Abstract
Obese women diagnosed with breast cancer have an increased risk for metastasis, and the underlying mechanisms are not well established. Within the mammary gland, adipose-derived stromal cells (ASCs) are heterogeneous cells with the capacity to differentiate into multiple mesenchymal lineages. To study the effects of obesity on ASCs, mice were fed a control diet (CD) or high-fat diet (HFD) to induce obesity, and ASCs were isolated from the mammary glands of lean and obese mice. We observed that obesity increased ASCs proliferation, decreased differentiation potential, and upregulated expression of α-smooth muscle actin, a marker of activated fibroblasts, compared to ASCs from lean mice. To determine how ASCs from obese mice impacted tumor growth, we mixed ASCs isolated from CD- or HFD-fed mice with mammary tumor cells and injected them into the mammary glands of lean mice. Tumor cells mixed with ASCs from obese mice grew significantly larger tumors and had increased invasion into surrounding adipose tissue than tumor cells mixed with control ASCs. ASCs from obese mice demonstrated enhanced tumor cell invasion in culture, a phenotype associated with increased expression of insulin-like growth factor-1 (IGF-1) and abrogated by IGF-1 neutralizing antibodies. Weight loss induced in obese mice significantly decreased expression of IGF-1 from ASCs and reduced the ability of the ASCs to induce an invasive phenotype. Together, these results suggest that obesity enhances local invasion of breast cancer cells through increased expression of IGF-1 by mammary ASCs, and weight loss may reverse this tumor-promoting phenotype.
Abbreviations: ASC, adipose-derived stromal cells; BMI, body mass index; CD, control diet; GAPDH, glyceraldehyde 3-phosphate dehydrogenase; GFP, green fluorescent protein; HFD, high fat diet; IGF-1, insulin-like growth factor-1; SMA, alpha-smooth muscle actin; SVF, stromal vascular fraction; WL, weight loss
Introduction
Obesity rates in the United States and around the world have more than doubled in the last 40 years [1]. Obesity is associated with increased risk for development of several types of cancer, including breast cancer in postmenopausal women [2], [3], [4]. Regardless of menopausal status, breast cancer patients with an obese body mass index (BMI) are more frequently diagnosed with poorly differentiated, larger primary tumors and lymph node metastases than lean patients [5], [6]. Increased BMI is significantly correlated with elevated rates of breast cancer–related mortality [7]. Understanding how obesity promotes the growth of aggressive breast tumors is of great clinical importance in order to develop targeted therapies for obese patients.
In obesity, adipose tissue expansion results in a chronic inflammatory state that contributes to obesity-related insulin resistance [8], [9], [10]. This inflammatory state leads to elevated circulating levels of proinflammatory cytokines, leptin, and insulin; many of these factors have been implicated in breast cancer progression [11], [12], [13], [14], [15]. Given the complexity of dysregulated factors in obesity, understanding how local changes within the adipose tissue of the breast may contribute to breast tumor growth and progression has been challenging to define.
Within the mammary gland, white adipose tissue is capable of undergoing expansion and retraction in response to changes in energy balance. Adipose-derived stromal cells (ASCs) are a heterogeneous group of cells within the extracellular matrix of the adipose tissue surrounding mature adipocytes [16]. ASCs, which are present in normal breast adipose tissue, have been shown to induce tissue remodeling through angiogenesis [17], proliferation [18], and deposition of extracellular matrix proteins [19]. One cell type within the ASC population is adipose stem cells which have the ability to differentiate into mature adipocytes in vivo as well as into multiple mesenchymal lineages in response to lineage-specific stimuli in vitro [20]. The differentiation potential of adipose stem cells in culture is influenced by multiple factors including fat depot–specific origin [21], [22], [23], alterations in the extracellular matrix [24], sex-specific hormones [25], and increasing BMI [26], [27], [28], [29]. Multiple studies have shown that secreted factors from ASCs promote growth of breast cancer cells within the tumor microenvironment [30], [31], [32], [33]. However, the effects of obesity on breast ASCs and their ability to promote cancer have not been well explored.
Here, we sought to address how obesity impacts the function of ASCs and how these cells within the microenvironment of the breast may impact tumor progression. We show that obesity increases ASC proliferation and reduces adipose stem cell differentiation potential in vitro. The ASCs from obese mice promote rapid mammary tumor growth and invasion into surrounding mammary adipose tissue. In addition, we demonstrate that some, but not all, obesity-induced changes to ASCs are reversible. Following weight loss, ASCs have less capacity to promote mammary tumor cell invasion. Overall, our findings suggest that obesity-induced changes in ASCs may contribute to the increased local invasion observed clinically in the breast tumors of obese women.
Materials and Methods
Animal Studies
All procedures involving animals were approved by the University of Wisconsin-Madison Institutional Animal Care and Use Committee. Female C57BL/6 (000664) and FVB/N (001800) mice were purchased from Jackson Laboratories and maintained according to the Guide for Care and Use of Laboratory Animals in AAALAC-accredited facilities. Eight-week-old female C57BL/6 and three-week-old FVB/N mice were fed control diet (CD, 10% kcal from fat, Test Diet 58Y1) or high-fat diet (HFD, 60% kcal from fat, Test Diet 58Y2) for 16 weeks to induce obesity. Purified diets contained equal amounts of vitamins and micronutrients. Body weights were measured weekly. For weight loss experiments, mice were fed HFD for 15 weeks and then switched to the CD for 5 weeks. Following euthanasia, thoracic and inguinal mammary glands were collected. Mammary tissue was minced and digested with collagenase I (Sigma; 1148089) for 1 hour. The mammary organoids, which are enriched for epithelial cells, were separated from the stromal vascular fraction (SVF) as described [34], and the SVF was cryopreserved for use in studies. SVF cells were plated in DMEM (Corning, 10-017-CV) containing 10% FBS (Gibco, 10437-28) and 1% antibiotic/antimycotic solution (Mediatech, 30-004-CI), and adherent cells were expanded in culture for no more than three passages prior to use in assays.
Human Tissue Isolation
All human breast tissues were obtained in compliance with the law and institutional guidelines as approved by the Institutional Review Board at the University of Wisconsin-Madison. Disease-free, deidentified breast tissues were obtained from patients undergoing elective reduction mammoplasty with informed consent through the Translational Science BioCore BioBank at the Carbone Cancer Center at the University of Wisconsin-Madison. This research study was approved by Institutional Review Board as Not Human Subject Research with a limited patient data set including patient age, date of service, and BMI. Tissue samples from patients aged 18-45 were included in these studies. Breast tissue from reduction mammoplasty surgeries was enzymatically dissociated for 8 hours using collagenase I as described [35], [36]. The digested tissue was allowed to settle at room temperature for 10 minutes. The lipid-rich fraction was removed, and the SVF was isolated, incubated with red blood cell lysis buffer (ACK Lysing Buffer, Lonza, 10-548E), and plated in DMEM supplemented with 10% FBS and 1% antibiotic/antimycotic solution to generate adherent stromal cells.
Cell Lines
EO771 cells were derived from a spontaneous mammary adenocarcinoma from a C57Bl/6 mouse [37] and were provided by Dr. Mikhail Kolonin. Met-1 cells were derived from metastasis from a MMTV-PyMT tumor from an FVB/N female mouse [38] and were provided by Dr. Alexander Borowsky. Met-1 cells were transduced with lentivirus encoding green fluorescent protein (GFP), and GFP+ cells were selected using fluorescence-activated cell sorting. MCF-7 cells were derived from pleural effusion from a metastatic estrogen receptor alpha–positive breast carcinoma [39] and were purchased from ATCC (30-2101). All tumor cell lines were cultured in DMEM supplemented with 10% FBS and 1% antibiotic/antimycotic solution at 37°C at 5% CO2.
Tumor and Stromal Cell Transplantations
To generate tumors, 1×106 EO771 or 5×105 Met-1 cells were mixed with 2.5×105 ASCs isolated from obese or lean C57Bl/6 or FVB/N mice, respectively. These ratios of tumor cells to stromal cells were based on previous studies examining tumor and stromal cell interactions [40], [41], [42]. Tumor cells and ASCs were pelleted and resuspended in 2:1 Matrigel (Corning, 354234):DMEM and injected bilaterally into the inguinal mammary glands of 8-week-old C57Bl/6 or FVB/N female mice fed CD. Tumor diameters were measured using calipers three times each week. Tumor volume was calculated using the formula 4/3πr3. When tumors reached the humane endpoint of 1.5 cm in diameter, mice were euthanized. Tumors were weighed and then sectioned for fixation in formalin or collagenase digestion. To isolate single tumor cells, tumors were minced, incubated in DMEM:F12 (Corning, 10-090-CV) supplemented with collagenase I, and further treated 0.25% trypsin-EDTA (Corning, 25-053-CI) as described [43].
Conditioned Media Collection and Treatment
Human and murine ASCs were grown on 100-mm tissue culture plates (Greiner, 664170) until confluency. Cells were washed with PBS then grown in DMEM supplemented with 0.5% FBS and 1% antibiotic/antimycotic solution. After 24 hours, conditioned media were collected, filtered through 0.22-μm filters (Fisher Scientific, 09-720-004), aliquoted, and stored at −80°C for use in experiments. For conditioned media experiments, 1×105 Met-1, EO771, or MCF-7 cells were plated on 6-well plates and treated for 7 days with conditioned media from murine or human ASCs. Cells were fed with conditioned media every 2 days. To assess the effects of insulin-like growth factor-1 (IGF-1) on invasion, Met-1 cells were treated with serum-free DMEM supplemented with 60 ng/ml recombinant mouse IGF-1 (R&D Systems, 791-MG) or PBS vehicle control for 24 hours. Met-1 cells were also treated with serum-free conditioned media from ACS isolated from HFD-fed mice supplemented with 1 μg/ml of either IGF-1 blocking antibody (R&D Systems, AF791) or control goat IgG control (R&D Systems, AB-108-C).
Invasion Assay
To assess invasion, 2.5×104 Met-1, 5×104 EO771, or 5×104 MCF-7 conditioned media–treated cells or 5×104 primary tumor cells isolated from Met-1 or EO771 tumors were plated in triplicate in serum-free media on growth factor–depleted Matrigel-coated invasion chambers (Corning, 354483) with 3 biological replicates. Invasion toward DMEM supplemented with 10% FBS was measured after 48 hours. Invasion inserts were formalin fixed and stained with 0.1% crystal violet. Four images of each invasion insert were taken at 100× magnification on a Nikon Eclipse E600 Microscope with a QICAM Fast 1394 camera and quantified using ImageJ (NIH) with cell counter plug-in.
Tumorsphere Assay
To assess changes in cancer stem-like cells, 500 Met-1 or 1500 MCF-7 cells treated with conditioned media from ASCs or 500 primary tumor cells from Met-1 or EO771 tumors were plated in triplicate on 24-well ultra-low adherent plates (Costar, 3473) in DMEM with 10% FBS and 1% antibiotic/antimycotic for 5 days. After 5 days, tumorspheres were collected, spun down at 17×g for 5 minutes, and counted using a light microscope in a 96-well plate. Primary tumorspheres were trypsinized with 0.05% trypsin (Corning, 25-052-CI) for 5 minutes at 37°C, spun down at 193×g for 5 minutes, and plated on a 24-well ultra-low-adherent plate for secondary tumorsphere formation. Secondary tumorspheres were incubated at 37°C in 5% CO2 and counted after 5 days. Tumorsphere assays were plated in triplicate with three biological replicates.
Proliferation Assay
To quantify differences in proliferation among ASCs, 1×105 CD, HFD, or weight loss (WL) ASCs were plated in triplicate on 35-mm tissue culture plates (Greiner, 627-160) in DMEM supplemented with 10% FBS and 1% antibiotic/antimycotic solution. After 3 days, cells were washed with PBS, trypsinized, centrifuged, and counted using a hemocytometer, then replated on 100-mm tissue culture plates. ASCs were incubated for an additional 3 days and counted. To quantify differences in proliferation among tumor cells, 1×105 primary tumor cells isolated from Met-1 or EO771 tumors were plated in DMEM supplemented with 10% FBS and 1% antibiotic/antimycotic solution on 35-mm tissue culture plates in triplicate. On day 3, cells were washed in PBS, trypsinized, centrifuged, and counted using a hemocytometer, then replated on 100-mm tissue culture plates. Primary tumor cells were incubated for an additional 3 days and counted. Met-1 tumor cells were replated on 100-mm plates and counted after 4 additional days.
Differentiation Assays and Quantification
To assess differentiation potential, 1×105 murine ASCs were plated on 6-well plates with DMEM supplemented with 10% FBS and 1% antibiotic/antimycotic until confluent. Adipocytes were differentiated in culture using DMEM supplemented with 10% FBS, 1% antibiotic/antimycotic solution, 0.1 μM dexamethasone (Sigma, D4902), 0.5 mM 3-isobutyl-methyl xanthine (IBMX, Sigma, I7018), and 0.5 μg/ml insulin (Sigma, I5500). ASCs were treated with vehicle-supplemented or adipocyte differentiation media for 3 weeks, and supplemented media were replaced three times weekly. Adipocyte differentiation was assessed using Oil Red O staining and quantified by extracting Oil Red O using isopropanol and measuring absorbance at 510 nm as previously described [42]. For bone differentiation, DMEM was supplemented with 10% FBS, 1% antibiotic/antimycotic solution, 100 mM ascorbic acid (Sigma, A4544), and 0.1 M β-glycerol phosphate (Sigma, 50020). ASCs were treated with bone differentiation media or vehicle-containing media for 3 weeks, and supplemented media were replaced three times weekly. Following differentiation, bone differentiation was detected and quantified using Alizarin Red staining as described [44].
Histology, Immunohistochemistry, and Immunofluorescence
Paraffin-embedded tissues were sectioned and stained with hematoxylin and eosin by the Experimental Pathology Laboratory (Carbone Cancer Center, University of Wisconsin-Madison). Tissue staining for Ki67 (Abcam, ab15580), CD31 (Biolegend, clone 390, 102401), smooth muscle actin (SMA, Sigma, A5228), GFP (Invitrogen, A-11122), and F4/80 (Biolegend, clone BM8, 123102) was performed as previously published [45]. Tissue sections were imaged using a Nikon Eclipse E600 Microscope and QICAM Fast 1394 camera. To quantify Ki67 and F4/80, images were divided into four quadrants, and the number of positive and negative cells in the top right quadrant for each image was counted. Five images were taken and quantified per slide from six tumors/group. The area of CD31+ and SMA+ staining was quantified using ImageJ from three images/tumor from six mice/group.
Tumor Invasion
Hematoxylin and eosin–stained slides of the edges of tumors surrounded by normal mammary tissue were imaged at 1000× magnification on a Nikon Eclipse E600 Microscope with a QICAM Fast 1394 camera. A border was drawn between the tumor and the mammary adipose tissue using the freehand selection tool on ImageJ. Tumor areas protruding past border line into the surrounding tissue were quantified as invasive foci. The number of invasive foci per image was averaged and analyzed using Prism.
Quantitative RT-PCR
RNA was isolated from cell pellets and tissue with TRIzol (Life Technologies, 15596026) and purified using Qiagen RNeasy Mini Kit (Qiagen, 74104). The RNA was reverse transcribed using the High Capacity cDNA Reverse Transcription Kit (Applied Biosciences, 4368814) and Techne Thermal Cycler (Techne). Quantitative PCR was performed using iTaq SYBR Green Supermix (Bio-Rad, 172-5121) with a Bio-Rad CFX Connect Real-Time PCR Detection System (Bio-Rad). Data were analyzed using the ∆Cq method, and transcripts were normalized to cyclophilin (mouse) or glyceraldehyde 3-phosphate (GAPDH; human). Primer sequences are listed in Supplementary Table 1.
Western Analysis
HFD and CD ASCs cells were grown to confluency on 10-cm plates in DMEM supplemented with 10% FBS and 1% antibiotic/antimycotic. Media were removed, and cells were washed with PBS twice. Proteins were extracted in RIPA buffer including protease and phosphatase inhibitors. Electrophoresis was performed with 4%-20% gel (Bio-Rad, 456-8093) with Tris/Glycine/SDS running buffer (Bio-Rad, 161-0772). Proteins were transferred to Amersham Hybond-ECL membrane (GE Healthcare, RPN303D). Membranes were blocked for 1 hour with 5% dry milk powder and 1% BSA (Sigma, A4503) in TBST. Membranes were probed for antibodies against SMA (Sigma-Aldrich, A5228, 1:5000), collagen I (abcam, ab34710, 1:5000), IGF-1 (R&D Systems, AF791, 1:250), or GAPDH (Invitrogen, MA5-15738, 1:5000). Secondary antibodies conjugated to horseradish peroxidase were goat anti-rabbit IgG (Invitrogen, 31460, 1:10,000), goat anti-mouse IgG (Invitrogen, 31430, 1:10,000), or rabbit anti-goat IgG (Invitrogen, 31402, 1:5000). Detection substrate used was SuperSignal West Pico Chemiluminescent Substrate (Thermo Scientific, 34080) or Clarity Western ECL Substrate (BioRad, 1705060). Amersham Hyperfilm ECL (GE Healthcare, 28-9068-38) film was used for development on the All-Pro Imaging Corp 100 Plus Automatic X-Ray Film Processor (All-Pro).
Cell Contractility Assay
Type I rat tail collagen (Corning, 354236) was diluted and neutralized in an equal volume of filter sterilized HEPES neutralization solution (2× PBS pH = 7.4, 0.1 M HEPES pH = 7.4, Sigma H4034). A total of 5×104 CD and HFD ASCs were trypsinized, counted, and added to the collagen to bring the final concentration to 1 mg/ml. The neutralized collagen and cell mixture was plated on six-well plates and incubated at 37°C. After 4 hours, the gels were released and floated in 2 ml of DMEM supplemented with 10% FBS and 1% antibiotic/antimycotic solution. The gel diameter was measured with a ruler at the time of gel release at day 0 and on days 1, 3, 5, and 6. Gels were fed after measurement on day 3. Gels were digested with collagenase (Roche, 11088793001) for 10 minutes at 37°C, and ASCs were counted to normalize the contracted area of the gel to the number of total cells on day 6. The assay was run in triplicate with three biological replicates for both CD and HFD ASCs. Contracted area was calculated using A = πr2 and subtracting the area from the beginning area at day 0. The difference in area of contraction was divided by the number of cells in the gel at day 6.
Statistics
Results were expressed as mean ± SD unless stated. Statistical differences were determined using Mann-Whitney U test or Kruskal-Wallis analysis of variance (ANOVA). Tumor growth and body weight differences were detected using two-way ANOVA analysis. P values of .05 or less were considered significant. Statistical analyses were conducted using GraphPad Prism 7.03 (GraphPad Software).
Results
Effects of HFD Feeding on ASCs
To understand the effects of obesity on stromal cells within the mammary adipose tissue, 3-week-old FVB/N and 8-week-old C57Bl/6 female mice were fed a control diet (CD) or high-fat diet (HFD) for 16 weeks. We have previously shown that HFD-fed FVB/N and C57Bl/6 mice have significantly greater weight gain than those fed the CD, resulting in increased mammary gland weight and adipocyte size [46]. We also observed the formation of crown-like structures of macrophages surrounding dying adipocytes in obese fat [46]. Consistent with our previous study, mice fed the HFD gained significantly more weight than mice fed the CD (Figures 1A, S1A). After 16 weeks, we isolated the SVF from the mammary glands, and cultured the cells to select for adherent cells. Consistent with recommendations [47], we refer to these short-term cultured SVF cells as adipose tissue–derived stromal cells (ASCs). To identify the cellular populations present in the ASC, we examined markers for endothelial cells (CD31), immune cells (CD45), epithelial cells (cytokeratins 18 and 5), and pericytes (CD31, calponin) using qRT-PCR. Transcripts for CD31, CD45, cytokeratin 5, and cytokeratin 18 were not detected (Figure S1B). Calponin expression was detectable at significantly reduced levels compared with controls; however, expression levels were not different in ASCs from CD and HFD mice (Figure S1B). These results suggest that short-term culture depleted the ASCs of endothelial cells, immune cells, and epithelial cells and did not significantly enrich for pericytes.
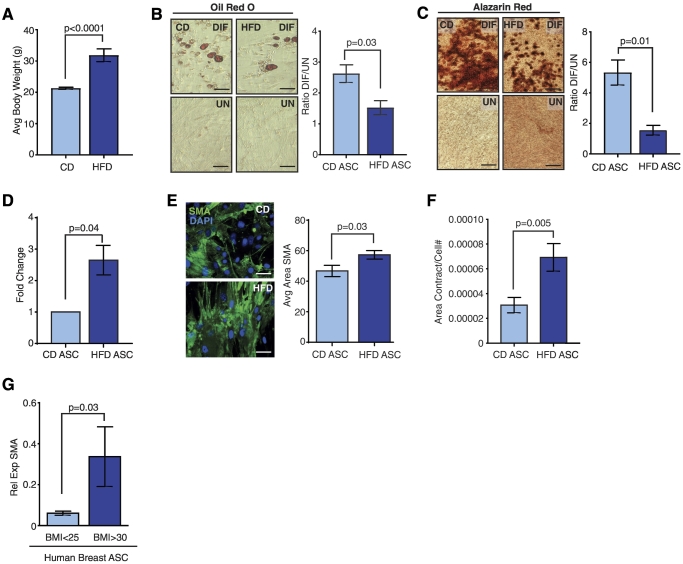
Figure 1.
HFD feeding alters normal, noncancerous mammary ASCs in mice and humans. (A) Average body weight of FVB/N female mice fed a CD or HFD for 16 weeks (n = 5 mice/group). ASCs from CD- or HFD-fed mice were differentiated in culture into (B) adipocytes or (C) osteoblasts. Representative images of cells treated with differentiation media (DIF) compared to vehicle-treated cells (UN). Data are represented as the ratio between differentiated and undifferentiated cells (n = ASCs from 5 mice/group). (D) Cellular proliferation of isolated HFD ASCs compared to CD ASCs. Data are shown as a fold change of HFD ASC proliferation compared to CD ASC proliferation (n = ASC from 3 mice/group). (E) Cultured CD and HFD ASCs were stained for SMA using immunofluorescence. SMA expression was quantified from three images (n = 3/group). (F) Average area of collagen contracted from ASCs isolated from CD or HFD-fed mice (n = 3 mice/group). Area of collagen contraction was divided by the total number of cells present at day 6 in each gel. (G) ASCs were isolated from reduction mammoplasty tissue from women with a defined BMI. Relative expression of SMA in cultured obese (BMI >30) and lean (BMI <25) ASCs was determined using qRT-PCR (n = ASCs isolated from 3 tissue samples/group). Bars represent mean ± SD. Magnification bars = 50 μm.
ASCs are a heterogeneous cell population, including multipotent adipose stem cells with the ability to differentiate into adipocytes and osteoblasts, among other lineage pathways in vitro [48], [49], [50]. Guidelines suggest that adipose stem cells are identified by expression of CD90, CD105, and CD73, with reduced expression of marker CD49f [47]. To assess how HFD feeding altered adipose stem cells within the ASC population, we quantified expression of these characterized markers in ASCs and uncultured SVF cells [47]. We observed no significant differences in CD90, CD105, and CD73 among CD and HFD ASCs and uncultured SVF cells (Figure S1C). The ASCs expressed very low levels of CD49f compared to uncultured SVF cells (Figure S1C). Together, these results suggest that ASCs from HFD-fed mice do not have significantly different expression of adipose stem cell markers compared to ASCs from lean mice. Since adipose stem cells within the ASCs have potential for multipotent differentiation in vitro, we tested the ability of ASCs from CD- or HFD-fed mice to differentiate in response to adipogenic or bone differentiation media. HFD ASCs displayed significantly decreased ability to differentiate into adipocytes containing lipid droplets (P = .03, Figure 1B) or osteoblasts secreting bone matrix (P = .01, Figure 1C) compared to CD ASCs. This reduced differentiation capacity is consistent with observations from human ASCs isolated from obese visceral and subcutaneous fat depots [27], [28], [51], [52], [53], [54]. These results suggest that obesity reduces the differentiation potential of ASCs in normal, noncancerous mammary adipose tissue.
Myofibroblasts have recently been described in the mammary adipose tissue of both obese mice and women [19], [55]. Since we observed decreased differentiation potential of HFD ASCs compared with CD ASCs, we hypothesized that the ASCs from HFD-fed mice may have increased numbers of myofibroblasts [19], [55]. HFD ASCs demonstrated significantly increased proliferation rates compared to those isolated from CD mice (P = .04, Figure 1D). HFD ASCs also had significantly increased expression of myofibroblast markers, SMA and collagen I (Col1), compared to ASCs from CD-fed mice (Figures 1E, S1D and E). To test functionally test for myofibroblasts, we plated CD and HFD ASCs into collagen and measured cellular contractility. After 6 days in culture, HFD ASCs demonstrated significantly increased contraction of the collagen gels compared to CD ASCs (Figure 1F). Together, these data suggest that obesity increases myofibroblasts within the HFD ASC population.
To examine how obesity alters ASCs in obese and lean women, we collected reduction mammoplasty tissue, isolated, and cultured the SVF. Similar to ASCs from HFD-fed mice, ASCs from obese women (BMI <30) expressed significantly higher levels of SMA transcripts compared to ASCs from lean women (BMI <25, Figure 1G). These data taken together suggest that obesity reduces the differentiation potential of adipose stem cells within ASCs and increases myofibroblasts under normal, noncancerous conditions.
ASCs from HFD-fed Mice Enhance Mammary Tumor Growth
To investigate how ASCs from HFD-fed mice impact mammary tumor progression, Met-1 mammary tumor cells were mixed with ASCs from CD or HFD-fed mice and transplanted into the inguinal mammary glands of 8-week-old FVB/N female mice fed CD. After 1 month, Met-1 tumor cells mixed with HFD ASCs formed tumors that were significantly larger than those mixed with CD ASCs (P < .001, Figures 2A, S2A). To determine whether this observed difference was specific to Met-1 cells, we repeated this experiment using C57Bl/6-derived EO771 mammary tumor cells transplanted into 8-week-old C57Bl/6 female mice fed the CD. Similar to our observations with Met-1 cells, EO771 cells mixed with HFD ASCs formed significantly larger tumors than those mixed with CD ASCs (P < .0001, Figures 2B, S2B). Consistent with increased growth rates, tumor sections from both Met-1 and EO771 tumor cells mixed with HFD ASCs demonstrated significantly increased expression of proliferation marker Ki67 (Figure 2, C and D). To assess which cells in the tumors were proliferating, tumor sections from Met-1 mixed tumors were stained with antibodies to detect the GFP-labeled tumor cells. In both CD and HFD ASC mixed tumors, 80%-90% of the cells expressed GFP (Figure S2C), suggesting that the Ki67+ cells were Met-1 cells. Together, these results suggest that ASCs from HFD-fed mice have enhanced ability to promote tumor cell growth.
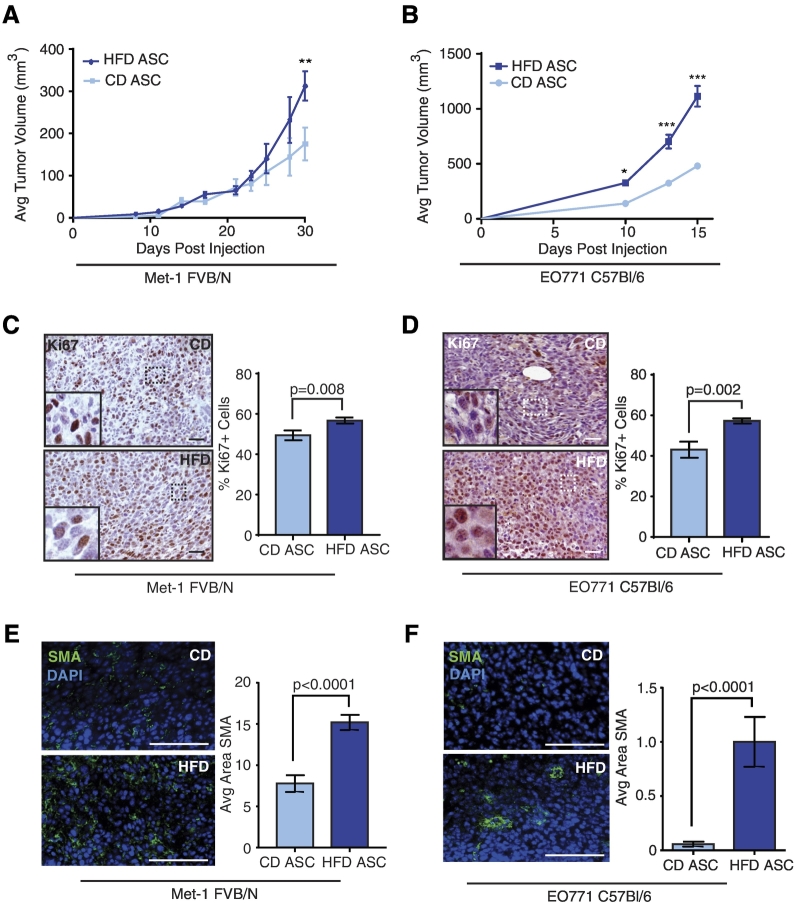
Figure 2.
ASCs from HFD-fed mice promote tumor growth. Met-1 (A) or EO771 tumor cells (B) were mixed with CD or HFD ASCs and transplanted into the inguinal mammary glands of FVB/N or C57Bl/6 female mice, respectively (n = 10 mice/group). Tumor growth is represented as mean ± SEM. Representative images of immunohistochemistry (IHC) stain for Ki67 on mixed Met-1 (C) and EO771 tumors (D). The percentage of Ki67 positive nuclei was determined from total nuclei on three images/tumor (n = 6 tumors/group). Representative images of IF stain for SMA on mixed Met-1 (E) and EO771 tumors (F). The average SMA+ area was quantified from three images/tumor (n = 6 tumors/group). Bars represent mean ± SD. Magnification bars = 50 μm.
Consistent with increased SMA expression in HFD ASCs, we observed significantly increased SMA expression in tumors from Met-1 and EO771 tumor cells mixed with HFD ASCs compared to those mixed with CD ASCs (Figure 2, E and F). We also detected collagen I–expressing cells within tumors from Met-1 tumor cells mixed with CD or HFD ASCs (Figure S2D). These results suggest that ASCs remain within the mixed tumors, consistent with other ASC mixed tumor models [56], [57]. No significant differences were observed in adipocyte numbers within the tumors of either Met-1 or EO771 tumor cells mixed with CD or HFD ASCs (Figure S2, E and F). Adipocytes present within the tumors may result from engulfed endogenous adipocytes due to rapid tumor growth.
ASCs have been shown to alter the tissue microenvironment through macrophage recruitment and angiogenesis [57], [58], [59], [60]. To determine whether HFD ASCs enhanced blood vessel density, we examined CD31 expression in endothelial cells within mixed tumors. In both Met-1 and EO771 tumors, no differences were observed in CD31 expression in tumors mixed with either CD or HFD ASCs (Figure S2, G and H). We also did not observe differences in numbers of F4/80+ tumor-associated macrophages present in either Met-1 or EO771 mixed tumors (Figure S2, I and J). These results suggest that HFD ASCs promote tumor cell proliferation rates but do not significantly recruit other stromal cell types within the tumor microenvironment compared to CD ASCs.
ASCs from HFD-Fed Mice Enhance Tumor Cell Invasion
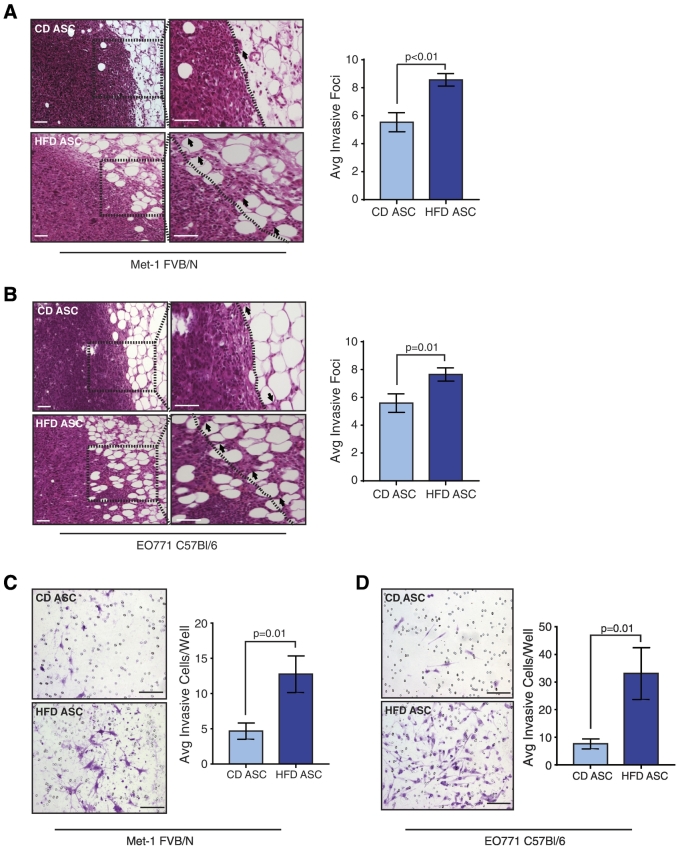
To identify differences in the morphology of the tumors, we examined tissues sections from Met-1 and EO771 mixed tumors. In both Met-1 and EO771 tumors mixed with HFD ASCs, we observed significantly increased invasive foci of tumor cells extending from the tumor border into the surrounding adipose tissue compared to controls (Figure 3, A and B). To examine the effects of the ASCs to promote tumor cell invasion, we isolated Met-1 and EO771 tumor cells from the primary tumors, plated the single cells on Matrigel-coated Transwells, and measured tumor cell invasion in response to media containing serum. Isolated tumor cells from the HFD ASC mixed tumors demonstrated significantly enhanced invasion compared to those from CD ASC mixed tumors (Figure 3, C and D). Together, these results suggest that ASCs from HFD-fed mice promote the growth of invasive tumor cells.
Figure 3.
HFD ASCs induce an invasive phenotype in mammary tumor cells. Invasive foci into the surrounding adipose tissue were quantified from Met-1 (A) or EO771 (B) tumor cells mixed with CD or HFD ASCs (n = 4 tumors/group). Dotted lines represent the edge of the tumor surrounded by adipose tissue; arrows mark invasive foci. Tumor cells from either Met-1 (C) or EO771 (D) mixed tumors were isolated and plated on Matrigel-coated Transwells. Invading cells were quantified from three images/well in triplicate (n = 3 tumors/group). Bars represent mean ± SD. Magnification bars = 50 μm.
Populations of cancer stem-like cells have been identified in tumors; these cells have acquired the ability for both local invasion as well as metastasis [61], [62], [63]. Adipose stem cells have been shown to promote the expansion of cancer stem-like cells in cancer cell lines [64]. To examine the effect of HFD ASCs on cancer stem-like cells within the mixed tumors, we plated isolated single tumor cells from the mixed HFD or CD Met-1 and EO771 tumors in limiting dilution on nonadherent plates to form tumorspheres. Similar numbers of primary Met-1 and EO771 tumorspheres were observed from both HFD and CD ASC mixed tumors (Figure S3, A and B). To assess self-renewal, we dissociated primary tumorspheres to single cells and replated the cells on nonadherent plates to form secondary tumorspheres. Similar to the primary tumorspheres, we did not observe any differences in secondary tumorsphere formation among the mixed tumors (Figure S3, A and B). Unlike our observations within sections from the primary tumors (Figure 2, C and D), we also did not observe increased proliferation rates of the isolated tumor cells from HFD ASC mixed tumors compared with CD ASC mixed tumors (Figure S3, C and D). These results suggest that HFD ASCs promote an invasive phenotype in tumor cells and other factors from within the microenvironment may be necessary to enhance CSC formation.
Promotion of invasive phenotype by secreted IGF-1
To elucidate how factors secreted by HFD ASCs promote tumor cell invasion, we plated equal numbers of HFD and CD ASCs and collected conditioned media. Met-1 tumor cells treated with conditioned media from HFD ASCs demonstrated significantly greater invasion through Matrigel Transwells than those treated with conditioned media from CD ASCs (P = .004, Figure 4A). We also plated conditioned media-treated Met-1 cells in limiting dilution on nonadherent plates to test ability to form tumorspheres. Similar to the tumor cells isolated from mixed tumors, no significant differences were detected in tumorsphere-forming ability in Met-1 cells treated with conditioned media from either HFD or CD ASCs (Figure S3E). These results suggested that secreted factors from HFD ASCs enhance an invasive tumor cell phenotype but not expansion of cancer stem-like cells.
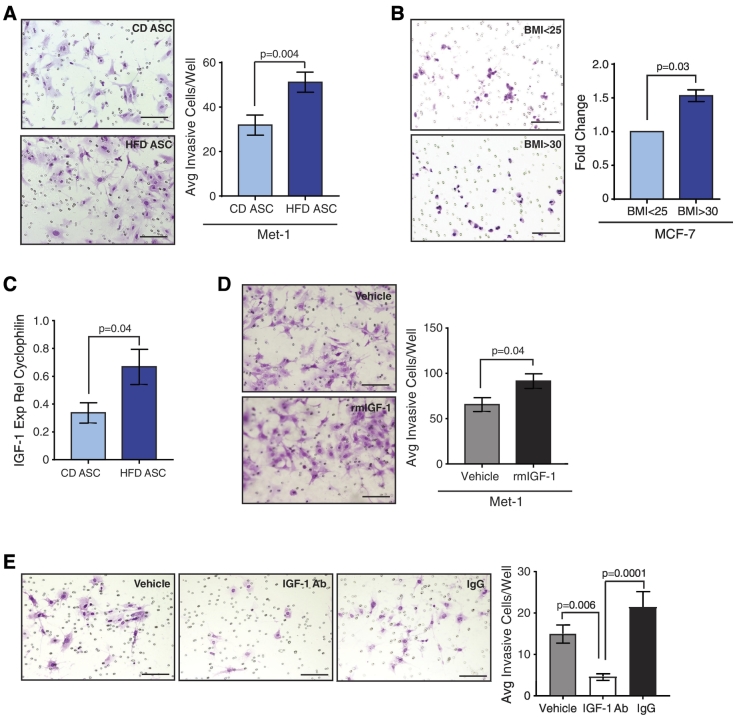
Figure 4.
IGF-1 secretion from HFD ASCs induces mammary cancer cell invasion. (A) Met-1 tumor cells were cultured in conditioned media from CD or HFD ASCs then plated on Matrigel-coated Transwells. Invasion was quantified in triplicate (n = 3 tumors/group). (B) MCF-7 cells were cultured in conditioned media from ASCs isolated from obese (BMI >30) or lean (BMI <25) patient reduction mammoplasty tissue (n = ASCs isolated from 3 tissue samples/group). Invasion was quantified in triplicate and represented as a fold change compared with invasion induced by lean BMI ASCs. (C) Relative IGF-1 expression detected using qRT-PCR from CD or HFD ASCs from FVB mice (n = 5 mice/group). (D) Met-1 cells were treated with recombinant mouse (rm) IGF-1 or vehicle and plated on Matrigel-coated Transwells. Invasion was quantified in triplicate (n = 3 experiments). (E) Met-1 cells were treated with conditioned media from HFD ASCs supplemented with control IgG or blocking antibody for IGF-1 and then plated on Matrigel-coated Transwells. Invasion was quantified in triplicate (n = 3 experiments). Bars represent mean ± SD. Magnification bars = 50 μm.
To elucidate whether ASCs from obese women also promote an invasive tumor cell phenotype, we collected conditioned media from ASCs isolated from reduction mammoplasty tissue from obese and lean women. We then treated MCF-7 tumor cells with conditioned media. Similar to HFD ASCs, conditioned media from ASCs from obese women enhanced MCF-7 cell invasion (P = .03, Figure 4B). Conditioned media–treated MCF-7 cells were also plated in limiting dilution on nonadherent plates to quantify changes in tumorsphere formation. No significant differences in primary or secondary tumorsphere formation were observed in MCF-7 cells treated with conditioned media from ASCs from obese or lean women (Figure S3F). Together, these results suggest that secreted factors from ASCs isolated from obese breast tissue promote breast cancer cell invasion.
In gene transcription studies investigating the effects of obesity on breast cancer, an IGF-1 gene signature was correlated with increased tumor aggressiveness [65], [66]. Although the major circulating source of IGF-1 is from the liver [67], we hypothesized that local synthesis of IGF-1 may be increased by ASCs in obesity. HFD ASCs had significantly increased expression of IGF-1 compared to CD ASCs (Figures 4C, S3G). To assess the effects of increased IGF-1 on an invasive phenotype of Met-1 cells, we treated Met-1 cells with recombinant mouse IGF-1 (rmIGF-1) or vehicle. Met-1 cells treated with rmIGF-1 were significantly more invasive than vehicle-treated cells (Figure 4D). To determine the role of secreted IGF-1 in the invasive phenotype induced by HFD ASCs, Met-1 cells were treated with conditioned media from HFD ASCs supplemented with vehicle, blocking antibody for IGF-1, or IgG control antibody. No significant differences were observed between vehicle-treated or IgG-treated control cells; however, treatment with the IGF-1 blocking antibody significantly abrogated invasion of Met-1 cells (Figure 4E). Together, these results suggest that IGF-1 secreted by HFD ASCs induces an invasive phenotype in tumor cells.
Weight Loss Reverses Activation of ASCs
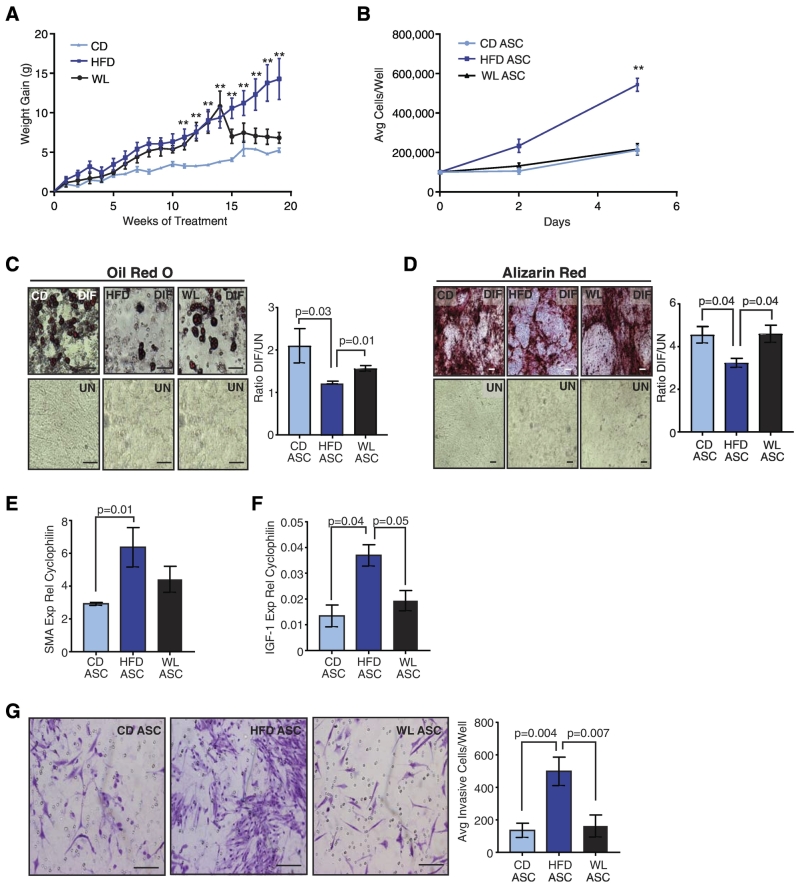
Since obesity promoted a myofibroblast phenotype in ASCs, we hypothesized that weight loss may reverse these changes. To induce weight loss, we fed 8-week-old C57Bl/6 female mice either CD or HFD for 15 weeks. After 15 weeks, half of the HFD-fed mice were switched to CD, and weight was monitored for an additional 5 weeks. Mice that were switched to the CD lost weight (5.9 ± 2.6 g; mean ± SD), and the resulting weight in these mice was similar to those fed the CD mice for 20 weeks (Figure 5A).
Figure 5.
Weight loss ameliorates changes in ASCs from HFD-fed mice. (A) C57Bl/6 mice were fed CD (n = 5 mice) or HFD (n = 10 mice). After 15 weeks, a group of HFD-fed mice was switched to the CD to induce WL for 5 weeks (n = 5 mice). Weight was measured weekly, and weight gain was calculated from difference of the total weight each week and the starting weight of each mouse. Differences in weight gain were determined using two-way ANOVA analysis (**P < .01). (B) Cellular proliferation of ASCs isolated from CD, HFD, and WL mice (n = 3 mice/group). Proliferation was quantified in duplicate, and differences were determined using two-way ANOVA (**P < .01). ASCs from CD, HFD, and WL mice were differentiated in culture into (C) adipocytes or (D) osteoblasts. Representative images of cells treated with differentiation media (DIF) compared to vehicle-treated cells (UN). Staining was quantified as described in Materials and Methods, and data are represented as the ratio between differentiated and undifferentiated cells (n = 3 mice/group). (E) Relative expression of SMA from HFD, CD, and WL ASCs measured by qRT-PCR (n = 5 mice/group). (F) Relative expression of IGF-1 from HFD, CD, and WL ASCs measured by qRT-PCR (n = 5 mice/group). (G) EO771 cells were treated with conditioned media from ASCs isolated from CD, HFD, and WL mice and then plated on Matrigel-coated Transwells. Invading cells were quantified in triplicate (n = 3 mice/group). Bars represent mean ± SD. Magnification bars = 50 μm.
To assess the effects of weight loss, ASCs were isolated from the mammary glands of CD, HFD, and WL mice. When plated in culture, HFD ASCs proliferated significantly faster than CD ASCs, while WL ASCs proliferated at a similar rate to CD ASCs (P < .001, Figure 5B). When exposed to adipogenic or bone-differentiating media in culture, HFD ASCs demonstrated reduced ability to form lipid droplets or mineralize compared to CD ASCs (Figure 5, C and D). In contrast, WL ASCs differentiated to fat or bone at a similar level to CD ASCs (Figure 5, C and D), suggesting that WL restored ability of ASCs to differentiate into fat and bone. To assess myofibroblasts, we quantified SMA expression. Similar to our observations in HFD ASCs from FVB/N mice, HFD ASCs from C57Bl/6 mice had significantly increased SMA expression compared to CD ASCs (Figure 5E). However, WL did not decrease SMA levels to those of CD ASCs (Figure 5E). These results suggest that weight loss reverses some, but not all, obesity-induced changes in mammary ASCs.
Since we observed that IGF-1 expression was significantly increased in HFD ASCs and promoted an invasive tumor cell phenotype, we examined the impact of weight loss on IGF-1 expression by ASCs. Similar to our observations in FVB/N mice, HFD ASCs from C57Bl/6 mice demonstrated significantly increased expression of IGF-1 compared to CD ASCs (Figure 5F). WL reduced the levels of IGF-1 expression to the level observed in CD ASCs (Figure 5F). These results suggest that weight loss reduces local expression of IGF-1 within mammary adipose tissue.
To test how reduction of IGF-1 expression in response to weight loss altered tumor cell invasion, we collected conditioned media from HFD, CD, and WL ASCs. EO771 cells treated with conditioned media from HFD ASCs were significantly more invasive when plated on Matrigel-coated Transwells than those treated with conditioned media from CD ASCs (Figure 5G). EO771 cells treated with conditioned media from WL ASCs reduced the number of invasive cells to the levels observed with conditioned media from CD ASCs (Figure 5G). These results suggest that weight loss reverses the ability of HFD ASCs to promote tumor cell invasion.
Discussion
Obese women diagnosed with breast tumors have a worse prognosis than lean women; an elevated BMI is correlated with increased local breast tumor invasion, incidence of recurrence, and presence of lymph node metastases [5], [6], [7]. Obesity is a complex systemic condition, resulting in increased circulating insulin and inflammatory mediators [68], [69], [70], [71], as well as localized macrophage recruitment within adipose tissue [8], [72], [73]. Here we examined how HFD feeding altered the behavior of stromal cells within the breast microenvironment. We observed that weight gain selected for ASCs with decreased ability for multipotent differentiation and increased expression of myofibroblast marker SMA. When mixed with tumor cells, HFD ASCs promoted rapid tumor growth and increased invasion into the surrounding mammary tissue compared to ASCs from CD mice. These results are consistent with those observed from ASCs derived from the visceral fat depot of obese women [14], [74], [75]. This promotion of tumor growth and invasion when implanted into the mammary glands of lean mice suggests that microenvironmental changes within the breast in response to obesity may contribute to the aggressive local invasion and lymph node metastasis observed clinically in obese breast cancer patients.
We observed that obesity decreased the ability of cells within ASCs from the mammary gland to differentiate into adipocytes and osteoblasts. Decreased plasticity in adipose stem cells isolated from visceral and subcutaneous fat has also been observed [27], [51], [53], [54], [76], [77], [78]. We also observed a significant increase in SMA expression within ASCs from obese mammary glands, suggesting that obesity promotes differentiation of ASCs toward a myofibroblast lineage. Consistent with our observations, recent studies have demonstrated increased SMA expression within adipose tissue of the mammary gland in both diet-induced obese mice as well as genetically obese mice [19]. These observations are reminiscent of changes in ASCs that were co-cultured with tumor cells; ASCs lost their ability to differentiate and promoted the growth of aggressive breast tumor xenografts [56]. This loss of differentiation potential of ASCs in obesity may result from exposure to chronic inflammatory signaling by macrophages recruited to obese adipose tissue. Macrophage-secreted factors have been shown to induce a profibrotic phenotype in preadipocytes [79], [80]. Recently, increased transforming growth factor beta within the obese mammary gland has also been shown to promote a myofibroblast phenotype in mammary ASCs [55]. The activation of ASCs toward a myofibroblast phenotype prior to tumor formation may promote the rapid growth of invasive breast tumors in obese women.
ASCs interact with other stromal cells both within the normal adipose tissue and during tumor formation. In obese adipose tissue, blood vessel density is increased [45], and ASCs promote angiogenesis in noncancerous mammary tissue through the secretion of angiogenic factors including IL-8 and VEGF [81], [82]. During tumor formation, ASCs also enhance angiogenesis [57], [83]; however, our study suggests that HFD ASCs do not further enhance this phenotype compared to CD ASCs. ASCs have also been shown to secrete cytokines, which may alter the immune microenvironment [14], [59], [60], [84]. Although we did not observe a significant increase in macrophage recruitment within the tumor microenvironment, ASCs from obese mammary glands may alter macrophage polarization. Further studies are necessary to delineate interactions of ASCs with other stromal cells within the tumor microenvironment in the context of obesity.
We observed that HFD ASCs expressed increased levels of IGF-1. Systemically, the major source of circulating IGF-1 is from the liver. In a mouse model with a liver-specific IGF-1 deletion, reduction in IGF-1 in obese mice led to significant delays in mammary tumor growth [85], suggesting that circulating IGF-1 enhances tumor growth in obesity. Our observations suggest that localized IGF-1 is also enhanced within obese breast tissue. When we inhibited IGF-1 activity in conditioned media from obese ASCs, the invasive phenotype of the mammary tumor cell lines was reversed. Consistent with our observations, treatment of human breast cancer cell lines with IGF-1 stimulated breast cancer cell growth and invasion [86], [87], as well as a gene signature associated with tumor aggressiveness [66]. This IGF-1 gene signature also correlates with an obesity-associated cancer signature generated from breast tumors from obese patients [65]. Although we observed an invasive phenotype in response to IGF-1, we did not observe significant increases in cancer stem-like cells, as measured using tumorsphere assays. This was surprising, as the IGF-1 receptor has been shown to be enriched in breast cancer stem cells identified by cell surface markers in a xenograft model of human breast cancer [88]. One possibility is that continued exposure to elevated IGF-1 is necessary to stimulate cancer stem-like cells. However, other factors that are increased in obesity have also been shown to enhance cancer stem-like cell behavior, including leptin [89], [90], [91] and inflammatory mediators increased in obese adipose tissue [84], [92]. Further studies are necessary to understand the interplay among dysregulated factors in obesity and the resulting breast tumor cell aggressiveness.
Although weight loss is recommended for obese breast cancer patients, the effects of weight loss on the risk for breast cancer recurrence are currently under investigation in long-term, clinical trials [93]. The effects of weight loss on the microenvironment of the breast are not well known but may impact breast cancer risk and recurrence. Within the visceral fat of morbidly obese patients, weight loss led to decreased expression of inflammatory genes and reduced macrophage infiltration [94], [95]. Similarly, in a mouse model of postmenopausal obesity, weight loss through calorie restriction resulted in a significant reduction in mammary gland inflammation [96]. Our study suggests that weight loss decreased ASC proliferation rates and restored multipotent differentiation potential. We also observed significantly decreased IGF-1 expression levels and diminished ability to promote tumor cell invasion. These results suggest that weight loss following obesity reduces some of the tumor promoting characteristics of the mammary microenvironment. Consistent with this idea, weight loss prior to tumor onset reduced tumor progression in a C3(1)-Tag transgenic mouse model of mammary tumorigenesis [97]. However, in a C57Bl/6 mouse model of weight loss, formerly obese mice demonstrated persistent growth of aggressive tumors, similar to that observed in obese mice [98], [99]. Mammary glands of formerly obese mice retained epigenetic changes observed in obese mice, resulting in persistent expression of inflammatory cytokines associated with obesity [98]. These results suggest that the changes in the microenvironment in response to weight loss may be time dependent. Future research is necessary to understand how weight loss may impact the breast microenvironment during treatment as well as in long-term breast cancer risk.
The following are the supplementary data related to this article.
Characterization of ASCs isolated from CD or HFD-fed mice. (A) Average body weight of C57Bl/6 female mice fed CD or HFD for 16 weeks (n=5 mice/group). (B) Relative expression of markers for endothelial cells (CD31), immune cells (CD45), epithelial cells [cytokeratin (CK) 5 and 18], and pericytes (CD31, calponin) from ASCs isolated from CD- or HFD-fed mice compared with control tissues. Expression was quantified using qRT-PCR (n=3 mice/group). (C) Relative expression of cell surface markers enriched (CD90, CD105, CD73) or depleted (CD49f) in adipose stem cell populations in CD or HFD ASCs or uncultured SVF cells. Expression was quantified using qRT-PCR (n=3 mice/group). Western analysis for (D) SMA and (E) collagen I (Col I) from CD and HFD ASCs (n=4 mice/group). GAPDH was used as a loading control, and SMA and Col I expression was normalized to GAPDH using densitometry. Bars represent mean±SD.
ASCs from HFD-fed mice do not enhance angiogenesis or tumor-associated macrophages into co-mixed tumors. Average tumor weight at end stage of Met-1 (A) or EO771 tumor cells (B) mixed with CD or HFD ASCs (n=10 tumors/group). (C) GFP expression in Met-1 tumor cells in mixed CD or HFD ASC tumors. (D) Collagen I expression in ASC from tumors of Met-1 cells mixed with CD or HFD ASCs. Adipocytes were identified within tumors from Met-1 (E) and EO771 (F) tumor cells mixed with either CD or HFD ASCs (n=6 tumors/group). CD31 expression was detected using immunofluorescence (IF) and quantified in tumors from Met-1 (G) or EO771 (H) tumor cells mixed with CD or HFD ASCs (n=6 tumors/group). F4/80+ macrophages were detected using IF and quantified from tumors from Met-1 (I) or EO771 (J) tumor cells mixed with CD or HFD ASCs (n=6 tumors/group). Bars represent mean±SD. Magnification bars=50 μm.
ASCs from HFD-fed mice do not enhance cancer stem-like cells in co-mixed tumors. Met-1 (A) or EO771 (B) tumor cells isolated from mixed tumors were plated in limiting dilution on nonadherent plates to form tumorspheres. Tumorspheres were quantified in triplicate (n=3 tumors/group). Tumorspheres were dissociated and replated to form secondary tumorspheres. Met-1 (C) or EO771 (D) tumor cells isolated from mixed tumors were plated in culture, and proliferation was quantified (n=3 tumors/group) in duplicate. Met-1 (E) and MCF-7 (F) tumor cell lines were treated with conditioned media from CD and HFD ASCs or ASCs from obese (BMI >30) and lean (BMI <25) reduction mammoplasty samples, respectively. Tumor cells were plated in limiting dilution on nonadherent plates to form tumorspheres. Tumorspheres were quantified in triplicate (n=3 tumors/group). Tumorspheres were dissociated and replated to form secondary tumorspheres. Tumorspheres from MCF-7 cells are represented as a fold change with respect to treatment with conditioned media from lean ASCs. (G) Western analysis of protein for IGF-1 and GAPDH from CD and HFD ASCs (n=4 mice/group). IGF-1 expression was normalized to GAPDH using densitometry. Bars represent mean±SD. Magnification bars=50 μm.
Primers Used for qRT-PCR Analyses
Acknowledgments
Acknowledgements
The authors would like to thank Victoria Thompson for technical assistance and Dr. Robert Lipinski for critically reading the manuscript.
Footnotes
Conflict of Interest: The authors have no conflicts of interest to disclose.
Funding: This work was supported by Susan G. Komen Career Catalyst Grant (CCR1532611) to L. M. A., NIH (T32OD010423) to T. C., NIH (T32GM007215) to L. E. H., and research funds from the Department of Comparative Biosciences and Expedition Inspiration.
References
- 1.WHO . WHO; 2017. Obesity and overweight. [doi: /entity/mediacentre/factsheets/fs311/en/index.html] [Google Scholar]
- 2.de Waard F, Baanders-van Halewijn EA. A prospective study in general practice on breast-cancer risk in postmenopausal women. Int J Cancer. 1974;14:153–160. doi: 10.1002/ijc.2910140203. [DOI] [PubMed] [Google Scholar]
- 3.IARC Working Group on the Evaluation of Cancer . IARC Press; 2002. Preventive strategies, weight control and physical activity. [Google Scholar]
- 4.Whiteman DC, Wilson LF. The fractions of cancer attributable to modifiable factors: a global review. Cancer Epidemiol. 2016;44:203–221. doi: 10.1016/j.canep.2016.06.013. [DOI] [PubMed] [Google Scholar]
- 5.Neuhouser ML, Aragaki AK, Prentice RL, Manson JE, Chlebowski R, Carty CL, Ochs-Balcom HM, Thomson CA, Caan BJ, Tinker LF. Overweight, obesity, and postmenopausal invasive breast cancer risk: a secondary analysis of the Women's Health Initiative Randomized Clinical Trials. JAMA Oncol. 2015;1:611–621. doi: 10.1001/jamaoncol.2015.1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ewertz M, Jensen MB, Gunnarsdottir KA, Hojris I, Jakobsen EH, Nielsen D, Stenbygaard LE, Tange UB, Cold S. Effect of obesity on prognosis after early-stage breast cancer. J Clin Oncol. 2011;29:25–31. doi: 10.1200/JCO.2010.29.7614. [DOI] [PubMed] [Google Scholar]
- 7.Reeves GK, Pirie K, Beral V, Green J, Spencer E, Bull D. Cancer incidence and mortality in relation to body mass index in the Million Women Study: cohort study. BMJ. 2007;335:1134. doi: 10.1136/bmj.39367.495995.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Asghar A, Sheikh N. Role of immune cells in obesity induced low grade inflammation and insulin resistance. Cell Immunol. 2017;315:18–26. doi: 10.1016/j.cellimm.2017.03.001. [DOI] [PubMed] [Google Scholar]
- 9.Rose DP, Gracheck PJ, Vona-Davis L. The interactions of obesity, inflammation and insulin resistance in breast cancer. Cancers (Basel) 2015;7:2147–2168. doi: 10.3390/cancers7040883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Olefsky JM, Glass CK. Macrophages, inflammation, and insulin resistance. Annu Rev Physiol. 2010;72:219–246. doi: 10.1146/annurev-physiol-021909-135846. [DOI] [PubMed] [Google Scholar]
- 11.Osborne CK, Monaco ME, Lippman ME, Kahn CR. Correlation among insulin binding, degradation, and biological activity in human breast cancer cells in long-term tissue culture. Cancer Res. 1978;38:94–102. [PubMed] [Google Scholar]
- 12.Milazzo G, Giorgino F, Damante G, Sung C, Stampfer MR, Vigneri R, Goldfine ID, Belfiore A. Insulin receptor expression and function in human breast cancer cell lines. Cancer Res. 1992;52:3924–3930. [PubMed] [Google Scholar]
- 13.Delort L, Rossary A, Farges MC, Vasson MP, Caldefie-Chezet F. Leptin, adipocytes and breast cancer: Focus on inflammation and anti-tumor immunity. Life Sci. 2015;140:37–48. doi: 10.1016/j.lfs.2015.04.012. [DOI] [PubMed] [Google Scholar]
- 14.Walter M, Liang S, Ghosh S, Hornsby PJ, Li R. Interleukin 6 secreted from adipose stromal cells promotes migration and invasion of breast cancer cells. Oncogene. 2009;28:2745–2755. doi: 10.1038/onc.2009.130. [onc2009130 [pii]] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ghosh S, Ashcraft K. An IL-6 link between obesity and cancer. Front Biosci (Elite Ed) 2013;5:461–478. doi: 10.2741/e628. [DOI] [PubMed] [Google Scholar]
- 16.Gimble JM, Bunnell BA, Frazier T, Rowan B, Shah F, Thomas-Porch C, Wu X. Adipose-derived stromal/stem cells: a primer. Organogenesis. 2013;9:3–10. doi: 10.4161/org.24279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Song YH, Shon SH, Shan M, Stroock AD, Fischbach C. Adipose-derived stem cells increase angiogenesis through matrix metalloproteinase–dependent collagen remodeling. Integr Biol (Camb) 2016;8:205–215. doi: 10.1039/c5ib00277j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Strong AL, Burow ME, Gimble JM, Bunnell BA. Concise review: the obesity cancer paradigm: exploration of the interactions and crosstalk with adipose stem cells. Stem Cells. 2015;33:318–326. doi: 10.1002/stem.1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Seo BR, Bhardwaj P, Choi S, Gonzalez J, Andresen Eguiluz RC, Wang K, Mohanan S, Morris PG, Du B, Zhou XK. Obesity-dependent changes in interstitial ECM mechanics promote breast tumorigenesis. Sci Transl Med. 2015;7 doi: 10.1126/scitranslmed.3010467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Caplan AI. Mesenchymal stem cells. J Orthop Res. 1991;9:641–650. doi: 10.1002/jor.1100090504. [DOI] [PubMed] [Google Scholar]
- 21.Wu Y, Kim JY, Zhou S, Smas CM. Differential screening identifies transcripts with depot-dependent expression in white adipose tissues. BMC Genomics. 2008;9:397. doi: 10.1186/1471-2164-9-397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vohl MC, Sladek R, Robitaille J, Gurd S, Marceau P, Richard D, Hudson TJ, Tchernof A. A survey of genes differentially expressed in subcutaneous and visceral adipose tissue in men. Obes Res. 2004;12:1217–1222. doi: 10.1038/oby.2004.153. [DOI] [PubMed] [Google Scholar]
- 23.Linder K, Arner P, Flores-Morales A, Tollet-Egnell P, Norstedt G. Differentially expressed genes in visceral or subcutaneous adipose tissue of obese men and women. J Lipid Res. 2004;45:148–154. doi: 10.1194/jlr.M300256-JLR200. [DOI] [PubMed] [Google Scholar]
- 24.Grandl G, Muller S, Moest H, Moser C, Wollscheid B, Wolfrum C. Depot specific differences in the adipogenic potential of precursors are mediated by collagenous extracellular matrix and Flotillin 2 dependent signaling. Mol Metab. 2016;5:937–947. doi: 10.1016/j.molmet.2016.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jeffery E, Wing A, Holtrup B, Sebo Z, Kaplan JL, Saavedra-Pena R, Church CD, Colman L, Berry R, Rodeheffer MS. The adipose tissue microenvironment regulates depot-specific adipogenesis in obesity. Cell Metab. 2016;24:142–150. doi: 10.1016/j.cmet.2016.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Frazier TP, Gimble JM, Devay JW, Tucker HA, Chiu ES, Rowan BG. Body mass index affects proliferation and osteogenic differentiation of human subcutaneous adipose tissue-derived stem cells. BMC Cell Biol. 2013;14:34. doi: 10.1186/1471-2121-14-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Strong AL, Hunter RS, Jones RB, Bowles AC, Dutreil MF, Gaupp D, Hayes DJ, Gimble JM, Levi B, McNulty MA. Obesity inhibits the osteogenic differentiation of human adipose-derived stem cells. J Transl Med. 2016;14:27. doi: 10.1186/s12967-016-0776-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Silva KR, Liechocki S, Carneiro JR, Claudio-da-Silva C, Maya-Monteiro CM, Borojevic R, Baptista LS. Stromal-vascular fraction content and adipose stem cell behavior are altered in morbid obese and post bariatric surgery ex-obese women. Stem Cell Res Ther. 2015;6:72. doi: 10.1186/s13287-015-0029-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Onate B, Vilahur G, Camino-Lopez S, Diez-Caballero A, Ballesta-Lopez C, Ybarra J, Moscatiello F, Herrero J, Badimon L. Stem cells isolated from adipose tissue of obese patients show changes in their transcriptomic profile that indicate loss in stemcellness and increased commitment to an adipocyte-like phenotype. BMC Genomics. 2013;14:625. doi: 10.1186/1471-2164-14-625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kamat P, Schweizer R, Kaenel P, Salemi S, Calcagni M, Giovanoli P, Gorantla VS, Eberli D, Andres AC, Plock JA. Human adipose-derived mesenchymal stromal cells may promote breast cancer progression and metastatic spread. Plast Reconstr Surg. 2015;136:76–84. doi: 10.1097/PRS.0000000000001321. [DOI] [PubMed] [Google Scholar]
- 31.Muehlberg FL, Song YH, Krohn A, Pinilla SP, Droll LH, Leng X, Seidensticker M, Ricke J, Altman AM, Devarajan E. Tissue-resident stem cells promote breast cancer growth and metastasis. Carcinogenesis. 2009;30:589–597. doi: 10.1093/carcin/bgp036. [bgp036 [pii]] [DOI] [PubMed] [Google Scholar]
- 32.Zhao M, Sachs PC, Wang X, Dumur CI, Idowu MO, Robila V, Francis MP, Ware J, Beckman M, Rizki A. Mesenchymal stem cells in mammary adipose tissue stimulate progression of breast cancer resembling the basal-type. Cancer Biol Ther. 2012;13:782–792. doi: 10.4161/cbt.20561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Prantl L, Muehlberg F, Navone NM, Song YH, Vykoukal J, Logothetis CJ, Alt EU. Adipose tissue-derived stem cells promote prostate tumor growth. Prostate. 2010;70:1709–1715. doi: 10.1002/pros.21206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McCready J, Arendt LM, Rudnick JA, Kuperwasser C. The contribution of dynamic stromal remodeling during mammary development to breast carcinogenesis. Breast Cancer Res. 2010;12:205. doi: 10.1186/bcr2578. [bcr2578 [pii]] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Proia DA, Kuperwasser C. Reconstruction of human mammary tissues in a mouse model. Nat Protoc. 2006;1:206–214. doi: 10.1038/nprot.2006.31. [DOI] [PubMed] [Google Scholar]
- 36.Kuperwasser C, Chavarria T, Wu M, Magrane G, Gray JW, Carey L, Richardson A, Weinberg RA. Reconstruction of functionally normal and malignant human breast tissues in mice. Proc Natl Acad Sci U S A. 2004;101:4966–4971. doi: 10.1073/pnas.0401064101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Casey AE, Laster WR, Jr., Ross GL. Sustained enhanced growth of carcinoma EO771 in C57 black mice. Proc Soc Exp Biol Med. 1951;77:358–362. doi: 10.3181/00379727-77-18779. [DOI] [PubMed] [Google Scholar]
- 38.Borowsky AD, Namba R, Young LJ, Hunter KW, Hodgson JG, Tepper CG, McGoldrick ET, Muller WJ, Cardiff RD, Gregg JP. Syngeneic mouse mammary carcinoma cell lines: two closely related cell lines with divergent metastatic behavior. Clin Exp Metastasis. 2005;22:47–59. doi: 10.1007/s10585-005-2908-5. [DOI] [PubMed] [Google Scholar]
- 39.Soule HD, Vazguez J, Long A, Albert S, Brennan M. A human cell line from a pleural effusion derived from a breast carcinoma. J Natl Cancer Inst. 1973;51:1409–1416. doi: 10.1093/jnci/51.5.1409. [DOI] [PubMed] [Google Scholar]
- 40.Kojima Y, Acar A, Eaton EN, Mellody KT, Scheel C, Ben-Porath I, Onder TT, Wang ZC, Richardson AL, Weinberg RA. Autocrine TGF-beta and stromal cell-derived factor-1 (SDF-1) signaling drives the evolution of tumor-promoting mammary stromal myofibroblasts. Proc Natl Acad Sci U S A. 2010;107:20009–20014. doi: 10.1073/pnas.1013805107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rudnick JA, Arendt LM, Klebba I, Hinds JW, Iyer V, Gupta PB, Naber SP, Kuperwasser C. Functional heterogeneity of breast fibroblasts is defined by a prostaglandin secretory phenotype that promotes expansion of cancer-stem like cells. PLoS One. 2011;6 doi: 10.1371/journal.pone.0024605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.McCready J, Arendt LM, Glover E, Iyer V, Briendel JL, Lyle SR, Naber SP, Jay DG, Kuperwasser C. Pregnancy-associated breast cancers are driven by differences in adipose stromal cells present during lactation. Breast Cancer Res. 2014;16 doi: 10.1186/bcr3594. [bcr3594 [pii];] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Phillips S, Prat A, Sedic M, Proia T, Wronski A, Mazumdar S, Skibinski A, Shirley SH, Perou CM, Gill G. Cell-state transitions regulated by SLUG are critical for tissue regeneration and tumor initiation. Stem Cell Rep. 2014;2:633–647. doi: 10.1016/j.stemcr.2014.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gregory CA, Grady Gunn W, Peister A, Prockop DJ. An Alizarin red-based assay of mineralization by adherent cells in culture: comparison with cetylpyridinium chloride extraction. Anal Biochem. 2004;329:77–84. doi: 10.1016/j.ab.2004.02.002. [DOI] [PubMed] [Google Scholar]
- 45.Arendt LM, McCready J, Keller PJ, Baker DD, Naber SP, Seewaldt V, Kuperwasser C. Obesity promotes breast cancer by CCL2-mediated macrophage recruitment and angiogenesis. Cancer Res. 2013;73:6080–6093. doi: 10.1158/0008-5472.CAN-13-0926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chamberlin T, D'Amato JV, Arendt LM. Obesity reversibly depletes the basal cell population and enhances mammary epithelial cell estrogen receptor alpha expression and progenitor activity. Breast Cancer Res. 2017;19:128. doi: 10.1186/s13058-017-0921-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bourin P, Bunnell BA, Casteilla L, Dominici M, Katz AJ, March KL, Redl H, Rubin JP, Yoshimura K, Gimble JM. Stromal cells from the adipose tissue-derived stromal vascular fraction and culture expanded adipose tissue-derived stromal/stem cells: a joint statement of the International Federation for Adipose Therapeutics and Science (IFATS) and the International Society for Cellular Therapy (ISCT) Cytotherapy. 2013;15:641–648. doi: 10.1016/j.jcyt.2013.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zuk PA, Zhu M, Mizuno H, Huang J, Futrell JW, Katz AJ, Benhaim P, Lorenz HP, Hedrick MH. Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng. 2001;7:211–228. doi: 10.1089/107632701300062859. [DOI] [PubMed] [Google Scholar]
- 49.Planat-Benard V, Silvestre JS, Cousin B, Andre M, Nibbelink M, Tamarat R, Clergue M, Manneville C, Saillan-Barreau C, Duriez M. Plasticity of human adipose lineage cells toward endothelial cells: physiological and therapeutic perspectives. Circulation. 2004;109:656–663. doi: 10.1161/01.CIR.0000114522.38265.61. [DOI] [PubMed] [Google Scholar]
- 50.Li H, Zimmerlin L, Marra KG, Donnenberg VS, Donnenberg AD, Rubin JP. Adipogenic potential of adipose stem cell subpopulations. Plast Reconstr Surg. 2011;128:663–672. doi: 10.1097/PRS.0b013e318221db33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Isakson P, Hammarstedt A, Gustafson B, Smith U. Impaired preadipocyte differentiation in human abdominal obesity: role of Wnt, tumor necrosis factor-alpha, and inflammation. Diabetes. 2009;58:1550–1557. doi: 10.2337/db08-1770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Permana PA, Nair S, Lee YH, Luczy-Bachman G, Vozarova De Courten B, Tataranni PA. Subcutaneous abdominal preadipocyte differentiation in vitro inversely correlates with central obesity. Am J Physiol Endocrinol Metab. 2004;286:E958–E962. doi: 10.1152/ajpendo.00544.2003. [DOI] [PubMed] [Google Scholar]
- 53.Onate B, Vilahur G, Ferrer-Lorente R, Ybarra J, Diez-Caballero A, Ballesta-Lopez C, Moscatiello F, Herrero J, Badimon L. The subcutaneous adipose tissue reservoir of functionally active stem cells is reduced in obese patients. FASEB J. 2012;26:4327–4336. doi: 10.1096/fj.12-207217. [DOI] [PubMed] [Google Scholar]
- 54.Mitterberger MC, Lechner S, Mattesich M, Zwerschke W. Adipogenic differentiation is impaired in replicative senescent human subcutaneous adipose-derived stromal/progenitor cells. J Gerontol A Biol Sci Med Sci. 2014;69:13–24. doi: 10.1093/gerona/glt043. [DOI] [PubMed] [Google Scholar]
- 55.Wolfson B, Zhang Y, Gernapudi R, Duru N, Yao Y, Lo PK, Zhou Q. High-fat diet promotes mammary gland myofibroblast differentiation through miR-140 downregulation. Mol Cell Biol. 2016 doi: 10.1128/MCB.00461-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chandler EM, Seo BR, Califano JP, Andresen Eguiluz RC, Lee JS, Yoon CJ, Tims DT, Wang JX, Cheng L, Mohanan S. Implanted adipose progenitor cells as physicochemical regulators of breast cancer. Proc Natl Acad Sci U S A. 2012;109:9786–9791. doi: 10.1073/pnas.1121160109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wang Y, Liu J, Jiang Q, Deng J, Xu F, Chen X, Cheng F, Zhang Y, Yao Y, Xia Z. Human adipose-derived mesenchymal stem cell-secreted CXCL1 and CXCL8 facilitate breast tumor growth by promoting angiogenesis. Stem Cells. 2017;35:2060–2070. doi: 10.1002/stem.2643. [DOI] [PubMed] [Google Scholar]
- 58.Kaplan JL, Marshall MA, CM C, Harmon DB, Garmey JC, Oldham SN, Hallowell P, McNamara CA. Adipocyte progenitor cells initiate monocyte chemoattractant protein-1-mediated macrophage accumulation in visceral adipose tissue. Mol Metab. 2015;4:779–794. doi: 10.1016/j.molmet.2015.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Reggiani F, Labanca V, Mancuso P, Rabascio C, Talarico G, Orecchioni S, Manconi A, Bertolini F. Adipose progenitor cell secretion of GM-CSF and MMP9 promotes a stromal and immunological microenvironment that supports breast cancer progression. Cancer Res. 2017;77:5169–5182. doi: 10.1158/0008-5472.CAN-17-0914. [DOI] [PubMed] [Google Scholar]
- 60.Bowles AC, Wise RM, Gerstein BY, Thomas RC, Ogelman R, Febbo I, Bunnell BA. Immunomodulatory effects of adipose stromal vascular fraction cells promote alternative activation macrophages to repair tissue damage. Stem Cells. 2017;35:2198–2207. doi: 10.1002/stem.2689. [DOI] [PubMed] [Google Scholar]
- 61.Shibue T, Weinberg RA. EMT, CSCs, and drug resistance: the mechanistic link and clinical implications. Nat Rev Clin Oncol. 2017;14:611–629. doi: 10.1038/nrclinonc.2017.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mani SA, Guo W, Liao MJ, Eaton EN, Ayyanan A, Zhou AY, Brooks M, Reinhard F, Zhang CC, Shipitsin M. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell. 2008;133:704–715. doi: 10.1016/j.cell.2008.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Peitzsch C, Tyutyunnykova A, Pantel K, Dubrovska A. Cancer stem cells: the root of tumor recurrence and metastases. Semin Cancer Biol. 2017;44:10–24. doi: 10.1016/j.semcancer.2017.02.011. [DOI] [PubMed] [Google Scholar]
- 64.Anjanappa M, Burnett R, Zieger MA, Merfeld-Clauss S, Wooden W, March K, Tholpady S, Nakshatri H. Distinct effects of adipose-derived stem cells and adipocytes on normal and cancer cell hierarchy. Mol Cancer Res. 2016;14:660–671. doi: 10.1158/1541-7786.MCR-16-0055. [DOI] [PubMed] [Google Scholar]
- 65.Creighton CJ, Sada YH, Zhang Y, Tsimelzon A, Wong H, Dave B, Landis MD, Bear HD, Rodriguez A, Chang JC. A gene transcription signature of obesity in breast cancer. Breast Cancer Res Treat. 2012;132:993–1000. doi: 10.1007/s10549-011-1595-y. [DOI] [PubMed] [Google Scholar]
- 66.Creighton CJ, Casa A, Lazard Z, Huang S, Tsimelzon A, Hilsenbeck SG, Osborne CK, Lee AV. Insulin-like growth factor-I activates gene transcription programs strongly associated with poor breast cancer prognosis. J Clin Oncol. 2008;26:4078–4085. doi: 10.1200/JCO.2007.13.4429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Belardi V, Gallagher EJ, Novosyadlyy R, LeRoith D. Insulin and IGFs in obesity-related breast cancer. J Mammary Gland Biol Neoplasia. 2013;18:277–289. doi: 10.1007/s10911-013-9303-7. [DOI] [PubMed] [Google Scholar]
- 68.Campbell KL, Landells CE, Fan J, Brenner DR. A systematic review of the effect of lifestyle interventions on adipose tissue gene expression: implications for carcinogenesis. Obesity (Silver Spring) 2017;25(Suppl. 2):S40–s51. doi: 10.1002/oby.22010. [DOI] [PubMed] [Google Scholar]
- 69.Grassmann S, Wirsching J, Eichelmann F, Aleksandrova K. Association between peripheral adipokines and inflammation markers: a systematic review and meta-analysis. Obesity (Silver Spring) 2017;25:1776–1785. doi: 10.1002/oby.21945. [DOI] [PubMed] [Google Scholar]
- 70.Barazzoni R, Gortan Cappellari G, Ragni M, Nisoli E. Insulin resistance in obesity: an overview of fundamental alterations. Eat Weight Disord. 2018 doi: 10.1007/s40519-018-0481-6. [DOI] [PubMed] [Google Scholar]
- 71.Stafeev IS, Vorotnikov AV, Ratner EI, Menshikov MY, Parfyonova YV. Latent inflammation and insulin resistance in adipose tissue. Int J Endocrinol. 2017;2017 doi: 10.1155/2017/5076732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Thomas D, Apovian C. Macrophage functions in lean and obese adipose tissue. Metabolism. 2017;72:120–143. doi: 10.1016/j.metabol.2017.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Boutens L, Stienstra R. Adipose tissue macrophages: going off track during obesity. Diabetologia. 2016;59:879–894. doi: 10.1007/s00125-016-3904-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Strong AL, Strong TA, Rhodes LV, Semon JA, Zhang X, Shi Z, Zhang S, Gimble JM, Burow ME, Bunnell BA. Obesity associated alterations in the biology of adipose stem cells mediate enhanced tumorigenesis by estrogen dependent pathways. Breast Cancer Res. 2013;15:R102. doi: 10.1186/bcr3569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Strong AL, Ohlstein JF, Biagas BA, Rhodes LV, Pei DT, Tucker HA, Llamas C, Bowles AC, Dutreil MF, Zhang S. Leptin produced by obese adipose stromal/stem cells enhances proliferation and metastasis of estrogen receptor positive breast cancers. Breast Cancer Res. 2015;17:112. doi: 10.1186/s13058-015-0622-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Pachon-Pena G, Serena C, Ejarque M, Petriz J, Duran X, Oliva-Olivera W, Simo R, Tinahones FJ, Fernandez-Veledo S, Vendrell J. Obesity determines the immunophenotypic profile and functional characteristics of human mesenchymal stem cells from adipose tissue. Stem Cells Transl Med. 2016;5:464–475. doi: 10.5966/sctm.2015-0161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wu CL, Diekman BO, Jain D, Guilak F. Diet-induced obesity alters the differentiation potential of stem cells isolated from bone marrow, adipose tissue and infrapatellar fat pad: the effects of free fatty acids. Int J Obes. 2013;37:1079–1087. doi: 10.1038/ijo.2012.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zhang Y, Nowicka A, Solley TN, Wei C, Parikh A, Court L, Burks JK, Andreeff M, Woodward WA, Dadbin A. Stromal cells derived from visceral and obese adipose tissue promote growth of ovarian cancers. PLoS One. 2015;10 doi: 10.1371/journal.pone.0136361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lacasa D, Taleb S, Keophiphath M, Miranville A, Clement K. Macrophage-secreted factors impair human adipogenesis: involvement of proinflammatory state in preadipocytes. Endocrinology. 2007;148:868–877. doi: 10.1210/en.2006-0687. [DOI] [PubMed] [Google Scholar]
- 80.Keophiphath M, Achard V, Henegar C, Rouault C, Clement K, Lacasa D. Macrophage-secreted factors promote a profibrotic phenotype in human preadipocytes. Mol Endocrinol. 2009;23:11–24. doi: 10.1210/me.2008-0183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Han TT, Toutounji S, Amsden BG, Flynn LE. Adipose-derived stromal cells mediate in vivo adipogenesis, angiogenesis and inflammation in decellularized adipose tissue bioscaffolds. Biomaterials. 2015;72:125–137. doi: 10.1016/j.biomaterials.2015.08.053. [DOI] [PubMed] [Google Scholar]
- 82.Han YD, Bai Y, Yan XL, Ren J, Zeng Q, Li XD, Pei XT, Han Y. Co-transplantation of exosomes derived from hypoxia-preconditioned adipose mesenchymal stem cells promotes neovascularization and graft survival in fat grafting. Biochem Biophys Res Commun. 2018;497:305–312. doi: 10.1016/j.bbrc.2018.02.076. [DOI] [PubMed] [Google Scholar]
- 83.Razmkhah M, Jaberipour M, Erfani N, Habibagahi M, Talei AR, Ghaderi A. Adipose derived stem cells (ASCs) isolated from breast cancer tissue express IL-4, IL-10 and TGF-beta1 and upregulate expression of regulatory molecules on T cells: do they protect breast cancer cells from the immune response? Cell Immunol. 2011;266:116–122. doi: 10.1016/j.cellimm.2010.09.005. [DOI] [PubMed] [Google Scholar]
- 84.Pinilla S, Alt E, Abdul Khalek FJ, Jotzu C, Muehlberg F, Beckmann C, Song YH. Tissue resident stem cells produce CCL5 under the influence of cancer cells and thereby promote breast cancer cell invasion. Cancer Lett. 2009;284:80–85. doi: 10.1016/j.canlet.2009.04.013. [S0304–3835(09)00271–7 [pii]] [DOI] [PubMed] [Google Scholar]
- 85.Ford NA, Nunez NP, Holcomb VB, Hursting SD. IGF1 dependence of dietary energy balance effects on murine Met1 mammary tumor progression, epithelial-to-mesenchymal transition, and chemokine expression. Endocr Relat Cancer. 2013;20:39–51. doi: 10.1530/ERC-12-0329. [DOI] [PubMed] [Google Scholar]
- 86.Walsh LA, Damjanovski S. IGF-1 increases invasive potential of MCF 7 breast cancer cells and induces activation of latent TGF-beta1 resulting in epithelial to mesenchymal transition. Cell Commun Signal. 2011;9:10. doi: 10.1186/1478-811X-9-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Sarkissyan S, Sarkissyan M, Wu Y, Cardenas J, Koeffler HP, Vadgama JV. IGF-1 regulates Cyr61 induced breast cancer cell proliferation and invasion. PLoS One. 2014;9 doi: 10.1371/journal.pone.0103534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Chang WW, Lin RJ, Yu J, Chang WY, Fu CH, Lai A, Yu JC, Yu AL. The expression and significance of insulin-like growth factor-1 receptor and its pathway on breast cancer stem/progenitors. Breast Cancer Res. 2013;15 doi: 10.1186/bcr3423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Bowers LW, Rossi EL, McDonell SB, Doerstling SS, Khatib SA, Lineberger CG, Albright JE, Tang X, deGraffenried LA, Hursting SD. Leptin signaling mediates obesity-associated CSC enrichment and EMT in preclinical TNBC models. Mol Cancer Res. 2018 doi: 10.1158/1541-7786.MCR-17-0508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Mishra AK, Parish CR, Wong ML, Licinio J, Blackburn AC. Leptin signals via TGFB1 to promote metastatic potential and stemness in breast cancer. PLoS One. 2017;12 doi: 10.1371/journal.pone.0178454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zheng Q, Banaszak L, Fracci S, Basali D, Dunlap SM, Hursting SD, Rich JN, Hjlemeland AB, Vasanji A, Berger NA. Leptin receptor maintains cancer stem-like properties in triple negative breast cancer cells. Endocr Relat Cancer. 2013;20:797–808. doi: 10.1530/ERC-13-0329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wei HJ, Zeng R, Lu JH, Lai WF, Chen WH, Liu HY, Chang YT, Deng WP. Adipose-derived stem cells promote tumor initiation and accelerate tumor growth by interleukin-6 production. Oncotarget. 2015;6:7713–7726. doi: 10.18632/oncotarget.3481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.McTiernan A. Weight, physical activity and breast cancer survival. Proc Nutr Soc. 2018:1–9. doi: 10.1017/S0029665118000010. [DOI] [PubMed] [Google Scholar]
- 94.Cancello R, Henegar C, Viguerie N, Taleb S, Poitou C, Rouault C, Coupaye M, Pelloux V, Hugol D, Bouillot JL. Reduction of macrophage infiltration and chemoattractant gene expression changes in white adipose tissue of morbidly obese subjects after surgery-induced weight loss. Diabetes. 2005;54:2277–2286. doi: 10.2337/diabetes.54.8.2277. [DOI] [PubMed] [Google Scholar]
- 95.Clement K, Viguerie N, Poitou C, Carette C, Pelloux V, Curat CA, Sicard A, Rome S, Benis A, Zucker JD. Weight loss regulates inflammation-related genes in white adipose tissue of obese subjects. FASEB J. 2004;18:1657–1669. doi: 10.1096/fj.04-2204com. [DOI] [PubMed] [Google Scholar]
- 96.Bhardwaj P, Du B, Zhou XK, Sue E, Harbus MD, Falcone DJ, Giri D, Hudis CA, Kopelovich L, Subbaramaiah K. Caloric restriction reverses obesity-induced mammary gland inflammation in mice. Cancer Prev Res (Phila) 2013;6:282–289. doi: 10.1158/1940-6207.CAPR-12-0467. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 97.Sundaram S, Freemerman AJ, Johnson AR, Milner JJ, McNaughton KK, Galanko JA, Bendt KM, Darr DB, Perou CM, Troester MA. Role of HGF in obesity-associated tumorigenesis: C3(1)-TAg mice as a model for human basal-like breast cancer. Breast Cancer Res Treat. 2013;142:489–503. doi: 10.1007/s10549-013-2741-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Rossi EL, de Angel RE, Bowers LW, Khatib SA, Smith LA, Van Buren E, Bhardwaj P, Giri D, Estecio MR, Troester MA. Obesity-associated alterations in inflammation, epigenetics, and mammary tumor growth persist in formerly obese mice. Cancer Prev Res (Phila) 2016;9:339–348. doi: 10.1158/1940-6207.CAPR-15-0348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.De Angel RE, Conti CJ, Wheatley KE, Brenner AJ, Otto G, Degraffenried LA, Hursting SD. The enhancing effects of obesity on mammary tumor growth and Akt/mTOR pathway activation persist after weight loss and are reversed by RAD001. Mol Carcinog. 2013;52:446–458. doi: 10.1002/mc.21878. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Characterization of ASCs isolated from CD or HFD-fed mice. (A) Average body weight of C57Bl/6 female mice fed CD or HFD for 16 weeks (n=5 mice/group). (B) Relative expression of markers for endothelial cells (CD31), immune cells (CD45), epithelial cells [cytokeratin (CK) 5 and 18], and pericytes (CD31, calponin) from ASCs isolated from CD- or HFD-fed mice compared with control tissues. Expression was quantified using qRT-PCR (n=3 mice/group). (C) Relative expression of cell surface markers enriched (CD90, CD105, CD73) or depleted (CD49f) in adipose stem cell populations in CD or HFD ASCs or uncultured SVF cells. Expression was quantified using qRT-PCR (n=3 mice/group). Western analysis for (D) SMA and (E) collagen I (Col I) from CD and HFD ASCs (n=4 mice/group). GAPDH was used as a loading control, and SMA and Col I expression was normalized to GAPDH using densitometry. Bars represent mean±SD.
ASCs from HFD-fed mice do not enhance angiogenesis or tumor-associated macrophages into co-mixed tumors. Average tumor weight at end stage of Met-1 (A) or EO771 tumor cells (B) mixed with CD or HFD ASCs (n=10 tumors/group). (C) GFP expression in Met-1 tumor cells in mixed CD or HFD ASC tumors. (D) Collagen I expression in ASC from tumors of Met-1 cells mixed with CD or HFD ASCs. Adipocytes were identified within tumors from Met-1 (E) and EO771 (F) tumor cells mixed with either CD or HFD ASCs (n=6 tumors/group). CD31 expression was detected using immunofluorescence (IF) and quantified in tumors from Met-1 (G) or EO771 (H) tumor cells mixed with CD or HFD ASCs (n=6 tumors/group). F4/80+ macrophages were detected using IF and quantified from tumors from Met-1 (I) or EO771 (J) tumor cells mixed with CD or HFD ASCs (n=6 tumors/group). Bars represent mean±SD. Magnification bars=50 μm.
ASCs from HFD-fed mice do not enhance cancer stem-like cells in co-mixed tumors. Met-1 (A) or EO771 (B) tumor cells isolated from mixed tumors were plated in limiting dilution on nonadherent plates to form tumorspheres. Tumorspheres were quantified in triplicate (n=3 tumors/group). Tumorspheres were dissociated and replated to form secondary tumorspheres. Met-1 (C) or EO771 (D) tumor cells isolated from mixed tumors were plated in culture, and proliferation was quantified (n=3 tumors/group) in duplicate. Met-1 (E) and MCF-7 (F) tumor cell lines were treated with conditioned media from CD and HFD ASCs or ASCs from obese (BMI >30) and lean (BMI <25) reduction mammoplasty samples, respectively. Tumor cells were plated in limiting dilution on nonadherent plates to form tumorspheres. Tumorspheres were quantified in triplicate (n=3 tumors/group). Tumorspheres were dissociated and replated to form secondary tumorspheres. Tumorspheres from MCF-7 cells are represented as a fold change with respect to treatment with conditioned media from lean ASCs. (G) Western analysis of protein for IGF-1 and GAPDH from CD and HFD ASCs (n=4 mice/group). IGF-1 expression was normalized to GAPDH using densitometry. Bars represent mean±SD. Magnification bars=50 μm.
Primers Used for qRT-PCR Analyses