Significance
The pupil regulates the amount of light entering the eyes to optimize visual sensitivity and sharpness. The visual system selects objects of interest for future fixation, and pupil size can be adjusted for object luminance before fixation. This study demonstrates that the intermediate layers of the superior colliculus (SC), a phylogenetically conserved structure for controlling eye movements and spatial attention, coordinates this predictive pupil response. By manipulating intermediate SC (SCi) excitability via microstimulation and lidocaine microinjection, we show that, although global luminance remained unchanged, pupil size was modulated by local luminance at the next fixated location. These results highlight a causal role of the SCi to prepare the pupil for local luminance conditions at the saccadic goal.
Keywords: superior colliculus, attention, saccade preparation, inactivation, microstimulation
Abstract
Spatial attention enables us to focus visual processing toward specific locations or stimuli before the next fixation. Recent evidence has suggested that local luminance at the spatial locus of attention or saccade preparation influences pupil size independent of global luminance levels. However, it remains to be determined which neural pathways produce this location-specific modulation of pupil size. The intermediate layers of the midbrain superior colliculus (SC) form part of the network of brain areas involved in spatial attention and modulation of pupil size. Here, we demonstrated that pupil size was altered according to local luminance level at the spatial location corresponding to a microstimulated location in the intermediate SC (SCi) map of monkeys. Moreover, local SCi inactivation through injection of lidocaine reversed this local luminance modulation. Our findings reveal a causal role of the SCi in preparing pupil size for local luminance conditions at the next saccadic goal.
When bombarded by excessive input from the environment, observers can selectively prioritize objects, ignore most stimuli, and move their eyes to selected objects of interest. Processing of a selected object can start before movement of the eyes to foveate the object, enabling faster and more efficient processing of information at the location of saccade goal and attention locus than at other locations (1, 2). Objects in the natural environment often have levels of luminance that are independent of global luminance. Pupil size changes according to the amount of light projected onto the retina, with constriction in response to global luminance increase and dilation to global luminance decrease (3) (referred to as global luminance modulation) to regulate the trade-off between image acuity and sensitivity (4, 5). If processing of a visual object starts during the planning of the saccade to foveate that object, these preparatory processes could also modulate pupil size to optimize the quality of the to-be-foveated image. This compelling idea, referred to as local luminance modulation, receives support from recent investigations. When simultaneously presented with bright and dark circular patch stimuli in the periphery, human subjects have smaller pupil size when the locus of spatial attention is guided to the bright, compared with the dark, patch stimulus, despite the fact that global luminance and eye position remains unchanged (6–9). The neural mechanisms underlying this preparatory local luminance modulation remain poorly understood.
The superior colliculus (SC), a subcortical sensorimotor structure, has anatomically differentiated superficial and intermediate layers. The intermediate SC (SCi) receives direct projections from the frontal eye field (FEF) and the lateral intraparietal cortex (LIP) and projects directly to the brainstem and the spinal cord to execute the orienting movement, including saccades and pupil dilation (10, 11). The SCi, together with the FEF and LIP, has been causally implicated in the shifts of gaze and spatial attention (12–15). SCi activity increases, before saccade initiation, in the neurons coding the next saccade vector (16, 17), and the sources of these preparatory signals are likely the FEF and LIP (18–20). Electrical microstimulation in the SCi triggers saccades and biases attention toward corresponding stimulated locations in the SCi saccade map (21–23). Recent research has shown that pupil light responses evoked by a bright stimulus are enhanced when it is presented at the location corresponding to the area where FEF microstimulation is applied (24), further linking this circuit to the modulation of pupil luminance responses (25). Given the role of the SCi in saccade planning and spatial attention and the link between the SCi and pupil control (11), we hypothesize that observers not only shift their attention to a potential to-be-foveated location but also scale pupil size to optimize visual processing at the next fixation target, and that the SCi plays an important role in mediating these location-specific preparatory pupil responses.
To establish a causal role for the SCi in mediating the local luminance modulation of pupil size, we altered SCi activity locally with electrical microstimulation or chemical microinjection of lidocaine to respectively enhance or depress neural activity in a localized region. Weak electrical microstimulation below the threshold for triggering any saccades or pupil dilation (26) and injection of lidocaine, a Na+ chemical blocker that temporarily depresses neural activity in the region of the injection, were used. Two visual stimuli, small bright and dark luminance patches, were presented simultaneously in opposite hemifields: one spatially aligned with the corresponding site where we altered SCi activity and the other at the opposite location relative to fixation. We hypothesize that microstimulation will enhance (27), whereas lidocaine injection will reduce, visuospatial processing toward the corresponding location, resulting in the alternation of an interaction with local luminance modulation.
Results
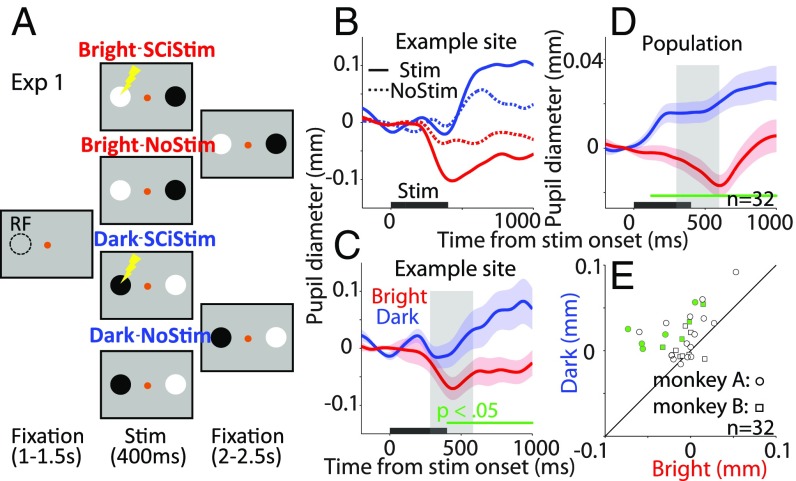
If the local pupil luminance responses are mediated through the SCi, microstimulation should alter pupil size according to local luminance changes at the spatial location coded by the stimulated site in the SCi. To demonstrate this effect, monkeys were trained to fixate upon a spot in the center of a computer monitor while task-irrelevant bright and dark patch stimuli were presented in opposite hemifields (Fig. 1A; detailed in Methods). On 50% of the trials, we delivered a subthreshold train of microstimulation (400 ms, 70–90 Hz) to a specific site in the SCi. One patch stimulus was presented at the location corresponding to the stimulated site in the SC map, and the other was presented at the opposite location. To isolate the pupil local luminance responses, stimulation parameters were set below saccade and pupil initiation thresholds (detailed in Methods).
Fig. 1.
Effect of SCi microstimulation on local luminance modulation. (A) Trials began with appearance of a central fixation point on a gray background. Subthreshold microstimulation was applied for 400 ms on 50% of trials, with the presentation of the patch stimuli. (B and C) Effects from (B) an example site and (C) after normalization between microstimulation and no-stimulation trials in the bright and dark conditions. (D and E) Summary of microstimulation effects collapsed across monkeys and stimulation sites (n = 32) on (D) pupil dynamics and (E) size (300–600-ms epoch poststimulation, gray shading in C and D). In B–D, the black bar on the x-axis indicates the time of microstimulation. In C and D, the shaded colored regions surrounding the pupillary response represent ±SE range for different conditions. The green bar on the x-axis indicates the time line at which differences between the bright and dark conditions were statistically significant (P < 0.05). In E, filled dots indicate sites with statistically significant differences (P < 0.05). RF, response field.
Experiment 1: Pupil Size Modulated by Local Luminance at the Microstimulated Location.
When subthreshold microstimulation was applied to the SCi, the resulting effect on pupil size was modulated by patch location (Fig. 1B). Transient pupillary responses were evoked by the presentation of patch stimuli alone (Fig. 1B, dashed traces), with a decrease in pupil size (i.e., constriction) at approximately 250 ms after presentation, followed by an increase in pupil size (i.e., dilation). Although the pupillary responses were modestly different between the bright and dark conditions, microstimulation exaggerated the pupillary responses evoked by the patch stimuli (Fig. 1B, solid traces). There was greater pupil constriction when the bright patch overlapped the microstimulation site and greater dilation when the dark patch was aligned with the stimulated site. Differences between the bright and dark patch conditions on no-stimulation trials could be results of the influence of microstimulation on 50% of the trials. To eliminate this bias, we normalized the data by contrasting pupil diameter values between the stimulation and no-stimulation conditions (SI Appendix). Fig. 1C shows the normalized pupil diameter in the bright and dark conditions at the same example stimulation site, demonstrating significantly smaller pupil size in the bright compared with the dark condition (significant differences denoted by a green horizontal bar in Fig. 1C). An epoch of 300–600 ms after stimulation onset (highlighted in gray area; Fig. 1C) was used to characterize transient differences between the two conditions (bright, mean, −0.056 ± 0.019; dark, mean, 0.0022 ± 0.024; t19 = 1.86, P = 0.039, d = 0.85). This pattern of results was significant in the population average of 32 stimulation sites from 2 monkeys (Fig. 1D; original and normalized population data from each monkey are shown in SI Appendix, Fig. S1). Immediately after microstimulation, pupil size decreased when the bright patch overlapped with the stimulated location and increased when the dark patch overlapped with the stimulated location (significant differences between two conditions denoted by a green horizontal bar in Fig. 1D; mean pupil size from 300 ms to 600 ms, bright, −0.0102 ± 0.0047; dark, 0.017 ± 0.0045; t31 = 5.53, P = 4.7 × 10−6, d = 1.04). Almost all stimulation sites in our sample (26 of 32) produced numerically smaller pupil sizes in the bright condition than in the dark condition (300–600-ms epoch, significant differences in 9 of 32 stimulation sites; Fig. 1E). Although the effects observed were relatively small and not significant in all stimulation sites, likely because of implementation of very weak microstimulation and goal-irrelevance of patch stimuli, these results nonetheless support our hypothesis: the alteration of pupil size changed according to local luminance at the SCi stimulated location.
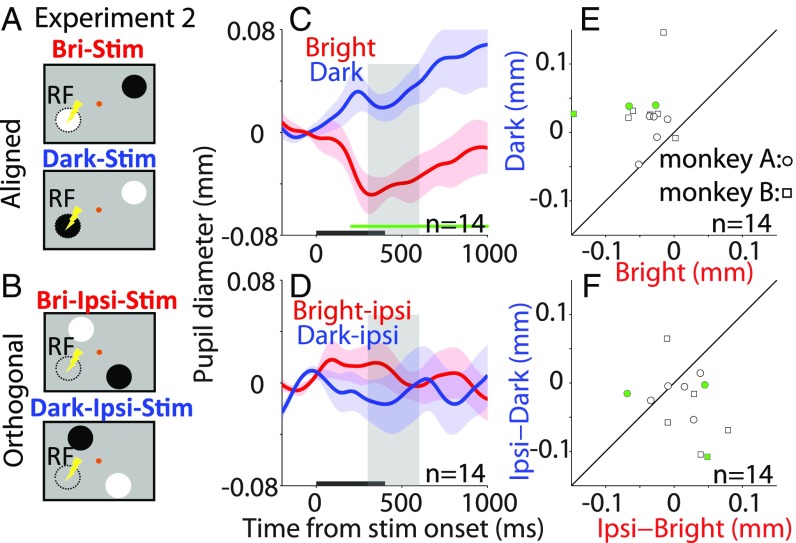
Experiment 2: Local Luminance Modulation Was Spatially Confined to the Microstimulated Location.
To further investigate the spatial specificity between the patch luminance effect and stimulation location, we conducted a follow-up experiment. More specifically, we tested whether the patch luminance effect is present only when the patch and stimulation locations align, or if this effect can still be generated as long as the patch and stimulation location are within the same hemifield. We introduced four configurations of the patch stimuli relative to the SCi stimulation site: the patch stimuli were presented aligned and opposite the SCi stimulation site (same as experiment 1; Fig. 2A) or orthogonal to this configuration (Fig. 2B). Because the orthogonal patch stimuli did not match with the corresponding stimulated site in the SCi, we predicted no systematic pupillary modulation. Consistent with the previous results, pupil constriction was observed after microstimulation when the bright patch spatially overlapped with the corresponding stimulated site, and pupil dilation was observed when the dark patch spatially overlapped with the stimulated site (Fig. 2 C and E: two-sided t test, mean pupil size from 300 ms to 600 ms, bright, −0.042 ± 0.011; dark, 0.026 ± 0.01; t13 = 4.77, P = 3.7 × 10−4, d = 1.68; SI Appendix, Fig. S2 shows results in individual monkeys). However, in the orthogonal condition, the pupillary responses were not different when the bright or dark patch was presented ipsilateral or contralateral to the stimulation site (two-sided t test, mean pupil size from 300 ms to 600 ms poststimulation, same example site, bright, 0.0072 ± 0.013; dark, −0.011 ± 0.023; t13 = 0.58, P = 0.57, d = 0.27; Fig. 2 D and F). Together, these results suggest that local luminance modulation was spatially confined to the corresponding stimulated location.
Fig. 2.
Effect of SCi microstimulation on different patch location conditions. (A and B) Patch stimuli were presented at the corresponding stimulated location (aligned) or orthogonal. (C and E) Effects of SCi microstimulation on the normalized pupil diameter in the bright and dark conditions collapsed across monkeys and stimulation sites (n = 14) on (C) pupil dynamics and (E) pupil size (300–600-ms epoch poststimulation). (D and F) Effects of SCi microstimulation on the normalized pupil diameter in the orthogonal condition collapsed across monkeys and stimulation sites on (D) pupil dynamics and (F) pupil size (300–600-ms epoch poststimulation). Symbols as in Fig. 1.
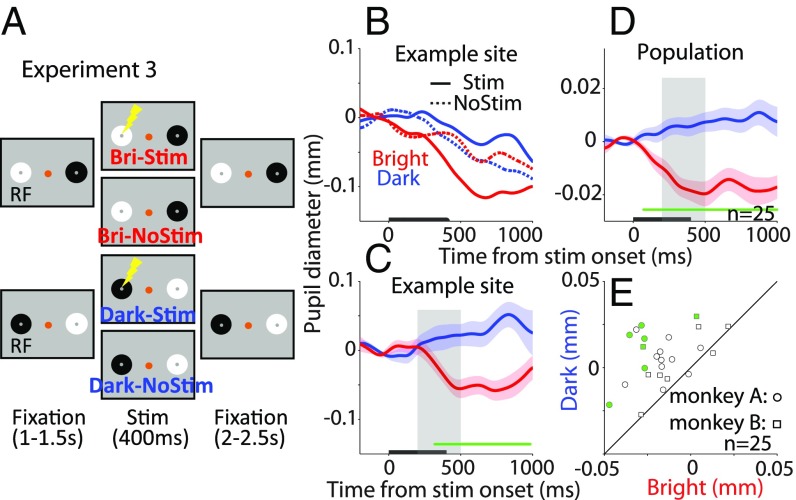
Experiment 3: Pupil Size Modulated by Local Luminance Independent of Transient Changes in the Visual Field.
The temporal overlap of the patch presentation and SCi microstimulation in experiments 1 and 2 may suggest that local luminance effect mediated via SCi microstimulation was only effective with sudden changes in the visual environment. To determine whether local luminance modulation by SCi microstimulation also occurs in the absence of simultaneous changes in the visual environment, we presented the patch stimuli 1,000–1,500 ms before applying SC microstimulation (Fig. 3A). Fig. 3 B and C show the effects of SCi microstimulation on pupil diameter in different patch conditions at an example stimulation site. Once again, microstimulation changed pupil size as a function of the patch condition: there was a decrease in pupil size when the bright patch was aligned with the stimulated site and an increase in pupil size when the dark patch was aligned with the stimulated site. Similar to previous results, we found smaller pupil sizes in the bright condition compared with the dark condition (t test, mean pupil size between 200 ms and 500 ms, bright, −0.027 ± 0.011; dark, 0.017 ± 0.016; t21 = 2.41, P = 0.013, d = 0.98).
Fig. 3.
Effect of SCi microstimulation on local luminance modulation. (A) Patch stimuli were presented before microstimulation. (B and C) Effects from (B) an example site and (C) after normalization between stimulation and no-stimulation trials in the bright and dark conditions. (D and E) Summary of microstimulation effects collapsed across monkeys and stimulation sites (n = 25) on (D) pupil dynamics and (E) pupil size (200–500-ms epoch poststimulation).
Fig. 3D summarizes the effects of SCi microstimulation on normalized pupil dynamics collapsed across 2 monkeys and 25 stimulation sites (SI Appendix, Fig. S3 shows original and normalized population data from each monkey; the differences in pupil size between the bright and dark conditions were insignificant on trials without microstimulation: mean pupil size from 200 ms to 500 ms, bright, −0.012 ± 0.0055; dark, −0.015 ± 0.0055; two-sided t test, t24 = 0.67, P = 0.51, d = 0.12). The effects of pupil local luminance responses through SCi microstimulation were evident, with pupil constriction after microstimulation when the bright patch overlapped with the SCi stimulated site and pupil dilation when the dark patch overlapped with the SCi stimulated site (significant differences between the two conditions, t test, P < 0.05 denoted by green horizontal bar in Fig. 3D; mean pupil size from 200 ms to 500 ms, bright, −0.016 ± 0.0034; dark, 0.0058 ± 0.003; t24 = 6.45, P = 1.1 × 10−6, d = 1.39; note that the timing of the selected epoch was earlier in this experiment because luminance signals were already available at the time of microstimulation). Almost all stimulation sites in our sample produced numerically smaller pupil size in the bright condition than in the dark condition (200–500 ms epoch, 23 of 25 stimulation sites; significant differences in 7 of 25 stimulation sites; Fig. 3E). These results are consistent with the previous microstimulation experiment (Fig. 1), suggesting that the mediation of the local luminance effect through the SCi does not require a transient change in the environment, but rather the onset of microstimulation at the SCi site aligned with the patch stimulus.
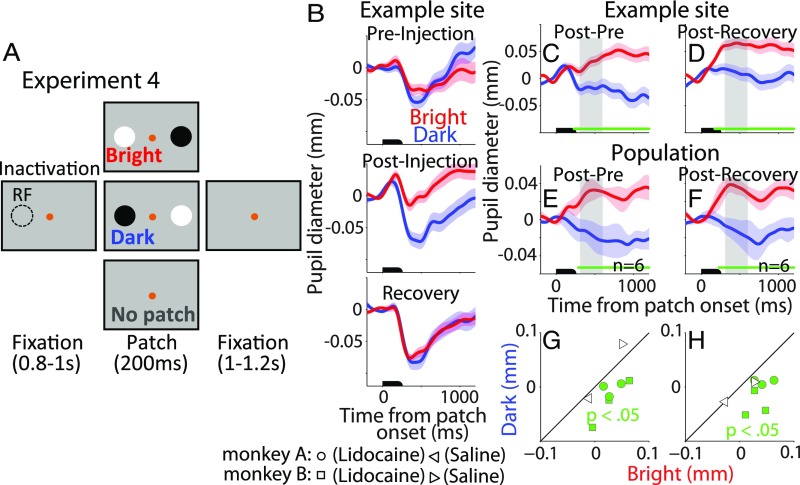
Experiment 4: Modulation of Pupil Local Luminance by Lidocaine Microinjection.
To further establish a causal role of the SCi in mediating the local luminance modulation, we microinjected small quantities of lidocaine (six sites) into the SCi of two monkeys to temporally inactivate a small portion of the SC (Materials and Methods). Lidocaine inactivates neural tissue through blocking sodium channels, and we hypothesized that this manipulation would produce the opposite effect of microstimulation. We examined performance before injection (preinjection), immediately after injection (postinjection), and after recovery from the injection. Effects of SC inactivation on saccade execution were investigated by presenting, on a subset of trials, a visual target in the region of the visual field that aligned with the SCi site. As demonstrated in earlier studies (28–30), the peak velocities of saccades made into the affected part of the visual field were slightly decreased after injecting small amounts of lidocaine (SI Appendix, Fig. S4A shows normalized mean peak velocity data; saccades made to the injection location, pre, 98 ± 0.53%; post, 90 ± 3.58%; recovery, 97 ± 1.63%; post and pre, t5 = 2.33, P = 0.034, d = 1.26; post and recovery, t5 = 1.86, P = 0.061, d = 0.99; saccades made to the opposite location, pre, 99 ± 0.38%; post, 95 ± 3%; recovery, 98 ± 2.87%; two-sided t test, post and pre, t5 = 1.24, P = 0.27, d = 0.62; post and recovery, t5 = 0.92, P = 0.39, d = 0.33).
Transient pupil responses were evoked by patch stimuli in the period of preinjection, postinjection, and recovery (Fig. 4B shows an example site). To directly examine the local luminance modulation, we normalized the data by contrasting pupil diameter values in the bright and dark conditions between the postinjection and preinjection or recovery period separately (SI Appendix). Fig. 4C shows the normalized pupil diameter (postinjection minus preinjection) in the bright and dark condition at the same example injection site, demonstrating significantly larger pupil size in the bright compared with the dark condition (significant differences between two conditions denoted by a green horizontal bar in Fig. 4C; epoch of 300–580 ms, highlighted in gray area, was used to characterize transient differences between the two conditions: mean size, bright, 0.027 ± 0.0093; dark, −0.018 ± 0.011; t76 = 3.09, P = 0.0014, d = 0.69). The normalized pupil diameter between the postinjection and recovery period at the same example injection site showed similar results (bright, 0.063 ± 0.0093; dark, 0.012 ± 0.011; t76 = 3.54, P = 3.4 × 10−4, d = 0.79; Fig. 4D). Moreover, this pattern of results was significant in the sample average of six injection sites from two monkeys (Fig. 4 E–H; SI Appendix, Fig. S4B shows data from each monkey population). After injection, pupil size between post and preinjection increased when the bright patch overlapped with the injected location, whereas pupil size decreased when the dark patch overlapped with the injected location (significant differences between two conditions denoted by a green horizontal bar in Fig. 4E; mean pupil size from 200 ms to 580 ms, bright, 0.027 ± 0.01; dark, −0.005 ± 0.016; t5 = 6.22, P = 7.8 × 10−4, d = 1.5). This pattern is opposite of microstimulation effect (compare Figs. 1–4). All injection sites in our sample produced statistically larger pupil sizes in the bright condition than in the dark condition (200–580-ms epoch, significant differences in six of six injection sites; Fig. 4G). Similar patterns of results were observed in the comparison of postinjection vs. recovery period, with significantly larger pupil size when the bright patch, but smaller pupil size when the dark patch, overlapped with the injected location (significant differences between two conditions denoted by a green horizontal bar in Fig. 4F; 200–580-ms epoch, bright, 0.027 ± 0.01; dark, −0.011 ± 0.0098; t5 = 4.46, P = 0.0033, d = 1.86, significant differences in six of six injection sites; Fig. 4H). These results could also be interpreted as spatial attention being biased toward the opposite location of the injection site as a result of inactivation of the SCi at the injection location, thus producing pupil dilation in the bright condition and constriction in the dark condition.
Fig. 4.
Effect of SCi inactivation on local luminance modulation. (A) Experimental paradigm. (B) Effects of lidocaine injection on pupil size following the patch presentation at an example site during preinjection, postinjection, or recovery period. (C and D) Effects of SCi inactivation on the normalized pupil diameter [differences between postinjection and preinjection (C) or recovery (D) trials] in the bright and dark condition at the same example site. (E and F) Summary of lidocaine injection effects collapsed across monkeys (n = 6) on (E) Post − Pre condition and (F) Post − Recovery condition. (G and H) Summary of injection effects on pupil size (300–580-ms epoch poststimulation), including two saline control injections (n = 2) on (G) postpre condition and (H) postrecovery condition. In B–F, the black bar on the x-axis indicates the time of patch stimuli. Symbols as in Fig. 1.
In the control experiment, a comparable volume of saline solution was injected into the SCi of two monkeys (two injections). Transient pupil responses between the bright and dark conditions after saline solution injection were not significantly different from preinjection or recovery responses (Fig. 4 G and H; monkey A, post and pre, bright, −0.011 ± 0.013; dark, −0.021 ± 0.013; t34 = 0.55, P = 0.58, d = 0.18; post and recovery, bright, −0.027 ± 0.013; dark, −0.027 ± 0.013; t34 = 0.004, P = 0.99, d = 0.0014; monkey B, post and pre, bright, 0.051 ± 0.014; dark, 0.079 ± 0.012; t73 = 1.58, P = 0.12, d = 0.36; post and recovery, bright, 0.027 ± 0.014; dark, 0.0092 ± 0.012; t73 = 0.98, P = 0.33, d = 0.22; SI Appendix, Fig. S4C shows saline solution injection data for each monkey), indicating that the reversed local luminance modulation depends on the injection of an inactivating substance into the SC. Overall, these results support our hypothesis that the SCi plays a causal role in mediating the local luminance modulation.
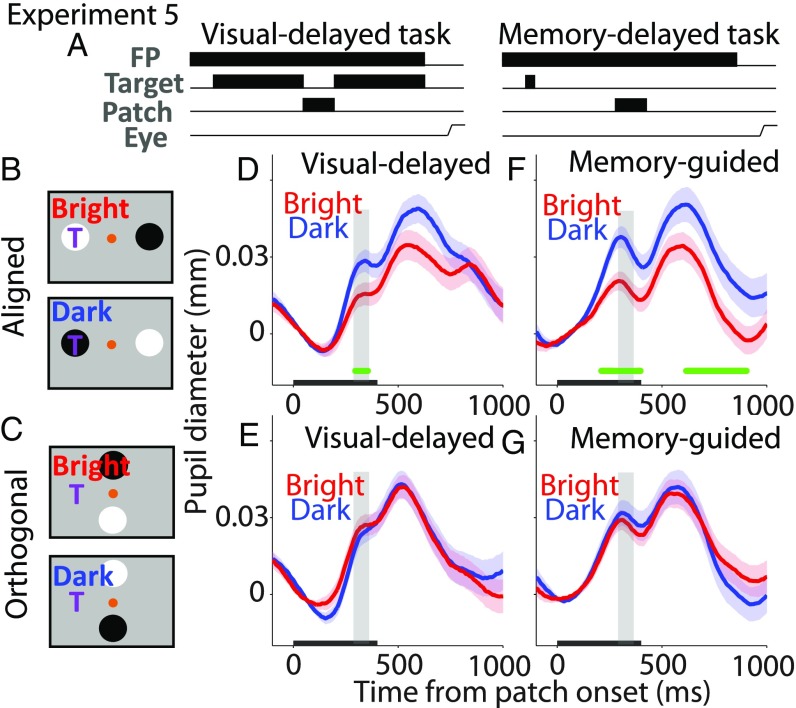
Experiment 5: Pupil Size Modulated by Local Luminance at the Saccade Goal.
To link the aforementioned local luminance modulation with human pupil local luminance responses observed during cognitive tasks (6, 7), monkeys were trained to perform a version of the visual-delayed and memory-guided saccade tasks that included presentation of patch stimuli during the delay period (Fig. 5A). Monkeys were required to prepare a saccade toward a visible or remembered target location (one of four possible locations in each block; two different blocks) but maintain central fixation until the disappearance of the fixation point, which instructed them to initiate a saccade to the target location. During the delay period, two task-irrelevant bright and dark patches were presented briefly. The patch stimuli were presented either aligned and opposite the saccade goal (Fig. 5B) or orthogonal to this configuration (Fig. 5C; similar to experiment 2; SI Appendix). Because neural activity in the SCi is sustained during the delay period (16, 31–33), we hypothesized that pupil size after the patch presentation should be smaller when the bright patch stimulus, compared with the dark patch stimulus, is spatially aligned with the future saccade goal. Moreover, because the orthogonal patch stimuli did not match with the saccade goal, we predicted no systematic pupillary modulation. Visual-delayed and memory-guided tasks were performed separately, and trials in each task were collapsed across two to five daily recording sessions. Similar local luminance modulations by the saccade goal were observed in both tasks across two monkeys (SI Appendix, Fig. S5 shows data from another monkey). As shown in an example monkey (Fig. 5 D and F), transient pupil responses after the patch presentation decreased significantly when the bright patch stimulus was presented at the saccade goal, compared with the dark patch stimulus, in the visual-delayed task (epoch of postpresentation 290–365 ms, selected arbitrarily to capture differences between two conditions, monkey A, t203 = 2.06, P = 0.041, d = 0.29; monkey B, t634 = 2.67, P = 0.0076, d = 0.21; SI Appendix, Fig. S5E) and the memory-guided task (epoch of postpresentation 290–365 ms, monkey A, t208 = 2.86, P = 0.0023, d = 0.4; monkey B, t386 = 2.26, P = 0.012, d = 0.23; SI Appendix, Fig. S5E). These local-luminance effects were consistent with the human behavioral effects showing smaller pupil size when the locus of attention overlaps with a bright patch compared with a dark patch (6, 7). Similar pupil responses were evoked after orthogonal patch stimuli in an example monkey (Fig. 5 E and G), but the differences between two orthogonal conditions (90° or 270° radial angles from target location) were indifferent (visual-delayed task, two-sided t test, epoch of postpresentation 290–365 ms, monkey A, t214 = 0.52, P = 0.6, d = 0.07; monkey B, t515 = 0.59, P = 0.55, d = 0.05; memory-guided task, two-sided t test, epoch of postpresentation 290–365 ms, monkey A, t210 = 0.49, P = 0.63, d = 0.067; monkey B, t438 = 1.64, P = 0.1, d = 0.16; SI Appendix, Fig. S5F). Note that, although pupil dynamics induced by the patch stimuli were different between two monkeys, the same local luminance modulation was observed.
Fig. 5.
Effect of saccade planning on local luminance modulation. (A–C) Visual-delayed and memory-guided saccade tasks. The bright (or dark) patch was spatially aligned with the target location in the bright (or dark) condition in B. Neither patch was aligned with the target location (orthogonal) in C. (D and F) Pupil responses from an example monkey in the bright and dark aligned conditions in (D) visual-delayed and (F) memory-guided saccade tasks. (E and G) Pupil responses from an example monkey in the bright and dark orthogonal conditions in (E) visual-delayed and (G) memory-guided saccade tasks. In B and C, “T” indicates the target location. In D–G, the black bar on the x-axis indicates the time of the patch presentation.
Discussion
The goal of this study was to investigate the role of the SCi in mediating pupil local luminance responses before movement of the eyes. We found that pupil size changed according to a luminance level at the location corresponding to the site where we applied weak microstimulation in the SCi, with constriction when the bright patch stimulus spatially matched with the stimulated site and dilation when the dark patch stimulus spatially matched with the stimulated site. Moreover, these observed effects were reversed when we injected lidocaine into the SCi to temporarily inactivate neurons in those regions. These results suggest that the SCi is not only causally involved in spatial attention and saccade preparation to bias visuospatial processing toward the selected location, but this bias also modulates pupil size potentially to optimize visual processing at the location before the next fixation.
Functional Role of the Local Luminance Modulation.
Pupil size changes according to the global luminance level to optimize the trade-off between image acuity and sensitivity (4, 5). Visual processing at the location of attention and saccade planning starts well before the eye movement (1, 2). Correspondingly, pupil size was also modulated by these presaccadic processes (6, 7), with smaller pupil size when the locus of spatial attention was directed to a bright stimulus compared with a dark stimulus (8) or when preparation for an eye movement toward a stimulus embedded in a bright compared with a dark background (34). Here, we found comparable local luminance effects in monkeys performing the visual-delayed and memory-guided saccade tasks: pupil size was smaller after the patch presentation when the bright stimulus overlapped with the target location, suggesting the involvement of similar local luminance mechanisms between humans and monkeys. Most importantly, the similar local luminance modulation was produced following SCi microstimulation and the effects were reversed by SCi inactivation through lidocaine injection. Together, these observations suggest that the effects of microstimulation on pupil responses were similar to those of spatial attention and saccade planning. Although research exploring the behavioral advantages of the local luminance modulation have shown that performance probed at the attended location correlated with the degree of local luminance modulation (9, 35), a functional role of the local luminance modulation on this behavioral advantage is still lacking. Does the local luminance modulation play any causal role on the behavioral advantage or does it simply reflects a degree of attentional processing (or saccade preparation) on the attended target, thus producing correlation between behavioral advantage and local luminance modulation size? Future research is required to directly examine the functional role of this local luminance modulation.
Depth perception is essential to our everyday life, and the near response is known to include three motor acts: vergence, accommodation, and pupillary constriction (36). This raises an interesting question: does this principle generalize to the near response of pupillary constriction or even to other motor acts of the near response? Although the present study is not able to answer this question directly, we argue that the pupil control system should be able to change according to shifts of attention in depth. That is, if attention is guided from a distant to a near object, the pupil should slightly constrict to provide a better focus on the attended target. Future studies are needed to investigate this important question.
Neural Mechanisms Underlying the Local Luminance Modulation.
A neural network that involves the SCi, FEF, and LIP is regularly attributed to effects of spatial attention and saccade planning (12, 13, 37). Anatomically, the SCi receives direct input from the FEF and LIP (18–20, 31), and neurons in the SCi project directly to the brainstem circuit to initiate various orienting responses such as saccades and pupil dilation (10, 11). Weak microstimulation of the SCi or FEF evokes pupil dilation without triggering saccades (26, 38, 39). Moreover, a recent study has shown greater pupil constriction evoked by a bright stimulus when it was presented at the corresponding FEF microstimulated location (24), suggesting that pupil light responses can be spatially enhanced by FEF microstimulation. Taken together, the SCi could integrate signals from the FEF and LIP, and luminance signals could be modulated in a spatially selective manner through the efferent projections from the SCi to the pupil control circuit.
The SCi has anatomical connections to the parasympathetic and sympathetic pathways to change pupil size (11). The SCi connects to a downstream parasympathetic structure, the Edinger–Westphal (EW) nucleus, mainly via projections through the central mesencephalic reticular formation (40, 41). The EW nucleus projects to the ciliary ganglion with excitatory and inhibitory connections (42) and can therefore produce constriction and dilation. Additionally, the EW nucleus receives projections from topographically organized the pretectal olivary nucleus (PON), which receives luminance signals directly from the retina (43–45). Because the EW nucleus receives projections from topographically organized SCi and PON, the efferent projections from the SCi and PON to the EW nucleus could interact in a spatially selective manner. Luminance signals carried by the PON could thus be enhanced by spatial attention signals delivered through the SCi, with increasing or decreasing EW activity according to the local luminance level at the stimulated location, resulting in pupil constriction or dilation, respectively. Therefore, enhancing or suppressing neurons SCi activity via microstimulation or microinjection of lidocaine respectively increased or decreased local luminance effects at the location corresponding to the site of SCi stimulation. In summary, we argue that the SCi–EW pathway is likely underlying the observed local luminance modulation.
It is still possible that other pathways also contribute to the local luminance modulation. The first possibility is that the alternation in pupil size is computed within the SCi itself because the SCi also receives direct retinal projections from the intrinsically photosensitive retinal ganglion cells, which are considered as luminance encoders and important for the pupil light reflex (46, 47). However, there is no evidence suggesting that luminance increase or decrease signals are processed differently in the SC. Another possibility is that the projections from the SCi to the SCs (48) play a role. The PON receives direct projections from the SCs (40, 44) and projects to the suprachiasmatic nucleus of the hypothalamus in the sympathetic system (49). It is possible that the luminance signals could be spatially modulated by SCi microstimulation through SCi projections to the SCs or by SCi microinjection through proximal spreading of lidocaine to the SCs. However, because of the low stimulation parameters used here (70–90 Hz, 2–25 µA), the SCi-to-SCs connection is less likely to be activated to influence the PON. In addition, microstimulation of the SCs failed to initiate any pupil response (26). In summary, we argue that the most likely scenario requires the SCi integrating the signals from the FEF and LIP and sending the spatially selective signals to the EW nucleus that merge with luminance signals from the PON to coordinate the location and direction (constriction or dilation)-sensitive pupil modulation.
Materials and Methods
Data were collected from two male Rhesus monkeys. All procedures were approved by the Queen’s University Animal Care Committee in accordance with the guidelines of the Canadian Council on Animal Care.
When the SCi had been localized, the SCi was microstimulated (300-Hz pulse train for 100 ms with alternating 0.3-ms anode plus 0.3-ms cathode pulses) and saccade threshold was determined (evoked saccades 50% of the time; range, 5–50 µA). In microstimulation experiments, we reduced the frequency of stimulation to 70–90 Hz and used 25–45% of the saccade threshold current to activate the target area in the SC without evoking saccades or pupil dilation (detailed in SI Appendix). Microinjections of lidocaine or saline solution were made through a metal cannula with an attached microelectrode. Injections consisted of 1–1.3 μL of 2% lidocaine or saline solution alone, delivered at a rate of 0.5 μL/min by using a Hamilton syringe at a depth that was 1–2.5 mm below the SC surface, defined by previous microelectrode recordings. Testing before (preinjection) and after injection (postinjection, ∼1–20 min after the injection) and after recovery (25+ min after the injection) was usually conducted in the same session (detailed in SI Appendix).
Monkeys were trained to perform fixation tasks, at which they had to maintain gaze within 1.5° of a fixation point (0.5° diameter; 20 cd/m2, isoluminant color of the background) at the center of the screen on a gray background (20 cd/m2) for a few seconds to obtain a liquid reward (details of all experiments are provided in SI Appendix). After the monkey maintained fixation for 1–1.5 s, a train of stimulation pulses was delivered (400 ms, 70–90 Hz, 25–45% saccade threshold) on 50% of the trials (Fig. 1A), coincident with the presentation of two task-irrelevant patch stimuli (3–9° in radius; one bright and the other dark, both with 95% contrast relative to the gray background). Monkeys had to maintain fixation for another 1.5–2.5 s for reward. Two patch stimulus conditions were used: in the bright condition (50% of trials), the center of the bright patch stimulus location was spatially aligned with the SCi stimulated location determined by the saccade vector evoked via suprathreshold SCi microstimulation, and the dark patch was presented at the opposite location of the bright patch. In the dark condition (50% of trials), the center of the dark patch stimulus location was spatially aligned with the SCi stimulated location, and the bright patch was presented at the opposite location of the dark patch. All conditions were randomly interleaved.
SI Appendix includes extended details about stimuli, equipment, procedure, and data analyses. The data, materials, and code that support the findings of this study are available from C.-A.W. upon reasonable request.
Supplementary Material
Acknowledgments
We thank Ann Lablans, Brittney Armitage-Brown, and Mike Lewis for outstanding technical assistance, as well as members of the laboratory of D.P.M. This work was supported by Canadian Institutes of Health Research Grant MOP-FDN-148418 (to D.P.M.) and the Canada Research Chair Program (D.P.M.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1809668115/-/DCSupplemental.
References
- 1.Kowler E. Eye movements: The past 25 years. Vision Res. 2011;51:1457–1483. doi: 10.1016/j.visres.2010.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Carrasco M. Visual attention: The past 25 years. Vision Res. 2011;51:1484–1525. doi: 10.1016/j.visres.2011.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McDougal DH, Gamlin PD. Autonomic control of the eye. Compr Physiol. 2015;5:439–473. doi: 10.1002/cphy.c140014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Laughlin SB. Retinal information capacity and the function of the pupil. Ophthalmic Physiol Opt. 1992;12:161–164. doi: 10.1111/j.1475-1313.1992.tb00281.x. [DOI] [PubMed] [Google Scholar]
- 5.Woodhouse JM. The effect of pupil size on grating detection at various contrast levels. Vision Res. 1975;15:645–648. doi: 10.1016/0042-6989(75)90278-3. [DOI] [PubMed] [Google Scholar]
- 6.Binda P, Murray SO. Keeping a large-pupilled eye on high-level visual processing. Trends Cogn Sci. 2015;19:1–3. doi: 10.1016/j.tics.2014.11.002. [DOI] [PubMed] [Google Scholar]
- 7.Mathôt S, Van der Stigchel S. New light on the mind’s eye: The pupillary light response as active vision. Curr Dir Psychol Sci. 2015;24:374–378. doi: 10.1177/0963721415593725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Binda P, Pereverzeva M, Murray SO. Attention to bright surfaces enhances the pupillary light reflex. J Neurosci. 2013;33:2199–2204. doi: 10.1523/JNEUROSCI.3440-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mathôt S, van der Linden L, Grainger J, Vitu F. The pupillary light response reveals the focus of covert visual attention. PLoS One. 2013;8:e78168. doi: 10.1371/journal.pone.0078168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Corneil BD, Munoz DP. Overt responses during covert orienting. Neuron. 2014;82:1230–1243. doi: 10.1016/j.neuron.2014.05.040. [DOI] [PubMed] [Google Scholar]
- 11.Wang C-A, Munoz DP. A circuit for pupil orienting responses: Implications for cognitive modulation of pupil size. Curr Opin Neurobiol. 2015;33:134–140. doi: 10.1016/j.conb.2015.03.018. [DOI] [PubMed] [Google Scholar]
- 12.Krauzlis RJ, Lovejoy LP, Zénon A. Superior colliculus and visual spatial attention. Annu Rev Neurosci. 2013;36:165–182. doi: 10.1146/annurev-neuro-062012-170249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bisley JW, Goldberg ME. Attention, intention, and priority in the parietal lobe. Annu Rev Neurosci. 2010;33:1–21. doi: 10.1146/annurev-neuro-060909-152823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wardak C, Olivier E, Duhamel J-R. A deficit in covert attention after parietal cortex inactivation in the monkey. Neuron. 2004;42:501–508. doi: 10.1016/s0896-6273(04)00185-0. [DOI] [PubMed] [Google Scholar]
- 15.Thompson KG, Bichot NP. A visual salience map in the primate frontal eye field. Prog Brain Res. 2005;147:251–262. doi: 10.1016/S0079-6123(04)47019-8. [DOI] [PubMed] [Google Scholar]
- 16.Glimcher PW, Sparks DL. Movement selection in advance of action in the superior colliculus. Nature. 1992;355:542–545. doi: 10.1038/355542a0. [DOI] [PubMed] [Google Scholar]
- 17.Dorris MC, Munoz DP. Saccadic probability influences motor preparation signals and time to saccadic initiation. J Neurosci. 1998;18:7015–7026. doi: 10.1523/JNEUROSCI.18-17-07015.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Paré M, Wurtz RH. Monkey posterior parietal cortex neurons antidromically activated from superior colliculus. J Neurophysiol. 1997;78:3493–3497. doi: 10.1152/jn.1997.78.6.3493. [DOI] [PubMed] [Google Scholar]
- 19.Sommer MA, Wurtz RH. Composition and topographic organization of signals sent from the frontal eye field to the superior colliculus. J Neurophysiol. 2000;83:1979–2001. doi: 10.1152/jn.2000.83.4.1979. [DOI] [PubMed] [Google Scholar]
- 20.Wurtz RH, Sommer MA, Paré M, Ferraina S. Signal transformations from cerebral cortex to superior colliculus for the generation of saccades. Vision Res. 2001;41:3399–3412. doi: 10.1016/s0042-6989(01)00066-9. [DOI] [PubMed] [Google Scholar]
- 21.Cavanaugh J, Wurtz RH. Subcortical modulation of attention counters change blindness. J Neurosci. 2004;24:11236–11243. doi: 10.1523/JNEUROSCI.3724-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kustov AA, Robinson DL. Shared neural control of attentional shifts and eye movements. Nature. 1996;384:74–77. doi: 10.1038/384074a0. [DOI] [PubMed] [Google Scholar]
- 23.Müller JR, Philiastides MG, Newsome WT. Microstimulation of the superior colliculus focuses attention without moving the eyes. Proc Natl Acad Sci USA. 2005;102:524–529. doi: 10.1073/pnas.0408311101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ebitz RB, Moore T. Selective modulation of the pupil light reflex by microstimulation of prefrontal cortex. J Neurosci. 2017;37:5008–5018. doi: 10.1523/JNEUROSCI.2433-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Binda P, Gamlin PD. Renewed attention on the pupil light reflex. Trends Neurosci. 2017;40:455–457. doi: 10.1016/j.tins.2017.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang C-A, Boehnke SE, White BJ, Munoz DP. Microstimulation of the monkey superior colliculus induces pupil dilation without evoking saccades. J Neurosci. 2012;32:3629–3636. doi: 10.1523/JNEUROSCI.5512-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Carello CD, Krauzlis RJ. Manipulating intent: Evidence for a causal role of the superior colliculus in target selection. Neuron. 2004;43:575–583. doi: 10.1016/j.neuron.2004.07.026. [DOI] [PubMed] [Google Scholar]
- 28.Hikosaka O, Wurtz RH. Saccadic eye movements following injection of lidocaine into the superior colliculus. Exp Brain Res. 1986;61:531–539. doi: 10.1007/BF00237578. [DOI] [PubMed] [Google Scholar]
- 29.McPeek RM, Keller EL. Deficits in saccade target selection after inactivation of superior colliculus. Nat Neurosci. 2004;7:757–763. doi: 10.1038/nn1269. [DOI] [PubMed] [Google Scholar]
- 30.McPeek RM. Reversal of a distractor effect on saccade target selection after superior colliculus inactivation. J Neurophysiol. 2008;99:2694–2702. doi: 10.1152/jn.00591.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Paré M, Wurtz RH. Progression in neuronal processing for saccadic eye movements from parietal cortex area lip to superior colliculus. J Neurophysiol. 2001;85:2545–2562. doi: 10.1152/jn.2001.85.6.2545. [DOI] [PubMed] [Google Scholar]
- 32.Kojima J, Matsumura M, Togawa M, Hikosaka O. Tonic activity during visuo-oculomotor behavior in the monkey superior colliculus. Neurosci Res. 1996;26:17–28. doi: 10.1016/0168-0102(96)01067-x. [DOI] [PubMed] [Google Scholar]
- 33.Munoz DP, Wurtz RH. Saccade-related activity in monkey superior colliculus. I. Characteristics of burst and buildup cells. J Neurophysiol. 1995;73:2313–2333. doi: 10.1152/jn.1995.73.6.2313. [DOI] [PubMed] [Google Scholar]
- 34.Mathôt S, van der Linden L, Grainger J, Vitu F. The pupillary light response reflects eye-movement preparation. J ExpPsychol Hum Percept Perform. 2015;41:28–35. doi: 10.1037/a0038653. [DOI] [PubMed] [Google Scholar]
- 35.Mathôt S, Dalmaijer E, Grainger J, Van der Stigchel S. The pupillary light response reflects exogenous attention and inhibition of return. J Vis. 2014;14:7. doi: 10.1167/14.14.7. [DOI] [PubMed] [Google Scholar]
- 36.Mays LE, Gamlin PD. Neuronal circuitry controlling the near response. Curr Opin Neurobiol. 1995;5:763–768. doi: 10.1016/0959-4388(95)80104-9. [DOI] [PubMed] [Google Scholar]
- 37.Gandhi NJ, Katnani HA. Motor functions of the superior colliculus. Annu Rev Neurosci. 2011;34:205–231. doi: 10.1146/annurev-neuro-061010-113728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lehmann SJ, Corneil BD. Transient pupil dilation after subsaccadic microstimulation of primate frontal eye fields. J Neurosci. 2016;36:3765–3776. doi: 10.1523/JNEUROSCI.4264-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Joshi S, Li Y, Kalwani RM, Gold JI. Relationships between pupil diameter and neuronal activity in the locus coeruleus, colliculi, and cingulate cortex. Neuron. 2016;89:221–234. doi: 10.1016/j.neuron.2015.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.May PJ. The mammalian superior colliculus: Laminar structure and connections. Prog Brain Res. 2006;151:321–378. doi: 10.1016/S0079-6123(05)51011-2. [DOI] [PubMed] [Google Scholar]
- 41.Bohlen MO, Warren S, May PJ. A central mesencephalic reticular formation projection to medial rectus motoneurons supplying singly and multiply innervated extraocular muscle fibers. J Comp Neurol. 2017;525:2000–2018. doi: 10.1002/cne.24187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Barnerssoi M, May PJ, Horn AKE. GABAergic innervation of the ciliary ganglion in macaque monkeys–A light and electron microscopic study. J Comp Neurol. 2017;525:1517–1531. doi: 10.1002/cne.24145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Scalia F, Arango V. Topographic organization of the projections of the retina to the pretectal region in the rat. J Comp Neurol. 1979;186:271–292. doi: 10.1002/cne.901860210. [DOI] [PubMed] [Google Scholar]
- 44.Gamlin PD. The pretectum: Connections and oculomotor-related roles. Prog Brain Res. 2006;151:379–405. doi: 10.1016/S0079-6123(05)51012-4. [DOI] [PubMed] [Google Scholar]
- 45.Do MTH, Yau K-W. Intrinsically photosensitive retinal ganglion cells. Physiol Rev. 2010;90:1547–1581. doi: 10.1152/physrev.00013.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hannibal J, et al. Central projections of intrinsically photosensitive retinal ganglion cells in the macaque monkey. J Comp Neurol. 2014;522:2231–2248. doi: 10.1002/cne.23588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gamlin PDR, et al. Human and macaque pupil responses driven by melanopsin-containing retinal ganglion cells. Vision Res. 2007;47:946–954. doi: 10.1016/j.visres.2006.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Phongphanphanee P, et al. A circuit model for saccadic suppression in the superior colliculus. J Neurosci. 2011;31:1949–1954. doi: 10.1523/JNEUROSCI.2305-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Moga MM, Moore RY. Organization of neural inputs to the suprachiasmatic nucleus in the rat. J Comp Neurol. 1997;389:508–534. doi: 10.1002/(sici)1096-9861(19971222)389:3<508::aid-cne11>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.