Abstract
Background
It was previously found that Korean Red Ginseng water extract (KRGE) inhibits the histamine-induced itch signaling pathway in peripheral sensory neurons. Thus, in the present study, we investigated whether KRGE inhibited another distinctive itch pathway induced by chloroquine (CQ); a representative histamine-independent pathway mediated by MrgprA3 and TRPA1.
Methods
Intracellular calcium changes were measured by the calcium imaging technique in the HEK293T cells transfected with both MrgprA3 and TRPA1 (“MrgprA3/TRPA1”), and in primary culture of mouse dorsal root ganglia (DRGs). Mouse scratching behavior tests were performed to verify proposed antipruritic effects of KRGE and ginsenoside Rg3.
Results
CQ-induced Ca2+ influx was strongly inhibited by KRGE (10 μg/mL) in MrgprA3/TRPA1, and notably ginsenoside Rg3 dose-dependently suppressed CQ-induced Ca2+ influx in MrgprA3/TRPA1. Moreover, both KRGE (10 μg/mL) and Rg3 (100 μM) suppressed CQ-induced Ca2+ influx in primary culture of mouse DRGs, indicating that the inhibitory effect of KRGE was functional in peripheral sensory neurons. In vivo tests revealed that not only KRGE (100 mg) suppressed CQ-induced scratching in mice [bouts of scratching: 274.0 ± 51.47 (control) vs. 104.7 ± 17.39 (KRGE)], but also Rg3 (1.5 mg) oral administration significantly reduced CQ-induced scratching as well [bouts of scratching: 216.8 ± 33.73 (control) vs. 115.7 ± 20.94 (Rg3)].
Conclusion
The present study verified that KRGE and Rg3 have a strong antipruritic effect against CQ-induced itch. Thus, KRGE is as a promising antipruritic agent that blocks both histamine-dependent and -independent itch at peripheral sensory neuronal levels.
Keywords: ginsenoside Rg3, itch, Korean Red Ginseng, MrgprA3, TRPA1
1. Introduction
Red ginseng – a steamed root of Panax ginseng Meyer – has long been used in many Asian countries as a traditional medicine to recover strength or health. Indeed, several studies show that red ginseng holds a wide range of beneficial effects such as anti-inflammatory [1], antiviral [2], and antioxidative [3] effects. Lately, its usage has expanded to alleviate pruritus; a sensation that evokes a desire to scratch. Indeed, the administration of red ginseng or saponin fraction inhibits scratching behavior in mice induced by either compound 48/80 or histamine [4]. Furthermore, in an experimental mouse model with atopic dermatitis, red ginseng extract reduces frequent scratching behavior and improves severe skin lesions [5], [6]. More importantly, our group previously found that Korean Red Ginseng water extract (KRGE) blocks histamine-dependent itch pathways in sensory neuronal levels [7]. Thus, it appears that red ginseng is a promising antipruritic agent with numerous supportive experimental data.
Itch is a sensation felt on the skin that causes a desire to scratch; a major symptom of many skin-related diseases. Although an acute itch sensation is believed to play a role as an alerting system for allergic reactions, chronic itch causes unwanted, debilitating, uncontrollable scratching, which further intensifies itch sensation, creating a vicious cycle. For a long time, histamine has been thought to evoke itch sensations in the sensory neurons by binding to H1R (histamine receptor subtype 1) and subsequently activating TRPV1 (transient receptor potential cation channel subfamily V member 1) [8]. Indeed, one study found that histamine-induced itch behavior was significantly reduced in the TRPV1−/− mice [8].
Researchers have proposed the notion that histamine is not the exclusive pruritogen that evokes itch sensation. In fact, it is now clear that there exists a histamine-independent itch pathways as well. For instance, chloroquine (CQ), an antimalarial agent with a severe itch side effect, evokes itch sensation independent of histamine [9]. In fact, severe itch was a major cause of noncompliance to malaria treatment, showing ∼30% of African patients declined further CQ treatment [10], [11]. Although detailed molecular mechanisms underlying CQ-induced itch was elusive for decades, Wilson et al. [12] recently clarified that CQ binds to a receptor known as MrgprA3 (Mas-related G-protein coupled receptor member A3) and further relays itch signal via activation of TRPA1 (transient receptor potential cation channel, subfamily A, member 1).
The importance of the histamine-independent pathway is evident. Patients with itch-related diseases such as atopic dermatitis, xeroderma, and psoriasis often complain that conventional antihistamine agents do not alleviate their severe difficult-to-tolerate itch [13], [14]. Of course, this is because itch from these diseases are mostly histamine independent [15], [16], [17]. Not surprisingly, the CQ-induced itch also cannot be suppressed by antihistamine agents as well [18], [19]. Thus, if a novel antipruritic agent for a histamine-independent pathway is developed, it could be a milestone improvement to cure these diseases by suppressing the difficult-to-tolerate itch.
In this regard, the present study examined whether KRGE has any inhibitory effect on CQ-induced itch, a representative histamine-independent pathway mediated by MrgprA3 and TRPA1.
2. Materials and methods
2.1. Materials
KRGE was manufactured and provided by the Korea Ginseng Corporation (Seoul, Korea). Briefly, KRG was made by steaming fresh ginseng at 90–100°C for 3 h and then drying it at 50–80°C. KRGE was prepared from red ginseng water extract, which was prepared by circulating hot water (85–90°C) three times. The water content of the pooled extract was 34.41% of total weight. HPLC analysis showed that KRGE contained the following ginsenosides: Rb1, 7.53 mg/g; Rc, 2.98 mg/g; Rb2, 2.86 mg/g; Rg3, 2.09 mg/g; Re, 1.90 mg/g; Rg1, 1.78 mg/g; Rf, 1.12 mg/g; Rg2s, 1.12 mg/g; Rd, 0.89 mg/g; and Rh1, 0.84 mg/g. Ginsenosides (Rb1, Rb2, Rc, Rd, Re, Rf, Rg1, Rg3, and Rg5) were prepared by Professor Young-Sik Kim at Seoul National University, College of Pharmacy.
2.2. Cell culture and transfection of cDNA
HEK293T cells were cultured in Dulbecco's modified Eagle's medium (Life Technologies, St. Louis, MO, USA) supplemented with 10% fetal bovine serum, 100 U/mL of penicillin/streptomycin (Hyclone, Thermo Scientific, South Logan, UT, USA) at 37°C in a humidified atmosphere of 5% CO2. Cells were cotransfected with mouse MrgprA3 (NM_153067) and human TRPA1 (NM_007332) cDNAs using FuGENE HD Transfection Reagent (Promega, Madison, WI, USA). Further experiments were performed 24 h later after transfection.
2.3. Primary culture of dorsal root ganglia
Mouse dorsal root ganglia (DRGs) were primarily cultured as described previously [20]. Briefly, DRGs were dissected and collected from adult mice and cultured in Neurobasal medium (Gibco, Life Technologies, Greenland, NY, USA), which contained 10% fetal bovine serum, 50–100 ng/mL nerve growth factor (Invitrogen, Gaithersburg, MD, USA), and 100 U/mL penicillin–streptomycin solution (Hyclone, Thermo Scientific). Dissected DRGs were incubated with 1 mg/mL collagenase (Worthington Biochemical, Lakewood, NJ, USA) for 30 min at 37°C, followed by incubation for an additional 30 min at 37°C with 2.5 mg/mL trypsin (Gibco, Life Technologies). Dissociated cells were plated on poly-L-lysine-coated eight-well chambers (Lab-Tek; Thermo Scientific) and grew for ≥ 3 d at 37°C in 95% air/5% CO2.
2.4. Calcium imaging
Intracellular free Ca2+ was identified by the calcium imaging technique as previously described [21]. Briefly, the culture medium was replaced with normal bath solution [140 mM NaCl, 5.0 mM KCl, 2 mM CaCl2, 0.5 mM MgCl2, 10 mM glucose, and 5.5 mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES) (pH 7.4)] containing Fluo-3 acetoxymethyl ester (2 μM, Invitrogen) and 0.1% pluronic F-127 (Invitrogen). After incubation for 40 min, the solution containing Fluo-3 acetoxymethyl ester was washed out with normal bath solution, and 1 mM CQ was applied to the cells to elicit calcium influx. For cases with KRGE and Rg3, these compounds were preincubated for 5 min prior to CQ application. The fluorescent intensities were measured at 488 nm with interval of 1.5 s under an inverted microscope (ECLIPSE Ti-U, Nikon, Tokyo, Japan). Intracellular Ca2+ changes were expressed as F/F0 ratios, where F0 was the initial fluorescence intensity. Image analysis was performed using ImageJ (NIH, Bethesda, MD, USA) with custom-made scripts for automatic cell counting, florescence intensity calculation and ratiometric image production.
2.5. In vivo scratching behavior test
All animals were maintained according to protocols approved by the Institutional Animal Care and Use Committee of the Lee Gil Ya Cancer and Diabetes Institute (Incheon, Korea). Six-week-old male imprinting control region mice were purchased from Orient (Gyeonggi-do, Korea) and an additional 1 wk was given for accommodation. To induce scratching, 200 μg CQ dissolved in saline was injected subcutaneously in the nape of the mice. To compare the effect of KRGE and Rg3, 100 mg of KRGE (n = 6) or 1.5 mg of Rg3 (n = 10) were orally given 30 min before CQ administration in mice. After injection of CQ, behaviors were video recorded using a camcorder (HDR-CX560, Sony, Japan) for 1 h. The recorded videos were played back later, and bouts of scratching were counted by experienced observer. Mice using their hind limbs to scratch near the injected area was counted as a bout of scratching [22].
2.6. Statistical analysis
Data were presented by mean ± standard error of the mean for calcium imaging experiments, and mean ± standard deviation for in vivo experiments. For calcium imaging data, total cell numbers from at least three separate experiments were acquired and represented as n. Student's t-test was applied for comparison between two groups. For comparison of > 3 groups, one-way analysis of variance with Dunnett's multiple comparison test was used. Fisher's exact test was used to compare the cell responsiveness of the primarily cultured DRG neurons. Statistical analysis was performed by Prism 6 (GraphPad Software Inc., San Diego, CA).
3. Results
3.1. KRGE and some ginsenosides have inhibitory effects on CQ-induced MrgprA3/TRPA1 activation
In order to verify whether KRGE has inhibitory effects on CQ-induced responses, in vitro experiments were performed. Since CQ binds to MrgprA3 receptor and further relays its signal to TRPA1 ion channel allowing calcium influx [12], HEK293T cell lines transiently transfected with both MrgprA3 and TRPA1 (“MrgprA3/TRPA1”) were prepared and CQ-induced responses were measured by calcium imaging technique.
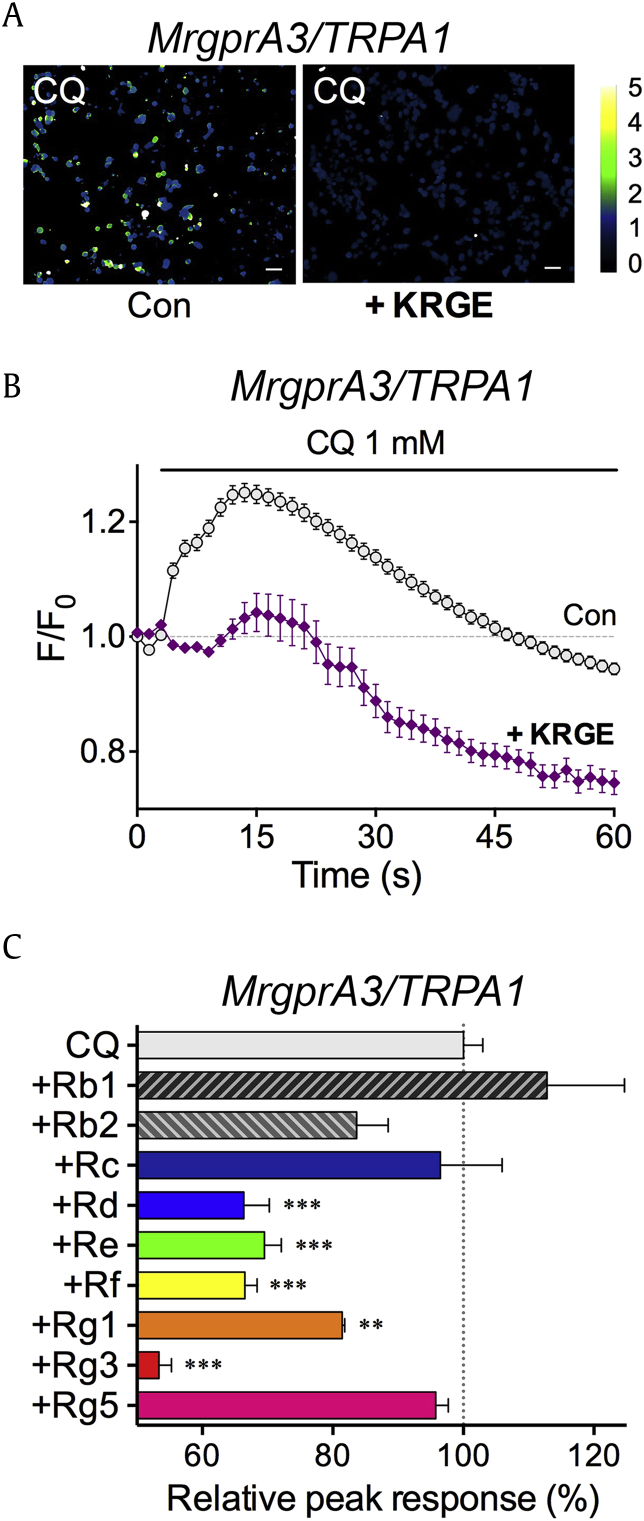
Treatment with CQ (1 mM, control, n = 336) induced intracellular calcium increase, suggesting that the expression of MrgprA3 and TRPA1 was functional (Figs. 1A, 1B). When cells were pretreated with KRGE (10 μg/mL, “+KRGE”, n = 436), however, CQ-induced calcium influx greatly decreased in MrgprA3/TRPA1 (Figs. 1A, 1B). These data indicated that KRGE had strong inhibitory effects on CQ-induced calcium influx in MrgprA3/TRPA1.
Fig. 1.
KRGE and ginsenosides inhibit CQ-induced calcium influx via MrgprA3 and TRPA1. (A) Representative ratiometric pseudocolor images of peak responses induced by CQ (1 mM) in the HEK293T cells transiently transfected with both MrgprA3 and TRPA1 (“MrgprA3/TRPA1”). “+KRGE” indicates cells pretreated with 100 μg/mL KRGE for 5 min. Scale bar indicates 50 μm. (B) A time-course graph of CQ-induced calcium influx in MrgprA3/TRPA1. F/F0 indicates fluorescence intensity (F) divided by F0, which refers to the initial fluorescence intensity at 0 s. Notice that KRGE pretreatment significantly decreased CQ-induced calcium influx. Dotted line indicates responses without CQ treatment in MrgprA3/TRPA1. (C) A comparison of various ginsenosides for their peak responses. “CQ” indicates the peak F/F0 response induced by 100 μM CQ in MrgprA3/TRPA1, regarded as 100%. Other peak responses were obtained by 100 μM CQ as well, with pretreatment of designated ginsenosides. “+” symbol in the y axis indicates that CQ was applied after pretreatment of ginsenosides. **p < 0.01, ***p < 0.001. Con, control; CQ, chloroquine; KRGE, Korean Red Ginseng Extract.
Since KRGE contains a mixture of various ginsenosides, we conducted further investigation to verify which ginsenoside had the most potent inhibitory effects against CQ-induced calcium influx. Ginsenosides Rd (66.36 ± 3.903%, n = 662, p < 0.001), Re (69.52 ± 2.602%, n = 689, p < 0.001), Rf (66.54 ± 1.859%, n = 798, p < 0.001), Rg1 (81.43 ± 0.372%, n = 541, p < 0.01), and most strongly Rg3 (53.35 ± 1.920%, n = 950, p < 0.001) showed reduced relative peak responses (Fig. 1C), indicating inhibitory effects against the CQ-induced responses in MrgprA3/TRPA1. Overall, these in vitro experiments have proved that KRGE indeed had an inhibitory effect against CQ-induced calcium influx in MrgprA3/TRPA1. It appears that a range of ginsenosides including Rg3 could be key components in terms of the inhibitory actions of KRGE over CQ-induced responses.
3.2. Rg3 dose-dependently blocks CQ-induced calcium influx in MrgrpA3/TRPA1
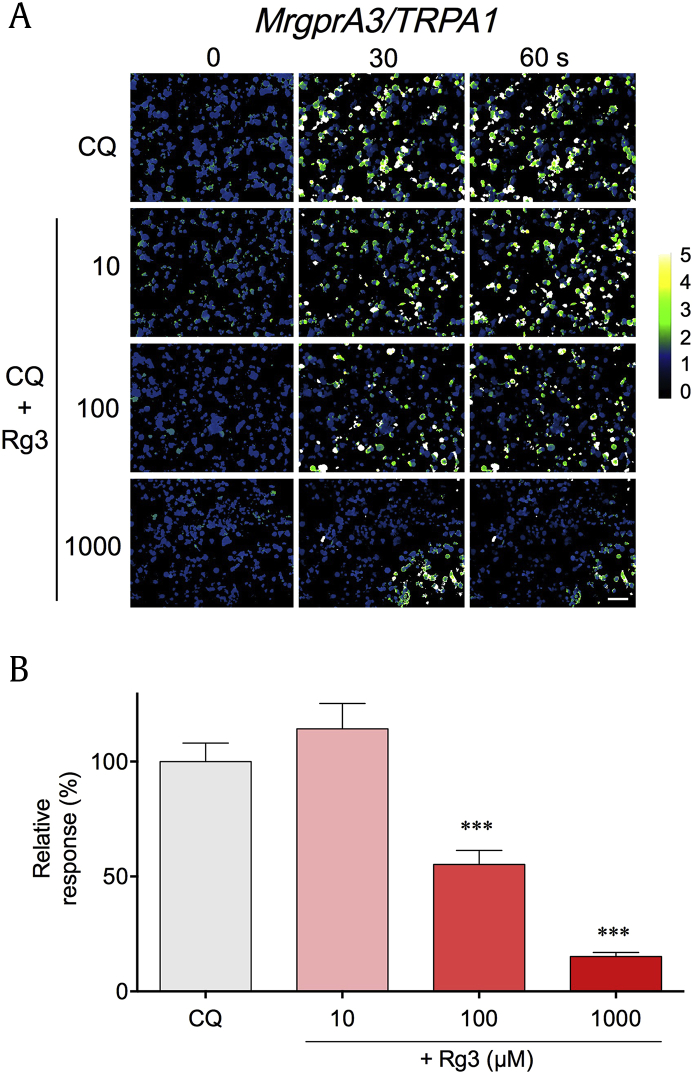
Since Rg3 showed the most potent inhibitory effects among the ginsenosides that were tested, we decided to focus on Rg3 for further investigation. To verify whether the observed inhibition achieved by Rg3 was nonspecific, different concentrations (10 μM, 100 μM, 1000 μM) of Rg3 were pretreated in MrgprA3/TRPA1 and CQ-induced calcium-specific fluorescent microscopic images were recorded. CQ-induced responses gradually decreased inversely proportional to Rg3 concentration (Fig. 2A). Indeed, the relative peak responses were significantly reduced when pretreated with 100 μM (55.23 ± 6.098%, n = 584, p < 0.001) or 1000 μM (15.16 ± 1.742%, n = 238, p < 0.001) Rg3 before CQ application (Fig. 2B). Therefore, we confirmed that Rg3 dose-dependently inhibited CQ-induced calcium influx in MrgprA3/TRPA1.
Fig. 2.
Rg3 dose-dependently inhibits CQ-induced calcium influx in MrgprA3/TRPA1. (A) Representative ratiometric pseudocolor images after CQ (1 mM) treatment with varying concentrations (10, 100, 1000 μM) of pretreated Rg3 (CQ + Rg3) in the HEK293T cells transfected with both MrgprA3 and TRPA1 (“MrgprA3/TRPA1”). Scale bar indicates 50 μm. (B) Comparison among relative peak responses with different Rg3 concentration used for pretreatment before CQ application in MrgprA3/TRPA1. ***p < 0.001. CQ, chloroquine; KRGE, Korean Red Ginseng Extract.
3.3. KRGE and Rg3 blocks CQ-induced calcium influx in mouse DRG neuron
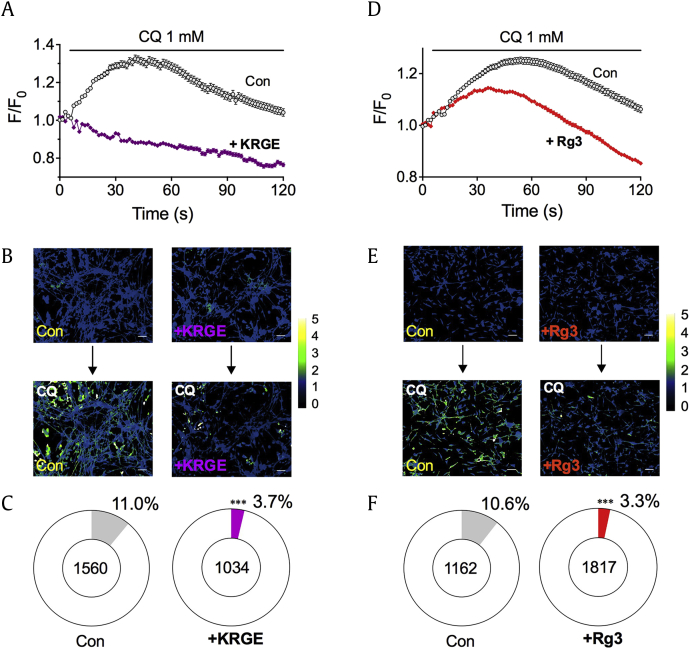
Since CQ-induced itch sensation is mediated by MrgprA3 and TRPA1 in itch-transmitting sensory neurons [12], validating whether KRGE and Rg3 blocked calcium influx induced by CQ in sensory neurons was mandatory as well. For this reason, mouse DRGs were primarily cultured and 1 mM CQ was applied. As a control, treatment of CQ elicited a calcium influx (Figs. 3A, 3B) (control, n = 1560). However, when cells were pretreated with KRGE (100 μg/mL), CQ did not evoke a calcium influx in primary culture of mouse DRGs (+KRGE, n = 1034, Figs. 3A, 3B). KRGE pretreatment not only decreased the averaged F/F0 responses, but also the total number of CQ-responsive DRGs (control: 11.0% of responsive cells among total 1162 cells vs. +KRGE: 3.7% of responsive cells among total 1034 cells, p < 0.001 by Fisher's exact test) (Fig. 3C).
Fig. 3.
KRGE and Rg3 inhibit CQ-induced calcium influx in primary culture of mouse DRGs. (A) Time-course graph of CQ-induced calcium influx in primary cultures of mouse DRGs, and comparison with pretreatment with 100 μg/mL KRGE. F/F0 indicates fluorescence intensity (F) divided by F0, which refers to the initial fluorescence intensity at 0 s. (B) Representative ratiometric pseudocolor images obtained by treatment with 1 mM CQ alone or pretreated with 100 μg/mL KRGE (“+KRGE”) in primary culture of mouse DRGs. Scale bar indicates 50 μm. (C) Comparison of responsive DRG cells between CQ (11.0% among 1560 cells) or “+KRGE” (3.7% among 1034 cells). *** p < 0.001 by Fisher's exact test. Similar data were obtained with pretreatment with 100 μM Rg3. (D) Time-course graph of CQ-induced calcium influx in primary cultures of mouse DRGs, and comparison with pretreatment with 100 μM Rg3. (E) Representative ratiometric pseudocolor images obtained by treatment with 1 mM CQ alone or pretreated with 100 μM Rg3 (“+Rg3”) in primary culture of mouse DRGs. Scale bar indicates 50 μm. (F) Comparison of responsive DRG cells between CQ (10.6% among 1162 cells) or “+Rg3” (3.3% among 1817 cells). ***p < 0.001 by Fisher's exact test. Con, control; CQ, chloroquine; DRGs, dorsal root ganglia; KRGE, Korean Red Ginseng Extract.
Similar results were also found when cells were pretreated with 100 μM Rg3. Pretreatment with 100 μM Rg3 clearly suppressed overall CQ-induced responses (“+Rg3”, n = 1193) compared to controls (control, n = 488) (Figs. 3D, 3E). Furthermore, Rg3 pretreatment not only decreased average F/F0 responses, but also reduced the number of CQ-responsive DRGs (control: 10.6% of responsive cells among total 1162 cells vs. +Rg3: 3.3% of responsive cells among total 1817 cells, p < 0.001 by Fischer's exact test) (Fig. 3F). Taken together, these data clearly indicate that both KRGE and Rg3 have strong inhibitory effects for CQ-induced calcium influx in primary culture of mouse DRG.
3.4. KRGE and Rg3 inhibited mouse scratching behaviors induced by CQ
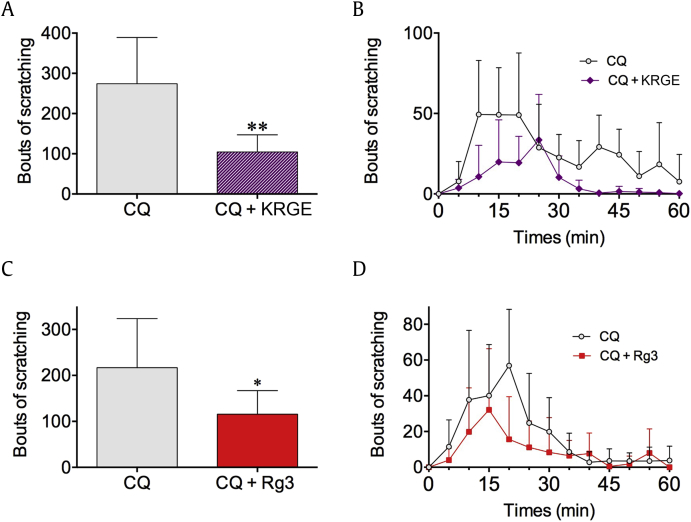
Lastly, the effects of KRGE and Rg3 on CQ-induced scratching behavior were investigated. Oral administration of KRGE (100 mg/animal) successfully decreased the bouts of scratching induced by subcutaneous CQ (200 μg/site) injection (274.0 ± 51.47 vs. 104.7 ± 17.39, n = 5 or 6, p < 0.01) (Figs. 4A, 4B). Furthermore, oral administration of Rg3 (1.5 mg/animal) also showed marked reduction of scratching behaviors (216.8 ± 33.73 vs. 115.7 ± 20.94, n = 6–10, p < 0.05) (Figs. 4C, 4D).
Fig. 4.
KRGE and Rg3 inhibit scratching behaviors induced by CQ. Both KRGE (100 mg/animal) and Rg3 (1.5 mg/animal) were administered orally 30 min before CQ subcutaneous injection (200 μg/site) in the nape. Bouts of scratching were counted right after CQ injection for 1 h. Both KRGE (A, n = 5–6) and Rg3 (C, n = 6–10) pretreatment significantly reduced total bouts of scratching. (B) and (D) show time-course graphs of bouts of scratching at 5-min intervals. Error bars indicate standard deviation. *p < 0.05, **p < 0.01. CQ, chloroquine; KRGE, Korean Red Ginseng Extract.
4. Discussion
The present study proved for the first time that KRGE is able to inhibit CQ-induced itch by blocking the MrgprA3/TRPA1 pathway. Moreover, ginsenoside Rg3 has the most potent inhibitory effect of all ginsenosides tested in the MrgprA3/TRPA1 pathway. However, it is still unclear how KRGE and some ginsenosides were able to inhibit MrgprA3/TRPA1 pathway. A plausible speculation is that TRPA1 activity might have been inhibited due to the antioxidative effect of KRGE and Rg3. TRPA1 is an ion channel that senses various pungent compounds such as mustard oil, cinnamaldehyde, formalin, acrolein, and acetaldehyde [23]. More importantly, TRPA1 can also be directly activated by H2O2 and other reactive oxygen species [24]. Since KRGE has antioxidative effects in human keratinocytes [25], KRGE may inhibit reactive oxygen species signaling in sensory neurons near the skin as well. Moreover, one stereoisomer of Rg3, 20(S)-Rg3 has antioxidative effects on cultured mammalian cell lines as well [26]. Thus, it is possible that the inhibitory effect of KRG and Rg3 were caused partially by their antioxidative effects that may further inhibit TRPA1 in the MrgprA3/TRPA1 pathway.
KRGE and Rg3 may also suppress activation of MrgprA3 activation rather than TRPA1, although the exact molecular mechanisms are elusive at the moment. Considering that activated MrgprA3 stimulates TRPA1 through its Gβγ complex [12], it is likely that KRGE and Rg3 may alter or interfere with Gβγ functions of MrgprA3. Also, a possibility cannot be ruled out that KRGE and Rg3 may interfere with binding of CQ to MrgprA3. Therefore, these possibilities imply that further in-depth study is warranted in order to figure out the exact molecular mechanisms of KRGE and Rg3 on MrgprA3.
Another plausible theory could be related to nitric oxide (NO). NO is a short-lived messenger involved in various activities such as muscle relaxation, inhibition of platelet activation, neurotransmission, and cytotoxicity [27]. Interestingly, CQ is known to stimulate NO synthesis in certain cell types [28], [29]. More importantly, CQ-induced scratching is mediated by an NO/cyclic guanosine monophosphate pathway in mice [30]. Thus, it appears that CQ induces itch in part by producing NO. Alternatively, fractions of red ginseng are able to suppress NO production in certain pathophysiological conditions [31], [32]. Moreover, ginsenoside Rg3 is believed to suppress NO production in inflammasomes [33]. Thus, it is expected that KRGE and Rg3 may exhibit antipruritic effects by inhibition of NO production, which could be promoted by CQ. However, this explanation needs to be proven by further experiments because there are some contradictory results related to NO. For instance, there are reports that KRGE and Rg3 are inducing NO production, especially in blood vessels and endothelial cells, thereby resulting in vasodilation and blood pressure decrease [34], [35]. Taken together, one might assume that the inhibition of NO production by KRGE and Rg3 may have suppressed CQ-induced itch, but further study is required to clarify the discrepancy between NO and KRGE and/or Rg3.
Cytotoxicity is always a concern when high concentrations of compounds are used in experiments. In the case of Rg3, however, the treatment time is short (5 min) when compared to conventional cytotoxicity experiments, which often last at ≥ 24 h. Although we have not tested cytotoxicity of Rg3, it is expected that the cytotoxic impact of Rg3 in the present experimental conditions would be negligible for two reasons. First, throughout the experiments, there were no morphological changes in HEK293T cells at all after incubation, even with 1000 μM Rg3. In general, morphology changes when cells are treated with cytotoxic compounds, but such a change was not observed in the current study. Secondly, previous studies have indicated that treatment of various cancer cells with 100 μM Rg3 for 24 h did not significantly affect cell survival [36], [37], implying that there will be negligible cytotoxic impact for 5-min treatment. Therefore, it appears that the inhibitory effect of Rg3 is not derived from nonspecific cytotoxicity.
The anti-pruritic effect of KRGE and Rg3 is not new. For instance, Rg3 reduced the skin severity scores of lesions induced by 2-chloro-1,3,5-trinitrobenzene in an atopic dermatitis mouse model [38]. Likewise, KRGE showed a therapeutic effect on atopic dermatitis-like lesions in mice [39]. In other words, it has already been proven that KRGE has strong antipruritic effects against various kinds of itch sensations. Considering that KRGE also possesses antipruritic effects on the histamine-dependent H1R/TRPV1 pathway [7], our current findings suggest that KRGE is a dual potent antipruritic candidate agent that can block both H1R/TRPV1 and MrgprA3/TRPA1 pathways.
In conclusion, the present study found that KRGE and its constituent Rg3 have antipruritic effects against a CQ-induced scratching mouse model. Moreover, the underlying mechanism of inhibitory action by KRGE and/or Rg3 could be inhibition of the MrgprA3/TRPA1 pathway responsible for CQ-induced itch at a sensory neuronal level. Considering that the MrgprA3/TRPA1 pathway represents histamine-independent itch, KRGE and specifically Rg3 could be used as novel histamine-independent antipruritic agents.
Conflicts of interest
Authors have declared that there are no conflicts of interest.
Acknowledgments
This work was supported by 2012 and 2014 grants from the Korean Society of Ginseng funded by Korea Ginseng Corporation (2012-5068, 2014-5173) and by Gachon Institute of Pharmaceutical Sciences Research Fund (2015-5131).
References
- 1.Baek K.S., Yi Y.S., Son Y.J., Yoo S., Sung N.Y., Kim Y., Hong S., Aravinthan A., Kim J.H., Cho J.Y. In vitro and in vivo anti-inflammatory activities of Korean Red Ginseng-derived components. J Ginseng Res. 2016;40:437–444. doi: 10.1016/j.jgr.2016.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Im K., Kim J., Min H. Ginseng, the natural effectual antiviral: protective effects of Korean Red Ginseng against viral infection. J Ginseng Res. 2016;40:309–314. doi: 10.1016/j.jgr.2015.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee C.H., Kim J.H. A review on the medicinal potentials of ginseng and ginsenosides on cardiovascular diseases. J Ginseng Res. 2014;38:161–166. doi: 10.1016/j.jgr.2014.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Trinh H.T., Shin Y.W., Han S.J., Han M.J., Kim D.H. Evaluation of antipruritic effects of red ginseng and its ingredients in mice. Planta Med. 2008;74:210–214. doi: 10.1055/s-2008-1034313. [DOI] [PubMed] [Google Scholar]
- 5.Samukawa K., Izumi Y., Shiota M., Nakao T., Osada-Oka M., Miura K., Iwao H. Red ginseng inhibits scratching behavior associated with atopic dermatitis in experimental animal models. J Pharmacol Sci. 2012;118:391–400. doi: 10.1254/jphs.11182fp. [DOI] [PubMed] [Google Scholar]
- 6.Lee J.H., Cho S.H. Korean red ginseng extract ameliorates skin lesions in NC/Nga mice: an atopic dermatitis model. J Ethnopharmacol. 2011;133:810–817. doi: 10.1016/j.jep.2010.11.020. [DOI] [PubMed] [Google Scholar]
- 7.Jang Y., Lee W.J., Hong G.S., Shim W.S. Red ginseng extract blocks histamine-dependent itch by inhibition of H1R/TRPV1 pathway in sensory neurons. J Ginseng Res. 2015;39:257–264. doi: 10.1016/j.jgr.2015.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shim W.S., Tak M.H., Lee M.H., Kim M., Kim M., Koo J.Y., Lee C.H., Kim M., Oh U. TRPV1 mediates histamine-induced itching via the activation of phospholipase A2 and 12-lipoxygenase. J Neurosci. 2007;27:2331–2337. doi: 10.1523/JNEUROSCI.4643-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu Q., Tang Z., Surdenikova L., Kim S., Patel K.N., Kim A., Ru F., Guan Y., Weng H.J., Geng Y. Sensory neuron-specific GPCR Mrgprs are itch receptors mediating chloroquine-induced pruritus. Cell. 2009;139:1353–1365. doi: 10.1016/j.cell.2009.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mnyika K.S., Kihamia C.M. Chloroquine-induced pruritus: its impact on chloroquine utilization in malaria control in Dar es Salaam. J Trop Med Hyg. 1991;94:27–31. [PubMed] [Google Scholar]
- 11.Sowunmi A., Fehintola F.A., Adedeji A.A., Falade A.G., Falade C.O., Akinyinka O.O., Oduola A.M. Comparative efficacy of chloroquine plus chlorpheniramine alone and in a sequential combination with sulfadoxine-pyrimethamine, for the treatment of acute, uncomplicated, falciparum malaria in children. Ann Trop Med Parasitol. 2000;94:209–217. doi: 10.1080/00034980050006375. [DOI] [PubMed] [Google Scholar]
- 12.Wilson S.R., Gerhold K.A., Bifolck-Fisher A., Liu Q., Patel K.N., Dong X., Bautista D.M. TRPA1 is required for histamine-independent, Mas-related G protein-coupled receptor-mediated itch. Nat Neurosci. 2011;14:595–602. doi: 10.1038/nn.2789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Patel T., Yosipovitch G. Therapy of pruritus. Expert Opin Pharmacother. 2010;11:1673–1682. doi: 10.1517/14656566.2010.484420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bigby M. A thorough systematic review of treatments for atopic eczema. Arch Dermatol. 2001;137:1635–1636. doi: 10.1001/archderm.137.12.1635. [DOI] [PubMed] [Google Scholar]
- 15.Reich A., Szepietowski J.C. Mediators of pruritus in psoriasis. Mediators Inflamm. 2007;2007:64727. doi: 10.1155/2007/64727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wilson S.R., Nelson A.M., Batia L., Morita T., Estandian D., Owens D.M., Lumpkin E.A., Bautista D.M. The ion channel TRPA1 is required for chronic itch. J Neurosci. 2013;33:9283–9294. doi: 10.1523/JNEUROSCI.5318-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shim W.S., Oh U. Histamine-induced itch and its relationship with pain. Mol Pain. 2008;4:29. doi: 10.1186/1744-8069-4-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Abila B., Ezeamuzie I.C., Igbigbi P.S., Ambakederemo A.W., Asomugha L. Effects of two antihistamines on chloroquine and histamine induced weal and flare in healthy African volunteers. Afr J Med Med Sci. 1994;23:139–142. [PubMed] [Google Scholar]
- 19.Ezeamuzie C.I., Igbigbi P.S., Asomugha L., Ambakederemo A.W., Abila B., Assem E.S. Urine methylhistamine concentrations before and after chloroquine in healthy black subjects. J Trop Med Hyg. 1990;93:423–425. [PubMed] [Google Scholar]
- 20.Pradhananga S., Shim W.S. Caffeic acid exhibits anti-pruritic effects by inhibition of multiple itch transmission pathways in mice. Eur J Pharmacol. 2015;762:313–321. doi: 10.1016/j.ejphar.2015.06.006. [DOI] [PubMed] [Google Scholar]
- 21.Jang Y., Kim S.W., Oh J., Hong G.S., Seo E.K., Oh U., Shim W.S. Ghrelin receptor is activated by naringin and naringenin, constituents of a prokinetic agent Poncirus fructus. J Ethnopharmacol. 2013;148:459–465. doi: 10.1016/j.jep.2013.04.039. [DOI] [PubMed] [Google Scholar]
- 22.Kuraishi Y., Nagasawa T., Hayashi K., Satoh M. Scratching behavior induced by pruritogenic but not algesiogenic agents in mice. Eur J Pharmacol. 1995;275:229–233. doi: 10.1016/0014-2999(94)00780-b. [DOI] [PubMed] [Google Scholar]
- 23.Bang S., Hwang S.W. Polymodal ligand sensitivity of TRPA1 and its modes of interactions. J Gen Physiol. 2009;133:257–262. doi: 10.1085/jgp.200810138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sawada Y., Hosokawa H., Matsumura K., Kobayashi S. Activation of transient receptor potential ankyrin 1 by hydrogen peroxide. Eur J Neurosci. 2008;27:1131–1142. doi: 10.1111/j.1460-9568.2008.06093.x. [DOI] [PubMed] [Google Scholar]
- 25.Hong C.E., Lyu S.Y. Anti-inflammatory and anti-oxidative effects of Korean Red Ginseng Extract in human keratinocytes. Immune Netw. 2011;11:42–49. doi: 10.4110/in.2011.11.1.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shin Y.M., Jung H.J., Choi W.Y., Lim C.J. Antioxidative, anti-inflammatory, and matrix metalloproteinase inhibitory activities of 20(S)-ginsenoside Rg3 in cultured mammalian cell lines. Mol Biol Rep. 2013;40:269–279. doi: 10.1007/s11033-012-2058-1. [DOI] [PubMed] [Google Scholar]
- 27.Gross S.S., Wolin M.S. Nitric oxide: pathophysiological mechanisms. Annu Rev Physiol. 1995;57:737–769. doi: 10.1146/annurev.ph.57.030195.003513. [DOI] [PubMed] [Google Scholar]
- 28.Chen T.H., Chang P.C., Chang M.C., Lin Y.F., Lee H.M. Chloroquine induces the expression of inducible nitric oxide synthase in C6 glioma cells. Pharmacol Res. 2005;51:329–336. doi: 10.1016/j.phrs.2004.10.004. [DOI] [PubMed] [Google Scholar]
- 29.Ghigo D., Aldieri E., Todde R., Costamagna C., Garbarino G., Pescarmona G., Bosia A. Chloroquine stimulates nitric oxide synthesis in murine, porcine, and human endothelial cells. J Clin Invest. 1998;102:595–605. doi: 10.1172/JCI1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Foroutan A., Haddadi N.S., Ostadhadi S., Sistany N., Dehpour A.R. Chloroquine-induced scratching is mediated by NO/cGMP pathway in mice. Pharmacol Biochem Behav. 2015;134:79–84. doi: 10.1016/j.pbb.2015.04.016. [DOI] [PubMed] [Google Scholar]
- 31.Bak M.J., Hong S.G., Lee J.W., Jeong W.S. Red ginseng marc oil inhibits iNOS and COX-2 via NFkappaB and p38 pathways in LPS-stimulated RAW 264.7 macrophages. Molecules. 2012;17:13769–13786. doi: 10.3390/molecules171213769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cho S.O., Lim J.W., Kim H. Red ginseng extract inhibits the expression of MCP-1 and iNOS in Helicobacter pylori-infected gastric epithelial cells by suppressing the activation of NADPH oxidase and Jak2/Stat3. J Ethnopharmacol. 2013;150:761–764. doi: 10.1016/j.jep.2013.09.013. [DOI] [PubMed] [Google Scholar]
- 33.Yoon S.J., Park J.Y., Choi S., Lee J.B., Jung H., Kim T.D., Yoon S.R., Choi I., Shim S., Park Y.J. Ginsenoside Rg3 regulates S-nitrosylation of the NLRP3 inflammasome via suppression of iNOS. Biochem Biophys Res Commun. 2015;463:1184–1189. doi: 10.1016/j.bbrc.2015.06.080. [DOI] [PubMed] [Google Scholar]
- 34.Hien T.T., Kim N.D., Pokharel Y.R., Oh S.J., Lee M.Y., Kang K.W. Ginsenoside Rg3 increases nitric oxide production via increases in phosphorylation and expression of endothelial nitric oxide synthase: essential roles of estrogen receptor-dependent PI3-kinase and AMP-activated protein kinase. Toxicol Appl Pharmacol. 2010;246:171–183. doi: 10.1016/j.taap.2010.05.008. [DOI] [PubMed] [Google Scholar]
- 35.Jeon B.H., Kim C.S., Kim H.S., Park J.B., Nam K.Y., Chang S.J. Effect of Korean red ginseng on blood pressure and nitric oxide production. Acta Pharmacol Sin. 2000;21:1095–1100. [PubMed] [Google Scholar]
- 36.Zhang Y.H., Li H.D., Li B., Jiang S.D., Jiang L.S. Ginsenoside Rg3 induces DNA damage in human osteosarcoma cells and reduces MNNG-induced DNA damage and apoptosis in normal human cells. Oncol Rep. 2014;31:919–925. doi: 10.3892/or.2013.2914. [DOI] [PubMed] [Google Scholar]
- 37.Wu K., Li N., Sun H., Xu T., Jin F., Nie J. Endoplasmic reticulum stress activation mediates Ginseng Rg3-induced anti-gallbladder cancer cell activity. Biochem Biophys Res Commun. 2015;466:369–375. doi: 10.1016/j.bbrc.2015.09.030. [DOI] [PubMed] [Google Scholar]
- 38.Kim H.S., Kim D.H., Kim B.K., Yoon S.K., Kim M.H., Lee J.Y., Kim H.O., Park Y.M. Effects of topically applied Korean red ginseng and its genuine constituents on atopic dermatitis-like skin lesions in NC/Nga mice. Int Immunopharmacol. 2011;11:280–285. doi: 10.1016/j.intimp.2010.11.022. [DOI] [PubMed] [Google Scholar]
- 39.Sohn E.H., Jang S.A., Lee C.H., Jang K.H., Kang S.C., Park H.J., Pyo S. Effects of korean red ginseng extract for the treatment of atopic dermatitis-like skin lesions in mice. J Ginseng Res. 2011;35:479–486. doi: 10.5142/jgr.2011.35.4.479. [DOI] [PMC free article] [PubMed] [Google Scholar]