Abstract
Background
Korean ginseng has been widely evaluated to treat human diseases; however, most studies on Korean ginseng have focused on its root. In this study, polysaccharides [acidic-polysaccharide-linked glycopeptide (APGP) extracted with 90% ethanol and hot water] were prepared from Korean ginseng berries, and their effect on immunosenescence was explored.
Methods
The effect of APGP on thymic involution was evaluated by measuring the size of thymi dissected from aged mice. The effect of APGP on populations of immune cells, including natural killer (NK) cells, dendritic cells, age-correlated CD11c-positive B cells, and several subtypes of T cells [CD4-positive, CD8-positive, and regulatory (Treg) T cells] in the thymi and spleens of aged mice was analyzed by fluorescence-activated cell sorting analysis. Serum levels of interleukin (IL)-2 and IL-6 were evaluated by enzyme-linked immunosorbent assay analysis. Profiles of APGP components were evaluated by high-performance liquid chromatography (HPLC) analysis.
Results
APGP suppressed thymic involution by increasing the weight and areas of thymi in aged mice. APGP increased the population of NK cells, but showed no effect on the population of dendritic cells in the thymi and spleens of aged mice. APGP decreased the population of age-correlated CD11c-positive B cells in the spleens of aged mice. APGP showed no effect on the populations of CD4- and CD8-positive T cells in the thymi of aged mice, whereas it increased the population of Treg cells in the spleens of aged mice. APGP further decreased the reduced serum levels of IL-2 in aged mice, but serum levels of IL-6 were not statistically changed by APGP in aged mice. Finally, HPLC analysis showed that APGP had one major peak at 15 min (a main type of polysaccharide) and a long tail up to 35 min (a mixture of a variety of types of polysaccharides).
Conclusion
These results suggested that APGP exerted an anti-immunosenescent effect by suppressing thymic involution and modulating several types of immune cells.
Keywords: APGP, ginseng berry, immunosenescence, NK cell, Treg cell
1. Introduction
Aging is a complex biological and physiological process and is one of the most critical risk factors for the development of various human diseases [1], [2]. Recently, nine specific hallmarks of aging have been described [3], and among these nine hallmarks, a change in the immune system known as immunosenescence is regarded as one of the most critical determinants [4], [5], [6]. Because immunosenescence reflects changes in the immune system with age, it describes systemic alterations in both innate and adaptive immunity [4], [5], [6]. These alterations of innate and adaptive immunity, consequently, lead to a low-grade inflammatory response known as inflammaging. Inflammaging reduces efficient immune responses against infection by invading pathogens, against the development of cancers, and against injury to endogenous tissues, and compromises immunity to previously recognized pathogens [7], [8].
Korean ginseng (Panax ginseng) is a slow-growing perennial plant found mostly in countries with cool climates (such as Korea and northeast China) and has been regarded as an herbal medicine traditionally used to treat a variety of human diseases in Asia. Many components have been isolated from Korean ginseng and identified, including various types of ginsenosides and acidic polysaccharides. These have been considered to be pharmaceutically active ingredients that exert modulatory activities in many human diseases, including cancer, diabetes mellitus, cardiovascular disease, and immune-related disease including inflammatory/autoimmune diseases [9], [10], [11], [12], [13], [14]. However, although the pharmaceutical effect of Korean ginseng has been successfully proved by many research groups globally, most of these studies have extensively focused on the root of Korean ginseng. Studies on the pharmaceutical effects of other parts of the Korean ginseng plant, such as the berry and leaf, have been limited.
Previous studies have shown various biological activities of Korean ginseng berry on immunity [15] and several disease conditions, including erectile dysfunction [16], [17], cancer [18], [19], [20], and diabetes mellitus [21], [22]. The berry has also been useful for cosmetic purposes, including skin whitening [23]. In spite of these studies, it still remains to be understood which ingredients in the Korean ginseng berry exert biological activities against the diseases and conditions mentioned above. Moreover, it is also necessary to investigate the regulatory role of the Korean ginseng berry in immune responses with age, representing a new area of Korean ginseng berry research. In this study, we extracted a new type of polysaccharide, named acidic-polysaccharide-linked glycopeptide (APGP), from a Korean ginseng berry and explored their biological effect on immunosenescence using a mouse model of aging.
2. Materials and methods
2.1. Materials
Polysaccharide-K (PSK) was purchased from Sigma-Aldrich Chemical Co. (St. Louis, MO, USA). Old (17 mo) and young (2 mo) male C57BL/6J mice were purchased from Central Laboratory Animal Inc. (Seoul, Korea). Fluorochrome-labeled monoclonal primary antibodies specific for CD-11c (CD11c-PE), NK1.1 (NK1.1-APC), B220 (B220-APC), Foxp3 (Foxp3-PE), and CD25 (CD25-biotin) and streptavidin–fluorescein isothiocyanate for fluorescence-activated cell sorting (FACS) analysis were purchased from BD Biosciences (San Jose, CA, USA) and ThermoFisher Scientific (Waltham, MA, USA). Enzyme-linked immunosorbent assay (ELISA) kits for mouse interleukin (IL)-2 (Mouse IL-2 ELISA Ready-SET Go) and mouse IL-6 (Mouse IL-6 ELISA Ready-SET Go) were purchased from ThermoFisher Scientific. Fetal bovine serum and RPMI 1640 medium were purchased from Gibco (Grand Island, NY, USA).
2.2. Mice and compound administration
Old (17 mo) and young (2 mo) male C57BL/6J mice were maintained under standard conditions in plastic cages. Water and a pellet diet (Samyang, Daejeon, Korea) were provided ad libitum, and all studies were performed according to the guidelines established by the Institutional Animal Care and Use Committee at Sungkyunkwan University, Suwon, Korea.
The mice were orally administered the indicated doses of APGP or PSK daily for 20 d, and the amounts of administered compounds were determined based on the body weights of the mice.
2.3. Preparation of APGP from Korean ginseng berries
The berries were first refluxed with 90% ethanol for 5 h. Then, the residues that remained were extracted with water at 100°C for 5 h to obtain the polysaccharide mixture. The supernatant of the extracted mixture was collected. By adding 95% ethanol to the supernatant, the berry polysaccharides were precipitated and then lyophilized to ginseng berry polysaccharide extract. The ginseng berry polysaccharide portion was dissolved in water and dialyzed with molecular weight cut off at 20,000. The dried powder was collected after lyophilization and stored at −20°C until use.
2.4. Measurement of thymic involution
The thymi were dissected from the old (17 mo) and young (2 mo) mice administered APGP or PSK, and the thymic involutions of these mice were determined by photographing the thymi and measuring the weights and areas of the thymi.
2.5. Flow cytometry analysis
Old and young mice administered APGP or PSK were sacrificed, and total cells were prepared from the spleens and thymi as reported previously [24]. The cells were washed with FACS buffer (phosphate buffer saline containing 2% fetal calf serum and 0.1% sodium azide) and then incubated in 50 μL FACS buffer containing 10% rabbit serum for 10 min on ice. The cells were incubated with the indicated dye-conjugated antibodies specific for CD11c, NK1.1, B220, Foxp3, and CD25 for 45 min on ice and washed three times with ice-cold FACS buffer. The stained cells were analyzed by a FACSCalibur instrument (Becton Dickinson, Mountain View, CA, USA).
2.6. Enzyme-linked immunosorbent assay
The sera were prepared by centrifuging the blood of the old and young mice administered APGP and PSK, and the serum levels of IL-2 and IL-6 were determined by ELISA using the antibodies specific for IL-2 and IL-6 according to the manufacturer's instructions.
2.7. High-performance liquid chromatography analysis
A high-performance liquid chromatography (HPLC)-9500 instrument (Young-Lin Co., Gyeonggi, Korea) equipped with a Superdex 200 GL (GE Healthcare) was performed to high-performance size exclusion chromatography. First, 20 μL of each polysaccharide solution was analyzed by an isocratic mobile phase (50mM ammonium formate buffer, pH 5.5) at a flow rate of 0.5 mL/min at room temperature. Then the molecular weights of the purified polysaccharides were calculated from a calibration curve constructed with Pullulan (P-800, 400, 200, 100, 50, 20, 10 and 5; Showa Denko Co., Ltd., Tokyo, Japan).
2.8. Statistical analysis
All data are expressed as means ± standard deviation of experiments performed with six samples. All other data presented are representative of three different experiments that yielded similar results. Similar experimental data were also obtained in an additional independent set of in vitro experiments that were performed with the same number of samples. For statistical comparisons, results were analyzed with analysis of variance, Scheffe's post hoc test, Kruskal–Wallis test, and Mann–Whitney test. A p value <0.05 was considered statistically significant. All statistical tests were performed with the SPSS software package (Version 22.0, 2013; IBM Corp., Armonk, NY, USA).
3. Results and discussion
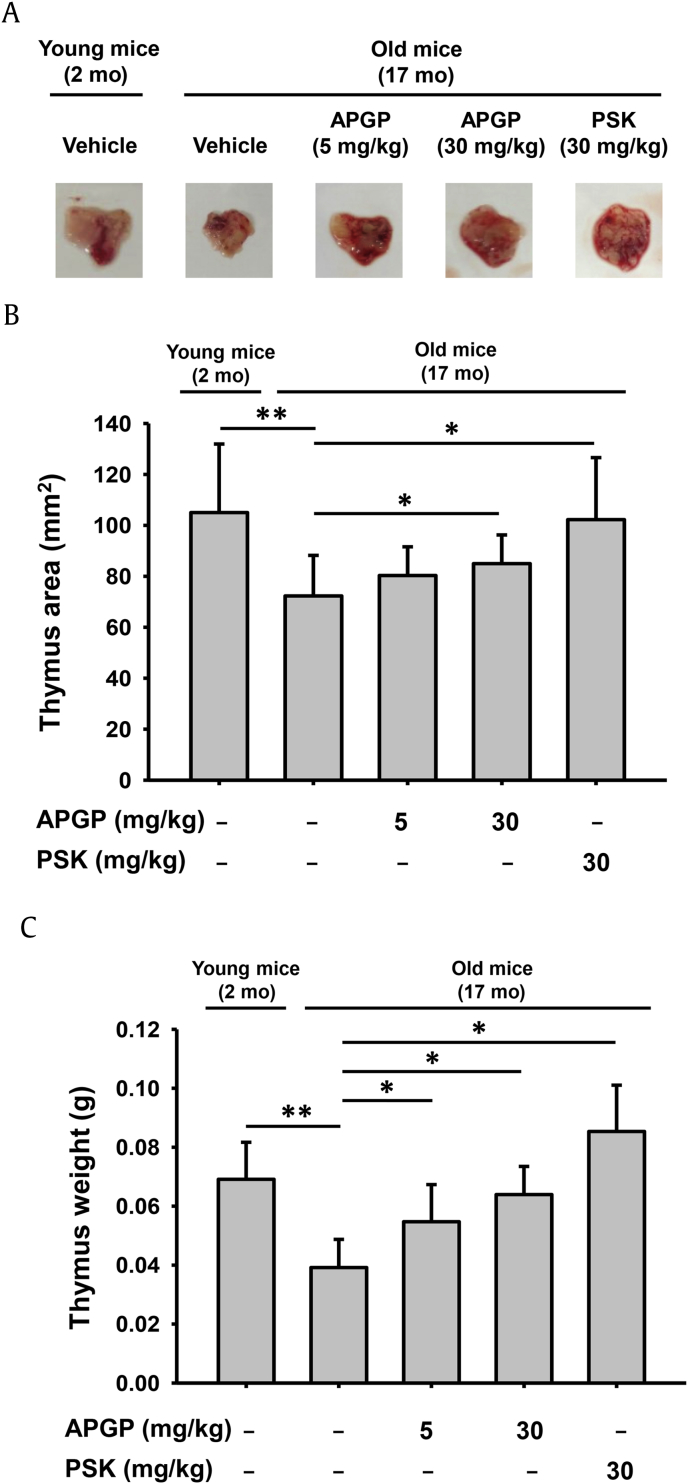
The thymus is a critical primary immune system organ in the body that initiates immune responses against various invading pathogens. Therefore, appropriate development and proper size of the thymus are required for active immune functions. One of the representative characteristics of immunosenescence is shrinking of the thymus with age. This process is known as thymic involution, resulting in changes in the thymic architecture and a decrease in tissue mass [25]. Therefore, thymic involution with age is regarded as one biological process of immunosenescence that causes a significant reduction in immune function [26], [27], [28]. We investigated the protective role of APGP, a polysaccharide extracted from a Korean ginseng berry, on immunosenescence in aged mice. We first evaluated the effect of APGP on thymic involution in aged mice. To examine whether APGP had an anti-immunosenescent effect on thymic involution in aged mice, old (17 mo) mice were orally administered APGP, and the sizes of their thymi were measured. The sizes of the thymi dissected from young (2 mo) and old mice administered APGP were compared by taking photographs. The sizes of the thymi from old mice were smaller than those from young mice, but were increased by APGP in a manner similar to PSK, which is a positive control polysaccharide (Fig. 1A). We further confirmed the anti-immunosenescent effect of APGP on thymic involution in aged mice by measuring the weights and areas of the thymi. As expected, both the weights (Fig. 1B) and areas (Fig. 1C) of thymi from old mice were smaller than those from young mice, but were dose-dependently increased by APGP in a manner similar to PSK. These results indicated that APGP exerted a protective effect on age-induced thymic involution in mice.
Fig. 1.
Effect of APGP on thymic involution in aged mice. (A) Thymi were dissected from old (17 mo) and young (2 mo) mice administered either APGP or PSK, and photographs were taken with a digital camera. (B) The area of each dissected thymus was measured by a ruler. (C) The weight of each dissected thymus was measured by an electronic scale. Data are presented as the means ± SD of one biological experiment performed with six technical replicates (n = 6). *p < 0.05, **p < 0.01 compared to a control group. APGP, acidic-polysaccharide-linked glycopeptide; PSK, polysaccharide-K; SD, standard deviation.
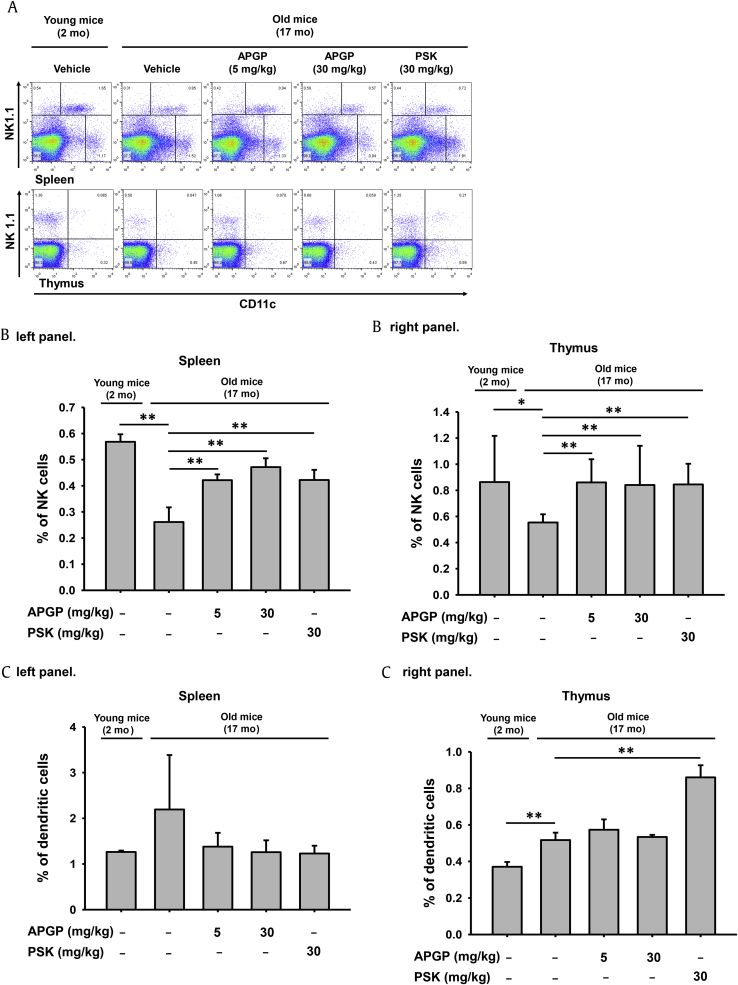
There are two groups of immune system organs, primary and secondary immune organs. Primary immune organs, such as the bone marrow and thymus, are organs where immature immune cells develop, whereas secondary immune organs, such as the lymph nodes, spleen, and tonsils, are organs where immune reactions occur via the recognition of antigens exposed to mature immune cells. Therefore, we investigated the effect of APGP on the population of natural killer (NK) cells (cytotoxic immune cells that recognize and directly remove invading pathogens) and on the population of dendritic cells (professional antigen presenting cells that initiate adaptive immune reactions in both primary and secondary immune organs). The populations of NK cells and dendritic cells in total thymic and splenic cell samples were determined by FACS analysis after staining the cells with a specific marker for each cell type (NK1.1 [29] and CD11c [30], respectively). FACS analysis revealed that the population of NK cells decreased in both the thymi and spleens of old mice compared to those of young mice, but was increased by APGP. Unlike NK cells, the population of dendritic cells was not increased by APGP in thymi and spleens of old mice (Fig. 2A). Unexpectedly, the dendritic cell population was not decreased in the thymi and spleens of old mice, but rather was increased in the thymi of old mice compared to those of young mice. The percentages of NK cells and dendritic cells in each organ, obtained from FACS analysis, were calculated and plotted (Figs. 2B and 2C). Of note, APGP increased the population of NK cells in the immune organs of aged mice in a manner similar to PSK (Fig. 2B) but did not have this effect on the population of dendritic cells in the immune organs of aged mice (Fig. 2C). These results indicate that APGP may exert an anti-immunosenescent role by facilitating cytotoxic activity against invading pathogens rather than by directly inducing adaptive immune responses through antigen presentation.
Fig. 2.
Effect of APGP on the populations of NK cells and dendritic cells in aged mice. (A) Total thymic and splenic cells (5 × 106 cells/mL) isolated from old and young mice administered the indicated compounds were analyzed by flow cytometry using antibodies specific for NK1.1 (a marker of NK cells) and CD11c (a marker of dendritic cells). The NK cell population in (B, left panel) total splenic cells and (B, right panel) total thymic cells was measured and plotted. The dendritic cell population in (C, left panel) total splenic cells and (C, right panel) total thymic cells was measured and plotted. Data are presented as the means ± SD of one biological experiment performed with six technical replicates (n = 6). *p < 0.05, **p < 0.01 compared to a control group. APGP, acidic-polysaccharide-linked glycopeptide; NK, nakural killer; PSK, polysaccharide-K; SD, standard deviation.
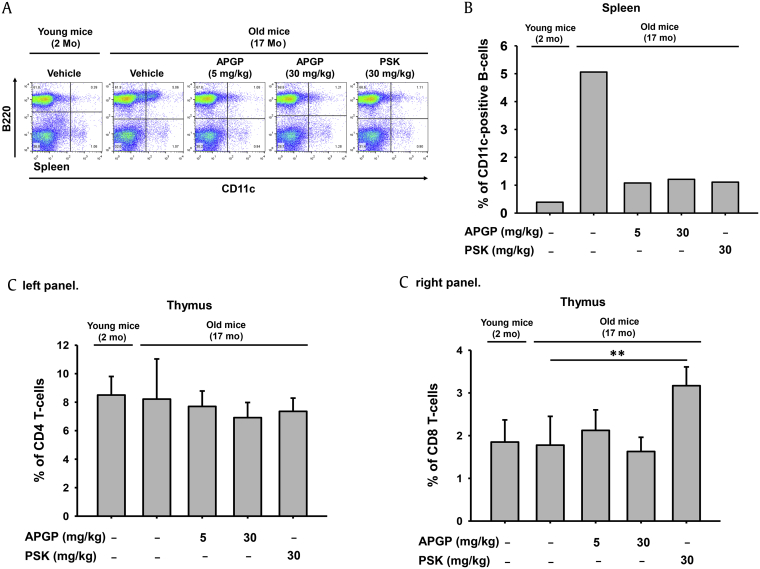
Recent studies have successfully identified a novel subset of CD11c-positive B cells in the spleens of aged mice [31], [32], possibly implying that the population of CD11c-positive B cells increased with age. Therefore, we investigated the effect of APGP on the population of CD11c-positive B cells in aged mice. The population of CD11c-positive B cells in total splenic cell samples of aged mice was determined by FACS analysis after staining the cells for CD11c and the B cell-specific marker, B220 [33]. FACS analysis revealed that the population of B220-positive/CD11c-positive B cells was increased in the spleens of old mice compared to those of young mice, but was decreased by APGP in a manner similar to PSK (Fig. 3A). The percentage of B220-positive/CD11c-positive B cells in the spleens, obtained from FACS analysis, was calculated and plotted (Fig. 3B). These results strongly suggest that APGP exerts an anti-immunosenescent activity by reducing the population of age-correlated CD11c-positive/B220-positive B cells in aged mice.
Fig. 3.
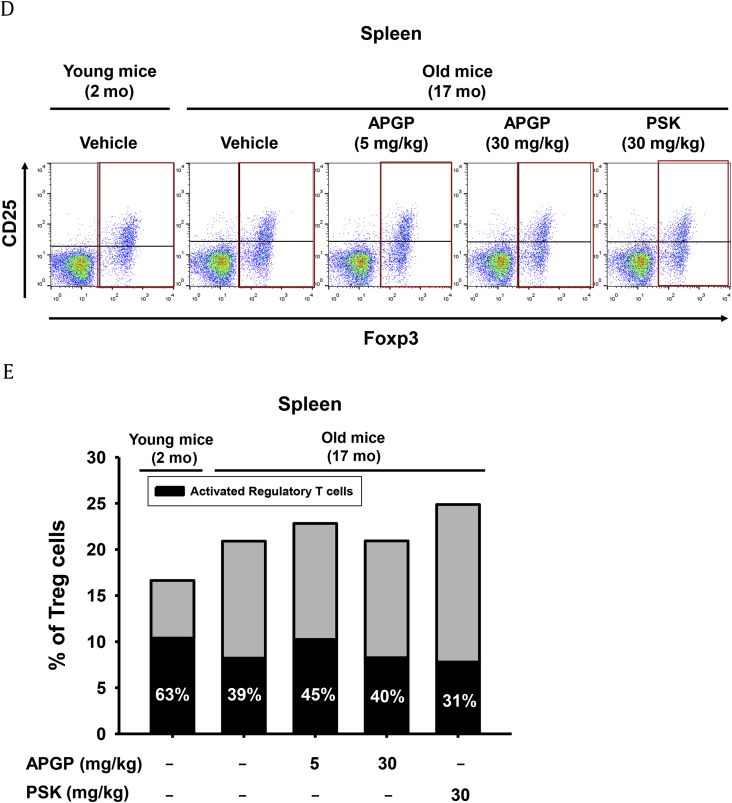
Effect of APGP on age-correlated CD11c-positive B cells and on T cell subpopulations in aged mice. (A) Total splenic cells (5 × 106 cells/mL) isolated from old and young mice administered the indicated doses of compounds were analyzed by flow cytometry using antibodies specific for CD11c and B220, a marker of B cells. (B) The population of age-correlated CD11c-positive B cells in total splenic cells was measured and plotted. (C) Total thymic cells (5 × 106 cells/mL) isolated from old and young mice administered the indicated compounds were analyzed by flow cytometry using antibodies specific for CD4, a marker of helper T cells, and CD8, a marker of cytotoxic T cells. The populations of (C, left panel) CD4-positive helper T cells and (C, right panel) CD8-positive cytotoxic T cells in total thymic cells were measured and plotted. (D) Total splenic cells (5 × 106 cells/mL) isolated from old and young mice administered the indicated compounds were analyzed by flow cytometry using antibodies specific for Foxp3 and CD25, markers of activated Treg cells. (E) The populations of Foxp3-positive Treg cells and activated double-positive (Foxp3 and CD25) Treg cells in total splenic cells were measured and plotted. Data are presented as the means ± SD of one biological experiment performed with six technical replicates (n = 6). *p < 0.05, **p < 0.01 compared to a control group. APGP, acidic-polysaccharide-linked glycopeptide; NK, natural killer; PSK, polysaccharide-K; SD, standard deviation; Treg, regulatory T cells.
Because the size of the thymus, where T cells mature, was reduced in aged mice (Fig. 1), the effect of APGP on populations of thymic T cells was further investigated. To examine this, total thymic cells obtained from old mice were stained for the helper T cell marker CD4 and the cytotoxic T cell marker CD8, and the population of each T cell type was determined by FACS analysis. Unexpectedly, populations of CD4-positive and CD8-positive T cells were not reduced in the thymi of aged mice, and APGP also failed to show any effect on populations of these cell types (Fig. 3C). These results indicated that thymic involution with age may have resulted from changes in thymic architecture owing to a reduction of cell types other than T cells, and studies to identify the types of cells that directly correlate with thymic involution will be required. Moreover, studies on the effect of APGP on helper and cytotoxic T cells in the thymi of aged mice are needed. The effect of APGP on the population of splenic regulatory T cells (Treg cells) in aged mice was also investigated. Treg cells, also known as suppressor T cells, comprise a subpopulation of T cells that play crucial roles in providing tolerance to self-antigens and preventing autoimmunity by suppressing the activation of effector T cells [34]. Treg cells are thought to be derived from the same lineage as CD4-positive T cells. Therefore, naturally occurring Treg cells express both CD4 and another specific marker, Foxp3 [35], [36], [37], [38]. Because effector T cells express another cell surface marker, CD25 (which is an IL-2 receptor alpha chain that interacts with CD4), functionally activated Treg cells express three different cell surface markers: CD4, CD25, and Foxp3 [36], [37], [38]. We determined the population of Treg cells in total splenic cell samples of aged mice administered APGP. CD4-gated total T cells were stained for CD25 and Foxp3, and the population of Foxp3-positive Treg cells was analyzed by FACS. FACS analysis revealed that the population of activated triple-positive (CD4-positive/CD25-positive/Foxp3-positive) Treg cells decreased in the total splenic cell samples of old mice compared to those of young mice, which were increased after 5 mg/kg APGP administration. In old mice, this Treg cell population was not increased by a high dose (5 mg/kg) of APGP, and even decreased after PSK administration (Figs. 3D and 3E). Interestingly, in contrast to our results, populations of functional Treg cells have been reported to increase with age [39], [40]. Vianna et al [41] reported that the frequency of Treg cells progressively increased in thymectomized young mice, whereas grafting of a functional thymus in aged mice decreased the frequency of Treg cells. It is not clear why our results are different from the previous observations. Clearly, additional studies designed to explain why the population of splenic activated Treg cells in aged mice was decreased (and increased on APGP administration) will be required.
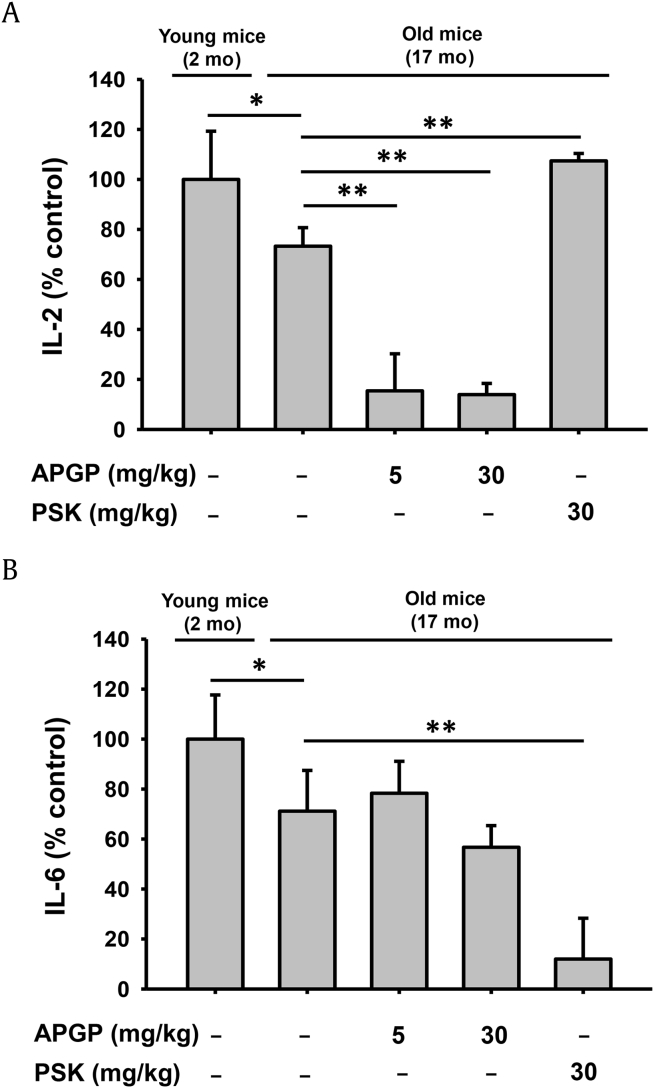
Cytokines are small proteins secreted mainly from immune cells and play critical roles in immune response modulation. Among these cytokines, IL-2 and IL-6 have been shown to exert particular roles in immunosenescence. Previous studies using mouse models of aging have demonstrated that IL-2 and IL-6 restore the generation of and improve the memory and responses of CD4-positive effector T cells activated from naïve CD4-positive T cells in a manner superior to that observed in younger animals [42], [43], [44], [45]. Moreover, dendritic cells activated by a ligand of a Toll-like receptor highly produced IL-6 during cognate T cell interactions, resulting in the induction of aged mouse CD4-positive naïve T cell expansion and survival, as well as the production of high levels of IL-2 from T cells [46]. A recent study also has demonstrated that the generation of CD8-positive cytotoxic T cells responding to live viruses was significantly enhanced by the cooperation of IL-2 and IL-6 in both aged mice and humans [47]. Therefore, we investigated the effect of APGP on the production of IL-2 and IL-6 in the sera of aged mice by ELISA analysis. IL-2 production was reduced in aged mice compared to young mice and also decreased in the aged mice receiving APGP treatment, whereas PSK increased IL-2 production in aged mice (Fig. 4A). It has been reported that IL-2 production decreased in CD4-positive T cells of aged mice [42], and our result that IL-2 production was reduced in aged mice compared to young mice was consistent with this study. However, contrary to our expectation, IL-2 production was even further decreased in the aged mice receiving APGP treatment. In addition, IL-6 production was also reduced in aged mice compared to young mice, whereas IL-6 production levels were not statistically changed in the aged mice receiving APGP treatment (Fig. 4B). It is still unclear why APGP decreased the production of IL-2 but exerted no effect on the production of IL-6 in aged mice, and further studies in this regard need to be conducted.
Fig. 4.
Effect of APGP on the serum levels of IL-2 and IL-6 in aged mice. Sera were obtained from the blood of old and young mice administered the indicated compounds, and serum levels of IL-2 and IL-6 in these mice were determined by ELISA. (A) IL-2. (B) IL-6. Data are presented as the means ± SD of one biological experiment performed with six technical replicates (n = 6). *p < 0.05, **p < 0.01 compared to a control group. APGP, acidic-polysaccharide-linked glycopeptide; ELISA, enzyme-linked immunosorbent assay; IL, interleukin PSK, polysaccharide-K; SD, standard deviation.
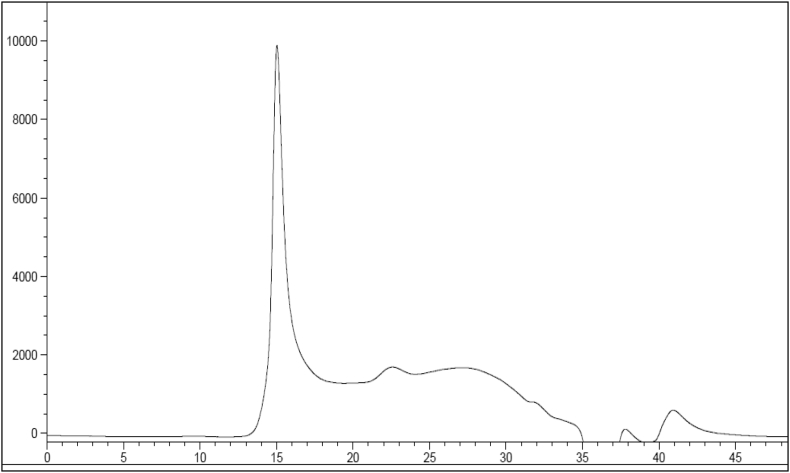
Lastly, we analyzed APGP HPLC profiles. HPLC analysis showed that APGP had one major sharp peak around 15 min (corresponding to the main form of polysaccharide in APGP) and a long tail up to 35 min (corresponding to a mixture of different polysaccharides in APGP) (Fig. 5). Identification of the major peak at 15 min will be required.
Fig. 5.
HPLC chromatogram of APGP extracted from Korean ginseng berries. HPLC analysis showed that APGP had one major peak at 15 min (a main type of polysaccharide) and a long tail up to 35 min (a mixture of a variety of types of polysaccharides). HPLC, high-performance liquid chromatography.
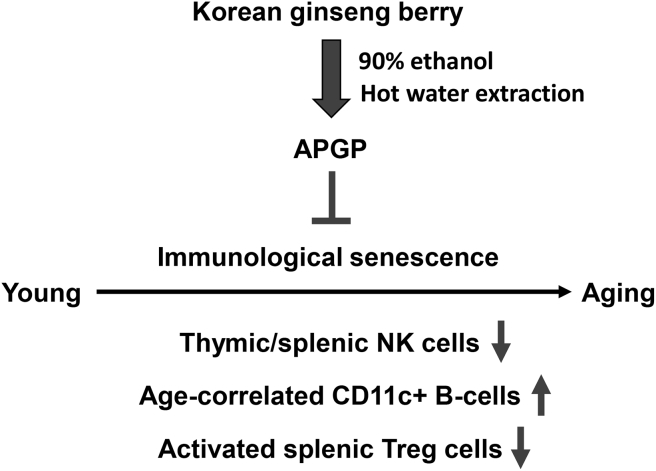
In summary, APGP polysaccharides extracted from a Korean ginseng berry were associated with recovery of thymic involution. APGP also increased the reduced population of thymic and splenic NK cells in aged mice and reduced the increased population of age-correlated splenic CD11c-positive B cells in aged mice. Moreover, APGP restored the population of functionally activated splenic Treg cells in aged mice. Taken together, these results strongly suggest that APGP suppresses immunosenescence by suppressing thymic involution (induced by aging) and by modulating populations of various types of immune cells that are critical for active immune responses (Fig. 6). Our findings also indicate that APGP could be useful as a novel antiaging agent by strengthening the body's immune functions.
Fig. 6.
Schematic description of the regulatory roles of APGP extracted from Korean ginseng berries on immunosenescence in aged mice. APGP was associated with recovery of thymic involution and also increased the reduced population of thymic and splenic NK cells in aged mice, and reduced the increased population of age-correlated splenic CD11c-positive B cells in aged mice. In addition, APGP restored the population of functionally activated splenic Treg cells in aged mice. APGP, acidic-polysaccharide-linked glycopeptide; NK, natural killer; Treg, regulatory T cells.
Conflicts of interest
The authors have no financial conflicts of interest.
Acknowledgments
This study was supported by BK21 program from NRF, Korea.
Contributor Information
Jae Youl Cho, Email: jaecho@skku.edu.
Song Seok Shin, Email: ssshin@amorepacific.com.
References
- 1.Lipsky M.S., King M. Biological theories of aging. Dis Mon. 2015;61:460–466. doi: 10.1016/j.disamonth.2015.09.005. [DOI] [PubMed] [Google Scholar]
- 2.Robert L., Fulop T. Aging. Facts and theories. Preface. Interdiscip Top Gerontol. 2014;39:VI–VIII. doi: 10.1159/000358894. [DOI] [PubMed] [Google Scholar]
- 3.Lopez-Otin C., Blasco M.A., Partridge L., Serrano M., Kroemer G. The hallmarks of aging. Cell. 2013;153:1194–1217. doi: 10.1016/j.cell.2013.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Solana R., Tarazona R., Gayoso I., Lesur O., Dupuis G., Fulop T. Innate immunosenescence: effect of aging on cells and receptors of the innate immune system in humans. Semin Immunol. 2012;24:331–341. doi: 10.1016/j.smim.2012.04.008. [DOI] [PubMed] [Google Scholar]
- 5.Montgomery R.R., Shaw A.C. Paradoxical changes in innate immunity in aging: recent progress and new directions. J Leukoc Biol. 2015;98:937–943. doi: 10.1189/jlb.5MR0315-104R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Castelo-Branco C., Soveral I. The immune system and aging: a review. Gynecol Endocrinol. 2014;30:16–22. doi: 10.3109/09513590.2013.852531. [DOI] [PubMed] [Google Scholar]
- 7.Franceschi C., Bonafe M., Valensin S., Olivieri F., De Luca M., Ottaviani E., De Benedictis G. Inflamm-aging. An evolutionary perspective on immunosenescence. Ann N Y Acad Sci. 2000;908:244–254. doi: 10.1111/j.1749-6632.2000.tb06651.x. [DOI] [PubMed] [Google Scholar]
- 8.Giunta S. Exploring the complex relations between inflammation and aging (inflamm-aging): anti-inflamm-aging remodelling of inflamm-aging, from robustness to frailty. Inflamm Res. 2008;57:558–563. doi: 10.1007/s00011-008-7243-2. [DOI] [PubMed] [Google Scholar]
- 9.Hasegawa H. Proof of the mysterious efficacy of ginseng: basic and clinical trials: metabolic activation of ginsenoside: deglycosylation by intestinal bacteria and esterification with fatty acid. J Pharmacol Sci. 2004;95:153–157. doi: 10.1254/jphs.fmj04001x4. [DOI] [PubMed] [Google Scholar]
- 10.Byeon S.E., Lee J., Kim J.H., Yang W.S., Kwak Y.S., Kim S.Y., Choung E.S., Rhee M.H., Cho J.Y. Molecular mechanism of macrophage activation by red ginseng acidic polysaccharide from Korean red ginseng. Mediators Inflamm. 2012;2012:732860. doi: 10.1155/2012/732860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yayeh T., Jung K.H., Jeong H.Y., Park J.H., Song Y.B., Kwak Y.S., Kang H.S., Cho J.Y., Oh J.W., Kim S.K. Korean red ginseng saponin fraction downregulates proinflammatory mediators in LPS stimulated RAW264.7 cells and protects mice against endotoxic shock. J Ginseng Res. 2012;36:263–269. doi: 10.5142/jgr.2012.36.3.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nabavi S.F., Sureda A., Habtemariam S., Nabavi S.M. Ginsenoside Rd and ischemic stroke; a short review of literatures. J Ginseng Res. 2015;39:299–303. doi: 10.1016/j.jgr.2015.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yang W.S., Jeong D., Yi Y.S., Lee B.H., Kim T.W., Htwe K.M., Kim Y.D., Yoon K.D., Hong S., Lee W.S. Myrsine seguinii ethanolic extract and its active component quercetin inhibit macrophage activation and peritonitis induced by LPS by targeting to Syk/Src/IRAK-1. J Ethnopharmacol. 2014;151:1165–1174. doi: 10.1016/j.jep.2013.12.033. [DOI] [PubMed] [Google Scholar]
- 14.Jung J.S., Lee S.Y., Kim D.H., Kim H.S. Protopanaxatriol ginsenoside Rh1 upregulates phase II antioxidant enzyme gene expression in rat primary astrocytes: involvement of MAP kinases and Nrf2/ARE signaling. Biomol Ther (Seoul) 2016;24:33–39. doi: 10.4062/biomolther.2015.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang W., Cho S.Y., Xiang G., Min K.J., Yu Q., Jin J.O. Ginseng berry extract promotes maturation of mouse dendritic cells. PLoS One. 2015;10:e0130926. doi: 10.1371/journal.pone.0130926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cho K.S., Park C.W., Kim C.K., Jeon H.Y., Kim W.G., Lee S.J., Kim Y.M., Lee J.Y., Choi Y.D. Effects of Korean ginseng berry extract (GB0710) on penile erection: evidence from in vitro and in vivo studies. Asian J Androl. 2013;15:503–507. doi: 10.1038/aja.2013.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Choi Y.D., Park C.W., Jang J., Kim S.H., Jeon H.Y., Kim W.G., Lee S.J., Chung W.S. Effects of Korean ginseng berry extract on sexual function in men with erectile dysfunction: a multicenter, placebo-controlled, double-blind clinical study. Int J Impot Res. 2013;25:45–50. doi: 10.1038/ijir.2012.45. [DOI] [PubMed] [Google Scholar]
- 18.Jung H., Bae J., Ko S.K., Sohn U.D. Ultrasonication processed Panax ginseng berry extract induces apoptosis through an intrinsic apoptosis pathway in HepG2 cells. Arch Pharm Res. 2016;39:855–862. doi: 10.1007/s12272-016-0760-6. [DOI] [PubMed] [Google Scholar]
- 19.Jang H.J., Han I.H., Kim Y.J., Yamabe N., Lee D., Hwang G.S., Oh M., Choi K.C., Kim S.N., Ham J. Anticarcinogenic effects of products of heat-processed ginsenoside Re, a major constituent of ginseng berry, on human gastric cancer cells. J Agric Food Chem. 2014;62:2830–2836. doi: 10.1021/jf5000776. [DOI] [PubMed] [Google Scholar]
- 20.Zhao J.M., Li N., Zhang H., Wu C.F., Piao H.R., Zhao Y.Q. Novel dammarane-type sapogenins from Panax ginseng berry and their biological activities. Bioorg Med Chem Lett. 2011;21:1027–1031. doi: 10.1016/j.bmcl.2010.12.035. [DOI] [PubMed] [Google Scholar]
- 21.Park E.Y., Kim H.J., Kim Y.K., Park S.U., Choi J.E., Cha J.Y., Jun H.S. Increase in insulin secretion induced by Panax ginseng berry extracts contributes to the amelioration of hyperglycemia in streptozotocin induced diabetic mice. J Ginseng Res. 2012;36:153–160. doi: 10.5142/jgr.2012.36.2.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dey L., Xie J.T., Wang A., Wu J., Maleckar S.A., Yuan C.S. Anti-hyperglycemic effects of ginseng: comparison between root and berry. Phytomedicine. 2003;10:600–605. doi: 10.1078/094471103322331908. [DOI] [PubMed] [Google Scholar]
- 23.Kim M.S., Lee Y., Sung G.H., Kim J.H., Park J.G., Kim H.G., Baek K.S., Cho J.H., Han J., Lee K.H. Pro-apoptotic activity of 4-Isopropyl-2-(1-Phenylethyl) aniline isolated from cordyceps bassiana. Biomol Ther (Seoul) 2015;23:367–373. doi: 10.4062/biomolther.2015.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Choi J.M., Kim S.H., Shin J.H., Gibson T., Yoon B.S., Lee D.H., Lee S.K., Bothwell A.L., Lim J.S., Lee S.K. Transduction of the cytoplasmic domain of CTLA-4 inhibits TcR-specific activation signals and prevents collagen-induced arthritis. Proc Natl Acad Sci USA. 2008;105:19875–19880. doi: 10.1073/pnas.0805198105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shanley D.P., Aw D., Manley N.R., Palmer D.B. An evolutionary perspective on the mechanisms of immunosenescence. Trends Immunol. 2009;30:374–381. doi: 10.1016/j.it.2009.05.001. [DOI] [PubMed] [Google Scholar]
- 26.Muller L., Pawelec G. Aging and immunity — impact of behavioral intervention. Brain Behav Immun. 2014;39:8–22. doi: 10.1016/j.bbi.2013.11.015. [DOI] [PubMed] [Google Scholar]
- 27.Palmer D.B. The effect of age on thymic function. Front Immunol. 2013;4:316. doi: 10.3389/fimmu.2013.00316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shen S.S., Kim J.S., Weksler M.E. Effect of age on thymic development, T cell immunity, and helper T cell function. Rev Physiol Biochem Pharmacol. 1999;139:123–139. doi: 10.1007/BFb0033650. [DOI] [PubMed] [Google Scholar]
- 29.Kitaichi N., Kotake S., Morohashi T., Onoe K., Ohno S., Taylor A.W. Diminution of experimental autoimmune uveoretinitis (EAU) in mice depleted of NK cells. J Leukoc Biol. 2002;72:1117–1121. [PubMed] [Google Scholar]
- 30.Zhang J., Kawashima N., Suda H., Nakano Y., Takano Y., Azuma M. The existence of CD11c+ sentinel and F4/80+ interstitial dendritic cells in dental pulp and their dynamics and functional properties. Int Immunol. 2006;18:1375–1384. doi: 10.1093/intimm/dxl070. [DOI] [PubMed] [Google Scholar]
- 31.Hao Y., O'Neill P., Naradikian M.S., Scholz J.L., Cancro M.P. A B-cell subset uniquely responsive to innate stimuli accumulates in aged mice. Blood. 2011;118:1294–1304. doi: 10.1182/blood-2011-01-330530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rubtsov A.V., Rubtsova K., Fischer A., Meehan R.T., Gillis J.Z., Kappler J.W., Marrack P. Toll-like receptor 7 (TLR7)-driven accumulation of a novel CD11c(+) B-cell population is important for the development of autoimmunity. Blood. 2011;118:1305–1315. doi: 10.1182/blood-2011-01-331462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Coffman R.L. Surface antigen expression and immunoglobulin gene rearrangement during mouse pre-B cell development. Immunol Rev. 1982;69:5–23. doi: 10.1111/j.1600-065x.1983.tb00446.x. [DOI] [PubMed] [Google Scholar]
- 34.Bettelli E., Carrier Y., Gao W., Korn T., Strom T.B., Oukka M., Weiner H.L., Kuchroo V.K. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441:235–238. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- 35.Curiel T.J. Tregs and rethinking cancer immunotherapy. J Clin Invest. 2007;117:1167–1174. doi: 10.1172/JCI31202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hori S., Nomura T., Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003;299:1057–1061. doi: 10.1126/science.1079490. [DOI] [PubMed] [Google Scholar]
- 37.Fontenot J.D., Gavin M.A., Rudensky A.Y. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat Immunol. 2003;4:330–336. doi: 10.1038/ni904. [DOI] [PubMed] [Google Scholar]
- 38.Khattri R., Cox T., Yasayko S.A., Ramsdell F. An essential role for Scurfin in CD4+CD25+ T regulatory cells. Nat Immunol. 2003;4:337–342. doi: 10.1038/ni909. [DOI] [PubMed] [Google Scholar]
- 39.Gregg R., Smith C.M., Clark F.J., Dunnion D., Khan N., Chakraverty R., Nayak L., Moss P.A. The number of human peripheral blood CD4+ CD25 high regulatory T cells increases with age. Clin Exp Immunol. 2005;140:540–546. doi: 10.1111/j.1365-2249.2005.02798.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sharma S., Dominguez A.L., Lustgarten J. High accumulation of T regulatory cells prevents the activation of immune responses in aged animals. J Immunol. 2006;177:8348–8355. doi: 10.4049/jimmunol.177.12.8348. [DOI] [PubMed] [Google Scholar]
- 41.Vianna P.H., Canto F.B., Nogueira J.S., Nunes C.F., Bonomo A.C., Fucs R. Critical influence of the thymus on peripheral T cell homeostasis. Immun Inflamm Dis. 2016;4:474–486. doi: 10.1002/iid3.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Haynes L., Linton P.J., Eaton S.M., Tonkonogy S.L., Swain S.L. Interleukin 2, but not other common gamma chain-binding cytokines, can reverse the defect in generation of CD4 effector T cells from naive T cells of aged mice. J Exp Med. 1999;190:1013–1024. doi: 10.1084/jem.190.7.1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Haynes L., Eaton S.M., Burns E.M., Randall T.D., Swain S.L. CD4 T cell memory derived from young naive cells functions well into old age, but memory generated from aged naive cells functions poorly. Proc Natl Acad Sci U S A. 2003;100:15053–15058. doi: 10.1073/pnas.2433717100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Maue A.C., Eaton S.M., Lanthier P.A., Sweet K.B., Blumerman S.L., Haynes L. Proinflammatory adjuvants enhance the cognate helper activity of aged CD4 T cells. J Immunol. 2009;182:6129–6135. doi: 10.4049/jimmunol.0804226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Haynes L., Eaton S.M., Burns E.M., Rincon M., Swain S.L. Inflammatory cytokines overcome age-related defects in CD4 T cell responses in vivo. J Immunol. 2004;172:5194–5199. doi: 10.4049/jimmunol.172.9.5194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jones S.C., Brahmakshatriya V., Huston G., Dibble J., Swain S.L. TLR-activated dendritic cells enhance the response of aged naive CD4 T cells via an IL-6-dependent mechanism. J Immunol. 2010;185:6783–6794. doi: 10.4049/jimmunol.0901296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhou X., Hopkins J.W., Wang C., Brahmakshatriya V., Swain S.L., Kuchel G.A., Haynes L., McElhaney J.E. IL-2 and IL-6 cooperate to enhance the generation of influenza-specific CD8 T cells responding to live influenza virus in aged mice and humans. Oncotarget. 2016;7:39171–39183. doi: 10.18632/oncotarget.10047. [DOI] [PMC free article] [PubMed] [Google Scholar]