Significance
Retinoic acid (RA) is an important transcriptional regulator during both vertebrate and invertebrate body pattern formation. The Homeobox (Hox) gene family is activated by a gradient of RA formed along the length of the embryo at specific time points during fetal development. Generation of a genetically modified mouse harboring mutations in the SMRT repressor demonstrated that SMRT-dependent repression of retinoic acid receptor (RAR) is critical to establish and maintain the somitic Hox code and segmental identity during fetal development via epigenetic marking of target loci.
Keywords: retinoic acid receptor, SMRT, somitogenesis, transcriptional repression, homeotic transformation
Abstract
Nuclear hormone receptors (NRs), such as retinoic acid receptors (RARs), play critical roles in vertebrate development and homeostasis by regulating target gene transcription. Their activity is controlled by ligand-dependent release of corepressors and subsequent recruitment of coactivators, but how these individual receptor modes contribute to development are unknown. Here, we show that mice carrying targeted knockin mutations in the corepressor Silencing Mediator of Retinoid and Thyroid hormone receptor (SMRT) that specifically disable SMRT function in NR signaling (SMRTmRID), display defects in cranial neural crest cell-derived structures and posterior homeotic transformations of axial vertebrae. SMRTmRID embryos show enhanced transcription of RAR targets including Hox loci, resulting in respecification of vertebral identities. Up-regulated histone acetylation and decreased H3K27 methylation are evident in the Hox loci whose somitic expression boundaries are rostrally shifted. Furthermore, enhanced recruitment of super elongation complex is evident in rapidly induced non-Pol II-paused targets in SMRTmRID embryonic stem cells. These results demonstrate that SMRT-dependent repression of RAR is critical to establish and maintain the somitic Hox code and segmental identity during fetal development via epigenetic marking of target loci.
The ability of nuclear receptors (NRs) to mediate repression of positively regulated target genes depends largely on their association with corepressors such as SMRT and NCoR (1–4). In the classic model, corepressors bind to NRs in the absence of cognate ligands and dissociate from them upon ligand binding (5–7) providing a regulated “on-off” switch. NR signaling has been demonstrated to be critical during mouse development by numerous receptor knockout models and agonist treatments. Nonetheless, the contribution from corepressor-associated signaling has been unclear at best. Among NRs, two receptor families, retinoic acid receptors (RARs) and thyroid hormone receptors (TRs) stand out in terms of strong ligand-independent corepressor binding affinities and clear roles in vertebrate development.
During vertebrate development, retinoic acid (RA) plays critical roles in hindbrain and mesoderm segmentation (6, 8–10). Spatially complex expression of RA synthesis (Aldh1a2) and degradation (Cyp26) enzymes produces a RA gradient whose distinct thresholds along the axis have been demonstrated as critical events in rhombomere specification (11). In addition to the RA gradient, mutually inhibitory FGF/Wnt signaling has been shown to form an opposing gradient during somitogenesis (12). During this developmental window, segmental identity is conferred to newly formed somites at the “determination front” of caudal presomitic mesoderm, and once acquired, this positional information is maintained throughout axis elongation (13). This segmental identity is thought to be encoded by a combination of Hox gene expression pattern, known as the Hox code. During somitogenesis, Hox genes are directionally activated in a specific temporal sequence. This temporal colinearity has been closely associated with dynamic progression of chromatin modifications by increasing activation marks in conjunction with decreasing repressive marks (14). While RAR is known to directly regulate Hox promoters, the mechanism producing its spatially graded transcription is poorly understood (15). Here, we show that SMRT-dependent repression is a key determinant of the Hox code specification and maintenance by a direct effect on RA degradation and transcriptional repression of RAR target Hox genes.
The crucial role of RARs in neural crest cell (NCC)-derived craniofacial structures has long been established by genetic and pharmacological studies (16), yet the contributions of RAR-directed repression versus activation to this developmental process are unknown. Compound deletion of RAR α and β does not affect the early stages of NCC development up to embryonic day 10.5 (E10.5) (16). Nonetheless, the presence of misshapen bones in NCC-derived structures illustrates the importance of RAR-directed signaling in NCCs during late developmental processes, such as the modeling of skeletal elements (17). However, a previously insurmountable difficulty in studying the role of repression in RA-dependent pattern formation has been that the complete SMRT knockout is embryonic lethal, in part due to its association with numerous transcription factors, including p53, Myc, MyoD, PLZF, RelA/p65, and Stat5 (18, 19).
Previously, it has been demonstrated in mouse ES cells that RA also rapidly recruits super elongation complex (SEC) components to nonpaused target genes such as RA-degrading enzyme, Cyp26a1 (20). This class of genes is unique in comparison with the RA-induced Hox gene clusters which possess paused-Pol II near their transcription start sites. Currently, it is unknown how these SEC components are rapidly recruited and regulated (20).
In the present study, by combining molecular, cellular, and gene targeting approaches, we demonstrated that the corepressor SMRT interactions with RARs are critical for specification and maintenance of axial Hox codes. We also show that repressive histone acetylation and methylation marks in Hox loci are established by histone deacetylases (HDACs) and Polycomb repressive complex (PRC) that are recruited by SMRT. Finally, we show that RA availability during development is regulated by SMRT-dependent transcriptional repression of the RA-degrading enzyme, Cyp26a1. These findings highlight unexpected roles for corepressor SMRT and importance of NR-dependent repression in murine axial development.
Results
SMRTmRID Mutant Mouse Phenotype.
Importantly, newborn SMRTmRID mice are born at expected Mendelian ratios and appear grossly normal except for a kinked tail, potential neural tube closure defect, as well as a 10% reduction in body size and weight (SI Appendix, Fig. S1 A and B). This smaller body size and weight reduction persist until weaning. Postnatal metabolic phenotypes of SMRTmRID mice have been discussed (21).
Normal Hindbrain Segmentation and Defective Cranial Neural Crest-Derived Structures.
In contrast to reported RAR-mediated forebrain and neural stem cell phenotypes of SMRT null mice (19), we did not observe any major defects in the SMRTmRID cortex (21). As RAR signaling plays critical roles in hindbrain patterning (16), we examined rhombomere segmentation in E8.5–10.5 SMRTmRID embryos (SI Appendix, Fig. S1 C and D). Although we did not observe abnormal expression of rhombomere marker genes during hindbrain segmentation in SMRTmRID animals, 2 of 10 embryos exhibited fused branches of the 9th and 10th cranial ganglia (SI Appendix, Fig. S1E). Furthermore, we observed malformations in the retrotympanic processes of the squamous temporal bone and thyroid cartilage with 100% penetrance (SI Appendix, Fig. S1 F and G). Interestingly, the spinous processes of the first and second cervical vertebrae were dramatically reduced in SMRTmRID animals (SI Appendix, Fig. S2A). In addition, cervical spina bifida was prevalent in SMRTmRID animals (SI Appendix, Fig. S2B). As these structures are formed by cells of cranial neural crest origin, we speculate that SMRTmRID mice might suffer from NCC defects by either cell-autonomous or surrounding mesodermal RAR derepression (22). Together, these findings demonstrate that NR-dependent SMRT-directed repression is critical for the normal formation of NCC-derived skeletal elements rather than forebrain and neural progenitor developments as in whole-body SMRT null animals (19). Interestingly, the malformation of NCC-derived structures seen in SMRTmRID mice was highly reminiscent of findings in RaraNCC−/− mutants, suggesting that a SMRT/RAR pathway may direct other developmental programs (17).
Homeotic Transformation of Axial Vertebrae.
Next, we examined the expression pattern of SMRT in developing mouse embryos. Using whole mount in situ hybridization (WISH), the expression of SMRT was ubiquitously detected in the developing somites, with highest signals occurring in the tail somites (SI Appendix, Fig. S2C). qPCR analyses of the somite regions confirmed the SMRT WISH results (SI Appendix, Fig. S2D). The expression of the related corepressor NCoR in the somites closely resembled the somitic expression pattern for SMRT at E10.5 (SI Appendix, Fig. S2D). These results suggested that SMRT and/or NCoR could play an important role in axial development.
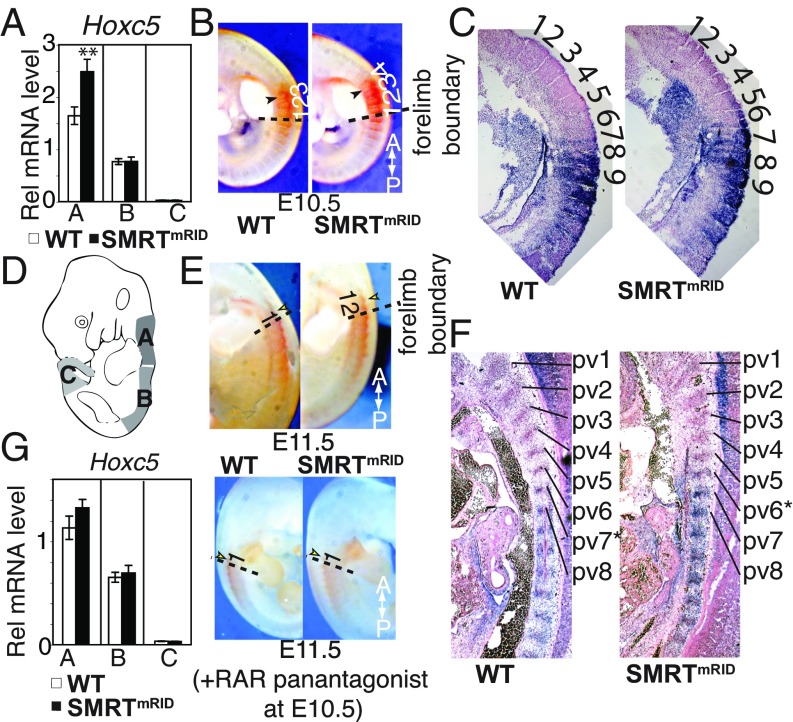
An examination of embryos at multiple stages revealed a striking posterior homeotic transformation of C5–7 cervical vertebrae and anterior transformation of L1 lumbar vertebrae in SMRTmRID mice, similar to those found in embryos exposed to a high dose of systemic RA at E10.5–11 (Fig. 1 A–C). Tuberculi anterior (TA) flanges, which should normally be unique to C6, appeared on both C5 and C6 vertebrae (Fig. 1B). In some cases, C6 lost the foramen transversarium (FT) which, in the upper six vertebrae, provides passage to a plexus of sympathetic nerves and vertebral blood vessels (SI Appendix, Fig. S2E). In addition, we found the frequent formation of either one or two incomplete ribs on C7, which are fused to the first thoracic rib (SI Appendix, Fig. S2 F and G). In 18% of SMRTmRID mutants, the first lumbar vertebra (L1) acquires a thoracic identity with acquisition of a supernumerary 14th rib (Fig. 1C). The aforementioned axial pattern is highly reminiscence of that observed in Cyp26a1 null mice (23). No significant defects in somite size or overall number were observed in SMRTmRID embryos as shown by WISH with somite markers, Myogenin and Uncx4.1 (SI Appendix, Fig. S2H), indicating that the homeotic shifts are not due to the global defects in somitogenesis. The above findings indicate that SMRT/NR-directed repression is an integral component in axial somite specification and that this repression cannot be compensated by another NR corepressor, NCoR.
Fig. 1.
Axial homeotic transformation, its rescue by retinoid antagonist, and enhanced sensitivity to RA-induced abnormality in SMRTmRID. (A) Schematic diagram of axial transformations in SMRTmRID. Vertebrae are depicted as ovals. Yellow ovals with vv indicate presence of tuberculi anterior. Location of the 14th rib in SMRTmRID is indicated in red. (B) Posterior transformation in cervical vertebrae of SMRTmRID. Filled circles and blue arrows indicate position of tuberculum anterior (TA). Incomplete rib anlages on C7 (54% of SMRTmRID) are indicated by black arrowheads. (C) Anterior transformation of lumbar vertebrae to thoracic. Presence of the 14th rib is indicated by arrowhead. (D) Rescue of cervical transformation in SMRTmRID by RARα antagonist (BMS 204493) treatment at E10.5. WT and SMRTmRID cervical columns are shown after antagonist treatment. (E) Typical cranial abnormality evident in SMRTmRID after exogenous RA exposure at E10 with single dose of 100 mg/kg. Fusion between basioccipital (bo) and exoccipital (eo) bones in SMRTmRID (white arrowheads). (F) Severe caudal truncations in SMRTmRID after RA exposure as in E.
Rescue of Homeotic Transformation by RAR Antagonist at E10.5.
These homeotic effects demonstrate that SMRT is a required mechanistic component to accurately translate the chemical RA agonist gradient into an appropriate somitic Hox code during somitogenesis. Indeed, the axial phenotype of SMRTmRID mice is virtually identical to the previously described vertebral transformation seen with ectopic all-trans RA exposure, which is thought to disrupt axial positional information at late somitogenesis (24). This led us to wonder whether loss of repression in SMRTmRID embryos could be rescued with a chemical retinoid antagonist such as BMS 493 (a pan-RAR antagonist). We chose the lower BMS 493 dose that is not teratogenic in wild-type (WT) embryos. Remarkably, this antagonist treatment at E10.5 led to a near complete reversal of posterior cervical transformations back into a WT pattern (Fig. 1D and SI Appendix, Table S1), indicating that a mechanism (chemical or genetic) to oppose RAR activation is critical for establishing or maintaining positional information. Together these results provide genetic evidence that somitic identity is dependent on genetic repression mediated by SMRT/RAR corepressor complexes during the E10.5–11 time window. Nonetheless, we could not rule out possible involvement of other corepressors such as NCoR or inhibition of coactivator recruitments upon RAR antagonist treatment. In addition, lack of phenotypic changes in WT control with BMS 493 treatment indicates that SMRT-dependent repression is required for stable RA-signaling gradient during late somitogenesis.
Furthermore, the above results suggest that SMRTmRID mice may be generally more sensitive to exogenous RA exposure than wild-type littermates. While “standard” teratogenic doses of RA are 100 mg/kg (Fig. 1 E and F), in SMRTmRID mice “nonteratogenic” doses of 5 mg/kg at E10.5 provoked cervical homeotic transformations (SI Appendix, Table S1). This lack of protection against low-dose RA suggests that SMRT repression enhances the robustness of the gene expression gradient by shielding it from exposure to potential external perturbance such as fluctuations in RA concentrations (SI Appendix, Table S1) during late somitogenesis. This enhanced ability of RA to change somatic identities in already specified somites suggests that SMRT, as a platform for chromatin modifiers, could be required to maintain the chromatin modification status of relevant RAR targets such as Hox genes, at least for the duration of their patterning functions.
Shifted Hox Gene Expression Boundary in SMRTmRID Mice.
Posterior cervical transformations are a classic example in which anterior expansion of Hox gene expression results in specification of anterior segments to more posterior ones (25–27). As retinoids are known to activate the Hox cluster, we assessed the expression boundaries of posterior Hox genes normally associated with cervical regions in SMRTmRID embryos (Fig. 2 A and B and SI Appendix, Fig. S3 A and B). Interestingly, the increased expression of Hoxc5 was detected in the anterior dorsal axis by qPCR (Fig. 2A). Notably, in situ hybridization assays confirmed that the anterior boundary of Hoxc5 expression was shifted forward by one segment in SMRTmRID embryos (Fig. 2 B–F). Additionally, after antagonist treatment at E10.5, the Hoxc5 expression boundary was normalized to WT levels (Fig. 2G). This demonstrates that in addition to vertebral patterning, SMRT-dependent repression is required to maintain the Hox expression boundary for somite identity at late somitogenesis.
Fig. 2.
Altered Hox expression domains in SMRTmRID. (A) Relative abundance of Hoxc5 mRNA along the body axis at E11.5. A, B, and C denote the regions of the embryo depicted in D. The data are shown as the mean ± SEM. n = 5, **P < 0.005 by Student’s t test. (B) Whole mount in situ hybridization showing anterior expansion of Hoxc5 expression boundary at E10.5. Somites are numbered from the forelimb level and arrow indicates the boundary of Hoxc5 expression. (C) Section in situ of E10.5 axial somites. Note Hoxc5 expression boundary is shifted to the seventh somite in comparison with WT embryos. (D) Anterior, A; middle, B; and caudal, C regions containing somites were used for mRNA quantitation. (E) Anterior expansion of Hoxc5 expression boundary at E11.5. (F) Section in situ showing anterior expansion of Hoxc5 expression domain in prevertebrae of E11.5 SMRTmRID embryo. * indicates anterior prevertebrae expressing Hoxc5. (G) Relative abundance of Hoxc5 mRNA and in situ hybridization showing normalization of anterior Hoxc5 expression boundary at E11.5 by RAR antagonist (BMS 493) treatment at E10.5. The data are shown as the mean ± SEM. n = 5.
Epigenetic Spatial Control of Hox Genes in SMRTmRID Mice.
SMRT has been identified as a component of histone deacetylase-containing protein complexes (28, 29). We wished to examine whether SMRT complexes play a role in the epigenetic control of Hox C loci in developing embryos at E10.5. We examined spatially distinct somite regions designated as front (F), middle (M), and rear (R) (Fig. 3B and SI Appendix, Fig. S4A) in ChIP assays. In the mouse Hox C loci, RAREs are located both 3′ and 5′ to the Hoxc4 locus. We first examined the occupancy of RAR and HDAC3, a component of SMRT complexes in the 5′ RARE (Fig. 3A). Occupancy of RAR was slightly reduced in all three somite regions of SMRTmRID embryos. It has been suggested in prior ChIP studies that activation of RAR leads to reduction in its occupancy to its binding sites in certain target genes (30, 31). It is possible that SMRT derepression mimics receptor activation by decreasing RAR promoter occupancy. Occupancy of a SMRT complex component, HDAC3, was dramatically reduced in SMRTmRID somites especially in M and R regions where most of the axial defects were identified. It is tempting to speculate that in the F region, other corepressors could compensate for SMRT in RAR-mediated repression as we did not find major defects in hindbrain specification.
Fig. 3.
Epigenetic spatial control of Hox genes in SMRTmRID mice. (A) Recruitment of RAR and HDAC3 to the Hoxc4 RARE in three axial somites regions (F, front; M, middle; R, rear depicted in B). The data are shown as the mean + SEM. n ≥ 3. Locations of ChIP primer pairs are indicated as arrowheads in HoxC loci diagram. (B) Epigenetic histone modification status of HoxC loci in three axial somites regions (F, front; M, middle; R, rear depicted). The data are shown as the mean + SEM. n ≥ 3. (C) Recruitment of Jmjd3 and Suz12 containing PRC2 complex to Hoxc5 exon 1 region in three axial somite regions (F, M, R, depicted in B). The data are shown as the mean + SEM. n ≥ 3. (D) Hoxc5 derepression in PC19 EC cells by SMRT-C overexpression and 50 nM HDAC inhibitor, TSA. The data are shown as the mean + SEM. n = 5. (E) siRNA-mediated knockdown of SMRT or Ezh2 expression resulted in Hoxc5 derepression in P19 EC cells. The data are shown as the mean ± SEM. n = 5. ns, not statistically significant; *P < 0.05; **P < 0.005 by Student’s t test.
Next, we looked at three chromatin marks (AcH3, H3K4me3, and H3K27me3) in exon 1 of each Hox C locus in spatially distinct somites of E10.5 embryos (Fig. 3B). Front somite regions (F), which are specified earlier than middle regions (M), had higher enrichments of AcH3 and H3K4me3 in the proximal Hox C loci. While H3K27me3 repressive marks were reduced in proximal Hox genes, they were increased in distal Hox C loci. Middle regions, which are specified earlier than rear regions (R) but later than front regions, displayed higher enrichment of activation marks in more distal Hox genes. At the same time, enrichment of repressive marks was not observed proximal to middle Hox genes. In distal Hox C-expressing rear regions, enrichment of repressive marks and absence of activation marks in proximal Hox were evident. Thus, spatial epigenetic marks closely resemble the known Hox C loci expression pattern along the rostral-to-caudal somites. Recently, it has been reported that NCoR occupancy was low to absent in Hox C loci during RA activation of ES cells (32). It is possible that Hox C loci are predominantly under the control of the SMRT/RAR complex.
As the SMRTmRID mutation abrogates RAR-dependent recruitment of SMRT/HDAC corepressor complexes, we predicted an increase in histone acetylation of Hox C loci in SMRTmRID embryos. Indeed, in SMRTmRID somites, increased AcH3 marks were detected in certain Hox C loci in M and R regions (Fig. 3B). Unexpectedly, a reciprocal decrease in H3K27me3 marks was detected, a modification known to be mediated by Ezh2 methyltransferase as a part of the PRC2 complex (33). These repressive marks can be removed by opposing demethylases such as Jmjd3 and UTX (34). Therefore, we examined Jmjd3 and Suz12 (a PRC2 component) recruitment to the Hoxc5 locus (Fig. 3C). Consistent with the reduced levels of H3K27me3, there was decreased occupancy of PRC2 methyltransferase complex (Suz12) only in SMRTmRID somites, suggesting SMRT plays a role in maintaining the H3K27 trimethylation status of this Hox locus. There was a trend in increased occupancy of Jmj3 demethylase but did not reach statistical significance. Although, Jmjd3 has been suggested as a direct RAR target in neural stem cells, we did not detect derepression of Jmjd3 expression in SMRTmRID somites (SI Appendix, Fig. S4 B and C) (19).
We further examined the role of SMRT in Hox gene regulation in P19 embryonal carcinoma (EC) cells, a model system for RA-dependent activation of Hox genes (Fig. 3D). In transient transfection assays, overexpression of a SMRTmRID construct did not change either basal or RA-dependent activation of endogenous Hoxc5 expression, as expected from the lack of phenotype in heterozygous SMRTmRID mice. In contrast, expression of a C-terminal portion of SMRT-containing RID but lacking repression domain (SMRT-C) resulted in derepression and enhanced RA-dependent activation of the Hox C5 locus, suggesting SMRT recruitment is required for repression of the Hox C5 locus (Fig. 3D). Knockdown of SMRT expression via siRNA also resulted in derepression and enhanced activation of Hoxc5 genes (Fig. 3E). Furthermore, pharmacological inhibition of HDAC activity by trichostatin A (TSA) resulted in dramatic increase in Hoxc5 expression, providing evidence for the importance of histone deacetylation in Hoxc5 regulation. We further examined the role of H3K27 trimethylation in Hoxc5 regulation by knocking down Ezh2 methyltransferase by siRNA (Fig. 3E). Ezh2 knockdown resulted in derepression of Hoxc5 similar to SMRT knockdown. Interestingly, SMRT knockdown or overexpression of SMRT-C in P19 EC cells resulted in increased expression of NCoR and Ezh2. Ezh2 knockdown resulted in reciprocally enhanced expression of SMRT and NCoR, suggesting a dynamic link between SMRT and the polycomb repressive complex, PRC2 (SI Appendix, Fig. S4 D and E). These results also implicate that SMRT is a preferential corepressor for RAR action in Hoxc5 gene expression.
Enhanced RAR Activity and RA Clearance in SMRTmRID Mice.
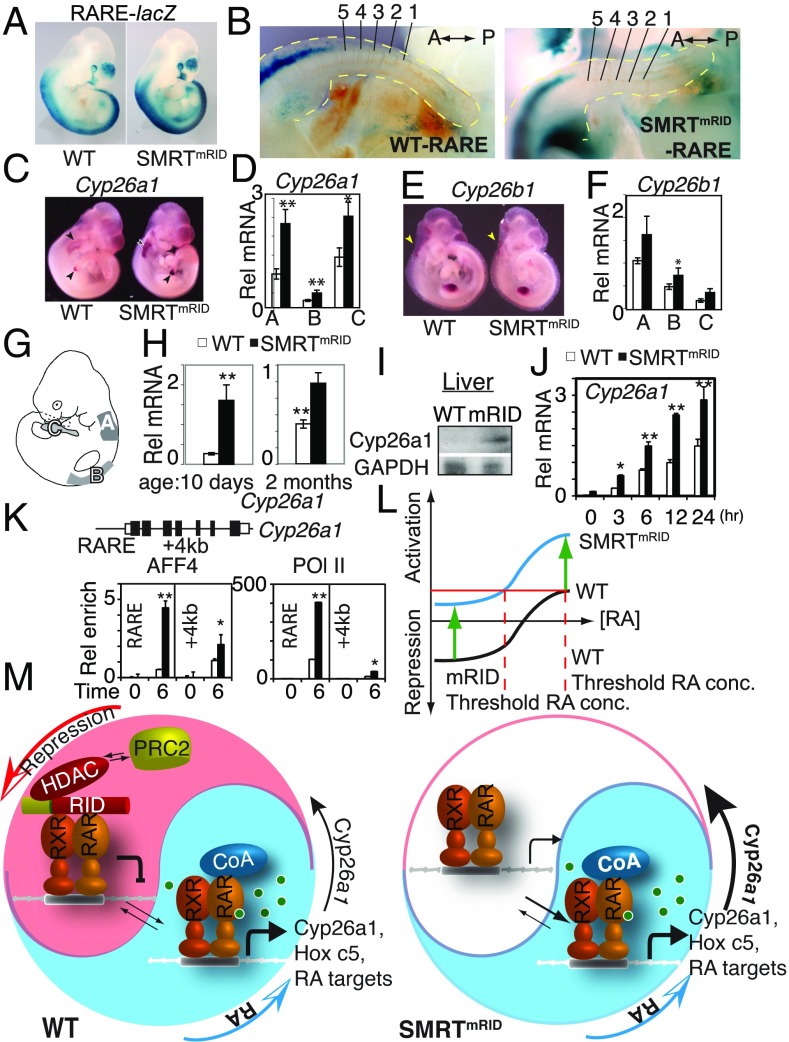
To further examine derepression of RAR in this mouse model, we crossed the SMRTmRID mice with a transgenic reporter line (RARE-hsp-lacZ) in which in vivo activation of RAR can be visualized by β-galactosidase (β-gal) activity (SMRTmRID-RARE) (35). In SMRTmRID-RARE embryos, higher β-gal activity was present all along the dorsal axis as well as the otic and optic vesicles (Fig. 4A). Interestingly, the caudal boundary of reporter expression along the dorsal axis was unchanged relative to WT, suggesting that retinoic acid degrading activity is undiminished or potentially enhanced in the tailbud region of the presomitic mesoderm (Fig. 4B). Thus, despite presumptively increased RA signaling, a relatively normal embryonic development suggests that this process is not significantly perturbed in this region. This relatively undisturbed RARE-lacZ caudal boundary in the SMRTmRID could be due to the higher expression of retinoid degrading activities.
Fig. 4.
Derepression of RA-degradation enzyme Cyp26a1 in SMRTmRID and enhanced recruitment of SEC to its regulatory region. (A) Enhanced RAR-dependent β-galactosidase activity in SMRTmRID: RAREhsplacZ at E10.5. (B) Preserved caudal boundary of RAR transcriptional activity visualized by LacZ staining (blue). Somites from the tail to the boundary of LacZ signals are numbered. (C and D) Increased Cyp26a1 expression in SMRTmRID by in situ hybridization in C and qPCR in D. The data are shown as the mean ± SEM. n = 5. (E and F) Expression levels of Cyp26b1 at E10.5 by in situ hybridization in E and qPCR in F. Arrows indicate sites of high Cyp26 expression. The data are shown as the mean ± SEM. n = 5. (G) Embryonic tissue regions, A–C, used for mRNA quantitation by qPCR in D and F. (H) Increased mRNA RA-degradation enzyme Cyp26a1 in the livers of 10-d-old and 2-mo-old SMRTmRID mice. The data are shown as the mean ± SEM. n = 10. (I) Increased Cyp26a1 protein levels in liver of 2-mo-old SMRTmRID. (J) Temporal kinetics of RA-activated Cyp26a1 mRNA in established WT and SMRTmRID ES cells. SEC-recruited, not Pol II-paused genes including Cyp26a1 are rapidly super induced by RA in SMRTmRID ES cells. Time appears in hour scale. The data are shown as the mean ± SEM. n = 5. (K) Enhanced enrichment of SEC component AFF4 at Cyp26a1 RARE in SMRTmRID ES cells. The data are shown as the mean + SEM. n = 5. (L) Loss of SMRT/RAR association in mRID results in decreased threshold RA concentration for RAR target gene activation. (M) Model for SMRT as a genetic antagonist to RA-transcriptional gradient. SMRT also plays a role in retinoid homeostasis by repressing the RA-degradation pathway. Derepression of RAR targets such as Hoxc5 in cervical somites results in anterior expansion of the Hoxc5 boundary, ultimately resulting in posterior homeotic transformation of cervical vertebrae. Derepression of Cyp26a1 counteracts to increased RA-transcriptional activity by degrading RA in SMRTmRID. *P < 0.05; **P < 0.005 by Student’s t test.
To the extent that a gene expression boundary is generated by the balance of a source and a sink (36), we wished to explore the impact of SMRTmRID on potential changes in RA synthetic and metabolizing enzymes. qPCR analysis revealed that the expression of the critical RA-generating enzyme, Aldh1a2, was unchanged in SMRTmRID embryos (SI Appendix, Fig. S4F) (37). In contrast, analysis of the Cyp26 genes encoding the RA-degrading enzymes (23) in the isolated somites revealed enhanced Cyp26a1 expression in E10.5 SMRTmRID embryos (Fig. 4 C–E). Cyp26a1 is known to control RA-mediated signaling by limiting RA levels in a spatiotemporal manner (38). Cyp26a1 deregulation was confirmed by WISH at different developmental stages (Fig. 4 C and E and SI Appendix, Fig. S4 G and H) and suggests that SMRT-dependent repression is required for normal RA metabolism. The enhanced expression of Cyp26a1 would result in more rapid degradation of RA and partially limit the teratogenic impact of derepressed RAR activity. Despite this compensatory RA clearance, the developmental phenotypes in SMRTmRID clearly implicate that SMRT/RAR-dependent repression is a major player in RAR signaling. Since the robustness of the RA gradient heavily relies on the auto-induced ligand degradation (39), the inability to precisely compensate for the derepressed RAR activity in SMRTmRID mice results in expanded Hox gene expression and aberrant specification of somitic identity.
Cyp26a1 derepression persists postnatally as indicated by the dramatic sevenfold increase in the livers of 10-d-old and 2-mo-old SMRTmRID mice (Fig. 4 H and I). To confirm that SMRT-dependent repression is a critical factor in Cyp26a1 regulation, we examined the SMRT occupancy and acetylation status of Cyp26a1 promoters in both WT and SMRTmRID livers under vitamin A deficiency (SI Appendix, Fig. S4I). In vitamin A-deficient WT livers, SMRT is enriched on the Cyp26a1 RARE while SMRT occupancy is compromised in SMRTmRID livers, suggesting that Cyp26a1 is a target of SMRT-dependent repression. Accordingly, in mutant mice, loss of SMRT recruitment to the Cyp26a1 RARE results in increased acetylated histone marks and strongly suggests that SMRT helps to prevent excessive retinoid metabolism, which in nutrient-deficient environments is potentially life sparing.
Rapidly Induced RA Targets and Super Elongation Complex Recruitment in SMRTmRID ES Cells.
SMRT has been identified as a cofactor that affects transcriptional initiation of NR target genes by recruiting corepressor complexes. Previously, A.S. and coworkers found that RA-induced targets in ES cells are also under the control of the SEC complex (20). Many of developmentally regulated genes such as Hox clusters are Pol II paused and ready to be activated by RA (20). As SMRT is a strong RAR protein interactor, we do observe derepression of RAR-regulated developmental target genes in SMRTmRID animals. We wanted to know whether the derepression seen in SMRTmRID embryos is also associated with the SEC complex in SMRTmRID ES cells.
In SMRTmRID ES cells, RA induction rapidly induces RAR-regulated Hox genes as early as 3 h posttreatment in comparison with WT ES cells (SI Appendix, Fig. S5A). Interestingly, the Hoxc5 gene was dramatically induced in SMRTmRID, strongly supporting our derepression view on posterior homeotic transformation and boundary shifting (SI Appendix, Fig. S5B). Another notable difference is with Cyp26a1 mRNA induction (Fig. 4J). This gene has been shown to be non-Pol II paused, but rapidly induced by RA with rapid RA-dependent recruitment of SEC complex (20). To see whether SEC recruitment is altered in SMRTmRID ES cells, we performed a ChIP assay. At the Cyp26a1 transcriptional start site, SEC components AFF4 and Pol II were detected after the RA treatment but greater enrichment was observed in SMRTmRID compared with WT (Fig. 4K). The Cyp26a1 locus has been shown to still retain repressive histone codes while it is induced by RA (20). It is possible that in SMRTmRID ES cells, the recruitment of SEC by RA and decreased H3K27 trimethylation synergistically enhance its target expression (Fig. 3B). We also looked at the expression of RA-induced but SEC not-recruited genes and SEC-recruited Pol II-paused genes (SI Appendix, Fig. S6A). Expression of these genes was not consistently altered over the time course except for two Hox genes in SMRTmRID ES cells, suggesting enhanced recruitment of SEC is only specific in certain classes of RA-controlled target genes. Furthermore, we found that SMRT is also important in regulation of the recruitment of SEC in non-Pol II paused, rapidly induced RA targets such as Cyp26a1.
Discussion
Our observations are summarized in the model shown in Fig. 4 L and M. While in WT animals, the SMRT complex is recruited to RAR/RXR target promoters in the absence of hormone, in SMRTmRID mutants, derepression can enhance target gene activity in the absence of ligand and “superactivate” in the presence of ligand. Thus, loss of nuclear receptor-SMRT binding decouples the apo-receptor status and target silencing. While the depression affects many target genes at the transcriptional initiation steps, certain classes of genes are additionally derepressed at the elongation steps, such as Cyp26a1. These derepressions affect the Hox code expression and ultimately alter the somitic identity. Furthermore, we provide evidence that SMRT-dependent repression is critical for regulation of RA-metabolizing enzyme Cyp26a1 in both developing embryos and the postnatal liver.
Importantly, we demonstrated that SMRT has a nonredundant role with NCoR and that loss of SMRT interaction with nuclear receptors produces clear developmental phenotypes that can be rescued by the retinoid antagonist. Derepression in mutant mice sensitizes the RA-signaling axis, resulting in posterior homeotic transformation and enhanced teratogenic susceptibility to retinoic acid. This enhanced sensitivity to retinoid agonist and antagonist in already specified cervical somites at late somitogenesis in SMRTmRID indicates that SMRT repression is critical for refining and maintaining the spatial boundaries in the developing somites until the formation of sclerotomes.
During somitogenesis, once Hox codes are established for newly formed somites, Hox expression domains must be maintained at least for the duration of their patterning period. To mediate the necessary long-term repression of Hox genes, components of PRC2 trimethylate H3K27. Mutations in Polycomb genes result in aberrant Hox gene expression, increased sensitivity to the teratogenic effect of RA, and posterior homeotic transformations similar to that observed in SMRTmRID animals (40). The present study provides evidence that the SMRT corepressor complex is a critical component in the maintenance of this transcriptional cellular memory during late somitogenesis. Together these results lead us to propose that SMRT complex-dependent dynamic epigenetic antagonism is the mechanism through which the RA morphogenic field is translated to an epigenetic and gene expression gradient during murine axial development.
Methods
Animals.
SMRTmRID knockin mice were generated by standard homologous recombination using an inserted neo cassette for positive selection and a tk cassette for negative selection. Chimeric mice were generated by microinjection of two independently targeted ES clones into blastocysts. Two independent mouse mutant lines were established. SMRTmRID mice were backcrossed for at least four generations to sv129. Homozygous SMRTmRID mice are viable in Sv129 or Sv129/C57BL6 mixed background, indicating that the previously described lethality of the SMRTmRID is background specific (41). All mice were fed a Labdiet 5002 containing 18 IU/g vitamin A. All experiments involving mice were approved by the Committee on Animal Care at the Salk Institute for Biological Studies.
Further details on materials and methods used in skeletal preparations, in situ hybridization, transient transfection, siRNA-mediated knockdown, gene expression analysis, chromatin immunoprecipitation assays, pharmacological treatments, and vitamin A deficiency study are included in SI Appendix, Materials and Methods.
Supplementary Material
Acknowledgments
We thank M. Capecchi and M. Petkovich for providing in situ probe constructs of Hox and Cyp26, respectively; J. Rossant and H. Sucov for providing RARE-hsp-lacZ mice; M. L. Privalsky for providing pSG5 GAL4AD-hRARs; A. Atkins for helpful suggestions and critical review of the manuscript; and Lita Ong and Christi Brondos for administrative assistance. Work in the J.C.I.B. laboratory was supported by the G. Harold and Leila Y. Mathers Charitable Foundation and Universidad Católica San Antonio de Murcia (UCAM). R.M.E. is an investigator of the Howard Hughes Medical Institute at the Salk Institute and the March of Dimes Chair in Molecular and Developmental Biology. This work was supported by the Howard Hughes Medical Institute and the National Institutes of Health Grants (DK057978 and HL105278).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1809480115/-/DCSupplemental.
References
- 1.Cavaillès V, et al. Nuclear factor RIP140 modulates transcriptional activation by the estrogen receptor. EMBO J. 1995;14:3741–3751. doi: 10.1002/j.1460-2075.1995.tb00044.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen JD, Evans RM. A transcriptional co-repressor that interacts with nuclear hormone receptors. Nature. 1995;377:454–457. doi: 10.1038/377454a0. [DOI] [PubMed] [Google Scholar]
- 3.Hörlein AJ, et al. Ligand-independent repression by the thyroid hormone receptor mediated by a nuclear receptor co-repressor. Nature. 1995;377:397–404. doi: 10.1038/377397a0. [DOI] [PubMed] [Google Scholar]
- 4.Ordentlich P, et al. Unique forms of human and mouse nuclear receptor corepressor SMRT. Proc Natl Acad Sci USA. 1999;96:2639–2644. doi: 10.1073/pnas.96.6.2639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lonard DM, O’malley BW. Nuclear receptor coregulators: Judges, juries, and executioners of cellular regulation. Mol Cell. 2007;27:691–700. doi: 10.1016/j.molcel.2007.08.012. [DOI] [PubMed] [Google Scholar]
- 6.Niederreither K, Dollé P. Retinoic acid in development: Towards an integrated view. Nat Rev Genet. 2008;9:541–553. doi: 10.1038/nrg2340. [DOI] [PubMed] [Google Scholar]
- 7.Ordentlich P, Downes M, Evans RM. Corepressors and nuclear hormone receptor function. Curr Top Microbiol Immunol. 2001;254:101–116. doi: 10.1007/978-3-662-10595-5_5. [DOI] [PubMed] [Google Scholar]
- 8.Lohnes D, et al. Function of retinoic acid receptor gamma in the mouse. Cell. 1993;73:643–658. doi: 10.1016/0092-8674(93)90246-m. [DOI] [PubMed] [Google Scholar]
- 9.Lohnes D, et al. Function of the retinoic acid receptors (RARs) during development (I). Craniofacial and skeletal abnormalities in RAR double mutants. Development. 1994;120:2723–2748. doi: 10.1242/dev.120.10.2723. [DOI] [PubMed] [Google Scholar]
- 10.Lufkin T, et al. High postnatal lethality and testis degeneration in retinoic acid receptor alpha mutant mice. Proc Natl Acad Sci USA. 1993;90:7225–7229. doi: 10.1073/pnas.90.15.7225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Niederreither K, et al. Genetic evidence that oxidative derivatives of retinoic acid are not involved in retinoid signaling during mouse development. Nat Genet. 2002;31:84–88. doi: 10.1038/ng876. [DOI] [PubMed] [Google Scholar]
- 12.Aulehla A, Pourquié O. Signaling gradients during paraxial mesoderm development. Cold Spring Harb Perspect Biol. 2010;2:a000869. doi: 10.1101/cshperspect.a000869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Iimura T, Denans N, Pourquié O. Establishment of Hox vertebral identities in the embryonic spine precursors. Curr Top Dev Biol. 2009;88:201–234. doi: 10.1016/S0070-2153(09)88007-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Soshnikova N, Duboule D. Epigenetic temporal control of mouse Hox genes in vivo. Science. 2009;324:1320–1323. doi: 10.1126/science.1171468. [DOI] [PubMed] [Google Scholar]
- 15.Pearson JC, Lemons D, McGinnis W. Modulating Hox gene functions during animal body patterning. Nat Rev Genet. 2005;6:893–904. doi: 10.1038/nrg1726. [DOI] [PubMed] [Google Scholar]
- 16.Mark M, Ghyselinck NB, Chambon P. Function of retinoic acid receptors during embryonic development. Nucl Recept Signal. 2009;7:e002. doi: 10.1621/nrs.07002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dupé V, Pellerin I. Retinoic acid receptors exhibit cell-autonomous functions in cranial neural crest cells. Dev Dyn. 2009;238:2701–2711. doi: 10.1002/dvdy.22087. [DOI] [PubMed] [Google Scholar]
- 18.Jepsen K, Rosenfeld MG. Biological roles and mechanistic actions of co-repressor complexes. J Cell Sci. 2002;115:689–698. doi: 10.1242/jcs.115.4.689. [DOI] [PubMed] [Google Scholar]
- 19.Jepsen K, et al. SMRT-mediated repression of an H3K27 demethylase in progression from neural stem cell to neuron. Nature. 2007;450:415–419. doi: 10.1038/nature06270. [DOI] [PubMed] [Google Scholar]
- 20.Lin C, et al. Dynamic transcriptional events in embryonic stem cells mediated by the super elongation complex (SEC) Genes Dev. 2011;25:1486–1498. doi: 10.1101/gad.2059211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nofsinger RR, et al. SMRT repression of nuclear receptors controls the adipogenic set point and metabolic homeostasis. Proc Natl Acad Sci USA. 2008;105:20021–20026. doi: 10.1073/pnas.0811012105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Matsuoka T, et al. Neural crest origins of the neck and shoulder. Nature. 2005;436:347–355. doi: 10.1038/nature03837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Abu-Abed S, et al. The retinoic acid-metabolizing enzyme, CYP26A1, is essential for normal hindbrain patterning, vertebral identity, and development of posterior structures. Genes Dev. 2001;15:226–240. doi: 10.1101/gad.855001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kessel M. Respecification of vertebral identities by retinoic acid. Development. 1992;115:487–501. doi: 10.1242/dev.115.2.487. [DOI] [PubMed] [Google Scholar]
- 25.Capecchi MR. Hox genes and mammalian development. Cold Spring Harb Symp Quant Biol. 1997;62:273–281. [PubMed] [Google Scholar]
- 26.Hunt P, Krumlauf R. Hox codes and positional specification in vertebrate embryonic axes. Annu Rev Cell Biol. 1992;8:227–256. doi: 10.1146/annurev.cb.08.110192.001303. [DOI] [PubMed] [Google Scholar]
- 27.Wellik DM. Hox patterning of the vertebrate axial skeleton. Dev Dyn. 2007;236:2454–2463. doi: 10.1002/dvdy.21286. [DOI] [PubMed] [Google Scholar]
- 28.Fischle W, et al. Enzymatic activity associated with class II HDACs is dependent on a multiprotein complex containing HDAC3 and SMRT/N-CoR. Mol Cell. 2002;9:45–57. doi: 10.1016/s1097-2765(01)00429-4. [DOI] [PubMed] [Google Scholar]
- 29.Nagy L, et al. Nuclear receptor repression mediated by a complex containing SMRT, mSin3A, and histone deacetylase. Cell. 1997;89:373–380. doi: 10.1016/s0092-8674(00)80218-4. [DOI] [PubMed] [Google Scholar]
- 30.Kashyap V, Gudas LJ. Epigenetic regulatory mechanisms distinguish retinoic acid-mediated transcriptional responses in stem cells and fibroblasts. J Biol Chem. 2010;285:14534–14548. doi: 10.1074/jbc.M110.115345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mahony S, et al. Ligand-dependent dynamics of retinoic acid receptor binding during early neurogenesis. Genome Biol. 2011;12:R2. doi: 10.1186/gb-2011-12-1-r2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.De Kumar B, et al. Analysis of dynamic changes in retinoid-induced transcription and epigenetic profiles of murine Hox clusters in ES cells. Genome Res. 2015;25:1229–1243. doi: 10.1101/gr.184978.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Soshnikova N, Duboule D. Epigenetic regulation of Hox gene activation: The waltz of methyls. BioEssays. 2008;30:199–202. doi: 10.1002/bies.20724. [DOI] [PubMed] [Google Scholar]
- 34.Agger K, et al. UTX and JMJD3 are histone H3K27 demethylases involved in HOX gene regulation and development. Nature. 2007;449:731–734. doi: 10.1038/nature06145. [DOI] [PubMed] [Google Scholar]
- 35.Rossant J, Zirngibl R, Cado D, Shago M, Giguère V. Expression of a retinoic acid response element-hsplacZ transgene defines specific domains of transcriptional activity during mouse embryogenesis. Genes Dev. 1991;5:1333–1344. doi: 10.1101/gad.5.8.1333. [DOI] [PubMed] [Google Scholar]
- 36.Lander AD. Morpheus unbound: Reimagining the morphogen gradient. Cell. 2007;128:245–256. doi: 10.1016/j.cell.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 37.Duester G. Retinoic acid synthesis and signaling during early organogenesis. Cell. 2008;134:921–931. doi: 10.1016/j.cell.2008.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.MacLean G, et al. Cloning of a novel retinoic-acid metabolizing cytochrome P450, Cyp26B1, and comparative expression analysis with Cyp26A1 during early murine development. Mech Dev. 2001;107:195–201. doi: 10.1016/s0925-4773(01)00463-4. [DOI] [PubMed] [Google Scholar]
- 39.Eldar A, Shilo BZ, Barkai N. Elucidating mechanisms underlying robustness of morphogen gradients. Curr Opin Genet Dev. 2004;14:435–439. doi: 10.1016/j.gde.2004.06.009. [DOI] [PubMed] [Google Scholar]
- 40.Bel-Vialar S, et al. Altered retinoic acid sensitivity and temporal expression of Hox genes in polycomb-M33-deficient mice. Dev Biol. 2000;224:238–249. doi: 10.1006/dbio.2000.9791. [DOI] [PubMed] [Google Scholar]
- 41.Pei L, et al. Thyroid hormone receptor repression is linked to type I pneumocyte-associated respiratory distress syndrome. Nat Med. 2011;17:1466–1472. doi: 10.1038/nm.2450. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.