Abstract
Background
Korean Red Ginseng (steamed and dried white ginseng, Panax ginseng Meyer) is well known for enhancing vital energy and immune capacity and for inhibiting cancer cell growth. Some clinical studies also demonstrated a therapeutic potential of ginseng extract for treating lung inflammatory disorders. This study was conducted to establish the therapeutic potential of ginseng saponins on the lung inflammatory response.
Methods
From Korean Red Ginseng, 11 ginsenosides (Rb1, Rb2, Rb3, Rc, Rd, Re, Rf, Rg1, Rg2, Rg3, and Rh2) were isolated. Their inhibitory potential and action mechanism were evaluated using a mouse model of lung inflammation, acute lung injury induced by intranasal lipopolysaccharide administration. Their anti-inflammatory activities were also examined in lung epithelial cell line (A549) and alveolar macrophage (MH-S).
Results
All ginsenosides orally administered at 20 mg/kg showed 11.5–51.6% reduction of total cell numbers in bronchoalveolar lavage fluid (BALF). Among the ginsenosides, Rc, Re, Rg1, and Rh2 exhibited significant inhibitory action by reducing total cell numbers in the BALF by 34.1–51.6% (n = 5). Particularly, Re showed strong and comparable inhibitory potency with that of dexamethasone, as judged by the number of infiltrated cells and histological observations. Re treatment clearly inhibited the activation of mitogen-activated protein kinases, nuclear factor-κB, and the c-Fos component in the lung tissue (n = 3).
Conclusion
Certain ginsenosides inhibit lung inflammatory responses by interrupting these signaling molecules and they are potential therapeutics for inflammatory lung diseases.
Keywords: Panax ginseng, ginsenoside, lung inflammation, MAPK, NF-κB
1. Introduction
Lung diseases including acute bronchitis and chronic obstructive pulmonary disease (COPD) are essentially inflammatory disorders. Especially, many inflammatory parameters are involved in provoking and exacerbating COPD which comprises chronic asthma, chronic bronchitis, and emphysema. Oxidative stress and nitric oxide induce inflammatory responses in the lungs. Proinflammatory cytokines and chemokines are released from lung epithelial cells and infiltrated inflammatory cells in the affected area. Extracellular matrix degradation enzymes such as matrix metalloproteinases are also involved [1], [2]. Because of these complex pathological processes, it is difficult to control COPD. The currently available drugs such as steroids and bronchodilators only relieve the symptoms of COPD. The newly developed drugs may not give satisfactory results since identification of critical target molecules in COPD has not yet been successful [3], [4]. Thus, herbal drugs and plant-originating compounds have been continuously explored in the hope to find novel potential agents.
The roots of Panax ginseng Meyer (white ginseng) are popular for enhancing vital energy and immune capacity and for inhibiting cancer cell growth. Its major constituents are various ginsenosides that exhibit numerous pharmacological activities, including enhanced vitality, immune modulation, anticancer activity, and a cartilage protective action [5], [6], [7], [8]. Ginseng has been used in Korean traditional herbal medicine for treating cough (Docksamtang) as a sole ingredient or as one of the ingredients in several complex prescriptions, such as Insamyeunpewhan [9]. Ginseng extracts and several ginsenosides possess anti-inflammatory activities. For example, the ginsenoside Rg1 possesses anti-inflammatory action in vitro and in vivo in a glucocorticoid receptor-dependent manner [10]. Various ginsenosides regulate the production of proinflammatory molecules, such as cyclooxygenase-2, cytokines, and chemokines. They also prevent oxidative and nitrosative stress. These anti-inflammatory actions of ginsenosides have been well summarized [11], [12]. Particularly, some ginsenosides inhibit activation of the p38 mitogen-activated protein kinase (MAPK) pathway in vitro [5], [10], [13]. As p38 MAPK signaling pathway is involved in provoking COPD [14], [15], [16], regulating p38 MAPK activation by some ginsenosides may provide a new therapeutic strategy for controlling inflammatory lung disorders.
The therapeutic effects of ginseng extracts and some ginsenosides against asthma have been well documented [17], [18], [19]. However, only the therapeutic potential of ginsenoside Rg5 has been reported for inflammatory lung disorders [20]. Small limited clinical studies have demonstrated potential therapeutic efficacy of ginseng extract for patients with COPD [21], [22]. Korean Red Ginseng (steamed and dried white ginseng) extract was also found to prevent acute respiratory illness in a clinical study [23]. In our preliminary study, a hot water extract of Korean Red Ginseng possessed strong inhibitory activity against a mouse model of acute lung injury. These findings led us to examine the effects of ginsenosides on lung inflammation and the MAPK pathway. To the best of our knowledge, this is the first report showing in vivo inhibition of lung inflammation by various ginsenosides and their therapeutic potential.
2. Materials and methods
2.1. Chemicals
2-Amino-5,6-dihydro-6-methyl-4H-1,3-thiazine hydrochloride (AMT) was purchased from Tocris Cookson Ltd. (Avonmouth, Bristol, UK). 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT), dexamethasone, interleukin (IL)-1β, and lipopolysaccharide (LPS, Escherichia coli 0127:B8) were purchased from Sigma-Aldrich Inc. (St. Louis, MO, USA). Compound K-enriched fraction (5%) was obtained from Fleton Natural Products Co. (Chengdu, China). The protein assay kit was purchased from Bio-Rad Lab (Hercules, CA, USA). All antibodies relating to MAPK and nuclear transcription factor-κB (NF-κB) signaling were purchased from Cell Signaling Technologies (Dancers, MA, USA). β-actin antibody was obtained from Bethyl Laboratories, Inc. (Montgomery, TX, USA). Lamin B1 antibody was purchased from Bioworld technology (Minneapolis, MN, USA).
2.2. Preparation of ginseng extracts and isolation of the ginsenosides
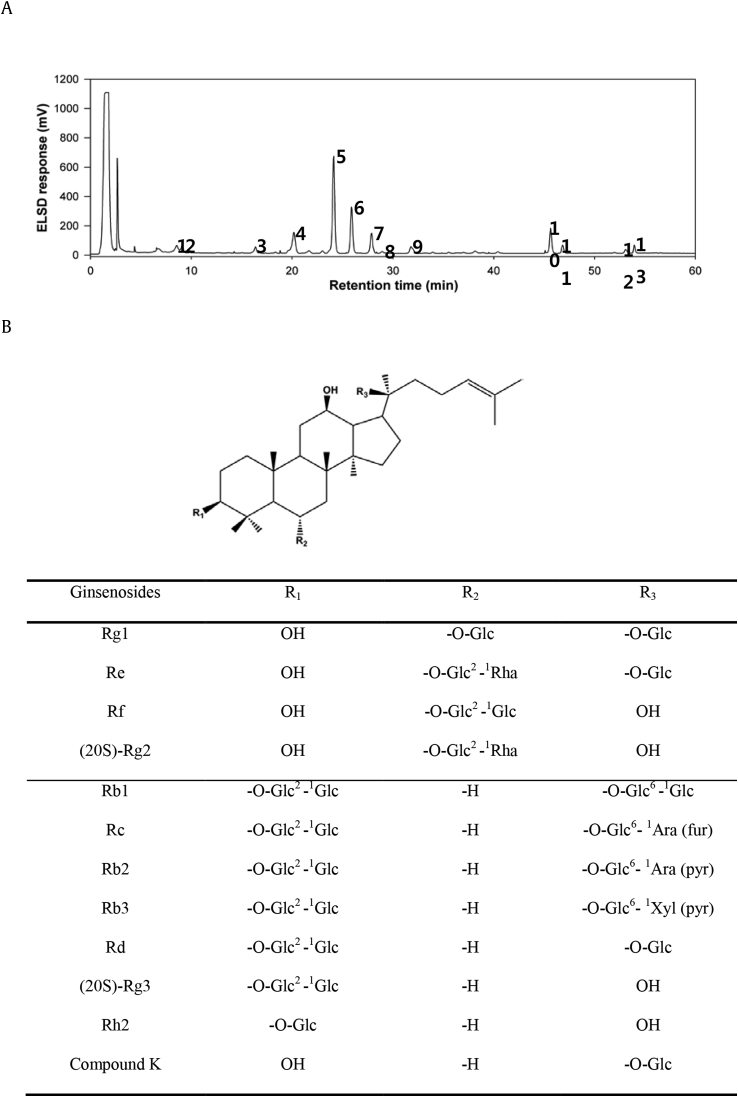
Panax ginseng Meyer was cultivated for 6 yrs in Korean peninsula under the supervision of the experts of KT&G Corporation (KT&G, Korea). The roots (white ginseng) were collected and dried. Korean Red Ginseng was manufactured by steaming and drying white ginseng in KT&G. The hot water extract was prepared (February 2015) and provided from KT&G. The dried extract was subsequently dissolved in distilled water and fractionated with n-butanol three times. The combined butanol fraction was again centrifuged to remove traces of large polar particles (sugar), evaporated, and freeze-dried to yield the saponin-enriched fraction for the subsequent counter-current column chromatographic separation. HPLC chromatogram of saponin-enriched fraction is shown in Fig. 1A. The counter-current column chromatography was carried out using solvent gradients composed of methylene chloride–methanol–isopropanol–water (different ratios, v/v). Finally, 11 ginsenosides were purified from red ginseng following the reported counter-current column chromatographic technique [24], including Rb1, Rb2, Rb3, Rc, Rd, Re, Rf, Rg1, Rg2, Rg3, and Rh2. Compound K was also purified using the same counter-current column chromatography. Methylene chloride–methanol–isopropanol–water (10:6:1:4, v/v/v/v) was used as the two-phase solvent system for counter-current column chromatography. The sample solution was prepared by dissolving the compound K-enriched fraction (200 mg) in 10 mL of the lower and upper phases (1:1, v/v) of the solvent system used. The apparatus was then rotated at 880 revolutions per minute (rpm), and the lower mobile phase was pumped into the head end of the column at a flow rate of 1 mL/min. The eluent was continuously monitored by a connecting tail outlet of the coiled column that was coupled with the evaporative light scattering detection (ELSD) system through a split valve with a 1:16 split ratio. The ELSD system was set to a probe temperature of 60°C and a gain of 3.0, and the nebulizer N2 gas was adjusted to 2.5 bar. Each peak fraction corresponding to compound K was collected and evaporated to yield the pure compound K. The chemical structures of ginsenosides studied in the present investigation are shown in Fig. 1B.
Fig. 1.
The HPLC chromatogram of the saponin-enriched fraction and chemical structures of the ginsenosides used in this study. (A) HPLC chromatogram of the saponin-enriched fraction of Korean Red Ginseng, The enriched saponin fraction was analyzed by HPLC using a Hitachi L-6200 instrument equipped with a Sedex 75 evaporative light scattering detector (ELSD), a SIL-9A autoinjector (Shimadzu, Kyoto, Japan), and a Zorbax SB-Aq C18 column (150 × 4.6 mm, 3.5 μm particle size) from Agilent Technologies (Palo Alto, CA, USA). HPLC conditions were as follows: eluent A, water; eluent B, acetonitrile; gradient, 0–6 (18–23% B), 6–48.5 (23–40% B), and 48.5–55 min (40–100% B); and equilibrated with 18% B for 5 min at a flow of 1 mL/min. The ELSD system was set to a probe temperature of 60°C and a gain of 9.0, and the nebulizer N2 gas was adjusted to 2.5 bar. 1, Rg1; 2, Re; 3, Rf; 4, Rg2; 5, Rb1; 6, Rc; 7, Rb2; 8, Rb3; 9, Rd; 10, Rg3(S); 11, Rg3(R); 12, Rk1; 13, Rg5. (B) The chemical structures of ginsenosides, Glc, β-D-glucopyranosyl; Ara (pyr), α-L-arabinopyranosyl; Ara (fur), α-L-arabinofuranosyl; Rha, α-L-rhamnopyranosyl; Xyl, β-D-xylopyranosyl.
2.3. Animals
Male Imprinting Control Region (ICR) mice (male, 18–22 g, specific pathogen-free) were obtained from KOATECH (Pyeongtaek, Gyeonggi, Korea). Animals were fed with standard laboratory chow and water ad libitum. The animals were maintained in an animal facility of Kangwon National University (KNU) at 20–22°C, under 40–60% relative humidity, and a 12 h/12 h (light/dark) cycle for at least 7 d prior to the experiment. The experimental design using the animals was approved by the local committee for animal experimentation, KNU (KW-150902-1). In addition, the ethical guideline described in the Korean Food and Drug Administration guide for the care and use of laboratory animals was followed throughout the experiments.
2.4. Inhibition of ginsenosides against LPS-induced airway inflammation in mice
For evaluating the pharmacological activity of ginsenosides on lung inflammation, an animal model of LPS-induced acute lung injury (ALI) was used [25], [26]. Mice were divided into the groups including control, LPS-treated, LPS/Korean Red Ginseng extract-treated, LPS/ginsenosides (20 mg/kg)-treated and LPS/dexamethasone (30 mg/kg)-treated (n = 5). After dissolving in 0.3% carboxymethylcellulose, the test compounds and dexamethasone were orally administered to mice. The same amounts of vehicle were also treated to the control and LPS-treated groups. After 1 hr, LPS (2 mg/kg in phosphate-buffered saline) was administered intranasally to mice (10 μL/mouse, 5 times, total 50 μL) to induce bronchitis according to a previous study [23]. At 16 h after LPS treatment, mice were sacrificed, and bronchoalveolar lavage fluid (BALF) was collected via intratracheal cannulation after 700 μL infusion of phosphate-buffered saline three times. The BALF collected was approximately 2,000 μL/mouse. Total cell number in the BALF regarded as an inflammatory marker was counted using a hemocytometer, and the cells were differentially counted with the fluorescence-activated cell sorter (FACS) (BD Biosciences, San Jose, CA, USA).
2.5. Inhibition of ginsenoside Rc and Re against LPS-induced lung inflammation
For the dose-dependent study and elucidation of action mechanism, mice were treated with LPS and different doses of ginsenosides Rc and Re (6–50 mg/kg, n = 8). After sacrifice, BALF was obtained, as described above (n = 5). The lungs of remaining mice were excised, and three samples were used for histological observation. The lungs were fixed in formalin solution, and conventional hematoxylin and eosin (H&E) staining was performed.
2.6. Action mechanism study in vivo
For measuring the levels of MAPK activation, mice were treated with LPS and ginsenoside Re, as described above (20 and 50 mg/kg, n = 3). After 2 hrs, mice were sacrificed, and the lungs were excised [27]. A part of the lungs (20 mg each) in 300 μL of Pro-Prep solution (iNtRON Biotechnology, Seongnam, Korea) containing 1mM phenylmethanesulfonyl fluoride (PMSF) and 1:100 dilution of phosphatase inhibitor cocktail (Sigma, St. Louis, MO) were homogenized, and cellular homogenates were obtained. Electrophoresis was performed in 12% gels. The transferred polyvinylidene difluoride (PVDF) membranes were incubated for 1 h in 5% skim milk for blocking and treated with primary antibodies overnight at 4°C. The membranes were incubated with secondary antibody (Enzo, Farmingdale, NY, USA) at 1:5,000 dilution for 1 h. The bands were detected by enhanced chemiluminescence (BioNote, Hwaseong, Korea). The intensities of each blot were analyzed by ImageJ (NIH, Bethesda, MA, USA).
For measuring the activation levels of transcription factors, nuclear transcription factor–κB (NF-κB), c-Jun, and c-Fos were identified in nuclear form. To obtain nuclear proteins, the remaining parts of the lung tissues (50 mg) were homogenized in 500 μL of buffer A (10mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES), pH 7.9, 10mM potassium chloride (KCl), 0.1mM ethylenediaminetetraacetic acid (EDTA), 1mM dithiothreitol (DTT), 0.5mM phenylmethylsulfonyl fluoride (PMSF), 0.5mM sodium fluoride (NaF), 0.5mM sodium orthovanadate, 0.1mM aprotinin, 0.1mM leupeptin). After adding 25 μL of 10% Nonidet P-40 (NP-40), the solution was vortexed for 10 sec and centrifuged at 15,000 rpm for 5 min at 4°C. The nuclear pellet was dissolved in buffer B (20mM HEPES, pH 7.9, 0.4M sodium chloride, 1mM EDTA, 1mM DTT, 1mM PMSF, 1mM phosphatase inhibitor cocktail, 1mM protease inhibitor cocktail) and centrifuged at 13,000 rpm for 10 min at 4°C. The bicinchoninic protein assay (Thermo Scientific Hyclone, Logan, UT, USA) was used to determine the protein concentration in the nuclear fraction. The supernatant was subjected to electrophoresis in 10% gels. Western blot analysis was performed using the same procedures as described above. The bands were detected by enhanced chemiluminescence (West Femto Luminol/Enhancer Solution, Thermo Scientific, Rockford, IL, USA).
2.7. Inhibition of ginsenosides against the inflammatory responses of lung epithelial cells and alveolar macrophages in vitro
For evaluating the inhibitory action on cell levels, lung-related cell lines were used. A549 cells, a human lung epithelial cell line, were obtained from American Type Culture Collection (ATCC, Rockville, VA). Cells were cultured and stimulated with IL-1β (10 ng/mL) according to previously reported procedures [28]. After 4 hrs, media was collected, and the concentration of IL-6 was determined from the media using the enzyme linked immunosorbent assay (ELISA) kit (eBioscience, San Diego, CA, USA), according to the manufacturer's recommendations. In addition, MH-S cells, a mouse alveolar macrophage cell line, obtained from ATCC were cultured and stimulated with LPS (0.1 μg/mL), according to the previously described procedures [29]. After 24 h of incubation, media was collected and nitric oxide (NO) concentration was determined as the stable conversion product of NO, nitrite (NO2-) using Griess reagent.
The cell viability was checked using an MTT bioassay, as previously described [30]. The test compounds including reference drug agents were dissolved in dimethylsulfoxide and properly diluted with complete media. The final concentration of dimethylsulfoxide in the cell culture was adjusted to 0.1% (v/v), and this concentration of dimethylsulfoxide did not affect the cell viability and the levels of IL-6 and NO production (data not shown).
2.8. Statistical analysis
Experimental values were represented as arithmetic mean ± SD. The one way analysis of variance followed by Dunnett's test was used to determine the statistical significance. For the analysis, IBM SPSS Statistics version 22 (IBM, Armonk, NY, USA) was used.
3. Results
3.1. Effects of ginsenosides on LPS-induced lung inflammation
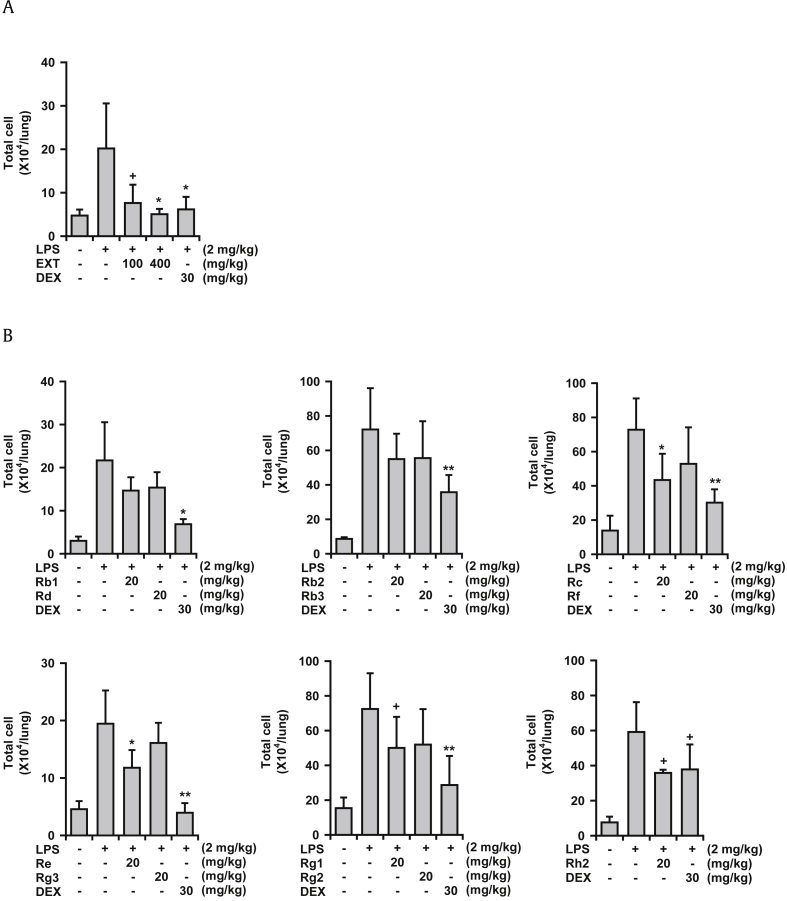
In an animal model of ALI, LPS administration to mice via intranasal route produces bronchitis-like symptoms in the lung tissue, including infiltration of inflammatory cells, upregulation of proinflammatory cytokine/chemokine concentrations, and histological changes in the lung tissue [26]. In this study, LPS treatment elevated the total number of cells in the BALF, as expected. Under this condition, Korean Red Ginseng extract clearly reduced the total number of cells in the BALF by 81.3% and 98.0% at 100 mg/kg and 400 mg/kg, respectively (Fig. 2A). Dexamethasone (30 mg/kg) was used as a reference drug and also strongly inhibited number of cells in the BALF (90.8%). All 11 ginsenosides tested showed some inhibitory activity (11.5–51.6% reduction in total cell numbers) when administered orally at 20 mg/kg (Fig. 2B). Among the ginsenosides, Rc, Re, Rg1, and Rh2 significantly inhibited total cell numbers by 49.0%, 51.6%, 39.2%, and 45.2%, respectively, at 20 mg/kg in the BALF.
Fig. 2.
Effects of Korean Red Ginseng extract and 11 ginsenosides on lipopolysaccharide (LPS)-induced lung inflammation (ALI) in mice. LPS was administered to mice intranasally. The BALF was obtained at 16 h after LPS treatment. Total cell numbers in the BALF were counted using a hemocytometer. (A) Effects of Korean Red Ginseng extract. (B) Effects of various ginsenosides on LPS-induced lung inflammation. +p < 0.1, * p < 0.05, ** p < 0.01; Significantly different from the LPS-treated group (n = 5). ALI, acute lung injury; BALF, bronchoalveolar lavage fluid; EXT, Korean Red Ginseng extract; DEX, dexamethasone; LPS, lipopolysaccharide.
3.2. Dose-dependent inhibition of lung inflammation by ginsenosides, Rc and Re
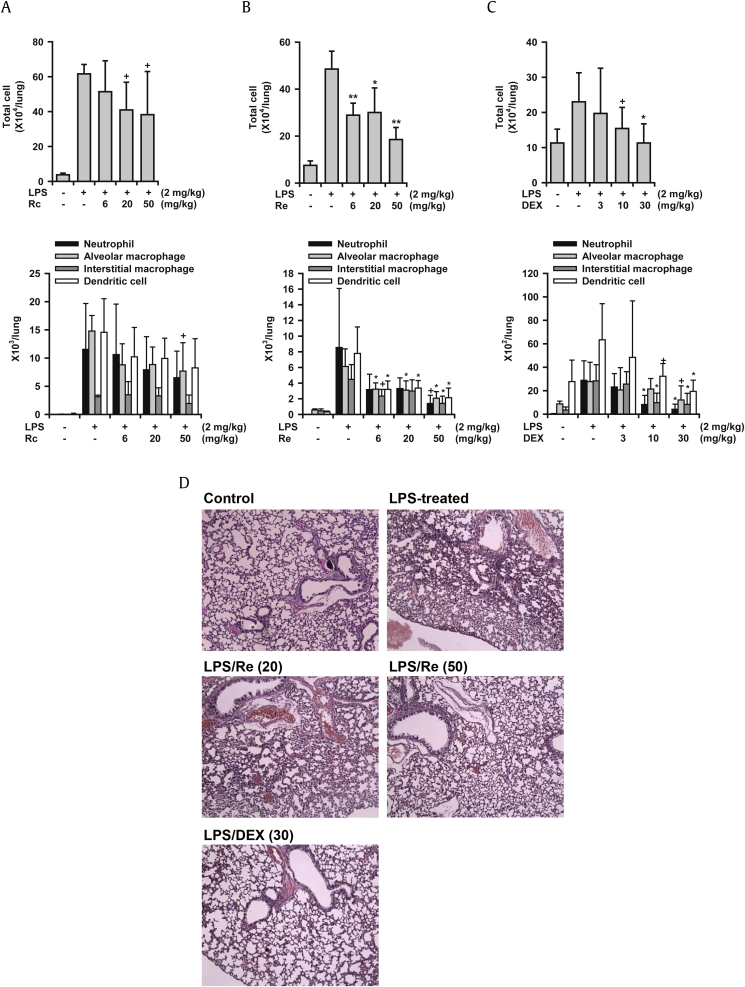
Next, the dose-dependent inhibitory activity of the most active ginsenosides (Rc and Re) was examined. Oral administration of Rc and Re reduced total cell numbers in the BALF at doses of 6–50 mg/kg (Figs. 3A and 3B). Rc reduced total cell numbers by 17.7%, 35.7%, and 39.6% at 6 mg/kg, 20 mg/kg, and 50 mg/kg, respectively. The percent inhibition values by Re were 46.1%, 39.1%, and 72.3% at 6 mg/kg, 20 mg/kg, and 50 mg/kg, respectively. FACS analysis also revealed that Rc and Re strongly reduced neutrophil and macrophage infiltration into the lungs. Histological observations of inflamed lung tissues demonstrated that LPS-treated lungs showed hyperplasia of the alveolar walls and infiltration of inflammatory cells by H&E staining (Fig. 3D). Re (50 mg/kg) strongly inhibited these lung inflammatory responses. Re reduced hyperplasia and recruitment of inflammatory cells. These histological observations confirm the FACS findings that Re reduced infiltration of inflammatory cells into the lung tissue. Dexamethasone (3–30 mg/kg) also strongly reduced these inflammatory markers including the histological changes (Figs. 3C and 3D). Notably, the ginsenosides Rc and Re showed comparable potency with that of dexamethasone.
Fig. 3.
Dose-dependent inhibition of ginsenosides Rc and Re on LPS-induced lung inflammation (ALI) in mice. LPS was administered to mice intranasally, LPS was administered to mice intranasally. Mice were sacrificed 16 h after LPS treatment. (A) Effects of ginsenoside Rc on LPS-induced lung inflammation. (B) Effects of ginsenoside Re on LPS-induced lung inflammation. (C) Effects of dexamethasone on LPS-induced lung inflammation. (D) Histology of the lung tissues, H&E staining, ×100, one of three samples is represented here. + p < 0.1, * p < 0.05, ** p < 0.01; Significantly different from the LPS-treated group (n = 5). LPS, lipopolysaccharide; DEX, dexamethasone.
3.3. Cellular mechanism of action of the ginsenoside Re
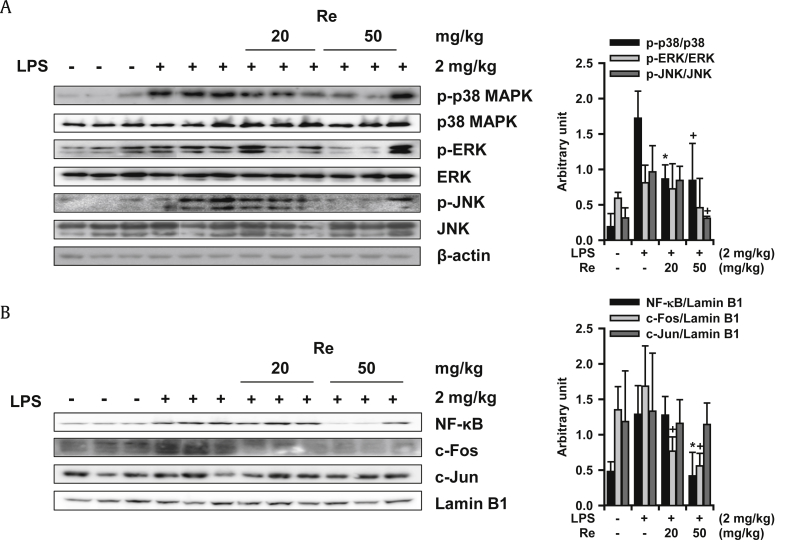
The most active ginsenoside, Re, was administered orally to LPS-treated mice, and the lung tissues were excised to elucidate the mechanism of inhibitory action(s) for the inflammatory lung responses. The MAPK activation level was examined in cellular homogenates by Western blotting analysis. As shown in Fig. 4A, the activation of p38 MAPK and c-Jun-activated protein kinase (JNK) among the three principal MAPKs [p38 MAPK, extracellular signal-regulated kinase (ERK), and JNK] examined was significantly inhibited by Re treatment. The activation of ERK tended to be inhibited, but not significantly. ERK was only slightly activated in LPS administrated lung tissue when compared to that of the basal level.
Fig. 4.
Action mechanisms of ginsenoside Re on inhibition against LPS-induced lung inflammation. (A) Effects of Re on MAPK activation. (B) Effects of Re on the activation of transcription factors. +p < 0.1, * p < 0.05; Significantly different from the LPS-treated group (n = 3). ERK, extracellular signal-regulated kinase; JNK, c-Jun-activated protein kinase; LPS, lipopolysaccharide; MAPK, mitogen activated protein kinase; NF-KB, nuclear transcription factor-κB; p-ERK, phospho-extracellular signal-regulated kinase; p-JNK, phospho-c-Jun-activated protein kinase.
Nuclear levels of transcription factor components, such as NF-κB and activator protein-1 (AP-1), were examined by Western blotting analysis of the nuclear fraction of the lung homogenate. Fig. 4B clearly shows that Re significantly inhibited translocation of NF-κB and c-Fos. However, nuclear c-Jun level in the lung tissue did not change in response to LPS-treatment. These findings suggest that Re inhibits lung inflammation, at least in part, by blocking MAPKs/NF-κB/c-Fos activation leading to the generation of proinflammatory molecules.
3.4. Effects of ginsenosides Rc, Re, and compound K on the inflammatory responses of A549 and MH-S cells
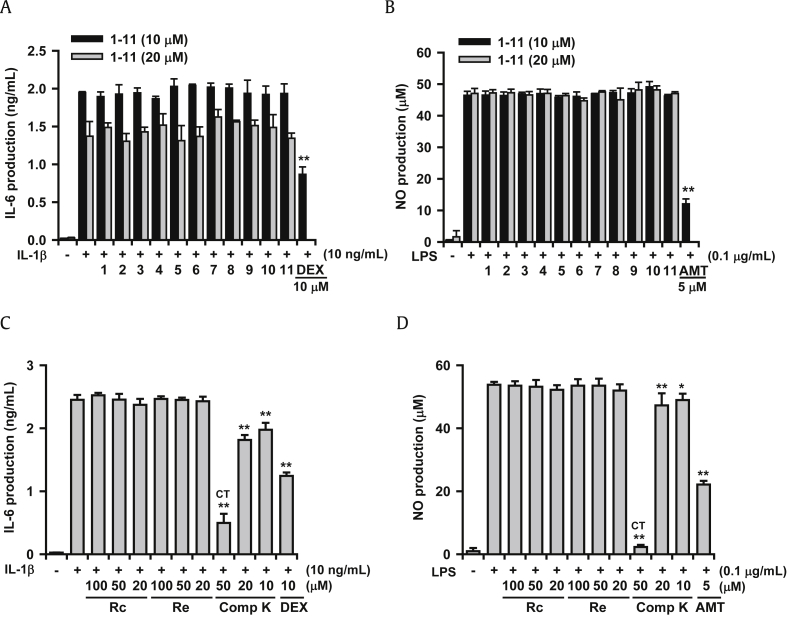
A549 and MH-S cells were used to evaluate inhibitory activity of the inflammatory response in lung cells. The A549 lung epithelial cell line produces IL-6 when challenged with IL-1β (Fig. 5A). However, when the cells were treated with 11 ginsenosides at non-cytotoxic concentrations of 10μM and 20μM, they did not significantly inhibit IL-6 production. The ginsenosides did not inhibit inducible nitric oxide synthase (iNOS)-catalyzed NO production in LPS-treated MH-S cells, which is an alveolar macrophage cell line (Fig. 5B). The reference compounds, dexamethasone and AMT (an iNOS inhibitor), potently inhibited IL-6 and NO production, respectively. In order to establish the role of the ginsenoside metabolite, the inhibitory action of compound K along with Rc and Re was examined in the same cell lines. As shown in Figs. 5C and 5D, compound K, an active metabolite of ginsenosides, showed significant inhibitory action on these two cell lines. Ginsenosides Rc and Re did not exert inhibitory action at 20–100μM as expected.
Fig. 5.
In vitro effects of ginsenosides on the inflammatory responses of IL-1β-treated A549 cells and LPS-induced MH-S cells. Ginsenosides were treated simultaneously with IL-1β or LPS. IL-6 concentration was measured using enzyme linked immunosorbent assay and NO concentration was measured by Griess assay. (A) Effects of ginsenosides on IL-6 production in A549 cells. (B) Effects of ginsenosides on NO production in MH-S cells. (C) Effects of compound K on IL-6 production in A549 cells. (D) Effects of compound K on NO production in MH-S cells. DEX (10μM) and AMT (5μM) were used as reference agents. * p < 0.05, ** p < 0.01; Significantly different from the IL-1β or LPS-treated group (n = 3). 1, Rb1; 2, Rb2; 3, Rb3; 4, Rc; 5, Rd; 6, Re; 7, Rf; 8, Rg1; 9, Rg2; 10, Rg3; 11, Rh2; AMT, 2-Amino-5,6-dihydro-6-methyl-4H-1,3-thiazine hydrochloride; Comp K, compound K; CT, cytotoxic; DEX, dexamethasone; IL, interleukin; LPS, lipopolysaccharide; MH-S, alveolar macrophage; NO, nitric oxide.
4. Discussion
The present investigation clearly demonstrates that Korean Red Ginseng extract (100–400 mg/kg) and various ginsenosides (20 mg/kg) possessed inhibitory actions on lung inflammatory responses in an animal model of ALI after oral administration. Particularly, ginsenosides Rc and Re (6–50 mg/kg) possessed strong and significant inhibitory actions in the same animal model. Re inhibited MAPKs, NF-κB, and c-Fos activation in the lung tissues. These findings support the notion that ginsenosides have the potential to inhibit lung inflammation, at least in part, by interrupting these activation pathways.
On the other hand, all of the ginsenosides isolated from Korean Red Ginseng did not significantly inhibit the inflammatory responses of lung-related cells in vitro under non-cytotoxic concentrations (10μM and 20μM). However, the reason for their inefficacy on these cells is unclear. We speculate that the ginsenosides may need to be transformed to active metabolites by intestinal and liver metabolism before exerting their activity in the body. Compound K is known to be one of the main active metabolites of ginsenosides formed by intestinal metabolism [31]. Contrary to the inactive nature of the ginsenosides isolated, compound K was found to inhibit inflammatory responses of lung-related cells. Thus, it may be suggested that orally administered ginsenosides may be transformed to active metabolites, such as compound K, which exert inhibitory activity in cells and in the lungs. Based on these findings, it should be mentioned that in vitro finding of pharmacological activity of ginsenosides needs a cautious interpretation since certain ginsenosides may show negative results in vitro while they are possibly active intrinsically by oral administration in vivo.
Ginseng and Korean Red Ginseng have been widely used to enhance vitality and immune function, prevent the common cold, and improve skin condition. Ginseng has been prescribed in traditional herbal medicine as an ingredient of various antitussive remedies. Of these, Docksamtang, in which ginseng is the only ingredient, has been used for treating cough [9]. These traditional uses suggest the effectiveness of ginseng against lung inflammatory disorders. The therapeutic potential of ginseng for COPD has also been suggested [12]. Our present results clearly provide supporting evidence from an in vivo experiment.
Ginsenosides affect various signal transduction molecules involved in inflammation and immune regulation, including MAPKs, NF-κB, AP-1, and signal transducer and activation of transcription [7]. In the present study, ginsenoside Re inhibited the activation of MAPKs and transcription factors, such as NF-κB and c-Fos, in vivo. In particular, inhibition of the p38 MAPK pathway by ginsenosides is important as this kinase is critically related to lung inflammation [32]. Additionally, NF-κB is a well-known transcription factor that stimulates cytokine production leading to the inflammatory response. Thus, the inhibitory potential of Re and probably other ginsenosides in these pathways could contribute to their in vivo lung anti-inflammatory action. Notably, activation of c-Fos in the lung tissue by LPS is reported for the first time in this study. We detected c-Fos bands using the enhanced chemiluminescent technique, although the band intensities were very weak.
We tested 11 ginsenosides, and some showed action comparable with that of dexamethasone, which is a potent steroid. Among the ginsenosides tested, the most active were Rc, Re, Rg1, and Rh2. Rc belongs to panaxadiol class, whereas ginsenosides, such as Re and Rg1, are panaxatriols. Due to their diverse chemical structures, it was not possible to establish the structural-activity relationships of the ginsenosides against lung inflammation. Nevertheless, the present study has merits in that the in vivo inhibitory actions of major ginsenosides against lung inflammation were demonstrated for the first time, except Rg5 was previously shown to inhibit lung inflammation [26]. It is also important to note that the inhibition was demonstrated after oral treatment in mice. The doses showing activity were 6–50 mg/kg of Rc and Re, which are approximately equivalent to 360 mg–3 g/person. Administering a particular ginsenoside (360 mg/person) may be feasible when pharmacologically treated. Korean Red Ginseng extract showed inhibitory activity at 100 mg/kg in the present study. It is possible that an equivalent dose of ginseng extracts (6 g/person) could be ingested orally. Thus, Korean Red Ginseng extract and some ginsenosides may be effective against some lung inflammatory disorders, such as bronchitis and COPD. A well-designed clinical study is strongly suggested to demonstrate the clinical effectiveness of ginseng and ginsenosides in the near future.
In conclusion, the inhibitory action of Korean Red Ginseng and 11 major ginsenosides on lung inflammation was studied. Certain ginsenosides and Korean Red Ginseng extract inhibited lung inflammation after oral treatment. In particular, the ginsenoside Re showed strong and significant inhibitory action against lung inflammation, at least in part, by interrupting the MAPKs/NF-κB/c-Fos signaling pathways.
Conflicts of interest
All authors have no conflicts of interest to declare.
Acknowledgments
This study was financially supported by a grant from Korea Ginseng Society (2014) and BK21-plus from the Ministry of Education (Korea). Bioassay facility of New Drug Development Inst. (KNU) was used and greatly acknowledged.
References
- 1.Al-Kassimi F.A., Alhamad E.H. A challenge to the seven widely believed concepts of COPD. Int J Chron Obstruct Pulmon Dis. 2013;8:21–30. doi: 10.2147/copd.s38714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barnes P.J. Cellular and molecular mechanisms of chronic obstructive pulmonary disease. Clin Chest Med. 2014;35:71–86. doi: 10.1016/j.ccm.2013.10.004. [DOI] [PubMed] [Google Scholar]
- 3.Mulhall A.M., Droege C.A., Ernst N.E., Panos R.J., Zafar M.A. Phosphodiesterase 4 inhibitors for the treatment of chronic obstructive pulmonary disease: a review of current and developing drugs. Expert Opin Investig Drugs. 2015;30:1–15. doi: 10.1517/13543784.2015.1094054. [DOI] [PubMed] [Google Scholar]
- 4.Yonetomi Y., Sekioka T., Kadode M., Kitamine T., Kamiya A., Nakao T., Nomura H., Murata M., Nakao S., Nambu F. Effects of ONO-6950, a novel dual cysteinyl leukotriene 1 and 2 receptors antagonist, in a Guinea pig model of asthma. Eur J Pharmacol. 2015;765:242–248. doi: 10.1016/j.ejphar.2015.08.041. [DOI] [PubMed] [Google Scholar]
- 5.Lee J.H., Lim H., Shehzad O., Kim Y.S., Kim H.P. Ginsenosides from Korean red ginseng inhibit matrix metalloproteinase-13 expression in articular chondrocytes and prevent cartilage degradation. Eur J Pharmacol. 2014;724:145–151. doi: 10.1016/j.ejphar.2013.12.035. [DOI] [PubMed] [Google Scholar]
- 6.Mochizuki M., Yo YungChoon, Matsuzawa K., Saiki I., Tonooka S., Samukawa K., Azuma I. Inhibitory effect of tumor metastasis in mice by saponins, ginsenosides Rb2, 20(R)- and 20(S)-ginsenoside Rg3, of red ginseng. Biol Pharm Bull. 1995;18:1197–1202. doi: 10.1248/bpb.18.1197. [DOI] [PubMed] [Google Scholar]
- 7.Nag S.A., Qin J.J., Wang M.H., Wang H., Zhang R. Ginsenosides as anticancer agents: in vitro and in vivo activities, structure-activity relationship, and molecular mechanisms of action. Front Pharmacol. 2012;3:25. doi: 10.3389/fphar.2012.00025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wu J.Y., Gardner B.H., Murphy C.I., Seals J.R., Kensil C.R., Recchia J., Beltz G.A., Newman G.W., Newman M.J. Saponin adjuvant enhancement of antigen-specific immune responses to an experimental HIV-1 vaccine. J Immunol. 1992;148:1519–1525. [PubMed] [Google Scholar]
- 9.Huh J. Ryo-gang Publishing; Seoul: 1994. Dongeui Bogam (1613) Translated version (translated by Dongeui Research Institute) [Google Scholar]
- 10.Du J., Cheng B., Zhu X., Ling C. Ginsenoside Rg1, a novel glucocorticoid receptor agonist of plant origin, maintains glucocorticoid efficacy with reduced side effects. J Immunol. 2011;187:942–950. doi: 10.4049/jimmunol.1002579. [DOI] [PubMed] [Google Scholar]
- 11.Lee D.C., Lau A.S. Effects of Panax ginseng on tumor necrosis factor-α-mediated inflammation: a mini-review. Molecules. 2011;16:2802–2816. doi: 10.3390/molecules16042802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shergis J.L., Di Y.M., Zhang A.L., Vlahos R., Helliwell R., Ye J.M., Xue C.C. Therapeutic potential of Panax ginseng and ginsenosides in the treatment of chronic obstructive pulmonary disease. Complement Ther Med. 2014;22:944–953. doi: 10.1016/j.ctim.2014.08.006. [DOI] [PubMed] [Google Scholar]
- 13.Joh E.H., Lee I.A., Jung I.H., Kim D.H. Ginsenoside Rb1 and its metabolite compound K inhibit IRAK-1 activation-the key step of inflammation. Biochem Pharmacol. 2011;82:278–286. doi: 10.1016/j.bcp.2011.05.003. [DOI] [PubMed] [Google Scholar]
- 14.Gaffey K., Reynolds S., Plumb J., Kaur M., Singh D. Increased phosphorylated p38 mitogen-activated protein kinase in COPD lungs. Eur Respir J. 2013;42:28–41. doi: 10.1183/09031936.00170711. [DOI] [PubMed] [Google Scholar]
- 15.Renda T., Baraldo S., Pelaia G., Bazzan E., Turato G., Papi A., Maestrelli P., Maselli R., Vatrella A., Fabbri L.M. Increased activation of p38 MAPK in COPD. Eur Respir J. 2008;31:62–69. doi: 10.1183/09031936.00036707. [DOI] [PubMed] [Google Scholar]
- 16.Singh D., Smyth L., Borrill Z., Sweeney L., Tal-Singer R. A randomized, placebo-controlled study of the effects of the p38 MAPK inhibitor SB-681323 on blood biomarkers of inflammation in COPD patients. J Clin Pharmacol. 2010;50:94–100. doi: 10.1177/0091270009347873. [DOI] [PubMed] [Google Scholar]
- 17.Babayigit A., Olmez D., Karaman O., Bagriyanik H.A., Yilmaz O., Kivcak B., Erbil G., Uzuner N. Ginseng ameliorates chronic histopathologic changes in a murine model of asthma. Allergy Asthma Proc. 2008;29:493–498. doi: 10.2500/aap.2008.29.3137. [DOI] [PubMed] [Google Scholar]
- 18.Chen T., Xiao L., Zhu L., Ma S., Yan T., Ji H. Anti-asthmatic effects of ginsenoside Rb1 in a mouse model of allergic asthma through relegating Th1/Th2. Inflammation. 2015;38:1814–1822. doi: 10.1007/s10753-015-0159-4. [DOI] [PubMed] [Google Scholar]
- 19.Li L.C., Piao H.M., Zheng M.Y., Lin Z.H., Choi Y.H., Yan G.H. Ginsenoside Rh2 attenuates allergic airway inflammation by modulating nuclear factor-κB activation in a murine model of asthma. Mol Med Rep. 2015;12:6946–6954. doi: 10.3892/mmr.2015.4272. [DOI] [PubMed] [Google Scholar]
- 20.Kim T.W., Joh E.H., Kim B., Kim D.H. Ginsenoside Rg5 ameliorates lung inflammation in mice by inhibiting the binding of LPS to toll-like receptor-4 on macrophages. Int Immunopharmacol. 2012;12:110–116. doi: 10.1016/j.intimp.2011.10.023. [DOI] [PubMed] [Google Scholar]
- 21.Gross D., Shenkman Z., Bleiberg B., Dayan M., Gittelson M., Efrat R. Ginseng improves pulmonary functions and exercise capacity in patients with COPD. Monaldi Arch Chest Dis. 2002;57:242–246. [PubMed] [Google Scholar]
- 22.Scaglione F., Weiser K., Alessandria M. Effects of the standardized ginseng extract G115 (TM) in patients with chronic bronchitis: a nonblinded, randomized, comparative pilot study. Clin Drug Investig. 2001;21:41–45. [Google Scholar]
- 23.Lee C.S., Lee J.H., Oh M., Choi K.M., Jeong M.R., Park J.D., Kwon D.Y., Ha K.C., Park E.O., Lee N. Preventive effect of Korean red ginseng for acute respiratory illness: A randomized and double-blind clinical trial. J Korean Med Sci. 2012;27:1472–1478. doi: 10.3346/jkms.2012.27.12.1472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shehzad O., Kim H.P., Kim Y.S. State-of-the-art separation of ginsenosides from Korean white and red ginseng by countercurrent chromatography. Anal Bioanal Chem. 2013;405:4523–4530. doi: 10.1007/s00216-012-6609-z. [DOI] [PubMed] [Google Scholar]
- 25.Chapman R.W., Minnicozzi M., Celly C.S., Phillips J.E., Kung T.T., Hipkim R.W., Fan X., Rindgen D., Deno G., Bond R. A novel orally active CXCR1/2 receptor antagonist, Sch527123, inhibits neutrophil recruitment, mucus production, and goblet cell hyperplasia in animal models of pulmonary inflammation. J Pharmacol Exp Ther. 2007;322:486–493. doi: 10.1124/jpet.106.119040. [DOI] [PubMed] [Google Scholar]
- 26.Lim H.J., Jin H.G., Woo E.R., Lee S.K., Kim H.P. The root barks of Morus alba and the flavonoid constituents inhibit airway inflammation. J Ethnopharmacol. 2013;149:169–175. doi: 10.1016/j.jep.2013.06.017. [DOI] [PubMed] [Google Scholar]
- 27.Blackwell T., Yull F.E., Chen C.L., Venkatakrishnan A., Blackwell T.R., Hicks D.J., Lancaster L.H., Christman J.W., Kerr L.D. Multiorgan nuclear factor kappa B activation in a transgenic mouse model of systemic inflammation. Am J Respir Crit Care Med. 2000;162:1095–1101. doi: 10.1164/ajrccm.162.3.9906129. [DOI] [PubMed] [Google Scholar]
- 28.Lee J.H., Lim H.J., Lee C.W., Son K.H., Son J.K., Lee S.K., Kim H.P. Methyl protodioscin from the roots of Asparagus cochinchinensis attenuates airway inflammation by inhibiting cytokine production. Evid Based Complement Alternat Med. 2015;2015:640846. doi: 10.1155/2015/640846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ko H.J., Jin J.H., Kwon O.S., Kim J.T., Son K.H., Kim H.P. Inhibition of experimental lung inflammation and bronchitis by phytoformula containing Broussonetia papyrifera and Lonicera japonica. Biomol Ther (Seoul) 2011;19:324–330. doi: 10.4062/biomolther.2012.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxic assays. J Immunol Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 31.Park E.K., Shin Y.W., Lee H.U., Kim S.S., Lee Y.C., Lee B.Y., Kim D.H. Inhibitory effect of ginsenoside Rb1 and compound K on NO and prostaglandin E2 biosyntheses of RAW264.7 cells induced by lipopolysaccharide. Biol Pharm Bull. 2005;28:652–656. doi: 10.1248/bpb.28.652. [DOI] [PubMed] [Google Scholar]
- 32.Millan D.S., Bunnage M.E., Burrows J.L., Butcher K.J., Dodd P.G., Evans T.J., Fairman D.A., Hughes S.J., Kilty I.C., Lemaitre A. Design and synthesis of inhaled p38 inhibitors for the treatment of chronic obstructive pulmonary disease. J Med Chem. 2011;54:7797–7814. doi: 10.1021/jm200677b. [DOI] [PubMed] [Google Scholar]