Abstract
Key points
Chronic (psychosocial) stress changes gut microbiota composition, as well as inducing behavioural and physiological deficits.
The microbial metabolites short‐chain fatty acids (SCFAs) have been implicated in gastrointestinal functional, (neuro)immune regulation and host metabolism, but their role in stress‐induced behavioural and physiological alterations is poorly understood.
Administration of SCFAs to mice undergoing psychosocial stress alleviates enduring alterations in anhedonia and heightened stress‐responsiveness, as well as stress‐induced increases in intestinal permeability.
In contrast, chronic stress‐induced alterations in body weight gain, faecal SCFAs and the gene expression of the SCFA receptors FFAR2 and FFAR3 remained unaffected by SCFA supplementation.
These results present novel insights into mechanisms underpinning the influence of the gut microbiota on brain homeostasis, behaviour and host metabolism, informing the development of microbiota‐targeted therapies for stress‐related disorders.
Abstract
There is a growing recognition of the involvement of the gastrointestinal microbiota in the regulation of physiology and behaviour. Microbiota‐derived metabolites play a central role in the communication between microbes and their host, with short‐chain fatty acids (SCFAs) being perhaps the most studied. SCFAs are primarily derived from fermentation of dietary fibres and play a pivotal role in host gut, metabolic and immune function. All these factors have previously been demonstrated to be adversely affected by stress. Therefore, we sought to assess whether SCFA supplementation could counteract the enduring effects of chronic psychosocial stress. C57BL/6J male mice received oral supplementation of a mixture of the three principle SCFAs (acetate, propionate and butyrate). One week later, mice underwent 3 weeks of repeated psychosocial stress, followed by a comprehensive behavioural analysis. Finally, plasma corticosterone, faecal SCFAs and caecal microbiota composition were assessed. SCFA treatment alleviated psychosocial stress‐induced alterations in reward‐seeking behaviour, and increased responsiveness to an acute stressor and in vivo intestinal permeability. In addition, SCFAs exhibited behavioural test‐specific antidepressant and anxiolytic effects, which were not present when mice had also undergone psychosocial stress. Stress‐induced increases in body weight gain, faecal SCFAs and the colonic gene expression of the SCFA receptors free fatty acid receptors 2 and 3 remained unaffected by SCFA supplementation. Moreover, there were no collateral effects on caecal microbiota composition. Taken together, these data show that SCFA supplementation alleviates selective and enduring alterations induced by repeated psychosocial stress and these data may inform future research into microbiota‐targeted therapies for stress‐related disorders.
Keywords: Gut Microbiota, Behaviour, Chronic Stress
Key points
Chronic (psychosocial) stress changes gut microbiota composition, as well as inducing behavioural and physiological deficits.
The microbial metabolites short‐chain fatty acids (SCFAs) have been implicated in gastrointestinal functional, (neuro)immune regulation and host metabolism, but their role in stress‐induced behavioural and physiological alterations is poorly understood.
Administration of SCFAs to mice undergoing psychosocial stress alleviates enduring alterations in anhedonia and heightened stress‐responsiveness, as well as stress‐induced increases in intestinal permeability.
In contrast, chronic stress‐induced alterations in body weight gain, faecal SCFAs and the gene expression of the SCFA receptors FFAR2 and FFAR3 remained unaffected by SCFA supplementation.
These results present novel insights into mechanisms underpinning the influence of the gut microbiota on brain homeostasis, behaviour and host metabolism, informing the development of microbiota‐targeted therapies for stress‐related disorders.
Introduction
Chronic stress is a considerable health concern for society, associated with various disease states including an increased risk of neuropsychiatric disorders like depression (Yang et al. 2015; Ramirez et al. 2017) and functional gastrointestinal disorders such as irritable bowel syndrome (Stam et al. 1997; Klooker et al. 2009; Moloney et al. 2016). Stress‐related disorders have complex and multifactorial aetiologies and trajectories. Recent years has seen a growing interest in the potential contribution of microbiota–gut–brain axis signalling in both the aetiology and treatment of stress‐related disorders (Foster et al. 2017; Rea et al. 2017). For instance, preclinical studies have revealed that chronic stress results in an altered gut microbial composition (Bailey et al. 2011; Bharwani et al. 2017; Burokas et al. 2017; Galley et al. 2017; Marin et al. 2017; Szyszkowicz et al. 2017; Dunphy‐Doherty et al. 2018). In addition, various microbiota‐targeted interventions are able to ameliorate chronic stress‐induced deficits in mice, which include the administration of live bacterial strains (probiotics) (Bharwani et al. 2017; Marin et al. 2017), as well as the supplementation of host‐indigestible dietary fibres that are fermented by the gut microbiota (prebiotics) (Burokas et al. 2017). Importantly, predictive analysis of functional microbiota pathways reveals that chronic social defeat stress resulted in a lower representation of pathways involved in fatty acid metabolism and biosynthesis, particularly involved in the metabolism of propanoate– and butanoate–conjugate bases of the short‐chain fatty acids (SCFAs) propionate and butyrate (Bharwani et al. 2016).
The primary source of endogenous SCFAs is the gut microbial fermentation of host‐indigestible dietary fibres, with the principal SCFAs being acetate, propionate and butyrate (Koh et al. 2016). These SCFAs have been shown to affect the host through multiple mechanisms including the regulation of histone acetylation and methylation (Krautkramer et al. 2016; Stilling et al. 2016), G‐protein coupled receptors (GPCRs) (Milligan et al. 2017), facilitating the secretion of various hormones (e.g. GLP‐1 and PYY) and neurochemicals (e.g. serotonin) (Chambers et al. 2015; Reigstad et al. 2015), and the induction of vagus nerve signalling (De Vadder et al. 2014; Li et al. 2017). SCFAs are also used as a mitochondrial energy source resulting in an extremely rapid uptake of these compounds in both humans and rodents (Egorin et al. 1999; Schonfeld & Wojtczak, 2016; Boets et al. 2017). It is therefore essential to emphasise that physiologically relevant levels of acetate and propionate have been reported to directly influence the brain (Perry et al. 2016; Hoyles et al. 2018). Considering the plethora of ways in which they can affect the host, it's not surprising that SCFAs have been implicated in numerous physiological functions such as gastrointestinal functionality, host metabolism, blood‐pressure regulation, circadian rhythm and (neuro)immune function (Koh et al. 2016; Erny et al. 2017; Pluznick, 2017; Tahara et al. 2018). Gut microbial‐derived SCFAs are also increasingly implicated in emotional processing and behaviour, as butyrate has been shown to ameliorate cognitive impairments in a model of vascular dementia and in mid‐adult high‐fat‐diet‐induced obese mice (Liu et al. 2015; Arnoldussen et al. 2017), and propionate is able to reduce anticipatory reward responses to high‐energy foods in the human striatum (Byrne et al. 2016). Finally, correlations between SCFA levels and depressive‐, anxiety‐ and stress‐related behaviours have been reported after prebiotic administration (Burokas et al. 2017). Considering that these behavioural parameters are negatively affected by chronic (psychosocial) stress (Yang et al. 2015; Ramirez et al. 2017), we hypothesised that SCFA supplementation could ameliorate these psychosocial stress‐induced behavioural deficits.
As such, we investigated whether oral SCFA supplementation could ameliorate psychosocial stress‐induced behavioural deficits in mice, specifically in sociability, anxiety‐ and depressive‐like behaviours, cognition and stress‐responsiveness. In parallel, we assessed changes in the expression of associated genes in discrete brains regions. Finally, we investigated the role of gut permeability and gut microbiota composition in SCFA‐induced amelioration of psychosocial stress‐induced behavioural deficits.
Methods
Ethical approval
All experiments were approved by the Animal Experimentation Ethics Committee of University College Cork and conducted in accordance with the European Directive 86/609/EEC and the Recommendation 2007/526/65/EC. The animal experiments conducted also comply with the policy and regulations of The Journal of Physiology (Grundy, 2015). All efforts were made to reduce the number of animals used and minimise animal suffering. Samples sizes were based on previous studies (Burokas et al. 2017).
Animals
This study used male C57Bl/6J mice (n = 40; 8 weeks of age on arrival; Harlan, UK; n = 10 per group). Animals were habituated for 4 weeks after which single housing began. Food and water were provided ad libitum throughout the study. The holding room was under a 12‐hour light/dark cycle (lights on at 07.00 h), with a temperature of 21 ± 1°C and humidity of 55 ± 10%. Body weight was monitored twice per week starting at the onset of psychosocial stress.
Experimental timeline and behavioural testing
Oral SCFA treatment commenced 1 week after the single housing, both of which continued throughout the entire study (Fig. 1). After 1 week of SCFA treatment, animals underwent 3 weeks of psychosocial stress followed by a behavioural assessment. Behavioural tests were performed in order of least stressful to most stressful to lessen the likelihood of prior behavioural tests influencing subsequent ones. In addition, there was a minimum of 36 h in between each test. Testing was performed in the following order: (1) social interaction test; (2) sucrose preference; (3) three‐chamber social interaction; (4) female urine sniffing; (5) open field; (6) novel object recognition; (7) marble burying; (8) elevated plus maze; (9) stress‐induced hyperthermia; (10) tail suspension; (11) fear conditioning; (12) hot plate; (13) in vivo FITC‐dextran intestinal permeability; (14) forced swim. These tests were followed by a 1 week washout period, after which mice were killed by decapitation.
Figure 1. Experimental design of the short‐chain fatty acid treatment, psychosocial stress and behavioural assessment.
After 1 week of short‐chain fatty acid (SCFA) treatment, animals underwent a 3‐week psychosocial stress protocol containing intermittent social defeat and overcrowding procedures. Mice subsequently underwent behavioural assessment, starting with the least stressful test to the most stressful test. The order of the behavioural tests was as follows. Week 4: social interaction test (SIT) and sucrose preference test (SPT); week 5: three‐chamber sociability test (3‐CT) and female urine sniffing test (FUST); week 6: open field (OF), novel object recognition test (NOR) and marble burying test (MB); week 7: elevated plus maze (EPM), stress‐induced hyperthermia test (SIH) and tail‐suspension test (TST); week 8: fear conditioning (FC) and hot plate test (HPT); week 9: FITC‐dextran intestinal permeability test (FITC); week 10: forced swim test (FST); week 11: sacrifice. SCFA treatment was for the entire duration. [Color figure can be viewed at http://wileyonlinelibrary.com]
Oral short‐chain fatty acid administration
A mix of the three principal short‐chain fatty acids – sodium acetate (67.5 mm, Sigma‐Aldrich, S7545), sodium propionate (25 mm, Sigma‐Aldrich, P1880) and sodium butyrate (40 mm, Sigma‐Aldrich, 303410) – was administered via drinking water (pH 7.6) as described previously (Smith et al. 2013; Erny et al. 2015). Drinking water was refreshed two times per week.
Psychosocial stress – social defeat and overcrowding procedures
The social defeat and overcrowding procedure schedule was performed for 3 weeks as previously described (see Fig. 1 Finger et al. 2011; Burokas et al. 2017). For the social defeat procedure, age‐matched male CD1 mice (n = 30; Harlan, UK) were tested for aggression on three individual days. Briefly, CD1 mice were exposed to another CD1 intruder mouse in their home cage until the first attack commenced. The 25 CD1 mice with the shortest attack latencies were used for the social defeat procedure, while the rest were used in the social interaction test. Test mice undergoing psychosocial stress were pseudo‐randomly assigned to a different CD1 mouse on each day, counterbalanced by the SCFA group. During the social defeat procedure, stress mice were gently placed in the home cage of the CD1 aggressor and allowed to interact until the first attack of the CD1 mouse took place, which was followed by a defeat posture of the stress mouse. Mice were then separated for 2 h by a perforated Plexiglass divider allowing for auditory, olfactory and visual, but not physical contact. The divider was subsequently removed, after which another social defeat took place, and test mice were placed in their original home cage. For the overcrowding procedure, stress mice of one group (n = 10) were housed in a standard holding cage for 24 h. During this period, control mice were left undisturbed.
Social interaction test
The social interaction test was used to assess avoidance of the CD1 aggressors 1 day following the final psychosocial stressor and was conducted as previously described (Burokas et al. 2017). The test was performed in an open arena (40 × 32 × 24 cm, L × W × H) containing an empty wire mesh cage (9.5 × 7.5 × 7.0 cm) opposed to one side. The test consisted of two 2.5 min trials, with a 1 min intertrial interval, wherein mice were allowed to explore the arena freely. In the first trial (‘no target’), the wire mesh cage was left empty, whereas an unfamiliar CD1 aggressor was placed in the wire mesh cage in the second trial. Both mice were returned to their homecage after the test, and the arena was cleaned with 70% ethanol. To reduce potential anxiogenic factors, all mice were habituated 45 min before testing, and testing was conducted in red light (5 lux). All trials were videotaped using a ceiling camera and analysed for time spent in the interaction zone using Ethovision version 13 software (Noldus). Social avoidance was calculated by dividing the time spent in the interaction zone in trial 2 by the time spent in the interaction zone in trial 1.
Sucrose preference test
Mice were assessed for hedonic‐like behaviour using the sucrose preference test, which was conducted as previously described (Kelly et al. 2016). Mice were presented with two drinking water bottles, one containing regular drinking water and the other one a sucrose solution (2% w/v), for 48 h. The side to which each drinking water bottle was fixed was randomised and alternated every 12 h. Mice were kept in their home cages throughout the test, and the same type of drinking water bottles were used in subsequent tests. Sucrose preference was calculated using the formula: (total sucrose intake/total fluid intake) × 100%.
Three‐chamber sociability test
The three‐chamber sociability test was used to assess social preference and recognition and was conducted as previously described (Desbonnet et al. 2014). The testing apparatus was a three‐chambered, rectangular box. The dividing walls between each chamber (20 × 40 × 22 cm, L × W × H) had small circular openings (5 cm diameter), allowing for access to all chambers. The two outer chambers contained wire cup‐like cages (10 cm bottom diameter, 13 cm H), allowing for auditory, olfactory and visual, but not physical contact. The test consisted of three 10 min phases: (1) habituation, (2) social preference, (3) social recognition. In the first phase (habituation), mice were allowed to explore the entire box with both wire cup‐like cages left empty to allow for habituation to the novel environment. In the second phase (social preference), one wire cup‐like cage contained a novel, age‐matched, conspecific, male mouse, whereas the other cage contained an object (rubber duckie). In the third phase (social recognition), the mouse of the previous trial was left in the wire cup‐like cage (familiar mouse), while the object was replaced with a conspecific mouse (novel mouse). The test mouse was held in the middle chamber while the conspecific mouse and object were placed in the cup wire‐like cages. The location of the conspecific mice and object were systemically altered between test mice. The three‐chamber test apparatus and wire cup‐like cages were cleaned with 10% ethanol after each test mouse and left to dry for a few minutes. To reduce potential anxiogenic factors, all mice were habituated to the testing room 40 min before the test, the floor of the testing arena was covered with sawdust and testing was performed under dim light (60 lux). All experiments were videotaped using a ceiling camera and were scored blinded for the time interacted with the wire cup‐like cages.
Female urine sniffing test
Mice were assessed for hedonic and reward‐seeking behaviour in the female urine sniffing test, which was performed as previously described (Finger et al. 2011). Before this experiment, vaginal smears from age‐matched female C57Bl/6 mice (n = 12; Harlan, UK) were taken and assessed for the oestrous cycle. Urine from female mice in the oestrous stage was collected and pooled. Male mice were habituated 45 min before the start of the test to the test room, with a cotton bulb attached to the lid of their housing cage. The test mice were subsequently introduced to a new cotton bulb containing 30 μl sterile water. After a 45 min intertrial interval, mice were introduced to a new cotton bulb containing 30 μl urine from a female mouse in oestrous for 3 min. The experiment was conducted in red light (5 lux). All tests were videotaped using a ceiling camera for 3 min and interaction time with the cotton bulbs was scored blinded.
Open field test
Mice were assessed for their locomotor activity and response to a novel environment in the open field test, which was conducted as previously described (Burokas et al. 2017). Mice were placed in an open arena (40 × 32 × 24 cm, L × W × H) and were allowed to explore the arena for 10 min. Animals were habituated to the room 1 h before the test. Testing was performed under dim light (60 lux). Experiments were videotaped using a ceiling camera and were analysed for time spend in the virtual centre zone (defined as 50% away from the edges) and total distance travelled using Ethovision version 13 software (Noldus).
Novel object recognition test
The day after the open field test, mice were allowed to explore two identical objects (acquisition phase), after which one object was replaced on the third day (retention phase). Like the open field test, testing was performed under dim light (60 lux) and lasted 10 min. Mice were habituated to the room 1 h before the test. Experiments were videotaped using a ceiling camera and time of directed contact of the mouse with the objects on day three (retention phase) was scored blinded by an independent researcher. Object preference was calculated using the following formula: (novel object exploration time – familiar object exploration time)/total exploration time × 100%.
Marble burying test
Mice were tested for repetitive and anxious behaviour with the marble burying test, which was conducted as previously described (Burokas et al. 2017). Animals were individually placed in a novel Plexiglas cage (35 × 28 × 18.5 cm, L × W × H), which was filled with sawdust (5 cm) and had 20 marbles placed on top (5 × 4 rows, equally spread between the walls and individual marbles). After mice had spent 30 min in the cage, the number of buried marbles was counted by two researchers and averaged, which was defined as 2/3 of the marble not anymore being visible.
Elevated plus maze
The elevated plus maze test was used to assess anxiety‐like behaviour, which was conducted as previously described (Burokas et al. 2017). The elevated plus maze apparatus consisted of a grey cross‐shaped maze with two open arms and two closed arms (50 × 5 × 15 cm walls or 1 cm no wall), elevated 1 m from the floor. Mice were allowed to explore the maze for 5 min. Experiments were conducted in red light (5 lux). Mice were habituated to the room 1 h before the test. Experiments were videotaped using a ceiling camera and videos were scored blinded for time spent in the open arms, which was defined as all paws in the open arm.
Stress‐induced hyperthermia test
The stress‐induced hyperthermia test was used to assess stress‐responsiveness, which was conducted as previously described (Burokas et al. 2017). Body temperature was taken at baseline (T1) and 15 min later (T2) by gently inserting a sterile, Vaseline‐covered thermometer 2.0 cm into the rectum. The temperature was noted to the nearest 0.1°C after it stabilised (∼10 s). Mice were restrained by scruffing during this procedure which was the stressor. Animals were habituated to the testing room 30 min before the test. The difference between T1 and T2 reflected the stress‐induced hyperthermia.
Tail‐suspension test
The tail‐suspension test was used to assess depressive‐like behaviour, which was conducted as previously described (Burokas et al. 2017). Mice were hung by their tail using adhesive tape (2 cm from the tip of the tail) to a 30 cm‐elevated grid bar for 6 min. Experiments were videotaped using a numeric tripod‐fixed camera and videos were scored blinded by an independent researcher for the time spent immobile.
Fear conditioning
Mice were assessed for fear‐associated contextual and cued learning in the fear conditioning test, which was conducted as previously described (Burokas et al. 2017). This test consisted of three phases: (1) acquisition, (2) retention, (3) extinction, each of which was carried out on successive days with a 24 h interval. The acquisition phase consisted of a 3 min baseline recording, followed by 6 tone‐conditioned stimuli (70 dB, 20 s) and shock pairings (0.6 mA, 2 s) with a 1 min interval. During the retention and acquisition phases, the same experimental procedure was carried out in the absence of the shocks. The fear conditioning apparatus was cleaned with 70% ethanol and left to dry for a few minutes between runs.
Hot plate test
The hot plate test was used to assess somatic pain response, which was conducted as previously described (Tramullas et al. 2012). Animals were gently placed on a hot plate (Stoelting) set at 52°C. After mice licked their front paw, they were immediately removed from the hot plate and returned to their homecage. Animals were habituated 60 min to the room before the test. Experiments were videotaped using a numeric tripod‐fixed camera and videos were scored blinded for the time it took the mice to lick either of their front paws.
In vivo FITC‐dextran intestinal permeability test
Gastrointestinal permeability was assessed using the FITC‐dextran intestinal permeability test, which was conducted as previously described (Scott et al. 2017). Mice were fastened overnight and received an oral gavage of FITC‐dextran dissolved in sterile PBS (Sigma, FD4) at 09.00 h. The administered dosage was 600 mg kg−1 body weight, and the approximate volume administered per mouse was 0.21 ml. Two hours after the FITC‐dextran was administered, approximately 90 μl of whole blood was taken by tail‐tip. For this procedure, the end of the tail was held with two fingers without restraining the mouse. A 2–4 mm long diagonal incision was made at the end of the tail using a single edge razor blade, and blood was collected in an EDTA‐containing capillary. Blood was then transferred to a tube, centrifuged for 15 min at 3500 g at 4°C, and plasma was collected and stored at −80°C for later analysis. Plasma FITC‐dextran concentrations were assessed with a multi‐mode plate reader (Synergy HT, BioTek Instruments) with an excitation of 490 nm and emission of 520 nm.
Forced swim test
The forced swim test was used to assess depressive‐like behaviour, which was conducted as previously described (Cryan & Mombereau, 2004). Mice were individually placed in a transparent glass cylinder (24 × 21 cm diameter) containing 15‐cm‐depth water (23–25°C) for 6 min. Mice were gently dried after the test, and water was discarded and renewed after each animal. Experiments were videotaped using a ceiling camera and videos were scored blinded by an independent researcher for immobility time in the last 4 min of the test.
Plasma sampling for corticosterone
Plasma from each animal was sampled by tail‐tip 5 min before the forced swim test and repeatedly after the test in 30 min intervals up to 120 min. For the tail‐tip, the end of the tail was held with two fingers without restraining the mouse. A 2–4 mm long diagonal incision was made at the end of the tail using a single edge razor blade. Approximately 40 μl of whole blood was taken per time point using an EDTA‐containing capillary, deposited in an Eppendorf and centrifuged for 15 min at 10,000 g at 4°C. Plasma was collected and stored at −80°C for later analysis.
Tissue collection
Animals were killed by decapitation in a random fashion regarding testing groups between 09.00 h and 15.00 h. Trunk blood was collected in EDTA‐containing tubes and centrifuged for 15 min at 10,000 g at 4°C. Plasma was collected and stored at −80°C for later analysis. The caecum, spleen, adrenals and thymus were removed, weighed, snap‐frozen on dry ice and stored at −80°C. Proximal colon tissue was either immediately frozen on dry ice and stored at −80°C for later noradrenalin quantification by HPLC, or stored in RNAlater (Sigma, R0901) for 48 h at 4°C, after which the RNAlater was removed, and tissues were stored at −80°C for later gene expression analysis. The brain was excised and gross‐dissected as described previously (Schellekens et al. 2012). Brain tissues were then stored in RNAlater and processed in the same manner as colon tissues.
Plasma corticosterone and lipopolysaccharide‐binding protein quantification
Corticosterone quantification of plasma samples (15 μl) obtained in the forced swim test was performed using a corticosterone ELISA (Enzo Life Sciences, ADI‐901‐097), and was carried out according to the manufacturer's guidelines. Lipopolysaccharide‐binding protein (LBP) was quantified in plasma (10 μl) obtained when animals were killed using an ELISA (Enzo Life Sciences, ALX‐850‐305), and was also performed according to the manufacturers’ instructions. A multi‐mode plate reader (Synergy HT, BioTek Instruments) was used to measure light absorbance in both assays.
Gene expression analysis
Total mRNA was isolated using the mirVana miRNA Isolation Kit (ThermoFisher Scientific, AM1560), after which samples were DNase treated (ThermoFisher Scientific, AM1907). RNA was determined using the NanoDrop spectrophotometer (ThermoFisher Scientific) and synthesised into cDNA using the High Capacity Reverse Transcription cDNA kit (ThermoFisher Scientific, 4368814). Gene expression analysis was finally carried out by quantitative real‐time polymerase chain reaction (qRT‐PCR) using various probes designed by Applied Biosystems and Integrated DNA Technologies (Table 1). The TaqMan Universal Master Mix II, no UNG (ThermoFisher Scientific, 4440040) was used. Singleplex amplification reactions were performed in duplicates in 384‐well plates on the LightCycler 480 system. Data were normalised using Actb as endogenous control and transformed using the 2−ΔΔCT method. All procedures were carried out according to the manufacturers’ instructions.
Table 1.
qRT‐PCR probes used in this study
| Gene symbol | Common gene name | Probe ID | Supplier |
|---|---|---|---|
| Actb | β‐Actin | 4352341E | Applied Biosystems |
| BDNF IV | Brain‐derived neurotrophic factor, Exon IV | Mm.pt.58.13575048 | Integrated DNA Technologies |
| Cldn1 | Claudin‐1 | Mm.PT.58.6163880 | Integrated DNA Technologies |
| CRF | Corticotrophin‐releasing factor | Mm01293920_s1 | Applied Biosystems |
| CRFR1 | Corticotrophin‐releasing factor receptor 1 | Mm.PT.58.13604366 | Integrated DNA Technologies |
| CRFR2 | Corticotrophin‐releasing factor receptor 2 | Mm.PT.58.12499462 | Integrated DNA Technologies |
| DRD1a | Dopamine receptor D1a | Mm01353211_m1 | Applied Biosystems |
| DRD2 | Dopamine receptor D2 | Mm00438545_m1 | Applied Biosystems |
| FFAR2/GPR43 | Free fatty acid receptor 2 | Mm.02620654_s1 | Applied Biosystems |
| FFAR3/GPR41 | Free fatty acid receptor 3 | Mm.02621638_s1 | Applied Biosystems |
| GR/Nr3c1 | Glucocorticoid receptor | Mm.PT.58.42952901 | Integrated DNA Technologies |
| Tjp1 | Tight junction protein ZO‐1 | Mm.PT.58.12952721 | Integrated DNA Technologies |
| MR/Nr3c2 | Mineralocorticoid receptor | Mm.PT.58.30752774 | Integrated DNA Technologies |
| Muc2 | Mucin 2 | Mm.PT.58.29496069 | Integrated DNA Technologies |
| Ocln | Occludin | Mm.PT.58.30118962 | Integrated DNA Technologies |
| TrkB/NTRK2 | Tropomyosin receptor kinase B | Mm.PT.58.11070732 | Integrated DNA Technologies |
DNA extraction from caecal contents and Illumina MiSeq sequencing
Total DNA was extracted from thawed caecal contents using the QIAamp Fast DNA Stool Mini Kit (Qiagen, 51604), coupled with an initial bead‐beating step (Fouhy et al. 2015). Briefly, a 0.2 g aliquot of caecal sample was added to 0.4 g of zirconia beads together with 1 ml InhibitEx buffer supplied in the kit. Samples were subsequently homogenised using the Biospec Minibeadbeater for 3 min. Removal of RNA and protein, as well as purification of the DNA, was completed according to the manufacturer's instructions. Finally, DNA was stored at ‐20°C until further analysis.
The 16S metagenomic sequencing library protocol (Illumina) was used to amplify the V3‐V4 variable region of the 16S rRNA gene from the DNA extracts. Briefly, DNA was amplified with V3‐V4 region‐specific to the 16S rRNA gene which also incorporates the Illumina overhang adaptor (forward primer 5′ TCGTCGGCAGCGTCAGATGTGTATAAGAGACAGCCTACGGGNGGCWGCAG; reverse primer 5′ GTCTCGTGGGCTCGGAGATGTGTATAAGAGACAGGACTACHVGGGTATCTAATCC). Each PCR reaction contained DNA template, 5 μl forward primer (1 μm), 5 μl reverse primer (1 μm), 12.5 μl 2× Kapa HiFi Hotstart ready mix (Anachem) and PCR grade water to a final volume of 25 μl. PCR amplification was carried out as follows: initial denaturation at 95°C for 3 min; 25 cycles of 95°C for 30 s, 55°C for 30 s, 72°C for 30 s; 72°C for 5 min and held at 4°C. PCR products were then visualised using gel electrophoresis (1× TAE buffer, 1.5% agarose, 100 V) and cleaned using AMPure XP magnetic bead‐based purification (Labplan). A second PCR reaction was performed on the purified DNA (5 μl) to index each of the samples, allowing samples to be pooled for sequencing on the one flow cell and finally demultiplexed for analysis. Two indexing primers (Illumina Nextera XT) were used per sample. Each PCR reaction contained 5 μl index 1 primer (N7xx), 5 μl index 2 primer (S5xx), 25 μl 2× Kapa HiFi Hot Start Ready mix and 10 μl PCR grade water. PCR was mostly carried out as described above, although only eight amplification cycles were completed instead of 25. PCR products were visualised using gel electrophoresis and subsequently cleaned (as described above). Samples were quantified using the Qubit (Bio‐Sciences) along with the high sensitivity DNA quantification assay kit (Bio‐Sciences), and samples were pooled in an equimolar fashion. The pooled samples were run on the Agilent Bioanalyser for quality analysis. The sample pool was prepared following Illumina guidelines. Samples were sequenced on the MiSeq sequencing platform (Clinical Microbiomics, Denmark), using a 2 × 250 cycle kit, following standard Illumina sequencing protocols.
Bioinformatic sequence analysis was performed as described previously (Murphy et al. 2017). Briefly, paired‐end sequences were assembled using FLASH (Magoc & Salzberg, 2011), and analysed using QIIME v1.8.0 (Quantitative Insights Into Microbial Ecology) (Caporaso et al. 2010). Sequences were then quality checked, and the remaining sequences were clustered into operational taxonomic units using USEARCH (v7‐64bit) (Edgar, 2010). Taxonomic ranks were assigned with a BLAST search against the SILVA SSURef database release 116 (Quast et al. 2013). Alpha and beta diversities were generated in QIIME and calculated based on weighted Unifrac distance matrices (Lozupone et al. 2011). Principal coordinate analysis (PCoA) plots were made using EMPeror v0.9.3‐dev (Vazquez‐Baeza et al. 2013). The relative abundance of bacterial taxa was expressed as a percentage of identified sequences.
Faecal SCFA quantification
Faecal pellets were collected 6 weeks post psychosocial stress. Faecal water was prepared by homogenising the faecal samples (approx. 200 mg) with 800 μl of 0.003 m HCl and 3 zirconia beads for 10 min using a vortex mixer. The use of HCl results in the acidification of samples ensuring protonation of SCFAs before analysis, which prevents the occurrence of split peaks in chromatograms (Zhao et al. 2006). Samples were centrifuged at 15,000 g for 5 min, after which supernatants were syringe filtered with 0.22 μm filters (Corning) and duplicate aliquots of 270 μL of filtrate were mixed with 30 μL of 10 mm internal standard. Samples were subsequently homogenised briefly and were centrifuged at 15,000 g for 3 min. The supernatant was transferred to 250 μL inserts (Agilent) placed in amber glass 2 mL GC vials (Agilent) sealed with silicone/PTFE screw caps (Agilent).
Samples were analysed by gas chromatography flame ionisation detection (GC‐FID) using a Varian 3800 GC system, fitted with a Zebron ZB‐FFAP column (30 mL × 0.3 2 mm ID × 0.25 μm d f; Phenomenex) and a flame ionisation detector with a CP‐8400 auto‐sampler. Helium was employed as the carrier gas at an initial flow rate of 1.3 mL min−1. The initial oven temperature was 50°C and was maintained for 30 s, then raised to 140°C at 10°C min−1 and held for 30 s. The temperature was finally increased to 240°C at 20°C min−1 and held for 5 min. The temperatures of the detector and the injection port were 300°C and 240°C respectively. A split‐less injection of 0.2 μL was carried out for each sample or standard using a 10 μL syringe (Agilent) installed to a CP‐8400 auto‐sampler (Varian). Peak integration was carried out using Varian Star Chromatography Workstation version 6.0 software. Vials containing 1800 μL of water were run between each sample duplicates as blanks to check for any potential carryover. Standards were included in each run to maintain the calibration.
Statistical analysis
All data were assessed for normality using the Shapiro‐Wilk test and Levene's test for equality of variances. Normally distributed data were analysed using a two‐way ANOVA, followed by Fisher's least significant difference (LSD) post hoc test. Non‐parametric datasets were analysed using the Kruskal‐Wallis test, followed by the Mann‐Whitney U test. Statistical analysis was performed using SPSS software version 24 (IBM Corp). Data are expressed as mean ± SEM. A P value <0.05 was deemed significant.
Results
Social behaviour
Three weeks of psychosocial stress induced a trend towards decreased social interaction with a CD1 mouse used in the social defeat sessions (Fig. 2 A), independently of SCFA treatment (Χ2(1) = 2.875, P = 0.090). Mice were later assessed for their preference between a conspecific mouse or an object in the three‐chamber sociability test. All groups exhibited a significant preference towards the conspecific mouse compared to the object (Fig. 2 B). Changing the object to a novel conspecific mouse to test for social recognition, resulted in a significant preference towards interacting with the novel conspecific mouse for all test groups (Fig. 2 C).
Figure 2. Chronic stress induces aggressor‐specific social avoidance.
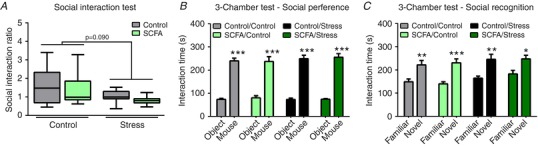
Social interaction with a CD1 mouse used in the social defeat procedure was assessed in the social interaction test 1 day after the last stressor (A). Mice were additionally assessed for social preference (B) and recognition (C) with a conspecific mouse in the three‐chamber sociability test 1 week post stress. The social interaction test was non‐parametrically distributed and analysed using the Kruskal‐Wallis test. Significant differences are depicted as: * P < 0.05; Control compared to Stress. In the three‐chamber sociability test, differences between ‘object’ versus ‘mouse’ and ‘familiar mouse’ versus ‘novel mouse’ were assessed using a Student's unpaired t test. Significant differences are depicted as: * P < 0.05, ** P < 0.01 and *** P < 0.001. All data are expressed as means ± SEM (n = 9–10). [Color figure can be viewed at http://wileyonlinelibrary.com]
Anxiety‐ and depressive‐like behaviour
Assessment of anxiety‐like behaviour using the open field test showed that SCFA supplementation increased the time spent in the centre of the open field (X2(3) = 15.622, P = 0.001) (Fig. 3 A). This effect was abolished when mice underwent psychosocial stress. These effects were independent of locomotor activity, as no differences in the total distance travelled were found in the open field test (Fig. 3 B). Nonetheless, no effects were found in other tests assessing anxiety‐like behaviours like the marble burying test (Table 2) and the elevated plus maze (Table 2). Assessment of depressive‐like behaviour using the tail‐suspension test did not reveal any significant differences (Table 2), even though mice supplemented with SCFAs exhibited a decreased immobility time in the forced swim test (F(1,38) = 5.548, P = 0.024) (Fig. 3 C). Surprisingly, no significant stress effects, independently of SCFAs, were found in any of the tests assessing anxiety‐ and depressive‐like behaviour.
Figure 3. SCFAs decrease specific anxiety‐ and depressive‐like behaviours in control but not stressed animals.

Anxiety‐like behaviour was assessed in the open field test 2 weeks post stress (A and B), whereas depressive‐like behaviour was determined using the forced swim test 6 weeks post stress (C). The open field test was non‐parametrically distributed and analysed using the Kruskal‐Wallis test, followed by the Mann‐Whitney test. The forced swim test was analysed using a two‐way ANOVA, followed by an LSD post hoc test. Significant differences are depicted as: * P < 0.05 and ** P < 0.01; Control/Control compared to SCFA/Control, $$ P < 0.01; SCFA/Control compared to SCFA/Stress. All data are expressed as means ± SEM (n = 9–10). Open circles on each graph represent individual animals. [Color figure can be viewed at http://wileyonlinelibrary.com]
Table 2.
Behavioural measurements
| Factor | Control/Control | SCFA/Control | Control/Stress | SCFA/Stress | |
|---|---|---|---|---|---|
| Marble burying test | |||||
| Marbles buried (#) | 13.7 ± 1.5 | 12.6 ± 1.8 | 11.0 ± 1.6 | 16.1 ± 0.5 | |
| Elevated plus maze | |||||
| Time spend in open arm (s) | 7.6 ± 2.8 | 5.9 ± 2.3 | 9.1 ± 2.6 | 12.6 ± 3.3 | |
| Entries into open arm (#) | 1.6 ± 0.5 | 1.6 ± 0.5 | 2.3 ± 0.4 | 2.2 ± 0.5 | |
| Tail suspension test | |||||
| Immobility time (s) | 127.0 ± 18.5 | 169.6 ± 4.0 | 144.8 ± 8.5 | 131.4 ± 12.2 | |
| Sucrose preference test | |||||
| Sucrose preference (%) | 81.5 ± 1.6 | 73.4 ± 3.8 | 86.4 ± 0.6* | 80.6 ± 2.1## | |
| Water consumption (mL) | 3.53 ± 0.14 | 4.10 ± 0.39 | 3.28 ± 0.07 | 3.90 ± 0.37 | |
| Sucrose consumption (mL) | 16.6 ± 1.6 | 12.4 ± 1.4 | 21.4 ± 1.5 | 17.0 ± 1.6 | |
| Novel object recognition | |||||
| Object preference (%) | 65.2 ± 3.5 | 58.6 ± 4.8 | 63.3 ± 5.6 | 65.7 ± 3.7 | |
| Hot plate test | |||||
| Time until frontpaw lick (s) | 17.6 ± 1.2 | 17.7 ± 0.7 | 16.0 ± 0.6 | 15.6 ± 0.9 | |
Tests for anxiety‐like behaviour were the marble burying test, which was performed 2 weeks post stress and the elevated plus maze which was done 3 weeks post stress. Depression was assessed using the tail‐suspension test 3 weeks post stress. Reward‐seeking behaviour was tested using the sucrose preference test 4 days post stress. Cognition was determined using the novel object recognition test 2 weeks post stress. Somatic pain response was assessed with the hot plate test 4 weeks post stress. Data from the sucrose preference test were non‐parametrically distributed and analysed using the Kruskal‐Wallis test, followed by the Mann‐Whitney test. Data from the hot plate test were normally distributed and analysed using a two‐way ANOVA. Significant differences are depicted as: * P < 0.05; Control/Control compared to Control/Stress, ## P < 0.01; Control/Stress compared to SCFA/Stress. All data are expressed as means ± SEM (n = 8–10).
Hedonic and reward‐seeking behaviour
Assessment of hedonic and reward‐seeking behaviour using the sucrose preference test revealed that stressed mice had an increased sucrose preference (Table 2), which was not seen following SCFA treatment (X2(3) = 12.839, P = 0.005). Conversely, in the female urine sniffing test, stressed mice spent less time interacting with the cotton bulb containing the urine of a female mouse in oestrus stage (Fig. 4 A), which was not present when mice additionally received SCFAs (X2(3) = 12.785, P = 0.005). We subsequently pursued potentially underlying mechanisms by investigating the expression of genes associated with reward‐signalling in the striatum, part of the mesolimbic reward pathway (Fig. 4 B). SCFA treatment increased dopamine receptor D1a expression (DRD1a) independent of psychosocial stress (F(1,36) = 13.416, P = 0.024), whereas dopamine receptor D2 (DRD2) gene expression remained unaffected. As brain‐derived neurotrophic factor (BDNF) has been shown to essential for dopaminergic signalling during psychosocial stress (Berton et al. 2006), we subsequently analysed the expression of BDNF (exon IV) and its receptor tropomyosin receptor kinase B (TrkB). Although no significant alterations were found in BDNF expression, the combination of SCFA treatment and stress decreased TrkB expression (F(1,37) = 4.264, P = 0.047).
Figure 4. Psychosocial stress induces long‐term anhedonia, which is absent after SCFA supplementation.
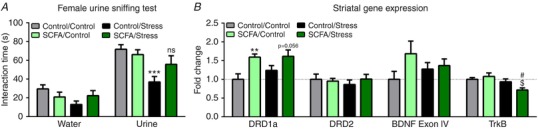
A, hedonic and reward‐seeking behaviour was assessed using the female urine sniffing test 2 weeks post stress. B, the expression of genes involved in reward signalling was investigated in the striatum, which were the dopamine receptor D1a (DRD1a), dopamine receptor D2 (DRD2), brain‐derived neurotrophic factor (BDNF Exon IV) and tropomyosin receptor kinase B (TrkB). Data from the female urine sniffing test and BDNF gene expression was non‐parametrically distributed and analysed using the Kruskal‐Wallis test, followed by the Mann‐Whitney test. Gene expression data from all other genes were normally distributed and analysed using a two‐way ANOVA, followed by an LSD post hoc test. Significant differences are depicted as: ** P < 0.01 and *** P < 0.001; Control/Control compared to SCFA/Control or Control/Stress, $ P < 0.05; SCFA/Control compared to SCFA/Stress, # P < 0.05; Control/Stress compared to SCFA/Stress. All data are expressed as means ± SEM (n = 8–10). [Color figure can be viewed at http://wileyonlinelibrary.com]
Memory and learning
Neither stress nor SCFA administration affected the discrimination index for memory in the novel object recognition test (Table 2). In addition, no effect of SCFAs or stress on the acquisition, retention and extinction in the fear conditioning test were found (Fig. 5).
Figure 5. Neither stress, nor SCFA treatment affected the acquisition, retention and extinction phase data from the fear conditioning test.
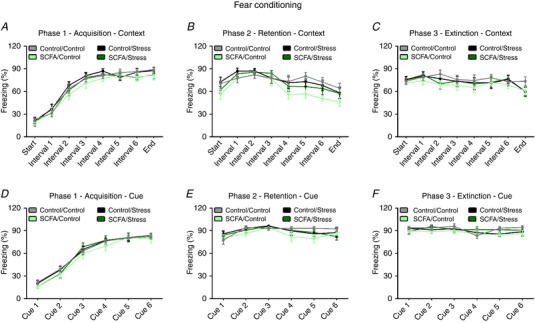
Cue‐ and context‐associative learning induced by foot shock was evaluated using fear conditioning 4 weeks post stress. At phase 1 (acquisition), mice were presented with a tone, followed by a foot chock. The context‐associative learning was assessed by measuring freezing behaviour in between tones (A), whereas cue‐associative learning was determined during the presentation of the tone (D). At phase 2 (retention), mice underwent the same protocol without the shocks to assess context‐ and cue‐associative retention (B and E, respectively). At phase 3 (extinction), mice were assessed for context‐ and cue‐associative extinction (C and F, respectively). All data are expressed as means ± SEM (n = 9–10). Open circles on each graph represent individual animals. [Color figure can be viewed at http://wileyonlinelibrary.com]
Stress responsiveness and HPA‐axis reactivity
Assessment of stress responsiveness and HPA‐axis reactivity in the stress‐induced hyperthermia test revealed a potentiated increase in body temperature after an acute stressor in psychosocially stressed mice (Fig. 6 A), which was restored by SCFAs (X2(3) = 8.676, P = 0.034). Additionally, SCFAs ameliorated the stress‐induced corticosterone potentiation after an acute stressor observed in the psychosocially stressed group (X2(3) = 11.058, P = 0.011) (Fig. 6 B and C). To further understand how SCFAs affect HPA‐axis reactivity, we investigated the expression of hypothalamic genes involved in stress signalling (Fig. 6 D). SCFAs induced a trend towards decreased corticotrophin‐releasing factor (CRF) expression (P = 0.122), which was significantly upregulated by psychosocial stress (X2(3) = 9.530, P = 0.023). In addition, SCFAs downregulated mineralocorticoid receptor (MR) expression, which was reversed by stress (X2(3) = 14.805, P = 0.002). Finally, no differences were found in glucocorticoid receptor (GR) expression. As glucocorticoid signalling in the hippocampus has previously been shown to affect behaviour (Kim et al. 2015), we investigated the gene expression of various glucocorticoid receptors in the hippocampus (Fig. 6 E). SCFAs induced a trend towards decreased corticotrophin‐releasing factor receptor 1 (CRFR1) expression, which was upregulated when mice also underwent stress (X2(3) = 7.426, P = 0.059). In accordance with the hypothalamic gene expression analysis, SCFAs downregulated MR expression, whereas stress increased MR expression (X2(3) = 19.185, P < 0.001). Finally, no differences were found in hippocampal GR expression.
Figure 6. Psychosocial stress induces increased responsiveness to acute stress, which was ameliorated by SCFAs.
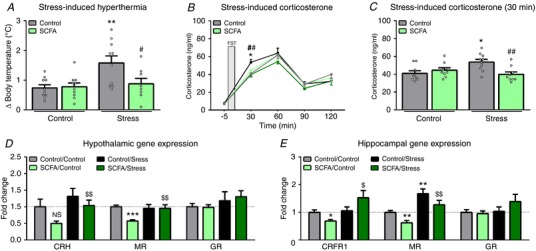
Stress responsiveness and HPA‐axis reactivity were assessed using the stress‐induced hyperthermia test 3 weeks after psychosocial stress (A), and by assessing the corticosterone levels in response to acute stress 6 weeks after psychosocial stress (B and C). The stressor used for the latter was the forced swim test. Hypothalamic genes involved in HPA‐axis signalling of which expression was investigated were corticotrophin‐releasing factor (CRF), mineralocorticoid receptor (MR) and glucocorticoid receptor (GR) (D). For the hippocampus, these were corticotrophin‐releasing factor receptor 1 (CRFR1), MR and GR (E). All data were non‐parametrically distributed and analysed using the Kruskal‐Wallis test, followed by the Mann‐Whitney test. Significant differences are depicted as: * P < 0.05, ** P < 0.01 and *** P < 0.001; Control/Control compared to SCFA/Control or Control/Stress, $ P < 0.05, $$ P < 0.01; SCFA/Control compared to SCFA/Stress, # P < 0.05 and ## P < 0.01; Control/Stress compared to SCFA/Stress. All data are expressed as means ± SEM (n = 8–10). [Color figure can be viewed at http://wileyonlinelibrary.com]
Intestinal permeability
In the intestinal permeability test, we found increased levels of plasma FITC‐dextran in psychosocially stressed mice (Fig. 7 A), which wasn't present when mice were also supplemented with SCFAs (X2(3) = 10.024, P = 0.018). Surprisingly, further analysis of intestinal permeability by plasma lipopolysaccharide‐binding protein (LBP) quantification, a marker for systemic exposure to the intestinal bacterial product lipopolysaccharide, didn't reveal any difference between groups (Table 3). As intestinal permeability is largely dependent on proper functioning of tight junctions (Zihni et al. 2016), we investigated gene expression of tight junction proteins in the colon (Fig. 7 B). Here we found increased expression of claudin‐1 (Cldn1) and tight junction protein 1 (Tjp1) in stressed mice, which was ameliorated by SCFAs (X2(3) = 15.311, P = 0.002 and X2(3) = 10.927, P = 0.012, respectively). No significant differences were found in occludin (Ocln) expression. We further hypothesised that mucus layer thickness could explain some of the observed findings, as this layer is impermeable to luminal bacteria (Johansson et al. 2011). And similar to the observed plasma LBP levels, no changes were found in mucin 2 (Muc2) expression, the primary marker for colonic mucus thickness. Finally, as stress signalling has been shown to induce intestinal permeability (Rodino‐Janeiro et al. 2015), we investigated colonic gene expression markers involved in glucocorticoid signalling (Fig. 7 C). Similar to the hippocampus, SCFAs decreased the expression of CRFR1, which was upregulated by stress (X2(3) = 11.003, P = 0.012). And surprisingly, both stress and SCFAs decreased CRFR2 expression (X2(3) = 10.280, P = 0.016). Stress also increased MR expression, whereas SCFA administration resulted in a downregulation (X2(3) = 20.076, P < 0.001). No differences were found in GR expression.
Figure 7. Stress induces intestinal permeability, which was rescued by SCFA treatment.
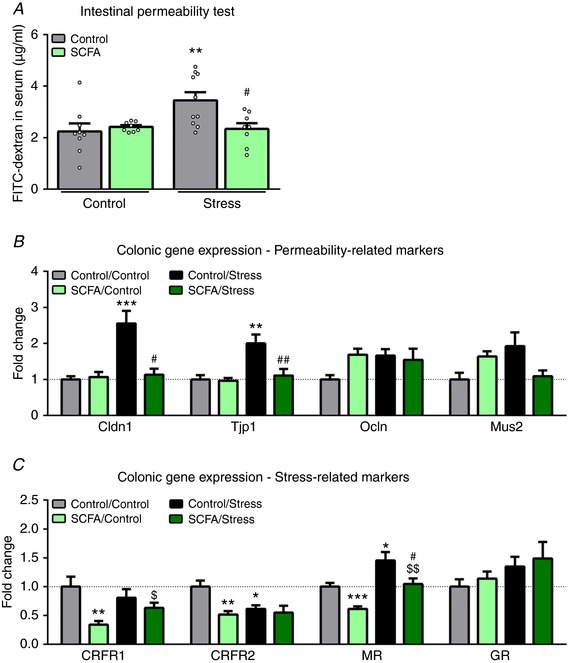
A, in vivo intestinal permeability was assessed 5 weeks post stress. B, colonic genes involved in intestinal permeability of which gene expression was investigated were claudin‐1 (Cldn1), tight junction protein 1 (Tjp1), occludin (Ocln) and mucus 2 (Muc2). C, gene expression analysis of genes involved glucocorticoid signalling were corticotrophin‐releasing factor receptors 1 and 2 (CRFR1 and CRFR2 respectively), mineralocorticoid receptor (MR) and glucocorticoid receptor (GR). All data were non‐parametrically distributed and analysed using the Kruskal‐Wallis test, followed by the Mann‐Whitney test. Significant differences are depicted as: * P < 0.05, ** P < 0.01 and *** P < 0.001; Control/Control compared to SCFA/Control or Control/Stress, $ P < 0.05 and $$ P < 0.01; SCFA/Control compared to SCFA/Stress, # P < 0.05 and ## P < 0.01; Control/Stress compared to SCFA/Stress. All data are expressed as means ± SEM (n = 8–10). Open circles on each graph represent individual animals. [Color figure can be viewed at http://wileyonlinelibrary.com]
Table 3.
Physiological measurements
| Factor | Control/Control | SCFA/Control | Control/Stress | SCFA/Stress |
|---|---|---|---|---|
| Plasma lipopolysaccharide‐binding protein | ||||
| LBP (μg mL−1) | 7.6 ± 0.3 | 8.0 ± 0.2 | 8.5 ± 0.2 | 7.8 ± 0.3 |
| Body weight change | ||||
| Day 3 of stress (Δg) | 0.24 ± 0.13 | 0.15 ± 0.05 | −0.54 ± 0.20** | −0.48 ± 0.21$ |
| Day 9 of stress (Δg) | 0.41 ± 0.16 | 0.39 ± 0.11 | −0.25 ± 0.17* | 0.18 ± 0.25 |
| Day 16 of stress (Δg) | 0.21 ± 0.25 | 0.28 ± 0.16 | 1.82 ± 0.28** | 1.85 ± 0.31$$ |
| 6 weeks post stress (Δg) | 0.39 ± 0.42 | 0.90 ± 0.17 | 1.63 ± 0.30* | 2.26 ± 0.27$$ |
| End of study (Δg) | 1.48 ± 0.29 | 1.44 ± 0.25 | 1.91 ± 0.40 | 2.61 ± 0.29 |
| Daily water intake at end of the study | ||||
| Drinking water consumed (mL) | 5.4 ± 0.2 | 4.8 ± 0.4 | 5.0 ± 0.2 | 4.7 ± 0.3 |
| Organ measurements | ||||
| Left adrenal (mg g−1 BW) | 0.052 ± 0.006 | 0.060 ± 0.006 | 0.067 ± 0.006 | 0.066 ± 0.005 |
| Right adrenal (mg g−1 BW) | 0.037 ± 0.006 | 0.047 ± 0.006 | 0.041 ± 0.006 | 0.043 ± 0.007 |
| Spleen (mg g−1 BW) | 2.48 ± 0.09 | 2.44 ± 0.05 | 2.60 ± 0.08 | 2.60 ± 0.07 |
| Thymus (mg g−1 BW) | 1.52 ± 0.08 | 1.64 ± 0.05 | 1.61 ± 0.08 | 1.67 ± 0.06 |
| Caecum (mg g−1 BW) | 17.2 ± 0.5 | 16.7 ± 0.7 | 17.2 ± 1.0 | 15.9 ± 0.4 |
| Colon (cm) | 7.3 ± 0.2 | 7.5 ± 0.2 | 7.3 ± 0.18 | 7.7 ± 0.3 |
| Faecal SCFAs and BCFAs | ||||
| Acetate (μmol g−1 wet mass) | 32.2 ± 5.6 | 35.0 ± 5.7 | 64.8 ± 11.4** | 54.1 ± 9.0 |
| Propionate (μmol g−1 wet mass) | 2.5 ± 0.3 | 3.2 ± 0.4 | 4.4 ± 0.8 | 4.0 ± 0.6 |
| Butyrate (μmol g−1 wet mass) | 2.0 ± 0.4 | 1.7 ± 0.2 | 2.3 ± 0.6 | 1.7 ± 0.2 |
| Valerate (μmol g−1 wet mass) | 0.85 ± 0.17 | 0.91 ± 0.21 | 1.00 ± 0.18 | 0.84 ± 0.14 |
| Total SCFAs (μmol g−1 wet mass) | 38.3 ± 5.7 | 42.0 ± 5.8 | 74.0 ± 12.6** | 62.2 ± 8.8 |
| Isobutyrate (μmol g−1 wet mass) | 0.53 ± 0.14 | 0.49 ± 0.10 | 0.85 ± 0.26 | 0.95 ± 0.19 |
| Isovalerate (μmol g−1 wet mass) | 0.52 ± 0.09 | 0.52 ± 0.07 | 0.58 ± 0.07 | 0.62 ± 0.08 |
| Total BCFAs (μmol g−1 wet mass) | 1.05 ± 0.18 | 0.99 ± 0.09 | 1.43 ± 0.29 | 1.57 ± 0.24 |
| SCFA GPCR expression | ||||
| FFAR2 (fold change) | 1.00 ± 0.17 | 0.89 ± 0.10 | 1.79 ± 0.23* | 1.89 ± 0.30$ |
| FFAR3 (fold change) | 1.00 ± 0.09 | 0.92 ± 0.04 | 1.14 ± 0.14 | 1.36 ± 0.12$ |
Lipopolysaccharide‐binding protein (LBP) was quantified from plasma obtained at the end of the experiment from trunk blood. Body weights and drinking water consumed were assessed throughout the study and normalised to the starting body weight to calculate body weight change (Δ body weight). Organs were collected at the end of the study and weighted. Thymus, adrenals, spleen and caecum weights were normalised to body weight, whereas the colon is expressed in total colon length. Faecal pellets were collected 6 weeks post psychosocial stress and analysed for SCFA and BCFA levels. Colonic gene expression analysis for SCFA GPCRs was carried out for free fatty acid receptors 2 and 3 (FFAR2 and FFAR3, respectively). Body weight data and gene expression data were non‐parametrically distributed and analysed using the Kruskal‐Wallis test, followed by the Mann‐Whitney test. Data from SCFA levels were normally distributed and analysed using a two‐way ANOVA, followed by an LSD post hoc test. Significant differences are depicted as: * P < 0.05 and ** P < 0.01; Control/Control compared to Control/Stress, $ P < 0.05 and $$ P < 0.01; SCFA/Control compared to SCFA/Stress. Data are expressed as means ± SEM (n = 8–10).
Somatic pain response
Mice were assessed for their response to a noxious thermal stimulus using the hot plate test 4 weeks post stress (Table 2). Animals that previously underwent psychosocial stress showed a slight, but significant, increase in somatic response (F(1,36) = 4.677, P = 0.038) as measured by the time until mice licked their front paw. Further post hoc tests did not reveal any differences between groups.
Body weight change
We sought to further understand the underlying causes of the behavioural phenotypes seen in the previous analyses. Considering that chronic psychosocial stress has previously been shown to affect host metabolism and body weight (Finger et al. 2011; Tramullas et al. 2012), and the crucial role of gut microbial‐derived SCFAs in host energy metabolism (Byrne et al. 2015), we hypothesized that SCFAs might ameliorate stress‐induced alterations in body weight, which was monitored throughout the study (Table 3). Interestingly, psychosocial stress significantly decreased body weight in the first 2 weeks of stress (day 3; X2(3) = 13.601, P = 0.004, to day 9; X2(3) = 8.854, P = 0.031). This decrease in body weight subsequently reversed, and a significant increase in body weight was observed in week 3 (day 16; X2(3) = 22.537, P < 0.001). The increase in body weight persisted throughout the rest study (6 weeks post stress; X2(3) = 14.991, P = 0.002), even though the effect size seemed to lessen over time and no significant differences were found at the endpoint of the study. In accordance with this, no differences were observed in organ measurements like the thymus, adrenal, spleen and caecum weight, as well as colon length (Table 3) Overall, SCFA treatment did not affect any stress‐induced changes in body weight, nor organ weights.
Short‐chain fatty acid levels and GPCR signalling
As an increased body weight is associated with elevated SCFA levels (Turnbaugh et al. 2006; Schwiertz et al. 2010; Fernandes et al. 2014; Rahat‐Rozenbloom et al. 2014), we investigated whether stress affected faecal SCFA level as well as body weight (Table 3). Stress increased total SCFAs (F(1,37) = 9.946, P = 0.003), which was probably due to an increase in acetate (F(1,37) = 9.470, P = 0.004), but not propionate, butyrate and valerate levels, all of which were unaffected by SCFA administration. Notable, branched‐chain fatty acids also remained unaffected. An increased body weight, as seen in high‐fat‐diet‐induced obese mice, has been shown to result in an elevated colonic gene expression of the SCFA GPCRs free fatty acid receptors 2 and 3 (FFAR2 and FFAR3, respectively) (Lu et al. 2016), indicating increased SCFA signalling. Similar to the faecal SCFA levels, stress increased the expression of FFAR2 (X2(3) = 11.610, P = 0.009), whereas FFAR3 was only significantly upregulated in mice receiving both stress and SCFAs (X2(3) = 7.986, P = 0.046) (Table 3).
Gut microbiota composition
16S sequencing revealed no apparent changes in caecal bacterial species diversity between groups (Fig. 8 A). In addition, principal coordinate analysis did not show any clear separation of microbiota composition (Fig. 8 B). Stress, as well as SCFA treatment, did induce some minor changes in caecal microbiota composition at the family and genus level (Fig. 9 A and B). Most of the differences in microbiota composition detected were induced by the combination of SCFAs and stress (Fig. 9 C).
Figure 8. Neither stress nor SCFAs affected caecal microbiota diversity.
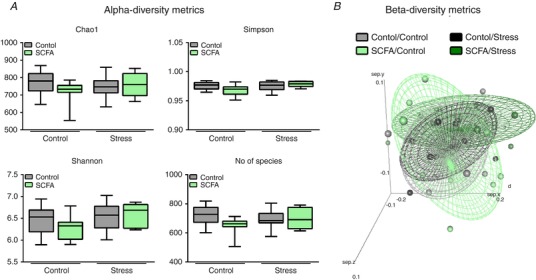
A, microbial alpha‐diversity was determined using Chao1, Simpson, Shannon metrics, as well as the number of species detected. B, beta‐diversity of the overall composition was depicted using principle component analysis. Alpha‐diversity metrics were non‐parametrically distributed and analysed using the Kruskal‐Wallis test. Data are depicted as median with IQR and minimum/maximum values as error bars (n = 9–10). [Color figure can be viewed at http://wileyonlinelibrary.com]
Figure 9. Subtle differences in caecum microbiota at family, genus and phylum levels were detected.
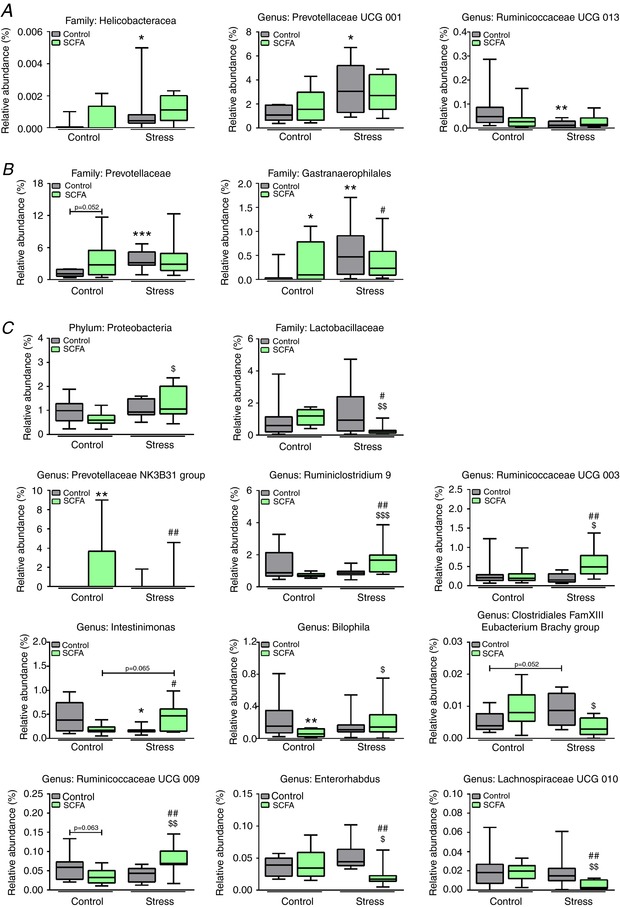
The 16S sequencing revealed few significant differences in microbiota composition on phylum, family and genus level. Data are organised on stress‐effect (A), concurrent SCFA‐ and stress‐effect (B), and combined SCFA and stress effect (C). Data were non‐parametrically distributed and analysed using the Kruskal‐Wallis test, followed by the Mann‐Whitney test. Significant differences are depicted as: * P < 0.05, ** P < 0.01 and *** P < 0.001; Control/Control compared to SCFA/Control or Control/Stress, $ P < 0.05, $$ P < 0.01 and $$$ P < 0.001; SCFA/Control compared to SCFA/Stress, # P < 0.05 and ## P < 0.01; Control/Stress compared to SCFA/Stress. Data are depicted as median with IQR and minimum/maximum values as error bars (n = 9–10). [Color figure can be viewed at http://wileyonlinelibrary.com]
Discussion
Microbial metabolites are receiving increased attention for their ability to modify brain and behaviour (Shultz et al. 2015; Sampson et al. 2016; Gronier et al. 2018). In the present study, we found that psychosocial stress affected reward‐seeking behaviour and increased both stress‐responsivity and in vivo intestinal permeability, all of which were ameliorated by SCFA treatment. SCFA supplementation also induced a decrease in anxiety‐like behaviour in the open field test and decreased depressive‐like behaviour in the forced swim test, both of which were absent following stress. Similarly, we found that the SCFA group showed decreased gene expression of receptors involved with stress‐signalling in the hypothalamus, hippocampus and colon.
The goal of these experiments was to characterise the enduring effects of chronic psychosocial stress on behavioural, physiological and microbiome parameters in the mouse and to assess if supplementation with SCFAs could counter such effects. In line with previous reports using a continuous stress exposure model, we report psychosocial stress‐induced anhedonia in the female urine sniffing test, increased stress‐responsiveness and increased in in vivo intestinal permeability (Burokas et al. 2017). Interestingly, the previously reported effects of chronic stress on cognition, anxiety‐ and depressive‐like behaviour, as well as alterations in gut microbiota composition, failed to endure in our model (Burokas et al. 2017). Indeed, it is well known that many stress‐induced physiological and behavioural deficits dissipate over time (Fanous et al. 2010; Ver Hoeve et al. 2013; Wohleb et al. 2014). For instance, 24 days after the last psychosocial stressor, monocyte infiltration into the brain, microglia inflammation and anxiety‐like behaviour revert back to baseline (Wohleb et al. 2014). In particular, microglia inflammation has been implicated in cognition, depression‐ and anxiety‐like behaviour (Wohleb & Delpech, 2016), which probably explains the absence of differences in these behaviours. The absence of long‐term stress‐induced differences gut microbiota might also be explained by dissipation, suggesting that the caecal microbiota composition does not contribute to the enduring effects of psychosocial stress, even though more research investigating the microbiota at different time points post stress, as well as gut microbiota‐derived metabolites, is warranted to validate this hypothesis.
Stress induced a long‐term increase in body weight, which is in line with the fact that stress exposure can lead to overeating and excessive body weight (Finger et al. 2012; Vancampfort et al. 2015; Razzoli & Bartolomucci, 2016; Razzoli et al. 2017). Stress also increased faecal acetate and total SCFA levels, as well as colonic FFAR2 and FFAR3 gene expression. This is perhaps not surprising, as increased body weight in obesity is associated with increased SCFA levels (Turnbaugh et al. 2006; Schwiertz et al. 2010; Fernandes et al. 2014; Rahat‐Rozenbloom et al. 2014), as well as increased colonic FFAR2 and FFAR3 gene expression (Lu et al. 2016). Surprisingly, oral SCFA supplementation did not ameliorate stress‐induced changes in body weight and SCFA levels, even though SCFA supplementation has previously been shown to reduce body weight in rodent models of obesity (Lin et al. 2012; Vinolo et al. 2012; Lu et al. 2016). Interestingly, the discrepancy between the fact that obesity is generally associated with increased SCFA levels, whereas SCFA supplementation ameliorates symptoms of obesity, has been left unexplained (Byrne et al. 2015; van de Wouw et al. 2017). As such, our data demonstrated a novel interaction between chronic stress, host metabolism and SCFAs, even though more research into the mechanisms involved is warranted in order to understand the exact role of chronic stress in SCFA–host metabolism interactions. Finally, oral SCFA administration itself did not affect faecal SCFA levels, which is probably explained by the rapid uptake of SCFAs (Pouteau et al. 2003; van der Beek et al. 2015).
We found that stress induced anhedonia in the female urine sniffing test, which was not present in combination with SCFA supplementation. However, the increased sucrose preference observed following stress is at odds with studies showing that chronic stress reduces reward‐seeking behaviour in the sucrose preference test (Iniguez et al. 2014; Veeraiah et al. 2014), even though this is not consistently reported (Van Bokhoven et al. 2011; O'Leary et al. 2014). This may be explained by the fact that it is a stress‐withdrawal‐induced effect we are modelling. Together, these data support the concept of SCFAs affecting reward‐processes. In this regard, a human brain imaging study showed that colonic propionate reduces anticipatory reward responses to high‐energy foods in the human striatum (Byrne et al. 2016). Such findings indicate that gut microbial‐derived SCFAs could provide target neural circuits underpinning hedonic food intake, in addition to being implicated in the regulation of homeostatic food intake (van de Wouw et al. 2017).
As previously reported, psychosocial stress induced long‐term increased responsiveness to acute stress challenges (Ver Hoeve et al. 2013). Here, we show that acute stress‐induced hyperthermia and corticosterone levels in chronically stressed mice are ameliorated by SCFA supplementation. Similar changes were found in colonic MR gene expression, even though the stress‐induced increased gene expression in hippocampal MR remained unaffected. In addition, no differences in GR gene expression were found in any of the investigated tissues which, similar to the absence of chronic stress‐induced anxiety‐ and depressive‐like behaviour and impaired cognition, could have dissipated over time. A more robust effect was observed in the group solely receiving SCFA administration, where there was a consistent decrease in CRFR1, CRFR2 and MR gene expression across all investigated tissues. Overall, these results reveal that SCFAs can downregulate stress‐signalling and HPA‐axis responsiveness, a crucial pathway in microbiota–gut–brain axis communication (Wiley et al. 2017). The sympathetic nervous system could play a key role in the mechanisms involved, as FFAR2 is expressed on autonomic and somatic sensory ganglia and activation hereof results in sympathetic nervous system activation (Kimura et al. 2011; Nohr et al. 2015), even though more research is warranted to validate this hypothesis. This has particular implications in pathophysiology of chronic anxiety disorders (e.g. PTSD), where a recurrent stressor after the long‐term cessation of chronic stress rapidly results in behavioural and neuroinflammatory sequelae similar to the ones observed immediately after the cessation of chronic stress (McKim et al. 2016), indicating that SCFA‐targeted therapies might be a suitable dietary target for such disorders.
Psychosocial stress induced an enduring increase in in vivo intestinal permeability 5 weeks after the last stressor, indicating long‐term stress‐induced changes in gastrointestinal barrier function. This was reversed by SCFA treatment, which is not surprising as SCFAs have previously been shown to restore intestinal permeability (Kelly et al. 2015; Tong et al. 2016; Simeoli et al. 2017). Surprisingly, stress induced an increased gene expression of tight junction proteins Cldn1 and Tjp1, even though increased intestinal permeability doesn't necessarily correlate to decreased tight junction gene expression (Golubeva et al. 2017). In addition, no differences were seen in plasma LBP levels, an indirect marker of systemic exposure to the intestinal bacterial product lipopolysaccharide. This might be attributable to the fact that Muc2 gene expression remained unaffected, indicating that the mucus barrier was still functional and impermeable to bacteria (Johansson et al. 2011). It is also important to note that gene expression does not necessarily correlate with protein levels. Another explanation for the discrepancy between in vivo intestinal permeability and LBP levels could be handling stress induced by the administration of FITC‐Dextran in the in vivo intestinal permeability test, as stress‐responsiveness was increased in the stress group, and as stress signalling has been shown to induce intestinal permeability (Rodino‐Janeiro et al. 2015). These findings have particular relevance for chronic stress‐induced gastrointestinal dysfunction, as chronic stress has previously been shown to promote colitis in mice (Gao et al. 2018), and chronic stress is associated with an increased risk of functional gastrointestinal disorders in humans, as well as increased rates of relapse and surgery for inflammatory bowel diseases (Stam et al. 1997; Klooker et al. 2009; Bernstein, 2017). It must be noted though that heightened right colonic fermentation has been shown to be a potential pathophysiological factor in irritable bowel syndrome (Farmer et al. 2014; Ringel‐Kulka et al. 2015).
Interestingly, although SCFAs decreased anxiety‐like behaviour in the open field test and depressive‐like behaviour in the forced swim test in control mice, the presence of a stressful history prevented such effects from being manifested. In a similar vein, we have shown that dietary manipulations with n‐3 polyunsaturated fatty acids, which alter the composition of the gut microbiota (Pusceddu et al. 2015a), have behavioural effects in control, but not early‐life stressed animals (Pusceddu et al. 2015b).
It is important to note that SCFAs were administered as a sodium salts and controls were sodium matched, which could result in yet unstudied changes in behaviour and physiology. This could have contributed to the unexpected findings in the sucrose preference test, even though it is important to note that drinking water consumption was the same between groups. As such, it has to be pointed out that a recent study showed that a high salt diet increases the susceptibility to colitis and alters gut microbiota composition (Miranda et al. 2018).
Taken together, these results present novel insights into how the gut microbial‐derived metabolites SCFAs influences brain homeostasis and behaviour, as well as host metabolism. Finally, as SCFAs are one of the main products of bacterial fermentation of dietary fibres, this study supports the importance of potential novel nutrition‐based therapeutic targets for stress‐related disorders.
Additional information
Competing interests
The authors have no competing interests to declare.
Author contributions
M.V.D.W., G.C., C.S., T.G.D. and J.F.C. contributed to the conception and design of the work, as well as critically revising it for intellectual content. Data acquisition, analysis and interpretation were performed by M.V.D.W., M.B., J.M.L., N.W., C.S. and O.S. Animal experiments, ELISAs and gene expression analysis were performed in APC Microbiome Institute Ireland, University College Cork, Cork, Ireland by M.V.D.W., M.B. and J.M.L. SCFA quantification and Illumina MiSeq sequencing were performed by N.W., C.S. and O.S. in Teagasc Food Research Centre, Moorepark, Fermoy, Cork, Ireland. All authors approve the final version of the manuscript and agree to be accountable for all aspects of the work. All individuals designated as author qualify for authorship and all individuals qualified for authorship are listed.
Funding
The APC Microbiome Institute is a research institute funded by Science Foundation Ireland (SFI) through the Irish Government's National Development Plan. J.F.C., T.G.D. and C.S. are supported by SFI (Grant Nos. SFI/12/RC/2273). In addition, M.B. is supported by supported by an educational grant from Science Foundation Ireland (SFI), Ireland (15/JP‐HDHL/3270; JPI‐HDHL‐NutriCog project ‘AMBROSIAC’). J.F.C., T.G.D. and C.S. have research support from Mead Johnson, Cremo, 4D Pharma, Suntory Wellness, and Nutricia. J.F.C., T.G.D. and C.S. have spoken at meetings sponsored by food and pharmaceutical companies. All other authors report no financial interests or potential conflicts of interest.
Acknowledgements
We thank P. Fitzgerald, C. Manly, M. Calis and D. Kandil for their invaluable technical assistance and Drs G. Moloney, K. Rea, A. Golubeva and K. O'Riordan for their assistance with data analysis and study design.
Biography
Marcel van de Wouw is a Dutch PhD student in his third year working at APC Microbiome Ireland at University College Cork under the supervision of Professors John Cryan and Ted Dinan. His research involves the investigation of how short‐chain fatty acids can affect microbiota‐gut‐brain axis signalling and how this relates to behaviour. He is also interested in the role of nutrition and in particular fermented foods on gut‐brain signaling. He completed his undergraduate training focused on how ghrelinergic signalling can influence food intake, which involved a 10‐month internship at University College Cork.

Edited by: Kim Barrett & David Grundy
This is an Editor's Choice article from the 15 October 2018 issue.
References
- Arnoldussen IAC, Wiesmann M, Pelgrim CE, Wielemaker EM, van Duyvenvoorde W, Amaral‐Santos PL, Verschuren L, Keijser BJF, Heerschap A, Kleemann R, Wielinga PY & Kiliaan AJ (2017). Butyrate restores HFD‐induced adaptations in brain function and metabolism in mid‐adult obese mice. Int J Obes 41, 935–944. [DOI] [PubMed] [Google Scholar]
- Bailey MT, Dowd SE, Galley JD, Hufnagle AR, Allen RG & Lyte M (2011). Exposure to a social stressor alters the structure of the intestinal microbiota: implications for stressor‐induced immunomodulation. Brain Behav Immun 25, 397–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein CN ( 2017). The brain‐gut axis and stress in inflammatory bowel disease. Gastroenterol Clin North Am 46, 839–846. [DOI] [PubMed] [Google Scholar]
- Berton O, McClung CA, Dileone RJ, Krishnan V, Renthal W, Russo SJ, Graham D, Tsankova NM, Bolanos CA, Rios M, Monteggia LM, Self DW & Nestler EJ (2006). Essential role of BDNF in the mesolimbic dopamine pathway in social defeat stress. Science 311, 864–868. [DOI] [PubMed] [Google Scholar]
- Bharwani A, Mian MF, Foster JA, Surette MG, Bienenstock J & Forsythe P (2016). Structural and functional consequences of chronic psychosocial stress on the microbiome & host. Psychoneuroendocrinology 63, 217–227. [DOI] [PubMed] [Google Scholar]
- Bharwani A, Mian MF, Surette MG, Bienenstock J & Forsythe P (2017). Oral treatment with Lactobacillus rhamnosus attenuates behavioural deficits and immune changes in chronic social stress. BMC Med 15, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boets E, Gomand SV, Deroover L, Preston T, Vermeulen K, De Preter V, Hamer HM, Van den Mooter G, De Vuyst L, Courtin CM, Annaert P, Delcour JA & Verbeke KA (2017). Systemic availability and metabolism of colonic‐derived short‐chain fatty acids in healthy subjects: a stable isotope study. J Physiol 595, 541–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burokas A, Arboleya S, Moloney RD, Peterson VL, Murphy K, Clarke G, Stanton C, Dinan TG & Cryan JF (2017). Targeting the microbiota‐gut‐brain axis: prebiotics have anxiolytic and antidepressant‐like effects and reverse the impact of chronic stress in mice. Biol Psychiatry 82, 472–487. [DOI] [PubMed] [Google Scholar]
- Byrne CS, Chambers ES, Alhabeeb H, Chhina N, Morrison DJ, Preston T, Tedford C, Fitzpatrick J, Irani C, Busza A, Garcia‐Perez I, Fountana S, Holmes E, Goldstone AP & Frost GS (2016). Increased colonic propionate reduces anticipatory reward responses in the human striatum to high‐energy foods. Am J Clin Nutr 104, 5–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrne CS, Chambers ES, Morrison DJ & Frost G (2015). The role of short chain fatty acids in appetite regulation and energy homeostasis. Int J Obes 39, 1331–1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caporaso JG, Kuczynski J, Stombaugh J, Bittinger K, Bushman FD, Costello EK, Fierer N, Pena AG, Goodrich JK, Gordon JI, Huttley GA, Kelley ST, Knights D, Koenig JE, Ley RE, Lozupone CA, McDonald D, Muegge BD, Pirrung M, Reeder J, Sevinsky JR, Turnbaugh PJ, Walters WA, Widmann J, Yatsunenko T, Zaneveld J & Knight R (2010). QIIME allows analysis of high‐throughput community sequencing data. Nat Methods 7, 335–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers ES, Viardot A, Psichas A, Morrison DJ, Murphy KG, Zac‐Varghese SE, MacDougall K, Preston T, Tedford C, Finlayson GS, Blundell JE, Bell JD, Thomas EL, Mt‐Isa S, Ashby D, Gibson GR, Kolida S, Dhillo WS, Bloom SR, Morley W, Clegg S & Frost G (2015). Effects of targeted delivery of propionate to the human colon on appetite regulation, body weight maintenance and adiposity in overweight adults. Gut 64, 1744–1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cryan JF & Mombereau C (2004). In search of a depressed mouse: utility of models for studying depression‐related behavior in genetically modified mice. Mol Psychiatry 9, 326–357. [DOI] [PubMed] [Google Scholar]
- De Vadder F, Kovatcheva‐Datchary P, Goncalves D, Vinera J, Zitoun C, Duchampt A, Backhed F & Mithieux G (2014). Microbiota‐generated metabolites promote metabolic benefits via gut‐brain neural circuits. Cell 156, 84–96. [DOI] [PubMed] [Google Scholar]
- Desbonnet L, Clarke G, Shanahan F, Dinan TG & Cryan JF (2014). Microbiota is essential for social development in the mouse. Mol Psychiatry 19, 146–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunphy‐Doherty F, O'Mahony SM, Peterson VL, O'Sullivan O, Crispie F, Cotter PD, Wigmore P, King MV, Cryan JF & Fone KCF (2018). Post‐weaning social isolation of rats leads to long‐term disruption of the gut microbiota‐immune‐brain axis. Brain Behav Immun 68, 261–273. [DOI] [PubMed] [Google Scholar]
- Edgar RC (2010). Search and clustering orders of magnitude faster than BLAST. Bioinformatics 26, 2460–2461. [DOI] [PubMed] [Google Scholar]
- Egorin MJ, Yuan ZM, Sentz DL, Plaisance K & Eiseman JL (1999). Plasma pharmacokinetics of butyrate after intravenous administration of sodium butyrate or oral administration of tributyrin or sodium butyrate to mice and rats. Cancer Chemother Pharmacol 43, 445–453. [DOI] [PubMed] [Google Scholar]
- Erny D, Hrabe de Angelis AL, Jaitin D, Wieghofer P, Staszewski O, David E, Keren‐Shaul H, Mahlakoiv T, Jakobshagen K, Buch T, Schwierzeck V, Utermohlen O, Chun E, Garrett WS, McCoy KD, Diefenbach A, Staeheli P, Stecher B, Amit I & Prinz M (2015). Host microbiota constantly control maturation and function of microglia in the CNS. Nat Neurosci 18, 965–977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erny D, Hrabe de Angelis AL & Prinz M (2017). Communicating systems in the body: how microbiota and microglia cooperate. Immunology 150, 7–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanous S, Hammer RP Jr & Nikulina EM (2010). Short‐ and long‐term effects of intermittent social defeat stress on brain‐derived neurotrophic factor expression in mesocorticolimbic brain regions. Neuroscience 167, 598–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farmer AD, Mohammed SD, Dukes GE, Scott SM & Hobson AR (2014). Caecal pH is a biomarker of excessive colonic fermentation. World J Gastroenterol 20, 5000–5007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandes J, Su W, Rahat‐Rozenbloom S, Wolever TM & Comelli EM (2014). Adiposity, gut microbiota and faecal short chain fatty acids are linked in adult humans. Nutr Diabetes 4, e121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finger BC, Dinan TG & Cryan JF (2011). High‐fat diet selectively protects against the effects of chronic social stress in the mouse. Neuroscience 192, 351–360. [DOI] [PubMed] [Google Scholar]
- Finger BC, Dinan TG & Cryan JF (2012). The temporal impact of chronic intermittent psychosocial stress on high‐fat diet‐induced alterations in body weight. Psychoneuroendocrinology 37, 729–741. [DOI] [PubMed] [Google Scholar]
- Foster JA, Rinaman L & Cryan JF (2017). Stress and the gut‐brain axis: Regulation by the microbiome. Neurobiol Stress 7, 124–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fouhy F, Deane J, Rea MC, O'Sullivan O, Ross RP, O'Callaghan G, Plant BJ & Stanton C (2015). The effects of freezing on faecal microbiota as determined using MiSeq sequencing and culture‐based investigations. PLoS One 10, e0119355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galley JD, Mackos AR, Varaljay VA & Bailey MT (2017). Stressor exposure has prolonged effects on colonic microbial community structure in Citrobacter rodentium‐challenged mice. Sci Rep 7, 45012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao X, Cao Q, Cheng Y, Zhao D, Wang Z, Yang H, Wu Q, You L, Wang Y, Lin Y, Li X, Wang Y, Bian JS, Sun D, Kong L, Birnbaumer L & Yang Y (2018). Chronic stress promotes colitis by disturbing the gut microbiota and triggering immune system response. Proc Natl Acad Sci U S A 115, E2960–E2969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golubeva AV, Joyce SA, Moloney G, Burokas A, Sherwin E, Arboleya S, Flynn I, Khochanskiy D, Moya‐Perez A, Peterson V, Rea K, Murphy K, Makarova O, Buravkov S, Hyland NP, Stanton C, Clarke G, Gahan CGM, Dinan TG & Cryan JF (2017). Microbiota‐related changes in bile acid & tryptophan metabolism are associated with gastrointestinal dysfunction in a mouse model of autism. EBioMedicine 24, 166–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gronier B, Savignac HM, Di Miceli M, Idriss SM, Tzortzis G, Anthony D & Burnet PWJ (2018). Increased cortical neuronal responses to NMDA and improved attentional set‐shifting performance in rats following prebiotic (B‐GOS((R))) ingestion. Eur Neuropsychopharmacol 28, 211–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grundy D (2015). Principles and standards for reporting animal experiments in The Journal of Physiology and Experimental Physiology . J Physiol 593, 2547–2549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoyles L, Snelling T, Umlai UK, Nicholson JK, Carding SR, Glen RC & McArthur S (2018). Microbiome‐host systems interactions: protective effects of propionate upon the blood‐brain barrier. Microbiome 6, 55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iniguez SD, Riggs LM, Nieto SJ, Dayrit G, Zamora NN, Shawhan KL, Cruz B & Warren BL (2014). Social defeat stress induces a depression‐like phenotype in adolescent male c57BL/6 mice. Stress 17, 247–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson ME, Ambort D, Pelaseyed T, Schutte A, Gustafsson JK, Ermund A, Subramani DB, Holmen‐Larsson JM, Thomsson KA, Bergstrom JH, van der Post S, Rodriguez‐Pineiro AM, Sjovall H, Backstrom M & Hansson GC (2011). Composition and functional role of the mucus layers in the intestine. Cell Mol Life Sci 68, 3635–3641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly CJ, Zheng L, Campbell EL, Saeedi B, Scholz CC, Bayless AJ, Wilson KE, Glover LE, Kominsky DJ, Magnuson A, Weir TL, Ehrentraut SF, Pickel C, Kuhn KA, Lanis JM, Nguyen V, Taylor CT & Colgan SP (2015). Crosstalk between microbiota‐derived short‐chain fatty acids and intestinal epithelial HIF augments tissue barrier function. Cell Host Microbe 17, 662–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly JR, Borre Y, C OB, Patterson E, El Aidy S, Deane J, Kennedy PJ, Beers S, Scott K, Moloney G, Hoban AE, Scott L, Fitzgerald P, Ross P, Stanton C, Clarke G, Cryan JF & Dinan TG (2016). Transferring the blues: Depression‐associated gut microbiota induces neurobehavioural changes in the rat. J Psychiatr Res 82, 109–118. [DOI] [PubMed] [Google Scholar]
- Kim EJ, Pellman B & Kim JJ (2015). Stress effects on the hippocampus: a critical review. Learn Mem 22, 411–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura I, Inoue D, Maeda T, Hara T, Ichimura A, Miyauchi S, Kobayashi M, Hirasawa A & Tsujimoto G (2011). Short‐chain fatty acids and ketones directly regulate sympathetic nervous system via G protein‐coupled receptor 41 (GPR41). Proc Natl Acad Sci U S A 108, 8030–8035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klooker TK, Braak B, Painter RC, de Rooij SR, van Elburg RM, van den Wijngaard RM, Roseboom TJ & Boeckxstaens GE (2009). Exposure to severe wartime conditions in early life is associated with an increased risk of irritable bowel syndrome: a population‐based cohort study. Am J Gastroenterol 104, 2250–2256. [DOI] [PubMed] [Google Scholar]
- Koh A, De Vadder F, Kovatcheva‐Datchary P & Backhed F (2016). From dietary fiber to host physiology: short‐chain fatty acids as key bacterial metabolites. Cell 165, 1332–1345. [DOI] [PubMed] [Google Scholar]
- Krautkramer KA, Kreznar JH, Romano KA, Vivas EI, Barrett‐Wilt GA, Rabaglia ME, Keller MP, Attie AD, Rey FE & Denu JM (2016). Diet‐microbiota interactions mediate global epigenetic programming in multiple host tissues. Mol Cell 64, 982–992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Yi CX, Katiraei S, Kooijman S, Zhou E, Chung CK, Gao Y, van den Heuvel JK, Meijer OC, Berbee JFP, Heijink M, Giera M, Willems van Dijk K, Groen AK, Rensen PCN & Wang Y (2017). Butyrate reduces appetite and activates brown adipose tissue via the gut‐brain neural circuit. Gut 67, 1269–1279. [DOI] [PubMed] [Google Scholar]
- Lin HV, Frassetto A, Kowalik EJ, Jr. , Nawrocki AR, Lu MM, Kosinski JR, Hubert JA, Szeto D, Yao X, Forrest G & Marsh DJ (2012). Butyrate and propionate protect against diet‐induced obesity and regulate gut hormones via free fatty acid receptor 3‐independent mechanisms. PLoS One 7, e35240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Sun J, Wang F, Yu X, Ling Z, Li H, Zhang H, Jin J, Chen W, Pang M, Yu J, He Y & Xu J (2015). Neuroprotective effects of clostridium butyricum against vascular dementia in mice via metabolic butyrate. BioMed Res Int 2015, 412946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lozupone C, Lladser ME, Knights D, Stombaugh J & Knight R (2011). UniFrac: an effective distance metric for microbial community comparison. ISME J 5, 169–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y, Fan C, Li P, Lu Y, Chang X & Qi K (2016). Short chain fatty acids prevent high‐fat‐diet‐induced obesity in mice by regulating G protein‐coupled receptors and gut microbiota. Sci Rep 6, 37589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKim DB, Patterson JM, Wohleb ES, Jarrett BL, Reader BF, Godbout JP & Sheridan JF (2016). Sympathetic release of splenic monocytes promotes recurring anxiety following repeated social defeat. Biol Psychiatry 79, 803–813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magoc T & Salzberg SL (2011). FLASH: fast length adjustment of short reads to improve genome assemblies. Bioinformatics 27, 2957–2963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marin IA, Goertz JE, Ren T, Rich SS, Onengut‐Gumuscu S, Farber E, Wu M, Overall CC, Kipnis J & Gaultier A (2017). Microbiota alteration is associated with the development of stress‐induced despair behavior. Sci Rep 7, 43859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milligan G, Shimpukade B, Ulven T & Hudson BD (2017). Complex pharmacology of free fatty acid receptors. Chem Rev 117, 67–110. [DOI] [PubMed] [Google Scholar]
- Miranda PM, De Palma G, Serkis V, Lu J, Louis‐Auguste MP, McCarville JL, Verdu EF, Collins SM & Bercik P (2018). High salt diet exacerbates colitis in mice by decreasing Lactobacillus levels and butyrate production. Microbiome 6, 57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moloney RD, Johnson AC, O'Mahony SM, Dinan TG, Greenwood‐Van Meerveld B & Cryan JF (2016). Stress and the microbiota‐gut‐brain axis in visceral pain: relevance to irritable bowel syndrome. CNS Neurosci Ther 22, 102–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy K, Curley D, O'Callaghan TF, O'Shea CA, Dempsey EM, O'Toole PW, Ross RP, Ryan CA & Stanton C (2017). The composition of human milk and infant faecal microbiota over the first three months of life: a pilot study. Sci Rep 7, 40597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nohr MK, Egerod KL, Christiansen SH, Gille A, Offermanns S, Schwartz TW & Moller M (2015). Expression of the short chain fatty acid receptor GPR41/FFAR3 in autonomic and somatic sensory ganglia. Neuroscience 290, 126–137. [DOI] [PubMed] [Google Scholar]
- O'Leary OF, Felice D, Galimberti S, Savignac HM, Bravo JA, Crowley T, El Yacoubi M, Vaugeois JM, Gassmann M, Bettler B, Dinan TG & Cryan JF (2014). GABAB(1) receptor subunit isoforms differentially regulate stress resilience. Proc Natl Acad Sci U S A 111, 15232–15237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry RJ, Peng L, Barry NA, Cline GW, Zhang D, Cardone RL, Petersen KF, Kibbey RG, Goodman AL & Shulman GI (2016). Acetate mediates a microbiome‐brain‐beta‐cell axis to promote metabolic syndrome. Nature 534, 213–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pluznick JL (2017). Microbial short‐chain fatty acids and blood pressure regulation. Curr Hypertens Rep 19, 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pouteau E, Nguyen P, Ballevre O & Krempf M (2003). Production rates and metabolism of short‐chain fatty acids in the colon and whole body using stable isotopes. Proc Nutr Soc 62, 87–93. [DOI] [PubMed] [Google Scholar]
- Pusceddu MM, El Aidy S, Crispie F, O'Sullivan O, Cotter P, Stanton C, Kelly P, Cryan JF & Dinan TG (2015a). N‐3 Polyunsaturated fatty acids (PUFAs) reverse the impact of early‐life stress on the gut microbiota. PLoS One 10, e0139721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pusceddu MM, Kelly P, Ariffin N, Cryan JF, Clarke G & Dinan TG (2015b). N‐3 PUFAs have beneficial effects on anxiety and cognition in female rats: Effects of early life stress. Psychoneuroendocrinology 58, 79–90. [DOI] [PubMed] [Google Scholar]
- Quast C, Pruesse E, Yilmaz P, Gerken J, Schweer T, Yarza P, Peplies J & Glockner FO (2013). The SILVA ribosomal RNA gene database project: improved data processing and web‐based tools. Nucleic Acids Res 41, D590–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahat‐Rozenbloom S, Fernandes J, Gloor GB & Wolever TM (2014). Evidence for greater production of colonic short‐chain fatty acids in overweight than lean humans. Int J Obes 38, 1525–1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez K, Fornaguera‐Trias J & Sheridan JF (2017). Stress‐induced microglia activation and monocyte trafficking to the brain underlie the development of anxiety and depression. Curr Top Behav Neurosci 31, 155–172. [DOI] [PubMed] [Google Scholar]
- Razzoli M & Bartolomucci A (2016). The dichotomous effect of chronic stress on obesity. Trends Endocrinol Metab 27, 504–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razzoli M, Pearson C, Crow S & Bartolomucci A (2017). Stress, overeating, and obesity: insights from human studies and preclinical models. Neurosci Biobehav Rev 76, 154–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rea K, Dinan TG & Cryan JF (2017). The brain‐gut axis contributes to neuroprogression in stress‐related disorders. Mod Trends Pharmacopsychiatry 31, 152–161. [DOI] [PubMed] [Google Scholar]
- Reigstad CS, Salmonson CE, Rainey JF 3rd, Szurszewski JH, Linden DR, Sonnenburg JL, Farrugia G & Kashyap PC (2015). Gut microbes promote colonic serotonin production through an effect of short‐chain fatty acids on enterochromaffin cells. FASEB J 29, 1395–1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ringel‐Kulka T, Choi CH, Temas D, Kim A, Maier DM, Scott K, Galanko JA & Ringel Y (2015). Altered colonic bacterial fermentation as a potential pathophysiological factor in irritable bowel syndrome. Am J Gastroenterol 110, 1339–1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodino‐Janeiro BK, Alonso‐Cotoner C, Pigrau M, Lobo B, Vicario M & Santos J (2015). Role of corticotropin‐releasing factor in gastrointestinal permeability. J Neurogastroenterol Motil 21, 33–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sampson TR, Debelius JW, Thron T, Janssen S, Shastri GG, Ilhan ZE, Challis C, Schretter CE, Rocha S, Gradinaru V, Chesselet MF, Keshavarzian A, Shannon KM, Krajmalnik‐Brown R, Wittung‐Stafshede P, Knight R & Mazmanian SK (2016). Gut microbiota regulate motor deficits and neuroinflammation in a model of Parkinson's disease. Cell 167, 1469–1480 e1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schellekens H, Clarke G, Jeffery IB, Dinan TG & Cryan JF (2012). Dynamic 5‐HT2C receptor editing in a mouse model of obesity. PLoS One 7, e32266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schonfeld P & Wojtczak L (2016). Short‐ and medium‐chain fatty acids in energy metabolism: the cellular perspective. J Lipid Res 57, 943–954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwiertz A, Taras D, Schafer K, Beijer S, Bos NA, Donus C & Hardt PD (2010). Microbiota and SCFA in lean and overweight healthy subjects. Obesity 18, 190–195. [DOI] [PubMed] [Google Scholar]
- Scott KA, Ida M, Peterson VL, Prenderville JA, Moloney GM, Izumo T, Murphy K, Murphy A, Ross RP, Stanton C, Dinan TG & Cryan JF (2017). Revisiting metchnikoff: age‐related alterations in microbiota‐gut‐brain axis in the mouse. Brain Behav Immun 65, 20–32. [DOI] [PubMed] [Google Scholar]
- Shultz SR, Aziz NA, Yang L, Sun M, MacFabe DF & O'Brien TJ (2015). Intracerebroventricular injection of propionic acid, an enteric metabolite implicated in autism, induces social abnormalities that do not differ between seizure‐prone (FAST) and seizure‐resistant (SLOW) rats. Behav Brain Res 278, 542–548. [DOI] [PubMed] [Google Scholar]
- Simeoli R, Mattace Raso G, Pirozzi C, Lama A, Santoro A, Russo R, Montero‐Melendez T, Berni Canani R, Calignano A, Perretti M & Meli R (2017). An orally administered butyrate‐releasing derivative reduces neutrophil recruitment and inflammation in dextran sulphate sodium‐induced murine colitis. Br J Pharmacol 174, 1484–1496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith PM, Howitt MR, Panikov N, Michaud M, Gallini CA, Bohlooly YM, Glickman JN & Garrett WS (2013). The microbial metabolites, short‐chain fatty acids, regulate colonic Treg cell homeostasis. Science 341, 569–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stam R, Akkermans LM & Wiegant VM (1997). Trauma and the gut: interactions between stressful experience and intestinal function. Gut 40, 704–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stilling RM, van de Wouw M, Clarke G, Stanton C, Dinan TG & Cryan JF (2016). The neuropharmacology of butyrate: the bread and butter of the microbiota‐gut‐brain axis? Neurochem Int 99, 110–132. [DOI] [PubMed] [Google Scholar]
- Szyszkowicz JK, Wong A, Anisman H, Merali Z & Audet MC (2017). Implications of the gut microbiota in vulnerability to the social avoidance effects of chronic social defeat in male mice. Brain Behav Immun 66, 45–55. [DOI] [PubMed] [Google Scholar]
- Tahara Y, Yamazaki M, Sukigara H, Motohashi H, Sasaki H, Miyakawa H, Haraguchi A, Ikeda Y, Fukuda S & Shibata S (2018). Gut microbiota‐derived short chain fatty acids induce circadian clock entrainment in mouse peripheral tissue. Sci Rep 8, 1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong LC, Wang Y, Wang ZB, Liu WY, Sun S, Li L, Su DF & Zhang LC (2016). Propionate ameliorates dextran sodium sulfate‐induced colitis by improving intestinal barrier function and reducing inflammation and oxidative stress. Front Pharmacol 7, 253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tramullas M, Dinan TG & Cryan JF (2012). Chronic psychosocial stress induces visceral hyperalgesia in mice. Stress 15, 281–292. [DOI] [PubMed] [Google Scholar]
- Turnbaugh PJ, Ley RE, Mahowald MA, Magrini V, Mardis ER & Gordon JI (2006). An obesity‐associated gut microbiome with increased capacity for energy harvest. Nature 444, 1027–1031. [DOI] [PubMed] [Google Scholar]
- Van Bokhoven P, Oomen CA, Hoogendijk WJ, Smit AB, Lucassen PJ & Spijker S (2011). Reduction in hippocampal neurogenesis after social defeat is long‐lasting and responsive to late antidepressant treatment. Eur J Neurosci 33, 1833–1840. [DOI] [PubMed] [Google Scholar]
- van de Wouw M, Schellekens H, Dinan TG & Cryan JF (2017). Microbiota‐gut‐brain axis: modulator of host metabolism and appetite. J Nutr 147, 727–745. [DOI] [PubMed] [Google Scholar]
- van der Beek CM, Bloemen JG, van den Broek MA, Lenaerts K, Venema K, Buurman WA & Dejong CH (2015). Hepatic uptake of rectally administered butyrate prevents an increase in systemic butyrate concentrations in humans. J Nutr 145, 2019–2024. [DOI] [PubMed] [Google Scholar]
- Vancampfort D, Stubbs B, Mitchell AJ, De Hert M, Wampers M, Ward PB, Rosenbaum S & Correll CU (2015). Risk of metabolic syndrome and its components in people with schizophrenia and related psychotic disorders, bipolar disorder and major depressive disorder: a systematic review and meta‐analysis. World Psychiatry 14, 339–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vazquez‐Baeza Y, Pirrung M, Gonzalez A & Knight R (2013). EMPeror: a tool for visualizing high‐throughput microbial community data. Gigascience 2, 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veeraiah P, Noronha JM, Maitra S, Bagga P, Khandelwal N, Chakravarty S, Kumar A & Patel AB (2014). Dysfunctional glutamatergic and gamma‐aminobutyric acidergic activities in prefrontal cortex of mice in social defeat model of depression. Biol Psychiatry 76, 231–238. [DOI] [PubMed] [Google Scholar]
- Ver Hoeve ES, Kelly G, Luz S, Ghanshani S & Bhatnagar S (2013). Short‐term and long‐term effects of repeated social defeat during adolescence or adulthood in female rats. Neuroscience 249, 63–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinolo MA, Rodrigues HG, Festuccia WT, Crisma AR, Alves VS, Martins AR, Amaral CL, Fiamoncini J, Hirabara SM, Sato FT, Fock RA, Malheiros G, dos Santos MF & Curi R (2012). Tributyrin attenuates obesity‐associated inflammation and insulin resistance in high‐fat‐fed mice. Am J Physiol Endocrinol Metab 303, E272–E282. [DOI] [PubMed] [Google Scholar]
- Wiley NC, Dinan TG, Ross RP, Stanton C, Clarke G & Cryan JF (2017). The microbiota‐gut‐brain axis as a key regulator of neural function and the stress response: Implications for human and animal health. J Anim Sci 95, 3225–3246. [DOI] [PubMed] [Google Scholar]
- Wohleb ES & Delpech JC (2016). Dynamic cross‐talk between microglia and peripheral monocytes underlies stress‐induced neuroinflammation and behavioral consequences. Prog Neuropsychopharmacol Biol Psychiatry 79, 40–48. [DOI] [PubMed] [Google Scholar]
- Wohleb ES, McKim DB, Shea DT, Powell ND, Tarr AJ, Sheridan JF & Godbout JP (2014). Re‐establishment of anxiety in stress‐sensitized mice is caused by monocyte trafficking from the spleen to the brain. Biol Psychiatry 75, 970–981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L, Zhao Y, Wang Y, Liu L, Zhang X, Li B & Cui R (2015). The effects of psychological stress on depression. Curr Neuropharmacol 13, 494–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao G, Nyman M & Jonsson JA (2006). Rapid determination of short‐chain fatty acids in colonic contents and faeces of humans and rats by acidified water‐extraction and direct‐injection gas chromatography. Biomed Chromatogr 20, 674–682. [DOI] [PubMed] [Google Scholar]
- Zihni C, Mills C, Matter K & Balda MS (2016). Tight junctions: from simple barriers to multifunctional molecular gates. Nat Rev Mol Cell Biol 17, 564–580. [DOI] [PubMed] [Google Scholar]


