Abstract
Study Objectives
African Americans have been under-represented in obstructive sleep apnea (OSA) research. This study determined the prevalence and correlates of OSA overall and by sex among African Americans in the Jackson Heart Sleep Study.
Methods
Participants (N = 852) underwent a type 3 in-home sleep apnea study, 7 day wrist actigraphy and completed standardized measurements and questionnaires. OSA was defined as an apnea–hypopnea index (AHI) of ≥15, where hypopneas were defined as ≥ 4% associated desaturation. Physician diagnosis of OSA was self-reported. Logistic regression models were fit to determine the associations of demographics, socioeconomic status, sleep symptoms, actigraphy-based sleep, body mass index (BMI), and comorbidities with OSA.
Results
Average age was 63.1 (standard deviation = 10.7), 66% were female, and mean BMI was 32.0 (6.9) kg/m2. Approximately 24% had an AHI ≥ 15; of those, 5% had a physician diagnosis of OSA. Prevalence of OSA increased across BMI categories, but not age groups. Men had a 12% higher prevalence of OSA compared with women, p < 0.01. Older age, male sex, higher BMI, larger neck circumference, and report of habitual snoring were independently associated with higher odds of OSA, all p < 0.05. Associations between sleep symptoms and OSA were similar for men and women. Sleepiness and waist circumference were not associated with OSA.
Conclusions
There was a high prevalence of objectively measured but undiagnosed OSA in this sample of African Americans. Snoring, BMI, and neck circumference were important markers of OSA for men and women. Our results suggest that screening tools that incorporate information on sleepiness and waist circumference may be suboptimal in this population.
Keywords: home sleep apnea testing, undiagnosed, sleep apnea, African American, Jackson Heart Study, epidemiology
Statement of Significance
In this population of African Americans living in the southern United States, we found a high prevalence of moderate or severe obstructive sleep apnea (OSA) (24%), which was mainly undiagnosed (95%). Men had a higher prevalence of OSA compared with women. The strongest predictor of OSA was male sex. Snoring, body mass index and neck circumference were important markers of OSA for both men and women. Our results suggest that screening tools that incorporate information on neck rather than waist circumference and snoring but more so than sleepiness may perform better in some African American populations. Future screening procedures may need to more directly assess OSA (e.g. using oximetry or other objective testing) in populations with high rates of obesity, including under-represented minority groups.
Introduction
Sleep apnea is highly prevalent, estimated to affect 26% of adults, with moderate or severe sleep apnea affecting approximately 10% of adults [1, 2]. Moreover, an estimated 80 to 90% of individuals with sleep apnea are undiagnosed [2–9]. Undiagnosed and untreated sleep apnea is associated with daytime sleepiness, decreased daily functioning, cognitive deficits, hypertension, and cardiovascular disease [10–12].
Sleep disorders are prevalent among minority populations [3, 13]. African Americans in particular have a higher prevalence of sleep apnea syndrome (apnea–hypopnea index [AHI] ≥ 5 plus sleepiness), daytime sleepiness, poor sleep quality, and more severe sleep apnea compared with non-Hispanic Whites [3, 14]. Also, findings from the Multi-Ethnic Study of Atherosclerosis (MESA) demonstrated that only 16.2% of African Americans from 6 US cities who had polysomnography-defined moderate or severe sleep apnea reported a physician diagnosis [3]. The prior study had a limited sample of African Americans and did not explore the factors related to sleep apnea in that population. It is possible that factors affecting screening or diagnosis are different and therefore should be explored.
Epidemiological studies have identified demographic (e.g. male sex and older age), comorbidities (e.g., obesity and hypertension), and sleep symptoms (e.g. snoring and sleepiness), as risk factors and correlates of increased obstructive sleep apnea (OSA) prevalence [2, 7]. These prior studies consisted of predominately non-Hispanic white individuals. Predictors of OSA may be different among African Americans than non-Hispanic whites due to differences in body fat distribution and the higher prevalence of comorbidities [15–17] and potentially to differences in perception of symptoms. Since central fat distribution predicts OSA, differences in body fat distribution may influence associations of body mass index (BMI) and OSA [15]. Therefore, BMI may be a poor surrogate for central fat distribution. To understand potential risk factors for OSA among African Americans, it is important to evaluate established risk factors and correlates of sleep apnea (e.g. demographics, sleep habits, and comorbidities) as well as indices obtained from actigraphy, a tool increasingly used in population health to characterize sleep patterns.
There are also important sex differences in the prevalence of OSA. For instance, research among non-Hispanic whites has shown that men are more likely to have OSA than women, due to differences in body fat distribution, upper airway anatomy, breathing control, hormones, aging, and other differences in physiology [18]. Similarly, in a US Hispanic/Latino population, men compared with women had a two- to three-fold higher prevalence of OSA [19]. Because of the high prevalence of obesity among African American women, sex differences in the prevalence of OSA may be mitigated. Also, a higher percentage of women may have under-diagnosed sleep disorders than men [8], which may influence sex differences in OSA.
The goal of this paper was to describe the prevalence and correlates of OSA by sex among African Americans in the Jackson Heart Sleep Study (JHSS) and estimate the proportion of undiagnosed cases. We hypothesized the following: (1) there would be a high prevalence of OSA with few participants reporting a physician-diagnosis; (2) traditional OSA risk factors as well as sleep symptoms and sleep patterns would be associated with OSA; and (3) OSA severity would be similar between women and men.
Methods
Jackson Heart Study (JHS) is a longitudinal study of 5306 African American adults aged 21 to 95 at baseline from three counties (Hinds, Madison, and Rankin) that comprise the Jackson, Mississippi metropolitan area. JHS was designed to prospectively study the etiology of cardiovascular disease among African Americans. Participants were recruited from the Atherosclerosis Risk in Communities Study (30%), family members of participants (28%), random selection (17%), and community volunteers (25%) between September 2000 and March 2004 and three core examinations were conducted. The details of the JHS have been published [20]. The current analyses utilize data from the JHSS (see below). Institutional Review Board approval was obtained from the University of Mississippi and Partners Research Committee and written informed consent was obtained from all participants.
Between 2012 and 2016, 913 participants were enrolled in the JHSS. Eligible participants were those who participated in the third JHS follow-up exam (N = 3609) or who had participated in other follow-up ancillary studies. We attempted to contact potential participants by phone and/or mail (N = 3015) with an invitation to participate. Individuals who reported regular use of continuous positive airway pressure (N = 70) or were first-degree relatives of a consenting participant (N = 10) were not eligible for the study. Participants attended a clinic visit and underwent in-home sleep apnea testing, 1 week wrist actigraphy, fasting venipuncture, anthropometry, blood pressure, and other vascular studies, and completed interviewer administered sleep and health questionnaires. The analysis was restricted to 852 participants with valid sleep apnea test data (≥3 hr of data from the oximeter, nasal pressure, and one or more respiratory band), see Supplementary Figure S1 for a consort flow diagram.
Sleep measures
OSA was assessed with a validated type 3 home sleep apnea device (Embletta-Gold device; Embla, Broomfield, CO) [21, 22], recording nasal pressure (measuring airflow); thoracic and abdominal inductance plethysmography; finger pulse oximetry; body position; and ECG. The AHI was derived as the sum of all apneas plus hypopneas associated with ≥3% (AHI3P) or 4% (AHI4P) oxygen desaturation divided by the estimated sleep time, edited to exclude artifact and likely wake times [23]. Sleep time was estimated using a previously reported method [23]. Sleep onset was identified based on reduction of movement artifact, heart rate, and assumption of rhythmic breathing. Sleep offset was identified by the appearance of sustained movement activity and/or increased heart rate. Obstructive apneas were identified when the amplitude (peak to trough) of the nasal pressure signal was flat or nearly flat for >10 s. Hypopneas were identified if a ≥30% reduction of amplitude was visualized in the nasal pressure signal or, if unclear, in the respiratory inductance bands for ≥10 s. The desaturation associated with respiratory events was based on the nadir desaturation after the termination of the event (20 to 45 s). The end of events was identified at the beginning of a breath that exceeded the amplitude for breaths qualifying as hypopneas.
OSA severity was characterized by the following standard AHI categories: <5 (unaffected); ≥5 (mild), ≥15 to 30 (moderate), and ≥30 (severe). For the current study, we further categorized “moderate or severe” OSA as AHI ≥15, with no upper limit.
Sleep duration and efficiency were obtained from 7 day wrist actigraphy. Participants wore a GT3X+ Activity Monitor on the nondominant wrist for 7 consecutive days and completed a sleep diary [24]. Actigraphic data during 60 s epochs were scored as sleep or wake by ActiLife version 6.13 analysis software (ActiGraph Corp, Pensicola, FL) using the validated algorithm (Cole-Kripke) [25]. From valid nocturnal actigraphy data, we computed the average values for the following: total sleep duration and sleep efficiency (total sleep time from lights off to lights on; continuously and dichotomized at 85%). There were 811 participants with valid actigraphy measures.
From the sleep questionnaire, we assessed daytime sleepiness (Epworth Sleepiness Score [ESS]), insomnia symptoms (Women’s Health Initiative Insomnia Rating Scale [WHIIRS]), habitual snoring, and self-reported diagnosis of sleep apnea. Sleepiness was defined by an ESS of >10 [26]. Insomnia was identified as a WHIIRS of ≥10 [27]. Habitual snoring was defined as self-reported snoring at least 3 times a week.
Demographics
Participants’ age (date of birth) and sex (male or female) were self-reported. Age was categorized as <50, 50–59, 60–69, and ≥70 years.
Socioeconomic status
Education was selected from four categories (less than high school, high school or GED, some college, or college degree). Self-reported family income was selected from 15 categories and a continuous measure was derived using midpoints. Employment status was self-reported as employed or not employed.
Anthropometry
Height, weight, waist, and neck circumference were measured by trained staff following a standardized protocol. BMI was calculated in kg/m2 using measurements of weight and height. BMI was categorized as underweight/normal weight (<25 kg/m2), overweight (25–29.9 kg/m2), obese (30–39.9 kg/m2), and morbidly obese (≥40 kg/m2).
Comorbidities
Seated blood pressure measurements were obtained using an Omron HEM907XL blood pressure monitor after 5 min of rest. Three seated blood pressure readings were taken 1 min apart and the last two were averaged. Hypertension was defined as a systolic blood pressure of ≥130 mmHg or a diastolic blood pressure of ≥80 mmHg, use of antihypertensive medications (self-report or identified from a medication inventory), or self-reported history of hypertension [28]. Diabetes was defined as fasting glucose ≥126 mg/dL, use of antidiabetic medication, or self-reported diabetes diagnosis [29].
Statistical analyses
Means with standard deviations and proportions were calculated to describe the marginal and sex-specific distribution of covariates and OSA measures. We reported medians with interquartile ranges for family income, AHI, and WHIIRS, which had skewed distributions. Sex differences in OSA prevalence were determined by the chi-square test. The primary OSA analytic measure was AHI4P, required 4% or greater desaturations to accompany hypopneas [30]; results with AHI3P (requiring 3% or greater desaturations to define hypopneas) were also examined (corresponding tables are shown in Supplementary Material).
A population-based estimate of OSA prevalence among African Americans age of 50–80 years in the United States (US) was estimated using data from the JHSS and national survey data. We estimated the prevalence of African Americans in the US in age, sex, and BMI-specific strata using data from the US census and from combined data from the National Health and Nutrition Examination Survey (NHANES) 2011–2012, 2013–2014, and 2015–2016. We used the NHANES sampling weights and included African American participants who were 50–80 years of age. We then estimated the population-based prevalence of OSA by applying JHSS OSA prevalence rates to NHANES age, sex, and BMI distributions. We used a bootstrap procedure to obtain the 95% confidence intervals for the OSA prevalence estimates [2].
Marginal and sex-specific prevalence of OSA by age group and BMI category was generated separately. We used linear regression models to test the significance of the trend in the prevalence of OSA across age groups or BMI categories among the total sample and the significance of sex interactions with age groups or BMI categories, analyzed as ordinal variables. We calculated Spearman correlation coefficients to describe the associations of covariates and OSA (AHI ≥ 15) among the overall and sex-specific strata.
Among the analytic sample, there was at most 3% missing for each covariate, except for income, which had 15% missing data. To address the missingness in the dataset, we imputed 10 complete datasets using the MICE package in R (version 3.4.0). Each dataset was imputed with 20 iterations. We confirmed convergence of the MICE imputation using trace plots. Logistic regression models were fit based on 10 imputed datasets to estimate crude odds ratios of OSA associated with each covariate and the respective 95% confidence interval. Age, BMI, waist, and neck circumference were standardized. The odds ratios were further adjusted for age, sex, and BMI in the multivariate logistic regression analyses. We also conducted sex-stratified analyses adjusted for age and BMI.
Statistical significance was determined as p ≤ 0.05. Except for imputation, all analyses were conducted using SAS 9.4 (SAS Institute, Cary, NC).
Results
Participants in JHSS were comparable to those in Exam 3 in terms of distributions of age, sex, BMI, diabetes, and self-reported sleep duration. However, compared with Exam 3 participants not enrolled in JHSS, participants in JHSS (N = 913) had a higher proportion with a college degree and with hypertension, p < 0.01 (Supplementary Table S1).
The study sample had a mean age of 63.1 years (standard deviation [SD]: 10.7) and was mostly female (66.0%) and college educated (53.8%) (Table 1). Compared with men, women were slightly older, less likely to be employed, had a lower median household income, had a higher BMI, a higher prevalence of hypertension, and reported a higher frequency of symptoms of insomnia (Table 1). Daytime sleepiness and habitual snoring were reported by 20.5 and 37.4% of participants, respectively. The average sleep duration was 6.7 hr (SD: 1.1) and 61.5% had a short sleep duration of <7 hr. Women had a longer sleep duration compared with men, p = 0.012. Approximately 29.3% had a sleep efficiency of <85%.
Table 1.
Participants’ characteristics among total, male, and female samples of Jackson Heart Sleep Study, N = 852, 2012–2016
| Characteristics | Total (N = 852, 100%) |
Men (N = 290, 34%) |
Women (N = 562, 66%) |
p |
|---|---|---|---|---|
| Demographics | ||||
| Age (years), mean ± SD | 63.1 ± 10.7 | 62.0 ± 10.6 | 63.7 ± 10.7 | 0.030 |
| Age group, N (%) | 0.284 | |||
| <50 | 79 (9.3%) | 32 (11.0%) | 47 (8.4%) | |
| 50–59 | 245 (28.8%) | 87 (30.0%) | 158 (28.1%) | |
| 60–69 | 270 (31.7%) | 94 (32.4%) | 176 (31.3%) | |
| ≥70 | 258 (30.3%) | 77 (26.6%) | 181 (32.2%) | |
| Education, N (%) | 0.015 | |||
| < High school | 78 (9.4%) | 23 (8.2%) | 55 (10.0%) | |
| High school or GED | 118 (14.2%) | 35 (12.4%) | 83 (15.1%) | |
| Some college/training | 188 (22.6%) | 82 (29.1%) | 106 (19.3%) | |
| College degree* | 447 (53.8%) | 142 (50.4%) | 305 (55.6%) | |
| Employed, N (%) | 398 (47.3%) | 155 (54.4%) | 243 (43.6%) | 0.003 |
| Family income, median ± IQR | 43,000 ± 4,000 | 63,000 ± 5,800 | 43,000 ± 4,000 | <0.001 |
| Anthropometry | ||||
| BMI (kg/m2), mean ± SD | 32.0 ± 6.9 | 30.7 ± 5.9 | 32.7 ± 7.3 | <0.001 |
| BMI category, N (%) | 0.001 | |||
| <25 | 104 (12.3%) | 42 (14.6%) | 62 (11.0%) | |
| 25–29.9 | 269 (31.8%) | 103 (35.9%) | 166 (29.7%) | |
| 30–39.9 | 368 (43.5%) | 124 (43.2%) | 244 (43.6%) | |
| ≥40 | 105 (12.4%) | 18 (6.3%) | 87 (15.6%) | |
| Waist circumference, mean ± SD | 106.0 ± 16.1 | 107.7 ± 16.0 | 105.1 ± 16.0 | 0.036 |
| Neck circumference, mean ± SD | 38.6 ± 4.0 | 41.9 ± 3.6 | 36.9 ± 3.1 | <0.001 |
| Comorbidities | ||||
| Hypertension, N (%) | 722 (85.7%) | 232 (80.8%) | 490 (88.3%) | 0.003 |
| Diabetes, N (%) | 216 (26.0%) | 65 (23.1%) | 151 (27.5%) | 0.179 |
| Sleep symptoms | ||||
| Daytime sleepiness (ESS > 10), N (%) | 172 (20.5%) | 66 (23.3%) | 106 (19.1%) | 0.156 |
| Habitual snoring, N (%) | 314 (37.4%) | 111 (39.1%) | 203 (36.6%) | 0.478 |
| Actigraphy-based sleep | ||||
| Sleep duration (hours), mean ± SD | 6.7 ± 1.1 | 6.5 ± 1.2 | 6.8 ± 1.1 | 0.012 |
| Sleep duration < 7 hours, N (%) | 499 (61.5%) | 180 (65.5%) | 319 (59.5%) | 0.100 |
| Sleep efficiency < 85%, N (%) | 238 (29.3%) | 83 (30.2%) | 155 (28.9%) | 0.708 |
| Sleep apnea syndrome (AHI 4% ≥ 5 and ESS >10), N (%) |
101 (12.1%) | 42 (14.8%) | 59 (10.6%) | 0.078 |
| WHIIRS, median ± IQR | 5.0 ± 7.0 | 4.0 ± 6.0 | 5.0 ± 7.0 | <0.001 |
| Insomnia (WHIIRS ≥ 10), N (%) | 187 (22.2%) | 49 (17.2%) | 138 (24.8%) | 0.012 |
| AHI 4% (events/hour), median ± IQR | 6.2 ± 12.0 | 8.9 ± 18.4 | 5.4 ± 10.2 | <0.001 |
*bachelor degree or higher.
SD = standard deviation; IQR = interquartile range; BMI = body mass index; ESS = Epworth sleepiness scale; AHI = apnea–hypopnea index; WHIIRS = Women’s Health Initiative Insomnia Rating Scale.
Distribution and sex, age, and BMI-related prevalence of OSA
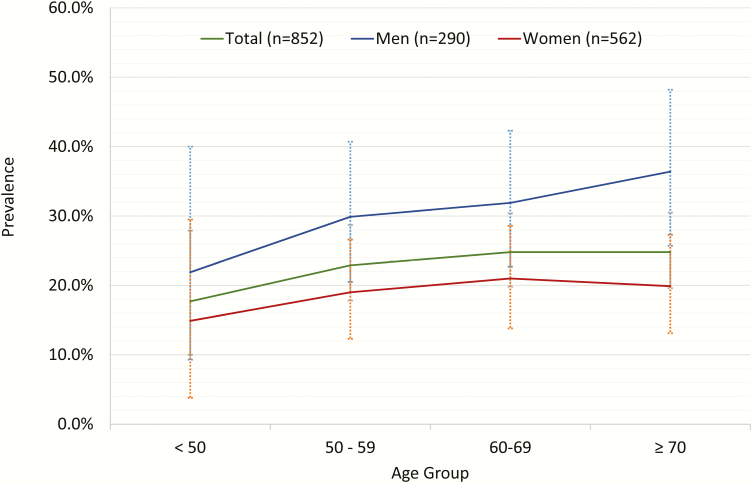
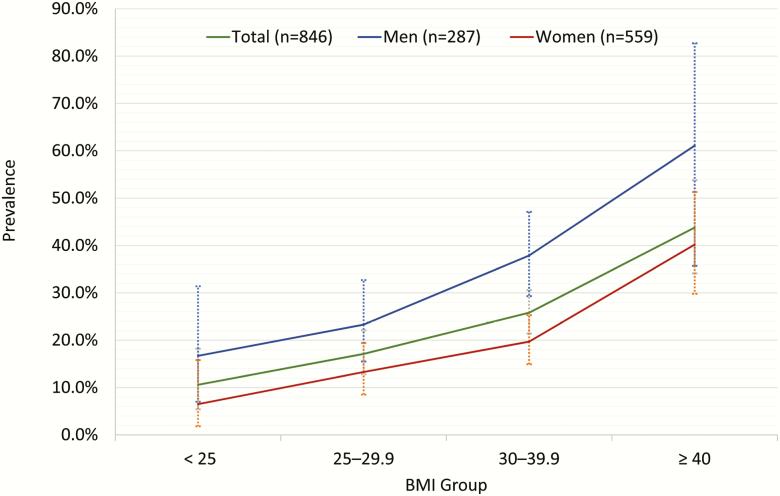
The prevalence of moderate or more severe OSA (AHI ≥ 15) was 23.6% based on the AHI4P. Prevalence of OSA syndrome, defined by an AHI of >5 and sleepiness (ESS ≥ 10), was 12.1%. Overall, the prevalence of OSA appeared to increase from 17.7% for those less than 50 years to 22.9% for those 50–59, then plateaued at 24.8% for those 60–69 years and older than 70 years old (Figure 1), but the trend was not statistically significant (ptrend = 0.23). The prevalence of OSA increased steeply across BMI categories from under/normal weight to morbidly obese, ptrend < 0.01 (Figure 2). Women had a lower median AHI4P compared with men, p < 0.001 (Table 1). Similarly, men had an approximately 11.8% higher prevalence of moderate or more severe OSA compared with women, p < 0.001. There were no sex differences in OSA prevalence across age groups (pinteraction = 0.34) or BMI categories (pinteraction = 0.40).
Figure 1.
Unadjusted prevalence of OSA (AHI4P) across age groups.
Figure 2.
Unadjusted prevalence of OSA (AHI4P) across BMI groups.
Supplementary Table S2 shows the OSA prevalence estimates that were determined by extrapolating JHSS age, sex, and BMI-specific OSA estimates to US national census and epidemiological data. The population-based OSA prevalence (AHI4P ≥ 15) was 20.4% (17.3, 23.4).
Correlates of OSA
In unadjusted models, male sex, higher BMI, larger waist and neck circumferences, hypertension, habitual snoring, and lower sleep efficiency were associated with higher odds of OSA, p < 0.05 (Table 2). After adjustment for age and BMI, male sex remained associated with higher odds of OSA, an odds ratio (OR) of 2.67 (95% confidence interval [CI]: 1.87, 3.80). In separate adjusted models, both BMI and neck circumference, but not waist circumference, were associated with OSA, OR of 2.06 (1.71, 2.47) and 1.55 (1.18, 2.05) per SD change in BMI and neck circumference, respectively. Hypertension, but not diabetes was associated with OSA in the unadjusted analysis; however, this association attenuated and was no longer significant after adjustments. Of the data on sleep symptoms and sleep patterns, habitual snoring but not excessive daytime sleepiness, insomnia symptoms nor actigraphy-based sleep (duration, efficiency) was associated with OSA after adjusting for covariates, OR of 1.94 (1.37, 2.75).
Table 2.
Unadjusted and adjusted odds ratio (95% CI) of sleep apnea (AHI4P ≥ 15) in Jackson Heart Sleep Study, 2012–2016
| Variable | Total N = 852 | Men N = 290 | Women N = 562 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Unadjusted model | Adjusted model† | Unadjusted model | Adjusted model‡ | Unadjusted model | Adjusted model‡ | |||||||
| OR | 95% CI | OR | 95% CI | OR | 95% CI | OR | 95% CI | OR | 95% CI | OR | 95% CI | |
| Demographics | ||||||||||||
| Age§ (1 SD) | 1.13 | 0.96, 1.32 | 1.43*** | 1.19, 1.71 | 1.19 | 0.93, 1.53 | 1.49*** | 1.12, 1.99 | 1.14 | 0.92, 1.40 | 1.39*** | 1.10, 1.75 |
| Sex (Male vs. Female) | 1.88*** | 1.36, 2.60 | 2.67*** | 1.87, 3.80 | — | — | — | — | — | — | — | — |
| Education (1–4) | 1.05 | 0.89, 1.23 | 1.17* | 0.98, 1.41 | 0.96 | 0.73, 1.25 | 1.10 | 0.83, 1.46 | 1.09 | 0.89, 1.35 | 1.23* | 0.98, 1.55 |
| Family income ($10000) | 1.03 | 0.98, 1.08 | 1.04 | 0.99, 1.10 | 1.00 | 0.92, 1.08 | 1.02 | 0.94, 1.11 | 1.02 | 0.95, 1.10 | 1.07 | 0.99, 1.15 |
| Employed (Yes vs. No) | 0.86 | 0.63, 1.19 | 1.04 | 0.69, 1.55 | 0.56** | 0.34, 0.94 | 0.54* | 0.28, 1.01 | 1.05 | 0.69, 1.59 | 1.64* | 0.96, 2.79 |
| Anthropometry | ||||||||||||
| BMI§ (1 SD) | 1.70*** | 1.45, 2.00 | 2.06*** | 1.71, 2.47 | 1.92*** | 1.45, 2.54 | 2.17*** | 1.60, 2.95 | 1.80*** | 1.47, 2.23 | 1.98*** | 1.58, 2.48 |
| Waist circumference§ (1 SD) | 1.86*** | 1.57, 2.21 | 1.26 | 0.94, 1.70 | 1.92*** | 1.45, 2.54 | 1.13 | 0.61, 2.09 | 1.81*** | 1.46, 2.24 | 1.26 | 0.89, 1.78 |
| Neck circumference§ (1 SD) | 1.96*** | 1.65, 2.33 | 1.55*** | 1.18, 2.05 | 2.02*** | 1.50, 2.72 | 1.53** | 1.00, 2.33 | 1.75*** | 1.41, 2.17 | 1.36** | 1.04, 1.78 |
| Comorbidities | ||||||||||||
| Hypertension (Yes vs. No) | 2.17*** | 1.25, 3.77 | 1.69* | 0.94, 3.05 | 2.35** | 1.12, 4.91 | 1.52 | 0.69, 3.35 | 2.54** | 1.07, 6.03 | 1.83 | 0.74, 4.54 |
| Diabetes (Yes vs. No) | 1.35* | 0.95, 1.93 | 1.20 | 0.83, 1.76 | 2.06** | 1.16, 3.64 | 1.72* | 0.94, 3.14 | 1.11 | 0.70, 1.77 | 1.11 | 0.64, 1.91 |
| Sleep symptoms | ||||||||||||
| Habitual snoring (Yes vs. No) | 1.89*** | 1.37, 2.60 | 1.94*** | 1.37, 2.75 | 1.84** | 1.11, 3.06 | 1.96** | 1.13, 3.41 | 1.91*** | 1.25, 2.92 | 1.94*** | 1.24, 3.04 |
| Daytime sleepiness (ESS 10 vs ESS ≤ 10) |
1.13 | 0.77, 1.67 | 1.08 | 0.71, 1.63 | 1.02 | 0.56, 1.85 | 1.04 | 0.56, 1.96 | 1.16 | 0.69, 1.94 | 1.11 | 0.64, 1.91 |
| Actigraphy-based sleep | ||||||||||||
| Sleep duration (minute) | 1.00 | 1.00, 1.00 | 1.00 | 1.00, 1.00 | 1.00 | 1.00, 1.00 | 1.00 | 1.00, 1.00 | 1.00 | 1.00, 1.00 | 1.00 | 1.00, 1.00 |
| Sleep efficiency (%) | 0.96** | 0.93, 0.99 | 0.97 | 0.94, 1.01 | 0.96* | 0.91, 1.00 | 0.96 | 0.91, 1.01 | 0.96 | 0.92, 1.01 | 0.98 | 0.94, 1.03 |
| Insomnia (WHIIRS ≥10 vs. WHIIRS <10) |
0.87 | 0.59, 1.29 | 1.08 | 0.71, 1.63 | 0.94 | 0.48, 1.83 | 1.09 | 0.53, 2.23 | 0.92 | 0.56, 1.53 | 1.06 | 0.64, 1.77 |
SD = standard deviation.
Adjusted model for BMI included age and sex. The bold values indicate the estimates that were statistically significant.
†Adjusted for age, sex, and BMI.
‡Adjusted for age and BMI.
§Standardized variable.
*p < 0.10; ** p < 0.05; ***p < 0.01.
Sex-stratified results are shown given the reported sex differences for OSA prevalence and risk factors in other populations (Table 2) [18]. There was an adjusted significant interaction between sex and employment, pinteraction = 0.02. The stratified analysis showed that the age- and BMI-adjusted odds of OSA tended to be lower for employed men, OR of 0.54 (0.28, 1.01) but higher for employed women, OR of 1.64 (0.96, 2.79). Sex-stratified associations with AHI3P are shown in Supplementary Table S3.
Undiagnosed OSA
Of the 23.6% of the study sample with OSA (AHI ≥ 15), only 5% reported a physician diagnosis of OSA; in other words, 95% of individuals with OSA were undiagnosed. Among those with an AHI4P > 15, the prevalence of a physician diagnosis was 4.5 and 5.8% among those with ESS > 10 vs. ≤10.
Prevalence using the AHI3P and an AHI ≥ 5
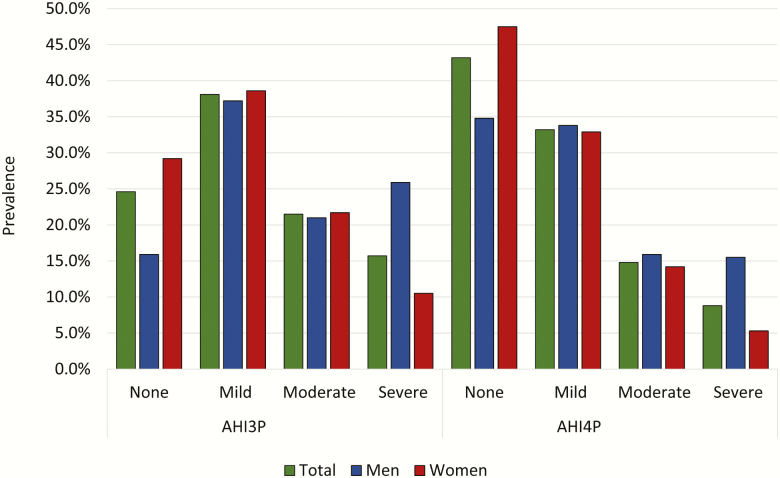
Prevalence of sleep apnea using an AHI ≥ 5 and AHI3P is shown in Supplementary Tables S4 and S5. As expected, the AHI3P and AHI4P were highly correlated, r = 0.72 (Supplementary Table S6) and had a κ coefficient of 0.69. The prevalence of moderate or more severe OSA (AHI ≥ 15) using the AHI3P was 37.2% (Figure 3). Of these, only 4% reported a physician diagnosis of OSA. Standardized estimates of prevalence of OSA based on an AHI4P ≥ 5 or AHI3P ≥ 15 are also shown in Supplementary Table S2. Associations with risk factors with AHI3P are shown in Supplementary Table S3.
Figure 3.
Total and sex-stratified prevalence of OSA by AHI3P and AHI4P.
Discussion
In this population of African Americans in the JHSS, we found a high prevalence of moderate or severe OSA, ranging from 23.6 to 37.2%, depending on the desaturation threshold used to define hypopneas. Furthermore, only 4%–5% with clinically significant levels of OSA reported a physician diagnosis of OSA. Consistent with the literature, OSA was more prevalent in men than women, with a 12%–15% higher prevalence in men, depending on the desaturation threshold used to define AHI. Although the trend test across ordinal age groups did not reveal a significant association between the prevalence of OSA and age groups, when age was analyzed as a continuous variable in a model adjusting for sex and BMI, older age was associated with higher odds of OSA. We observed a strong association of increasing BMI with OSA in both men and women. After adjusting for BMI, there was a significant association between neck, but not waist circumference in the entire sample and in men and women. Snoring, but not sleepiness, insomnia symptoms, or actigraphy measures of sleep duration or efficiency, was associated with OSA in both men and women.
The JHSS is a large study of African Americans aimed to understand sleep patterns and the role of sleep disorders in the health of this population. Insufficient sleep and sleep difficulties were common among our population. Unrecognized OSA was extremely prevalent, with 95% of participants with an AHI ≥ 15 reporting no prior history of diagnosed OSA. Short sleep duration affected 61% of participants, which is higher than observed in other studies with actigraphy among African Americans [3]. Almost a third of the population had low sleep efficiency and reported daytime sleepiness. Insomnia symptoms were also common, affecting almost 22% of the sample. However, insomnia and sleep patterns by actigraphy were not associated with OSA. There was a clear burden of sleep deficiency and sleep disorders among the population. The weak inter-relationships among the measured sleep parameters suggest that there are likely multiple factors that negatively affect sleep health in this population. Although some studies suggest that insomnia and OSA frequently overlap, particularly in women [31], we did not observe such a pattern in this population. The majority of research pertaining to OSA has been conducted among non-Hispanic white populations. Although data are limited and mixed, some prior studies have shown that African Americans have a high prevalence of OSA [3, 13, 14, 32]. Thus, it is important to understand the factors that contribute to this high burden. Limited data are available from prior studies of African Americans for comparison with our results. However, among participants in MESA (N = 2230) with an average age of 68, 30.3% had an AHI ≥ 15, which is similar to the distribution observed in the current study [3]. In the same study, Chen et al. reported that 84% of African American participants with polysomnography-defined OSA were undiagnosed [3]. In contrast, in the current study, we found a higher proportion (95%) of undiagnosed OSA among those with clinically significant OSA levels, and thus a higher proportion of individuals with untreated OSA. Study differences may be attributable to sociodemographic and/or geographic differences between the cohorts. Overall, however, the findings of prior studies are consistent with our own observation of a high prevalence of undiagnosed OSA among African Americans, emphasizing the need to understand the contributing factors.
Given the paucity of data addressing OSA in African Americans, we explored a variety of risk factors and correlates of OSA. Our findings were consistent with previous studies. For example, data from the Sleep Heart Health Study (predominately non-Hispanic white) demonstrated that male sex, age, BMI, neck girth, and snoring were independently related to OSA [33]. Habitual snoring strongly associates with OSA in both the prior and current studies. Snoring is commonly measured to assess OSA risk [33–35] and is reflective of dynamic changes in airway narrowing, a fundamental mechanism for OSA. Sleepiness, a symptom used to grade severity of OSA and reflective of sleep disruption, was unexpectedly not associated with OSA. Given the high prevalence of both sleepiness and short sleep duration, it is plausible that the specificity of sleepiness as a symptom for OSA was reduced in this sample of community-dwelling individuals. Despite the possibility that markers of disrupted sleep may correlate with OSA [36–38], we did not observe that either insomnia symptoms or actigraphy-based sleep duration or efficiency was associated with OSA after age, sex, and BMI. This also may be due to the multiple contributors to poor sleep in this sample. However, our findings on sleep symptoms and OSA are similar to prior research among Hispanics, which also demonstrated that snoring but not other sleep symptoms predicted OSA, which underscores the importance of habitual snoring as a marker of OSA [39].
In men and women, OSA prevalence increased in association with both increasing BMI and neck circumference. Furthermore, neck circumference but not waist circumference was associated with OSA even after adjusting for BMI. Although alternative OSA tools utilize either neck or waist circumference to predict individuals at high risk for OSA [40, 41], our findings suggest that among a generally obese African American adult sample, neck circumference has greater utility. Screening tools such as the NoSAS score [42] which incorporates neck circumference, BMI, snoring, age, and sex may perform better in identifying African Americans at risk for OSA and should be evaluated in this population.
After adjusting for age, sex, and BMI, we did not observe significant associations between OSA and hypertension or diabetes. However, the odds ratio for hypertension and diabetes in relationship to OSA in the current study was similar to the one observed in the Study of Latinos/Hispanic Community Health Study (SOL/HCHS) [19]. The significant association observed in SOL/HCHS is likely due to its larger sample size.
There is growing interest in sex differences in the prevalence and mechanisms of OSA. We observed a narrower difference in the prevalence of OSA between men and women (12%–15%) than reported previously. In the SOL/HCHS, the prevalence of OSA was approximately 30%–40% higher in men than in women, even among those older than 50 years of age [19]. Differences in the sex-stratified prevalence rates between the prior study and current study are likely due to the older age (predominately postmenopausal women) and higher BMI in the current study and may also be attributable to the racial/ethnic composition. Snoring was similarly associated with OSA in men and women, which is consistent with studies of other samples [43]. An association between diabetes and OSA appeared to be stronger in men than in women, but the interaction test was not significant. This result contrasts with a prior study (predominantly non-Hispanic white), which reported an association between OSA and diabetes in women, but not men; this may reflect differences due to ethnicity or other, unidentified factors [44]. Additional research is needed to clarify whether associations between cardiometabolic disease and OSA differ by sex.
To the best of our knowledge, this is the first study of its size to conduct objective OSA testing and administer validated questionnaires in a sample of African Americans. Study strengths included the availability of a comprehensive set of anthropometric, physiological, and sleep (including 7 day actigraphy) measurements for analysis. We scored hypopneas using alternative definitions, allowing us to explore the impact of AHI definition on prevalence rates and associations. The study also has several limitations. Use of a type 3 sleep apnea monitor precluded assessment of sleep stages; furthermore, these devices may underestimate AHI by 10 to 15% [23]. However, we edited sleep time using a validated approach that minimizes misclassification [23]. The sensitivity and specificity of the ESS may be limited among individuals with a high prevalence of short sleep duration and who may have had sleepiness for many years and thus may not adequately recognize this as a problem. Our population of African Americans in Jackson, MS may not be representative of others in the United States, due to geographic or cultural differences. The findings of this paper may not generalize to populations of lower education; fifty-four% of our sample has a college degree. It is important to consider that OSA prevalence rates will vary over time and across cohorts according to the sensors used to detect respiratory events (thermal sensors vs. nasal pressure) as well as the definition of hypopneas (e.g. level of desaturation). This is evidenced by the difference in prevalence rates (37.2% and 23.6%) observed in the current study when using a 3 or 4% definition, respectively. Nevertheless, it is evident that there is a substantial burden of OSA, predominantly undiagnosed and untreated, in this population, and a critical need for interventions to increase screening and treatment.
Conclusions
In conclusion, we found a high prevalence of OSA among a large population of African American men and women, the majority (95%) of whom were undiagnosed and untreated. Although male sex, BMI, neck circumference, and snoring were associated with OSA, neither sleepiness nor waist circumference (after adjusting for BMI) was associated with OSA. Our results suggest that screening tools [42] that incorporate information on neck rather than waist circumference and snoring more so than sleepiness may perform better in some community-dwelling African American populations. Moreover, future-screening procedures may need to more directly assess OSA (e.g. using oximetry or other objective testing) and be utilized in populations of men and women with high rates of overweight/obesity, including under-represented minority groups. Given the known association between OSA and poor health outcomes [45], intervention studies are needed to identify improved ways to screen and diagnose individuals, and evaluate the impact of OSA treatment on health outcomes and health disparities.
Supplementary Material
Supplementary material is available at SLEEP online.
Acknowledgments
The authors would like to thank the investigators and participants of the Jackson Heart Study for their valuable support and contributions.
Funding
Research reported in this publication was supported by the National Heart, Lung, and Blood Institute (NHLBI) R01HL110068, 3R01HL110068-03S2; T32HL007901-18; and K01HL138211. Dr. Wilson was supported by U54GM115428 from the National Institute of General Medical Sciences. Dr. Redline was supported in part by 5R35HL135818. This work was also conducted with support from Harvard Catalyst | The Harvard Clinical and Translational Science Center (National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health Award UL1 TR001102) and financial contributions from Harvard University and its affiliated academic healthcare centers. The content is solely the responsibility of the authors and does not necessarily represent the official views of Harvard Catalyst, Harvard University and its affiliated academic healthcare centers, or the National Institutes of Health. The Jackson Heart Study (JHS) is supported and conducted in collaboration with Jackson State University (HHSN268201300049C and HHSN268201300050C), Tougaloo College (HHSN268201300048C), and the University of Mississippi Medical Center (HHSN268201300046C and HHSN268201300047C) contracts from the NHLBI and the National Institute for Minority Health and Health Disparities (NIMHD). The authors also wish to thank the staffs and participants of the JHS. The views expressed in this manuscript are those of the authors and do not necessarily represent the views of the National Heart, Lung, and Blood Institute; the National Institutes of Health; or the US Department of Health and Human Services.
Conflict of interest statement. None declared.
References
- 1. Lee W, et al. . Epidemiology of obstructive sleep apnea: a population-based perspective. Expert Rev Respir Med. 2008;2(3):349–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Peppard PE, et al. . Increased prevalence of sleep-disordered breathing in adults. Am J Epidemiol. 2013;177(9):1006–1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Chen X, et al. . Racial/ethnic differences in sleep disturbances: the Multi-Ethnic Study of Atherosclerosis (MESA). Sleep. 2015;38(6):877–888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Rosen RC, et al. . Low rates of recognition of sleep disorders in primary care: comparison of a community-based versus clinical academic setting. Sleep Med. 2001;2(1):47–55. [DOI] [PubMed] [Google Scholar]
- 5. Altevogt BM, et al. (eds). Sleep Disorders and Sleep Deprivation: An Unmet Public Health Problem. Washington, DC: National Academies Press; 2006. [PubMed] [Google Scholar]
- 6. Kapur V, et al. . Underdiagnosis of sleep apnea syndrome in U.S. communities. Sleep Breath. 2002;6(2):49–54. [DOI] [PubMed] [Google Scholar]
- 7. Young T, et al. . Risk factors for obstructive sleep apnea in adults. JAMA. 2004;291(16):2013–2016. [DOI] [PubMed] [Google Scholar]
- 8. Young T, et al. . Epidemiological insights into the public health burden of sleep disordered breathing: sex differences in survival among sleep clinic patients. Thorax. 1998;53 (Suppl 3):S16–S19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Young T, et al. . Estimation of the clinically diagnosed proportion of sleep apnea syndrome in middle-aged men and women. Sleep. 1997;20(9):705–706. [DOI] [PubMed] [Google Scholar]
- 10. Jackson ML, et al. . Cognition and daytime functioning in sleep-related breathing disorders. Prog Brain Res. 2011;190:53–68. [DOI] [PubMed] [Google Scholar]
- 11. Newman AB, et al. . Daytime sleepiness predicts mortality and cardiovascular disease in older adults. The Cardiovascular Health Study Research Group. J Am Geriatr Soc. 2000;48(2):115–123. [DOI] [PubMed] [Google Scholar]
- 12. Marin JM, et al. . Long-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure: an observational study. Lancet. 2005;365(9464):1046–1053. [DOI] [PubMed] [Google Scholar]
- 13. Jean-Louis G, et al. . Evaluation of sleep apnea in a sample of black patients. J Clin Sleep Med. 2008;4(5):421–425. [PMC free article] [PubMed] [Google Scholar]
- 14. Redline S, et al. . Racial differences in sleep-disordered breathing in African-Americans and caucasians. Am J Respir Crit Care Med. 1997;155(1):186–192. [DOI] [PubMed] [Google Scholar]
- 15. Wagner DR, et al. . Measures of body composition in blacks and whites: a comparative review. Am J Clin Nutr. 2000;71(6):1392–1402. [DOI] [PubMed] [Google Scholar]
- 16. Mensah GA. Eliminating disparities in cardiovascular health: six strategic imperatives and a framework for action. Circulation. 2005;111(10):1332–1336. [DOI] [PubMed] [Google Scholar]
- 17. National Research Council, Committee on Population. Understanding Racial and Ethnic Differences in Health in Late Life: A Research Agenda. Washington, DC: National Academies Press; 2004. [PubMed] [Google Scholar]
- 18. Lin CM, et al. . Gender differences in obstructive sleep apnea and treatment implications. Sleep Med Rev. 2008;12(6):481–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Redline S, et al. . Sleep-disordered breathing in Hispanic/Latino individuals of diverse backgrounds. The Hispanic Community Health Study/Study of Latinos. Am J Respir Crit Care Med. 2014;189(3):335–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Fuqua SR, et al. . Recruiting African-American research participation in the Jackson Heart Study: methods, response rates, and sample description. Ethn Dis. 2005;15 (4 Suppl 6):S6–S18. [PubMed] [Google Scholar]
- 21. Oldenburg O, et al. . Cardiorespiratory screening for sleep-disordered breathing. Eur Respir J. 2006;28(5):1065–1067. [DOI] [PubMed] [Google Scholar]
- 22. Dingli K, et al. . Evaluation of a portable device for diagnosing the sleep apnoea/hypopnoea syndrome. Eur Respir J. 2003;21(2):253–259. [DOI] [PubMed] [Google Scholar]
- 23. Zhao YY, et al. . Effect of manual editing of total recording time: implications for home sleep apnea testing. J Clin Sleep Med. 2017;13(1):121–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Morgenthaler T, et al. ; Standards of Practice Committee; American Academy of Sleep Medicine. Practice parameters for the use of actigraphy in the assessment of sleep and sleep disorders: an update for 2007. Sleep. 2007;30(4):519–529. [DOI] [PubMed] [Google Scholar]
- 25. Cole RJ, et al. . Automatic sleep/wake identification from wrist activity. Sleep. 1992;15(5):461–469. [DOI] [PubMed] [Google Scholar]
- 26. Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991;14(6):540–545. [DOI] [PubMed] [Google Scholar]
- 27. Levine DW, et al. . Reliability and validity of the women’s health initiative insomnia rating scale. Psychol Assess. 2003;15(2):137–148. [DOI] [PubMed] [Google Scholar]
- 28. Muntner P, et al. . Potential U.S. population impact of the 2017 ACC/AHA high blood pressure guideline. J Am Coll Cardiol. 2018;71(2):109–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Association AD. Screening for type 2 diabetes. Diabetes Care. 2004;27(1):s11–s14. [DOI] [PubMed] [Google Scholar]
- 30. Kapur VK, et al. . Clinical practice guideline for diagnostic testing for adult obstructive sleep apnea: an American Academy of Sleep Medicine Clinical Practice Guideline. J Clin Sleep Med. 2017;13(3):479–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Krell SB, et al. . Insomnia complaints in patients evaluated for obstructive sleep apnea. Sleep Breath. 2005;9(3):104–110. [DOI] [PubMed] [Google Scholar]
- 32. Ancoli-Israel S, et al. . Sleep-disordered breathing in African-American elderly. Am J Respir Crit Care Med. 1995;152(6 Pt 1):1946–1949. [DOI] [PubMed] [Google Scholar]
- 33. Young T, et al. ; Sleep Heart Health Study Research Group. Predictors of sleep-disordered breathing in community-dwelling adults: the sleep heart health study. Arch Intern Med. 2002;162(8):893–900. [DOI] [PubMed] [Google Scholar]
- 34. Young T, et al. . Epidemiology of obstructive sleep apnea: a population health perspective. Am J Respir Crit Care Med. 2002;165(9):1217–1239. [DOI] [PubMed] [Google Scholar]
- 35. Pang KP, et al. . Screening for obstructive sleep apnea: an evidence-based analysis. Am J Otolaryngol. 2006;27(2):112–118. [DOI] [PubMed] [Google Scholar]
- 36. Luyster FS, et al. . Comorbid insomnia and obstructive sleep apnea: challenges for clinical practice and research. J Clin Sleep Med. 2010;6(2):196–204. [PMC free article] [PubMed] [Google Scholar]
- 37. Eckert DJ, et al. . Arousal from sleep: implications for obstructive sleep apnea pathogenesis and treatment. J Appl Physiol (1985). 2014;116(3):302–313. [DOI] [PubMed] [Google Scholar]
- 38. Eckert DJ, et al. . Defining phenotypic causes of obstructive sleep apnea. Identification of novel therapeutic targets. Am J Respir Crit Care Med. 2013;188(8):996–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Shah N, et al. . Sex-specific prediction models for sleep apnea from the Hispanic Community Health Study/Study of Latinos. Chest. 2016;149(6):1409–1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Nagappa M, et al. . Validation of the STOP-bang questionnaire as a screening tool for obstructive sleep apnea among different populations: a systematic review and meta-analysis. PLoS One. 2015;10(12):e0143697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Chai-Coetzer CL, et al. . A simplified model of screening questionnaire and home monitoring for obstructive sleep apnoea in primary care. Thorax. 2011;66(3):213–219. [DOI] [PubMed] [Google Scholar]
- 42. Marti-Soler H, et al. . The NoSAS score for screening of sleep-disordered breathing: a derivation and validation study. Lancet Respir Med. 2016;4(9):742–748. [DOI] [PubMed] [Google Scholar]
- 43. Young T, et al. . The gender bias in sleep apnea diagnosis. Are women missed because they have different symptoms?Arch Intern Med. 1996;156(21):2445–2451. [PubMed] [Google Scholar]
- 44. Newman AB, et al. ; Sleep Heart Health Study Research Group. Relation of sleep-disordered breathing to cardiovascular disease risk factors: the sleep heart health study. Am J Epidemiol. 2001;154(1):50–59. [DOI] [PubMed] [Google Scholar]
- 45. Golbidi S, et al. . Cardiovascular consequences of sleep apnea. Lung. 2012;190(2):113–132. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.