Abstract
Study Objectives
The purpose of this study is to examine the association of abnormal periodic limb movements during sleep (PLMS) with neurocognitive and behavioral outcomes in adolescents with attention-deficit/hyperactivity disorder (ADHD) from the general population.
Methods
Four hundred twenty-one adolescents (17.0 ± 2.3 years, 53.9% male) from the Penn State Child Cohort, a random general population sample, underwent 9 hr polysomnography, clinical history, physical examination, neurocognitive evaluation, and completed the Child or Adult Behavioral Checklist (C/ABCL). The presence of ADHD was ascertained by parent- or self-report of receiving a diagnosis of ADHD. PLMS were defined as a PLM index (PLMI) of ≥5 events per hour of sleep.
Results
Adolescents with ADHD (n = 98) had a significantly higher PLMI (5.4 ± 7.3) and prevalence of PLMS (35%) when compared with controls (3.4 ± 5.6, p = 0.006 and 21%, p = 0.004). Significant interactions between ADHD and PLMS showed that adolescents with both disorders (n = 35) were characterized by deficits in control interference, as measured by Stroop test, and elevated internalizing behaviors, as measured by C/ABCL. ADHD severity and externalizing behaviors were elevated in a dose-response manner across ADHD-alone (n = 63) and ADHD + PLMS groups. The association of ADHD with other neurocognitive functions did not vary as a function of PLMS.
Conclusions
PLMS are significantly more frequent in adolescents with ADHD. Importantly, adolescents with both disorders not only have worse neurobehavioral functioning than adolescents with ADHD-alone but specifically presented with executive deficits and anxiety symptoms. These data suggest that PLMS may be a marker of more severe underlying neurobiological deficits in adolescents with ADHD and comorbid internalizing problems.
Keywords: adolescents, attention-deficit/hyperactivity disorder, periodic limb movements, period limb movement disorder, neurobehavioral functioning
Statement of Significance
Attention-deficit/hyperactivity disorder (ADHD) has been associated with high rates of abnormal periodic limb movements during sleep (PLMS). Whether PLMS contribute to poor clinically significant outcomes in ADHD is not well-established, particularly in adolescents. The novel findings of this study indicated that adolescents with both ADHD and PLMS characteristically presented with greater executive functioning deficits and internalizing behavioral problems. These data suggest that PLMS may be a marker of more severe underlying neurobiological deficits that may put adolescents with ADHD at greater risk for anxiety and mood disorders. Clinicians should consider the potential for underlying PLMS in adolescents with ADHD, while we need to develop novel, targeted treatments for sleep and behavior in adolescents with ADHD and abnormal PLMS.
Introduction
Attention-deficit/hyperactivity disorder (ADHD) in children has been associated with sleep disturbances, including high rates of periodic limb movements during sleep (PLMS) [1]. PLMS can be diagnosed as a movement disorder characterized by brief, repetitive jerking of the extremities, primarily the limbs, associated with sleep disruption and daytime impairment [2]. The threshold for abnormal PLMS in children is a PLM index (PLMI) of ≥5 events per hour of sleep [2, 3]. The prevalence of abnormal PLMS in children and adolescents with ADHD ranges from 26% to 64% in clinical cohorts [4–7]. Conversely, in those diagnosed with abnormal PLMS, an estimated 91% met criteria for ADHD in a clinical sample [8] and 44% in a combined clinical and community-based sample [9]. However, little is known about the prevalence of abnormal PLMS in adolescents with ADHD, particularly those from the general population.
ADHD is a disorder marked by externalizing behaviors, including hyperactivity and impulsivity, as well as neurocognitive deficits in sustained attention, working memory and control interference [10–12]. Emerging evidence has suggested that sleep disruption, including abnormal PLMS, may also contribute to impairments in neurobehavioral functioning [13, 14]. One study indicated that children with significant parent-reported inattention and hyperactivity had a higher prevalence of PLMS, even after controlling for potential covariates [7]. Symptoms of ADHD, including distractibility, hyperactivity, and impulsive behaviors have been significantly correlated with PLMS, supporting an association between these two conditions [15].
In addition to associations with externalizing behaviors, there is also evidence to suggest a potential link between PLMS and internalizing symptoms. In adults, abnormal PLMS has been associated with increased depression and anxiety [16–18], whereas one study found that youth with PLMS had higher scores on measures of internalizing behaviors in comparison to youth with sleep-disordered breathing (SDB) [15]. However, no published study has specifically examined the emotional functioning of adolescents with ADHD and PLMS.
Despite high rates of comorbidity and similar neurobehavioral impairments, there is lack of data examining the association of ADHD and PLMS with neurocognitive and behavioral outcomes, particularly in adolescents from the general population. The few studies that have been published relied primarily on behavior questionnaires without the use of standardized neurocognitive measures of underlying cognitive processes. Thus, the purpose of this study was to address a key gap in the literature by investigating the associations of ADHD and PLMS with neurobehavioral functioning in a nonclinical sample of adolescents. Specifically, we examined the moderating role of PLMS in the association of ADHD with well-established neurocognitive and behavioral clinical outcomes.
Methods
Population
The overall sample consisted of 421 adolescents from the Penn State Child Cohort (PSCC), of whom 46.1% were female and 21.9% of African-American, Hispanic or Asian race/ethnicity. The PSCC was established to examine the prevalence of SDB and other sleep disorders in a general population sample of children and has been described in detail in previous studies [19, 20]. In brief, 18 elementary schools with approximately 1,500 students within three school districts of Dauphin County (Pennsylvania) were selected over the course of 5 years during phase I. A validated survey published by Ali and colleagues [21] assessing risk of SDB as well as height, weight, age, gender, race, and ethnicity was sent home with every child in the selected schools to be completed by a parent. We sent home 7,312 questionnaires and 5,740 were returned, for a 78.5% response rate. In phase II, 1000 children were randomly selected based on stratification for grade, gender, and risk for SDB, resulting in a sample of 700 children (aged 5–12 years old), for a response rate of 70.0%. More recently, all 700 children were invited to participate in a follow-up study approximately 8 years later. A total of 421 adolescents (aged 12–23 years old, average age = 17.0 ± 2.3 years) participated in the follow-up study (60.1% response rate) and were the focus of the present study. All these adolescents underwent a full clinical history, neurocognitive evaluation including behavioral measures, and a sleep study. The study protocol was approved by Penn State University College of Medicine Institutional Review Board. Written informed consent was obtained from all participants and their parents or legal guardians if younger than 18 years old.
Key measurements
Polysomnography and periodic limb movements
All participants’ sleep was continuously monitored for 9 hr with a seven-channel electroencephalography (EEG), electrooculography (EOG), and electromyography (EMG). The sleep records were scored according to standardized criteria by a registered polysomnography (PSG) technologist who was blinded to participant characteristics. Respiration was monitored with nasal pressure, thermocouple, and thoracic and abdominal strain gauges, whereas hemoglobin oxygen saturation (SpO2) was obtained from the finger. The average apnea–hypopnea index (AHI) was 2.7 ± 0.3 events per hour and SDB was defined as 2 ≤ AHI < 5 and AHI ≥ 5.
PLMs were recorded via tibial EMG and scored based on standardized criteria [2]. A PLM was defined as four leg movements within 90 s of at least 0.5 s in duration and 5 s apart [2]. The average PLMI was 3.9 ± 6.1 events per hour of sleep, with a distribution of 0.0, 1.6, and 4.9 events per hour for the 25%, 50%, and 75% percentile, respectively. This distribution was identical in young (i.e. 12–17 years) and older (i.e. 18–23 years) adolescents. Thus, given the mean age of the participants and the distribution of PLMI, we defined abnormal PLMS based on pediatric criteria as a PLMI ≥ 5 [2].
Parents of a subset of participants also completed a Pediatric Sleep Questionnaire, in which specific items assessed for symptoms of leg movements related to restless legs syndrome (RLS) [22]. However, it should be noted that parents were not asked if their children experienced the urge to move their legs, which is a specific diagnostic feature of RLS. Parents were asked “does your child describe restlessness of the legs when in bed?”, “does your child have brief kicks of one leg or both legs during sleep?” and “does your child have repeated kicks or jerks of the legs at regular intervals?”
Physical exam, clinical history, and ADHD
Tanner staging was measured by self-report using a standardized scale [23]. The age- and sex-adjusted body mass index (BMI) percentile for each participant was calculated based on growth charts for height and weight [24]. Participants or their parent completed questionnaires on sleep to assess sleep-wake patterns and other sleep symptoms, including the self-reported Morningness-Eveningness Questionnaire (MEQ) to ascertain circadian preference [25] and the parent-reported Pediatric Behavior Scale (PBS) to ascertain severity of global sleep and arousal problems [26].
During the clinical history and physical exam prior to the sleep study, the participant or their parent reported on the presence of a lifetime history of a diagnosis of a psychiatric or behavioral disorder with the question “Have you ever been treated for a psychiatric/behavioral disorder?”, if self-reported, and “Has your child ever been treated for a psychiatric/behavioral disorder?”, if parent-reported. These questions were followed by the option to specify whether that disorder was ADHD and whether it was being currently treated or it had been treated in the past. Current use of stimulants and other psychoactive medications such as antidepressants was recorded, whereas data on other treatments (e.g. behavioral, school-based) were not available.
Neurocognitive functioning
All participants underwent a 2.5 hr neurocognitive evaluation in the afternoon prior to the sleep study, at approximately the same time, and administered individually by a trained psychometrist. Neurocognitive outcomes included vigilance, distractibility, processing speed, working memory, control interference, achievement, and intelligence. The Gordon Diagnostic System (GDS), which is a continuous performance test and well-established measure of attention, was administered to measure vigilance and distractibility (subtests) [27]. Processing speed and working memory were assessed using two subtests from either the Wechsler Intelligence Scale for Children, Fourth Edition (WISC-IV) [28] or Wechsler Adult Intelligence Scale, Third Edition (WAIS-III) [29] depending on the participant’s age (i.e. younger or older than 16 years old). The Digit Span (Ds) backward subtest is a measure of working memory and the Coding (Cd) and Symbol Search (Ss) subtests are measures of processing speed. The Stroop Color and Word Test, Child [30] and Adult Version [31], was used as a measure of executive functioning that involves cognitive flexibility and control interference (i.e. response inhibition). The Stroop test comprises three trials of word (W), color (C), and color-word (CW); the difference score (ID) between the C and CW scores is calculated (e.g. ID = C – CW) and lower scores indicate less interference from incongruent W when naming the C in the CW condition and, thus, is a purer measure of control interference. The Wechsler Abbreviated Scales of Intelligence (WASI) is a measure of verbal, performance, and full-scale intelligence derived from four subtests: block design, matrix reasoning, vocabulary, and similarities [32]. Academic achievement was assessed using the Math Computation and Word Reading subtests from the Wide Range Achievement Test, Third Edition (WRAT-3) [33].
Behavioral functioning
Behavior was assessed via self-reported and parent-reported questionnaires. The Child Behavior Checklist (CBCL), which is a widely used tool for the assessment of behavioral problems, was completed by the parents of participants aged 12–17 years old; if participants were 18 years or older, they completed the self-reported Adult Behavior Checklist (ABCL). For each scale and subscale, T-scores with a mean of 50 and a standard deviation of 10 were obtained following standard scoring procedures [34, 35] and a clinically elevated score was defined as a T-score of ≥60. Global scale scores were calculated for internalizing and externalizing problems. Subscale scores were calculated for anxious-depressed, withdrawn-depressed, somatic complaints, thought problems, attention problems, rule-breaking behavior, and aggressive behavior as well as for DSM-oriented scales including anxious, somatic, and ADHD problems.
Statistical analysis
Categorical and continuous variables were analyzed with chi-square test and analysis of variance (ANOVA), respectively. As shown in Table 1, most adolescents with ADHD reported a current history of treatment (n = 71), whereas only about a third reported a past history of treatment (n = 27). Key clinical characteristics of adolescents with a past or current history of treatment for ADHD did not differ between each other; for example, there were no significant differences between those with a past or current treatment for ADHD on PLMI (6.4 ± 8.4 vs. 5.0 ± 6.7, p = 0.295), PLMS (33.3% vs. 36.6%, p = 0.762), processing speed (8.8 ± 1.9 vs. 8.8 ± 2.2, p = 0.984), working memory (5.6 ± 2.3 vs. 6.3 ± 2.3, p = 0.227), control interference (42.7 ± 6.7 vs. 41.2 ± 10.1, p = 0.448), or C/ABCL’s global behavioral problems (54.2 ± 7.8 vs. 56.1 ± 10.3, p = 0.394). Thus, these adolescents were combined together in the analyses. Full-factorial, multivariable-adjusted general linear models tested whether PLMS modified the association between ADHD and neurobehavioral outcomes after controlling for multiple potential confounders (i.e. sex, race, age, BMI percentile, MEQ, insomnia, EDS, sleep onset latency, number of awakenings, and AHI). Based on the combination of PLMS and ADHD, four groups consisting of ADHD + PLMS, ADHD-alone, PLMS-alone, and controls (i.e. the common reference group without ADHD or PLMS) were created. The critical statistical confidence level for all analyses was p < 0.05, two-tailed. All analyses were performed using SPSS Statistics version 23 (IBM, Armonk, NY, USA).
Table 1.
Demographic and clinical characteristics of the sample
| ADHD | P | PLMS | P | |||
|---|---|---|---|---|---|---|
| No (n = 323) | Yes (n = 98) | No (n = 318) | Yes (n = 103) | |||
| Male (%) | 49.2 | 69.4 | <0.001 | 51.3 | 62.1 | 0.054 |
| Ethnic minority (%) | 22.0 | 21.4 | 0.908 | 22.0 | 21.4 | 0.889 |
| White | 78.0 | 78.6 | 0.937 | 78.0 | 78.6 | 0.584 |
| Black | 12.7 | 12.2 | 13.2 | 10.7 | ||
| Hispanic | 6.2 | 7.1 | 5.7 | 8.7 | ||
| Other | 3.1 | 2.0 | 3.1 | 1.9 | ||
| Age (years) | 17.0 ± 2.3 | 16.8 ± 2.1 | 0.606 | 17.0 ± 2.3 | 16.8 ± 2.1 | 0.533 |
| Tanner stage (score) | 4.2 ± 0.8 | 4.1 ± 0.7 | 0.513 | 4.2 ± 0.8 | 4.2 ± 0.8 | 0.829 |
| Pre-pubertal (%) | 1.0 | 1.1 | 0.355 | 1.0 | 1.0 | 0.701 |
| Early pubertal (%) | 1.6 | 0.0 | 1.6 | 0.0 | ||
| Mid puberty (%) | 13.5 | 13.8 | 13.1 | 15.0 | ||
| Late puberty (%) | 44.6 | 54.3 | 46.1 | 49.0 | ||
| Adulthood (%) | 39.4 | 30.9 | 38.2 | 35.0 | ||
| BMI (percentile) | 65.6 ± 28.6 | 64.4 ± 27.9 | 0.709 | 67.3 ± 27.9 | 59.3 ± 29.1 | 0.014 |
| Normal weight (%) | 64.7 | 70.4 | 0.580 | 62.6 | 76.7 | 0.028 |
| Overweight (%) | 19.5 | 16.3 | 21.1 | 11.7 | ||
| Obesity (%) | 15.8 | 13.3 | 16.4 | 11.7 | ||
| ADHD (%) | 0.0 | 100.0 | N/A | 19.8 | 34.0 | 0.003 |
| None | 100.0 | 0.0 | 80.2 | 66.0 | 0.012 | |
| Past treatment | 0.0 | 27.6 | 5.7 | 8.7 | ||
| Current treatment | 0.0 | 72.4 | 14.2 | 25.2 | ||
| Stimulant medication (%) | 0.6 | 39.8 | <0.001 | 7.5 | 16.5 | 0.008 |
| Psychoactive medication (%) | 5.6 | 18.4 | <0.001 | 6.0 | 16.5 | 0.001 |
| Sleep medication (%) | 1.2 | 4.1 | 0.071 | 1.9 | 1.9 | 0.972 |
| Allergy/asthma medication (%) | 23.2 | 16.3 | 0.146 | 21.1 | 23.3 | 0.632 |
| PLMS (%) | 21.1 | 35.7 | 0.003 | 0.0 | 100.0 | |
| PLM (#) | 25.1 ± 40.8 | 38.7 ± 52.8 | 0.020 | 9.3 ± 10.8 | 86.8 ± 55.7 | <0.001 |
| PLMa (#) | 3.4 ± 5.7 | 4.0 ± 6.1 | 0.392 | 1.9 ± 3.1 | 8.7 ± 8.6 | <0.001 |
| PLMI (events per hour) | 3.4 ± 5.6 | 5.4 ± 7.2 | 0.006 | 1.3 ± 1.5 | 12.0 ± 7.5 | <0.001 |
BMI = body mass index percentile for age and sex; PLMS = abnormal periodic limb movements; PLMI = periodic limb movement index; PLMa = number of arousals associated with periodic limb movements. Bold values are statistically significant at P ≤ 0.05.
Results
Demographic and clinical characteristics
As shown in Table 1, adolescents with ADHD were significantly more likely to be male and on psychotropic medication. Importantly, adolescents with ADHD had a significantly higher PLMI (5.4 ± 7.3) and prevalence of PLMS (35%) when compared with adolescents without ADHD (3.4 ± 5.6 and 21%, respectively). Adolescents with PLMS were significantly more likely to be male, leaner, and on psychotropic medication and have received a diagnosis of ADHD (Table 1).
Polysomnographic and subjective sleep parameters
We present in Table 2 the PSG parameters across study groups, whereas self- or parent-reported sleep parameters are presented in Supplementary Table 1. The PLMS-alone group had significantly higher wake after sleep onset, number of awakenings and stage 1, shorter total sleep time, and lower sleep efficiency and were more likely to report being E-types and greater arousal problems, when compared with controls. In contrast, the ADHD-alone group did not significantly differ from controls in PSG characteristics, aside from being significantly more likely to report insomnia symptoms, a later bedtime on weekdays and greater specific sleep and arousal problems. The ADHD + PLMS group had significantly higher sleep onset latency when compared with all other study groups. Also, the ADHD + PLMS group had significantly more stage 1 than the control and ADHD-alone groups. Importantly, the ADHD + PLMS group did not significantly differ in terms of PLMI but had significantly less stage 2 and awakenings when compared with the PLMS-alone group. The ADHD + PLMS group also reported being more likely to be either I-types or E-types and more EDS when compared with controls or PLMS-alone; also, they reported significantly greater specific sleep and arousal problems when compared with all other study groups. Finally, only the ADHD + PLMS group had significantly higher parent reports of RLS symptoms.
Table 2.
Polysomnographic parameters across the study subgroups
| 1. None (n = 255) | 2. PLMS (n = 68) | 3. ADHD (n = 63) | 4. ADHD + PLMS (n = 35) | 1 vs. 2 | 1 vs. 3 | 1 vs. 4 | 2 vs. 4 | 3 vs.4 | |
|---|---|---|---|---|---|---|---|---|---|
| Sleep continuity | |||||||||
| Sleep onset latency (minutes) | 24.8 ± 1.5 | 26.1 ± 2.9 | 24.8 ± 3.1 | 38.9 ± 4.1 | 0.681 | 0.997 | 0.001 | 0.012 | 0.006 |
| Awakenings (#) | 35.8 ± 0.7 | 39.3 ± 1.4 | 37.1 ± 1.5 | 34.0 ± 2.0 | 0.031 | 0.425 | 0.403 | 0.032 | 0.209 |
| Wake after sleep onset (minutes) | 66.0 ± 2.7 | 79.5 ± 5.2 | 71.9 ± 5.4 | 70.1 ± 7.3 | 0.022 | 0.336 | 0.608 | 0.289 | 0.839 |
| Total sleep time (minutes) | 451.6 ± 3.5 | 436.3 ± 6.7 | 444.6 ± 6.9 | 435.8 ± 9.4 | 0.043 | 0.370 | 0.117 | 0.968 | 0.451 |
| Sleep efficiency (%) | 83.5 ± 0.6 | 80.8 ± 1.2 | 82.3 ± 1.3 | 80.5 ± 1.7 | 0.048 | 0.406 | 0.101 | 0.893 | 0.389 |
| Sleep architecture | |||||||||
| Stage 1 (%) | 0.8 ± 0.1 | 1.3 ± 0.2 | 1.0 ± 0.2 | 1.8 ± 0.2 | 0.010 | 0.401 | <0.001 | 0.090 | 0.006 |
| Stage 2 (%) | 53.4 ± 0.6 | 55.2 ± 1.1 | 53.1 ± 1.1 | 51.1 ± 1.5 | 0.145 | 0.776 | 0.157 | 0.028 | 0.302 |
| Stage 3 (%) | 27.2 ± 0.5 | 25.1 ± 1.0 | 27.6 ± 1.0 | 28.2 ± 1.4 | 0.076 | 0.700 | 0.485 | 0.076 | 0.731 |
| Stage R (%) | 18.6 ± 0.3 | 18.3 ± 0.6 | 18.3 ± 0.6 | 18.8 ± 0.9 | 0.712 | 0.696 | 0.822 | 0.661 | 0.648 |
| Sleep-disordered breathing | |||||||||
| AHI (events per hour) | 2.5 ± 0.3 | 2.7 ± 0.7 | 3.0 ± 0.7 | 3.0 ± 0.9 | 0.808 | 0.530 | 0.663 | 0.825 | 0.964 |
| AHI < 2 (%) | 64.7 | 63.2 | 49.2 | 65.7 | — | — | — | — | — |
| 2 ≤ AHI < 5 (%) | 26.3 | 23.5 | 34.9 | 28.6 | 0.832 | 0.195 | 0.967 | 0.857 | 0.404 |
| AHI ≥ 5 (%) | 9.0 | 13.2 | 15.9 | 5.7 | 0.254 | 0.117 | 0.608 | 0.274 | 0.185 |
| SpO2 | 91.5 ± 0.3 | 91.6 ± 0.6 | 91.7 ± 0.7 | 90.8 ± 0.9 | 0.813 | 0.808 | 0.451 | 0.415 | 0.416 |
| Periodic limb movements | |||||||||
| PLM (#) | 9.7 ± 1.9 | 84.4 ± 3.6 | 9.6 ± 3.8 | 92.4 ± 5.0 | <0.001 | 0.983 | <0.001 | 0.193 | <0.001 |
| PLMa (#) | 2.0 ± 0.3 | 8.9 ± 0.6 | 2.1 ± 0.6 | 7.6 ± 0.9 | <0.001 | 0.840 | <0.001 | 0.248 | <0.001 |
| PLMI (events per hour) | 1.3 ± 0.2 | 11.6 ± 0.5 | 1.3 ± 0.5 | 12.5 ± 0.7 | <0.001 | 0.995 | <0.001 | 0.371 | <0.001 |
Data are means ± standard error of the mean (SEM), adjusted for sex, race, age, and BMI percentile. p-Values are post hoc comparisons from multivariable-adjusted linear models. Bold values are statistically significant at P ≤ 0.05.
PLMA = periodic limb movement arousal index; PLMS = abnormal periodic limb movements; PLMI = periodic limb movement index.
Role of PLMS in the association of ADHD with neurobehavioral functioning
The results of full-factorial, multivariable-adjusted general linear models are presented in Supplementary Table 2 and Table 3. As expected, main effects showed that ADHD was significantly associated with more impaired scores in all measures of neurocognitive or behavioral functioning. Main effects for PLMS showed no significant associations with measures of neurocognitive functioning but rather with more impaired scores in C/ABCL behavioral scales. Importantly, a significant interaction revealed that the association between ADHD with control interference was modified by PLMS (p = 0.031), whereas no significant interactions were observed for other neurocognitive functions. In terms of behavioral outcomes, significant interactions were revealed for externalizing behaviors (p = 0.037), attention problems (p = 0.017), thought problems (p = 0.036), and internalizing problems (p = 0.024). This latter significant interaction was specific to the anxious-depressed (p = 0.007), somatic complaints (p = 0.035), and DSM-oriented anxious (p = 0.003) subscales.
Table 3.
Neurocognitive and behavioral functioning across the study subgroups
| 1. None (n = 255) |
2. PLMS (n = 68) |
3. ADHD (n = 63) |
4. ADHD + PLMS (n = 35) |
1 vs. 2 | 1 vs. 3 | 1 vs. 4 | 2 vs. 4 | 3 vs.4 | |
|---|---|---|---|---|---|---|---|---|---|
| Neurocognitive | |||||||||
| Vigilance | 103.7 ± 0.8 | 103.6 ± 1.6 | 100.4 ± 1.7 | 97.7 ± 2.4 | 0.957 | 0.087 | 0.022 | 0.042 | 0.367 |
| Processing speed | 10.1 ± 0.1 | 10.0 ± 0.2 | 9.3 ± 0.3 | 8.6 ± 0.4 | 0.689 | 0.007 | <0.001 | 0.001 | 0.102 |
| Distractibility | 108.2 ± 0.6 | 108.0 ± 1.2 | 106.7 ± 1.3 | 102.5 ± 1.8 | 0.911 | 0.320 | 0.004 | 0.012 | 0.058 |
| Working memory | 7.1 ± 0.2 | 7.1 ± 0.3 | 6.2 ± 0.3 | 6.2 ± 0.4 | 0.986 | 0.008 | 0.037 | 0.065 | 0.980 |
| Control interference | 38.6 ± 0.5 | 38.6 ± 1.0 | 40.2 ± 1.1 | 44.6 ± 1.4 | 0.951 | 0.195 | <0.001 | 0.001 | 0.013 |
| Achievement | 105.3 ± 0.6 | 104.9 ± 1.2 | 98.1 ± 1.2 | 95.0 ± 1.7 | 0.725 | <0.001 | <0.001 | <0.001 | 0.136 |
| Intelligence quotient | 105.7 ± 0.7 | 104.3 ± 1.3 | 101.8 ± 1.3 | 99.3 ± 1.8 | 0.318 | 0.009 | 0.001 | 0.022 | 0.248 |
| Behavioral | |||||||||
| Internalizing problems | 50.1 ± 0.7 | 50.8 ± 1.2 | 50.3 ± 1.3 | 57.0 ± 1.8 | 0.592 | 0.873 | <0.001 | 0.005 | 0.003 |
| Anxious depressed | 53.8 ± 0.4 | 53.2 ± 0.7 | 53.6 ± 0.7 | 57.1 ± 1.0 | 0.512 | 0.877 | 0.002 | 0.002 | 0.006 |
| Withdrawn depressed | 54.3 ± 0.4 | 55.5 ± 0.8 | 55.4 ± 0.9 | 59.3 ± 1.2 | 0.184 | 0.245 | <0.001 | 0.009 | 0.008 |
| Somatic complaints | 55.3 ± 0.4 | 55.8 ± 0.8 | 55.1 ± 0.9 | 59.2 ± 1.2 | 0.635 | 0.843 | 0.002 | 0.016 | 0.005 |
| Attention problems | 54.1 ± 0.4 | 54.1 ± 0.8 | 59.1 ± 0.8 | 63.1 ± 1.1 | 0.953 | <0.001 | <0.001 | <0.001 | 0.004 |
| Thought problems | 53.9 ± 0.4 | 54.7 ± 0.7 | 56.9 ± 0.8 | 60.9 ± 1.0 | 0.300 | <0.001 | <0.001 | <0.001 | 0.002 |
| Externalizing problems | 47.6 ± 0.6 | 47.3 ± 1.1 | 52.4 ± 1.2 | 57.2 ± 1.6 | 0.811 | <0.001 | <0.001 | <0.001 | 0.019 |
| Rule-breaking behaviors | 53.3 ± 0.3 | 53.0 ± 0.6 | 55.4 ± 0.7 | 58.3 ± 0.9 | 0.695 | 0.006 | <0.001 | <0.001 | 0.011 |
| Aggressive behaviors | 52.9 ± 0.3 | 52.8 ± 0.6 | 55.0 ± 0.7 | 58.5 ± 0.9 | 0.836 | 0.007 | <0.001 | <0.001 | 0.001 |
| DSM-oriented scales | |||||||||
| Anxious problems | 53.6 ± 0.3 | 53.3 ± 0.6 | 53.6 ± 0.7 | 57.2 ± 0.9 | 0.653 | 0.977 | <0.001 | <0.001 | 0.001 |
| Somatic problems | 54.9 ± 0.5 | 55.6 ± 0.9 | 54.6 ± 0.9 | 58.9 ± 1.2 | 0.439 | 0.823 | 0.003 | 0.031 | 0.006 |
| ADHD problems | 53.8 ± 0.4 | 53.5 ± 0.7 | 60.4 ± 0.8 | 63.7 ± 1.0 | 0.638 | <0.001 | <0.001 | <0.001 | 0.011 |
Data are means ± standard error of the mean (SEM), adjusted for sex, race, age, BMI percentile, MEQ, insomnia, EDS, sleep onset latency, number of awakenings, and AHI. p-Values are post hoc comparisons from multivariable-adjusted general linear models. Bold values are statistically significant at P ≤ 0.05.
PLMS = abnormal periodic limb movements.
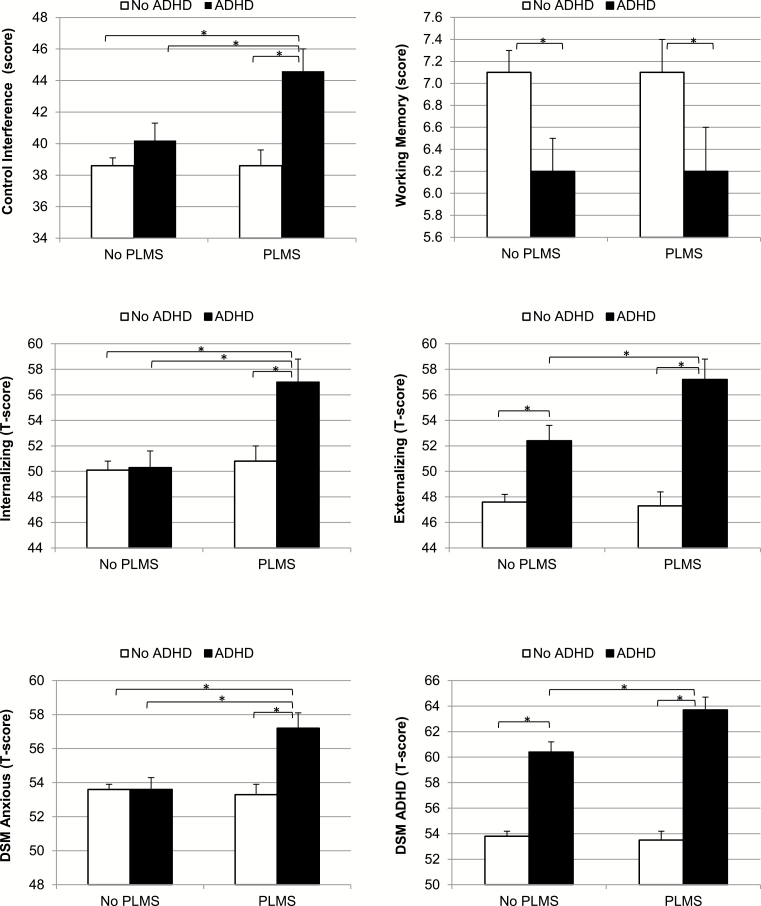
We depict in Figure 1 the significant synergistic effects (control interference and internalizing behaviors) and significant additive effects (externalizing behaviors and inattention/hyperactivity behaviors) resulting from the interactions mentioned above and presented in Table 3. In brief, higher scores in control interference and internalizing behaviors were significantly present in the ADHD + PLMS group but not in the ADHD-alone group. Internalizing behaviors were clinically elevated in about 37% of adolescents with ADHD + PLMS, whereas they were clinically elevated only in 19% and 18% of adolescents with ADHD-only and PLMD-only or without ADHD, respectively (Supplementary Figure 1). In contrast, externalizing behaviors and ADHD severity were clinically elevated in a dose-response manner in the ADHD-alone and ADHD + PLMS groups, as shown in Table 3 and Figure 1. Externalizing behavior was clinically elevated in 25% and 44% of adolescents with ADHD-only and ADHD + PLMS, respectively, compared with only 13% of those with PLMS-only and 12% of those without ADHD (Supplementary Figure 1). The lack of synergistic or additive effects on working memory is also depicted in Figure 1, indicating that both the ADHD-alone and ADHD + PLMS groups had lower working memory scores without differing between each other.
Figure 1.
Executive functioning and internalizing and externalizing behaviors. Significant synergistic effects (left) showed that adolescents with both ADHD and PLMS had significantly worse control interference and internalizing scores, whereas adolescents with ADHD alone did not. Significant additive effects (right) on externalizing behaviors and ADHD severity showed that adolescents with ADHD with or without PLMS had significantly worse aggressive behaviors and inattention/hyperactivity behaviors in a dose-response manner. These group differences were present despite similar working memory deficits in adolescents with ADHD with and without PLMS. ADHD = attention-deficit/hyperactivity disorder; PLMS = abnormal periodic limb movements; DSM = diagnostic and statistical manual of mental disorder.
Sensitivity analysis of spurious effects and specificity of the associations found
Neither SDB, total sleep time, nor insomnia symptoms significantly moderated the relationship between ADHD and internalizing (p for interaction = 0.645, 0.401, and 0.654, respectively) or externalizing (p for interaction = 0.712, 0.940, and 0.987, respectively) behaviors, which indicated that the role found for PLMS in the context of ADHD as it pertains to neurobehavioral functioning is specific, not spurious, and not generalizable to all sleep disorders. Also, the exclusion of participants on stimulants (n = 41) or other psychoactive medications including antidepressants (n = 36) provided similar data and in the same direction and did not affect the results presented herein (Supplementary Figure 2).
Discussion
This is the first population-based study in a nonclinical sample of adolescents to examine the role of PLMS in the association of ADHD with clinically meaningful neurobehavioral outcomes. Our data showed that the prevalence of abnormal PLMS was about 15% higher in adolescents with ADHD than those without ADHD. Importantly, our data showed that the presence of PLMS is associated not only with greater severity of ADHD but with a specific neurobehavioral profile consisting of clinically elevated internalizing problems and executive functioning deficits that were not present in adolescents with ADHD or PLMS alone. These novel findings address a gap in the literature by demonstrating the neurobehavioral significance of PLMS in the context of ADHD and suggest potential treatment targets at the sleep and neurobehavioral levels.
From a clinical standpoint, the results of this study suggest that the presence of PLMS in adolescents with ADHD may help identify those with more severe neurobehavioral outcomes. More specifically, the synergistic effects on control interference, an executive function tightly linked to the prefrontal cortex and subcortical connections to inhibit behavior, as well as on internalizing behaviors, specifically anxiety problems, indicate that these neurobehavioral deficits were present only in adolescents with ADHD and PLMS but not in those with ADHD alone. Importantly, these findings remained similar and in the same direction even after excluding those adolescents who were on psychoactive medication, including antidepressants, which are known to induce or aggravate PLMS [36–38]. Together, these results suggest that the executive dysfunction observed may be a potential neurocognitive underpinning of the increased anxiety in adolescents with ADHD and PLMS, given that control interference deficits, understood as inhibiting irrelevant information, are a shared neurocognitive process between ADHD and anxiety disorders. Alternatively, the observed control interference deficit may be an objective indicator of increased anxiety, given that the Stroop test is a reliable measure of actual behavior inhibition. Regardless of the direction of these associations, our data suggest that adolescents with ADHD and PLMS are a distinct phenotype associated with increased anxiety, physiological difficulty falling asleep, and control interference deficits. Future studies should examine whether there is an association with PLMS in adolescents with anxiety disorders that may require specific treatment needs. Also, the neural mechanisms of adolescents with ADHD and PLMS when compared to those with ADHD-alone need to be examined using combined neuroimaging and EEG methods.
Another interesting and novel finding of this study was that the sleep profiles were significantly different between adolescents with ADHD and PLMS and adolescents with PLMS alone. Although the latter group was characterized by significant sleep fragmentation and resulting hypersomnia (i.e. increased number of awakenings and stage 1 on the PSG and higher hypoarousal scores on the PBS), adolescents with ADHD and PLMS were characterized by physiological difficulty falling asleep and decreased sleep pressure (i.e. increased sleep onset latency and decreased stage 2 on the PSG). These physiological sleep differences further support the potential phenotyping of adolescents with ADHD based on the presence of PLMS and suggest different pathophysiological mechanisms for their sleep disturbances.
The prevalence of abnormal PLMS based on pediatric criteria was about 24% in this general population sample of 421 adolescents, which exceeds previous estimates. A previous study of 195 community-based children and adolescents, who were recruited as healthy, normal research volunteers without any other sleep disorder or chronic medical condition, reported a prevalence of PLMI ≥ 5 to be 5% in children (i.e. 5–12 years old) and 10.5% in adolescents (i.e. 13–17.9 years old) [3]. The baseline portion of our cohort revealed that only 2.6% of children 5–12 years old had an PLMI ≥ 5 compared with 24% in the current follow-up portion of the cohort, a distribution that did not differ among younger (i.e. 12–17 years) or older (i.e., 18–23 years) adolescents. The overall prevalence in this general population sample of adolescents based on pediatric criteria is, thus, closer to adult estimates of 28.6% [39] and may indicate that adolescence is a critical period for the increased onset of abnormal PLMS. In fact, the prevalence of abnormal PLMS was 35% in adolescents with ADHD in the present study, which falls within current estimates of 26%–64% for clinical samples of ADHD [4–7]. Furthermore, in only 26%–27% of those with ADHD and PLMS, their parents endorsed observing leg movements during their child’s sleep or symptoms of RLS, a disorder in which the individual experiences an urge to move their legs and uncomfortable sensations in their legs, typically during periods of inactivity, that is relieved by movement [40]. These results suggest that parent-reported symptoms of leg movements during sleep or RLS are not sufficient to identify abnormal PLMS and, thus, any potential RLS disorder should be thoroughly evaluated by clinical history, whereas PSG is required to detect those children in whom PLMS have true clinical significance. Considering that both groups with abnormal PLMS had an average PLMI of about 11–13 events per hour, it is worth noting that such level of PLMS was associated with significant sleep disturbance and daytime functioning outcomes. Currently, most children and adolescents with ADHD are referred for a PSG study when there is a suspicion of SDB (e.g. enlarged tonsils, obesity, and/or snoring). Based on our novel data, adolescents with ADHD and comorbid internalizing disorders, particularly anxiety disorders, should undergo a PSG study.
Some limitations of our study should be noted. This was a cross-sectional study and, therefore, we could not assess causality between PLMS and neurobehavioral outcomes. Thus, the data may also suggest that adolescents with ADHD with more severe executive dysfunction and internalizing symptoms may suffer from clinically significant PLMS. Nevertheless, our data indicate that adolescents with both ADHD and PLMS represent a clinically meaningful subgroup. Additionally, the diagnosis of ADHD was based on a clinical history and physical examination in which study participants indicated whether they had received a diagnosis for ADHD and whether it was currently treated or had been treated in the past. This definition together with the oversampling of symptoms and risk factors for SDB, based on a questionnaire that included an item on hyperactivity [21], in the recruitment of the original cohort [20], could explain that about 23% of participants had ADHD in this randomly selected population, which exceeds national estimates of 12% for adolescents [41], and should be interpreted with caution. Nevertheless, the use of standardized objective neurocognitive measures and self- and parent-reported behavioral scales confirmed the correct classification of the participants identified with ADHD and assures rigor and reproducibility of study findings. It should be noted that participants underwent only one night of PSG, which may not be representative of their habitual sleep in the home environment and cannot capture the night-to-night variability in PLMS [42], which may be greater in adolescents with ADHD. However, the PSG differences between groups were those expected for adolescents with ADHD, as well as for the sleep disruptive effects of abnormal PLMS. Furthermore, research has suggested an absence of a “first night effect” for PLMS to the extent observed for sleep continuity parameters [42]. Additionally, there is a need for validated actigraphy measures to assess PLMS in the child/adolescent’s home environment over multiple consecutive nights; however, current clinical practice guidelines recommend against using actigraphy for monitoring PLMS [43] and should be abided by until reliable and valid actigraphy methods are developed. Unfortunately, iron status was not assessed in the PSCC and, thus, we could not examine ferritin levels as a potential mediator of the associations found [44, 45]. Given that a significant proportion of adolescents with ADHD and PLMS reported RLS symptoms, it is very likely that iron deficiency may be a common underlying mechanism at play. Despite these limitations, this study expands the limited data and addresses current gaps on the neurobehavioral significance of PLMS in the context of ADHD.
In conclusion, PLMS were significantly more prevalent in adolescents with ADHD from a nonclinical, general population sample. Importantly, adolescents with both ADHD and PLMS not only had worse neurobehavioral outcomes than adolescents with ADHD-alone, but present specific executive functioning deficits and internalizing behaviors. These data suggest that PLMS may be a marker of more severe underlying neurobiological deficits that may put adolescents with ADHD at greater risk for anxiety and mood disorders. Alternatively, these data may suggest that PLMS may be a marker of ADHD comorbid with anxiety disorders. Nevertheless, it appears that novel targeted treatments for sleep may need to be developed for this phenotype of adolescents with ADHD and clinically significant PLMS.
Supplementary Material
Supplementary material is available at SLEEP online.
Funding
This research was funded by the National Institutes of Health grants R01 HL63772, R01 HL97165, UL1 RR033184, and C06 RR16499.
Note
Conflict of interest statement. None declared.
References
- 1. Cortese S, et al. Sleep and alertness in children with attention-deficit/hyperactivity disorder: a systematic review of the literature. Sleep. 2006;29(4):504–511. [PubMed] [Google Scholar]
- 2. American Academy of Sleep Medicine. International Classification of Sleep Disorders, 3rd ed Darien, IL: American Academy of Sleep Medicine; 2014. [Google Scholar]
- 3. Marcus CL, et al. Prevalence of periodic limb movements during sleep in normal children. Sleep. 2014;37(8):1349–1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Picchietti DL, et al. Periodic limb movement disorder and restless legs syndrome in children with attention-deficit hyperactivity disorder. J Child Neurol. 1998;13(12):588–594. [DOI] [PubMed] [Google Scholar]
- 5. Picchietti DL, et al. Further studies on periodic limb movement disorder and restless legs syndrome in children with attention-deficit hyperactivity disorder. Mov Disord. 1999;14(6):1000–1007. [DOI] [PubMed] [Google Scholar]
- 6. Chervin RD, et al. Clinical prediction of periodic leg movements during sleep in children. Sleep Med. 2001;2(6):501–510. [DOI] [PubMed] [Google Scholar]
- 7. Chervin RD, et al. Associations between symptoms of inattention, hyperactivity, restless legs, and periodic leg movements. Sleep. 2002;25(2):213–218. [PubMed] [Google Scholar]
- 8. Picchietti DL, et al. Moderate to severe periodic limb movement disorder in childhood and adolescence. Sleep. 1999;22(3):297–300. [DOI] [PubMed] [Google Scholar]
- 9. Crabtree VM, et al. Periodic limb movement disorder of sleep in children. J Sleep Res. 2003;12(1):73–81. [DOI] [PubMed] [Google Scholar]
- 10. Martinussen R, et al. A meta-analysis of working memory impairments in children with attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 2005;44(4):377–384. [DOI] [PubMed] [Google Scholar]
- 11. Willcutt EG, et al. Validity of the executive function theory of attention-deficit/hyperactivity disorder: a meta-analytic review. Biol Psychiatry. 2005;57(11):1336–1346. [DOI] [PubMed] [Google Scholar]
- 12. van Lieshout M, et al. Does neurocognitive functioning predict future or persistence of ADHD? A systematic review. Clin Psychol Rev. 2013;33(4):539–560. [DOI] [PubMed] [Google Scholar]
- 13. Smedje H, et al. Associations between disturbed sleep and behavioural difficulties in 635 children aged six to eight years: a study based on parents’ perceptions. Eur Child Adolesc Psychiatry. 2001;10(1):1–9. [DOI] [PubMed] [Google Scholar]
- 14. Chervin RD, et al. Symptoms of sleep disorders, inattention, and hyperactivity in children. Sleep. 1997;20(12):1185–1192. [DOI] [PubMed] [Google Scholar]
- 15. Gaultney JF, et al. Parent-reported periodic limb movement, sleep disordered breathing, bedtime resistance behaviors, and ADHD. Behav Sleep Med. 2005;3(1):32–43. [DOI] [PubMed] [Google Scholar]
- 16. Picchietti D, et al. Restless legs syndrome, periodic limb movements in sleep, and depression. Sleep. 2005;28(7):891–898. [PubMed] [Google Scholar]
- 17. Vandeputte M, et al. Sleep disorders and depressive feelings: a global survey with the Beck depression scale. Sleep Med. 2003;4(4):343–345. [DOI] [PubMed] [Google Scholar]
- 18. Aikens JE, et al. Differential rates of psychopathology symptoms in periodic limb movement disorder, obstructive sleep apnea, psychophysiological insomnia, and insomnia with psychiatric disorder. Sleep. 1999;22(6):775–780. [DOI] [PubMed] [Google Scholar]
- 19. Bixler EO, et al. Natural history of sleep disordered breathing in prepubertal children transitioning to adolescence. Eur Respir J. 2016;47(5):1402–1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bixler EO, et al. Sleep disordered breathing in children in a general population sample: prevalence and risk factors. Sleep. 2009;32(6):731–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ali NJ, et al. Snoring, sleep disturbance, and behaviour in 4-5 year olds. Arch Dis Child. 1993;68(3):360–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Chervin RD, et al. Pediatric sleep questionnaire (PSQ): validity and reliability of scales for sleep-disordered breathing, snoring, sleepiness, and behavioral problems. Sleep Med. 2000;1(1):21–32. [DOI] [PubMed] [Google Scholar]
- 23. Carskadon MA, et al. A self-administered rating scale for pubertal development. J Adolesc Health. 1993;14(3):190–195. [DOI] [PubMed] [Google Scholar]
- 24. Kuczmarski RJ, et al. 2000 CDC Growth Charts for the United States: methods and development. Vital Health Stat. 2002;246:1–190. [PubMed] [Google Scholar]
- 25. Carskadon MA, et al. Association between puberty and delayed phase preference. Sleep. 1993;16(3):258–262. [DOI] [PubMed] [Google Scholar]
- 26. Lindgren SD, Koeppl GK. Assessing child behavior problems in a medical setting: Development of the Pediatric Behavior Scale. In: Prinz RJ, ed. Advances in Behavioral Assessment of Children and Families. Greenwich, CT: JAI;1987:57–90. [Google Scholar]
- 27. Gordon M. The Gordon Diagnostic System. DeWitt, NY: Gordon Systems; 1983. [Google Scholar]
- 28. Wechsler D. Wechsler Intelligence Scale for Children. 4th ed San Antonio, TX: Psychological Corporation; 2003. [Google Scholar]
- 29. Wechsler D. Wechsler Adult Intelligence Scale.3rd ed San Antonio, TX: Psychological Corporation; 1997. [Google Scholar]
- 30. Golden CJ, et al. Stroop Color and Word Test Children’s Version. Wood Dale, IL: Stoelting; 2003. [Google Scholar]
- 31. Golden CJ., et al. The Stroop Color and Word Test: A Manual for Clinical and Experimental Uses. Chicago: Stoelting; 2002. [Google Scholar]
- 32. Wechsler D. Wechsler Abbreviated Scale of Intelligence. San Antonio, TX: Psychological Corporation; 1999. [Google Scholar]
- 33. Wilkinson GS. Wide Range Achievement Test–Revision 3. Wilmington, DE: Jastak Association; 1993. [Google Scholar]
- 34. Achenbach TM, et al. Manual for the ASEBA School-Age Forms and Profiles. Burlington, VT: ASEBA; 2001. [Google Scholar]
- 35. Achenbach TM, et al. Manual for the ASEBA Adult Forms and Profiles. Burlington, VT: ASEBA; 2003. [Google Scholar]
- 36. Yang C, et al. Antidepressants and periodic leg movements of sleep. Biol Psychiatry. 2005;58(6):510–514. [DOI] [PubMed] [Google Scholar]
- 37. Vendrame M, et al. Selective serotonin reuptake inhibitors and periodic limb movements of sleep. Pediatr Neurol. 2011;45(3):175–177. [DOI] [PubMed] [Google Scholar]
- 38. Sobanski E, et al. Sleep in adults with attention deficit hyperactivity disorder (ADHD) before and during treatment with methylphenidate: a controlled polysomnographic study. Sleep. 2008;31(3):375–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Haba-Rubio J, et al. Prevalence and determinants of periodic limb movements in the general population. Ann Neurol. 2016;79(3):464–474. [DOI] [PubMed] [Google Scholar]
- 40. Picchietti D, et al. Restless legs syndrome: prevalence and impact in children and adolescents–the Peds REST study. Pediatrics. 2007;120(2):253–266. [DOI] [PubMed] [Google Scholar]
- 41. Pastor PN, et al. Association between Diagnosed ADHD and Selected Characteristics among Children Aged 4–17 Years: United States, 2011–2013. NCHS Data Brief. Number 201 Atlanta, GA: Centers for Disease Control and Prevention; 2015. [PubMed] [Google Scholar]
- 42. Picchietti MA, et al. Children show individual night-to-night variability of periodic limb movements in sleep. Sleep. 2009;32(4):530–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Smith MT, McCrae CS, Cheung J, Martin JL, Harrod CG, Heald JL, Carden KA. Use of Actigraphy for the Evaluation of Sleep Disorders and Circadian Rhythm Sleep-Wake Disorders: An American Academy of Sleep Medicine Clinical Practice Guideline. J Clin Sleep Med. 2018;14(70):1231–1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Dye TJ, et al. Outcomes of long-term iron supplementation in pediatric restless legs syndrome/periodic limb movement disorder (RLS/PLMD). Sleep Med. 2017;32:213–219. [DOI] [PubMed] [Google Scholar]
- 45. Wang Y, et al. Iron status in attention-Deficit/Hyperactivity Disorder: a systematic review and meta-analysis. PLoS One. 2017;12(1):e0169145. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.