Significance
Pgrmc1 plays an important role in mediating progesterone’s protective effects in that it is a critical mediator of progesterone-induced BDNF release. Here, we identified the microRNA let-7i, which increased in stroke, as a negative regulator of Pgrmc1 and BDNF expression. Conversely, inhibition of let-7i enhanced progesterone’s protective effects against stroke. In addition to enhancing progesterone’s neuroprotective effects, the fact that let-7i also diminishes the expression of BDNF suggests that inhibition of let-7i may also be useful to any intervention that targets the enhancement of BDNF signaling and, as such, may be relevant to the treatment of a variety of brain disorders where BDNF is diminished, to include depression, traumatic brain injury, and Alzheimer’s disease.
Keywords: progesterone, BDNF, Pgrmc1, ischemic stroke, let-7i
Abstract
Progesterone (P4) is a potent neuroprotectant and a promising therapeutic for stroke treatment. However, the underlying mechanism(s) remain unclear. Our laboratory recently reported that brain-derived neurotrophic factor (BDNF) is a critical mediator of P4’s protective actions and that P4-induced BDNF release from cortical astrocytes is mediated by a membrane-associated progesterone receptor, Pgrmc1. Here, we report that the microRNA (miRNA) let-7i is a negative regulator of Pgrmc1 and BDNF in glia and that let-7i disrupts P4-induced BDNF release and P4’s beneficial effects on cell viability and markers of synaptogenesis. Using an in vivo model of ischemia, we demonstrate that inhibiting let-7i enhances P4-induced neuroprotection and facilitates functional recovery following stroke. The discovery of such factors that regulate the cytoprotective effects of P4 may lead to the development of biomarkers to differentiate/predict those likely to respond favorably to P4 versus those that do not.
Stroke has been reported as one of the leading causes of death and a major cause of disability in the United States (1), costing approximately $34 billion annually (according to the Centers for Disease Control). A number of studies have shown that the ovarian hormone progesterone (P4) is neuroprotective in a variety of experimental models of stroke (2–4). However, the underlying mechanisms for P4’s protective effects remain unclear.
One known mediator of P4’s protective function is brain-derived neurotrophic factor (BDNF) (5). A deficit in BDNF has been linked to a more severe stroke pathophysiology (6, 7). This neurotrophin also has an established role in promoting neuronal differentiation, survival, synaptic plasticity (8–10), and synaptogenesis (11–13). Synaptogenesis occurring in the penumbra is known to strongly contribute to enhanced functional recovery from stroke (14–17). Based on these observations, it is plausible that the P4/BDNF signaling-mediated enhanced synaptogenesis and neuroprotection may contribute to P4’s protective effects during poststroke brain repair.
We recently reported that P4 elicits the release of BDNF from primary astrocytes via a putative membrane progesterone receptor consisting of progesterone receptor membrane component 1 (Pgrmc1) (18). Our results also suggest that conditioned medium derived from P4-treated astrocytes, when applied to primary cortical neurons, increases the expression of synaptic markers in these neural cells and enhances their survival against oxidative stress. These findings support the model whereby P4 elicits its (neuro)protective effects through a mechanism that involves Pgrmc1-dependent BDNF release from glia.
While it is clear that Pgrmc1 plays an important role in P4’s protective effects on the brain, knowledge regarding the regulation of Pgrmc1 in the brain and the consequence of such regulation is limited. Studies suggest that microRNA (miRNA) might be involved (19, 20). MicroRNAs are a class of small noncoding RNAs with mature transcripts consisting of 18 to 25 nucleotides (21). Indeed, there exists support for the role of miRNA in stroke (21–23) where manipulation of miRNA in experimental models of stroke resulted in neuroprotection (19, 22–24).
In the present study, we aimed to investigate the role of miRNA let-7i in regulating the protective function of progesterone in ischemia. Using an in vitro two-cell model system, which consisted of astrocytes and neurons, we found that let-7i negatively regulates expression of both Pgrmc1 and BDNF in glia, leading to suppression of P4-induced BDNF release from glia and attenuation of P4’s beneficial effects on cell viability and markers of synaptogenesis. In our in vivo model of ischemia [middle cerebral artery occlusion (MCAo), followed by reperfusion], combined treatment of P4 and the let-7i inhibitor/antagomir led to reduced ischemic injury and complete recovery of motor function. These findings support the therapeutic value of let-7i inhibition as an approach to enhance P4’s protective efficacy in ischemic brain.
Results
BDNF and Pgrmc1 Are Negatively Regulated by Let-7i in Primary Cortical Astrocytes.
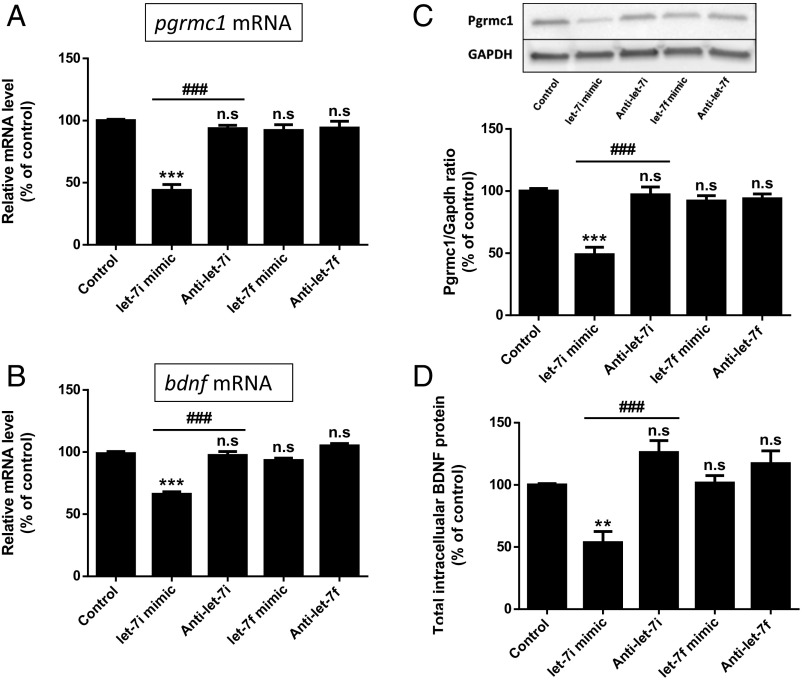
An in silico analysis using three prediction software programs (miRDB, TargetScan, and microRNA.org) revealed putative let-7 binding sites in the 3′ UTR of pgrmc1 and bdnf that were conserved in rat, mouse, and human sequences. Based on this observation, coupled with a prior report citing the regulation of Pgrmc1 by let-7i in ovarian cancer cells, we evaluated the effect of let-7i on both Pgrmc1 and BDNF. We evaluated the effect of let-7f as a control, recognizing that the Sohrabji laboratory had previously shown an inverse relationship between let-7f and BDNF (19). Our studies showed that overexpression of let-7i, by transfecting a let-7i mimic, led to decreased mRNA levels of both pgrmc1 (Fig. 1A) and BDNF (Fig. 1B). Interestingly, overexpression of let-7f had no effect. Additionally, inhibition of let-7i (anti–let-7i) and let-7f (anti–let-7f), using miRNA inhibitor, did not alter basal mRNA levels of pgrmc1 and bdnf (Fig. 1 A and B). Western blot analysis (Fig. 1C) showed a consistent finding in that reduction in Pgrmc1 protein level was only observed in the group transfected with let-7i mimic. Quantitative assessments of BDNF, using an ELISA (Fig. 1D), showed that BDNF levels were down-regulated in cultures transfected with let-7i mimic. These data suggested that let-7i, and not let-7f, negatively regulates BDNF/Pgrmc1 system in cortical astrocytes.
Fig. 1.
Let-7i negatively regulates the expression of Pgrmc1 and BDNF in primary cortical astrocytes. The effect of let-7 mimics and antagomirs on pgrmc1 (A) and bdnf (B) mRNA (n = 4). (C) Representative immunoblot for Pgrmc1 protein and associated quantitation depicting the signal (densitometric) intensity, expressed as the ratio of Pgrmc1 to GAPDH (n = 4). (D) Total cellular BDNF measured by ELISA (n = 5). n.s, not significant; ***P < 0.001 compared with control; ###P < 0.001 compared with let-7i mimic. Data are presented as the mean ± SEM.
Progesterone-Induced BDNF Release Is Inhibited in Cultures Overexpressing Let-7i.
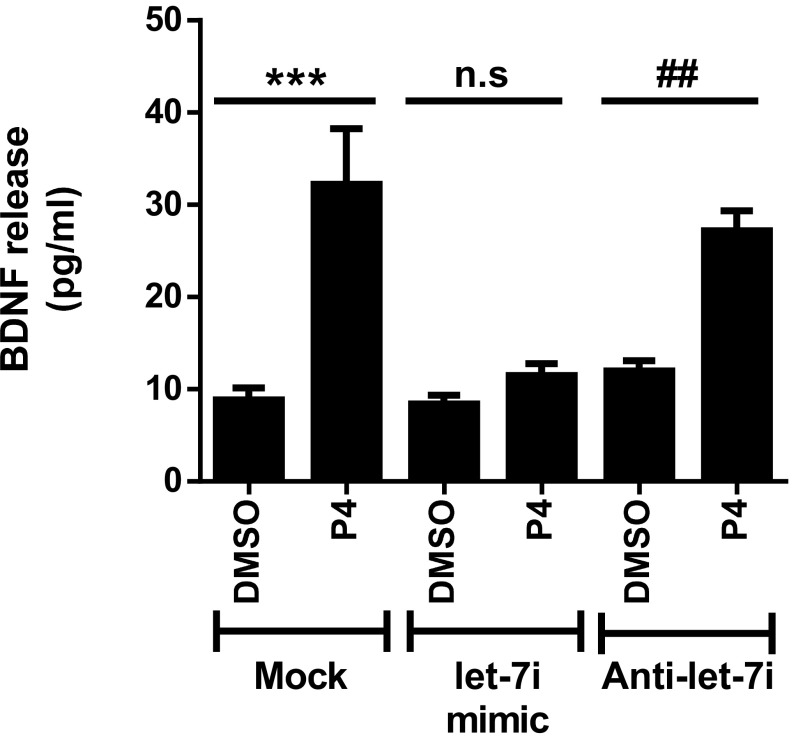
Since our laboratory has shown that Pgrmc1 plays a central role in mediating the effect of P4 on the release of BDNF from cortical astrocytes (18) and let-7i negatively regulates expression of this component (Fig. 1 A and C), we tested whether overexpression of let-7i inhibits P4-elicited BDNF release from these cells. In situ assessment of BDNF release (Fig. 2) showed that P4 (10 nM, 24 h) elicited a significant release of BDNF into the culture media compared with the vehicle control (DMSO), an effect that was not blocked by overexpressing the let-7i antagomir. In contrast, let-7i overexpression led to the inhibition of P4’s effect on BDNF release. These findings support our hypothesis that overexpression of let-7i, through the negative regulation of the Pgrmc1/BDNF axis, abolishes P4-induced BDNF release from primary cortical astrocytes.
Fig. 2.
Let-7i overexpression abolished progesterone (P4)-induced BDNF release from primary cortical astrocytes. Quantitation of BDNF release measured by BDNF in situ ELISA (n = 4). n.s, not significant; ***P < 0.001; ##P < 0.01 compared with corresponding DMSO groups. Data are presented as the mean ± SEM.
The Let-7i Antagomir Inhibits Oxygen–Glucose Deprivation-Induced Increase in Let-7i Expression.
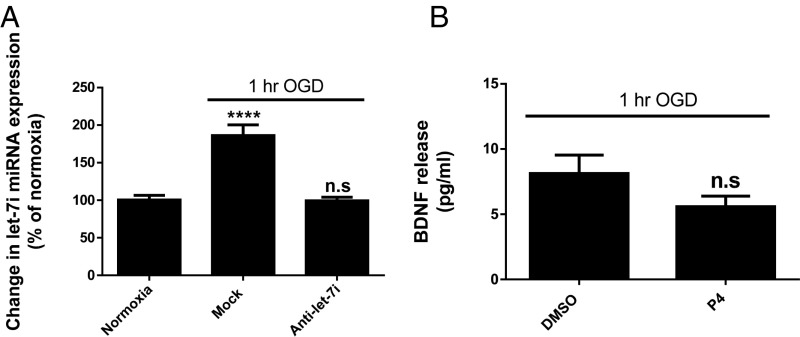
Oxygen–glucose deprivation (OGD), used in the primary cortical astrocytes as an in vitro model of ischemia, revealed an increase in let-7i expression. Importantly, the data also verified the effectiveness of the let-7i antagomir to attenuate the OGD-induced increase in let-7i expression (Fig. 3A). Moreover, as a complement to data presented in Fig. 2, data in Fig. 3B demonstrate that OGD (which increases let-7i expression) compromised the ability of progesterone (P4)-induced BDNF release from primary cortical astrocytes, similar to what was noted when let-7i was specifically overexpressed.
Fig. 3.
Oxygen–glucose deprivation (OGD) results in an increase in let-7i expression and suppresses progesterone (P4)-induced BDNF release from primary cortical astrocytes. Primary cortical astrocytes were exposed to 1 h of OGD. Immediately after reinstatement of normal oxygen and glucose concentrations, these cells were either mock transfected (control) or transfected with the let-7i antagomir. Twelve hours later, expression of let-7i was evaluated (A) (n = 4). n.s, not significant; ****P < 0.0001 compared with normoxic control. (B) Quantitation of BDNF release measured by BDNF in situ ELISA (n = 4). n.s, not significant compared with DMSO group. Data are presented as mean ± SEM.
Let-7i Represses Progesterone’s Neuroprotection and Its Enhancement on Synaptogenesis.
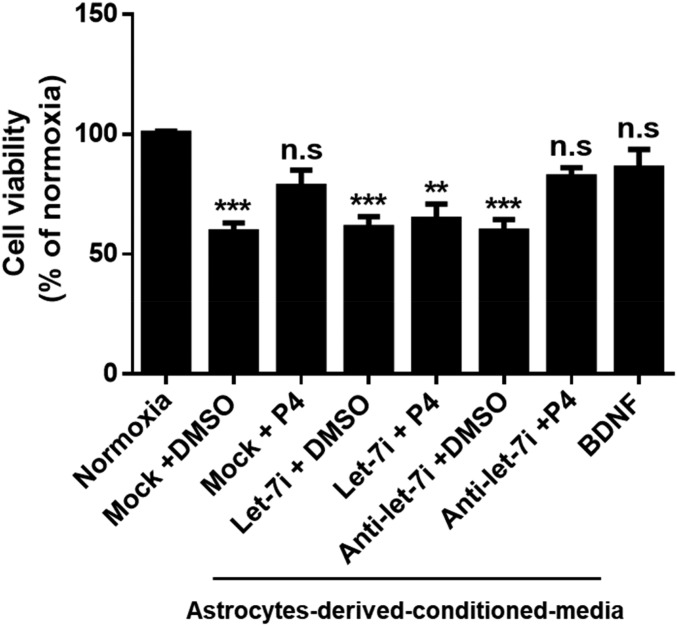
To investigate the role of let-7i in P4’s neuroprotective effects, we manipulated miRNA expression in primary cortical astrocytes and then treated them with either vehicle (DMSO) or P4, following which astrocyte-derived conditioned medium (ACM) was collected. The conditioned medium was then applied to primary cortical neurons [days in vitro (DIV)14] that had been exposed to oxygen–glucose deprivation (OGD). The neurons were then assessed for cell viability to ascertain if conditioned media from P4-treated astrocytes elicited greater neuroprotection relative to neurons treated with conditioned media from DMSO-treated astrocytes (Fig. 4). We found that conditioned media collected from P4-treated astrocytes conferred similar neuroprotection as seen in the positive control group [consisting of direct administration of BDNF (50 ng/mL) to the neuronal cultures]. However, conditioned media collected from P4-treated astrocytes that overexpressed let-7i failed to promote the protection of neurons from OGD.
Fig. 4.
Let-7i prevents progesterone (P4)-induced neuroprotection against oxygen–glucose deprivation (OGD). Conditioned media derived from hormone or control-treated astrocytes were applied to primary cortical neurons (DIV 14) after 1-h exposure to OGD. BDNF (50 ng/mL) was directly added to neurons after OGD to serve as positive control. Neuronal viability was measured by CellTiter-Glo viability assay (n = 5). n.s, not significant; ***P < 0.001 and **P < 0.01 compared with normoxia. Data are presented as the mean ± SEM.
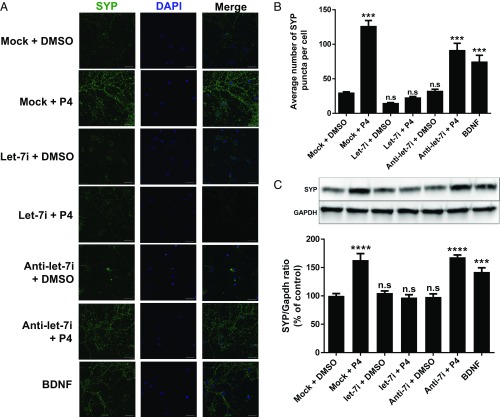
Next, we determined if conditioned media from the different experimental groups represented in Fig. 5 resulted in changes in expression of synaptophysin (SYP), a presynaptic marker closely linked to synaptogenesis (4). We observed that conditioned media derived from P4-treated astrocytes (P4-ACM) resulted in a robust increase in SYP (green) immunofluorescence (Fig. 5A), relative to neurons treated with conditioned media from DMSO-treated, and mock-transfected astrocytes. Quantitative analysis revealed that P4-ACM significantly increased both SYP protein level (Fig. 5C) and the number of SYP puncta (Fig. 5B). The same observations were seen in the positive control group [consisting of direct application of BDNF (50 ng/mL) to the primary neuronal cultures]. Application of conditioned media collected from P4-treated astrocytes that overexpressed let-7i (group label: let-7i + P4), however, failed to elicit the increase in synaptophysin expression.
Fig. 5.
Let-7i inhibits progesterone (P4)-induced synaptophysin (SYP) expression in primary cortical neurons. (A) Representative confocal images of primary cortical neurons (DIV 14) immunostained with synaptophysin (SYP, green) and DAPI (blue). (Magnification: 60×.) (Scale bars: 30 µm.) (B) Quantification of average number of SYP puncta per neuron (n = 3). n.s, not significant; ***P < 0.001 compared with mock transfected + DMSO group. (C) Representative immunoblots probed for SYP and quantification graph of relative SYP protein ratio to Gapdh (n = 4). n.s, not significant; ****P < 0.0001 and ***P < 0.001 compared with mock transfected + DMSO group. Data are presented as the mean ± SEM.
Combined Treatment of Progesterone and Let-7i Inhibition Alleviates Ischemia-Induced Suppression of Pgrmc1 and BDNF Expressions in the Penumbra of the Ischemic Brain.
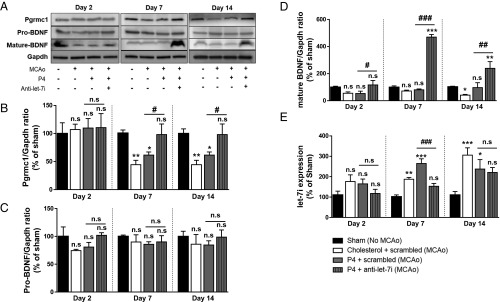
Since we determined that let-7i is a negative regulator of BDNF and Pgrmc1 (Fig. 1) and that OGD, as an in vitro model of ischemia, induced expression of let-7i (Fig. 3A), we next determined the expression of let-7i in the middle cerebral artery occlusion model of ischemic stroke, focusing on changes in the penumbra. Assessments of let-7i expression were conducted at different time points – 2, 7, and 14 d following stroke. Representative images of immunoblots probed for Pgrmc1, along with pro-BDNF and mature BDNF, are shown in Fig. 6A. We found that, compared with sham (nonstroked controls), ischemic injury resulted in an up-regulation of let-7i expression (Fig. 6E) starting at day 7 and remained elevated up to 14 d following stroke. P4 treatment alone [P4 + a control sequence for let-7i (scrambled)] did not attenuate the stroke-induced increase in let-7i. As expected, ischemia-induced increase in let-7i expression was repressed in the group receiving combined treatment of P4 and let-7i inhibition (P4 + –let-7i) (Fig. 6E). Importantly, along with up-regulating let-7i level, ischemia also resulted in a reduction of Pgrmc1 protein level observed at day 7 and day 14 (Fig. 6B). P4 treatment alone did not restore Pgrmc1 level at either of the two time points. Combined treatment (P4 + anti–let-7i), however, reversed ischemia-induced suppression of Pgrmc1 protein levels. Furthermore, expression of mature BDNF was reduced as a consequence of stroke at the 14-d poststroke evaluation period (Fig. 6D) while pro-BDNF levels (Fig. 6C) remained unchanged across all time points and all treatments. Compared with sham, the treatment of P4 alone was able to maintain the same level of mature BDNF, even at 14 d poststroke. Remarkably, combined treatment (P4 + anti–let-7i) led to a robust increase in expression of mature BDNF observed at day 7 and day 14.
Fig. 6.
Combined treatment with progesterone (P4) and the let-7i inhibitor reversed ischemia-induced suppression of Pgrmc1 and BDNF expression in the penumbra. (A) Representative immunoblots probed for Pgrmc1, pro-BDNF, and mature BDNF. (B) Quantitation graph of relative Pgrmc1 protein ratio to Gapdh (n = 4 to 5 per group). (C) Quantitation graph of relative pro-BDNF protein ratio to Gapdh (n = 4 to 5 per group). (D) Quantitation graph of relative mature BDNF protein ratio to Gapdh (n = 4 to 5 per group). (E) Quantitation graph of relative let-7i expression in ischemic brain (n = 4 to 5 per group). n.s, not significant; **P < 0.01 and *P < 0.05 compared with sham; ###P < 0.01 between the two indicated groups, ##P < 0.05 between the two indicated groups, and #P < 0.05 compared with P4 + scrambled. Data are presented as the mean ± SEM.
Combined Treatment of Progesterone and Let-7i Inhibition Reduces Ischemic Injury and Enhances Functional Recovery.
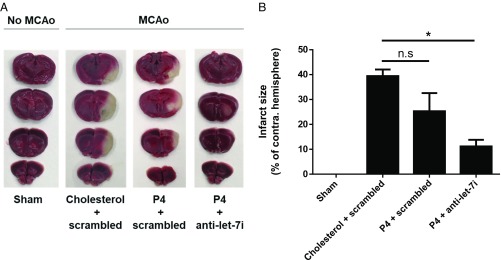
To examine the effect of P4 with or without the let-7i antagomir on the extent of ischemic injury, we utilized 2,3,5-triphenyltetrazolium chloride (TTC) staining to visualize the size of the ischemic lesion. Representative images of TTC stained are shown in Fig. 7A. Quantification of relative infarct size (Fig. 7B) revealed that the combined treatment (P4 + anti–let-7i) significantly reduced ischemic injury whereas P4 treatment alone did not.
Fig. 7.
Co-administration of let-7i antagomir (anti–let-7i) and progesterone (P4) reduces ischemic injury. (A) Representative images of serial coronal brain sections stained with triphenyltetrazolium chloride (TTC). (B) Quantification of infarct sizes of TTC-stained images (n = 4 per group). n.s, not significant; *P < 0.01 compared with cholesterol + scrambled group. Data are presented as the mean ± SEM.
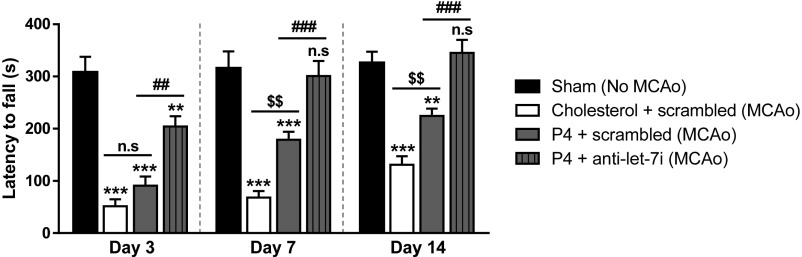
Motor function (grip strength) was also evaluated using the wire suspension test. Results (Fig. 8) showed that, compared with the vehicle group (DMSO + scrambled), treatment of P4 led to a partial recovery of motor function, observed on day 7 and day 14. Interestingly, the combined treatment of P4 and the let-7i antagomir resulted in a rapid, but partial, motor function recovery as early as 3 d posttreatment. By day 7, combined treatment led to complete functional recovery, and the improvement was still evident at day 14. Results from Figs. 7 and 8 support our hypothesis that let-7i inhibition enhances P4’s neuroprotective effects, and enhances functional recovery.
Fig. 8.
Co-administration of let-7i antagomir (anti–let-7i) and progesterone (P4) enhances recovery of motor function/grip strength following stroke. Results of wire suspension test at day 3, 7, and 14 poststroke (n = 15 to 20 per group). n.s, not significant; ***P < 0.001 and **P < 0.01 compared with sham; ###P < 0.001, ##P < 0.01 compared with P4 + scrambled; and $$P < 0.01 compared with cholesterol + scrambled. Data are presented as the mean ± SEM.
Inhibition of Let-7i Enhances Progesterone’s Effect on a Synaptogenic Marker.
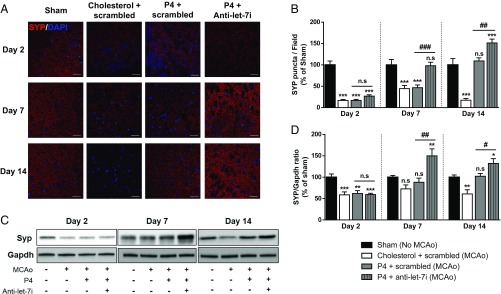
Synaptic plasticity in the ischemic penumbra region has long been known to influence the functional recovery after stroke (14, 16, 25). Therefore, to determine whether synaptogenesis occurring in the penumbra could be a factor contributing to functional recovery observed in Fig. 8, we extended our in vitro findings to evaluate the expression of synaptophysin (SYP), a synaptogenic marker, in the penumbra of stroked mice. To do so, we performed immunofluorescence to visualize SYP expression (red) (Fig. 9A) and quantified the relative number of SYP puncta, which is an indication of potential synapses (Fig. 9B). In parallel, Western blot analysis was performed to evaluate total SYP protein levels. Representative immunoblots probed for SYP are shown in Fig. 9C, and its relative quantification of protein level is depicted in Fig. 9D. Results revealed that ischemia resulted in a sustained down-regulation of synaptophysin puncta (Fig. 9B) in the penumbra at day 2, 7, and 14 poststroke. In addition, ischemic injury led to decreased SYP protein level at day 2 and 14. There was a transient increase in SYP expression at day 7, which could be due to a compensatory response to the ischemic injury. P4 treatment alone led to a delayed, but sustained, restoration in SYP total protein expression, observed at day 7 and day 14. With regard to the number of SYP puncta, the positive effect of P4 was only evident at day 14 posttreatment. Interestingly, at day 7 and 14, combined treatment (P4 + anti–let-7i) resulted in significantly higher expression of SYP, compared with sham controls and P4 treatment alone. This combined treatment also led to a complete restoration of synaptophysin puncta at day 7, an effect that was further enhanced at day 14. Taken together, these findings indicate that P4 induces synaptogenesis in the penumbra of ischemic brain and that let-7i inhibition further enhances this beneficial function of P4.
Fig. 9.
Inhibition of let-7i enhances progesterone (P4)’s effect on the expression of synaptophysin in the penumbra. (A) Representative confocal images of penumbra region staining for synaptophysin (SYP, red) and DAPI (blue). (Magnification: 60×.) (Scale bars: 30 µm.) (B) Quantification of average relative SYP puncta present in each field (n = 3 per group). n.s, not significant; ***P < 0.001 compared with sham; ###P < 0.001 and ##P < 0.01 compared with P4 + scrambled. (C) Representative immunoblots probed for SYP protein. (D) Quantification graph of Syp signal, expressed as a ratio of Gapdh (n = 4 to 5 per group). n.s., not significant; ***P < 0.001, **P < 0.01, *P < 0.05 compared with sham; ##P < 0.01 and #P < 0.05 compared with P4 + scrambled.
Discussion
An increasing number of publications infer the brain-protective effects of P4, including studies showing that P4 is neuroprotective in a variety of experimental models of stroke (2–4). However, the underlying mechanism(s) for P4’s protective effects remain unclear. In point of fact, our ability to optimize the effectiveness of P4 requires a better understanding of the factors that influence the expression of key mediators (e.g., receptors) of P4’s protective effects. In addition, most of the literature associated with P4’s protective effects has focused on a direct effect of P4 on neurons. The notion that glia may be an equally important target underlying P4’s protective effects on the brain has only been studied minimally. Indeed, astrocytes have been considered an important component of postischemic recovery as these cells are critical for regeneration and remodeling of neural circuits following stroke (26).
One mechanism that underlies the protective function of P4 is its ability to initiate intercellular cross-talk between astrocytes and neurons, where BDNF is a key mediator (27). For example, we have reported that P4 elicits a significant release of mature BDNF from astrocytes through a Pgrmc1-dependent ERK5 signaling mechanism. From this same study, BDNF from the conditioned media of P4-treated astrocytes was identified specifically as the protective factor that not only promoted neuronal viability but also increased the expression of markers of synaptogenesis (27). In this study, we identified an upstream regulator of this Pgrmc1/BDNF axis in glia and characterized its influence in a mouse model of ischemic stroke (MCAo). Our results support the role of let-7i as a negative regulator of Pgrmc1 and BDNF in primary cortical astrocytes. Interestingly, despite sharing a similar seeding sequence, let-7f did not have the same effect, suggesting that regulation of Pgrmc1/BDNF in astrocytes is specific to let-7i. Not only did increased expression of let-7i significantly reduce P4-induced BDNF release from cultured astrocytes, it prevented P4 from protecting neurons against oxygen–glucose deprivation (OGD) and from increasing the expression of synaptophysin, a surrogate marker for synaptogenesis. We also found that OGD, serving as an in vitro model of ischemia, resulted in an up-regulation of let-7i. Consistent with our working model that let-7i, by inhibiting the expression of Pgrmc1, inhibits P4-induced BDNF release from astrocytes (as shown in Fig. 2), OGD also inhibited the P4-induced BDNF release, an effect correlated with the effect of OGD on let-7i expression. Collectively, these findings suggest that let-7i and a condition that leads to the elevation of this miRNA, such as ischemia, repress P4-induced BDNF release from glia, thus leading to attenuation of the beneficial effects of P4 on neuronal survival and markers of synaptogenesis. It is noteworthy that inhibition of let-7i alone (using the let-7i antagomir/inhibitor) did not confer any protection against OGD. Rather, let-7i inhibition enhanced the protective effects of P4.
The results from our in vivo studies, using the MCAo model of stroke in mice, corroborated the results from our in vitro experiments. For example, like OGD, ischemic injury induced in the mouse led to an up-regulation of let-7i in the penumbra, which was correlated with decreased Pgrmc1 expression and a reduction in the level of mature BDNF which was seen at day 14 following stroke. Given that BDNF has such a vital role on brain function (8–10), the observed reduction in Pgrmc1 could not only compromise the protective efficacy of hormones like P4 but, more generally, could underlie the long term sequelae that lead to functional impairment over time in stroke victims. It is worth noting, however, that the reduction in BDNF levels was observed only at day 14. This could be due to a compensatory effort of the brain to maintain BDNF at earlier time points (day 2 and 7).
Treatment of P4 was able to restore the level of mature BDNF. Despite the reduction in Pgrmc1 levels in ischemic brain, P4’s mild restorative effect on BDNF expression might be due to its action via classical progesterone receptor (PR), as supported by our previous work (28). Importantly, combined treatment (P4 and let-7i inhibition) restored Pgrmc1 expression and resulted in a marked increase in mature BDNF level, despite pro-BDNF levels not changing appreciably. This interesting finding, where the let-7i antagomir, when coapplied with progesterone (P4), enhanced mature BDNF expression without affecting pro-BDNF, is somewhat consistent with our prior published studies where progesterone increased the ratio of mature to pro-BDNF released from glia (27). However, another possible consequence of elevated let-7i is to influence the expression of proneurotrophin convertases, such that increased let-7i may inhibit the convertases responsible for processing pro-BDNF to mature BDNF. Indeed, our data show that the reduced expression of mature BDNF (at day 14) in the stroke-only group (Fig. 6D, open bar) is a time point associated with a significant elevation of let-7i. Conversely, it is possible that inhibiting let-7i, which would alleviate inhibition of the convertases, could result in greater processing of the pro-BDNF molecule to mature BDNF. The striking increase in mature BDNF expression observed with the combined treatment could also be due to the effect of the intervention on the stability of mature BDNF (versus pro-BDNF). Nevertheless, taken together with our in vitro results, the observations from the in vivo studies support the hypothesis that inhibition of let-7i alleviates ischemia-induced suppression of Pgrmc1 expression, thereby allowing P4 to increase expression (and release) of mature BDNF via Pgrmc1 signaling.
In agreement with other published studies (2, 29), our results showed that treatment of P4 alone led to a modest and delayed improvement in functional recovery, along with an increase in synaptogenesis depicted by the increase in expression and number of synaptophysin puncta. However, treatment with P4 alone failed to reduce the size of the ischemic lesion. Interestingly, the combined treatment of the let-7i antagomir and P4 not only significantly reduced the size of the ischemic lesion but also led to a quicker and more pronounced functional recovery, as well as a more pronounced increase in markers of synaptogenesis in the penumbra region. These findings are consistent with other studies proposing the link between synaptogenesis in the penumbra and functional recovery following stroke (14, 30).
While progesterone has been shown to exert protective effects in animal models of ischemic stroke (3, 31), this effect is not always consistent (32, 33). Moreover, there is yet to be a clinical evaluation of the effectiveness of P4 intervention for ischemic stroke. We recognize, however, that prior efforts to translate robust preclinical data that support the neuroprotective effects of steroid hormones, like P4, have been somewhat disappointing. For example, a recent phase III clinical trial (ProTECT III) assessing the efficacy of P4 treatment for acute traumatic brain injury (TBI) showed rather disappointing results, with no favorable effects noted (34) despite significant support for the therapeutic efficacy of P4 from preclinical studies. Similarly, the SYNAPSE trial (35), which evaluated the efficacy of P4 against severe TBI, also failed to demonstrate improvement in functional outcomes. The apparent discrepancy between the preclinical studies and the clinical trials may be attributed to numerous variables, including the complexity and variability of the injuries noted in human subjects within the trials. In addition, we suggest that the biological heterogeneity of the subject cohort within multicenter trials, despite laudable efforts to define the study cohort with cited inclusionary and exclusionary criteria, may also play a role. Such heterogeneity may have its basis in the biology of the individual, through variable expression of factors that modify the effects of P4. Our data offer potential insight into how differences in the expression of microRNA let-7i could alter the efficacy of progesterone. Since some studies have reported an elevated expression of let-7i in traumatic brain injury (TBI) models (36, 37), we suggest that intersubject differences in let-7i expression may predict who may be responsive to P4. More specifically, our data would suggest that higher expression of let-7i leads to impaired Pgrmc1/BDNF signaling, thus dampening the protective function of P4.
Collectively, our findings reported herein identify let-7i as a negative upstream regulator of both Pgrmc1 and BDNF in glia, leading to suppression of P4-induced BDNF release from glia and attenuation of P4’s beneficial effects on neuronal viability and synaptogenesis in the ischemic brain. Furthermore, the increased expression of let-7i with stroke may explain why poststroke therapy may not be so effective. As such, dampening the up-regulation of let-7i may prove to be an effective strategy to enhance the efficacy of P4 and other therapeutic candidates that engage the BDNF system and, as such, could potentially extend the “window of opportunity” for stroke therapy.
Materials and Methods
Primary Cultures.
Dissociated cortical neurons were prepared and maintained as previously described (27). Briefly, cortices were removed from neonatal mouse brains (postnatal day 2 to 4, mixed gender) and dissociated with 0.25% trypsin. Cortical neurons were then plated on glass coverslips or plastic culture dishes coated with poly-d-lysine (Sigma). The culture medium used was Neurobasal (ThermoFisher Scientific), supplemented with Glutamax and B27 serum-free supplement (ThermoFisher Scientific). At day in vitro (DIV) 3, 5 µM final concentration of 1-β-arabinofuranosylcytosine (38) (Sigma) was added to the neuronal cultures to prevent glial proliferation. Half of the medium was replaced with fresh medium every 4 d. For viability assay, cortical neurons were plated onto 96-well plates (Corning) at the concentration of 1.2 × 105 cells per square centimeter. For immunocytochemistry, cortical neurons were plated onto 12-mm glass coverslips (Neuvitro) at the density of 4 × 104 cells per square centimeter. Treatments of primary cortical neurons started at DIV12.
Primary cortical astrocytes were prepared and maintained as previously described (39), with some modifications. Briefly, cortices of postnatal day 2 to 4 mouse pups were dissociated with 0.25% trypsin and plated onto 75-cm2 tissue culture flasks. The culture medium used was DMEM (ThermoFisher Scientific), supplemented with 10% FBS (GE Healthcare Life Sciences) and 10,000 U/mL penicillin-streptomycin (ThermoFisher Scientific). After reaching confluence, mixed glial cultures were placed on the shaker for 48 h to dislodge microglia, resulting in cultures enriched with astrocyte population.
Treatment of Primary Cultures.
To determine the miRNA regulation of downstream targets in primary cortical astrocytes, miRNA mimics and inhibitors were transfected into these cells for 48 h. After transfection, total RNA and proteins were isolated for gene and protein expression analysis. Mock transfection was used as the control for these experiments.
To study the effect of miRNA on P4-induced BDNF release from astrocytes, BDNF in situ ELISAs were performed. Expression of miRNA was first manipulated by transfection as described above. Twenty-four hours after transfection, 10 nM P4 was added to primary cortical astrocytes for an additional 24 h without changing media containing transfection complexes. Vehicle controls were performed in parallel such that control cultures were exposed to 0.1% dimethyl sulfoxide (DMSO). The 10-nM concentration of P4 used in studies described here was chosen because it has been reported to elicit a maximal release of BDNF from astrocytes (18).
In experiments evaluating the effect of miRNA on P4-induced neuroprotection and the synaptogenic marker synaptophysin, we first transfected miRNA mimic and inhibitor into primary cortical astrocytes for 24 h. Afterward, P4 (10 nM) was added to these cultures for an additional 24 h to generate P4-treated astrocyte-derived conditioned media (P4-ACM). In parallel, treatment of 0.1% DMSO was performed to generate DMSO-treated astrocyte-derived conditioned media (DMSO-ACM), which served as vehicle controls. Before applying to primary neurons, these conditioned media were filtered through a 10-kDa cutoff column to eliminate residual P4 and miRNA mimic or inhibitor. In the neuroprotection assay, astrocyte-conditioned media were added to primary cortical neurons with prior exposure to 1 h of oxygen–glucose deprivation (OGD), an in vitro model of ischemic-like insult. Based on our experience, 1 h of OGD was enough to induce 50% neuronal cell death. BDNF (50 ng/mL) was directly added to different groups after OGD to serve as positive control. Neuronal cultures exposed to normoxia were used as the control for these datasets. Twenty-four hours after the applications of BDNF or conditioned media, a CellTiter-Glo Luminescent cell viability assay (Promega) was performed to measure neuroprotection. In the synaptogenic marker measurement assay, BDNF and astrocyte-derived conditioned media were directly added to primary cortical neurons for 24 h. Synaptophysin expression and the number of synaptophysin puncta in these neuronal cultures were assessed by immunocytochemistry, followed by confocal imaging and analysis using ImageJ (National Institutes of Health) software (40).
Transfection.
Transfection of miRNA mimics and inhibitors was performed using the Hiperfect transfection reagent (Qiagen) according to the manufacturer’s instructions. Cells were transfected with miRNA mimics and inhibitors for 48 h. This duration was chosen since it resulted in an optimal effect on targets of interest. Synthetic miRNA mimics (Syn-mmu-let-7i-5p, Syn-mmu-let-7f-5p) and inhibitors (Anti-mmu-let-7i-5p, Anti-mmu-let-7f-5p) were purchased from Qiagen.
Quantitative RT-PCR.
Total RNA was isolated from primary cortical astrocytes and mouse brains using the MiRNeasy Mini Kit (Qiagen) according to the manufacturer’s instructions. Concentrations of extracted RNA were determined using absorbance values at 260 nm. The purity of RNA was assessed by ratios of absorbance values at 260 and 280 nm (A260/A280 ratios of 1.9 to 2.0 were considered acceptable).
For miRNA expression measurements, total RNA (10 ng) was reverse transcribed into cDNA in a total volume of 15 µL using the microRNA cDNA Archive Kit (ThermoFisher Scientific) according to the manufacturer’s instructions. The reaction mixture contained water, 2× quantitative PCR Master Mix (Eurogentec), and 20× Assay-On-Demand for each target gene. A separate reaction mixture was prepared for the endogenous control, U6. The reaction mixture was aliquotted in a 96-well plate, and cDNA was added to give a final volume of 20 µL. Each sample was analyzed in triplicate. The comparative cycle threshold (Ct) method (2−∆∆Ct) was used to calculate the relative changes in target miRNA expression.
For mRNA expression measurements, total RNA (1.6 µg) was reverse transcribed into cDNA in a total volume of 20 µL using the High-Capacity cDNA Archive Kit (ThermoFisher Scientific) according to the manufacturer’s instructions. The reaction mixture contained water, 2× quantitative PCR Master Mix (Eurogentec), and 20× Assay-On-Demand for each target gene. A separate reaction mixture was prepared for the endogenous control, gapdh. The reaction mixture was aliquotted in a 96-well plate, and cDNA (30 ng of RNA converted to cDNA) was added to give a final volume of 30 µL. Each sample was analyzed in triplicate. The comparative cycle threshold (Ct) method (2−∆∆Ct) was used to calculate the relative changes in target gene expression.
PCR primers were purchased as Assay-On-Demand from ThermoFisher Scientific. The assays were supplied as a 20 mix of PCR primers (900 nM) and TaqMan probes (200 nM). The let-7i (002221), U6 (001973), bdnf (Mm00432069_m1), gap-43 (Mm00500404_m1), gapdh (Mm99999915_g1), psd-95 (Mm00492193_m1), pgrmc1 (Mm00443985_m1), and syp (Mm00436850_m1) assays contained FAM (6-carboxy-fluorescein phosphoramidite) dye label at the 5′ end of the probes and minor groove binder and nonfluorescent quencher at the 3′ end of the probes.
CellTiter-Glo Luminescent Cell Viability Assay (Promega).
The CellTiter-Glo Luminescent Cell Viability Assay uses the level of adenosine triphosphate (41) as an indicator of metabolically active cells and is directly proportional to the number of living cells (42, 43). The assay was performed according to the manufacturer’s instructions. In brief, the cell plate was first equilibrated to room temperature for 30 min. A volume of the kit reagent equal to the volume of cell culture present was then added to each well. The plate was then placed on an orbital shaker for 2 min to induce cell lysis, followed by 10 min of incubation at room temperature. Luminescence was recorded using a plate reader.
BDNF Immunoassay in Situ.
To determine the amount of endogenous BDNF released with P4 treatment, we performed an ELISA in situ assay, as previously described (18). In brief, a 96-well Nunc MaxiSorp surface polystyrene flat-bottom immunoplate was precoated with an anti-BDNF monoclonal antibody [diluted 1:1,000 in coating buffer (25 mM sodium bicarbonate and 25 mM sodium carbonate, pH 9.7)]. After blocking nonspecific binding, primary cortical astrocytes were then plated, followed by appropriate treatment applications. BDNF standards, ranging in concentration from 1.95 to 500 pg/mL, were added to parallel wells. At the end of hormone treatment, cells were carefully washed with Tris-buffered saline containing 0.2% Tween 20 (TBS-T). The plate was then incubated with the polyclonal anti-human BDNF antibody. The amount of specifically bound polyclonal antibody was then detected through the use of the anti–IgY-horseradish peroxidase (HRP) tertiary antibody, which, when exposed to the chromogenic substrate (TMB reagent; Promega), changes color in proportion to the amount of BDNF present in the sample. The color intensity was quantified by measuring the absorbance at 450 nm with a Viktor3 ELISA plate reader (Perkin-Elmer). Only values within the linear range of the standard curve, and above the lowest standard, were considered valid. This method allowed detection of as little as 2 pg/mL BDNF release in control cultures to ∼250 pg/mL in P4-treated cultures.
Oxygen–Glucose Deprivation.
OGD was performed according to an established protocol, as described elsewhere, with minor modifications (44). Briefly, primary cortical neurons were carefully washed five times with HBSS (ThermoFisher Scientific) to remove residual glucose. Glucose-free DMEM (ThermoFisher Scientific) was then added to the cultures, and the plates were transferred into a hypoxic chamber (0.1% oxygen) for 1 h. At the end of hypoxia, glucose-free DMEM was replaced with regular maintaining media. Reoxygenation was initiated by transferring the cells to a normoxic 5% CO2 cell culture incubator.
Western Blotting.
Primary cortical astrocytes and mouse brains were lysed with radioimmunoprecipitation assay lysis buffer containing protease and phosphatase inhibitors, as previously described (27). After homogenization, samples were centrifuged at 100,000 × g for 30 min at 4 °C, and supernatants were collected. Total protein concentrations were determined using the Bio-Rad DC protein assay kit (Bio-Rad Laboratories). Cell lysates were separated by SDS/PAGE and transferred onto a polyvinylidene fluoride membrane (Bio-Rad Laboratories) by electroblotting. Membranes were blocked with 5% skim milk in Tris-buffered saline containing 0.2% Tween 20 (TBS-T) for 1 h at room temperature, followed by overnight incubations of primary antibodies at 4 °C. The following primary antibodies were used: rabbit polyclonal anti-PSD 95 (1:1,000, ab18258; Abcam), rabbit polyclonal anti-synaptophysin (1:1,000, ab14692; Abcam), rabbit monoclonal anti-GAP43 (1:200,000, ab75810; Abcam), rabbit monoclonal anti-GAPDH (1:1,000, 14C10; Cell Signaling), rabbit polyclonal anti-BDNF (1:300, sc546; Santa Cruz), and goat polyclonal anti-Pgrmc1 (1:500, ab48012; Abcam). After washing three times with TBS-T, membranes were incubated with anti-goat IgG or anti-rabbit IgG conjugated with horseradish peroxidase (Millipore) for 1 h at room temperature. After triple washes with TBS-T, immunoreactive bands were visualized with the ECL detection system (ThermoFisher Scientific) and were captured using a luminescent image analyzer (Alpha Innotech). Densitometric analysis was conducted using ImageJ (National Institutes of Health) software (40).
Immunofluorescence.
The cortical neurons were fixed in 4% paraformaldehyde (45) for 15 min, followed by incubation in 0.2% Triton X-100 in Tris-buffered saline (TBS) for 15 min at room temperature for permeabilization. Cultures were then blocked with 5% donkey serum/1% BSA in TBS for 1 h at room temperature and incubated with rabbit monoclonal anti-synaptophysin (1:500, ab32127; Abcam) for 48 h at 4 °C. After extensive rinsing with TBS-Tween 20, cultures were incubated with Alexa Fluor 647-conjugated secondary antibody (1:500; Jackson ImmunoResearch Laboratories) for 2 h at room temperature. After extensive washing with TBS to remove unbound secondary antibody, the coverslips were mounted onto glass slides (VWR Scientific) using Vectashield mounting medium with DAPI (Vector Laboratories). The slides were observed under a confocal fluorescence microscope (FV1200; Olympus) with a 60× objective.
Mouse brains were fixed in 4% paraformaldehyde overnight at 4 °C and subsequently cryoprotected in 30% sucrose solution. The brains were then sectioned into 40-µm-thick coronal slices and subjected to immunostaining using an established protocol described elsewhere, with some modifications (46). In brief, brain sections were blocked in 5% donkey serum/1%BSA/TBS solution for 2 h at room temperature. In staining using mouse primary antibody, sections were subsequently blocked in F(ab) fragment donkey anti-mouse IgG (50 µg/mL; Jackson ImmunoResearch Laboratories) for 2 h at room temperature to reduce background caused by secondary antibody binding to endogenous mouse IgG in the tissue. After the blocking step, brain sections were then incubated in primary antibody solution at 4 °C for 72 h. Primary antibodies used were as follow: mouse monoclonal anti-NeuN (1:500, ab104224; Abcam); rabbit polyclonal anti-GFAP (1:1,000, ab7260; Abcam); rabbit monoclonal anti-synaptophysin (1:500, ab32127; Abcam), and goat polyclonal anti-Pgrmc1 (1:200, ab48012; Abcam). Alexa Fluor 647, Alexa Fluor 594, or Rhodamine Red-conjugated secondary antibodies (Jackson ImmunoResearch Laboratories) were used at 1:500 dilution. After immunostaining, sections were mounted onto microscope slides with Vectashield mounting medium (Vector Laboratories) and observed under a confocal fluorescence microscope (FV1200; Olympus) with a 63× objective.
Mice and Treatments.
All procedures with animals were reviewed and approved by the Institutional Animal Care and Use Committee of the University of North Texas Health Science Center. All institutional and federal guidelines for the care and the use of animals were followed. Female C57BL/6J mice (18 wk old) were purchased from The Jackson Laboratory. Animals were habituated to housing conditions 1 wk before experiments.
All mice were first ovariectomized to deplete endogenous ovarian hormone levels. Two weeks after ovariectomy (OVX), P4 pellets were s.c. implanted into these animals to replenish their progesterone levels. In parallel, different groups received cholesterol pellet implantations to serve as vehicle control. One week after pellet implantation, stroke was induced in these mice using a middle cerebral artery occlusion (MCAo) procedure. In parallel, different groups received sham operation (nonstroke). Thirty minutes after MCAo, 5 µg of either scrambled or let-7i inhibitor was injected into each animal brain via intracerebroventricular (ICV) injection. Experimental groups included sham-operated mice with cholesterol pellet implantation (sham), stroked mice with cholesterol pellet implantation and scrambled ICV injection (cholesterol + scrambled), stroked mice with P4 pellet implantation and scrambled ICV injection (P4 + scrambled), and stroked mice with P4 pellet implantation and let-7i inhibitor ICV injection (P4 + scrambled).
Ovariectomy.
Bilateral ovariectomy (OVX) was performed using a dorsal approach under isoflurane anesthesia, as described elsewhere (47). Briefly, small incisions were made bilaterally to expose ovaries. The arteries adjacent to ovaries were ligated before removal of ovaries. Incisions were then closed using a 4-0 Vicryl absorbable suture.
Transient Middle Cerebral Artery Occlusion.
MCAo was performed to induce transient focal cerebral ischemia, as previously described (48). In brief, mice were anesthetized with isoflurane inhalation. A midline incision was made on the neck. The left common carotid artery (CCA), external carotid artery (49), and internal carotid artery (ICA) were dissected from the connective tissue. The left MCA was occluded by a 6-0 monofilament suture (Doccol Corporation) introduced via the internal carotid artery. After 45-min occlusion, the suture was withdrawn for reperfusion. In sham-operated animals, the monofilament was advanced to the MCA region and withdrawn immediately without MCA occlusion.
Intracerebroventricular Injection.
Five micrograms of either scrambled or let-7i inhibitor (GE Healthcare Dharmacon) was suspended in 0.5 µL of PBS and injected into lateral ventricles using a stereotaxic instrument, as previously described, with minor modifications (23). In brief, the solution was injected using a 5-µL Hamilton syringe attached to the Ultra Micro Pump UMP3 system (World Precision Instruments) at a flow rate of 0.2 μL/min. Coordinates used for ICV injection were anteroposterior (AP) −0.58 mm, mediolateral (ML) +1.2 mm, and dorsoventral (DV) −2.1 mm.
Assessment of Brain Tissue Damage: 2,3,5-Triphenyltetrazolium Chloride Staining.
TTC staining was performed to assess ischemic injury among groups, as described in an established protocol (50). Briefly, 24 h after MCAo, mouse brains were harvested and sectioned into 2-mm-thick coronal sections. These sections were immersed in 2% TTC solution for 30 min at 37 °C and then fixed in 10% formalin. The stained slices were photographed and subsequently measured for the surface area of the slices and the ischemic lesion (Image-Pro Plus 3.0.1; Silver Springs). Images of stained sections were captured, and infarct sizes were analyzed using ImageJ (National Institutes of Health) software (40).
Functional Recovery Assessment: Wire Suspension Test.
In ordered to assess motor function recovery with different treatments, a wire suspension test, a test of grip strength and endurance, was used, as described elsewhere (51). In brief, mice were allowed to suspend their bodies on a single wire that was elevated above a padded platform. The latency for animals to fall off the wire was recorded. Mice were trained 2 d before MCAo to establish a baseline across groups. Training was achieved with several rounds of habituation and trials. In the actual testing phase, each mouse was tested three times, and average performance was taken as final values. Performances of these mice was evaluated at day 3, 7, and 14 poststroke.
Synaptophysin Optical Density Analysis and Puncta Quantification.
For experiments using primary cortical neurons, mounted coverslips were imaged using a confocal fluorescence microscope (FV1200; Olympus) with a 63× objective. Healthy cells that were at least two cell diameters from their nearest neighbor were identified and selected at random by eye by DAPI fluorescence. Ten nonoverlapping fields per sample were imaged. Quantification of SYP immunoreactivity (IR) was performed using ImageJ (National Institutes of Health) software (40). Average IR was calculated by dividing total IR value by the number of cells presented in the captured image. Synaptophysin puncta quantification was analyzed with a custom plug-in [written by Barry Wark; available at https://elifesciences.org/articles/04047/figures#SD1-data (52)] for the ImageJ program. The details of this imaging and quantification method can be found in a previous publication (53).
To quantify SYP fluorescence intensity and number of puncta in mouse brain, three independent coronal brain sections per animal were stained with SYP. Five-micrometer confocal scans were performed (optical section width, 0.33 μm; 15 optical sections each) at 63× magnification, as previously described (54). Maximum projections of three consecutive optical sections corresponding to 1-μm sections were analyzed by using the ImageJ puncta analyzer option to quantify for numbers of SYP puncta (≥5 optical sections per brain section and ≥15 total images per brain). Average SYP puncta density per imaged area was calculated for each treatment group.
Statistical Analysis.
In vitro data obtained from no fewer than three independent experiments (where each independent experiment consisted of between five to eight replicates) and in vivo data obtained from at least four animals per group (as many as 20 animals per group for the functional recovery/motor function tests) were analyzed using an analysis of variance (ANOVA), followed by appropriate post hoc analyses for the assessment of group differences, and presented as a bar graph depicting the mean ± SEM, using GraphPad Software. The parameters used to inform sample size considered the following: detecting an effect size of at least 30%, α = 0.05, the variance of the end point measured, and achieving a statistical power of at least 0.8.
Acknowledgments
This work was supported in part by American Heart Association Grant 13SDG17050059 (to C.S.), National Institutes of Health Grant AG027956 (to M.S.), and a fellowship (to T.N.) through Neurobiology of Aging Training Grant T32 AG020494 (Program Director M.S.).
Footnotes
Conflict of interest statement: The authors of this manuscript are coinventors on a patent application (application no. PCT/US18/46456).
This article is a PNAS Direct Submission.
References
- 1.Mozaffarian D, et al. American Heart Association Statistics Committee and Stroke Statistics Subcommittee Heart disease and stroke statistics–2015 update: A report from the American Heart Association. Circulation. 2015;131:e29–e322, and erratum (2016) 133:e417. doi: 10.1161/CIR.0000000000000152. [DOI] [PubMed] [Google Scholar]
- 2.Atif F, et al. Combination treatment with progesterone and vitamin D hormone is more effective than monotherapy in ischemic stroke: The role of BDNF/TrkB/Erk1/2 signaling in neuroprotection. Neuropharmacology. 2013;67:78–87. doi: 10.1016/j.neuropharm.2012.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ishrat T, Sayeed I, Atif F, Stein DG. Effects of progesterone administration on infarct volume and functional deficits following permanent focal cerebral ischemia in rats. Brain Res. 2009;1257:94–101. doi: 10.1016/j.brainres.2008.12.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhao Y, et al. Progesterone influences postischemic synaptogenesis in the CA1 region of the hippocampus in rats. Synapse. 2011;65:880–891. doi: 10.1002/syn.20915. [DOI] [PubMed] [Google Scholar]
- 5.Kaur P, et al. Progesterone increases brain-derived neuroptrophic factor expression and protects against glutamate toxicity in a mitogen-activated protein kinase- and phosphoinositide-3 kinase-dependent manner in cerebral cortical explants. J Neurosci Res. 2007;85:2441–2449. doi: 10.1002/jnr.21370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu L, et al. The neuroprotective effects of Tanshinone IIA are associated with induced nuclear translocation of TORC1 and upregulated expression of TORC1, pCREB and BDNF in the acute stage of ischemic stroke. Brain Res Bull. 2010;82:228–233. doi: 10.1016/j.brainresbull.2010.04.005. [DOI] [PubMed] [Google Scholar]
- 7.Sato Y, et al. White matter activated glial cells produce BDNF in a stroke model of monkeys. Neurosci Res. 2009;65:71–78. doi: 10.1016/j.neures.2009.05.010. [DOI] [PubMed] [Google Scholar]
- 8.Causing CG, et al. Synaptic innervation density is regulated by neuron-derived BDNF. Neuron. 1997;18:257–267. doi: 10.1016/s0896-6273(00)80266-4. [DOI] [PubMed] [Google Scholar]
- 9.Coffey ET, Akerman KE, Courtney MJ. Brain derived neurotrophic factor induces a rapid upregulation of synaptophysin and tau proteins via the neurotrophin receptor TrkB in rat cerebellar granule cells. Neurosci Lett. 1997;227:177–180. doi: 10.1016/s0304-3940(97)00335-2. [DOI] [PubMed] [Google Scholar]
- 10.Heldt SA, Stanek L, Chhatwal JP, Ressler KJ. Hippocampus-specific deletion of BDNF in adult mice impairs spatial memory and extinction of aversive memories. Mol Psychiatry. 2007;12:656–670. doi: 10.1038/sj.mp.4001957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McAllister AK. Dynamic aspects of CNS synapse formation. Annu Rev Neurosci. 2007;30:425–450. doi: 10.1146/annurev.neuro.29.051605.112830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sheng M, Hoogenraad CC. The postsynaptic architecture of excitatory synapses: A more quantitative view. Annu Rev Biochem. 2007;76:823–847. doi: 10.1146/annurev.biochem.76.060805.160029. [DOI] [PubMed] [Google Scholar]
- 13.Waites CL, Craig AM, Garner CC. Mechanisms of vertebrate synaptogenesis. Annu Rev Neurosci. 2005;28:251–274. doi: 10.1146/annurev.neuro.27.070203.144336. [DOI] [PubMed] [Google Scholar]
- 14.Stroemer RP, Kent TA, Hulsebosch CE. Neocortical neural sprouting, synaptogenesis, and behavioral recovery after neocortical infarction in rats. Stroke. 1995;26:2135–2144. doi: 10.1161/01.str.26.11.2135. [DOI] [PubMed] [Google Scholar]
- 15.Liu HS, et al. Post-treatment with amphetamine enhances reinnervation of the ipsilateral side cortex in stroke rats. Neuroimage. 2011;56:280–289. doi: 10.1016/j.neuroimage.2011.02.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang L, et al. Delayed administration of human umbilical tissue-derived cells improved neurological functional recovery in a rodent model of focal ischemia. Stroke. 2011;42:1437–1444. doi: 10.1161/STROKEAHA.110.593129. [DOI] [PubMed] [Google Scholar]
- 17.Zhang L, et al. Intravenous administration of human umbilical tissue-derived cells improves neurological function in aged rats after embolic stroke. Cell Transplant. 2013;22:1569–1576. doi: 10.3727/096368912X658674. [DOI] [PubMed] [Google Scholar]
- 18.Su C, Cunningham RL, Rybalchenko N, Singh M. Progesterone increases the release of brain-derived neurotrophic factor from glia via progesterone receptor membrane component 1 (Pgrmc1)-dependent ERK5 signaling. Endocrinology. 2012;153:4389–4400. doi: 10.1210/en.2011-2177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Selvamani A, Sathyan P, Miranda RC, Sohrabji F. An antagomir to microRNA Let7f promotes neuroprotection in an ischemic stroke model. PLoS One. 2012;7:e32662. doi: 10.1371/journal.pone.0032662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wendler A, Keller D, Albrecht C, Peluso JJ, Wehling M. Involvement of let-7/miR-98 microRNAs in the regulation of progesterone receptor membrane component 1 expression in ovarian cancer cells. Oncol Rep. 2011;25:273–279. [PubMed] [Google Scholar]
- 21.Di Y, et al. MicroRNAs expression and function in cerebral ischemia reperfusion injury. J Mol Neurosci. 2014;53:242–250. doi: 10.1007/s12031-014-0293-8. [DOI] [PubMed] [Google Scholar]
- 22.Caballero-Garrido E, et al. In vivo inhibition of miR-155 promotes recovery after experimental mouse stroke. J Neurosci. 2015;35:12446–12464. doi: 10.1523/JNEUROSCI.1641-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ni J, et al. MicroRNA let-7c-5p protects against cerebral ischemia injury via mechanisms involving the inhibition of microglia activation. Brain Behav Immun. 2015;49:75–85. doi: 10.1016/j.bbi.2015.04.014. [DOI] [PubMed] [Google Scholar]
- 24.Pena-Philippides JC, Caballero-Garrido E, Lordkipanidze T, Roitbak T. In vivo inhibition of miR-155 significantly alters post-stroke inflammatory response. J Neuroinflammation. 2016;13:287. doi: 10.1186/s12974-016-0753-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sigler A, Murphy TH. In vivo 2-photon imaging of fine structure in the rodent brain: Before, during, and after stroke. Stroke. 2010;41(Suppl 1):S117–S123. doi: 10.1161/STROKEAHA.110.594648. [DOI] [PubMed] [Google Scholar]
- 26.Franke H, Verkhratsky A, Burnstock G, Illes P. Pathophysiology of astroglial purinergic signalling. Purinergic Signal. 2012;8:629–657. doi: 10.1007/s11302-012-9300-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sun F, et al. Pgrmc1/BDNF signaling plays a critical role in mediating glia-neuron cross talk. Endocrinology. 2016;157:2067–2079. doi: 10.1210/en.2015-1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jodhka PK, Kaur P, Underwood W, Lydon JP, Singh M. The differences in neuroprotective efficacy of progesterone and medroxyprogesterone acetate correlate with their effects on brain-derived neurotrophic factor expression. Endocrinology. 2009;150:3162–3168. doi: 10.1210/en.2008-1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yousuf S, Atif F, Sayeed I, Wang J, Stein DG. Post-stroke infections exacerbate ischemic brain injury in middle-aged rats: Immunomodulation and neuroprotection by progesterone. Neuroscience. 2013;239:92–102. doi: 10.1016/j.neuroscience.2012.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stroemer RP, Kent TA, Hulsebosch CE. Enhanced neocortical neural sprouting, synaptogenesis, and behavioral recovery with D-amphetamine therapy after neocortical infarction in rats. Stroke. 1998;29:2381–2393; discussion 2393–2395. doi: 10.1161/01.str.29.11.2381. [DOI] [PubMed] [Google Scholar]
- 31.Chen J, Chopp M, Li Y. Neuroprotective effects of progesterone after transient middle cerebral artery occlusion in rat. J Neurol Sci. 1999;171:24–30. doi: 10.1016/s0022-510x(99)00247-6. [DOI] [PubMed] [Google Scholar]
- 32.Toung TJ, Chen TY, Littleton-Kearney MT, Hurn PD, Murphy SJ. Effects of combined estrogen and progesterone on brain infarction in reproductively senescent female rats. J Cereb Blood Flow Metab. 2004;24:1160–1166. doi: 10.1097/01.WCB.0000135594.13576.D2. [DOI] [PubMed] [Google Scholar]
- 33.Murphy SJ, Traystman RJ, Hurn PD, Duckles SP. Progesterone exacerbates striatal stroke injury in progesterone-deficient female animals. Stroke. 2000;31:1173–1178. doi: 10.1161/01.str.31.5.1173. [DOI] [PubMed] [Google Scholar]
- 34.Wright DW, et al. NETT Investigators Very early administration of progesterone for acute traumatic brain injury. N Engl J Med. 2014;371:2457–2466. doi: 10.1056/NEJMoa1404304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Skolnick BE, et al. SYNAPSE Trial Investigators A clinical trial of progesterone for severe traumatic brain injury. N Engl J Med. 2014;371:2467–2476. doi: 10.1056/NEJMoa1411090. [DOI] [PubMed] [Google Scholar]
- 36.Johnson D, et al. Acute and subacute microRNA dysregulation is associated with cytokine responses in the rodent model of penetrating ballistic-like brain injury. J Trauma Acute Care Surg. 2017;83(Suppl 1):S145–S149. doi: 10.1097/TA.0000000000001475. [DOI] [PubMed] [Google Scholar]
- 37.Balakathiresan N, et al. MicroRNA let-7i is a promising serum biomarker for blast-induced traumatic brain injury. J Neurotrauma. 2012;29:1379–1387. doi: 10.1089/neu.2011.2146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Spartalis E, et al. The “yin and yang” of platelet-rich plasma in breast reconstruction after mastectomy or lumpectomy for breast cancer. Anticancer Res. 2017;37:6557–6562. doi: 10.21873/anticanres.12112. [DOI] [PubMed] [Google Scholar]
- 39.Roy Choudhury G, et al. Methylene blue protects astrocytes against glucose oxygen deprivation by improving cellular respiration. PLoS One. 2015;10:e0123096. doi: 10.1371/journal.pone.0123096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rueden CT, et al. ImageJ2: ImageJ for the next generation of scientific image data. BMC Bioinformatics. 2017;18:529. doi: 10.1186/s12859-017-1934-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jala VR, et al. The yin and yang of leukotriene B4 mediated inflammation in cancer. Semin Immunol. 2017;33:58–64. doi: 10.1016/j.smim.2017.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Crouch SP, Kozlowski R, Slater KJ, Fletcher J. The use of ATP bioluminescence as a measure of cell proliferation and cytotoxicity. J Immunol Methods. 1993;160:81–88. doi: 10.1016/0022-1759(93)90011-u. [DOI] [PubMed] [Google Scholar]
- 43.Parker CG, et al. Ligand and target discovery by fragment-based screening in human cells. Cell. 2017;168:527–541.e29. doi: 10.1016/j.cell.2016.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ryou MG, et al. Methylene blue-induced neuronal protective mechanism against hypoxia-reoxygenation stress. Neuroscience. 2015;301:193–203. doi: 10.1016/j.neuroscience.2015.05.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Krebs CJ, et al. A membrane-associated progesterone-binding protein, 25-Dx, is regulated by progesterone in brain regions involved in female reproductive behaviors. Proc Natl Acad Sci USA. 2000;97:12816–12821. doi: 10.1073/pnas.97.23.12816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jin K, et al. Increased hippocampal neurogenesis in Alzheimer’s disease. Proc Natl Acad Sci USA. 2004;101:343–347. doi: 10.1073/pnas.2634794100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Singh M, Meyer EM, Millard WJ, Simpkins JW. Ovarian steroid deprivation results in a reversible learning impairment and compromised cholinergic function in female Sprague-Dawley rats. Brain Res. 1994;644:305–312. doi: 10.1016/0006-8993(94)91694-2. [DOI] [PubMed] [Google Scholar]
- 48.Li W, et al. PTEN degradation after ischemic stroke: A double-edged sword. Neuroscience. 2014;274:153–161. doi: 10.1016/j.neuroscience.2014.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Salvi V, et al. Metabolic syndrome in Italian patients with bipolar disorder. Gen Hosp Psychiatry. 2008;30:318–323. doi: 10.1016/j.genhosppsych.2008.04.009. [DOI] [PubMed] [Google Scholar]
- 50.Yang SH, et al. 17-beta estradiol can reduce secondary ischemic damage and mortality of subarachnoid hemorrhage. J Cereb Blood Flow Metab. 2001;21:174–181. doi: 10.1097/00004647-200102000-00009. [DOI] [PubMed] [Google Scholar]
- 51.Hauben U, D’Hooge R, Soetens E, De Deyn PP. Effects of oral administration of the competitive N-methyl-D-aspartate antagonist, CGP 40116, on passive avoidance, spatial learning, and neuromotor abilities in mice. Brain Res Bull. 1999;48:333–341. doi: 10.1016/s0361-9230(99)00008-8. [DOI] [PubMed] [Google Scholar]
- 52.Risher WC. Astrocytes refine cortical connectivity at dendritic spines. eLife. 2014;3:e04047. doi: 10.7554/eLife.04047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ippolito DM, Eroglu C. Quantifying synapses: An immunocytochemistry-based assay to quantify synapse number. J Vis Exp. 2010;45:2270. doi: 10.3791/2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kucukdereli H, et al. Control of excitatory CNS synaptogenesis by astrocyte-secreted proteins Hevin and SPARC. Proc Natl Acad Sci USA. 2011;108:E440–E449. doi: 10.1073/pnas.1104977108. [DOI] [PMC free article] [PubMed] [Google Scholar]