Significance
Our previous work has shown that mild physical exercise can promote better memory in rodents. Here, we use functional MRI in healthy young adults to assess the immediate impact of a short bout of mild exercise on the brain mechanisms supporting memory processes. We find that this brief intervention rapidly enhanced highly detailed memory processing and resulted in elevated activity in the hippocampus and the surrounding regions, as well as increased coupling between the hippocampus and cortical regions previously known to support detailed memory processing. These findings represent a mechanism by which mild exercise, on par with yoga and tai chi, may improve memory. Future studies should test the long-term effects of regular mild exercise on age-related memory loss.
Keywords: physical exercise, hippocampus, episodic memory, pattern separation, functional MRI
Abstract
Physical exercise has beneficial effects on neurocognitive function, including hippocampus-dependent episodic memory. Exercise intensity level can be assessed according to whether it induces a stress response; the most effective exercise for improving hippocampal function remains unclear. Our prior work using a special treadmill running model in animals has shown that stress-free mild exercise increases hippocampal neuronal activity and promotes adult neurogenesis in the dentate gyrus (DG) of the hippocampus, improving spatial memory performance. However, the rapid modification, from mild exercise, on hippocampal memory function and the exact mechanisms for these changes, in particular the impact on pattern separation acting in the DG and CA3 regions, are yet to be elucidated. To this end, we adopted an acute-exercise design in humans, coupled with high-resolution functional MRI techniques, capable of resolving hippocampal subfields. A single 10-min bout of very light-intensity exercise (30%) results in rapid enhancement in pattern separation and an increase in functional connectivity between hippocampal DG/CA3 and cortical regions (i.e., parahippocampal, angular, and fusiform gyri). Importantly, the magnitude of the enhanced functional connectivity predicted the extent of memory improvement at an individual subject level. These results suggest that brief, very light exercise rapidly enhances hippocampal memory function, possibly by increasing DG/CA3−neocortical functional connectivity.
Physical exercise is an important lifestyle intervention for promoting mental health, including hippocampal-dependent memory. Wheel running has well-known effects on hippocampal neural plasticity and memory in rodents (1); however, the most effective exercise regimen (e.g., intensity level) for improving hippocampal function remains an open question. Exercise intensity can be assessed according to whether it induces a stress response based on the lactate threshold (LT). Our recent studies using an animal model of exercise that utilizes controlled treadmill running to distinguish stress-free mild exercise (below LT) from intense exercise (above LT) have shown that mild exercise increases hippocampal neuronal activity (2) and promotes adult neurogenesis in the dentate gyrus (DG) (3–5), improving spatial memory performance (6). Intriguingly, these effects were suppressed with intense exercise, i.e., follow a hormetic dose–response profile (3–6). Based on this evidence, we hypothesized that very light-intensity exercise can stimulate the human hippocampus, and improve episodic memory through functional activation in the hippocampal network.
To test this hypothesis in humans, we used an acute-exercise design based on our previous human studies (7–10), coupled with high-resolution functional magnetic resonance imaging (fMRI) techniques, capable of resolving hippocampal subfields, to examine the neural substrates of exercise-enhanced hippocampal function. We specifically hypothesized that mild exercise will enhance DG-mediated pattern separation, the process of differentiating among similar experiences to keep stored memories distinct from one another (11). We recently reported that acute moderate-intensity exercise (50% peak oxygen uptake []) improves mnemonic discrimination performance for highly similar items in a task that is thought to rely on DG-mediated pattern separation (12). Using the same experimental design, we conducted two experiments to investigate whether even acute very light, stress-free exercise similarly improves hippocampal memory, and, if so, to identify the underlying neural mechanisms using high-resolution fMRI of hippocampal subfields and cortical regions during task performance.
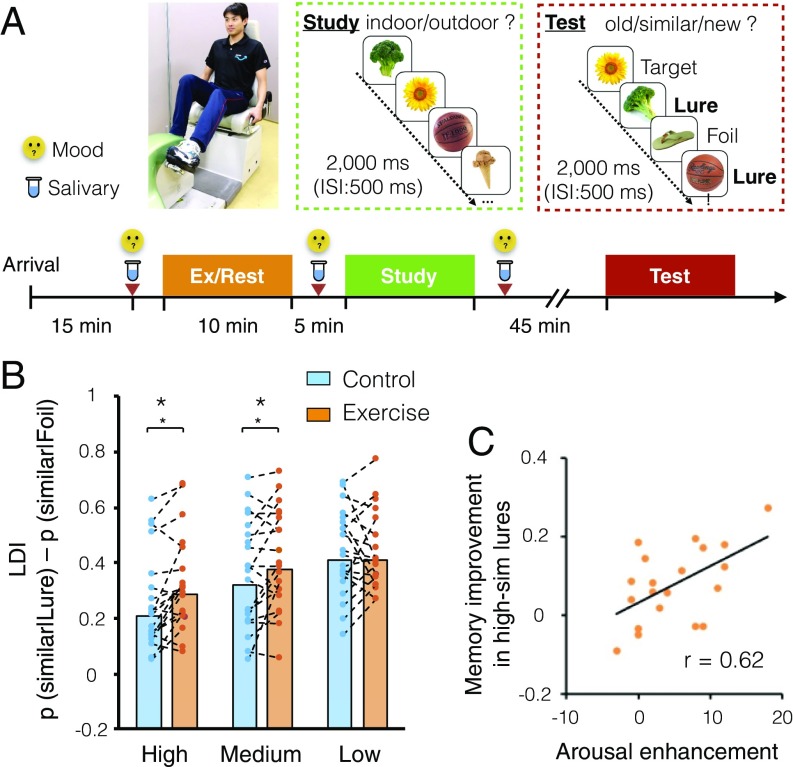
In experiment 1, we assessed the effect of 10 min of acute mild exercise (30% ; defined as “very light” by the American College of Sports Medicine [ACSM]) on performance in the mnemonic discrimination task. We set the exercise duration to 10 min because our past work has shown that a minimum of 10 min of exercise improves cognitive performance (13). Healthy young adults (SI Appendix, Table S1) were assessed under two experimental conditions, control (CTL) and exercise (EX), on separate days in randomized order (Fig. 1A). A within-subject design was applied to increase power and reduce the effects of intersubject variability in the response to exercise. In the EX condition, participants performed 10 min of very light-intensity exercise on a recumbent cycle ergometer, with an individualized load corresponding to 30% of the participant’s . In the CTL condition, participants sat quietly on the ergometer instead of performing exercise. All other conditions were held constant. After 10 min in the EX or CTL condition, participants performed the explicit version of the mnemonic discrimination task described previously (14, 15). During the study phase, participants were shown pictures of everyday objects and asked to indicate whether each item was an indoor or an outdoor item. This was followed by a recognition test in which participants were asked to identify each item as either “old” (targets: previously seen items), “similar” (lures: similar but not identical to previously viewed images), or “new” (foils: new items not previously seen). The lure stimuli varied in the degree of mnemonic similarity to the targets, thereby allowing us to parametrically manipulate the level of interference (12, 14). Parametric changes in discrimination performance dependent on mnemonic interference levels are strongly associated with age-related deficits (14), aerobic fitness-related memory improvement (16), and functional signals in the DG/CA3 (17). Thus, the task and its corresponding lure discrimination measure are appropriate for assessing changes in an individual’s capacity for DG-mediated pattern separation. In addition, we assessed exercise-induced psychological mood changes to examine whether an acute bout of mild exercise leads to increased arousal levels, which may, in turn, mediate improved hippocampal memory function.
Fig. 1.
(A) Outline of the experimental procedures. Participants performed 10 min of exercise or rested (CTL) on different experimental days. After that, the study phase of the mnemonic discrimination task was administered. Participants waited ∼45 min before performing the test phase, an old−similar−new judgment task using targets, foils, and similar lures to which hippocampal pattern separation is particularly sensitive. (B) Discrimination performance assessed by the LDI for high, medium, and low mnemonic similarity bins. Mild exercise improved the LDI for the high- and medium-similarity bins compared with the CTL condition. *P < 0.05. (C) Increased psychological arousal levels positively correlated with LDI improvement in high-similarity lures.
In experiment 2, we assessed the neural substrates of the observed behavioral effects using high-resolution fMRI. Participants performed a continuous recognition version (combining the study and test sessions into one continuous session) of a mnemonic discrimination task in the MRI scanner within ∼5 min after a 10-min mild exercise session (SI Appendix, Fig. S1). We compared neural activity during the critical pattern separation contrast [lure correct rejections (CRs) minus lure false alarms (FAs)] based on prior study (18) between the EX and CTL conditions. Moreover, we assessed functional correlations between hippocampal subfields and cortical regions using psychophysiological interaction (PPI) analysis.
Results
Physiological and Psychological Response to Acute Mild Exercise.
In both experiments, we first confirmed that mean heart rate (HR) at the end of the EX session was within the range of very light intensity according to the ACSM guidelines (SI Appendix, Table S1).
We measured salivary alpha amylase (sAA) and cortisol levels throughout the experiment. A repeated measures two-way ANOVA for sAA levels revealed a significant interaction between the condition and time-point factors [F(2, 36) = 6.73, P < 0.01; SI Appendix, Fig. S3C]. Bonferroni-corrected post hoc comparisons revealed that sAA level in the EX condition was significantly higher for the postexercise session [F(1, 18) = 12.99, P < 0.01]. Differences in salivary cortisol levels were not significant between conditions (SI Appendix, Fig. S3D).
We also measured psychological mood state (arousal and pleasure) by the Two-Dimensional Mood Scale. A repeated measures two-way ANOVA for arousal levels revealed a significant interaction between condition and time point [F(2, 38) = 14.01, P < 0.001; SI Appendix, Fig. S3A]. Bonferroni-corrected post hoc comparisons revealed that arousal level in the EX condition was significantly higher in the postexercise session [F(1, 19) = 30.11, P < 0.001], and there was no significant difference between the preexercise session [F(1, 19) = 2.29, P = 0.15] and the poststudy session [F(1, 19) = 3.74, P = 0.07]. Pleasure levels did not differ significantly between conditions and exhibited no interaction across time points (SI Appendix, Fig. S3B).
Mild Exercise Improves Discrimination Performance for Highly Similar Objects.
The response proportions of the mnemonic discrimination task for each condition in experiment 1 are shown in detail in SI Appendix, Fig. S2A. The statistical analyses methods applied were previously validated to extract response bias-corrected indices of performance (12, 14, 15). The key measure of discrimination (the behavioral correlate of pattern separation) is the lure discrimination index (LDI), which is defined as P(“similar”|lure) minus P(“similar”|foil), calculated separately for each level of similarity/interference (Fig. 1B) (12). A repeated measures two-way ANOVA for condition (EX, CTL) and similarity (high, medium, and low similarity) revealed a significant main effect of condition [F(1, 19) = 5.07, P < 0.05] and similarity [F(2, 38) = 32.96, P < 0.001], and a significant interaction [F(2, 38) = 3.80, P < 0.05]. Bonferroni-corrected post hoc comparisons revealed that the LDI in the EX condition was significantly higher than the LDI in the CTL condition for the high- [F(1, 19) = 13.08, P < 0.01] and medium-similarity lures [F(1, 19) = 5.04, P < 0.05]. No difference was detected between conditions, however, for the low-similarity lures [F(1, 19) < 0.01, P = 0.93]. In addition, exercise-induced arousal enhancement (SI Appendix, Fig. S3A) positively correlated with the LDI improvement for high-similarity lures (r = 0.54, P < 0.05), but not with medium- (r = 0.05, P = 0.83) or low- (r = −0.36, P = 0.12) similarity lures (adjusted significance threshold using the Bonferroni method; Fig. 1C).
For experiment 2, in an orthogonal sample, we observed that overall performance of the continuous version of the mnemonic discrimination task in the MRI scanner was comparable to that in experiment 1 (SI Appendix, Fig. S2C). In addition, performance differed significantly between the EX and CTL conditions [t(15) = 2.61, P < 0.05; SI Appendix, Fig. S2D], which served as an independent replication of the findings in experiment 1.
Exercise Enhances Pattern Separation Related Activity in the Hippocampal Network.
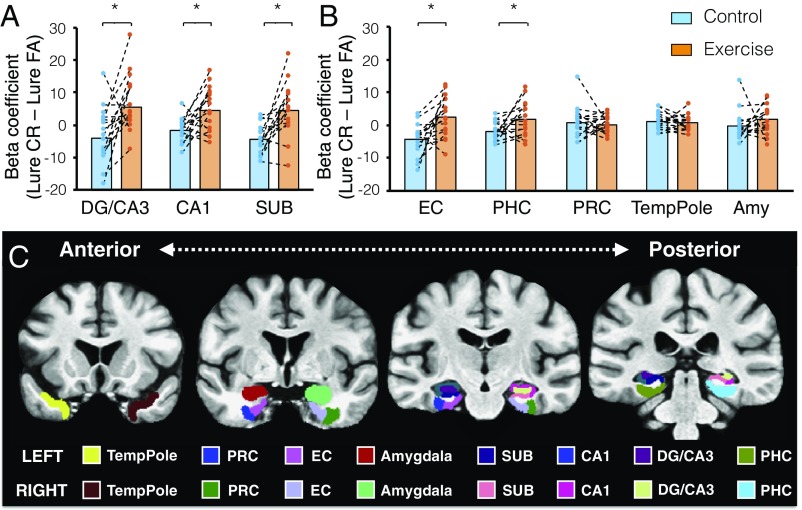
We further examined the critical contrast of fMRI signals (lure CRs minus lure FAs) with a repeated measures ANOVA, and limited our analysis of regions of interest (ROIs) to hippocampal subregions and the medial temporal lobe. We observed a main effect for condition [F(1, 15) = 18.88, P < 0.001] and a significant interaction between condition and region [F(7, 105) = 5.18, P < 0.001]. Holm−Bonferroni-corrected post hoc comparisons revealed higher levels of activation in the EX condition compared with the CTL condition across hippocampal subfields, including the DG/CA3 [F(1, 15) = 9.70, P < 0.01], CA1 [F(1, 15) = 10.26, P < 0.01], and subiculum [F(1, 15) = 16.98, P < 0.001; Fig. 2A]. We also observed similar increases in the entorhinal cortex (EC) [F(1, 15) = 10.92, P < 0.01] and parahippocampal cortex (PHC) [F(1, 15) = 5.71, P < 0.05; Fig. 2B].
Fig. 2.
Neural activity profiles in (A) the hippocampus and (B) other ROIs. Values indicate the critical pattern separation contrast of fMRI signals (lure CRs minus lure FAs). Of all hippocampal subfields, the EC and PHC exhibited higher levels of activation during the EX condition compared with the CTL condition. *P < 0.05. (C) Coronal view of ROI segmentation on a custom group template. Representative slices are shown from top to bottom in the anterior−posterior direction, and ROI demarcations are represented based on the color key displayed below. Note: PRC, perirhinal cortex; SUB, subiculum; TempPole, temporal pole.
Increase in Functional Connectivity Between DG/CA3 and Cortical Regions.
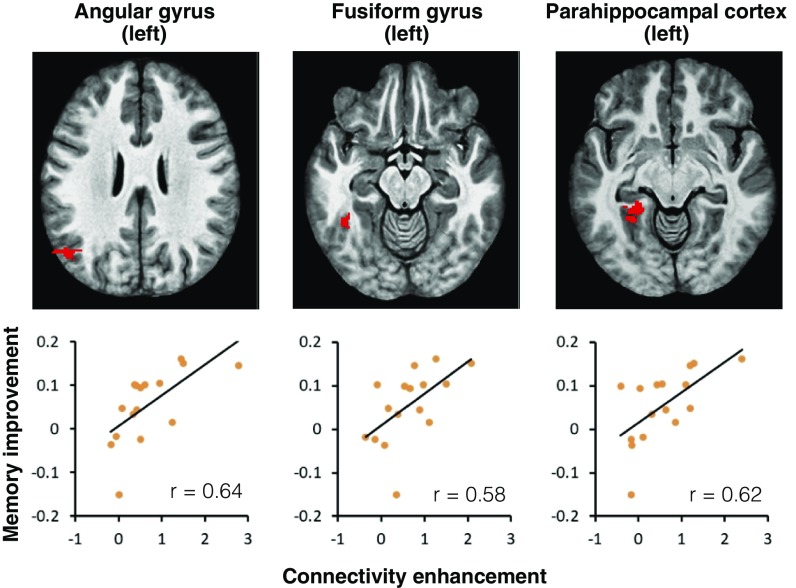
To assess whether exercise affected the functional connectivity between hippocampal and neocortical sites, we conducted a PPI analysis. Briefly, in both the EX and CTL conditions, we extracted the time series of activity from seed regions (DG/CA3, CA1, subiculum, and whole-hippocampus ROIs) during specific trials (lure CRs and lure FAs). Specifically, we tested the hypothesis that improved discrimination is mediated by increased functional connectivity between the DG/CA3 region and extrahippocampal/cortical regions involved in recall (see Materials and Methods for details). Seeding the DG/CA3 subfield bilaterally during the lure CRs condition, we found significant correlations with the left angular gyrus, left fusiform gyrus, and left PHC (P < 0.05 family-wise error corrected; Fig. 3). We further detected a significant positive correlation between the DG/CA3 and left primary visual cortex, and a significant negative correlation between the DG/CA3 and temporal pole (P < 0.05 family-wise error corrected; SI Appendix, Fig. S5). Conversely, when we seeded the whole hippocampus (including CA1 and subiculum in addition to DG/CA3), we found a significant correlation between the DG/CA3 region and bilateral PHC during lure CRs, but not the angular gyrus or fusiform gyrus. Neither seed region correlated significantly with the DG/CA3 region during lure FAs. Finally, seeding the CA1 or subiculum did not reveal significant correlations that survived the corrected threshold. Thus, the DG/CA3 appears to make somewhat specific contributions to cortical communication during lure CRs.
Fig. 3.
Results of PPI analyses. (Upper) Voxels within cortical regions with significantly higher context-dependent (lure CRs) correlations with the hippocampal DG/CA3 in the EX condition compared with the CTL condition. (Lower) Significant correlations between the extent of PPI connectivity of the DG/CA3 with the specified cortical region and the enhancement in the LDI resulting from exercise. These brain−behavior relationships are observed in the left angular gyrus, left fusiform gyrus, and left parahippocampal gyrus.
Enhanced Functional Connectivity Correlates with Memory Improvement.
We next evaluated whether these correlations were predictive of the behavioral benefit observed in the EX condition. We calculated a simple change in the performance metric from the behavioral data (LDIEX – LDICTL), and correlated this with the magnitude of the change in the correlation between the PPI seed and target regions. Behavioral improvement was predicted by higher correlations between the DG/CA3 and the angular gyrus (r = 0.64, P < 0.01), fusiform gyrus (r = 0.57, P < 0.05), and PHC (r = 0.62, P < 0.01) across participants (adjusted significance threshold using the Holm−Bonferroni method; Fig. 3). No significant correlations were detected between the DG/CA3 and primary visual cortex or temporal pole. Additionally, no significant correlations were detected when seeding the whole hippocampus. Thus, specific correlations between the DG/CA3 and cortical regions known to be involved in detailed forms of memory seem to predict the exercise-related improvement in behavior.
Discussion
The findings of the present study demonstrate that acute very light exercise improves hippocampal memory function, especially DG-mediated pattern separation. Furthermore, from the results of high-resolution fMRI analysis, involvement of hippocampal−cortical networks as an underlying neural basis of memory improvement has emerged. Although there is a large literature on exercise effects on the human brain (19, 20), including the impact of long-term moderate-intensity exercise interventions on hippocampal volume (21) and DG cerebral blood volume (22), this study demonstrates rapid enhancement of hippocampal memory function with acute very light exercise.
The results of experiment 1 revealed that 10 min of very light intensity (30%) exercise improved discrimination performance for high- and medium-similarity lures, the more difficult discrimination conditions. Because the DG/CA3 region is highly sensitive to small changes in sensory input (23), a hallmark feature of prior studies of pattern separation is the effect on discrimination performance for high-, but not necessarily low-, similarity items (11). The results of experiment 2 independently replicated the behavioral effect observed in experiment 1 under scanning conditions while using a continuous recognition variant of the same task. The short bout (10 min) of mild exercise increased activity specifically in hippocampal subregions, and in the entorhinal and parahippocampal cortices. Other regions within the scanning field of view, such as the perirhinal cortex, temporal pole, and amygdala, exhibited no change in activation, suggesting that this effect is specific to certain brain regions and not secondary to global brain changes induced by exercise. This particular network of brain subregions may be involved in processing sensory input together with the hippocampus, or representing recalled information as distinct from the current experience. Based on context-dependent PPI analysis, exercise increased the functional connectivity between the DG/CA3 and associated memory cortices (i.e., parahippocampal, angular, and fusiform gyri) during correct rejection of lures, and the magnitude of the enhancement correlated positively with the magnitude of the improvement in discrimination performance. These findings support the hypothesis that mild exercise improves hippocampal memory by facilitating DG/CA3 communication with surrounding neocortical regions.
The behavioral results in experiment 1 support and extend our previous findings that acute exercise at moderate intensity, which is around the LT, positively affects hippocampal memory (12). Mild exercise does not increase the release of lactate and adrenocorticotropic hormone, and is therefore considered stress-free exercise (24), as confirmed in the present study by the stable salivary cortisol levels (SI Appendix, Fig. S3D). Mild exercise is highly practical and feasible, especially for older adults and individuals with physical disabilities and low levels of physical fitness. We have previously shown that acute mild exercise positively improves prefrontal executive function (7), and here we provide evidence that mild exercise also improves hippocampal pattern separation, and propose a mechanistic account for this improvement at the level of hippocampal subfields and hippocampal−neocortical communication. Importantly, the rapid form of plasticity observed is distinct from previously reported neurogenesis-mediated effects of exercise interventions, which operate on a much longer timescale (1). It is possible that the increased connectivity we observed is associated with synaptogenesis that may provide a suitable niche for the subsequent integration of newborn granule cells. Past studies have shown that a large proportion of newborn granule cells die within a short period of time if not integrated within functional networks (25). Perhaps increasing connectivity via exercise allows for this integration to occur.
The increased context-dependent functional connectivity observed is consistent with the idea that detailed memories involve strengthening of shared representations across the hippocampus and neocortex (26). Correlations with the primary visual cortices, the angular gyrus, the fusiform gyrus, and the PHC are implicated in rich, vivid recollection processes of visual information, including the well-known contextual reinstatement effect (27–29). For instance, the angular gyrus is considered to be a convergence point between multisensory inputs (serving as an integrator) and top-down predictions, with a critical role in episodic memory retrieval, particularly during successful recollection (28, 30). The fusiform gyrus is a part of the ventral visual stream (higher-order visual cortex) and is a key region involved in functionally specialized computations of higher-level visual features such as object recognition and face perception (31, 32). Similar processing of complex features could be employed here in our object discrimination task, where stimuli are processed holistically (33). The PHC is thought to be part of a network of brain regions that processes contextual associations, and is involved in associative memory (27, 29). Interestingly, a negative correlation between DG/CA3 and the temporal pole (left) with exercise was observed. The temporal pole is implicated in general, scheme-based memory (34), and is often implicated in false memory (35). Thus, this negative relationship could also reflect a sharper, more accurate representation as a result of exercise. Taken together, we suggest that these brain regions play a role in representing high-precision memories, and enhanced communication with the DG/CA3 may contribute to improve memory discrimination.
Although the molecular, synaptic, and chemical bases of the transient modulation of pattern separation by mild exercise remain largely unclear, the observed correlation between the change in psychological arousal and improved cognitive performance, similar to our previous findings (7), suggests that mild exercise-related activation of the arousal system improves hippocampal memory. DG function is regulated by several neuromodulatory systems, including cholinergic input from the medial septum (36). Cholinergic modulation is also thought to be involved in switching hippocampal network modes between recall and storage (36). Mild exercise such as treadmill walking in animals increases hippocampal acetylcholine concentrations (37). Up-regulation of acetylcholine by exercise may increase arousal levels and improve DG-mediated pattern separation.
This study has several limitations. First, in the present experimental design, the effect of exercise on encoding could not be separated from the effect on storage/consolidation. A poststudy intervention design (15) is required to assess distinct contributions of exercise to facilitating encoding vs. storage mechanisms. Second, we adopted a high-resolution blood-oxygen-level−dependent fMRI sequence focusing on the medial temporal lobe and posterior parietal regions, and could therefore not adequately assess the activity of other brain regions that may be involved, e.g., the frontal lobes (38). Finally, the exercise intensity required to optimize this effect is unknown. Previous findings in rodents showed that mild exercise training, compared with intense exercise training, increases survival and maturation of newborn neurons, and induces the expression of a larger number of genes and proteins, suggesting that mild exercise has more molecular effects than explored here (4). Further studies are needed to evaluate these points.
In conclusion, the present study demonstrates that a single bout of very light-intensity exercise, comparable to walking at slow pace or traditional oriental bodywork such as yoga and tai chi, improves hippocampal pattern separation, possibly by enhancing functional activity levels across hippocampal subfields and bolstering DG/CA3-neocortical communication. These transient responses to acute exercise are a potential basis for hippocampal adaptation to chronic interventions observed in both humans and animals. This is of particular significance, since episodic memory loss is present in many conditions, including Alzheimer’s disease, and much less is currently known about the utility of milder interventions. Given physical capacity and activity limitations common to the elderly and vulnerable populations, the use of mild exercise to slow down or stave off cognitive decline is a crucial avenue of future exercise investigation.
Materials and Methods
For a full description of all materials and methods, see SI Appendix, SI Materials and Methods.
Participants.
A total of 36 healthy young adults participated in the study; 20 (mean age 20.6 ± 1.7 y, 8 women) participated in experiment 1, and 16 (mean age 21.1 ± 2.0 y, 12 women) participated in experiment 2. None of the subjects reported a history of neurological or psychiatric disorders, or had a disease requiring medical care. All participants had normal or corrected-to-normal vision and normal color vision. All participants provided written informed consent to participate in the study. The University of Tsukuba Ethics Committee approved the study protocol, which conformed to the ethical principles of the seventh revision (2013) of the Declaration of Helsinki. Participants’ demographic and physiological characteristics are presented in SI Appendix, Table S1. Based on our previous studies (7–10, 12) and sample size determination software G-power (39), 20 and 16 subjects were considered sufficient to detect a significant difference (dz = 0.9) between groups on a two-sided, 0.05 test of proportions (difference between two dependent means [matched pairs]) with >80% power.
Experiment 1 Procedures.
All participants underwent the CTL and EX experiments on separate days in a randomized order (Fig. 1A). All experiments were conducted at the same time of day for each participant, and the experiments were started between the hours of 1200 and 1800. The two experimental days were separated by at least 48 h. Participants were also asked to refrain from exercise and consuming alcohol and caffeine for at least 24 h before the experiment to control for outside factors that could affect cognitive function.
An outline of the experimental procedures is shown in Fig. 1A. Twenty minutes after arrival, participants performed 10 min of mild exercise on a recumbent cycle ergometer (Corival Recumbent; Lode), with an individualized load corresponding to 30% of the subject’s in the EX condition. We previously reported that a single 10-min bout of exercise enhances prefrontal activation and executive function in young adults (7–9); thus we used the same parameters for this experiment. Heart rate (HR) and Borg’s rating of perceived exertion (RPE) (40) were recorded once every minute during exercise. In the CTL condition, participants sat on the recumbent cycle ergometer for 10 min and did not pedal. Approximately 5 min after the 10-min exercise or rest period, participants began the encoding phase of the discrimination task. After completing the encoding phase, the participants rested for 45 min while they watched a movie (low-arousal stimulus) without sound to avoid falling asleep. After the rest period, participants performed the retrieval phase of the mnemonic discrimination task.
Mnemonic Discrimination Task.
The task used in this study consisted of an encoding and retrieval phase (Fig. 1A). In the encoding phase, participants viewed a series of 196 color photographs of everyday objects on a white background on a computer screen and were required to judge whether the item displayed represented an indoor or outdoor object. In the retrieval phase, participants viewed a series of 256 color photographs of various objects and were asked to identify each item as “old,” “similar,” or “new” by pressing buttons. Sixty-four (25%) of the presented items in the retrieval phase were “old,” or exact repetitions of those presented in the encoding phase (targets); 128 (50%) of the items were “similar” to those seen during the encoding phase, but not identical (lures); and 64 (25%) were “new” items not previously presented (foils). In both phases, each picture was presented for 2 s with a 0.5-s interstimulus interval. All participants underwent a practice session (four encoding items, eight retrieval items) to confirm their understanding of the task instructions and procedures, using photographs that were not included in the experimental task sets.
The task measures discrimination performance for lures with varying degrees of similarity. The lure stimuli were stratified into three bins, namely high-, medium-, and low-similarity lures, based on the discrimination rating for each similar object pair to the targets. The ratings were based on testing in an orthogonal data set with n > 100 adults to arrive at ratings that are highly stable and reliable (41). This is superior to using a simple perceptual similarity rating or an automated computer algorithm to determine similarity based on features, as it takes into account the level of familiarity and “confusability” of specific object classes. We have used the same approach in numerous studies in the past, and it has been replicated across multiple laboratories (see ref. 42 for recent review). The LDI was calculated as the probability of correctly responding “similar” when presented with similar lure objects minus the probability of incorrectly responding “similar” when presented with novel foil objects [p (similar|lure) − p (similar|new)] for each similarity bin. Subtraction was used to correct for any bias in selecting “similar” overall.
Experiment 2 Procedures.
The overall experimental design and procedure was the same as for experiment 1 (SI Appendix, Fig. S1). The recumbent ergometer was placed in the anteroom of the MRI scanner. After the 10-min period of exercise or rest, the participants were quickly placed into the scanner as instructed before the experiment. HR was calculated from the continuous signal derived from an MRI-compatible pulse oximeter (4500 MRI Pulse Oximeter; Invivo) placed over the left index finger (SI Appendix, Fig. S4). Before beginning the memory task, 12 images of high-speed echoplanar single shot (five images for coronal plane, seven images for sagittal plane) were obtained to fix the imaging area of the functional echoplanar imaging (EPI) scans. All participants started the task within ∼5 min after the end of the EX or CTL session (mean 5 min 31 s ± 17.2 s). The exercise−scan interval was set to 5 min because noncerebral hemodynamic variables such as middle cerebral artery mean blood velocity and skin blood flow increase following 10 min of very light-intensity exercise and return to basal levels within 5 min (43). Structural magnetization-prepared rapid gradient echo (MPRAGE) scans were collected for anatomical localization and cross-subject alignment, followed by functional EPI scans on the first experimental day for each participant.
MRI Data Acquisition.
Neuroimaging data were acquired on a 3.0 Tesla Philips scanner with a 32-channel sensitivity encoding (SENSE) head coil at the Center for Cybernics Research at the University of Tsukuba. Functional images were collected using a high-speed T2*-weighted EPI sequence with an acquisition matrix size of 64 × 64, repetition time of 2,000 ms, echo time of 35 ms, flip angle of 70°, field of view (FOV) of 96 × 96 mm, SENSE parallel reduction factor of 2, and in-plane resolution of 1.5 × 1.5 × 1.5 mm. Each volume comprised 19 oblique 1.5-mm-thick axial slices with no gap parallel to the principal axis of the hippocampus and covered the medial temporal lobe bilaterally. Each run comprised 144 trials, and each trial was presented for 2,000 ms with a 500-ms interstimulus interval. Four initial “dummy” volumes were acquired to ensure MR signal stabilization. Each subject completed four functional runs. We also collected a high-resolution structural scan using an MPRAGE T1-weighted sequence with an FOV of 384 × 384 mm, repetition time of 12 ms, echo time of 5.9 ms, and flip angle of 9°, comprising 250 oblique slices with 0.65-mm isotropic resolution after functional runs of the EX or CTL session. All images for each subject are uploaded in XNAT CENTRAL (https://www.re3data.org/repository/r3d100010874; Acute Mild Exercise).
fMRI Data Analysis: General Linear Model Regression.
Only test data are included in the analyses. Behavioral vectors based on the trial type (i.e., target hits and misses, lure CRs and FAs) were used to model the data using a deconvolution approach based on multiple linear regression (3dDeconvolve). Deconvolution of the hemodynamic response was achieved using tent functions covering stimulus onset to 12 s after onset with six estimator functions distributed across this time window. In addition to modeling trials of interest, motion parameters were entered into the model as explicit repressors to reduce the effect of motion on task-related parameter estimates. Additionally, global signals from white matter and ventricles were regressed from the modeled signal in the gray matter using ANATICOR (44), conforming to the rigorous data scrubbing procedures recommended by Power et al. (45). These scripts, in addition to those for preprocessing as outlined in MRI Data Acquisition, are available at https://github.com/yassalab/afni_proc_py_pipeline.
The statistical fit coefficients resulting from the regression analysis represent the difference in activity between trial types and the baseline (novel foil trials) for a given time-point in a voxel. The sum of the fit coefficients over the expected hemodynamic response (3–12 s after trial onset) was taken as the model’s estimate of the relative response to each trial type. Group analyses were performed using a two-way analysis of variance (ANOVA) with trial type and condition (EX vs. CTL) as fixed factors, and participant as a random factor, nested within condition. Each participant’s overall F-statistic (i.e., activity that was modulated by any aspect of the task) was thresholded at P < 0.05 with a cluster-corrected threshold of 19 voxels to create a mask of “task-active” voxels, which was then combined with anatomical ROIs to create new hybrid functional/structural ROIs. Importantly, use of the overall F-statistic eliminates concerns about circularity because the voxels were not selected based on the contrast of interest (17, 46). Voxels in these ROIs were collapsed and the mean activity in each ROI was extracted to conduct second-level analyses. This approach reduces voxel-selection biases and enhances the signal-to-noise ratio. This yielded eight bilateral ROIs in the hippocampus (DG/CA3, CA1, and subiculum), cortical regions (temporopolar cortex, PRC, EC, and PHC), and amygdala. A contrast of activity during lure CRs vs. lure FAs was calculated. Subsequent testing was conducted using a two-way repeated measures ANOVA with condition (EX and CTL) and region (DG/CA3, CA1, subiculum, temporopolar cortex, PRC, EC, PHC, amygdala). We kept all hippocampal subfield ROIs as bilateral ROIs and did not split them by hemisphere to reduce the number of comparisons and because we had no a priori reason to separate right from left in this particular task. When a significant main effect or interaction was detected by the ANOVA, we adjusted the significance threshold using the Holm-Bonferroni method to parse the effect with post hoc comparisons.
fMRI Data Analysis: Interregional Correlations and Interactions.
We performed a generalized PPI analysis, also termed context-dependent correlation analysis (47), with the test data. Details of these analysis steps in Analysis of Functional NeuroImages can be found here: (https://afni.nimh.nih.gov/CD-CorrAna). Briefly, a positive correlation indicates a positive relationship between significant voxels and a seed region in a given condition, whereas a negative correlation indicates a negative relationship.
In the generalized PPI analysis, we individually modeled correlations during target hits and misses, as well as lure CRs and FAs (i.e., the “psychological variables”). In these data, we were particularly interested in lure discrimination, so we focused our analyses on lure trials. Given that pattern separation performed by the DG/CA3 is involved in lure discrimination, we generated a seed time series in the bilateral DG/CA3 (3dmaskave; i.e., the “physiological variable”) and detrended the time series (3dDetrend; this seed time series features the same data scrubbing steps described previously). After transposing the detrended time series as a column vector (1dtranspose), we generated a canonical hemodynamic response function (waver) and used this to extract the expected contributions of blood-oxygen-level−dependent signaling to the time series and generate an up-sampled “neural time series” (3dTfitter). We next multiplied the resulting neural time series with stimulus timing files (timing_tool.py and 1deval), which were then convolved with the canonical hemodynamic response function to create the interaction regressor (waver). This regressor was entered into a general linear model (3dDeconvolve), where the ensuing beta weights reflect the degree of context-dependent correlations with the seeded region. To limit the effect of voxels on the edge of the functional acquisitions whose susceptibility to motion and partial volumes can induce spurious correlations, we removed the outermost-edge voxels from the correlation maps before the regression analysis (3dZeropad). To test whether any resultant significant correlations were specific to DG/CA3, we also repeated these steps using each bilateral whole-hippocampus, CA1, and subiculum seed.
To visualize correlational structures across the brain, PPI analyses were voxel-based rather than ROI-based. In these cases, we applied the appropriate statistical corrections. Individual subject maps were analyzed at the group level using t tests (3dttest++). Voxels in the group analysis were considered significant at P < 0.05 corrected for family-wise error rate (parameters are as reported in the t test over the general linear model analysis described in fMRI Data Analysis: General Linear Model Regression). Illustrative voxels in the statistical maps were thresholded as described in fMRI Data Analysis: General Linear Model Regression.
Supplementary Material
Acknowledgments
We thank members of the Laboratory of Exercise Biochemistry and Neuroendocrinology for their assistance with data collection. This work was supported, in part, by the Special Funds for Education and Research of the Ministry of Education, Culture, Sports, Science, and Technology 1111501004 (to H.S.); the Japan Society for the Promotion of Science Grants HFH27016 (to H.S.), 16H06405 [“Creation and promotion of WILLDYNAMICS” (to H.S.)], 18H04081 (to H.S.), and 16K20930 (to K.B.); the US National Institutes of Health Grants R01MH102392, R21AG049220, R01AG053555, and P50AG16573 (to M.A.Y.); and the Center for Exercise Medicine and Sport Sciences at the University of California, Irvine.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: All neuroimaging data were deposited with XNAT CENTRAL and are available at https://central.xnat.org (Acute Mild Exercise). The fMRI scripts were deposited on GitHub and are available at https://github.com/yassalab/afni_proc_py_pipeline.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1805668115/-/DCSupplemental.
References
- 1.van Praag H, Kempermann G, Gage FH. Running increases cell proliferation and neurogenesis in the adult mouse dentate gyrus. Nat Neurosci. 1999;2:266–270. doi: 10.1038/6368. [DOI] [PubMed] [Google Scholar]
- 2.Soya H, et al. BDNF induction with mild exercise in the rat hippocampus. Biochem Biophys Res Commun. 2007;358:961–967. doi: 10.1016/j.bbrc.2007.04.173. [DOI] [PubMed] [Google Scholar]
- 3.Okamoto M, et al. Mild exercise increases dihydrotestosterone in hippocampus providing evidence for androgenic mediation of neurogenesis. Proc Natl Acad Sci USA. 2012;109:13100–13105. doi: 10.1073/pnas.1210023109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Inoue K, et al. Long-term mild, rather than intense, exercise enhances adult hippocampal neurogenesis and greatly changes the transcriptomic profile of the hippocampus. PLoS One. 2015;10:e0128720. doi: 10.1371/journal.pone.0128720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Okamoto M, et al. Hormetic effects by exercise on hippocampal neurogenesis with glucocorticoid signaling. Brain Plast. 2015;1:149–158. doi: 10.3233/BPL-150012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Inoue K, et al. Long-term mild exercise training enhances hippocampus-dependent memory in rats. Int J Sports Med. 2015;36:280–285. doi: 10.1055/s-0034-1390465. [DOI] [PubMed] [Google Scholar]
- 7.Byun K, et al. Positive effect of acute mild exercise on executive function via arousal-related prefrontal activations: An fNIRS study. Neuroimage. 2014;98:336–345. doi: 10.1016/j.neuroimage.2014.04.067. [DOI] [PubMed] [Google Scholar]
- 8.Hyodo K, et al. Acute moderate exercise enhances compensatory brain activation in older adults. Neurobiol Aging. 2012;33:2621–2632. doi: 10.1016/j.neurobiolaging.2011.12.022. [DOI] [PubMed] [Google Scholar]
- 9.Yanagisawa H, et al. Acute moderate exercise elicits increased dorsolateral prefrontal activation and improves cognitive performance with Stroop test. Neuroimage. 2010;50:1702–1710. doi: 10.1016/j.neuroimage.2009.12.023. [DOI] [PubMed] [Google Scholar]
- 10.Kujach S, et al. A transferable high-intensity intermittent exercise improves executive performance in association with dorsolateral prefrontal activation in young adults. Neuroimage. 2018;169:117–125. doi: 10.1016/j.neuroimage.2017.12.003. [DOI] [PubMed] [Google Scholar]
- 11.Yassa MA, Stark CEL. Pattern separation in the hippocampus. Trends Neurosci. 2011;34:515–525. doi: 10.1016/j.tins.2011.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Suwabe K, et al. Acute moderate exercise improves mnemonic discrimination in young adults. Hippocampus. 2017;27:229–234. doi: 10.1002/hipo.22695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yanagisawa H, Soya H, Dan I. Effect of duration of acute moderate exercise on exercise-elicited cortical activation and cognitive performance on Stroop task: A preliminary examination. Int J Hum Mov Sci. 2009;3:111–132. [Google Scholar]
- 14.Stark SM, Yassa MA, Lacy JW, Stark CEL. A task to assess behavioral pattern separation (BPS) in humans: Data from healthy aging and mild cognitive impairment. Neuropsychologia. 2013;51:2442–2449. doi: 10.1016/j.neuropsychologia.2012.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Borota D, et al. Post-study caffeine administration enhances memory consolidation in humans. Nat Neurosci. 2014;17:201–203. doi: 10.1038/nn.3623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Suwabe K, et al. Aerobic fitness associates with mnemonic discrimination as a mediator of physical activity effects: Evidence for memory flexibility in young adults. Sci Rep. 2017;7:5140. doi: 10.1038/s41598-017-04850-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yassa MA, Mattfeld AT, Stark SM, Stark CEL. Age-related memory deficits linked to circuit-specific disruptions in the hippocampus. Proc Natl Acad Sci USA. 2011;108:8873–8878. doi: 10.1073/pnas.1101567108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yassa MA, et al. High-resolution structural and functional MRI of hippocampal CA3 and dentate gyrus in patients with amnestic mild cognitive impairment. Neuroimage. 2010;51:1242–1252. doi: 10.1016/j.neuroimage.2010.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Prakash RS, Voss MW, Erickson KI, Kramer AF. Physical activity and cognitive vitality. Annu Rev Psychol. 2015;66:769–797. doi: 10.1146/annurev-psych-010814-015249. [DOI] [PubMed] [Google Scholar]
- 20.Voss MW, Vivar C, Kramer AF, van Praag H. Bridging animal and human models of exercise-induced brain plasticity. Trends Cogn Sci. 2013;17:525–544. doi: 10.1016/j.tics.2013.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Erickson KI, et al. Exercise training increases size of hippocampus and improves memory. Proc Natl Acad Sci USA. 2011;108:3017–3022. doi: 10.1073/pnas.1015950108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pereira AC, et al. An in vivo correlate of exercise-induced neurogenesis in the adult dentate gyrus. Proc Natl Acad Sci USA. 2007;104:5638–5643. doi: 10.1073/pnas.0611721104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lacy JW, Yassa MA, Stark SM, Muftuler LT, Stark CEL. Distinct pattern separation related transfer functions in human CA3/dentate and CA1 revealed using high-resolution fMRI and variable mnemonic similarity. Learn Mem. 2010;18:15–18. doi: 10.1101/lm.1971111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Soya H, et al. Threshold-like pattern of neuronal activation in the hypothalamus during treadmill running: Establishment of a minimum running stress (MRS) rat model. Neurosci Res. 2007;58:341–348. doi: 10.1016/j.neures.2007.04.004. [DOI] [PubMed] [Google Scholar]
- 25.Deng W, Aimone JB, Gage FH. New neurons and new memories: How does adult hippocampal neurogenesis affect learning and memory? Nat Rev Neurosci. 2010;11:339–350. doi: 10.1038/nrn2822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Reagh ZM, Murray EA, Yassa MA. Repetition reveals ups and downs of hippocampal, thalamic, and neocortical engagement during mnemonic decisions. Hippocampus. 2017;27:169–183. doi: 10.1002/hipo.22681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Aminoff EM, Kveraga K, Bar M. The role of the parahippocampal cortex in cognition. Trends Cogn Sci. 2013;17:379–390. doi: 10.1016/j.tics.2013.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rugg MD, King DR. Ventral lateral parietal cortex and episodic memory retrieval. Cortex. July 25, 2017 doi: 10.1016/j.cortex.2017.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Elward RL, Rugg MD. Retrieval goal modulates memory for context. J Cogn Neurosci. 2015;27:2529–2540. doi: 10.1162/jocn_a_00878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Seghier ML. The angular gyrus: Multiple functions and multiple subdivisions. Neuroscientist. 2013;19:43–61. doi: 10.1177/1073858412440596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Weiner KS, Zilles K. The anatomical and functional specialization of the fusiform gyrus. Neuropsychologia. 2016;83:48–62. doi: 10.1016/j.neuropsychologia.2015.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Haxby JV, Hoffman EA, Gobbini MI. The distributed human neural system for face perception. Trends Cogn Sci. 2000;4:223–233. doi: 10.1016/s1364-6613(00)01482-0. [DOI] [PubMed] [Google Scholar]
- 33.Hanson SJ, Matsuka T, Haxby JV. Combinatorial codes in ventral temporal lobe for object recognition: Haxby (2001) revisited: Is there a “face” area? Neuroimage. 2004;23:156–166. doi: 10.1016/j.neuroimage.2004.05.020. [DOI] [PubMed] [Google Scholar]
- 34.Patterson K, Nestor PJ, Rogers TT. Where do you know what you know? The representation of semantic knowledge in the human brain. Nat Rev Neurosci. 2007;8:976–987. doi: 10.1038/nrn2277. [DOI] [PubMed] [Google Scholar]
- 35.Chadwick MJ, et al. Semantic representations in the temporal pole predict false memories. Proc Natl Acad Sci USA. 2016;113:10180–10185. doi: 10.1073/pnas.1610686113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hasselmo ME, Schnell E, Barkai E. Dynamics of learning and recall at excitatory recurrent synapses and cholinergic modulation in rat hippocampal region CA3. J Neurosci. 1995;15:5249–5262. doi: 10.1523/JNEUROSCI.15-07-05249.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nakajima K, Uchida S, Suzuki A, Hotta H, Aikawa Y. The effect of walking on regional blood flow and acetylcholine in the hippocampus in conscious rats. Auton Neurosci. 2003;103:83–92. doi: 10.1016/s1566-0702(02)00263-1. [DOI] [PubMed] [Google Scholar]
- 38.Pidgeon LM, Morcom AM. Cortical pattern separation and item-specific memory encoding. Neuropsychologia. 2016;85:256–271. doi: 10.1016/j.neuropsychologia.2016.03.026. [DOI] [PubMed] [Google Scholar]
- 39.Faul F, Erdfelder E, Lang A-G, Buchner A. G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods. 2007;39:175–191. doi: 10.3758/bf03193146. [DOI] [PubMed] [Google Scholar]
- 40.Borg GA. Psychophysical bases of perceived exertion. Med Sci Sports Exerc. 1982;14:377–381. [PubMed] [Google Scholar]
- 41.Yassa MA, et al. Pattern separation deficits associated with increased hippocampal CA3 and dentate gyrus activity in nondemented older adults. Hippocampus. 2011;21:968–979. doi: 10.1002/hipo.20808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Leal SL, Yassa MA. Integrating new findings and examining clinical applications of pattern separation. Nat Neurosci. 2018;21:163–173. doi: 10.1038/s41593-017-0065-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Byun K, et al. Possible influences of exercise-intensity-dependent increases in non-cortical hemodynamic variables on NIRS-based neuroimaging analysis during cognitive tasks: Technical note. J Exerc Nutrition Biochem. 2014;18:327–332. doi: 10.5717/jenb.2014.18.4.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jo HJ, Saad ZS, Simmons WK, Milbury LA, Cox RW. Mapping sources of correlation in resting state FMRI, with artifact detection and removal. Neuroimage. 2010;52:571–582. doi: 10.1016/j.neuroimage.2010.04.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Power JD, Barnes KA, Snyder AZ, Schlaggar BL, Petersen SE. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. Neuroimage. 2012;59:2142–2154. doi: 10.1016/j.neuroimage.2011.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Reagh ZM, Yassa MA. Object and spatial mnemonic interference differentially engage lateral and medial entorhinal cortex in humans. Proc Natl Acad Sci USA. 2014;111:E4264–E4273. doi: 10.1073/pnas.1411250111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.McLaren DG, Ries ML, Xu G, Johnson SC. A generalized form of context-dependent psychophysiological interactions (gPPI): A comparison to standard approaches. Neuroimage. 2012;61:1277–1286. doi: 10.1016/j.neuroimage.2012.03.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.