Abstract
Key points
Several behavioural studies have shown the sensory perceptions are reduced during movement; yet the neurophysiological reason for this is not clear.
Participants underwent stimulation of the median nerve when either sitting quietly (i.e. passive stimulation condition) or performing haptic exploration of a ball with the left hand.
Magnetoencephalographic brain imaging and advanced beamforming methods were used to identify the differences in somatosensory cortical responses.
We show that the neural populations active during the passive stimulation condition were strongly gated during the haptic exploration task.
These results imply that the reduced haptic perceptions might be governed by gating of certain somatosensory neural populations.
Abstract
Several behavioural studies have shown that children have reduced sensory perceptions during movement; however, the neurophysiological nexus for these altered perceptions remains unknown. We used magnetoencephalographic brain imaging and advanced beamforming methods to address this knowledge gap. In our experiment, a cohort of children (aged 10–18 years) underwent stimulation of the median nerve when either sitting quietly (i.e. passive stimulation condition) or performing haptic exploration of a ball with the left hand. Our results revealed two novel observations. First, there was a relationship between the child's age and the strength of the beta (18–26 Hz) response seen within the somatosensory cortices during the passive stimulation condition. This suggests that there may be an age‐dependent change in the processing of peripheral feedback by the somatosensory cortices. Second, all of the cortical regions that were active during the passive stimulation condition were almost completely gated during the haptic task. Instead, the haptic task involved neural oscillations within Brodmann area 2, which is known to convey less spatially precise tactile information but is involved in the processing of more complex somatosensations across the respective digits. These results imply that the reduced somatosensory perceptions seen during movements in healthy children may be related to the gating of certain neural generators, as well as activation of haptic‐specific neural generators within the somatosensory cortices. The utilization of such haptic‐specific circuits during development may lead to the enhanced somatosensory processing during haptic exploration seen in healthy adults.
Keywords: Hand, MEG, Sensory, Cortical Oscillations, Children
Key points
Several behavioural studies have shown the sensory perceptions are reduced during movement; yet the neurophysiological reason for this is not clear.
Participants underwent stimulation of the median nerve when either sitting quietly (i.e. passive stimulation condition) or performing haptic exploration of a ball with the left hand.
Magnetoencephalographic brain imaging and advanced beamforming methods were used to identify the differences in somatosensory cortical responses.
We show that the neural populations active during the passive stimulation condition were strongly gated during the haptic exploration task.
These results imply that the reduced haptic perceptions might be governed by gating of certain somatosensory neural populations.
Introduction
Children expand their cognitive and perceptual capacities through active exploration of objects in their environment. This exploration involves the integration of motor and sensory information to form internal haptic perceptions of an object's shape, contours and dynamics. The neurophysiological nexus for the development of such haptic perceptions partially resides in somatosensory cortical circuits. Several electroencephalographic (EEG) studies have shown that the somatosensory event‐related potentials (ERP) evoked by peripheral stimulations appear to rapidly mature and become adult‐like by 6 years of age (Boor et al. 1998; Boor & Goebel, 2000). Despite the relatively prompt ontogenesis of such somatosensory ERPs, behavioural outcomes indicate that the establishment of haptic precision does not fully develop until well into adulthood (Gori et al. 2008; Gori et al. 2012).
Several behavioural studies have shown that the perception of somatosensations is reduced during movement, and that haptic perceptions are inferior in children compared to adults (Angel & Malenka, 1982; Milne et al. 1988; Gori et al. 2012; Holst‐Wolf et al. 2016). This altered perception in adults and children is presumed to be partially attributable to differential suppression of somatosensory responses during the ongoing movement (Papakostopoulos et al. 1975; Jones et al. 1989; Kristeva‐Feige et al. 1996; Macerollo et al. 2016). Animal studies have indicated that the suppressed cortical responses during movement might be the result of attenuation (i.e. gating) of the efferent feedback at several levels of the ascending lemniscal pathway (e.g. dorsal column nuclei, medial lemniscus, thalamus), and through cortico‐cortical connections (Seki et al. 2003; Seki & Fetz, 2012). Potentially, developmental differences in somatosensory perception in children during movement may be partially a result of an uncharacteristic gating of somatosensory cortical activity during object exploration. Such gating may prevent important sensory information from reaching higher cortical levels, thus impacting the ongoing neural computations that are involved in the processing and comparison of haptic feedback.
Predominantly, our understanding of movement‐related somatosensory gating has been derived from ERP studies of peripheral nerve stimulation (Papakostopoulos et al. 1975; Jones et al. 1989; Kristeva‐Feige et al. 1996; Macerollo et al. 2016). Although the outcomes of these prior studies have been pivotal for advancing our understanding of sensorimotor integration and motor‐related gating, neural oscillatory activity is almost certain to play a computational role in such processing, and this domain remains completely unexplored. Focusing on the neural oscillations may provide unique and important insight about the cortical dynamics that are not directly phase‐locked to the peripheral stimulus. It is well recognized that peripheral stimulation of the hands when sitting quietly produces a desynchronization of the somatosensory cortical oscillations throughout the alpha (8–12 Hz) and beta (15–30 Hz) bands (Hirata et al. 2002; Gaetz & Cheyne, 2003; Della Penna et al. 2004; Dockstader et al. 2008). Furthermore, it has recently been shown that these neural oscillations are modulated across a broad frequency range (e.g. 10–75 Hz) following a pair of identical and successive peripheral somatosensations (Kurz et al. 2017; Wiesman et al. 2017; Spooner et al. 2018). However, whether these frequency‐specific somatosensory cortical oscillations are modulated by haptic exploration remains unknown.
In the present study, we begin to address this knowledge gap by applying electrical stimulation to the median nerve of healthy children who were performing a haptic ball exploration task with the hand, or sitting quietly with no motor activity, during magnetoencephalography (MEG). Our key hypotheses were: (i) that oscillatory responses in the somatosensory cortices would be diminished (i.e. gated) during the haptic exploration of a ball relative to a no‐exploration condition; (ii) that the strength of the somatosensory cortical oscillations during the no‐exploration condition would not scale with age; and (iii) that the strength of the somatosensory cortical oscillations during the haptic exploration of a ball would scale with age.
Methods
Participants
Fifteen typically‐developing right‐handed children (11 males; aged 10–18 years) with no neurological or musculoskeletal impairments participated in the present study. This experimental work conformed to the standards set by the Declaration of Helsinki, except for registration in a database. The Institutional Review Board at the University of Nebraska Medical Center reviewed and approved this investigation (IRB#557‐15‐EP). Informed consent was acquired from the parents and the children assented to participate in the experiment.
MEG data acquisition and experimental paradigm
The neuromagnetic responses were acquired with a bandwidth of 0.1–330 Hz and were sampled continuously at 1 kHz using a MEG system (Elekta, Helsinki, Finland) with 306 magnetic sensors (204 planar gradiometers and 102 magnetometers). All recordings were conducted in a one‐layer magnetically‐shielded room with active shielding engaged for advanced environmental noise compensation. During data acquisition, the children were monitored via real‐time audio‐video feeds from inside the shielded room. This monitoring was used to establish that the participants were complying with instructions for the passive vs. haptic exploration conditions.
During the experiment, participants were seated in a custom‐made non‐magnetic chair with their head positioned within the helmet‐shaped MEG sensor array. Unilateral electrical stimulation was applied to the median nerve at a location that was just proximal of the radial styloid of the left hand using an external cutaneous stimulator (Digitimer DS7A; HW Medical Products, Neuberg, Germany). The stimulator consisted of two stainless steel disk electrodes that had a 33 mm spacing. During the passive stimulation condition, participants sat quietly, looking at their left hand and not performing any movements. During the haptic exploration condition, the participants were instructed to looked at their hand, and continuously manipulate and explore the surface of a ball with the fingers of their left hand (Fig. 1). The passive and haptic exploration conditions were performed separately and presented in a random order. For each condition, 125 electrical stimulations were applied with an inter‐stimulus interval that varied randomly between 1800 and 2200 ms. Each condition lasted ∼4 min. The electrical stimulation consisted of 0.2 ms constant‐current square wave that was set to 10% above the motor threshold required to elicit a subtle but visible flexor twitch in the first digit when the child was sittiing passively. The threshold of the electrical stimulation was at the same level for both conditions. The epochs were defined offline and were of 1700 ms duration, including a 400 ms pre‐stimulus baseline.
Figure 1. Depiction of the haptic exploration condition where the child is seated with their head in the MEG and actively moving the ball within the fingers of the left hand.
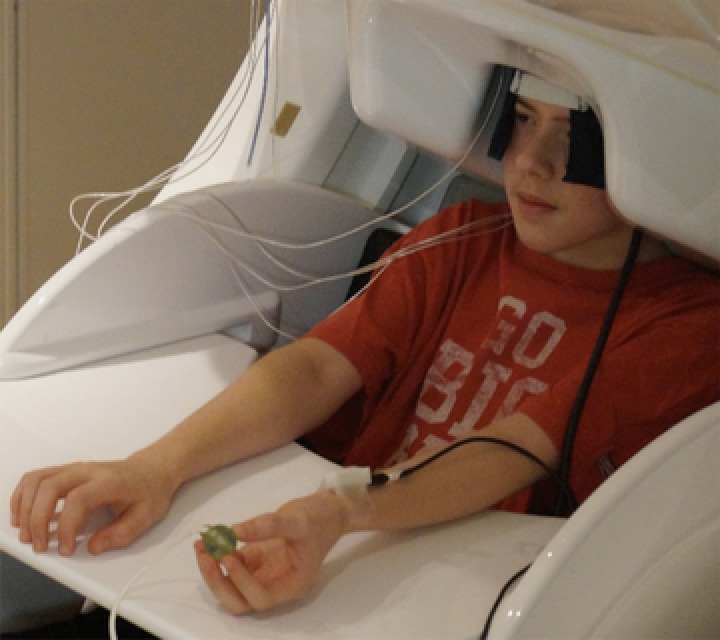
The string attached to the ball was taped to the table and used to prevent the ball from falling to the floor, in case the participant dropped it. Although this precaution was in place, none of the subjects dropped the ball during the experiment. [Color figure can be viewed at http://wileyonlinelibrary.com]
Structural magnetic resonance imaging (MRI) processing and MEG co‐registration
Structural MRI data were acquired using a Achieva 3T scanner (Philips, Eindhoven, The Netherlands). High‐resolution T1‐weighted sagittal images were obtained with an eight‐channel head coil using a 3‐D fast field echo sequence with the parameters: field of view: 24 cm; slice thickness: 1 mm; no gap; in‐plane resolution of 1.0 × 1.0 mm; and sense factor of 2.0.
Prior to the start of the MEG experiment, four coils were affixed to the child's head. The locations of these coils, three fiducial points and the scalp surface were digitized to determine their 3‐D position relative to each other (Fastrak 3SF0002; Polhemus Navigator Sciences, Colchester, VT, USA). Head localization was accomplished by continuously feeding an electric current with a unique frequency label (e.g. 322 Hz) to each of the coils during the data collection. This induced a measurable magnetic field and allowed for each coil to be localized in reference to the sensors throughout the recording session. Because the coil locations were also known in head co‐ordinates, all MEG measurements could be transformed into a common co‐ordinate system. With this co‐ordinate system, the MEG data for each child were co‐registered with their native space structural T1‐weighted MRI data. The structural MRI data were aligned parallel to the anterior and posterior commissures, and were transformed into standardized space following source imaging (i.e. beamforming; see below).
MEG preprocessing, time‐frequency transformation and sensor‐level statistics
Each raw MEG data set was individually corrected for head motion that may have occurred during task performance. Average head motion per participant was less than 2 mm across each condition, and this did not statistically differ between conditions (P > 0.9). All MEG data were also subjected to noise reduction using the signal space separation method with a temporal extension (Taulu & Simola, 2006). This method involves defining signal subspaces and eliminating signals that could not have originated in the head volume. In addition, cardiac artefacts were removed from the data using signal‐space projection and the projection operator was accounted for during source reconstruction (Uusitalo & Imoniemi, 1997).
Artefact rejection was based on a fixed threshold that was established based on the gradient and amplitude changes of the neuromagnetic signals, and supplemented with visual inspection. This procedure eliminated any large fluctuations in the signal that may have been generated by eye blinks and/or muscular activity. The continuous magnetic time series was divided into epochs of 1700 ms in duration, with the baseline being defined as –600 ms to –200 ms before initial stimulus onset.
The artefact‐free epochs were transformed into the time‐frequency domain using complex demodulation, and the resulting spectral power estimations per sensor were averaged over trials to generate time‐frequency plots of the mean spectral density. These sensor‐level data were then normalized per time‐frequency bin using the respective bin's baseline power. The specific time‐frequency windows used for imaging were determined by statistical analysis of the sensor‐level spectrograms across the entire array of gradiometers per condition. Each data point in the spectrogram was initially evaluated using a mass univariate approach based on the general linear model. To reduce the risk of false positive results at the same time as maintaining reasonable sensitivity, a two‐stage procedure was followed to control for type 1 error. In the first stage, one‐sample t tests were conducted on each data point and the output spectrogram of t values was thresholded at P < 0.05 to define time‐frequency bins containing potentially significant oscillatory deviations across all participants. In stage two, time‐frequency bins that survived the threshold were clustered with temporally and/or spectrally neighbouring bins that were also above the threshold (P < 0.05) and a cluster value was derived by summing all of the t values of all data points in the cluster. Non‐parametric permutation testing was then used to derive a distribution of cluster values and the significance level of the observed clusters (from stage one) were tested directly using this distribution (Ernst, 2004; Maris & Oostenveld, 2007). For each comparison, 10,000 permutations were computed to build a distribution of cluster values. Based on these analyses, the time‐frequency windows that contained significant oscillatory events across all participants were subjected to a beamforming analysis.
MEG source imaging and source‐level statistics
A minimum variance vector beamforming algorithm was employed to calculate the source power across the entire brain volume (Van Veen et al. 1997; Gross et al. 2001; Hillebrand et al. 2005) using spatial filters in the frequency domain and a spherical head model. The single images were derived from the cross‐spectral densities of all combinations of MEG sensors within the time‐frequency ranges of interest, and the solution of the forward problem for each location on a 4.0 × 4.0 × 4.0 mm grid specified by the input voxel space. Following convention, the source power in these images were normalized per subject using a separately averaged pre‐stimulus noise period of equal duration and bandwidth (Van Veen et al. 1997; Hillebrand et al. 2005). Such images are typically referred to as pseudo‐t maps, with units (pseudo‐t) that reflect noise‐normalized power differences (i.e. active vs. baseline) per voxel. Thus, the normalized power per voxel for each time‐frequency component (see below) was computed over the entire brain volume per participant. Each child's functional images, which were co‐registered to native space anatomical images prior to beamforming, were transformed into standardized space using the transformation that was previously applied to the structural MRI volume and spatially resampled. MEG imaging was performed using BESA, version 6.0 (BESA GmbH, Grafelfing, Germany).
The time‐frequency windows containing significant oscillatory responses (see Results below) were imaged using a beamformer in each condition, and the output images were averaged across the group of participants separately for each condition and oscillatory component. The peak voxels in these images were then extracted for correlation analyses using Pearson product moment correlations to determine the rank order relationships between the respective response amplitudes per condition and the child's age. All correlation analyses were performed with SPSS (IBM Corp., Armonk, NY, USA) using an alpha level of 0.05.
Finally, whole brain statistical analyses were conducted on each oscillatory response using voxel‐wise paired‐samples t tests. To control for type 1 error, a two‐stage procedure similar to that used in the sensor‐level time‐frequency analysis was followed. For the stage two permutation testing to control for type 1 error, 1000 permutations were computed to build a distribution of cluster values.
Results
Passive stimulation condition: sensor‐level and beamforming analysis
During the passive stimulation condition, a series of significant oscillations was detected in a cluster of gradiometers near the sensorimotor strip. Briefly, these sensor‐level spectrograms revealed prominent increases in theta (4–8 Hz) and low gamma (26–42 Hz) activity that began immediately after the onset of the stimulation, and lasted 200 ms and 75 ms, respectively (P < 0.001, corrected) (Fig. 2 A). In addition, significant decreases in beta (18–26 Hz) were observed during the latter 150–400 ms time window (P < 0.001, corrected) and these occurred in parallel with significant alpha (8–16 Hz) decreases during the 225–525 ms window (P < 0.001, corrected).
Figure 2. Time‐frequency spectrograms from the same gradiometer sensor located near the sensorimotor strip and the location of the peak activity in the beamformer images.
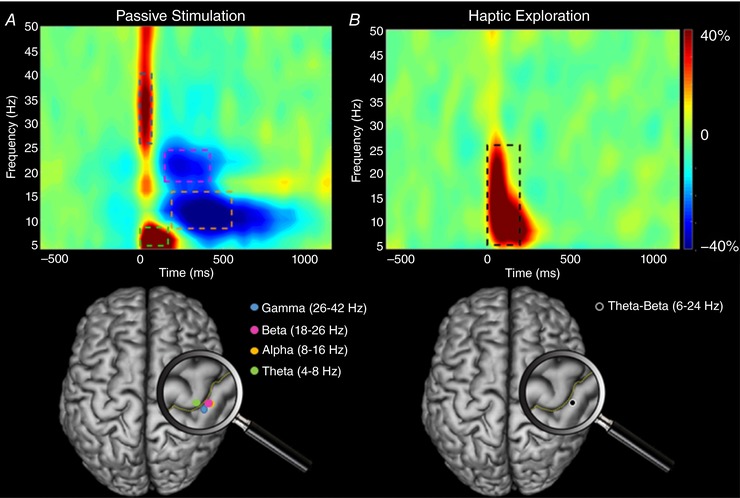
The time‐frequency spectrograms shown were averaged across participants for the passive (A) and haptic (B) conditions, respectively. Time (ms) is denoted on the x‐axis, with 0 ms defined as the onset of the stimulations. Spectral power is expressed as the percent difference from the baseline period (–600 ms to –200 ms) and the colour scale bar is shown to the far right. As shown in (A), neuronal activity strongly increased within the gamma (26–42 Hz) and theta (4–8 Hz) frequency ranges between 0 and 200 ms during the passive condition, and this was followed by a decrease in alpha (8–16 Hz) and beta (18–26 Hz) power from 150–525 ms. The location of neural generators for the alpha, beta and gamma oscillations resided in the hand region of the post‐central gyrus, whereas that for the theta oscillations resided in the precentral gyrus. As shown in (B), there was a stark difference in oscillatory activity during the haptic condition because there was a strong increase (synchronization) across the theta‐beta (6–24 Hz) frequency range from 0 to 175 ms. The location of the theta‐beta oscillations resided in the hand region of the postcentral gyrus. Talairach co‐ordinates for the peak of the respective responses are provided in Table 1. [Color figure can be viewed at http://wileyonlinelibrary.com]
These significant oscillations were individually subjected to a beamformer analysis to identify their cortical origins. This revealed that the theta increase during the 0–200 ms time window occurred within the hand region of the right sensorimotor cortices, with the peak activity residing in Brodmann area (BA) 4 of the right precentral gyrus (Fig. 2 A). The low gamma increase during the 0–75 ms time window also originated in the hand region of the right sensorimotor cortices, although the peak was slightly posterior in the right primary somatosensory cortices. Regarding the beta decrease (150–400 ms), this also originated in the hand area of the right sensorimotor cortices, with the strongest peak in BA 3 of the somatosensory cortices. Finally, the alpha decrease (225–525 ms) emanated from the same hand area of the right sensorimotor cortices, with the peak activity occurring in BA 3 of the somatosensory cortices. The Talairach co‐ordinates corresponding to the local maximums of each response are shown in Table 1.
Table 1.
Talairach co‐ordinates for peak voxels seen in the respective frequency bands and time windows
| Condition | Frequency bands | Time window (ms) | Co‐ordinates (x, y, z) |
|---|---|---|---|
| Passive | Theta (4‒8 Hz) | 0‒200 | 34, ‒28, 53 |
| Alpha (8‒16 Hz) | 225‒525 | 42, ‒24, 49 | |
| Beta (18‒26 Hz) | 150‒400 | 42, ‒24, 49 | |
| Gamma (26‒42 Hz) | 0‒75 | 38, ‒24, 49 | |
| Haptic | Theta‒Beta (6‒24 Hz) | 0‒175 | 38, ‒24, 45 |
Passive stimulation condition: correlations between age and peak activity
The peak voxels for each response in the passive stimulation condition were extracted from the beamformer images and correlated with participant age. There was a negative rank order correlation between the peak beta decrease seen in the somatosensory cortices (ρ = –0.51; P = 0.027) and the child's age (Fig. 3). This correlation implied that the older children tended to display a stronger beta decrease during the 150–400 ms time window of the passive stimulation condition. None of the other peak voxels identified during passive stimulation were correlated with age (P > 0.05).
Figure 3. Correlation between the age of the children and the strength of the somatosensory beta event related desynchronization (ERD).
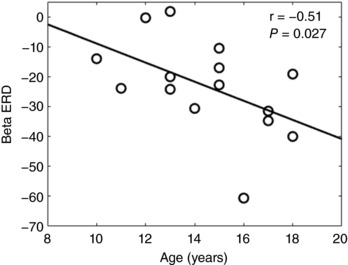
Correlation between the age of the children in years and the strength of the somatosensory beta (18–26 Hz) ERD during the 150–400 ms time window for the passive stimulation condition. This correlation implied that the older children tended to display a stronger decrease in the beta oscillations.
Haptic stimulation condition: sensor‐level and beamforming analysis
Unlike the passive condition, only one significant oscillatory response was detected during the haptic exploration condition. This response occurred in the same cluster of gradiometers as the oscillatory events detected in the passive condition, although it was more broadband, stretching from 6–24 Hz (P < 0.001, corrected) (Fig. 2 B). This 6–24 Hz synchronization (increase) began immediately after the onset of the stimulation and was sustained for 175 ms (i.e. 0–175 ms). Remarkably, the other time frequency components seen in the passive simulation condition were absent and appeared to have been completely gated when the children performed the haptic task.
The significant window (6–24 Hz, 0–175 ms) was imaged using a beamformer and the resulting images showed that the increased activity was centered on the hand region of the right sensorimotor cortices, with the peak activity located in BA 2 of the primary somatosensory cortices (Fig. 2 B). The Talairach co‐ordinates for the local maxima of this response are shown in Table 1.
Haptic stimulation condition: correlations between age and peak activity
The peak voxel seen in the haptic condition was extracted from the beamformer images and correlated with participant age. Unlike the passive stimulation condition, the results showed that the peak voxel amplitude did not correlate with the child's age (P > 0.05).
Passive vs. haptic stimulation: cortical oscillatory differences
Finally, we conducted whole‐brain statistical comparisons of the passive and haptic exploration conditions using the images from each oscillatory response. First, for the haptic condition, we imaged the four time‐frequency windows identified through the passive stimulation condition, and vice‐versa for the one oscillatory response identified in the haptic condition. Our findings indicated no statistical differences in the theta range (4–8 Hz, 0–200 ms) between passive and haptic conditions. By contrast, the alpha decrease (8–16 Hz, 225–525 ms) was significantly stronger in the right precentral (P < 0.000001; Cohen's d = 1.92) and postcentral gyri (P < 0.000001; Cohen's d = 2.23) during the passive condition, with the main peak being in the right postcentral gyrus (Fig. 4 A). Regarding the beta response (18–26 Hz, 150–400 Hz), the decrease in beta activity relative to the baseline period was significantly stronger in the passive relative to the haptic condition, with the peak difference being centered on the right postcentral gyrus (P < 0.000001; Cohen's d = 2.21) (Fig. 4 B). By contrast, the broadband response (6–24 Hz, 0–175 ms) was significantly elevated in the haptic compared to the passive condition (Fig. 4 C), with the increase in activity being statistically stronger in the left superior parietal (P < 0.000001; Cohen's d = 1.45) and the right postcentral gyrus (P < 0.000001; Cohen's d = 1.79). As with the theta response, there were no statistical differences between the passive and haptic conditions in the low gamma range (26–42 Hz, 0–75 ms). Lastly, we subjected the three maps shown in Fig. 4 to non‐parametric permutation testing to control for multiple comparisons, and all clusters shown in Fig. 4 survived at a corrected P value of 0.01.
Figure 4. Statistical parametric maps (SPMs) of the condition differences for alpha (8–16 Hz), beta (18‐26 Hz) and theta‐beta (6–24 Hz) oscillations.
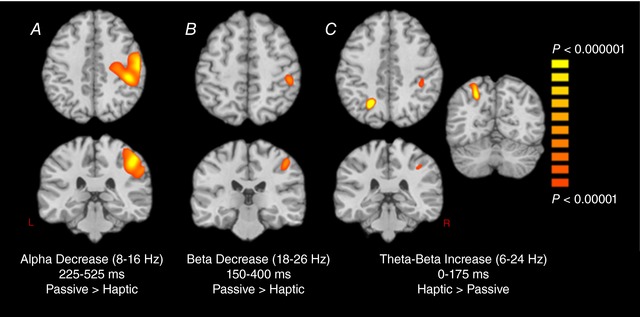
The images are shown following the neurological convention (R = R), with the significance colour bar shown to the right. As shown in (A), the alpha (8–16 Hz) decrease (desynchronization) was stronger in the right precentral and postcentral gyri during the passive condition. B, the beta (18–26 Hz) decrease was also stronger during the passive condition with the peak difference in the right postcentral gyrus. C, the theta‐beta (6–24 Hz) increase (synchronization) was stronger in the left superior parietal and the right postcentral gyrus during the haptic condition. [Color figure can be viewed at http://wileyonlinelibrary.com]
Discussion
There were major differences in cortical oscillatory activity between our passive and haptic exploration conditions, despite the peripheral stimulations being identical. Briefly, compared with the passive condition, cortical oscillations in the 6–24 Hz range were more synchronized from 0–175 ms within the somatosensory and parietal cortices during haptic exploration. These outcomes are aligned with prior functional MRI and EEG studies that have connected activity in these cortical regions with object localization during a haptic task (Reed et al. 2005; Neuper et al. 2006; Marangon et al. 2015). Conversely, the sharp decreases seen in alpha (8–16 Hz) and beta (18–26 Hz) activity were notably stronger and centralized to the somatosensory cortices during the passive stimulation condition. Altogether, these differences clearly show that the cortical processing of somatosensations is strongly modulated by haptic explorations.
Our beamforming results identified that the immediate increases in theta and low gamma activity during the passive stimulation condition resided within BA 4 and 3 of the right hemisphere, respectively. Numerous EEG and MEG studies have employed dipole modelling techniques and identified an early evoked somatosensory response (e.g. ∼20 ms) within the same regions (Kawamura et al. 1996; Jung et al. 2008). Our finding of cortical oscillations within the motor cortices (e.g. BA 4) was not surprising because prior animal and human lesion studies have also found that stimulation of the median nerve evokes activity within this area (Mauguiere et al. 1983; Mauguiere & Desmedt, 1991). Furthermore, it has been shown that activity in BA 4 persists even when the somatosensory cortices are ablated (Malis et al. 1953; Lemon, 1979; Lemon & van der Burg, 1979; Mauguiere et al. 1983). The current consensus is that some of the neural generators within the primary motor cortices are involved in the processing of cutaneous stimuli as a result of the extensive overlap of the thalamocortical tract terminations serving the cortical areas that represent the hand.
The later alpha and beta decreases seen during the passive stimulation condition were also consistent with what has been reported in previous EEG and MEG studies that have employed a median nerve stimulation paradigm (Nikouline et al. 2000; Della Penna et al. 2004; Svoboda et al. 2004; Chien et al. 2014; Boto et al. 2017). Although a delay in these responses has been recognized for several decades (Allison et al. 1989), the significance of this cortical activity has been somewhat elusive. It could be postulated that such late alpha and beta responses represent a rebound of the somatosensory cortical oscillations. However, we are skeptical of this interpretation because our data driven approach did not reveal a preceding power increase in the same frequency bands. Given the delay in these responses (∼150 ms), we suspect that these alpha and beta oscillations may be a result of sensory information arising from the electrical stimulation of peripheral alpha motor neurons and/or Ia afferents that interface with the muscle spindles. It is well established that excitation of the Ia afferents with a low‐grade electrical stimulation augments the Hoffman reflex (Zehr, 2002; Tucker et al. 2005; Grosset et al. 2007). This reflexive pathway generates a muscular twitch via the monosynaptic connections between the Ia afferents and alpha motor neurons in the anterior horn of the spinal cord. Our beamforming results support this conjecture because they showed that the late alpha and beta oscillations occurred in BA 3, which is known to be involved in the processing of sensory information generated from the muscle spindles (Costanzo & Gardner, 1980; Gardner & Costanzo, 1981). Hence, we propose that these alpha and beta neural oscillations were delayed because they represent the processing of sensory feedback that was generated after the muscular twitch.
Our correlational results implied that the older children tended to display a stronger beta decrease within BA 3 of the somatosensory cortices during the passive stimulation condition. Based on the model proposed above, we suggest that this age related correlation might be tied to the maturation of sensory pathways transmitting information about the muscle twitch. This notion is partially supported by a prior study showing that the magnitude of the Hoffman reflex scales with age throughout adolescence (Grosset et al. 2007). However, our finding that somatosensory activity scales throughout adolescence is in partial opposition to the ERP literature, which suggests that the somatosensory evoked potentials are adult‐like by 6 years of age (Boor et al. 1998; Boor & Goebel, 2000). Potentially, these conflicting results may be a result of differences in analysis methods (i.e. time‐domain averages of evoked vs. oscillatory activity), especially because somatosensory cortical oscillations that are not directly phase‐locked with the peripheral stimulus are lost in the time‐domain approach.
Interestingly, all of the neural oscillations that were observed during the passive stimulation condition were completely gated during haptic exploration. Somatosensory responses during the haptic exploration task consisted of an increase in activity stretched across the theta to beta range, which began immediately after the stimulation. By contrast to the passive stimulation condition, these 6–24 Hz neural oscillations resided in BA 2 of the somatosensory cortices. Outcomes from non‐human primate studies have revealed that the mechanoreceptive fields found in BA 2 are larger and respond to more complex somatosensation than those in BA 3 (Iwamura & Tanaka, 1978; Gardner, 1988). For example, receptive fields in BA 2 encapsulate multiple digits and are activated when objects are held or manipulated by the hand (Iwamura & Tanaka, 1978; Gardner, 1988). Experimental evidence has also shown that BA 2 specializes in the integration of information relayed across the mechanoreceptors of the digits and kinesthetic feedback from the joints (e.g. joint postures and speed of movement) (Costanzo & Gardner, 1980; Gardner & Costanzo, 1981; Gardner, 1988). Collectively, these prior results imply that the increase in 6–24 Hz activity most probably represented higher‐level sensory computations that are related to the integration of ongoing haptic feedback.
Our results are also well‐aligned with the outcomes of prior EEG and animal model studies that have shown that somatosensory ERPs are gated during movement (Papakostopoulos et al. 1975; Jones et al. 1989; Kristeva‐Feige et al. 1996; Seki et al. 2003; Houdayer et al. 2006; Seki & Fetz, 2012; Macerollo et al. 2016). However, our results suggest that such gating is not related to a basic decrease in cortical activity, but rather a ceasing of oscillatory activity in certain BAs of the sensorimotor region, along with increased oscillatory responses in other somatosensory cortical areas. The activity seen within BA 3 and 4 during the passive stimulation condition was probably important for the initial stages of identifying ‘what’ and ‘where’ the stimulation was occurring on the body. However, digit specific sensory information and fine grain ‘what’ and ‘where’ might be less important during a haptic task. Rather, the processing of sensory feedback that terminates in BA 2 may be important for integrating sensory information across the digits when moving an object in the hand. Although it is difficult to pinpoint where along the lemniscal pathway the gating of ‘what’ and ‘where’ sensory information occurs, a prior animal model suggests that it might originate at the spinal cord level where the corticospinal pathways have an inhibitory effect on the sensory spinal circuitry (Seki et al. 2003). It has been postulated that this inhibitory effect is necessary for gaining greater control of the spinal circuits that govern the desired motor action. We propose that this may have a corollary effect that also facilitates the concurrent transmission of important kinesthetic and haptic sensory information through sensory circuits during the motor action. Such sensory information is probably accentuated because it is important for comparing the difference between intended and ongoing motor actions.
We should note that a prior study has shown that the somatosensory alpha, beta and gamma band oscillations are stronger when an individual's attention is directed toward the electrical stimulation applied to the median nerve when sitting passively (Dockstader et al. 2010). Hence, it is possible that the gated somatosensory activity seen for the haptic exploration condition may be related to the selective attention towards the different type of sensory feedback occurring during the haptic exploration task. Effectively, attention may be more aligned with the sensory information from the mechanoreceptors of the digits and kinesthetic feedback from the joints, as opposed to information conveyed from the peripheral alpha motor neurons and Ia afferents. Although this conjecture is conceivable, it is difficult to challenge and support experimentally. Lastly, it would be useful to ensure that our results generalizes to females because our study sample was primarily composed of males.
Conclusions
Compared with adults, children have been noted to have a reduced perception of somatosensations during movement (Angel & Malenka, 1982; Milne et al. 1988; Gori et al. 2012; Holst‐Wolf et al. 2016). Despite this impression, we did not find a correlation between the strength of 6–24 Hz neural oscillations in the somatosensory cortices during the haptic exploration task and a child's age. This implies that the integration of complex somatosensory information in BA 2 might be mature by early adolescence. However, this position needs to be interrogated further in children who are younger than the participants in this investigation to clearly identify when these more complex somatosensory computations actually mature. Previous investigations have presumed that the altered perception of somatosensations during movement is attributable to a suppression of the somatosensory evoked potentials (Papakostopoulos et al. 1975; Jones et al. 1989; Kristeva‐Feige et al. 1996; Macerollo et al. 2016). By contrast to this viewpoint, our experimental results suggest that the altered perception is more probably related to the activation of different neuronal groups within the sensorimotor cortices. In other words, the activation of neural generators in BA 2, as opposed to those in BA 3 and 4, probably alters the perception of the ongoing somatosensations during movement as a result of the type of sensory information that these respective cortical areas process.
Additional information
Competing interests
The authors declare that they have no competing interests.
Author contributions
MJK and TWW contributed to the conceptual design of the experimental work, acquisition and interpretation of the data, and drafting/revising the intellectual content of the manuscript. AIW and NMC contributed to the analysis, interpretation of the data and assisted with the manuscript revisions. All authors approved the final version of the manuscript and are accountable for all aspects of the work related to the accuracy and integrity of any part of the work are appropriately investigated and resolved. All of the persons designated as authors on this manuscript qualify for authorship, and all those who qualify for authorship are listed.
Funding
This work was partially supported by grants from the National Institutes of Health (1R01‐HD086245) and the National Science Foundation (NSF 1539067).
Biography
Max Kurz received his doctorate in Psychology with a concentration in Neuroscience and Behaviour from the University of Nebraska. He is currently an Associate Professor in the Department of Physical Therapy at University of Nebraska Medical Center's Munroe‐Meyer Institute and the Director of the Sensorimotor Learning Laboratory. His research uses a blend of biomechanical engineering and brain imaging techniques to quantify how the sensorimotor cortices reach a decision threshold, integrate sensory feedback and produce motor actions. The long‐term goal of his research is to understand the developmental differences in how children learn new motor skills.

Edited by: Janet Taylor & Diego Contreras
References
- Allison T, McCarthy G, Wood CC, Williamson PD & Spencer DD (1989). Human cortical potentials evoked by stimulation of the median nerve. II. Cytoarchitectonic areas generating long‐latency activity. J Neurophysiol 62, 711–722. [DOI] [PubMed] [Google Scholar]
- Angel RW & Malenka RC (1982). Velocity‐dependent suppression of cutaneous sensitivity during movement. Exp Neurol 77, 266–274. [DOI] [PubMed] [Google Scholar]
- Boor R & Goebel B (2000). Maturation of near‐field and far‐field somatosensory evoked potentials after median nerve stimulation in children under 4 years of age. Clin Neurophysiol 111, 1070–1081. [DOI] [PubMed] [Google Scholar]
- Boor R, Goebel B & Taylor MJ (1998). Subcortical somatosensory evoked potentials after median nerve stimulation in children. Eur J Paediatr Neurol 2, 137–143. [DOI] [PubMed] [Google Scholar]
- Boto E, Meyer SS, Shah V, Alem O, Knappe S, Kruger P, Fromhold TM, Lim M, Glover PM, Morris PG, Bowtell R, Barnes GR & Brookes MJ (2017). A new generation of magnetoencephalography: room temperature measurements using optically‐pumped magnetometers. Neuroimage 149, 404–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chien JH, Liu CC, Kim JH, Markman TM & Lenz FA (2014). Painful cutaneous laser stimuli induce event‐related oscillatory EEG activities that are different from those induced by nonpainful electrical stimuli. J Neurophysiol 112, 824–833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costanzo RM & Gardner EP (1980). A quantitative analysis of responses of direction‐sensitive neurons in somatosensory cortex of awake monkeys. J Neurophysiol 43, 1319–1341. [DOI] [PubMed] [Google Scholar]
- Della Penna S, Torquati K, Pizzella V, Babiloni C, Franciotti R, Rossini PM & Romani GL (2004). Temporal dynamics of alpha and beta rhythms in human SI and SII after galvanic median nerve stimulation. A MEG study. Neuroimage 22, 1438–1446. [DOI] [PubMed] [Google Scholar]
- Dockstader C, Cheyne D & Tannock R (2010). Cortical dynamics of selective attention to somatosensory events. Neuroimage 49, 1777–1785. [DOI] [PubMed] [Google Scholar]
- Dockstader C, Gaetz W, Cheyne D, Wang F, Castellanos FX & Tannock R (2008). MEG event‐related desynchronization and synchronization deficits during basic somatosensory processing in individuals with ADHD. Behav Brain Funct 4, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst MD (2004). Permutation methods: a basis for exact inference. Stat Sci 19, 676–685. [Google Scholar]
- Gaetz WC & Cheyne DO (2003). Localization of human somatosensory cortex using spatially filtered magnetoencephalography. Neurosci Lett 340, 161–164. [DOI] [PubMed] [Google Scholar]
- Gardner EP (1988). Somatosensory cortical mechanisms of feature detection in tactile and kinesthetic discrimination. Can J Physiol Pharmacol 66, 439–454. [DOI] [PubMed] [Google Scholar]
- Gardner EP & Costanzo RM (1981). Properties of kinesthetic neurons in somatosensory cortex of awake monkeys. Brain Res 214, 301–319. [DOI] [PubMed] [Google Scholar]
- Gori M, Del Viva M, Sandini G & Burr DC (2008). Young children do not integrate visual and haptic form information. Curr Biol 18, 694–698. [DOI] [PubMed] [Google Scholar]
- Gori M, Squeri V, Sciutti A, Masia L, Sandini G & Konczak J (2012). Motor commands in children interfere with their haptic perception of objects. Exp Brain Res 223, 149–157. [DOI] [PubMed] [Google Scholar]
- Gross J, Kujala J, Hamalainen M, Timmermann L, Schnitzler A & Salmelin R (2001). Dynamic imaging of coherent sources: studying neural interactions in the human brain. Proc Natl Acad Sci U S A 98, 694–699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosset JF, Mora I, Lambertz D & Perot C (2007). Changes in stretch reflexes and muscle stiffness with age in prepubescent children. J Appl Physiol (1985) 102, 2352–2360. [DOI] [PubMed] [Google Scholar]
- Hillebrand A, Singh KD, Holliday IE, Furlong PL & Barnes GR (2005). A new approach to neuroimaging with magnetoencephalography. Hum Brain Mapp 25, 199–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirata M, Kato A, Taniguchi M, Ninomiya H, Cheyne D, Robinson SE, Maruno M, Kumura E, Ishii R, Hirabuki N, Nakamura H, Yoshimine T (2002). Frequency‐dependent spatial distribution of human somatosensory evoked neuromagnetic fields. Neurosci Lett 318, 73–76. [DOI] [PubMed] [Google Scholar]
- Holst‐Wolf JM, Yeh IL & Konczak J (2016). Development of proprioceptive acuity in typically developing children: normative data on forearm position sense. Front Hum Neurosci 10, 436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houdayer E, Labyt E, Cassim F, Bourriez JL & Derambure P (2006). Relationship between event‐related beta synchronization and afferent inputs: analysis of finger movement and peripheral nerve stimulations. Clin Neurophysiol 117, 628–636. [DOI] [PubMed] [Google Scholar]
- Iwamura Y & Tanaka M (1978). Postcentral neurons in hand region of area 2: their possible role in the form discrimination of tactile objects. Brain Res 150, 662–666. [DOI] [PubMed] [Google Scholar]
- Jones SJ, Halonen JP & Shawkat F (1989). Centrifugal and centripetal mechanisms involved in the ‘gating’ of cortical SEPs during movement. Electroencephalogr Clin Neurophysiol 74, 36–45. [DOI] [PubMed] [Google Scholar]
- Jung P, Baumgartner U, Magerl W & Treede RD (2008). Hemispheric asymmetry of hand representation in human primary somatosensory cortex and handedness. Clin Neurophysiol 119, 2579–2586. [DOI] [PubMed] [Google Scholar]
- Kawamura T, Nakasato N, Seki K, Kanno A, Fujita S, Fujiwara S & Yoshimoto T (1996). Neuromagnetic evidence of pre‐ and post‐central cortical sources of somatosensory evoked responses. Electroencephalogr Clin Neurophysiol 100, 44–50. [DOI] [PubMed] [Google Scholar]
- Kristeva‐Feige R, Rossi S, Pizzella V, Lopez L, Erne S, Edrich J & Rossini PM (1996). Neuromagnetic study of movement‐related somatosensory gating. Electroencephalogr Clin Neurophysiol Suppl 46, 337–342. [PubMed] [Google Scholar]
- Kurz MJ, Wiesman AI, Coolidge NM & Wilson TW (2017). Children with cerebral palsy hyper‐gate somatosensory stimulations of the foot. Cereb Cortex 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemon RN (1979). Short‐latency peripheral inputs to the motor cortex in conscious monkeys. Brain Res 161, 150–155. [DOI] [PubMed] [Google Scholar]
- Lemon RN & van der Burg J (1979). Short‐latency peripheral inputs to thalamic neurones projecting to the motor cortex in the monkey. Exp Brain Res 36, 445–462. [DOI] [PubMed] [Google Scholar]
- Macerollo A, Chen JC, Parees I, Sadnicka A, Kassavetis P, Bhatia KP, Kilner JM, Rothwell JC & Edwards MJ (2016). Abnormal movement‐related suppression of sensory evoked potentials in upper limb dystonia. Eur J Neurol 23, 562–568. [DOI] [PubMed] [Google Scholar]
- Malis LI, Pribram KH & Kruger L (1953). Action potentials in motor cortex evoked by peripheral nerve stimulation. J Neurophysiol 16, 161–167. [DOI] [PubMed] [Google Scholar]
- Marangon M, Kubiak A & Kroliczak G (2015). Haptically guided grasping. fMRI shows right‐hemisphere parietal stimulus encoding, and bilateral dorso‐ventral parietal gradients of object‐ and action‐related processing during grasp execution. Front Hum Neurosci 9, 691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maris E & Oostenveld R (2007). Nonparametric statistical testing of EEG‐ and MEG‐data. J Neurosci Methods 164, 177–190. [DOI] [PubMed] [Google Scholar]
- Mauguiere F & Desmedt JE (1991). Focal capsular vascular lesions can selectively deafferent the prerolandic or the parietal cortex: somatosensory evoked potentials evidence. Ann Neurol 30, 71–75. [DOI] [PubMed] [Google Scholar]
- Mauguiere F, Desmedt JE & Courjon J (1983). Astereognosis and dissociated loss of frontal or parietal components of somatosensory evoked potentials in hemispheric lesions. Detailed correlations with clinical signs and computerized tomographic scanning. Brain 106, 271–311. [DOI] [PubMed] [Google Scholar]
- Milne RJ, Aniss AM, Kay NE & Gandevia SC (1988). Reduction in perceived intensity of cutaneous stimuli during movement: a quantitative study. Exp Brain Res 70, 569–576. [DOI] [PubMed] [Google Scholar]
- Neuper C, Wortz M & Pfurtscheller G (2006). ERD/ERS patterns reflecting sensorimotor activation and deactivation. Prog Brain Res 159, 211–222. [DOI] [PubMed] [Google Scholar]
- Nikouline VV, Linkenkaer‐Hansen K, Wikstrom H, Kesaniemi M, Antonova EV, Ilmoniemi RJ & Huttunen J (2000). Dynamics of mu‐rhythm suppression caused by median nerve stimulation: a magnetoencephalographic study in human subjects. Neurosci Lett 294, 163–166. [DOI] [PubMed] [Google Scholar]
- Papakostopoulos D, Cooper R & Crow HJ (1975). Inhibition of cortical evoked potentials and sensation by self‐initiated movement in man. Nature 258, 321–324. [DOI] [PubMed] [Google Scholar]
- Reed CL, Klatzky RL & Halgren E (2005). What vs. where in touch: an fMRI study. Neuroimage 25, 718–726. [DOI] [PubMed] [Google Scholar]
- Seki K & Fetz EE (2012). Gating of sensory input at spinal and cortical levels during preparation and execution of voluntary movement. J Neurosci 32, 890–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seki K, Perlmutter SI & Fetz EE (2003). Sensory input to primate spinal cord is presynaptically inhibited during voluntary movement. Nat Neurosci 6, 1309–1316. [DOI] [PubMed] [Google Scholar]
- Spooner RK, Wiesman AI, Proskovec AL, Heinrichs‐Graham E & Wilson TW (2018). Rhythmic spontaneous activity mediates the age‐related decline in somatosensory function. Cereb Cortex, 10.1093/cercor/bhx349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svoboda J, Sovka P & Stancak A (2004). Effects of muscle contraction on somatosensory event‐related EEG power and coherence changes. Neurophysiol Clin 34, 245–256. [DOI] [PubMed] [Google Scholar]
- Taulu S & Simola J (2006). Spatiotemporal signal space separation method for rejecting nearby interference in MEG measurements. Phys Med Biol 51, 1759–1768. [DOI] [PubMed] [Google Scholar]
- Tucker KJ, Tuncer M & Turker KS (2005). A review of the H‐reflex and M‐wave in the human triceps surae. Hum Mov Sci 24, 667–688. [DOI] [PubMed] [Google Scholar]
- Uusitalo MA & Ilmoniemi RJ (1997). Signal‐space projection method for separating MEG or EEG into components. Med Biol Eng Comput 35, 135–140. [DOI] [PubMed] [Google Scholar]
- Van Veen BD, van Drongelen W, Yuchtman M & Suzuki A (1997). Localization of brain electrical activity via linearly constrained minimum variance spatial filtering. IEEE Trans Biomed Eng 44, 867–880. [DOI] [PubMed] [Google Scholar]
- Wiesman AI, Heinrichs‐Graham E, Coolidge NM, Gehringer JE, Kurz MJ & Wilson TW (2017). Oscillatory dynamics and functional connectivity during gating of primary somatosensory responses. J Physiol 595, 1365–1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zehr EP (2002). Considerations for use of the Hoffmann reflex in exercise studies. Eur J Appl Physiol 86, 455–468. [DOI] [PubMed] [Google Scholar]


