Abstract
Background
High-grade gliomas (HGGs) are a heterogeneous disease group, with variable prognosis, inevitably causing deterioration of the quality of life. The estimated 2-year overall survival is 20%, despite the best trimodality treatment consisting of surgery, chemotherapy, and radiotherapy.
Aim
To evaluate long-term survival outcomes and factors influencing the survival of patients with high-grade gliomas treated with radiotherapy.
Materials and methods
Data from 47 patients diagnosed with high-grade gliomas between 2009 and 2014 and treated with three-dimensional radiotherapy (3DRT) or intensity-modulated radiotherapy (IMRT) were analyzed retrospectively.
Results
Median survival was 16.6 months; 29 patients (62%) died before the time of analysis. IMRT was employed in 68% of cases. The mean duration of radiotherapy was 56 days, and the mean delay to the start of radiotherapy was 61.7 days (range, 27–123 days). There were no statistically significant effects of duration of radiotherapy or delay to the start of radiotherapy on patient outcomes.
Conclusions
Age, total amount of gross resection, histological type, and use of adjuvant temozolomide influenced survival rate (p < 0.05). The estimated overall survival was 18 months (Kaplan–Meier estimator). Our results corroborated those reported in the literature.
Keywords: Radiotherapy, Anaplastic, Glioma, Chemotherapy, Glioblastoma
1. Background and aim
High-grade gliomas (HGGs) are a heterogeneous disease group, both genetically and histologically, with variable prognosis, inevitably causing deterioration of the quality of life. The estimated 2-year overall survival is 20%, despite the best trimodality treatment consisting of surgery, chemotherapy, and radiotherapy. In our study, three-dimensional radiotherapy (3DRT) and intensity-modulated radiotherapy (IMRT) were used after surgery for HGG, with or without chemotherapy.
Radiotherapy for HGG can follow two protocols: the American Radiation Therapy Oncology Group (RTOG) and the European Organization for Research and Treatment of Cancer (EORTC) – Table 1. Chemoradiation with concurrent and adjuvant temozolomide (TMZ) is the standard treatment for glioblastoma and will be further discussed below. Patients with anaplastic astrocytoma (AA) have poorer survival than those with oligodendroglial tumors. Their tumors often progress to grade IV, which justifies the intensification of treatment of these lesions with chemoradiation. However, the results of randomized studies (NCT00887146 and NCT00626990) to evaluate the benefit of adding concomitant chemotherapy or adjuvant radiotherapy for treatment of 1p19q codeleted tumors (CODEL) or not-codeleted tumors (CATNON) are not yet available.
Table 1.
Target volume comparison between EORTC and RTOG guidelines.
| EORTC (EORTC 22981/22961, 26071/22072 (Centric), 26981-22981, and AVAglio) | RTOG (RTOG 0525, 0825, 0913, and AVAglio) |
|---|---|
| Phase 1 (60 Gy/30 fractions) | Phase 1 (46 Gy/23 fr) |
| GTV = surgical cavity + contrast T1 enhancing tissue | GTV1 = surgical cavity + contrast T1 enhancing tissue + perilesional edema in T2 or FLAIR |
| CTV = GTV + 2-cm margin* | CTV1 = GTV1 + 2-cm margin (if no peritumoral edema, CTV is contrast enhancing area + 2.5-cm margin) |
| PTV = CTV + 3–5-mm margin | PTV1 = CTV1 + 3–5 mm margin |
| Phase 2 (boost 14 Gy/7 fr) | |
| GTV2 = surgical cavity + contrast T1 enhancing tissue | |
| CTV2 = GTV2 + 2-cm margin | |
| PTV2 = CTV2 + 3–5 mm margin |
AVAglio; EORTC; RTOG; GTV, gross tumor volume; CTV, clinical target volume; PTV, planning target volume.
Margins up to 3 cm were allowed in EORTC 22981/22961 and from 1 to 1.5 cm in EORTC 26981-22981.
Source: Adapted from Niyazi M et al. ESTRO-ACROP guideline.29
2. Materials and methods
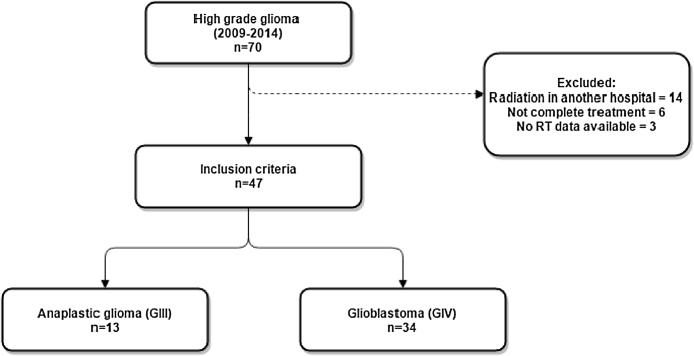
Patients diagnosed with primary HGG were selected by a search performed in our institution's database. The inclusion criterion was the treatment for anaplastic glioma or glioblastoma between 2009 and 2014 at the Hospital das Clínicas de Ribeirão Preto of the University of São Paulo (HCFMRP-USP). Patients who were under 18 years of age, had undergone radiotherapy in another facility, or had not completed radiotherapy were excluded.
The IMRT technique was implemented in the HCRP in 2010; previously, all patients were treated with 3DRT. The treatment was performed in a linear accelerator model Oncor Impression (Siemens) or Primus (Siemens), with 6 MV energy and a 1-cm thickness multileaf collimator or individualized protection for each course of treatment. All plans were non-coplanar, using the number of fields and sectors most suitable for a better compliance index and heterogeneity. Fractionation was 1.8–2.0 Gy per fraction (one fraction a day, 5 days a week) using total doses of 52–60 Gy in 26–30 fractions, following RTOG or EORTC guidelines. Quality controls were analyzed individually in IMRT with system ionization chamber arrangements (MATRIXX, MULTICube QA Software) (IBA Dosimetry, Bartlett, TN, USA). The treatment was permitted when the gamma function was below 3%.
Data collected included age, sex, histology, performance status, use of concomitant and/or adjuvant chemotherapy, surgical resection (total or subtotal macroscopic), date of treatment, final dose, treatment duration, irradiation technique (IMRT or 3DRT), first presenting symptoms, date of progression (if any) according to clinical and radiological magnetic resonance imaging (MRI) control, date of death, and date of last medical appointment (if the patient was alive at the time of data collection).
Data were analyzed with SAS software, version 9.2. Initially, an exploratory analysis of data was performed through measurements of central position and dispersion. Qualitative variables were described by absolute and relative frequencies. A Kaplan–Meier plot was constructed by the R program, SURVIVAL package, to estimate the overall survival. A Weibull regression model was adjusted to verify which factors influenced the time to death. The following variables were considered in the covariate model: sex, age, adjuvant TMZ, IMRT, time to initiation of radiotherapy, duration of radiotherapy, histology (glioblastoma or not glioblastoma), type of resection (total or subtotal), initial symptoms (convulsions, vomiting, headache, mental confusion, motor abnormalities, and visual disturbances). The model was implemented in the SAS version 9.2 by PROC LIFEREG.
The response to treatment was determined according to the standard strict criteria. Macdonald and RANO (Response Assessment in Neuro-Oncology Criteria) consider four types of magnetic resonance imaging (MRI) response to treatment: complete, partial, stable disease, and disease progression.1 The inflammatory reaction during and after treatment can change the permeability of the blood–brain barrier and thus change the response assessment. This became more evident after the introduction of chemoradiation with TMZ, through the pseudo-progression phenomenon: about 30% of patients showed an increase in contrast enhancement that was not necessarily related to the progression of the disease. Pseudo-progression is more frequent in patients with methylguanine methyltransferase (MGMT) methylation and those who present as clinically stable, with no signs of clinical disease progression, should remain with their current therapy until further evaluation.2, 3, 4
The study was approved by the Ethics Research Committee of HC-FMRP-USP.
3. Results
Our database included 70 eligible patients with primary HGG. Fourteen were excluded because they did not undergo radiotherapy at our facility, six were excluded because they did not complete the proposed radiotherapy treatment, and three were excluded for lack of data. Thus, 47 patients were included in the study (Fig. 1). Their mean age was 51 years (range, 27–85 years), and 28 (59%) were male. Histologically, 34 patients (72%) had glioblastoma, and the rest had various subtypes of grade III gliomas (anaplastic) (Fig. 2).
Fig. 1.
Selection of subjects.
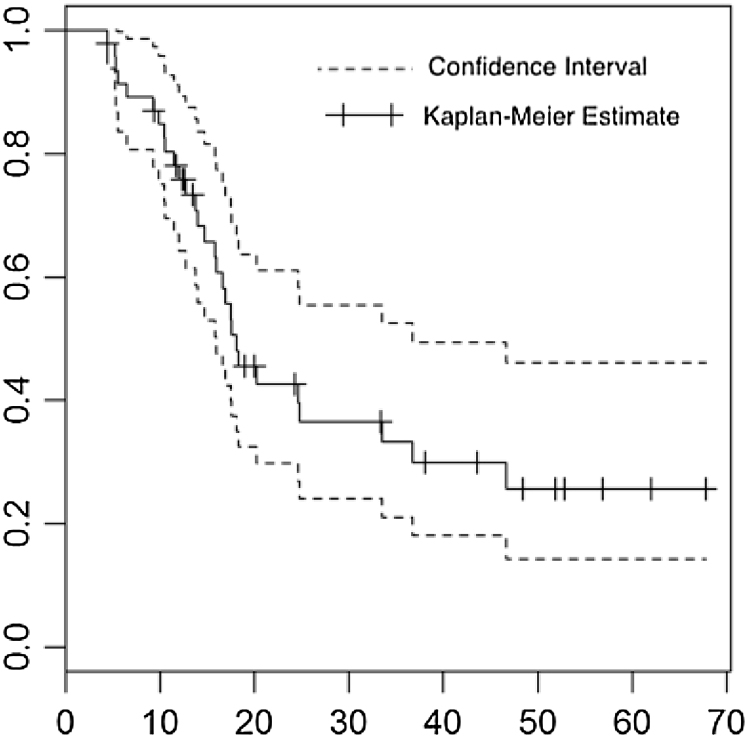
Fig. 2.
Kaplan–Meier graph: estimation of survival rate (survival probability × time in months) between confidence intervals.
The initial symptoms were headache (51%), visual disturbances (34%), seizures (34%), confusion and motor changes (23%), and vomiting (21%). The treatment was surgery, with maximum safe resection (total or subtotal), followed by adjuvant radiotherapy, with or without concomitant chemotherapy and adjuvant chemotherapy. Macroscopic resection was performed in 26 patients (55%). Chemoradiation with TMZ was prescribed for 37 patients (87%) in both the concomitant and the adjuvant settings. Thirty-two patients (68%) underwent IMRT; the average dose was 59 Gy (52–60 Gy) in 30 sessions (range, 25–33). The average duration of radiation was 48 days (range, 36–73 days), and the average interval (delay) between surgery and radiotherapy was 61.7 days (range, 27–123 days) (Table 2).
Table 2.
Measures of central position and dispersion of quantitative measures.
| Variable | N | Average | SD | Median | Q1 | Q3 | Minimum | Maximum |
|---|---|---|---|---|---|---|---|---|
| Survival (mo) | 47 | 22.37 | 16.64 | 16.63 | 11.47 | 33.37 | 4.43 | 67.77 |
| Age (yr) | 46 | 51.64 | 12.79 | 54.19 | 40.34 | 59.26 | 27.12 | 85 |
| Time to radiotherapy/delay (days) | 47 | 61.68 | 19.28 | 60 | 47 | 73 | 27 | 123 |
| Radiotherapy duration (days) | 47 | 48.42 | 6.98 | 48 | 45 | 51 | 36 | 73 |
| Radiotherapy dose (Gy) | 47 | 59.14 | 1.99 | 60 | 60 | 60 | 52 | 60 |
| Fractions | 44 | 29.95 | 1.31 | 30 | 30 | 30 | 25 | 33 |
Median survival was 16.6 months (range, 4.4–67.7 months); 29 patients (62%) had died by the time of data collection (Table 2, Table 3). Among the patients who died, 26 (90%) had glioblastoma, 19 (66%) were male, and most of them presented with seizures (28%), vomiting (22%), motor alterations (41%), or visual alterations (3%) at diagnosis. Total resection was performed in 17 cases (59%), and IMRT was performed in 18 cases (62%). Radiotherapy was administered with concurrent TMZ in 25 cases (86%) and adjuvant TMZ in 21 cases (72%) (Table 4).
Table 3.
Description of qualitative variables.
| Variable | No. (n = 47) | % |
|---|---|---|
| Death | 29 | 61.7 |
| Male sex | 28 | 59.57 |
| Glioblastoma | 34 | 72.34 |
| Complete resection | 26 | 55.32 |
| Seizures | 16 | 34.04 |
| Vomit | 10 | 21.28 |
| Headache | 24 | 51.06 |
| Mental confusion | 11 | 23.4 |
| Motor alteration | 11 | 23.4 |
| Visual alteration | 16 | 34.04 |
| TMZ concomitant | 41 | 87.23 |
| IMRT | 32 | 68.09 |
| TMZ adjuvant | 37 | 78.72 |
TMZ, temozolomide; IMRT, intensity-modulated radiotherapy.
Table 4.
Description of qualitative variables in relation to death.
| Variable | Death |
|
|---|---|---|
| No (n = 18) | Yes (n = 29) | |
| Male sex | 9 (50) | 19 (65.52) |
| Glioblastoma | 8 (44.44) | 26 (89.66) |
| Complete resection | 9 (50) | 17 (58.62) |
| Seizures | 8 (44.44) | 8 (27.59) |
| Vomit | 4 (22.22) | 6 (20.69) |
| Headache | 9 (50) | 15 (51.72) |
| Mental confusion | 5 (27.78) | 6 (20.69) |
| Motor alteration | 4 (22.22) | 12 (41.38) |
| Visual alteration | 2 (11.11) | 1 (3.45) |
| TMZ concomitant | 16 (88.89) | 25 (86.21) |
| IMRT | 14 (77.78) | 18 (62.07) |
| TMZ adjuvant | 16 (88.89) | 21 (72.41) |
TMZ, temozolomide; IMRT, intensity-modulated radiotherapy.
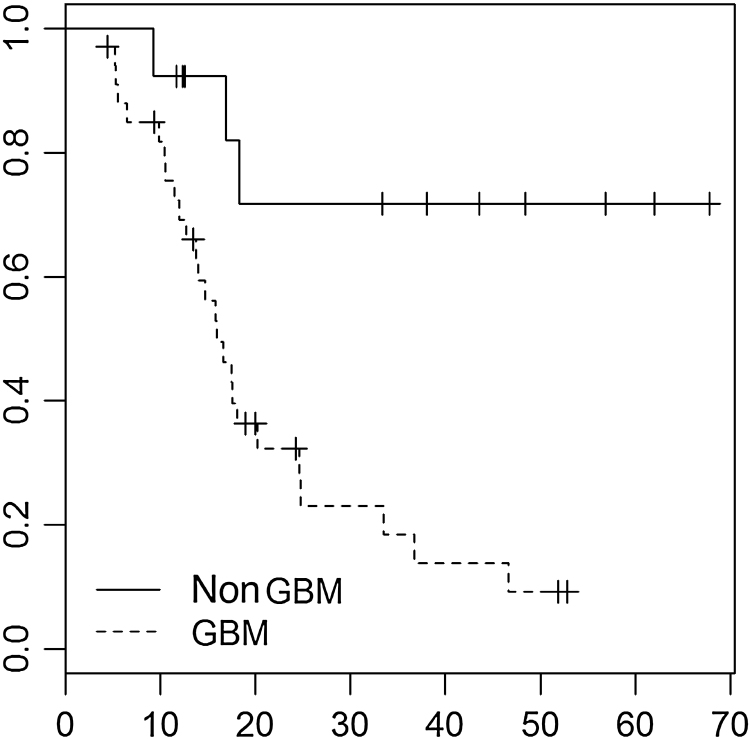
To analyze the factors that influence survival, we used the Weibull regression model, with statistical significance (p ≤ 0.05) for age (estimated risk [ER] –0.0248, 95% confidence interval [CI] –0.0481 to –0.0015, p = 0.0367), complete macroscopic resection (ER –0.4928, 95% CI –0.9741 to –0.0115, p = 0.0448), glioblastoma as histological type (ER 1.0763, 95% 0.338–1.8145, p = 0.0043), and performance of adjuvant TMZ (ER –0.5258, 95% CI –1.0418 to –0.0098, p = 0.0458) (Table 5). Fifty percent of patients died within 18 months, with a confidence interval ranging from 16 to 37 months. These results are obtained through the median and the confidence interval for the median (R result in the program through the command SURVFIT). Fig. 3 compares the survival curves of patients with anaplastic glioma and patients with glioblastoma (dotted line) (survival probability × time in months).
Table 5.
Estimations of Weibull regression model parameters.
| Parameter | ER | 95% CI | p value | |
|---|---|---|---|---|
| Intercept | 3.9136 | 1.8552 | 5.972 | 0.0002 |
| Sex | 0.4541 | –0.0833 | 0.9914 | 0.0977 |
| Age | –0.0248 | –0.0481 | –0.0015 | 0.0367 |
| TMZ adjuvant | –0.5258 | –1.0418 | –0.0098 | 0.0458 |
| IMRT | 0.0646 | –0.393 | 0.5223 | 0.7819 |
| Delay to radiotherapy | 0.0001 | –0.0123 | 0.0125 | 0.9867 |
| Radiotherapy duration | 0.0005 | –0.0032 | 0.0043 | 0.7755 |
| Glioblastoma | 1.0763 | 0.338 | 1.8145 | 0.0043 |
| Complete resection | –0.4928 | –0.9741 | –0.0115 | 0.0448 |
| Seizure | –0.1411 | –0.662 | 0.3798 | 0.5955 |
| Vomit | –0.1857 | –0.9142 | 0.5427 | 0.6173 |
| Headache | –0.0157 | –0.5005 | 0.4692 | 0.9495 |
| Mental confusion | –0.1527 | –0.7585 | 0.453 | 0.6212 |
| Motor alteration | 0.4241 | –0.0702 | 0.9184 | 0.0927 |
| Visual alteration | 0.749 | –0.4955 | 1.9936 | 0.2382 |
| Scale | 0.4981 | 0.3662 | 0.6775 | |
| Weibull shape | 2.0075 | 1.476 | 2.7304 | |
ER, estimated risk; CI, confidence interval; TMZ, temozolomide; IMRT, intensity-modulated radiotherapy.
Boldface type indicates parameters with established relation and statistical significance in relation to the time to death.
Fig. 3.
Kaplan–Meier graph: estimation of survival rate Grade III – dotted vs. IV (survival probability × time in months). Updated.
4. Discussion
HGG is usually treated as an incurable disease. The treatment goal is the longest control of the disease with minimum disease symptoms. In the past decade, Stupp and colleagues achieved a 2-month gain in survival compared with other historical series (median survival 14.6 vs. 12.1 months, p < 0.001).5, 6 TMZ concomitant with radiotherapy plus adjuvant TMZ (75 mg/m2 daily, followed by six cycles of 150–200 mg/m2 for 5 days every 28 days) produced significant improvement in patient survival compared with radiotherapy alone (15 vs. 12 months, hazard ratio [HR] 0.63, p < 0.001). Overall survival at 2 years was 27% and 10%, respectively.1, 7, 8 Our data show that patients who received adjuvant TMZ had a lower risk of death than patients who did not receive the drug (ER –0.5258, 95% CI –0.0481 to –0.0098, p = 0.0458).
Age and performance status are important prognostic factors of glioblastoma, as featured in survival estimation indexes, such as the “Recursive Partitioning Analysis” (RPA). The RPA is divided into six groups according to selected characteristics: age, performance status, histology, type of resection, and neurological function.8, 9 Our analysis shows a lower mortality risk estimate for younger patients (ER –0.0248, 95% CI –0.0481 to –0.0015, p = 0.0367), consistent with what has been reported.
Patients with histological grade IV tumors had poorer survival rates than those with grade III tumors (ER 1.0763, 95% CI 0.338–1.8145, p = 0.0043), a result in line with literature data. According to the RPA, patients with anaplastic glioma have a higher median survival (49 months) than glioblastoma (14 month) (p = 0.000001) – in the pre-TMZ era.10 Perhaps the present study should have been designed to distinguish these entities, due to notable differences in survival between patients with anaplastic and those with glioblastoma tumors.
The type of resection (subtotal or total gross) influenced the risk of death in our evaluation (ER –0.4928, 95% CI –0.9741 to –0.0115, p = 0.0448). Several studies have reported that total macroscopic resection is associated with longer survival.11, 12, 13, 14, 15 Possible reasons for this could be tumor debulking and increased oxygen flow to previously hypoxic or necrotic regions removed in surgery, allowing for better performance of chemotherapy and radiotherapy. The extent of resection was evaluated as an isolated predictor of survival: the volume of resection (> or <95% tumor) influenced survival results (16.3 vs. 11.6 months; p = 0.03).16 The equivalent analysis for anaplastic astrocytomas yielded similar results.11
We searched for other factors associated with mortality risk, including use of the intensity-modulated technique, early presenting symptoms, duration of radiotherapy, and delay from surgery to radiotherapy, but found no statistically significant relationships. We did not observe a relationship between the duration of radiation (ER 0.0005, 95% CI –0.0032 to 0.0043, p = 0.7755) or between the time delay/delay to start of radiotherapy (ER 0.0001, 95% CI –0.0123 to 0.0125, p = 0.9867) and the risk of death. A recent meta-analysis including 12 observational studies with 521 glioblastoma patients concluded that there was no overall impairment in survival from delay in the start of radiotherapy (ER 0.98, 95% CI 0.90–1.08, p = 0.70).17 A German retrospective study also reported that small delays at the start of adjuvant treatment with chemoradiation did not affect survival.18 This study showed that the median time to onset of radiotherapy was 61 days, and only 25% of patients started radiotherapy within 45 days.
In our study, the first symptoms (seizures, vomiting, headache, mental confusion, motor or visual changes) were not predictors of response to treatment. A 2006 Australian retrospective analysis of 132 patients with glioblastoma proposed that patients with early focal symptoms, such as seizures and motor and visual changes, were more likely to receive early treatment and hence had improved survival. Their results showed a trend toward improved survival in patients with acute symptoms, but the relation was not statistically significant (p = 0.07).19 Another retrospective study by Toledo et al. also evaluated seizures and epilepsy as independent predictors of increased survival (p < 0.001).20
Treatment with IMRT did not increase the survival rate in the present study (ER 0.0646, 95% CI –0.393 to 0.5223, p = 0.7819). Dosimetric analysis, however, reported increased coverage of the target volume (compliance rate) and reduction of doses in the brain, brainstem, and optical chiasm with IMRT (p < 0.05).21, 22, 23 Considering that high-grade tumors such as glioblastomas have a small alpha/beta ratio, it may be beneficial to use hypofractionated schemes to deliver higher doses to areas of potential recurrence, such as the lesion residue surgical cavity.24 Studies have evaluated the use of simultaneous integrated boost (SIB) to deliver these higher-dose hypofractionated schemes through IMRT.25, 26 In patients receiving TMZ, Cho et al. evaluated 25 fractions of 2.4 to a total 60 Gy to the gross tumor volume (GTV), while the planning target volume (PTV) received 25 daily doses of 2.0 Gy to 50 Gy total, they considered the SIB hypofractionated scheme safe and feasible, with the advantage of minimizing treatment time.27, 28 There was little toxicity obtained in hypofractionated IMRT schemes associated with chemotherapy and according to data provided on the dosimetric advantages of IMRT to reflect the clinical ability to deliver higher doses in shorter time, enabling satisfactory results without increased toxicity.21
We conclude from this retrospective analysis that there is an agreement between published data and data obtained from treatments recommended in the radiotherapy protocol of our institution. We emphasize that this was a retrospective study and subject to the biases inherent to this type of study such as records that were not designed for the study, and poor quality of available data recorded in the past.
We are far from a cure for HGG. Patients with anaplastic glioma or glioblastoma in its most aggressive form have overall 2-year survival of 40% and 20%, respectively, and we still lack a definitive and effective combination therapy to eradicate the disease without the possibility of recurrence or progression. There is a great expectation regarding the genetic and molecular analysis based on refined histological classification and allowing the possibility of customization of treatment with specific molecular therapy. We lack prospective studies to alter the natural course of this disease. We eagerly await the results of phase III trials NCT00887146 (CODEL) and NCT00626990 (CATNON).
In 2014, a report was elaborated by our unified health system, the National Technology Incorporation Commission (CONITEC) of Sistema Unico de Saude (SUS), concerning the incorporation of TMZ as a treatment for patients with HGGs. It states that TMZ is not cost-effective compared with nitrosoureas, and there is a lack of randomized clinical trials comparing the two drugs. Currently, only the state of São Paulo provides TMZ for public patients in Brazil.
Conflict of interest
None declared.
Financial disclosure
None declared.
References
- 1.Wen P.Y., Macdonald D.R., Reardon D.A. Updated response assessment criteria for high-grade gliomas: response assessment in neuro-oncology working group. J Clin Oncol. 2010;28(11):1963–1972. doi: 10.1200/JCO.2009.26.3541. [DOI] [PubMed] [Google Scholar]
- 2.Caroline I., Rosenthal M.A. Imaging modalities in high-grade gliomas: pseudoprogression, recurrence, or necrosis? J Clin Neurosci. 2012;19(5):633–637. doi: 10.1016/j.jocn.2011.10.003. [DOI] [PubMed] [Google Scholar]
- 3.Jahangiri A., Aghi M.K. Pseudoprogression and treatment effect. Neurosurg Clin N Am. 2012;23(2):277–287. doi: 10.1016/j.nec.2012.01.002. [DOI] [PubMed] [Google Scholar]
- 4.Young R.J., Gupta A., Shah A.D. MRI perfusion in determining pseudoprogression in patients with glioblastoma. Clin Imaging. 2013;37(1):41–49. doi: 10.1016/j.clinimag.2012.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stupp R., Mason W.P., van den Bent M.J. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352(10):987–996. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 6.Stupp R., Hegi M.E., Mason W.P. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC–NCIC trial. Lancet Oncol. 2009;10(5):459–466. doi: 10.1016/S1470-2045(09)70025-7. [DOI] [PubMed] [Google Scholar]
- 7.Hart M.G., Grant R., Garside R., Rogers G., Somerville M., Stein K. Temozolomide for high grade glioma. Cochrane Database Syst Rev. 2008;30(4) doi: 10.1002/14651858.CD007415. [DOI] [PubMed] [Google Scholar]
- 8.Stupp R., Brada M., van den Bent M.J., Tonn J.C., Pentheroudakis G. High-grade glioma: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2014;25(suppl 3):93–101. doi: 10.1093/annonc/mdu050. [DOI] [PubMed] [Google Scholar]
- 9.Scott C.B., Scarantino C., Urtasun R. Validation and predictive power of Radiation Therapy Oncology Group (RTOG) recursive partitioning analysis classes for malignant glioma patients: a report using RTOG 90-06. Int J Radiat Oncol Biol Phys. 1998;40(1):51–55. doi: 10.1016/s0360-3016(97)00485-9. [DOI] [PubMed] [Google Scholar]
- 10.Li J., Wang M., Won M. Validation and simplification of the radiation therapy oncology group recursive partitioning analysis classification for glioblastoma. Int J Radiat Oncol Biol Phys. 2011;81(3):623–630. doi: 10.1016/j.ijrobp.2010.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Curran W.J., Scott C.B., Horton J. Recursive partitioning analysis of prognostic factors in three radiation therapy oncology group malignant glioma trials. J Natl Cancer Inst. 1993;85(9):704–710. doi: 10.1093/jnci/85.9.704. [DOI] [PubMed] [Google Scholar]
- 12.Sanai N., Berger M.S. Extent of resection influences outcomes for patients with gliomas. Rev Neurol (Paris) 2011;167(10):648–654. doi: 10.1016/j.neurol.2011.07.004. [DOI] [PubMed] [Google Scholar]
- 13.Sanai N., Berger M.S. Glioma extent of resection and its impact on patient outcome. Neurosurgery. 2008;62(4):753–764. doi: 10.1227/01.neu.0000318159.21731.cf. [DOI] [PubMed] [Google Scholar]
- 14.Mcgirt M.J., Chaichana K.L., Gathinji M. Independent association of extent of resection with survival in patients with malignant brain astrocytoma. J Neurosurg. 2009;110(1):156–162. doi: 10.3171/2008.4.17536. [DOI] [PubMed] [Google Scholar]
- 15.Hardesty D.A., Sanai N. The value of glioma extent of resection in the modern neurosurgical era. Front Neurol. 2012;October:1–8. doi: 10.3389/fneur.2012.00140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sanai N., Berger M.S. Operative techniques for gliomas and the value of extent of resection. Neurotherapeutics. 2009;6(3):478–486. doi: 10.1016/j.nurt.2009.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chaichana K.L., Cabrera-Aldana E.E., Jusue-Torres I. When gross total resection of a glioblastoma is possible, how much resection should be achieved? World Neurosurg. 2014;82(1–2):e257–e265. doi: 10.1016/j.wneu.2014.01.019. [DOI] [PubMed] [Google Scholar]
- 18.Loureiro L.V.M., Victor E.D.S., Callegaro-Filho D. Minimizing the uncertainties regarding the effects of delaying radiotherapy for glioblastoma: a systematic review and meta-analysis. Radiother Oncol. 2016;118(1):1–8. doi: 10.1016/j.radonc.2015.11.021. [DOI] [PubMed] [Google Scholar]
- 19.Seidlitz A., Siepmann T., Löck S., Juratli T., Baumann M., Krause M. Impact of waiting time after surgery and overall time of postoperative radiochemotherapy on treatment outcome in glioblastoma multiforme. Radiat Oncol. 2015;10(1):172. doi: 10.1186/s13014-015-0478-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yuile P., Dent O., Cook R., Biggs M., Little N. Survival of glioblastoma patients related to presenting symptoms, brain site and treatment variables. J Clin Neurosci. 2006;13(7):747–751. doi: 10.1016/j.jocn.2005.10.011. [DOI] [PubMed] [Google Scholar]
- 21.Toledo M., Sarria-Estrada S., Quintana M. Prognostic implications of epilepsy in glioblastomas. Clin Neurol Neurosurg. 2015;139:166–171. doi: 10.1016/j.clineuro.2015.10.002. [DOI] [PubMed] [Google Scholar]
- 22.Amelio D., Lorentini S., Schwarz M., Amichetti M. Intensity-modulated radiation therapy in newly diagnosed glioblastoma: a systematic review on clinical and technical issues. Radiother Oncol. 2010;97(3):361–369. doi: 10.1016/j.radonc.2010.08.018. [DOI] [PubMed] [Google Scholar]
- 23.Hermanto U., Frija E.K., Lii M.J., Chang E.L., Mahajan A., Woo S.Y. Intensity-modulated radiotherapy (IMRT) and conventional three-dimensional conformal radiotherapy for high-grade gliomas: does IMRT increase the integral dose to normal brain? Int J Radiat Oncol Biol Phys. 2007;67(4):1135–1144. doi: 10.1016/j.ijrobp.2006.10.032. [DOI] [PubMed] [Google Scholar]
- 24.MacDonald S.M., Ahmad S., Kachris S. Intensity modulated radiation therapy versus three-dimensional conformal radiation therapy for the treatment of high grade glioma: a dosimetric comparison. J Appl Clin Med Phys. 2007;8(2):47–60. doi: 10.1120/jacmp.v8i2.2423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nieder C., Mehta M.P. Advances in translational research provide a rationale for clinical re-evaluation of high-dose radiotherapy for glioblastoma. Med Hypotheses. 2011;76(3):410–413. doi: 10.1016/j.mehy.2010.11.005. [DOI] [PubMed] [Google Scholar]
- 26.Piroth M.D., Pinkawa M., Holy R. Integrated boost IMRT with FET-PET-adapted local dose escalation in glioblastomas: results of a prospective phase II study. Strahlentherapie Onkol. 2012;188(4):334–339. doi: 10.1007/s00066-011-0060-5. [DOI] [PubMed] [Google Scholar]
- 27.Tsien C.I., Brown D., Normolle D. Concurrent temozolomide and dose-escalated intensity-modulated radiation therapy in newly diagnosed glioblastoma. Clin Cancer Res. 2012;18(1):273–279. doi: 10.1158/1078-0432.CCR-11-2073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cho K.H., Kim J.Y., Lee S.H. Simultaneous integrated boost intensity-modulated radiotherapy in patients with high-grade gliomas. Int J Radiat Oncol Biol Phys. 2010;78(2):390–397. doi: 10.1016/j.ijrobp.2009.08.029. [DOI] [PubMed] [Google Scholar]
- 29.Maximilian N., Brada M., Chalmers Anthony J. ESTRO-ACROP guideline “target delineation of glioblastomas”. Radiother Oncol. 2016;118(1):35–42. doi: 10.1016/j.radonc.2015.12.003. [DOI] [PubMed] [Google Scholar]