Abstract
Background
Korean ginseng (Panax ginseng) plays an anti-inflammatory role in a variety of inflammatory diseases such as gastritis, hepatitis, and colitis. However, inflammation-regulatory activity of the calyx of the P. ginseng berry has not been thoroughly evaluated. To understand whether the calyx portion of the P. ginseng berry is able to ameliorate inflammatory processes, an ethanolic extract of P. ginseng berry calyx (Pg-C-EE) was prepared, and lipopolysaccharide-activated macrophages and HEK293 cells transfected with inflammation-regulatory proteins were used to test the anti-inflammatory action of Pg-C-EE.
Methods
The ginsenoside contents of Pg-C-EE were analyzed by HPLC. Suppressive activity of Pg-C-EE on NO production, inflammatory gene expression, transcriptional activation, and inflammation signaling events were examined using the Griess assay, reverse transcription-polymerization chain reaction, luciferase activity reporter gene assay, and immunoblotting analysis.
Results
Pg-C-EE reduced NO production and diminished mRNA expression of inflammatory genes such as cyclooxygenase-2, inducible NO synthase, and tumor necrosis factor-α in a dose-dependent manner. This extract suppressed luciferase activity induced only by nuclear factor-κB. Interestingly, immunoblotting analysis results demonstrated that Pg-C-EE reduced the activities of protein kinase B (AKT)1 and AKT2.
Conclusion
These results suggest that Pg-C-EE may have nuclear-factor-κB-targeted anti-inflammatory properties through suppression of AKT. The calyx of the P. ginseng berry is an underused part of the ginseng plant, and development of calyx-derived extracts may be useful for treatment of inflammatory diseases.
Keywords: AKT, anti-inflammatory activity, calyx of berry, nuclear factor-κB, Panax ginseng
1. Introduction
Inflammation is an innate immune response requiring numerous biological processes to protect the body from infection by pathogens, including bacteria, viruses, and fungi [1], [2], [3]. Toll-like receptors (TLRs) greatly contribute to managing the innate immune system. The proteins are expressed in immune cells such as antigen-presenting cells (tissue macrophages and dendritic cells), which detect structurally conserved molecules derived from microbes [4], [5], [6]. Pathogen-activated ligands of TLRs include lipopolysaccharide (LPS) from Gram-negative bacteria, recognizing TLR4; poly(I:C) from virus-like particles, recognizing TLR3; and peptidoglycan/pam3CSK from Gram-positive bacteria, recognizing TLR2 [7], [8], [9]. The activation of TLRs in macrophages causes cells to trigger various signaling events managed by adaptive molecules such as myeloid differentiation primary response protein (MyD)88 and TRIF (TIR-domain-containing adapter-inducing interferon-β), tyrosine kinases (Janus kinase, spleen tyrosine kinase and Src), and serine–threonine kinases [protein kinase B (AKT), phosphoinositide-dependent kinase-1, mitogen-activated protein kinases (extracellular signal-regulated kinase, p38, and C-Jun N-terminal kinase), and inhibitor of κB kinase (IKK)] to activate various transcription factors such as nuclear factor (NF)-κB [p65 (Rel A) and p50], activator protein (AP)-1 (c-Fos, c-Jun, and activating transcription factor 2), cAMP response element-binding protein, and interferon regulatory transcription factor [10], [11], [12]. Due to the progress of inflammatory signaling pathways and the activation of transcription factors, new proteins involved in the inflammatory process are synthesized; these include platelet-activating factors, secretion of inflammatory mediators, NO, prostaglandin (PG)E2, chemokines, and cytokines including tumor necrosis factor (TNF)-α, interleukin (IL)-1β, and IL-6 [3], [13], [14], [15]. Although the inflammatory barrier is critically important in the defensive response, continuous inflammation can make the human body more prone to serious disease conditions such as cancer, diabetes, and cardiovascular and autoimmune diseases [16]. Thus, finding anti-inflammatory remedies targeted to decreasing prolonged inflammation could help us to treat such serious diseases [17], [18].
Korean ginseng has been widely used for a long time as an ethnopharmacologically valuable herb against numerous diseases in Korea, Japan, and China [19], [20]. Korean Red Ginseng (KRG) is known to contain ginsenosides, acid polysaccharides, and fatty acids as major components. Ginsenosides are composed of protopanaxadiol and protopanaxatriol [21], [22], [23], [24], [25], [26], [27], which have known pharmacological features and biological activities including immune enhancement, anti-inflammation effects, antioxidant effects, memory enhancement, platelet-aggregation inhibitory effects, improvement of menopausal disorders, and induction of metabolic energy [28], [29], [30], [31], [32]. The inflammation-regulatory effects of KRG have been reported in several papers [33], [34]; however, the immunopharmacological roles and cellular mechanisms of the Korean ginseng calyx (a peduncle of ginseng) is poorly understood. In this study, we evaluated the phytochemical profiles and anti-inflammatory cellular mechanisms of an ethanol extract of the Panax ginseng calyx (Pg-C-EE) by measuring inflammation-related cytokines, transcription factors, and expression levels of proteins under in vitro conditions.
2. Materials and methods
2.1. Materials
LPS, LY294002, Nω-nitro-l-arginine methyl ester (l-NAME), and polyethylenimine were from Sigma–Aldrich (St. Louis, MO, USA). LY294002 was purchased from Calbiochem (Darmstadt, Germany). Standard ginsenosides (G-Rg1, -Re, -Rb1, -Rc, -Rb2, and -Rd) were obtained from Ambo Institute (Daejeon, Korea). 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) was purchased from Amresco (Brisbane, Australia). TRIzol reagent was purchased from MRCgene (Cincinnati, OH, USA) and cDNA synthesis kit was purchased from Thermo Fisher Scientific (Waltham, MA, USA). The forward and reverse primer sets used for reverse transcription-polymerase chain reaction (RT-PCR) were synthesized by Macrogen (Seoul, Korea), and PCR premix was purchased from Bio-D Inc. (Seoul, Korea). Constructs for signaling proteins (FLAG–MyD88, cyan fluorescent protein (CFP)–TRIF, AKT1–hemagglutinin (HA), and AKT2–HA) and luciferase constructs with promoters with binding sites for NF-κB and AP-1 were used as reported previously [33], [34]. Antibodies were purchased from Cell Signaling Technology (Beverly, MA, USA). The luciferase assay system was purchased from Promega (Madison, WI, USA). RAW264.7 and HEK293 cells were purchased from the American Type Culture Collection (Manassas, VA, USA). RPMI 1640 medium and Dulbecco's modified Eagle's medium, fetal bovine serum (FBS), and penicillin–streptomycin were obtained from HyClone (Logan, UT, USA).
2.2. Preparation of Pg-C-EE
Korean ginseng calyx was harvested and dried in hot air and refluxed with 70% ethanol for 10 h. After the extract was filtered and concentrated under reduced pressure at 45°C, Pg-C-EE powder was finally prepared by lyophilization and stored at −20°C until it was used.
2.3. Cell culture
RAW264.7 cells were usually maintained in RPMI 1640 with 10% FBS and 1% penicillin–streptomycin, and HEK293 cells were cultured in Dulbecco's modified Eagle's medium with 5% FBS and 1% penicillin–streptomycin at 37°C, in a 5% CO2 humidified incubator.
2.4. Animals
Male, 6-wk-old Balb/C mice purchased from ORIENT (Gyeonggi, Korea) were housed under a 12-h light/dark cycle (lights on at 6:00 am). Animal care followed the guidelines from the National Institute of Health for the Care and Use of Laboratory Animals (NIH Publication 80-23, revised in 1996). This study was approved by the Institutional Animal Care and Use Committee at Sungkyunkwan University (Suwon, Korea; Approval ID: SKKUBBI 13-6-4).
2.5. Preparation of peritoneal macrophages
Peritoneal macrophages from Balb/C mice were prepared according to previously described methods [35].
2.6. NO production assay
RAW264.7 cells were seeded at 105 cells/well in 96-well plates for 24 h. After 24 h, RAW264.7 cells were pretreated with Pg-C-EE and l-NAME and induced with LPS (1 μg/mL) for 12 h. NO production level was measured using the Griess assay [36].
2.7. Cell viability assay
RAW264.7 cells were seeded at 105 cells/well in 96-well plates for 24 h. After 24 h, RAW264.7 cells were treated with Pg-C-EE and induced with LPS (1 μg/mL) for 12 h. Cell viability level was measured using the MTT assay [37].
2.8. HPLC
The content of ginsenosides in the ginseng calyx extract was determined using the HPLC-UV method, as reported previously [38], [39]. The mobile phase consisted of a mixture of acetonitrile (A) and water (B). The initial composition was 20% (A) and 80% (B) and gradient elution was as follows; 0–10 min, 20% A; 10–40 min, 32% A; 40–48 min, 42% A; 48–50 min, 100% A; 50–60 min, 100% A; 60–62 min, 20% A; and 62–70 min, 20% A. The flow rate was 1 mL/min, and the injection volume was 10 μL.
2.9. mRNA analysis by RT-PCR
Total RNA prepared from RAW264.7 cells treated with LPS (1 μg/mL) for 6 h in the presence or absence of Pg-C-EE was isolated using TRIzol reagent. cDNA from 1,000 ng total RNA was synthesized using a cDNA synthesis kit (Thermo Fisher Scientific). RT-PCR was conducted using the specific forward and reverse primers for inflammatory genes [40] as listed in Table 1.
Table 1.
Primers used for reverse transcription-polymerase chain reaction
| Name | Sequence (from 5′ to 3′) | |
|---|---|---|
| iNOS | F | GGAGCCTTTAGACCTCAACAGA |
| R | TGAACGAGGAGGGTGGTG | |
| TNF-α | F | TGCCTATGTCTCAGCCTCTTC |
| R | GAGGCCATTTGGGAACTTCT | |
| COX-2 | F | GGGAGTCTGGAACATTGTGAA |
| R | GCACATTGTAAGTAGGTGGACTGT | |
| GAPDH | F | CAATGAATACGGCTACAGCAAC |
| R | AGGGAGATGCTCAGTGTTGG |
COX-2, cyclooxygenase 2; iNOS, inducible NO synthase; TNF-α, tumor necrosis factor-α.
2.10. Luciferase reporter gene assay
HEK293 cells were transfected with β-galactosidase and NF-κB-Luc or AP-1-Luc (0.7 μg/mL each) with adaptor proteins (MyD88 or TRIF; 0.7 μg/mL each) using the polyethylenimine method in a 24-well plate as reported previously [41]. The cells were treated with Pg-C-EE for 24 h prior to termination. Luciferase assays were performed using the Luciferase Assay System (Promega) as reported previously [29]. Luciferase activity was normalized to β-galactosidase activity.
2.11. Preparation of total lysates and nuclear extracts and immunoblotting
RAW264.7 cells were treated with Pg-C-EE. HEK293 cells were independently transfected with each gene (AKT1-HA, AKT2-HA, or HA) for 24 h and treated with Pg-C-EE for 24 h. Total lysates and nuclear extracts were prepared as described previously [42]. Immunoblotting of phosphorylated or total levels of transcription factor (p65 and p50), IκBα, IKKα/β, AKT, and p85/PI3K (phosphoinositide 3-kinase) was performed as described previously [42]. β-Actin was used as a loading control.
2.12. Statistical analysis
All in vitro data are expressed as mean ± standard deviation of experiments performed with six samples each. All other data presented are representative of three independent experiments. Statistical significance of our results was evaluated by analysis of variance and Scheffe's post hoc test and the Kruskal–Wallis and Mann–Whitney U tests using SPSS version 22.0 (IBM Corp., Armonk, NY, USA).
3. Results
3.1. Effect of Pg-C-EE on NO production
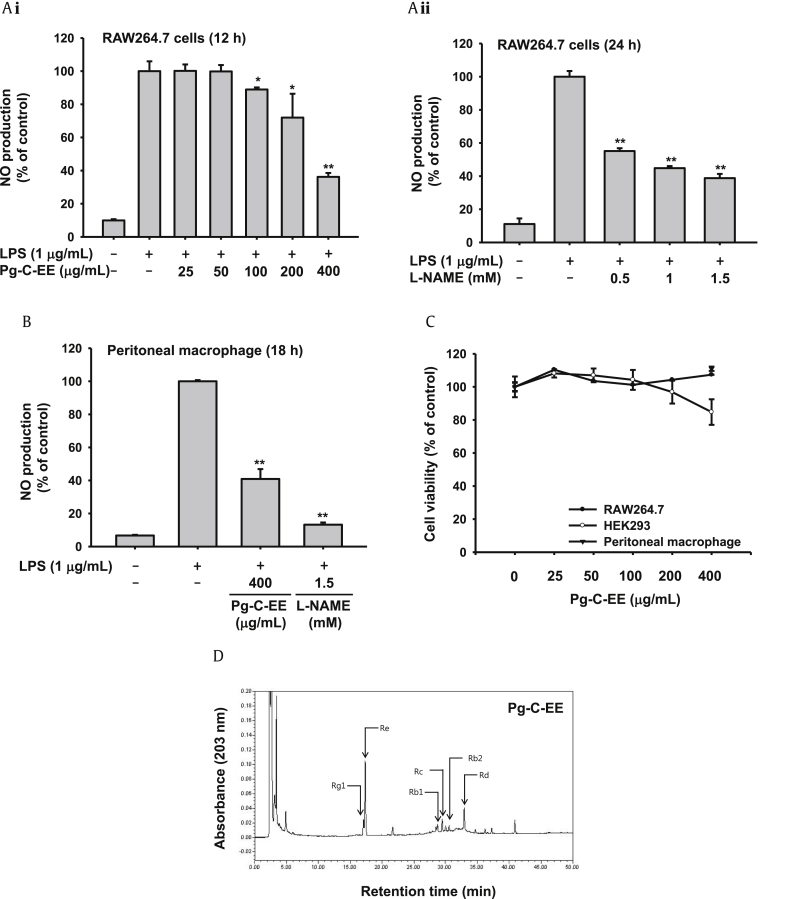
To evaluate whether Pg-C-EE is able to regulate inflammatory mediator production, the inhibitory level of Pg-C-EE on NO production in LPS-treated RAW264.7 cells was examined. Pg-C-EE dose-dependently suppressed NO production in LPS-stimulated RAW264.7 cells. The suppressive levels of Pg-C-EE in NO production displayed 11% (100 μg/mL), 28% (100 μg/mL), and 64% (400 μg/mL) (Fig. 1Ai). l-NAME, a standard drug with direct inhibitory activity against inducible NO synthase (iNOS) and constitutive NO synthase, strongly decreased NO production under the same conditions, exhibiting inhibition levels of 55.1% at 0.5mM, 44.8% at 1mM, and 38.8% at 1.5mM (Fig. 1Aii). Pg-C-EE also inhibited NO production in LPS-treated peritoneal macrophages. The suppressive level of Pg-C-EE in NO production was 59.1% at 400 μg/mL, while that of l-NAME (1.5mM) was 86.7% (Fig. 1B). Furthermore, Pg-C-EE did not display any cytotoxicity in RAW264.7 cells, HEK293 cells, or peritoneal macrophages (Fig. 1C). To check the phytochemical finger printing of Pg-C-EE, HPLC analysis was used. As Fig. 1D and Table 2 show, Pg-C-EE contains ginsenoside (G)-Rg1 (1.38%), G-Re (6%) of protopanaxatriol and G-Rb1 (0.61%), G-Rc (1.18%), G-Rb2 (0.5%), and G-Rd (0.93%) of protopanaxadiaol.
Fig. 1.
Effect of NO production of Pg-C-EE in RAW264.7 cells, and HPLC analysis of Pg-C-EE. (A) The level of NO production was determined from culture supernatants of LPS-activated RAW264.7 cells pretreated with Pg-C-EE (i) or l-NAME (ii). (B) The level of NO production was determined from peritoneal macrophage treated with LPS in the presence or absence of Pg-C-EE or l-NAME. (C) Viability of RAW264.7 cells and HEK293 cells incubated with different concentrations of Pg-C-EE determined using the MTT assay. (D) Phytochemical characteristics of ginsenosides in Pg-C-EE were examined by high performance liquid chromatography. *p < 0.05 and **p < 0.01 compared to the normal or control group. l-NAME, Nω-nitiro-l-arginine methyl ester; LPS, lipopolysaccharide; MTT, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide; Pg-C-EE, ethanolic extract of Panax ginseng berry calyx.
Table 2.
Ginsenoside components of Pg-C-EE by HPLC analysis
| Unit: % | PPT |
PPD |
PPT/PPD | ||||
|---|---|---|---|---|---|---|---|
| Rg1 | Re | Rb1 | Rc | Rb2 | Rd | ||
| Pg-C-EE | 1.38 | 6.00 | 0.61 | 1.18 | 0.5 | 0.93 | 2.29 |
Pg-C-EE, ethanolic extract of Panax ginseng berry calyx; PPD, protopanaxadiol; PPT, protopanaxatriol.
3.2. Effect of Pg-C-EE on expression of inflammatory genes and transcriptional activation of NF-κB
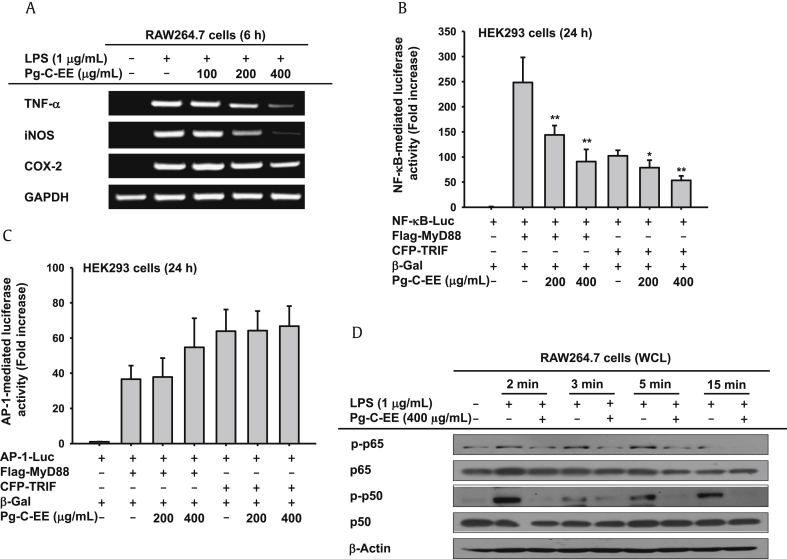
To confirm inhibition of Pg-C-EE on the expression of inflammation-regulating genes including TNF-α, iNOS, and COX-2, mRNA expression levels were measured by RT-PCR. Pg-C-EE (200 μg/mL and 400 μg/mL) significantly reduced the mRNA levels of TNF-α and iNOS under LPS-induced conditions (Fig. 2A). COX-2 was slightly decreased at 400 μg/mL (Fig. 2A). To further understand transcriptional regulation of Pg-C-EE, the luciferase reporter gene assay performed with HEK293 cells was used. HEK293 cells were cotransfected with the TLR adaptor molecules MyD88 and TRIF to activate both NF-κB and AP-1. Pg-C-EE dose-dependently inhibited NF-κB-mediated luciferase activities triggered by both TRIF (increased up to 144-fold) and MyD88 (248.4-fold), while Pg-C-EE did not suppress AP-1-mediated luciferase activity triggered by MyD88 and TRIF (Figs. 2B, 2C). Since Pg-C-EE inhibited only NF-κB-mediated luciferase activity (Fig. 2B), we next confirmed whether Pg-C-EE could block the phosphorylation of NF-κB subunits using immunoblotting analysis with whole cell lysates. As we expected, Pg-C-EE decreased the phosphorylation of p65 and p50 from 2 min to 15 min (Fig. 2D).
Fig. 2.
Effect of Pg-C-EE on mRNA expression of inflammatory genes and the activation of the NF-κB pathway. (A) mRNA expression of inflammatory genes (TNF-α, iNOS, and COX-2) was determined from total RNA extracts of RAW264.7 cells treated with Pg-C-EE (100 mg/mL, 200 mg/mL, and 400 μg/mL) and LPS (1 μg/mL) using reverse transcription-polymerase chain reaction. (B and C) HEK293 cells were cotransfected with NF-κB-Luc or AP-1-Luc and adaptor proteins (MyD88 or TRIF) as well as β-Gal plasmid constructs in the presence or absence of Pg-C-EE. Luciferase activity was measured. (D) Effects of Pg-C-EE on total and phosphorylated NF-κB subunits (p65, p50, p-p65, and p-p50) were determined using immunoblotting analysis. *p < 0.05 and **p < 0.01 compared to control group. COX-2, cyclooxygenase-2; β-Gal, β-galactosidase; iNOS, inducible NO synthase; LPS, lipopolysaccharide; MyD88, myeloid differentiation primary response protein 88; NF-κB, nuclear factor-κB; Pg-C-EE, ethanolic extract of Panax ginseng berry calyx; TNF-α, tumor necrosis factor-α; TRIF, TIR-domain-containing adapter-inducing interferon-β; WCL, whole cell lysate.
3.3. Effect of Pg-C-EE on upstream target of NF-κB
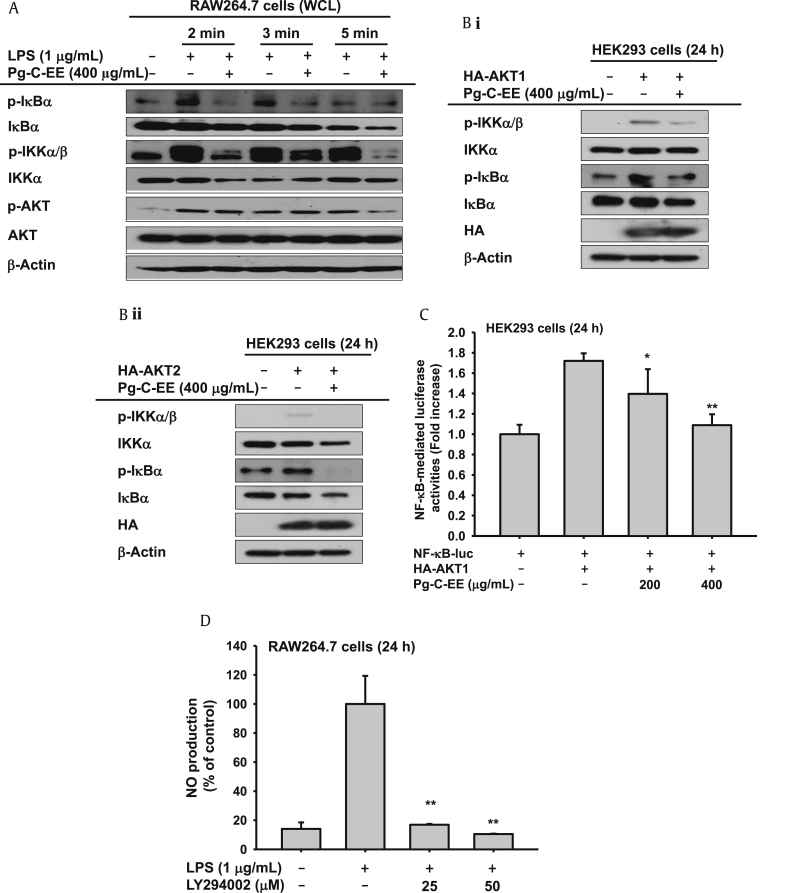
To know the exact target of Pg-C-EE in the NF-κB phosphorylation pathway, the activation patterns of upstream enzymes were examined by immunoblotting analysis. Pg-C-EE blocked the phosphorylation of IκBα and IKKα/β from 2 min and 15 min, but did not change the phospho-AKT level at 2 min and 3 min (Fig. 3A), indicating that AKT might be directly targeted by Pg-C-EE. To validate this possibility, additional biochemical experiments were performed using overexpression conditions with HA–AKT1 or HA–AKT2 constructs. Overexpression of AKT1 or AKT2 induced increased levels of phospho-IKKα/β and phospho-IκBα, whereas Pg-C-EE strongly reduced these upregulation patterns (Fig. 3B). To confirm transcriptional regulation of Pg-C-EE, luciferase reporter gene assay done with HEK293 cells was used. HEK293 cells were cotransfected with AKT1 and NF-κB. Similar to the results shown in Fig. 2B where Pg-C-EE inhibited only NF-κB-mediated luciferase activity, Pg-C-EE dose-dependently reduced NF-κB-mediated luciferase activities triggered under AKT1 transfected conditions (Fig. 3C).
Fig. 3.
Effect of Pg-C-EE and LPS on the activation of upstream signaling protein for the NF-κB pathway. (A) RAW264.7 cells were incubated with LPS (1 μg/mL) in the presence or absence of Pg-C-EE. After preparing WCLs, the expression of total or phosphorylated IκBα, IKKα/β, IKKα, and AKT was identified using immunoblotting analysis. (B) HEK293 cells were transfected with AKT1 (i) and AKT2 (ii) for 24 h and were then treated with Pg-C-EE for an additional 24 h. After preparing WCLs, expression of total or phosphorylated IκBα, IKKα/β, and AKT as well HA, a tagging protein of AKT1 and AKT2, was detected by immunoblotting analysis. (C) HEK293 cells were cotransfected with NF-κB-Luc and AKT1 as well as β-Gal plasmid constructs in the presence or absence of Pg-C-EE. (D) Level of NO production released from LPS-activated RAW264.7 cells treated with LPS in the presence or absence of LY294002 was measured by Griess assay. AKT, protein kinase B; β-Gal, β-galactosidase; HA, hemagglutinin; IKK, inhibitor of κB kinase; LPS, lipopolysaccharide; NF-κB, nuclear factor-κB; Pg-C-EE, ethanolic extract of Panax ginseng berry calyx; WCL, whole cell lysates.
To confirm the importance of the AKT pathway in the NO production response of LPS-treated RAW264.7 cells, the inhibitory activity of LY294002 was examined under the same conditions. LY294002 completely abrogated the release of NO in LPS-activated RAW264.7 cells (Fig. 3D).
4. Discussion
Numerous studies have indicated that Korean ginseng and KRG exhibit anti-inflammatory properties. However, Pg-C-EE has not yet been studied for its inflammatory regulatory activity or its phytochemical ingredients. In this study, therefore, we aimed to investigate phytochemical profiles and anti-inflammatory responses of Pg-C-EE by performing HPLC analysis and measuring NO production, inflammation-regulatory genes, transcription factor activation patterns, and expression level of regulatory proteins functioning transcription factor regulation under in vitro inflammatory conditions using LPS-activated RAW264.7 cells and HEK293 cells stimulated by inflammation regulatory genes.
We determined that Pg-C-EE can be used as an anti-inflammatory agent because it suppresses NO production (Fig. 1Ai) as much as l-NAME (Fig. 1Aii) without displaying cytotoxicity (Fig. 1C). We also determined that the mRNA levels of TNF-α, iNOS, and COX-2 were attenuated dose-dependently in the Pg-C-EE-treated group (Fig. 2A). Pg-C-EE inhibited transcriptional activation of NF-κB in LPS-induced macrophages; we also learned that Pg-C-EE blocked the nuclear and phosphorylation protein levels of NF-κB (Fig. 2, Fig. 3A) and that NF-κB-mediated luciferase activities were downregulated (Fig. 2B). The roles of AKT1 and AKT2 in the inflammatory process have been previously studied. The AKT signaling pathway is known to play a central role in causing various inflammation-mediated diseases, such as atherosclerosis, multiple sclerosis, asthma, psoriasis, and rheumatoid arthritis [32], [43], [44], [45], [46], [47], [48] and enhance NF-κB activation by directly phosphorylating IKKα in response by LPS or Pam3CSK [49]. Therefore, activated IKKα causes degradation of IκB and NF-κB and acts as a transcription factor during nuclear translocation where it promotes expression of IL-1β, IL-18, and TNF-α mRNA boosting the inflammatory response [50], [51]. Similarly, phosphorylation of PI3K/AKT was strongly enhanced by LPS treatment (Figs. 3A, 3B). To verify the role of PI3K/AKT in NO production, we used LY294002, a PI3K inhibitor (Fig. 3D), as it was reported to suppress activation of some target enzymes such as mammalian target of rapamycin, casein kinase 2, and glycogen synthase kinase 3β [52]. These results suggest that Pg-C-EE may be used as an anti-inflammatory agent specifically targeted to AKT1- and AKT2-mediated inflammation responses such as those seen in rheumatoid arthritis.
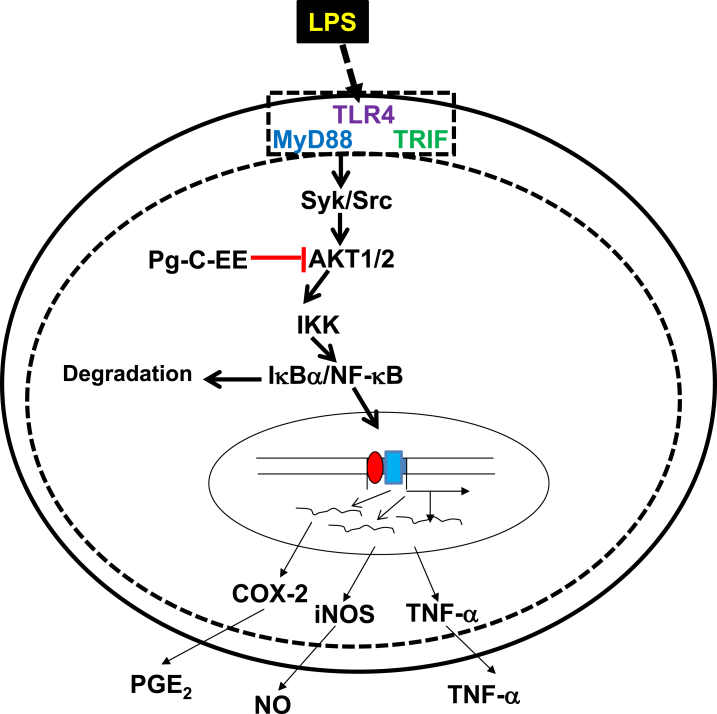
In summary, we found that Pg-C-EE inhibited inflammatory responses through the production of NO and expression of TNF-α, iNOS, and COX-2 in LPS-induced RAW264.7 cells. Additionally, Pg-C-EE inhibited NF-κB transcription factor and its upstream activation pathway. We demonstrated by immunoblotting that Pg-C-EE blocked both AKT1 and AKT2 enzymes in the NF-κB signal pathway. A brief summary of our results is shown in Fig. 4. Taken together, these results suggest that Pg-C-EE can attenuate macrophage-mediated inflammatory responses and thus could be developed as an AKT targeted anti-inflammatory agent.
Fig. 4.
Pg-C-EE inhibition pathway in macrophage-mediated inflammatory NF-κB. Pg-C-EE might interfere the AKT-mediated NF-κB activation pathway in its anti-inflammatory action linked to suppression of mRNA expression of COX-2, iNOS, and TNF-α. COX-2, cyclooxygenase 2; IKK, inhibitor of κB kinase; iNOS, inducible NO synthase; NF-κB, nuclear factor-κB; Pg-C-EE, ethanolic extract of Panax ginseng berry calyx; PGE2, prostaglandin E2; Syk, spleen tyrosine kinase; TLR4, Toll-like receptor 4; TNF-α, tumor necrosis factor-α.
Conflicts of interest
The authors report no conflict of interests.
Contributor Information
Jong-Hoon Kim, Email: jhkim1@chonbuk.ac.kr.
Song Seok Shin, Email: ssshin@amorepacific.com.
Jae Youl Cho, Email: jaecho@skku.edu.
References
- 1.Zou J., Guo P., Lv N., Huang D. Lipopolysaccharide-induced tumor necrosis factor-α factor enhances inflammation and is associated with cancer (Review) Mol Med Rep. 2015;12:6399–6404. doi: 10.3892/mmr.2015.4243. [DOI] [PubMed] [Google Scholar]
- 2.Ferrero−Miliani L., Nielsen O., Andersen P., Girardin S. Chronic inflammation: importance of NOD2 and NALP3 in interleukin-1β generation. Clin Exp Immunol. 2007;147:227–235. doi: 10.1111/j.1365-2249.2006.03261.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Roberts-Thomson I.C., Fon J., Uylaki W., Cummins A.G., Barry S. Cells, cytokines and inflammatory bowel disease: a clinical perspective. Expert Rev Gastroenterol Hepatol. 2011;5:703–716. doi: 10.1586/egh.11.74. [DOI] [PubMed] [Google Scholar]
- 4.Mahla R.S., Reddy C.M., Prasad D., Kumar H. Sweeten PAMPs: role of sugar complexed PAMPs in innate immunity and vaccine biology. Front Immunol. 2013;4:248. doi: 10.3389/fimmu.2013.00248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ribeiro-Gomes F., Silva M., Dosreis G. Neutrophils, apoptosis and phagocytic clearance: an innate sequence of cellular responses regulating intramacrophagic parasite infections. Parasitology. 2006;132:S61–S68. doi: 10.1017/S0031182006000862. [DOI] [PubMed] [Google Scholar]
- 6.Galluzzi L., Buqué A., Kepp O., Zitvogel L., Kroemer G. Immunogenic cell death in cancer and infectious disease. Nat Rev Immunol. 2016;17:97–111. doi: 10.1038/nri.2016.107. [DOI] [PubMed] [Google Scholar]
- 7.Fukata M., Abreu M.T. Pathogen recognition receptors, cancer and inflammation in the gut. Curr Opin Pharmacol. 2009;9:680–687. doi: 10.1016/j.coph.2009.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Akira S., Takeda K. Toll-like receptor signalling. Nat Rev Immunol. 2004;4:499–511. doi: 10.1038/nri1391. [DOI] [PubMed] [Google Scholar]
- 9.O'Neill L.A., Golenbock D., Bowie A.G. The history of Toll-like receptors redefining innate immunity. Nat Rev Immunol. 2013;13:453–460. doi: 10.1038/nri3446. [DOI] [PubMed] [Google Scholar]
- 10.Byeon S.E., Yi Y.S., Oh J., Yoo B.C., Hong S., Cho J.Y. The role of Src kinase in macrophage-mediated inflammatory responses. Mediators Inflamm. 2012;2012:512926. doi: 10.1155/2012/512926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yu T., Yi Y.S., Yang Y., Oh J., Jeong D., Cho J.Y. The pivotal role of TBK1 in inflammatory responses mediated by macrophages. Mediators Inflamm. 2012;2012:979105. doi: 10.1155/2012/979105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jeon J.W., Park B.C., Jung J.G., Jang Y.S., Shin E.C., Park Y.W. The soluble form of the cellular prion protein enhances phagocytic activity and cytokine production by human monocytes via activation of ERK and NF-κB. Immune Netw. 2013;13:148–156. doi: 10.4110/in.2013.13.4.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kang D.H., Kang S.W. Targeting cellular antioxidant enzymes for treating atherosclerotic vascular disease. Biomol Ther. 2013;21:89–96. doi: 10.4062/biomolther.2013.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wojdasiewicz P., Poniatowski Ł.A., Szukiewicz D. The role of inflammatory and anti-inflammatory cytokines in the pathogenesis of osteoarthritis. Mediators Inflamm. 2014;2014:561459. doi: 10.1155/2014/561459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kopf M., Bachmann M.F., Marsland B.J. Averting inflammation by targeting the cytokine environment. Nat Rev Drug Discov. 2010;9:703–718. doi: 10.1038/nrd2805. [DOI] [PubMed] [Google Scholar]
- 16.Turner M.D., Nedjai B., Hurst T., Pennington D.J. Cytokines and chemokines: at the crossroads of cell signalling and inflammatory disease. Biochim Biophys Acta. 2014;1843:2563–2582. doi: 10.1016/j.bbamcr.2014.05.014. [DOI] [PubMed] [Google Scholar]
- 17.Chiurchiu V., Maccarrone M. Chronic inflammatory disorders and their redox control: from molecular mechanisms to therapeutic opportunities. Antioxid Redox Signal. 2011;15:2605–2641. doi: 10.1089/ars.2010.3547. [DOI] [PubMed] [Google Scholar]
- 18.Przemyslaw L., Boguslaw H.A., Elzbieta S., Malgorzata S.M. ADAM and ADAMTS family proteins and their role in the colorectal cancer etiopathogenesis. BMB Rep. 2013;46:139–150. doi: 10.5483/BMBRep.2013.46.3.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Blumenthal M. Asian ginseng: potential therapeutic uses. Adv Nurse Pract. 2001;9:26–28. [PubMed] [Google Scholar]
- 20.Baeg I.H., So S.H. The world ginseng market and the ginseng (Korea) J Ginseng Res. 2013;37:1–7. doi: 10.5142/jgr.2013.37.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Peng D., Wang H., Qu C., Xie L., Wicks S.M., Xie J. Ginsenoside Re: its chemistry, metabolism and pharmacokinetics. Chin Med. 2012;7:2. doi: 10.1186/1749-8546-7-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Helmes S. Cancer prevention and therapeutics: Panax ginseng. Altern Med Rev. 2004;9:259–274. [PubMed] [Google Scholar]
- 23.Hu S.Y. A contribution to our knowledge of ginseng. Am J Chin Med. 1977;5:1–23. doi: 10.1142/s0192415x77000026. [DOI] [PubMed] [Google Scholar]
- 24.Attele A., Wu J., Yuan C. Multiple pharmacological effects of ginseng. Biochem Pharmacol. 1999;58:1685–1693. doi: 10.1016/s0006-2952(99)00212-9. [DOI] [PubMed] [Google Scholar]
- 25.Attele A.S., Zhou Y.P., Xie J.T., Wu J.A., Zhang L., Dey L., Pugh W., Rue P.A., Polonsky K.S., Yuan C.S. Antidiabetic effects of Panax ginseng berry extract and the identification of an effective component. Diabetes. 2002;51:1851–1858. doi: 10.2337/diabetes.51.6.1851. [DOI] [PubMed] [Google Scholar]
- 26.Lee T.K., Johnke R.M., Allison R.R., O'Brien K.F., Dobbs L.J. Radioprotective potential of ginseng. Mutagenesis. 2005;20:237–243. doi: 10.1093/mutage/gei041. [DOI] [PubMed] [Google Scholar]
- 27.Fuzzati N. Analysis methods of ginsenosides. J Chromatogr B Analyt Technol Biomed Life Sci. 2004;812:119–133. doi: 10.1016/j.jchromb.2004.07.039. [DOI] [PubMed] [Google Scholar]
- 28.Kim J.-H. Cardiovascular diseases and Panax ginseng: a review on molecular mechanisms and medical applications. J Ginseng Res. 2012;36:16–26. doi: 10.5142/jgr.2012.36.1.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim E.H., Son R.H., Myoung H.J., Mar W.C., Kim W.K., Nam K.W. The inhibitory effect of baicalin on the short-term food intake in C57BL/6J mice. Biomol Ther. 2010;18:171–177. [Google Scholar]
- 30.Kim M.Y., Yoo B.C., Cho J.Y. Ginsenoside-Rp1-induced apolipoprotein A-1 expression in the LoVo human colon cancer cell line. J Ginseng Res. 2014;38:251–255. doi: 10.1016/j.jgr.2014.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang D., Koh H.L., Hong Y., Zhu H.T., Xu M., Zhang Y.J., Yang C.R. Chemical and morphological variations of Panax notoginseng and their relationship. Phytochemistry. 2013;93:88–95. doi: 10.1016/j.phytochem.2013.03.007. [DOI] [PubMed] [Google Scholar]
- 32.Yang Y., Yang W.S., Yu T., Sung G.H., Park K.W., Yoon K., Son Y.J., Hwang H., Kwak Y.S., Lee C.M. ATF-2/CREB/IRF-3-targeted anti-inflammatory activity of Korean red ginseng water extract. J Ethnopharmacol. 2014;154:218–228. doi: 10.1016/j.jep.2014.04.008. [DOI] [PubMed] [Google Scholar]
- 33.Yang Y., Lee J., Rhee M.H., Yu T., Baek K.S., Sung N.Y., Kim Y., Yoon K., Kim J.H., Kwak Y.S. Molecular mechanism of protopanaxadiol saponin fraction-mediated anti-inflammatory actions. J Ginseng Res. 2015;39:61–68. doi: 10.1016/j.jgr.2014.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hossen M.J., Jeon S.H., Kim S.C., Kim J.H., Jeong D., Sung N.Y., Yang S., Baek K.S., Yoon D.H., Song W.O. In vitro and in vivo anti-inflammatory activity of Phyllanthus acidus methanolic extract. J Ethnopharmacol. 2015;168:217–228. doi: 10.1016/j.jep.2015.03.043. [DOI] [PubMed] [Google Scholar]
- 35.Park J.G., Son Y.J., Kim M.Y., Cho J.Y. Syk and IRAK1 contribute to immunopharmacological activities of anthraquinone-2-carboxlic acid. Molecules. 2016;21:E809. doi: 10.3390/molecules21060809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Green L.C., Wagner D.A., Glogowski J., Skipper P.L., Wishnok J.S., Tannenbaum S.R. Analysis of nitrate, nitrite, and [15N]nitrate in biological fluids. Anal Biochem. 1982;126:131–138. doi: 10.1016/0003-2697(82)90118-x. [DOI] [PubMed] [Google Scholar]
- 37.Jeong D., Yi Y.S., Sung G.H., Yang W.S., Park J.G., Yoon K., Yoon D.H., Song C., Lee Y., Rhee M.H. Anti-inflammatory activities and mechanisms of Artemisia asiatica ethanol extract. J Ethnopharmacol. 2014;152:487–496. doi: 10.1016/j.jep.2014.01.030. [DOI] [PubMed] [Google Scholar]
- 38.Vo H.T., Cho J.Y., Choi Y.E., Choi Y.S., Jeong Y.H. Kinetic study for the optimization of ginsenoside Rg3 production by heat treatment of ginsenoside Rb1. J Ginseng Res. 2015;39:304–313. doi: 10.1016/j.jgr.2015.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Park J.G., Son Y.J., Aravinthan A., Kim J.H., Cho J.Y. Korean Red Ginseng water extract arrests growth of xenografted lymphoma cells. J Ginseng Res. 2016;40:431–436. doi: 10.1016/j.jgr.2016.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lee H.J., Hyun E.A., Yoon W.J., Kim B.H., Rhee M.H., Kang H.K., Cho J.Y., Yoo E.S. In vitro anti-inflammatory and anti-oxidative effects of Cinnamomum camphora extracts. J Ethnopharmacol. 2006;103:208–216. doi: 10.1016/j.jep.2005.08.009. [DOI] [PubMed] [Google Scholar]
- 41.Shen T., Lee J.H., Park M.H., Lee Y.G., Rho H.S., Kwak Y.S., Rhee M.H., Park Y.C., Cho J.Y. Ginsenoside Rp 1, a ginsenoside derivative, blocks promoter activation of iNOS and Cox-2 genes by suppression of an IKKβ-mediated NF-κB pathway in HEK293 cells. J Ginseng Res. 2011;35:200–208. doi: 10.5142/jgr.2011.35.2.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Park J.G., Kang W.-S., Park K.T., Park D.J., Aravinthan A., Kim J.-H., Cho J.Y. Anticancer effect of joboksansam, Korean wild ginseng germinated from bird feces. J Ginseng Res. 2016;40:304–308. doi: 10.1016/j.jgr.2016.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rajaram M.V., Ganesan L.P., Parsa K.V., Butchar J.P., Gunn J.S., Tridandapani S. Akt/Protein kinase B modulates macrophage inflammatory response to Francisella infection and confers a survival advantage in mice. J Immunol. 2006;177:6317–6324. doi: 10.4049/jimmunol.177.9.6317. [DOI] [PubMed] [Google Scholar]
- 44.Kuuliala K., Kuuliala A., Hämäläinen M., Koivuniemi R., Kautiainen H., Moilanen E., Repo H., Leirisalo–Repo M. Impaired Akt phosphorylation in monocytes of patients with rheumatoid arthritis. Scand J Immunol. 2017;85:155–161. doi: 10.1111/sji.12521. [DOI] [PubMed] [Google Scholar]
- 45.Kumar S., Patel R., Moore S., Crawford D.K., Suwanna N., Mangiardi M., Tiwari-Woodruff S.K. Estrogen receptor β ligand therapy activates PI3K/Akt/mTOR signaling in oligodendrocytes and promotes remyelination in a mouse model of multiple sclerosis. Neurobiol Dis. 2013;56:131–144. doi: 10.1016/j.nbd.2013.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ogawa A., Firth A.L., Ariyasu S., Yamadori I., Matsubara H., Song S., Fraidenburg D.R., Yuan J.X.J. Thrombin-mediated activation of Akt signaling contributes to pulmonary vascular remodeling in pulmonary hypertension. Physiol Rep. 2013;1:e00190. doi: 10.1002/phy2.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mitra A.D., Raychaudhuri S.P., Abria C.J., Mitra A., Wright R., Ray R., Kundu-Raychaudhuri S. 1α, 25-Dihydroxyvitamin-D3-3-bromoacetate regulates AKT/mTOR signaling cascades: a therapeutic agent for psoriasis. J Invest Dermatol. 2013;133:1556–1564. doi: 10.1038/jid.2013.3. [DOI] [PubMed] [Google Scholar]
- 48.Luo Y., Sun G., Dong X., Wang M., Qin M., Yu Y., Sun X. Isorhamnetin attenuates atherosclerosis by inhibiting macrophage apoptosis via PI3K/AKT activation and HO-1 induction. PloS One. 2015;10:e0120259. doi: 10.1371/journal.pone.0120259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ozes O.N., Mayo L.D., Gustin J.A., Pfeffer S.R., Pfeffer L.M., Donner D.B. NF-κB activation by tumour necrosis factor requires the Akt serine–threonine kinase. Nature. 1999;401:82–85. doi: 10.1038/43466. [DOI] [PubMed] [Google Scholar]
- 50.Tang B., Tang F., Wang Z., Qi G., Liang X., Li B., Yuan S., Liu J., Yu S., He S. Upregulation of akt/NF-κB-regulated inflammation and akt/Bad-related apoptosis signaling pathway involved in hepatic carcinoma process: suppression by carnosic acid nanoparticle. Int J Nanomedicine. 2016;11:6401–6420. doi: 10.2147/IJN.S101285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nakano N., Matsuda S., Ichimura M., Minami A., Ogino M., Murai T., Kitagishi Y. PI3K/AKT signaling mediated by G protein-coupled receptors is involved in neurodegenerative Parkinson's disease (Review) Int J Mol Med. 2016;39:253–260. doi: 10.3892/ijmm.2016.2833. [DOI] [PubMed] [Google Scholar]
- 52.Gharbi S.I., Zvelebil M.J., Shuttleworth S.J., Hancox T., Saghir N., Timms J.F., Waterfield M.D. Exploring the specificity of the PI3K family inhibitor LY294002. Biochem J. 2007;404:15–21. doi: 10.1042/BJ20061489. [DOI] [PMC free article] [PubMed] [Google Scholar]