 72 hr in TTX, represented as mean ± SEM. ***p = 0.0008, Mann-Whitney. Number of spines (n) from independent neurons (N): control = 258 (6), TTX = 214 (6).
(J) Ranked control 72-hr mEPSC amplitudes plotted against TTX mEPSC amplitudes. Best fit line to the data: TTX = control × 1.10–0.01.
(K) Ranked control 72-hr spine volumes plotted against TTX 72-hr spine volumes. Best fit line to the data: TTX = control × 1.26–0.004.
See also Figures S1 and S2.
After 72 hr of activity blockade, synapses maintained the enhancement of mEPSC size (Figure 1H; mean ± SEM: control 72 hr = 20.35 ± 0.27 pA, TTX 72 hr = 22.18 ± 0.239 pA, p = 1.88 × 10−15, Mann-Whitney) and spine volume increases (Figure 1I; mean ± SEM: control = 0.16 ± 0.0073 μm³, TTX = 0.19 ± 0.0093 μm³, p = 0.0008 Mann-Whitney). However, at this time, scaling was instantiated in a linear fashion at both the level of mEPSC amplitude and spine volumes (Figures 1J and 1K). This suggests that the expression of functional and structural scaling evolves dynamically over time in response to prolonged activity blockade. We wanted to determine whether the prolonged activity blockade affected the total number of spines, and therefore we calculated spine density over days (Figure S1A). Spine density did not differ significantly over time between the control and TTX populations (p = 0.568, two-way ANOVA), indicating that activity blockade did not lead to an alteration in the total number of spines.
Reversibility of Homeostatic Plasticity-Mediated Structural Changes
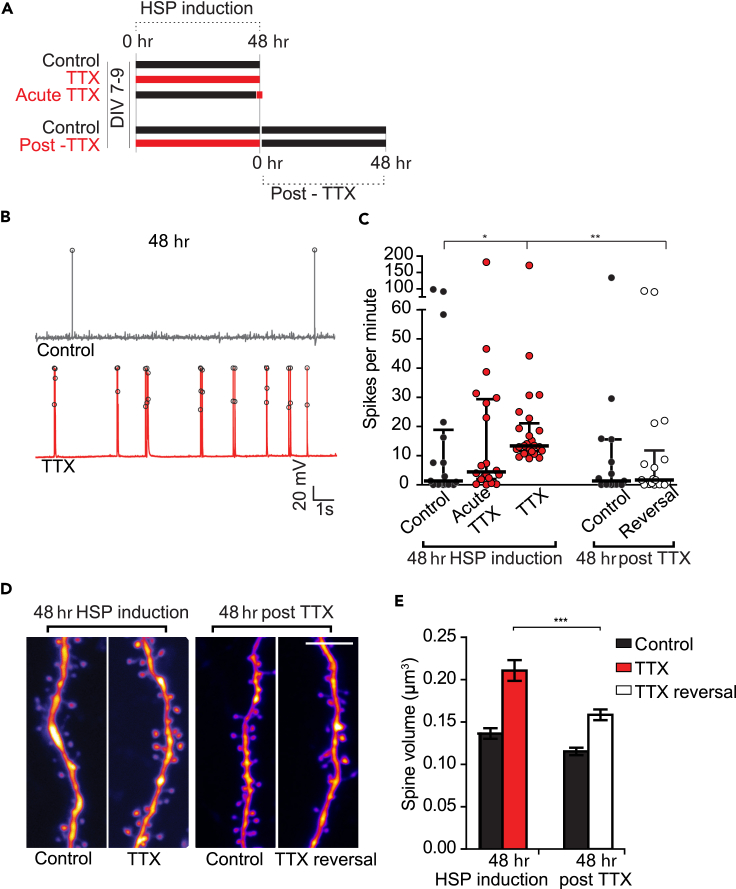
Synaptic strength modifications that occur in response to activity blockade are reversible upon re-exposure of a circuit to activity (Desai et al., 2002, Wallace and Bear, 2004). We tested whether the structural changes that resulted following activity blockade were also reversible when activity is restored. After 48 hr of homeostatic plasticity induction, by which time significant structural and functional scaling have occurred (Figure 1), we removed TTX allowing slices to resume activity and measured spontaneous firing using whole-cell patch-clamp recordings (Figures 2A and 2B). Activity blockade for 48 hr led to higher firing rates compared with untreated cells (Figure 2C; control [median] = 1.33, IQ range = 0.11:18.83 spikes/min; TTX [median] = 13.33, IQ range = 11.5:21.06 spikes/min; p = 0.03, Kruskal-Wallis post-hoc Dunn). To exclude the possibility that the higher firing rates we observed were due to a rebound after the acute withdrawal of TTX from the system, a set of control neurons were briefly incubated in TTX (2–4 hr, named “acute TTX condition”). This manipulation did not significantly alter firing rates, which were similar to untreated controls (Figure 2C; acute TTX: median = 4.44, IQ range = 2.0:29.33 spikes/min versus control no TTX: median = 1.33, IQ range = 0.11:18.83 spikes/min, p > 0.9 Kruskal-Wallis post-hoc Dunn). Thus the firing rate changes we observed were the result of HSP and did not arise from the acute withdrawal of TTX. We then investigated whether the circuit would return to its original level of activity once neurons were released from blockade. Indeed, after having removed TTX for 48 hr, firing rates were indistinguishable from controls (Figure 2C; control [median] = 1.33, IQ range = 0.11:15.56. TTX reversal [median] = 1.67, IQ range = 0.194:11.76. p > 0.05, Kruskal-Wallace post-hoc Dunn). We then examined whether structural modifications accompanied these firing rate changes, and observed a similar reversal in spine volumes (Figures 2D and 2E). TTX-treated spines significantly reduced in size 48 hr after TTX removal (Figure 2E; mean volume ± SEM: TTX 48 hr = 0.21 ± 0.0063 μm³; TTX 48 hr reversal = 0.16 ± 0.0064 μm³; p = 4.1852 × 10−7). These findings support the idea that functional plasticity is matched by structural plasticity and that spine sizes reversibly and accurately reflect the activity landscape.
Figure 2.
Structural and Functional Changes after HSP Are Reversible
(A) Experimental timeline for reversal experiments.
(B) Example traces showing cell firing following 48 hr of activity block. Spikes are demarcated with black circles.
(C) Quantification of spikes per minute throughout experiment, represented as median + IQ range. *p48 control–48 TTX = 0.03, Kruskal-Wallis post-hoc Dunn; **p48 TTX–48 post TTX = 0.010, Kruskal-Wallis post-hoc Dunn. Number of neurons: 48 hr TTX condition: 48 hr control = 17, 48 hr acute TTX = 20, 48 hr TTX = 26; 48 hr “post-TTX” control = 15, 48 hr post-TTX reversal = 18.
(D) Representative images from dendrites after 48 hr of HSP induction and at 48 hr post TTX. Scale bar, 5 μm.
(E) Quantification of spine sizes throughout the experiment, represented as mean ± SEM. All p values calculated using Kruskal-Wallis test, post-hoc Dunn's. ***p48 TTX–48 post TTX = 4.1852 × 10−7. Number of spines (n = first number) of neurons (N = in parentheses): 48 hr control = 285 (7), 48 hr TTX = 208 (6), 48 hr “post-TTX” control = 514 (14), 48 hr post-TTX = 430 (15).
Enhanced Hebbian Potentiation of Small Spines after Homeostatic Plasticity
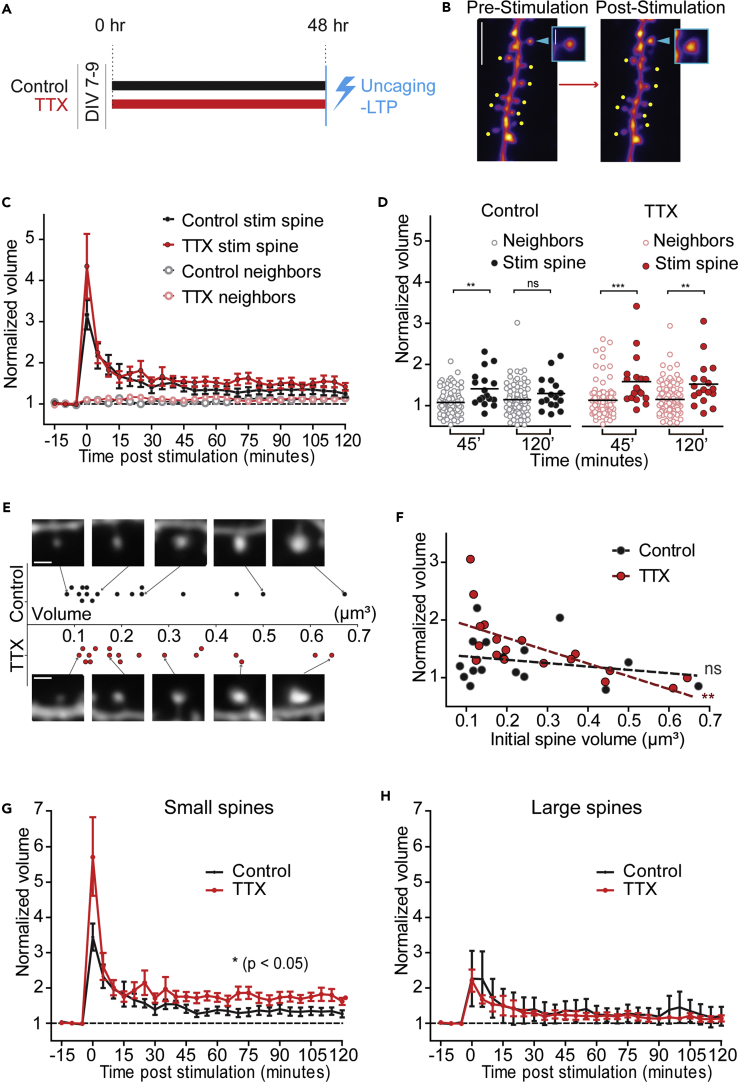
Functional plasticity can be enhanced upon homeostatic modulation in hippocampal slices (Arendt et al., 2013, Félix-Oliveira et al., 2014), but occlusion of LTP may also occur (Soares et al., 2017). Given our finding that robust growth of spines occurs during homeostatic plasticity, we wanted to determine whether individual inputs were able to undergo further activity-dependent structural plasticity. We therefore induced HSP in hippocampal slices for 48 hr and followed this with synaptic potentiation at visually identified dendritic spines through 2-photon-mediated glutamate uncaging (Figures 3A and 3B; see Transparent Methods). This paradigm elicits potentiation and growth of only the stimulated input, but not the nearby neighboring spines (Govindarajan et al., 2011, Harvey and Svoboda, 2007). We followed the growth of spines relative to their average baseline volumes, in both the TTX and control conditions (Figures 3C and 3D), and compared with the unstimulated neighboring spines. During the initial 45 min post-stimulation, both TTX-treated and control spines grew to a similar extent, indicating that homeostatic structural scaling does not occlude activity-dependent structural plasticity (mean ± SEM; control stimulated 45′ = 1.45 ± 0.10; control neighbors 45′ = 1.08 ± 0.04; TTX stimulated 45′ = 1.59 ± 0.14; TTX neighbors 45′ = 1.14 ± 0.04; p > 0.99) (Figure 3D). However, after 120 min, only the stimulated inputs in the TTX-treated neurons remain significantly larger than their neighbors (Figure 3D; mean ± SEM: TTX stimulated 120′ = 1.53 ± 0.13; TTX neighbors 120′ = 1.16 ± 0.04), as control spines begin to return to their initial volume (Figure 3D; control stimulated 120′ = 1.30 ± 0.01; control neighbors 120′ = 1.14 ± 0.04). Although the TTX and control spines are not significantly different at 120′ (p = 0.1345, Mann-Whitney), they begin to diverge, as seen relative to their respective neighbors. Thus, at scaled synapses, activity that would otherwise lead to short-lived structural plasticity now elicits long-lasting structural plasticity.
Figure 3.
Potentiation of Single Inputs Is Enhanced at Smaller Spines following HSP
(A) Timeline for uncaging experiments.
(B) Representative images of structural plasticity following single spine glutamate uncaging. Arrowheads and dots indicate stimulated and neighboring non-stimulated spines, respectively. Scale bar, 5 μm (main image), 1 μm (inset).
(C) Normalized volume of stimulated and neighboring spines for control and TTX-treated conditions over 2 hr of LTP induction, represented as mean + SEM. Volumes were calculated from Z-stacks taken in 5-min bins from time point 0 (thus the stack represented as 0 was collected between 0 and 5 min, for example). For all data, the total number of spines are represented by the first number (n), and followed by the number of independent neurons (N = in parentheses) from which they were collected. Control stimulated = 17 (17), TTX stimulated = 18 (18), control neighbors = 85 (17), TTX neighbors = 102 (18).
(D) All analyzed stimulated and neighboring spine volumes at time points 45 min and 120 min post-stimulation, with the mean marked. Significance was calculated using one-way ANOVA post-hoc Bonferroni. Control condition: ∗∗pstimulated45–neighbors45 = 8.4246 × 10−4; pstimulated120–neighbors120 = 0.6881. TTX condition: ∗∗∗pstimulated45–neighbors45 = 2.9383 × 10−4. ∗∗pstimulated120 – neighbors120 = 0.0046 [n same as in (C)].
(E) Distribution of all initial volumes of stimulated spines, with example spine images. The cutoff for defining “large” spines was the median volume off all spines multiplied by 1.5. Scale bar, 1 μm.
(F) Initial absolute spine size versus normalized spine volume at 120 min. TTX spines linear regression: r2 = 0.48, ∗∗p = 0.002. Control linear regression: r2 = 0.06, p = 0.346.
(G) Time course of the structural LTP experiment plotting only the small spines, represented as mean +SEM. ∗p = 0.0458, two-way repeated measures ANOVA. Number of spines (n) from neurons (N, in parentheses): control = 13 (13), TTX = 11 (11).
(H) Time course of structural LTP plotting only large spines, represented as mean +SEM. p = 0.699, two-way repeated measures ANOVA. Number of spines (n) from neurons (N, in parentheses): control = 4 (4), TTX = 17 (17).
See also Figure S2.
Structural plasticity at individual inputs varies with activity and spine size. Weak stimulations preferentially affect small spines (Matsuzaki et al., 2004), whereas strong stimuli can elicit structural plasticity across a variety of spine sizes (Govindarajan et al., 2011, Oh et al., 2013, Ramiro-Cortés and Israely, 2013). Our stimulated spines spanned a range of sizes, allowing us to test whether size interacted with prior expression of HSP when expressing synaptic potentiation and further spine growth (Figure 3E). We examined the amount of structural plasticity expressed by spines of different initial sizes after activity blockade. We found a negative correlation between a spine's initial average value and its normalized final volume when the spine population had first undergone homeostatic plasticity, but not in the control population, indicating that spine volume is an important modulator of the potential for plasticity after HSP (Figures 3E and 3F; r2 = 0.48 for TTX, p < 0.01, r2 = 0.06 for control, p > 0.3). To further examine how HSP modulates the size dependence of structural plasticity, we classified spines as “large” if their initial volume was more than 150% of the median initial volume of all spines, and the remainder were placed in the “small” category. This latter group did not fall below the 25th percentile of the entire distribution, as can be seen in the plot of all the experimental spines' initial volumes relative to the population from which they were drawn (Figures S2A and S2B). Among the small spines, we observed a significant increase in the magnitude of LTP in the TTX condition compared with the controls (Figure 3G; p = 0.046, 2-way repeated measures ANOVA). On the other hand, large spines expressed a short-lasting growth that quickly decayed to baseline and was not dependent on whether they had been subjected to activity blockade (Figure 3H; p = 0.70, 2-way repeated measures ANOVA). Therefore, small spines preferentially underwent long-lasting structural plasticity after HSP. We further observed that small spines, which had undergone HSP, tended to show a greater degree of initial growth in response to the induction of plasticity (Figure 3G, time point 0). To quantify the dynamic growth of these spines, we analyzed high-speed images of the spine head taken throughout the stimulation period (approximately 20 Hz) and did not observe a significant difference in the growth curves between the control and TTX-treated spines (Figures 4A and 4B). When we examined the rate of growth that each spine expressed in the first minute after the stimulation, however, we found that TTX-treated small spines grew more than control small spines (Figure 4C; p = 0.018, one-way ANOVA with post-hoc Tukey's), whereas large spines showed no difference between the two conditions. Therefore, glutamate stimulation leads to a sustained growth of homeostatically modified small spines, which may reflect prolonged signaling at these synapses. Together, these data indicate that HSP facilitates Hebbian plasticity and that this is accomplished at the individual spine level through preferential structural plasticity of small inputs.
Figure 4.
HSP Increases the Efficacy and Reduces the Threshold for Induction of LTP at Individual Spines
(A) Representative image of a single spine during the course of uncaging. Scale bar, 5 μm (main image), 1 μm (inset).
(B) Individual traces of spine growth during glutamate uncaging stimulation. Control spine growth versus TTX spine growth: p = 0.198, two-way repeated measures ANOVA. Number of spines (n): control = 16, TTX = 18.
(C) Growth rates of stimulated spines for 1 min after the end of stimulation, represented as mean +SEM. *psmall control–small TTX = 0.018, one-way ANOVA with post-hoc Tukey's. plarge control–large TTX < 0.99, one-way ANOVA with post-hoc Tukey's. Number of spines (n): control small = 13, TTX small = 11, control large = 4, TTX large = 7.
(D) Quantification of normalized spine volume with different stimulation pulse durations in the control condition, represented as mean +SEM. p1ms stimulated–1ms neighbors = 0.292, two-way repeated measures ANOVA post-hoc Bonferroni. Number of spines (n) from neurons from which they were collected (N, in parentheses): stimulated 1 ms = 11 (11), neighbors 1 ms = 52 (11), stimulated 4 ms = 17 (17). *p1msstimulated–4ms stimulated = 0.039; two-way rm ANOVA. Note 4 ms data are the same as shown in Figure 3.
(E) Quantification of normalized spine volume with different stimulation pulse durations in the TTX condition, represented as mean + SEM. **p1ms stimulated–1ms neighbors = 0.007, two-way rm ANOVA. p1ms stimulated– 4ms stimulated = 0.27, two-way rm ANOVA. Number of spines (n) from neurons (N): stimulated 1 ms = 11 (11), neighbors 1 ms = 52 (11), stimulated 4 ms = 18 (18). Note 4 ms data are the same as in Figure 3.
Homeostatic Plasticity Facilitates the Induction of Hebbian Structural Plasticity
Having observed that small spines showed enhanced responses to stimulation after homeostatic plasticity, as reflected by a higher rate of growth and longer lasting structural plasticity (Figures 3G and 4C), we reasoned that these inputs may respond more robustly to a weaker stimulation. This is also supported by previous findings that loss of activity at individual inputs lowers their threshold for subsequent plasticity (Lee et al., 2010). To test this possibility, we applied a subthreshold stimulation that utilizes a shorter laser pulse of 1 ms (instead of 4 ms) during the glutamate uncaging protocol, since this does not induce structural plasticity at spines (Govindarajan et al., 2011, Harvey and Svoboda, 2007). Indeed, control spines that underwent this subthreshold stimulation showed only a transient post-stimulus growth that lasted 5 min, resulting in no long-term plasticity (Figure 4D; control spines, 1 ms stimulated versus neighbors: p > 0.05, 2-way repeated measures ANOVA, post-hoc Bonferroni). These results were significantly different from what we had observed in response to the 4-ms stimulation (Figure 4D; 1 ms stimulated versus 4 ms stimulated; p = 0.04, 2-way repeated measures ANOVA; compared with data from Figure 3C). In contrast to this, HSP-modified spines grew significantly in response to the subthreshold stimulation and remained larger than their neighbors (Figure 4E; TTX spines, 1 ms stimulated versus neighbors, p = 0.01, 2-way repeated measures ANOVA, post-hoc Bonferroni). Following TTX treatment, both weak and strong stimulations elicited similar levels of long-lasting growth of spines (Figure 4E; 1 ms stimulated versus 4 ms stimulated: p = 0.27, 2-way repeated measures ANOVA). Thus, homeostatic plasticity facilitates structural plasticity by lowering the threshold for stimulation, without affecting the magnitude of plasticity. In this way, HSP acts locally, in conjunction with activity, to increase the likelihood that an individual synapse will participate in the encoding of information.
Homeostatic Plasticity Influences Structural Plasticity of Neighboring Spines
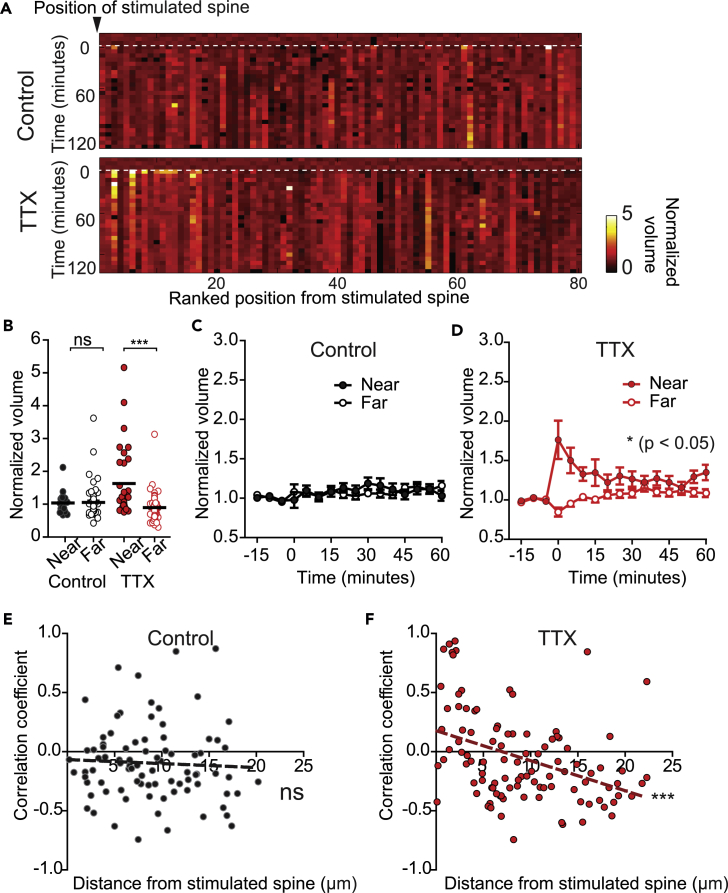
In addition to modifying synaptic inputs through scaling, homeostatic plasticity can also influence cellular firing rates by modulating the intrinsic excitability of neuronal membranes (Desai et al., 1999). We reasoned that such alterations could facilitate the expression of structural plasticity not only at stimulated spines but also at nearby inputs within the dendritic branch. To test this idea, we examined whether homeostatic plasticity altered neighboring spine volume dynamics following the potentiation of single inputs. We ranked neighboring spines according to their distance from the stimulated spine (with 1 representing the closest neighbor to a stimulated spine) and plotted their growth dynamics for 2 hr following the stimulation (Figure 5A). We found that spines located in close proximity to the stimulated spine increased in volume only in neurons that had first undergone homeostatic plasticity (compare the first 20 spines, from 0 to 60 min after stimulation, Figure 5A). We classified the unstimulated neighbors as “near” or “far”—located either within or beyond 5 μm of the stimulated spine, respectively—and quantified spine volume changes over time (Figures 5B–5D). As expected, none of the neighbors of stimulated spines changed significantly from their original size in untreated neurons (Figures 5B and 5C). However, after HSP, neighbors that were within 5 μm of the target spine exhibited significant growth in the 5 min following the stimulation (Figure 5B) and remained significantly larger than more distant spines (Figures 5B and 5D; p = 0.001, two-way repeated measures ANOVA). Interestingly, farther neighbors (located up to 20 μm away from the site of stimulation) tended to decrease in size for several minutes following activity, although this was not significantly different from baseline (Figure 5D). Given that neighboring spines grew, whereas distal spines tended to shrink, we did not find a significant difference in the mean spine volumes at the level of the dendritic branch between control and TTX-treated neighbors (Figure S3; p = 0.573, two-way ANOVA). We next investigated whether there was a correlation between the structural dynamics of each stimulated spine and its neighbors. We calculated correlation coefficients between the volume change of the stimulated spine and those of its neighbors across the time course of the experiment. Fluctuations in the volumes of neighboring spines in control conditions were independent of the stimulated input (Figure 5E; r2 = 0.00263, p = 0.61), whereas spine volumes in TTX-treated neurons changed in accordance with the stimulated spine (Figure 5F; r2 = 0.156, p < 0.001). Specifically, near-TTX neighbors had a positive correlation with the stimulated input, reflecting growth, which eventually became a negative correlation at further distances from the stimulated spine. These findings indicate that HSP reduces the input specificity of activity in a distance-dependent manner that is simultaneously cooperative with near and competitive with far inputs. This allows for homeostatic modulation to effect structural plasticity within a dendritic region through activity at a single spine, which favors clustered plasticity of synaptic inputs.
Figure 5.
Stimulation of Single Inputs Leads to Plasticity of Clustered Neighbors after HSP
(A) Heat maps of growth dynamics of neighboring spines over time. Neighbors from all experiments were pooled and ranked in order of distance from the stimulated spine. Index 1 is the spine nearest to its stimulated spine, and 80 is the furthest away. Heatmap = normalized spine volume. Number of spines (n) from neurons from which they were collected (N, in parentheses): control = 80 (17), TTX = 80 (18).
(B) Normalized spine volumes for the first 10 min following stimulation for the different neighboring conditions, with the mean marked. ***pTTX near–TTX far = 3.66 x 10-6; pControl near–Control far>0.99, Kruskal-Wallis. Number of spines (n) from neurons from which they were collected (N, in parentheses): control near = 22 (17), control far = 63 (17), TTX near = 32 (18), TTX far = 70 (18).
(C) Normalized volumes of near and far neighboring spines in the control condition, represented as mean + SEM. p = 0.987, two-way rm ANOVA. Number of spines same as above.
(D) Normalized volumes of neighboring spines in the TTX condition, represented as mean +SEM. *p = 0.0124, two-way repeated measures ANOVA. Number of spines same as above.
(E and F) Correlation coefficients of volume change of stimulated spines versus volume change of neighboring spine, plotted against the distance between the neighboring spine and the stimulated spine. (E) Linear regression control: r2 = 0.003, p = 0.641. (F) Linear regression TTX: r2 = 0.156, ***p = 3.91 × 10−5.
See also Figure S3.
Discussion
Synaptic networks must balance the need to enhance efficacy during learning without saturating their capacity for further changes. Homeostatic plasticity can achieve this regulatory role by effecting a gain modulation on neurons to maintain synaptic activity within an optimal target range. The consequences of implementing HSP for the network, and specifically, the effect of its interaction with Hebbian plasticity on the subsequent encoding of information at the level of single inputs, is unclear. Due to the linear relationship between synaptic structure and function, we used live 2-photon imaging and glutamate uncaging to probe the physical consequences of inducing homeostatic plasticity on a neuron, and to determine how this affects the ability of single synapses to undergo further changes in efficacy. We show that HSP, induced by 48 hr of activity blockade, leads to the reversible growth of dendritic spines. We demonstrate that this form of modulation facilitates subsequent structural plasticity at single synapses and that this effect preferentially occurs at smaller inputs. After HSP, activity elicits a faster growth rate at these spines and converts an otherwise subthreshold stimulation into one that is now capable of eliciting structural plasticity. Interestingly, we find that the induction of Hebbian plasticity on a background of homeostatic plasticity leads to compromised input specificity, as neighboring spines grow when they are located in close proximity to a stimulated synapse. Taken together, our results show that homeostatic plasticity can modulate a neuron's response to activity by facilitating the sensitivity of smaller inputs and inducing structural plasticity at neighboring synapses.
The structural scaling that we observe after 48 hr of activity blockade results in a non-linear upscaling of spines and an increased number of large spines (Figure 1G). By 72 hr, this structural scaling becomes linear (Figure 1K), suggesting that the initial changes may represent a physical overshooting of the target size. This has also been observed on short timescales following glutamate stimulation of single spines, where an initial large volume change is followed by stabilization of the spine at a more modest size (Matsuzaki et al., 2004). Of interest, an overshoot of cellular responses has been described during recovery from visual deprivation, suggesting that exaggerated responses of both structural and functional modifications may be a feature of the convergence of Hebbian and homeostatic plasticity (Toyoizumi et al., 2014). Upon the reinstatement of activity, we find that spines return to their original size as firing patterns normalize by 48 hr (Figures 2C–2E), indicating that homeostatic structural changes are plastic and reversible, similarly to homeostatic responses to alterations in activity levels.
We demonstrate that activity blockade increases spine sizes across the population, raising the question of whether further enhancement of potentiation and spine growth could be achieved across inputs of different sizes. Both enhancement and occlusion of plasticity have been reported following HSP at the population level (Arendt et al., 2013, Félix-Oliveira et al., 2014, Soares et al., 2017), but it is unclear how individual inputs are modulated. Furthermore, although larger synapses have been shown to undergo functional homeostatic modulation (Thiagarajan et al., 2005), inputs show a size-dependent variation in their ability to undergo Hebbian structural plasticity, large spines being more stable and requiring stronger stimulation to be potentiated (Govindarajan et al., 2011, Matsuzaki et al., 2004). We probed plasticity with glutamate uncaging and found that HSP leads to structural plasticity lasting over 2 hr preferentially at small spines, whereas control spines decay to baseline after 1 hr (Figures 3G and 3H). This indicates that after HSP a long-lasting, protein-synthesis-dependent form of Hebbian plasticity was induced at these inputs, whereas a shorter, protein-synthesis-independent form was induced in controls, based on the differential longevities of structural plasticity (Govindarajan et al., 2011). Several lines of evidence point to the involvement of new protein synthesis during homeostatic plasticity. The specific production of plasticity-related proteins has recently been shown to occur following activity blockade with TTX (Schanzenbacher et al., 2018), and a mechanistically distinct form of HSP acting through retinoic acid receptors is also known to induce new protein synthesis (Aoto et al., 2008, Sutton et al., 2006). Furthermore, activation of group 1 metabotropic glutamate receptors by Homer1A leads to ERK- and mammalian target of rapamycin-dependent protein translation during homeostatic scaling (Hu et al., 2010). These studies suggest that protein synthesis is likely to be a crucial component of homeostatic responses, similar to its role in the maintenance of long-term plasticity changes (Costa-Mattioli et al., 2009).
Closer examination of the structural plasticity we induced revealed that the population of small spines express the majority of the growth, whereas large spines remain stable (Figures 3G and 3H). This result implies that while homeostatic modifications may increase the absolute number of large spines, plasticity at these inputs is saturated to an equal extent irrespective of whether they had undergone activity blockade, and their threshold for structural plasticity is not further altered by HSP. Upon stimulation, we did not observe a significant difference in the magnitude of the potentiation that spines expressed, nor in their immediate response to the stimulation itself, but rather we found that spines post-HSP express a significantly faster growth rate compared with their counterparts in the first minute after stimulation (Figures 4A–4C). This enhanced response may be due to amplified signaling cascades shared between these forms of plasticity (Fernandes and Carvalho, 2016), which could facilitate the induction of long-lasting structural plasticity. It was therefore not surprising to find that a subthreshold stimulation elicits long-lasting growth only at inputs that have undergone homeostatic plasticity (Figures 4D and 4E), without affecting the initial magnitude of the response between the HSP-modified synapses and the control populations. Changes to N-methyl-D-aspartate receptor composition that occur following activity blockade could provide a means by which to enhance the calcium permeability of synapses and thus would allow for prolonged signaling responses (Barria and Malinow, 2005, Lee et al., 2010, Paoletti et al., 2013). Taken together, these data show that the effects of homeostatic plasticity preferentially affect small spines during Hebbian regulation of synaptic strength by predisposing them to undergo long-lasting structural changes. Such modulation may result in the conversion of weaker stimuli into more salient forms and proposes a more global role for HSP in information encoding beyond the optimization of neuronal activity.
A hallmark of activity-dependent changes during Hebbian plasticity is input specificity (Barrionuevo and Brown, 1983). In contrast, homeostatic plasticity is generally expressed over a wider synaptic range, although local homeostatic changes can also occur at the dendritic level (Branco et al., 2008, Ju et al., 2004, Liu, 2004, Sutton et al., 2006). How such modulation affects Hebbian learning rules at individual inputs is unknown (Vitureira and Goda, 2013), but computational studies have postulated that homeostatic plasticity can influence previous Hebbian events at a synapse (Rabinowitch and Segev, 2008). We therefore considered the analogous possibility that HSP may function to influence subsequent Hebbian plasticity, beyond the stimulated synapse. Indeed, we find that HSP induces the spillover of Hebbian structural plasticity within the dendrite, as seen by the growth of neighboring unstimulated spines following the activation of one input (Figure 5). Thus, homeostatic plasticity drives the local expression of Hebbian plasticity and reduces input specificity. Mechanistically, the high degree of overlap between pathways leading to the expression of Hebbian and homeostatic plasticity may facilitate such interactions. Most prominently among these is the modulation of synaptic strength through changes in AMPA receptor content in the postsynaptic density, which occurs during both forms of plasticity (Huganir and Nicoll, 2013, O'Brien et al., 1998). Furthermore, a variety of proteins that regulate AMPA receptor localization and retention in the post-synaptic density (PSD) have been shown to play a role in both Hebbian and homeostatic plasticity, such as the activity-dependent protein Arc/Arg2.1, PSD-95, and other PDZ domain-containing proteins such as PICK1 and GRIP1 (for review, see: Fernandes and Carvalho, 2016, Yee et al., 2017). These may serve to regulate either the number or the subtype of AMPA receptors present at the synapse in an activity-dependent manner, and if acting at the branch level, may influence subsequent Hebbian plasticity. In addition, pathways classically associated with the expression of Hebbian plasticity, such as CamKII activation of SynGAP and thus Ras (Komiyama et al., 2002), have recently been linked to homeostatic plasticity via SynGAP-mediated suppression of protein translation (Wang et al., 2013). By directly impinging on ERK-Ras activity, this may represent one mechanism by which homeostatic plasticity can influence Hebbian plasticity at neighboring inputs. It remains to be determined how these processes are regulated to achieve both neuronal stability and information storage.
Although cooperative interactions have been shown to occur between inputs when multiple synapses are stimulated (Govindarajan et al., 2011, Harvey and Svoboda, 2007), in the absence of such co-activation, structural changes at neighbors have thus far served to counterbalance the direction of plasticity (Oh et al., 2015). Our observations that HSP promotes activity-driven growth of a group of synapses within a 5-μm area is in alignment with the observed distance and time over which plasticity-related proteins, such as Ras, can spread between co-active synapses (Harvey et al., 2008, Harvey and Svoboda, 2007). Following activity at a single spine, the movement of Ras in particular has been shown to spread locally in the dendrite and to effect a reduction in the threshold of plasticity at neighbors. As signaling by brain-derived neurotrophic factor, which leads to Ras-ERK activation, plays a role in both Hebbian and homeostatic plasticity (Korte et al., 1998, Rutherford et al., 1998, Wang et al., 2013), it is possible that Ras activity represents a mechanistic point of convergence that allows for the spread of LTP following HSP. Some of the signaling events related to Ras and Rho activation can now begin to be elucidated by taking advantage of new fluorescence resonance energy transfer-based sensors that allow for the visualization of ERK and PKA kinases (Tang and Yasuda, 2017), which can travel for over 10-μm along the dendrite, matching well with the parameters over which we observe metaplasticity between spines. It will be interesting to assess whether the spatial and temporal spread of Ras is altered following activity blockade.
The interaction between homeostatic and Hebbian plasticity may coordinately delimit a region of clustered plasticity, which has been proposed to increase the memory capacity of a neural circuit (Chklovskii et al., 2004, Govindarajan et al., 2011, Govindarajan et al., 2006, Ramiro-Cortés et al., 2014). Although clustered activity and its structural correlates have begun to emerge (Fu et al., 2012, Govindarajan et al., 2011, Kleindienst et al., 2011, Makino and Malinow, 2011, Yadav et al., 2012), the mechanisms that drive the physical organization of inputs are still unknown. HSP may provide a basis for such organization by reducing the threshold for cooperativity between inputs, allowing primed synapses located within several microns of an active spine to be more easily potentiated. This may serve as a first step in the physical arrangement of synapses into clusters. The broader consequence of this could be the binding together of information of varying saliencies, within a delimited region, into the same engram.
Our finding that synaptic threshold modulation after HSP is implemented at small, rather than at large spines, and in a reversible manner, suggests that synaptic scaling mechanisms are separable from plasticity mechanisms. However, by promoting long-lasting plasticity at select spines, and potentiating unstimulated neighboring spines within a delimited dendritic region, homeostatic plasticity may interact with and reduce the input-specific nature of Hebbian plasticity, enhance the clustering of synaptic inputs, and shape the long-term organization of neural circuits. In this way, despite the global nature of homeostatic plasticity, inputs may be locally modulated in an activity-dependent manner.
Limitations of the Study
The linear correlation between the structural properties of a spine (i.e., volume) and the functional ones (i.e., conductance) have been repeatedly demonstrated (Arellano et al., 2007, Govindarajan et al., 2011, Harvey and Svoboda, 2007, Matsuzaki et al., 2004). This allows for changes in spine volume to be used as a proxy for quantifying changes in functional plasticity. Indeed, we have previously shown that when spine current amplitude is measured hours after the induction of structural plasticity, the linear scaling of size with current amplitude is maintained (Govindarajan et al., 2011). Nevertheless, we cannot formally exclude the possibility that some of the changes induced upon homeostatic plasticity may alter this structure-function relationship. Although it is not currently possible to follow functional changes at individual inputs over many days, it would be interesting to observe in real time the dynamic fluctuations in a spine's structural and functional properties in response to changes in activity. In addition, since the complete blockade of all activity in vivo cannot be achieved, we could not test the interplay between homeostatic and Hebbian plasticity at single spines in this context, something that would be of interest to evaluate in the future.
Methods
All methods can be found in the accompanying Transparent Methods supplemental file.
Acknowledgments
We thank Ali Ozgur Argunsah for advice and help with data analysis. We thank Gil Costa for design help with the figures and the graphical abstract. We thank Steven Kushner, Rui Costa, and members of the Israely laboratory for their support and critical reading of the manuscript. This work was supported by grants from the Fundação Bial (161/10-2010 to I.I.), Fundação para a Ciência e a Technologia (PTDC/SAU-NMC/122035/2010 to I.I.; SFRH/BD/51265/2010 to A.H.), and Consejo Nacional de Ciencia y Tecnología (254878 to Y.R.C.).
Author Contributions
A.F.H., Y.R.C., and I.I. designed the experiments and built the setup. A.F.H. performed the experiments and analyzed the data. A.F.H. and I.I. wrote the manuscript.
Declaration of Interests
The authors declare no competing interests.
Published: October 26, 2018
Footnotes
Supplemental Information includes Transparent Methods and three figures and can be found with this article online at https://doi.org/10.1016/j.isci.2018.09.015.
Supplemental Information
References
- Aoto J., Nam C.I., Poon M.M., Ting P., Chen L. Synaptic signaling by all-trans retinoic acid in homeostatic synaptic plasticity. Neuron. 2008;60:308–320. doi: 10.1016/j.neuron.2008.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arellano J.I., Benavides-piccione R., Defelipe J., Yuste R. Ultrastructure of dendritic spines: correlation between synaptic and spine morphologies. Front. Neurosci. 2007;1:131–143. doi: 10.3389/neuro.01.1.1.010.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arendt K.L., Sarti F., Chen L. Chronic inactivation of a neural circuit enhances LTP by inducing silent synapse formation. J. Neurosci. 2013;33:2087–2096. doi: 10.1523/JNEUROSCI.3880-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes S.J., Franzoni E., Jacobsen R.I., Erdelyi F., Szabo G., Clopath C., Keller G.B., Keck T. Deprivation-induced homeostatic spine scaling in vivo is localized to dendritic branches that have undergone recent spine loss. Neuron. 2017;96:871–882.e5. doi: 10.1016/j.neuron.2017.09.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barria A., Malinow R. NMDA receptor subunit composition controls synaptic plasticity by regulating binding to CaMKII. Neuron. 2005;48:289–301. doi: 10.1016/j.neuron.2005.08.034. [DOI] [PubMed] [Google Scholar]
- Barrionuevo G., Brown T.H. Associative long-term potentiation in hippocampal slices. Proc. Natl. Acad. Sci. U S A. 1983;80:7347–7351. doi: 10.1073/pnas.80.23.7347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Branco T., Staras K., Darcy K.J., Goda Y. Local dendritic activity sets release probability at hippocampal synapses. Neuron. 2008;59:475–485. doi: 10.1016/j.neuron.2008.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chklovskii D.B., Mel B.W., Svoboda K. Cortical rewiring and information storage. Nature. 2004;431:782–788. doi: 10.1038/nature03012. [DOI] [PubMed] [Google Scholar]
- Costa-Mattioli M., Sossin W.S., Klann E., Sonenberg N. Translational control of long-lasting synaptic plasticity and memory. Neuron. 2009;61:10–26. doi: 10.1016/j.neuron.2008.10.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desai N.S., Cudmore R.H., Nelson S.B., Turrigiano G.G. Critical periods for experience-dependent synaptic scaling in visual cortex. Nat. Neurosci. 2002;5:783–789. doi: 10.1038/nn878. [DOI] [PubMed] [Google Scholar]
- Desai N.S., Rutherford L.C., Turrigiano G.G. Plasticity in the intrinsic excitability of cortical pyramidal neurons. Nat. Neurosci. 1999;2:515–520. doi: 10.1038/9165. [DOI] [PubMed] [Google Scholar]
- Erdil E., Yagci A.M., Argunsah A.O., Ramiro-Cortes Y., Hobbiss A.F., Israely I., Unay D. A tool for automatic dendritic spine detection and analysis. Part I : dendritic spine detection using multi-level region-based segmentation. 3rd International Conference On Image Processing Theory, Tools and Applications, IPTA. 2012:167–171. [Google Scholar]
- Félix-Oliveira A., Dias R.B., Colino-Oliveira M., Rombo D.M., Sebastião A.M. Homeostatic plasticity induced by brief activity deprivation enhances long-term potentiation in the mature rat hippocampus. J. Neurophysiol. 2014;112:3012–3022. doi: 10.1152/jn.00058.2014. [DOI] [PubMed] [Google Scholar]
- Fernandes D., Carvalho A.L. Mechanisms of homeostatic plasticity in the excitatory synapse. J. Neurochem. 2016;139:973–996. doi: 10.1111/jnc.13687. [DOI] [PubMed] [Google Scholar]
- Fu M., Yu X., Lu J., Zuo Y. Repetitive motor learning induces coordinated formation of clustered dendritic spines in vivo. Nature. 2012;483:92–95. doi: 10.1038/nature10844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghani M.U., Mesadi F., Kanık S.D., Argunşah A.Ö., Hobbiss A.F., Israely I., Ünay D., Taşdizen T., Çetin M. Dendritic spine classification using shape and appearance features based on two-photon microscopy. J. Neurosci. Methods. 2017;279:13–21. doi: 10.1016/j.jneumeth.2016.12.006. [DOI] [PubMed] [Google Scholar]
- Govindarajan A., Israely I., Huang S.-Y.Y., Tonegawa S. The dendritic branch is the preferred integrative unit for protein synthesis-dependent LTP. Neuron. 2011;69:132–146. doi: 10.1016/j.neuron.2010.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Govindarajan A., Kelleher R.J., Tonegawa S. A clustered plasticity model of long-term memory engrams. Nat. Rev. Neurosci. 2006;7:575–583. doi: 10.1038/nrn1937. [DOI] [PubMed] [Google Scholar]
- Harvey C.D., Svoboda K. Locally dynamic synaptic learning rules in pyramidal neuron dendrites. Nature. 2007;450:1195–1200. doi: 10.1038/nature06416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey C.D., Yasuda R., Zhong H., Svoboda K. The spread of Ras activity triggered by activation of a single dendritic spine. Science. 2008;321:136–140. doi: 10.1126/science.1159675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu J.-H., Park J.M., Park S., Xiao B., Dehoff M.H., Kim S., Hayashi T., Schwarz M.K., Huganir R.L., Seeburg P.H. Homeostatic scaling requires group I mGluR activation mediated by Homer1a. Neuron. 2010;68:1128–1142. doi: 10.1016/j.neuron.2010.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huganir R.L., Nicoll R.A. AMPARs and synaptic plasticity: the last 25 years. Neuron. 2013;80:704–717. doi: 10.1016/j.neuron.2013.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ju W., Morishita W., Tsui J., Gaietta G., Deerinck T.J., Adams S.R., Garner C.C., Tsien R.Y., Ellisman M.H., Malenka R.C. Activity-dependent regulation of dendritic synthesis and trafficking of AMPA receptors. Nat. Neurosci. 2004;7:244–253. doi: 10.1038/nn1189. [DOI] [PubMed] [Google Scholar]
- Karmarkar U.R., Buonomano D.V. Different forms of homeostatic plasticity are engaged with distinct temporal profiles. Eur. J. Neurosci. 2006;23:1575–1584. doi: 10.1111/j.1460-9568.2006.04692.x. [DOI] [PubMed] [Google Scholar]
- Keck T., Keller G.B., Jacobsen R.I., Eysel U.T., Bonhoeffer T., Hübener M. Synaptic scaling and homeostatic plasticity in the mouse visual cortex in vivo. Neuron. 2013;80:327–334. doi: 10.1016/j.neuron.2013.08.018. [DOI] [PubMed] [Google Scholar]
- Kirov S.A., Harris K.M. Dendrites are more spiny on mature hippocampal neurons when synapses are inactivated. Nat. Neurosci. 1999;2:878–883. doi: 10.1038/13178. [DOI] [PubMed] [Google Scholar]
- Kleindienst T., Winnubst J., Roth-Alpermann C., Bonhoeffer T., Lohmann C. Activity-dependent clustering of functional synaptic inputs on developing hippocampal dendrites. Neuron. 2011;72:1012–1024. doi: 10.1016/j.neuron.2011.10.015. [DOI] [PubMed] [Google Scholar]
- Komiyama N.H., Watabe A.M., Carlisle H.J., Porter K., Charlesworth P., Monti J., Strathdee D.J.C., O’Carroll C.M., Martin S.J., Morris R.G.M. SynGAP regulates ERK/MAPK signaling, synaptic plasticity, and learning in the complex with postsynaptic density 95 and NMDA receptor. J. Neurosci. 2002;22:9721–9732. doi: 10.1523/JNEUROSCI.22-22-09721.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korte M., Kang H., Bonhoeffer T., Schuman E. A role for BDNF in the late-phase of hippocampal long-term potentiation. Neuropharmacology. 1998;37:553–559. doi: 10.1016/s0028-3908(98)00035-5. [DOI] [PubMed] [Google Scholar]
- Lee M.-C., Yasuda R., Ehlers M.D. Metaplasticity at single glutamatergic synapses. Neuron. 2010;66:859–870. doi: 10.1016/j.neuron.2010.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu G. Local structural balance and functional interaction of excitatory and inhibitory synapses in hippocampal dendrites. Nat. Neurosci. 2004;7:373. doi: 10.1038/nn1206. [DOI] [PubMed] [Google Scholar]
- Makino H., Malinow R. Compartmentalized versus global synaptic plasticity on dendrites controlled by experience. Neuron. 2011;72:1001–1011. doi: 10.1016/j.neuron.2011.09.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malenka R.C., Bear M.F. LTP and LTD: an embarrassment of riches. Neuron. 2004;44:5–21. doi: 10.1016/j.neuron.2004.09.012. [DOI] [PubMed] [Google Scholar]
- Matsuzaki M., Honkura N., Ellis-Davies G.C.R., Kasai H. Structural basis of long-term potentiation in single dendritic spines. Nature. 2004;429:761–766. doi: 10.1038/nature02617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murthy V.N., Schikorski T., Stevens C.F., Zhu Y. Inactivity produces increases in neurotransmitter release and synapse size. Neuron. 2001;32:673–682. doi: 10.1016/s0896-6273(01)00500-1. [DOI] [PubMed] [Google Scholar]
- O'Brien R.J., Kamboj S., Ehlers M.D., Rosen K.R., Fischbach G.D., Huganir R.L. Activity-dependent modulation of synaptic AMPA receptor accumulation. Neuron. 1998;21:1067–1078. doi: 10.1016/s0896-6273(00)80624-8. [DOI] [PubMed] [Google Scholar]
- Oh W.C., Hill T.C., Zito K. Synapse-specific and size-dependent mechanisms of spine structural plasticity accompanying synaptic weakening. Proc. Natl. Acad. Sci. U S A. 2013;110:E305–E312. doi: 10.1073/pnas.1214705110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh W.C.C., Parajuli L.K.K., Zito K. Heterosynaptic structural plasticity on local dendritic segments of hippocampal CA1 neurons. Cell Rep. 2015;10:162–169. doi: 10.1016/j.celrep.2014.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paoletti P., Bellone C., Zhou Q. NMDA receptor subunit diversity: impact on receptor properties, synaptic plasticity and disease. Nat. Rev. Neurosci. 2013;14:383–400. doi: 10.1038/nrn3504. [DOI] [PubMed] [Google Scholar]
- Rabinowitch I., Segev I. Two opposing plasticity mechanisms pulling a single synapse. Trends Neurosci. 2008;31:377–383. doi: 10.1016/j.tins.2008.05.005. [DOI] [PubMed] [Google Scholar]
- Ramiro-Cortés Y., Hobbiss A.F., Israely I. Synaptic competition in structural plasticity and cognitive function. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2014;369:20130157. doi: 10.1098/rstb.2013.0157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramiro-Cortés Y., Israely I. Long lasting protein synthesis- and activity-dependent spine shrinkage and elimination after synaptic depression. PLoS One. 2013;8:e71155. doi: 10.1371/journal.pone.0071155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutherford L.C., Nelson S.B., Turrigiano G.G. BDNF has opposite effects on the quantal amplitude of pyramidal neuron and interneuron excitatory synapses. Neuron. 1998;21:521–530. doi: 10.1016/s0896-6273(00)80563-2. [DOI] [PubMed] [Google Scholar]
- Schanzenbacher C.T., Langer J.D., Schuman E.M. Time- and polarity-dependent proteomic changes associated with homeostatic scaling at central synapses. Elife. 2018:1–20. doi: 10.7554/eLife.33322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soares C., Lee K.F.H., Béïque J.-C. Metaplasticity at CA1 synapses by homeostatic control of presynaptic release dynamics. Cell Rep. 2017;21:1293–1303. doi: 10.1016/j.celrep.2017.10.025. [DOI] [PubMed] [Google Scholar]
- Sutton M.A., Ito H.T., Cressy P., Kempf C., Woo J.C., Schuman E.M. Miniature neurotransmission stabilizes synaptic function via tonic suppression of local dendritic protein synthesis. Cell. 2006;125:785–799. doi: 10.1016/j.cell.2006.03.040. [DOI] [PubMed] [Google Scholar]
- Tang S., Yasuda R. Imaging ERK and PKA activation in single dendritic spines during structural plasticity. Neuron. 2017;93:1315–1324.e3. doi: 10.1016/j.neuron.2017.02.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiagarajan T.C., Lindskog M., Tsien R.W. Adaptation to synaptic inactivity in hippocampal neurons. Neuron. 2005;47:725–737. doi: 10.1016/j.neuron.2005.06.037. [DOI] [PubMed] [Google Scholar]
- Toyoizumi T., Kaneko M., Stryker M.P., Miller K.D. Modeling the dynamic interaction of hebbian and homeostatic plasticity. Neuron. 2014;84:497–510. doi: 10.1016/j.neuron.2014.09.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turrigiano G. Too many cooks? Intrinsic and synaptic homeostatic mechanisms in cortical circuit refinement. Annu. Rev. Neurosci. 2011;34:89–103. doi: 10.1146/annurev-neuro-060909-153238. [DOI] [PubMed] [Google Scholar]
- Turrigiano G.G., Leslie K.R., Desai N.S., Rutherford L.C., Nelson S.B. Activity-dependent scaling of quantal amplitude in neocortical neurons. Nature. 1998;391:892–896. doi: 10.1038/36103. [DOI] [PubMed] [Google Scholar]
- Vitureira N., Goda Y. The interplay between hebbian and homeostatic synaptic plasticity. J. Cell Biol. 2013;203:175–186. doi: 10.1083/jcb.201306030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace W., Bear M.F. A morphological correlate of synaptic scaling in visual cortex. J. Neurosci. 2004;24:6928–6938. doi: 10.1523/JNEUROSCI.1110-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C.C., Held R.G., Hall B.J. SynGAP regulates protein synthesis and homeostatic synaptic plasticity in developing cortical networks. PLoS One. 2013;8 doi: 10.1371/journal.pone.0083941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yadav A., Gao Y.Z., Rodriguez A., Dickstein D.L., Wearne S.L., Luebke J.I., Hof P.R., Weaver C.M. Morphologic evidence for spatially clustered spines in apical dendrites of monkey neocortical pyramidal cells. J. Comp. Neurol. 2012;520:2888–2902. doi: 10.1002/cne.23070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yee A.X., Hsu Y.-T., Chen L. A metaplasticity view of the interaction between homeostatic and Hebbian plasticity. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2017;372 doi: 10.1098/rstb.2016.0155. 20160155. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.