Significance
Bacteria, such as nontyphoidal Salmonella, are responsible for a large global burden of disease. Due to limited need in developed countries and consequent lack of commercial incentive, vaccines are unavailable against many bacteria. Glycoconjugates constitute the standard bacterial vaccine approach, but can be costly, particularly where multivalent preparations are required. This report compares a low-cost vesicle-based technology, known as Generalized Modules for Membrane Antigens (GMMA), with glycoconjugate in bivalent vaccines against nontyphoidal Salmonella. In head-to-head immunogenicity and infection studies in mice, GMMA performed at least as well as equivalent glycoconjugate vaccine, indicating good potential of this approach. Given that many bacteria are amenable to genetic engineering for GMMA production, the GMMA strategy could provide a breakthrough for a range of needed bacterial vaccines.
Keywords: nontyphoidal, Salmonella, vaccines, GMMA, vesicles
Abstract
Nontyphoidal Salmonellae cause a devastating burden of invasive disease in sub-Saharan Africa with high levels of antimicrobial resistance. Vaccination has potential for a major global health impact, but no licensed vaccine is available. The lack of commercial incentive makes simple, affordable technologies the preferred route for vaccine development. Here we compare equivalent Generalized Modules for Membrane Antigens (GMMA) outer membrane vesicles and O-antigen-CRM197 glycoconjugates to deliver lipopolysaccharide O-antigen in bivalent Salmonella Typhimurium and Enteritidis vaccines. Salmonella strains were chosen and tolR deleted to induce GMMA production. O-antigens were extracted from wild-type bacteria and conjugated to CRM197. Purified GMMA and glycoconjugates were characterized and tested in mice for immunogenicity and ability to reduce Salmonella infection. GMMA and glycoconjugate O-antigen had similar structural characteristics, O-acetylation, and glucosylation levels. Immunization with GMMA induced higher anti–O-antigen IgG than glycoconjugate administered without Alhydrogel adjuvant. With Alhydrogel, antibody levels were similar. GMMA induced a diverse antibody isotype profile with greater serum bactericidal activity than glycoconjugate, which induced almost exclusively IgG1. Immunization reduced bacterial colonization of mice subsequently infected with Salmonella. S. Typhimurium numbers were lower in tissues of mice vaccinated with GMMA compared with glycoconjugate. S. Enteritidis burden in the tissues was similar in mice immunized with either vaccine. With favorable immunogenicity, low cost, and ability to induce functional antibodies and reduce bacterial burden, GMMA offer a promising strategy for the development of a nontyphoidal Salmonella vaccine compared with established glycoconjugates. GMMA technology is potentially attractive for development of vaccines against other bacteria of global health significance.
Invasive nontyphoidal Salmonella (iNTS) disease is a leading cause of death and morbidity in developing countries (1–3). Nontyphoidal Salmonellae are responsible for up to 39% of community-acquired bloodstream infections in sub-Saharan Africa with an average case fatality rate of 19% (4). The effectiveness of antibiotic treatment is hampered by the difficulty in making a diagnosis, the sudden onset of the disease, and the growing frequency of multidrug resistance (1, 2, 5). Higher incidence and increased severity of iNTS disease have been observed in young children below 72 mo of age, in patients with malaria, anemia, malnutrition, HIV, sickle cell disease, and hemolysis (6–9). Moreover, the Global Burden of Disease Study 2015 estimated that NTS is the third commonest cause of diarrheal deaths at 90,300 (95% uncertainty interval, 34,100–183,100) (10).
Salmonella enterica serovars Typhimurium and Enteritidis are responsible for 91% of the cases of iNTS disease reported in Africa (4) and a similar proportion of NTS diarrheal disease. A bivalent vaccine against these two serovars could represent a valuable public health intervention. Several groups have been working on the development of glycoconjugate, protein-based, vesicle-based, and live attenuated vaccines against NTS (11), but none has entered clinical trials over the last 16 y. Hence, a licensed vaccine is still a long way off. This lack of progress relates primarily to the absence of a commercial incentive to develop such a vaccine. Hence, a technology that could produce large quantities of an effective vaccine simply and at low cost would be enormously valuable for advancing a vaccine against this devastating disease.
The serovar-specific O-antigen (OAg) moiety of Salmonella lipopolysaccharide (LPS) is the principal target of protective immunity (12–14). LPS molecules are composed of lipid A (endotoxin) attached to the 3-deoxy-d-manno-octulosonic acid (KDO) terminus of the conserved core region, which is linked to the variable OAg chain containing serogroup-specific repeating units. S. Typhimurium and S. Enteritidis OAg repeating units share a common backbone, consisting of mannose (Man), rhamnose (Rha), and galactose (Gal). A different 3,6-dideoxy-hexose residue is linked to Man in the two serovars: abequose (Abe), conferring O:4 specificity to S. Typhimurium OAg, and tyvelose (Tyv), conferring O:9 specificity to S. Enteritidis OAg. Both repeating units can be variably glucosylated and O-acetylated (15). Specific anti-OAg antibodies have been shown to mediate killing (12, 16) and confer protection against infection in animal models (13, 14, 17, 18).
The current state-of-the-art approach to polysaccharide-based vaccines is the glycoconjugate approach, where polysaccharide is covalently linked to a suitable carrier protein, enabling the induction of a T cell-dependent antibody response (19). To date, glycoconjugates have been the technology of choice for vaccine development against iNTS disease (11, 20). We have previously shown that O-antigen conjugated to the nontoxic recombinant form of the diphtheria toxin, CRM197, is immunogenic and reduces the tissue burden of Salmonella infection in mice (13, 21–23). However, glycoconjugate vaccines can be both expensive and complex to produce, particularly when multiple valencies are necessary, and require large capital investment on infrastructure. These represent major disadvantages for a vaccine that has no commercial high-income country application and where the final manufacturer is likely to be a developing country vaccine manufacturer with limited available expertise compared with large multinational vaccine companies.
As an alternative strategy to global health vaccines, we are pioneering the use of an outer membrane vesicle technology known as Generalized Modules for Membrane Antigens (GMMA). GMMA technology can be employed as a vehicle to deliver Salmonella lipopolysaccharide O-antigen to the immune system. GMMA represent a straightforward technology with the advantages of low-cost, high-production yields and ease of technology transfer to the end manufacturer (24, 25).
The integrity and attachment of the inner and outer bacterial cell wall membranes of Salmonella can be altered by disruption of the Tol-Pal system, through deletion of the tolR gene (26, 27), resulting in the release of large quantities of GMMA. GMMA are outer membrane vesicles of homogeneous size, typically in the range 40–250 nm, released from the surface of genetically mutated Gram-negative bacteria (24). GMMA constitute an enriched source of outer membrane antigens, including OAg, presented to the immune system in their native conformation. GMMA are optimally sized for uptake by antigen-presenting cells and have self-adjuvanting activity, delivering innate signals through toll-like receptor (TLR) ligands and other pathogen-associated molecular patterns. Unlike live attenuated vaccines, there is no possibility of infection. Work to date indicates that GMMA are highly immunogenic (28–31), but so far there has been no direct comparison of immunogenicity with equivalent glycoconjugate vaccines.
The aims of this study were to: (i) generate and characterize a bivalent GMMA and bivalent glycoconjugate vaccine against S. Typhimurium and S. Enteritidis, as alternative approaches to developing a vaccine against iNTS disease; and (ii) compare the immunogenicity and ability of the two vaccines to reduce the bacterial burden following infection with Salmonella in mice, to down-select a vaccine strategy for clinical development.
Results
OAg-CRM197 Conjugates.
S. Typhimurium and S. Enteritidis OAg, purified from 2189 and 618 wild-type bacteria (21), were independently linked to CRM197 as carrier protein. Terminal linkage of the sugar chains to the protein was selected in order not to impact on OAg chain structure and epitopes (22). Both conjugates were characterized by an OAg-to-protein weight ratio close to 2 (1.9 for S. Typhimurium OAg-CRM197 and 2.3 for S. Enteritidis OAg-CRM197), with <20% free saccharide. Analysis by HPLC-SEC (SI Appendix, Fig. S1) indicated the higher–molecular-weight peak expected for the conjugates, compared with unconjugated protein, and no detectable presence of unreacted CRM197. S. Typhimurium OAg-CRM197 was characterized by a more polydisperse population compared with S. Enteritidis OAg-CRM197. This is likely due to the presence of two different populations in 2189 wild-type OAg (average size of 80.8 and 31.6 kDa), compared with just one population of relatively lower molecular weight for 618 wild-type OAg (average size of 29.6 kDa).
GMMA.
GMMA were produced from ΔtolR mutants of the same strains used as the source of OAg for the glycoconjugate vaccine (26). S. Typhimurium and S. Enteritidis GMMA had similar size and OAg-to-protein weight ratio (0.72 and 0.61, respectively) (Table 1). The amount of lipid A per mg of protein was higher for S. Enteritidis compared with S. Typhimurium GMMA (Table 1).
Table 1.
Characterization of GMMA particles
| Characteristic | S. Typhimurium GMMA | S. Enteritidis GMMA |
| Percent soluble proteins | <5 | <5 |
| Particle size [weight average geometric radius (Rw) nm by multiangle light scattering] | 38.4 | 39.1 |
| Particle size (radius nm by trasmission electron microscopy) | 22.5 | 22.5 |
| OAg/protein wt/wt ratio | 0.72 | 0.61 |
| nmol lipid A/mg protein | 107 | 157 |
Characterization of OAg on GMMA and Free OAg for Conjugation to CRM197.
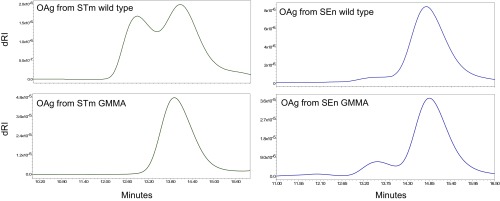
OAg is the main target for the immune response against both NTS GMMA and glycoconjugate vaccines. Therefore, the structural characteristics of OAg purified from wild-type bacteria for the synthesis of glycoconjugates and presented on GMMA from corresponding ΔtolR mutated strains were fully investigated and compared. Both S. Typhimurium and S. Enteritidis OAg from GMMA showed similar sugar composition, O-acetylation, and glucosylation levels to OAg from corresponding wild-type bacteria (Table 2). S. Enteritidis OAg were also similar for molecular size distribution, with OAg on GMMA showing an additional shoulder at higher molecular weight compared with S. Enteritidis wild-type OAg. For S. Typhimurium, OAg on GMMA was characterized as being one main population, while an additional population at relatively higher molecular weight was observed with OAg from wild-type bacteria (Fig. 1).
Table 2.
Sugar composition analysis of OAg on GMMA (from ΔtolR bacteria) and from wild-type bacteria for conjugation to CRM197
| Molar ratio to Rha | |||||
| OAg from | Tyv/Abe | Man | Gal | % Glc | % OAc |
| Salmonella Typhimurium | |||||
| Wild type | 0.95 | 1.01 | 1.09 | 51 | 58 |
| GMMA | 1.00 | 0.83 | 1.18 | 39 | 53 |
| Salmonella Enteritidis | |||||
| Wild type | 0.95 | 0.95 | 1.05 | 19 | 16 |
| GMMA | 1.00 | 0.99 | 1.12 | 24 | 4 |
Sugar composition analysis by HPAEC-PAD, but Tyv/Abe quantification and O-acetylation by 1H NMR.
Fig. 1.
HPLC-SEC chromatograms of OAg chains extracted from GMMA and compared with OAg purified from corresponding wild-type bacteria. SEn, S. Enteritidis; STm, S. Typhimurium.
Immunogenicity Studies in Mice.
GMMA and OAg-CRM197 conjugates were compared in mice at a 10-fold increasing range of OAg doses (1 ng to 10 μg for GMMA and 0.1–10 μg for OAg-CRM197, as OAg equivalents), all formulated with Alhydrogel (Fig. 2). Formulations of both GMMA and conjugates at 1 μg OAg dose were also tested without Alhydrogel to assess the impact of Alhydrogel on immune response. For S. Typhimurium, GMMA induced a significantly higher anti-OAg IgG response at day 42 than conjugate at all tested doses (P ≤ 0.007 for all comparisons) (Fig. 2A). For S. Enteritidis, the anti-OAg IgG response induced by GMMA was similar to that induced by the conjugate at day 42 for all tested OAg doses and only significantly higher with the GMMA 10 μg OAg dose at day 28 (P = 0.002) (Fig. 2B).
Fig. 2.
S. Typhimurium and S. Enteritidis GMMA and glycoconjugate vaccines compared in mice as monovalent formulations. Eight C57BL/6 mice per group were s.c. immunized at days 0 and 28, with different OAg doses on Alhydrogel. (A and B) Summary graphs of anti-OAg IgG geometric mean units (bars) and individual antibody levels (dots). (C and D) Serum bactericidal assay (SBA) titers of pooled sera from day 42 from each group against S. Typhimurium D23580 or S. Enteritidis CMCC4314 strains. SEn, S. Enteritidis; STm, S. Typhimurium.
Both for S. Typhimurium and S. Enteritidis GMMA, a dose–response was observed (Spearman rank correlation, day 42 samples: ρ = 0.839 and P < 0.0001 for S. Typhimurium; ρ = 0.879 and P < 0.0001 for S. Enteritidis). A rise in antibody titer following the second vaccine dose was observed for both GMMA (P = 0.008 in the range 0.1–10 μg dose for both S. Typhimurium and S. Enteritidis) and conjugates (P = 0.04 at 0.1 and 10 μg dose and P = 0.008 at 1 μg dose for S. Typhimurium; P = 0.008 at all doses tested for S. Enteritidis). When mice were immunized with S. Typhimurium GMMA in the absence of Alhydrogel, anti-OAg IgG response was higher than with Alhydrogel (P = 0.005 at day 42). With S. Enteritidis GMMA, addition of Alhydrogel did not affect the immune response (P = 0.5 at day 42). For both S. Typhimurium and S. Enteritidis conjugates at 1 μg dose, no IgG response was detected in the absence of Alhydrogel (at day 42, P = 0.0005 and P < 0.0001, respectively).
Day 42 pooled sera for each group were tested for functional activity. Serum bactericidal assay (SBA) titers induced by GMMA were higher than those induced by conjugates for both S. Typhimurium and S. Enteritidis, (Fig. 2 C and D). Sera from mice immunized with S. Typhimurium GMMA at 1 μg OAg dose were 75-fold times more bactericidal than sera from mice immunized with the corresponding conjugate at the same OAg dose. There was a 20-fold difference when comparing bactericidal titers of sera from S. Enteritidis GMMA and conjugate at 1 μg OAg dose. Sera from mice immunized with very low amounts of GMMA (1–10 ng OAg) also showed bactericidal activity. Alhydrogel did not increase SBA titers induced by the GMMA vaccines, whereas it enhanced those induced by the glycoconjugates (26-fold difference for S. Typhimurium and 18-fold difference for S. Enteritidis conjugates).
To better understand the reason for the differences observed, analysis of IgG subclasses and IgM was performed. Day 42 sera from mice immunized with GMMA and conjugate vaccines, at 1 μg OAg dose with Alhydrogel, were compared. IgG induced by the conjugates was almost exclusively IgG1, particularly for S. Enteritidis conjugate, with sera containing undetectable levels of OAg-specific IgG2a, IgG2b, and IgG3 (SI Appendix, Fig. S2). In contrast, GMMA induced all IgG subclasses, with the exception of IgG2a for S. Enteritidis. S. Enteritidis and S. Typhimurium GMMA induced significantly higher anti–OAg-specific IgG2b (P = 0.0006 for S. Typhimurium and P = 0.0014 for S. Enteritidis) and IgG3 (P = 0.0003 for S. Typhimurium and P = 0.0014 for S. Enteritidis) than conjugates. GMMA also induced anti-OAg IgM antibodies, which were undetectable in sera of mice immunized with conjugate vaccines (SI Appendix, Fig. S2).
Based on these immunogenicity results and with the aim of developing a vaccine against both S. Typhimurium and S. Enteritidis, bivalent formulations of GMMA and conjugates at 1 μg OAg dose were further tested in mice. In this second study, bivalent GMMA and OAg-CRM197 formulations induced similar S. Typhimurium and S. Enteritidis anti–OAg-specific IgG responses (at day 42, P = 0.07 for S. Typhimurium and P = 0.14 for S. Enteritidis) (Fig. 3 A and B). SBA performed with day 42 individual mouse sera showed markedly higher SBA titers following immunization with GMMA compared with conjugates (P = 0.008 and 0.0001 for S. Typhimurium and S. Enteritidis strains, respectively). Many sera from mice immunized with conjugate vaccines (17 out of 24) did not show any bactericidal activity (Fig. 3 C and D).
Fig. 3.
In vivo infection study in mice immunized with GMMA and conjugate in bivalent formulation. Twelve C57BL/6 mice per group were s.c. immunized at days 0 and 28 at 1 μg OAg/dose per each antigen with Alhydrogel. Seventeen days after the second injection, six mice of each group were challenged intraperitoneally with 104 cfu of S. Typhimurium D23580 or with S. Enteritidis D24954. Twenty-four hours after challenge, mice were killed and spleens and livers collected for bacterial plate counting. (A and B) Summary graphs of anti-OAg IgG geometric mean units (bars) and individual antibody levels (dots). (C and D) SBA titers of single sera collected at day 42 from each group against S. Typhimurium D23580 or S. Enteritidis CMCC4314 strains. (E and F) Bacterial cfu measured in spleens and livers postchallenge. SEn, S. Enteritidis; STm, S. Typhimurium.
To compare the in vivo efficacy of GMMA and conjugate vaccines, we performed a Salmonella infection study in mice immunized with bivalent formulations of either vaccine. After challenge with invasive strains of S. Typhimurium or S. Enteritidis, mice immunized with either bivalent GMMA or conjugate vaccines showed reduced bacterial colonization of the spleen and liver compared with control mice. The GMMA vaccine resulted in lower bacterial cfus than conjugates (Fig. 3 E and F) in liver (P = 0.009), although there was no significant difference in spleen after infection with S. Typhimurium. There was no difference in bacterial counts between mice immunized with S. Enteritidis GMMA and conjugates.
Discussion
In this study, we have compared glycoconjugate and GMMA OAg-based approaches to the development of a vaccine against iNTS disease. In view of the coendemicity of S. Typhimurium and S. Enteritidis in Africa, a bivalent vaccine covering both serovars is an obvious strategy (23). For this reason, we compared monovalent and bivalent GMMA and glycoconjugate formulations. The GMMA were able to (i) induce (a) high anti–OAg-specific IgG responses in mice, (b) broad IgG subclass and Ig isotype profile, and (c) strong bactericidal activity, and (ii) reduce bacterial colonization in mice infected with virulent endemic strains. The bivalent OAg-CRM197 conjugate formulation induced immune responses similar to the single-conjugate components, confirming previous results (23). Here we have gone on to demonstrate that the vaccine reduces bacterial colonization in mice following infection.
We found that GMMA and glycoconjugates induced similar anti-OAg IgG responses when administered with Alhydrogel. IgG levels induced by GMMA were high, irrespective of the presence of Alhydrogel, while conjugates were unable to induce a response without Alhydrogel. This lack of immunogenicity with unadjuvanted conjugates is in contrast with our previous findings and may be related to the lower OAg-equivalent dose used in the current study compared with previous studies (23) and to the different immunization schedule used, with two doses at days 0 and 28 compared with three doses at 2-wk intervals (21). In general, GMMA were able to induce higher serum bactericidal titers than conjugates, with sera from around 70% of conjugate-immunized mice unable to kill Salmonella.
Considering the impact that OAg structural modifications (saccharide size, O-acetylation, and glucosylation level) can have on the immune responses (13, 21), OAg from wild-type bacteria used to synthesize the glycoconjugates and OAg from the GMMA were fully characterized, and no major differences were identified. Therefore, OAg structure is unlikely to be a factor responsible for the difference in immunogenicity in mice between the two vaccine approaches. A more plausible factor is the different antibody class and subclass profile elicited, with GMMA inducing higher specific IgG2b, IgG3, and IgM levels than the glycoconjugate, which, in contrast, elicited IgG1 only. IgG2a and IgG2b are known to bind complement component C1q more efficiently than IgG1 (32), and IgM is the most bactericidal of all antibody classes. Another factor to account for the difference in immunogenicity and bactericidal activity is that GMMA may constitute a more immunogenic format for presenting OAg to the immune system. GMMA likely exert a self-adjuvanting effect. They also have the advantage of presenting multiple antigens to the immune system with the potential to induce killing through various effector mechanisms (complement-, phagocyte-, and T cell-mediated) directed at outer membrane proteins as well as OAg.
In the infection study, prior immunization with bivalent GMMA vaccine reduced the S. Typhimurium load more than corresponding glycoconjugate, but there was no difference between conjugates and GMMA in relation to S. Enteritidis counts. This finding may be partly explained by our previous observation that S. Enteritidis isolates are less susceptible to antibody-dependent complement-mediated killing than S. Typhimurium isolates (33). Hence, bactericidal activity of anti-OAg antibodies may be more critical for killing S. Typhimurium than S. Enteritidis. Other immune effector mechanisms, including antibody-mediated phagocytosis (34) and CD4+ and CD8+ T cell mechanisms, may be of greater importance for killing S. Enteritidis.
A potential concern with the use of GMMA vaccines is their reactogenicity, since GMMA contain LPS (34). Therefore, we have engineered S. Typhimurium and S. Enteritidis GMMA with reduced reactogenicity while maintaining adjuvanticity by modifying the LPS lipid A acylation pattern (26). A similar detoxification strategy was employed for our most advanced GMMA-based vaccine, a Shigella sonnei GMMA vaccine, which was well-tolerated in clinical trials (25, 29, 30). In the current study, we tested GMMA without modified lipid A structure. However, we have shown previously that introduction of genetic manipulations to reduce Salmonella GMMA reactogenicity, including pagP and msbB deletions, does not significantly impact anti–OAg-specific IgG response or ability of postvaccination sera to kill bacteria in vitro (28). While we have verified that use of Alhydrogel does not enhance immunogenicity induced in mice by GMMA, formulation with Alhydrogel is required for abrogation of pyrogenicity in rabbits (35). For this reason, GMMA and conjugates were for the most part compared when coadministered with Alhydrogel.
A key advantage of GMMA over conjugates is the simplicity and cost-effectiveness of the production process, making the GMMA approach particularly attractive for global health vaccines for low- and middle-income countries, where high cost of manufacture can be an obstacle to vaccine implementation (36, 37). Production yields are high, and following fermentation of the GMMA-producing bacterial strains, two simple tangential flow filtration steps allow a high level of purification of GMMA. These factors all contribute to potential very low cost of goods for GMMA vaccines, another critical factor for vaccines designed primarily for global health application.
In conclusion, we have designed equivalent bivalent S. Typhimurium and S. Enteritidis GMMA and glycoconjugate vaccines. By testing these vaccines in mice, we have demonstrated that the GMMA approach confers equal or enhanced immunogenicity and ability to reduce bacterial load compared with the standard glycoconjugate approach. Given the added advantages of simplicity of production and low cost of goods, these findings indicate that the GMMA strategy has excellent potential. GMP lots of the GMMA vaccine have now been produced with a view to an in-human study in the near future.
This is a direct comparison of the GMMA and glycoconjugate vaccine strategies for bacterial vaccines. Therefore, it will be important to determine whether the GMMA approach offers the same advantages for development of vaccines against other bacterial diseases. Similar comparative vaccinology studies should be conducted for other Gram-negative bacteria, which are particularly amenable to genetic manipulation for GMMA production.
Materials and Methods
SI Appendix, Materials and Methods feature additional information to the section provided here.
GMMA were produced from S. Typhimurium 2189 ΔtolR and from S. Enteritidis 618 ΔtolR strains (SI Appendix, Materials and Methods) and purified and characterized as previously described (26, 28, 38). For synthesis of glycoconjugates, OAg were purified from the same S. Typhimurium 2189 and S. Enteritidis 618 wild-type bacteria (21, 39) and characterized as previously described (15, 39). OAg chains isolated from GMMA were characterized by using the same methods for OAg from wild-type bacteria (15, 28).
For conjugation to CRM197 (provided by GSK Vaccines), OAg was derivatized with adipic acid dihydrazide (ADH) by reductive amination of the KDO terminal sugar and linked to the amino groups on the protein after attachment of a second linker, adipic acid bis(N-hydroxysuccinimide) (SIDEA), to ADH (40). Conjugation conditions and assays for characterization of OAg-CRM197 conjugates and intermediates were as previously described (13, 21–23). Two studies in mice were conducted to compare the immunogenicity of S. Typhimurium and S. Enteritidis GMMA and glycoconjugates. Details on the immunization schemes and sera analysis are reported in SI Appendix, Materials and Methods.
Supplementary Material
Acknowledgments
This work was supported by a Bill & Melinda Gates Foundation Grand Challenges Explorations Award (to C.A.M.): ‘A novel approach to manufacture of highly immunogenic and affordable polysaccharide vaccines for Global Health priority diseases.’ S.C. and C.B. are funded by Wellcome Trust Grant 206194.
Footnotes
Conflict of interest statement: F.M., S.R., R.A., L.L., F.N., O.R., R.R., and A.S. are employees of the GSK group of companies. A.N. and C.A.M. were employees of NVGH (now GVGH) during part of the study.
2This work was initiated at the Novartis Vaccines Institute for Global Health; in March 2015 the Novartis noninfluenza vaccines business was acquired by the GSK group of companies. Thereafter the company became GSK Vaccines Institute for Global Health, an affiliate of GlaxoSmithKline Biologicals SA.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1807655115/-/DCSupplemental.
References
- 1.Feasey NA, Dougan G, Kingsley RA, Heyderman RS, Gordon MA. Invasive non-typhoidal Salmonella disease: An emerging and neglected tropical disease in Africa. Lancet. 2012;379:2489–2499. doi: 10.1016/S0140-6736(11)61752-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.MacLennan CA, Levine MM. Invasive nontyphoidal Salmonella disease in Africa: Current status. Expert Rev Anti Infect Ther. 2013;11:443–446. doi: 10.1586/eri.13.27. [DOI] [PubMed] [Google Scholar]
- 3.Ao TT, et al. Global burden of invasive nontyphoidal Salmonella disease, 2010(1) Emerg Infect Dis. 2015;21:941–949. doi: 10.3201/eid2106.140999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Uche IV, MacLennan CA, Saul A. A systematic review of the incidence, risk factors and case fatality rates of invasive nontyphoidal Salmonella (iNTS) disease in Africa (1966 to 2014) PLoS Negl Trop Dis. 2017;11:e0005118. doi: 10.1371/journal.pntd.0005118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kariuki S, Gordon MA, Feasey N, Parry CM. Antimicrobial resistance and management of invasive Salmonella disease. Vaccine. 2015;33:C21–C29. doi: 10.1016/j.vaccine.2015.03.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gordon MA. Salmonella infections in immunocompromised adults. J Infect. 2008;56:413–422. doi: 10.1016/j.jinf.2008.03.012. [DOI] [PubMed] [Google Scholar]
- 7.MacLennan CA, et al. Dysregulated humoral immunity to nontyphoidal Salmonella in HIV-infected African adults. Science. 2010;328:508–512. doi: 10.1126/science.1180346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Feasey NA, et al. Modelling the contributions of malaria, HIV, malnutrition and rainfall to the decline in paediatric invasive non-typhoidal Salmonella disease in Malawi. PLoS Negl Trop Dis. 2015;9:e0003979. doi: 10.1371/journal.pntd.0003979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.MacLennan CA, et al. Presentation of life-threatening invasive nontyphoidal Salmonella disease in Malawian children: A prospective observational study. PLoS Negl Trop Dis. 2017;11:e0006027. doi: 10.1371/journal.pntd.0006027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Anonymous; GBD Diarrhoeal Diseases Collaborators Estimates of global, regional, and national morbidity, mortality, and aetiologies of diarrhoeal diseases: A systematic analysis for the global burden of disease study 2015. Lancet Infect Dis. 2017;17:909–948. doi: 10.1016/S1473-3099(17)30276-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.MacLennan CA, Martin LB, Micoli F. Vaccines against invasive Salmonella disease: Current status and future directions. Hum Vaccin Immunother. 2014;10:1478–1493. doi: 10.4161/hv.29054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rondini S, et al. Invasive African Salmonella Typhimurium induces bactericidal antibodies against O-antigens. Microb Pathog. 2013;63:19–23. doi: 10.1016/j.micpath.2013.05.014. [DOI] [PubMed] [Google Scholar]
- 13.Rondini S, et al. Design of glycoconjugate vaccines against invasive African Salmonella enterica serovar Typhimurium. Infect Immun. 2015;83:996–1007. doi: 10.1128/IAI.03079-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goh YS, et al. Monoclonal antibodies of a diverse isotype induced by an O-antigen glycoconjugate vaccine mediate in vitro and in vivo killing of African invasive nontyphoidal Salmonella. Infect Immun. 2015;83:3722–3731. doi: 10.1128/IAI.00547-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Micoli F, et al. Structural analysis of O-polysaccharide chains extracted from different Salmonella Typhimurium strains. Carbohydr Res. 2014;385:1–8. doi: 10.1016/j.carres.2013.12.003. [DOI] [PubMed] [Google Scholar]
- 16.Trebicka E, Jacob S, Pirzai W, Hurley BP, Cherayil BJ. Role of antilipopolysaccharide antibodies in serum bactericidal activity against Salmonella enterica serovar Typhimurium in healthy adults and children in the United States. Clin Vaccine Immunol. 2013;20:1491–1498. doi: 10.1128/CVI.00289-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Simon R, et al. Salmonella enterica serovar enteritidis core O polysaccharide conjugated to H:g,m flagellin as a candidate vaccine for protection against invasive infection with S. Enteritidis. Infect Immun. 2011;79:4240–4249. doi: 10.1128/IAI.05484-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Watson DC, Robbins JB, Szu SC. Protection of mice against Salmonella Typhimurium with an O-specific polysaccharide-protein conjugate vaccine. Infect Immun. 1992;60:4679–4686. doi: 10.1128/iai.60.11.4679-4686.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pollard AJ, Perrett KP, Beverley PC. Maintaining protection against invasive bacteria with protein-polysaccharide conjugate vaccines. Nat Rev Immunol. 2009;9:213–220. doi: 10.1038/nri2494. [DOI] [PubMed] [Google Scholar]
- 20.Simon R, Levine MM. Glycoconjugate vaccine strategies for protection against invasive Salmonella infections. Hum Vaccin Immunother. 2012;8:494–498. doi: 10.4161/hv.19158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lanzilao L, et al. Strain selection for generation of O-antigen-based glycoconjugate vaccines against invasive nontyphoidal Salmonella disease. PLoS One. 2015;10:e0139847. doi: 10.1371/journal.pone.0139847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stefanetti G, et al. Impact of conjugation chemistry on the immunogenicity of S. Typhimurium conjugate vaccines. Vaccine. 2014;32:6122–6129. doi: 10.1016/j.vaccine.2014.08.056. [DOI] [PubMed] [Google Scholar]
- 23.Fiorino F, et al. Immunogenicity of a bivalent adjuvanted glycoconjugate vaccine against Salmonella Typhimurium and Salmonella Enteritidis. Front Immunol. 2017;8:168. doi: 10.3389/fimmu.2017.00168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Berlanda Scorza F, et al. High yield production process for Shigella outer membrane particles. PLoS One. 2012;7:e35616. doi: 10.1371/journal.pone.0035616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gerke C, et al. Production of a Shigella sonnei vaccine based on generalized modules for membrane antigens (GMMA), 1790GAHB. PLoS One. 2015;10:e0134478. doi: 10.1371/journal.pone.0134478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rossi O, et al. Toll-like receptor activation by generalized modules for membrane antigens from lipid a mutants of Salmonella enterica serovars typhimurium and enteritidis. Clin Vaccine Immunol. 2016;23:304–314. doi: 10.1128/CVI.00023-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Meloni E, et al. Simplified low-cost production of O-antigen from Salmonella Typhimurium generalized modules for membrane antigens (GMMA) J Biotechnol. 2015;198:46–52. doi: 10.1016/j.jbiotec.2015.01.020. [DOI] [PubMed] [Google Scholar]
- 28.De Benedetto G, et al. Characterization of O-antigen delivered by generalized modules for membrane antigens (GMMA) vaccine candidates against nontyphoidal Salmonella. Vaccine. 2017;35:419–426. doi: 10.1016/j.vaccine.2016.11.089. [DOI] [PubMed] [Google Scholar]
- 29.Launay O, et al. Safety profile and immunologic responses of a novel vaccine against Shigella sonnei administered intramuscularly, intradermally and intranasally: Results from two parallel randomized phase 1 clinical studies in healthy adult volunteers in Europe. EBioMedicine. 2017;22:164–172. doi: 10.1016/j.ebiom.2017.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Obiero CW, et al. A phase 2a randomized study to evaluate the safety and immunogenicity of the 1790GAHB generalized modules for membrane antigen vaccine against Shigella sonnei administered intramuscularly to adults from a shigellosis-endemic country. Front Immunol. 2017;8:1884. doi: 10.3389/fimmu.2017.01884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Koeberling O, et al. A broadly-protective vaccine against meningococcal disease in sub-Saharan Africa based on generalized modules for membrane antigens (GMMA) Vaccine. 2014;32:2688–2695. doi: 10.1016/j.vaccine.2014.03.068. [DOI] [PubMed] [Google Scholar]
- 32.Leatherbarrow RJ, Dwek RA. Binding of complement subcomponent C1q to mouse IgG1, IgG2a and IgG2b: A novel C1q binding assay. Mol Immunol. 1984;21:321–327. doi: 10.1016/0161-5890(84)90103-2. [DOI] [PubMed] [Google Scholar]
- 33.Onsare RS, et al. Relationship between antibody susceptibility and lipopolysaccharide O-antigen characteristics of invasive and gastrointestinal nontyphoidal Salmonellae isolates from Kenya. PLoS Negl Trop Dis. 2015;9:e0003573. doi: 10.1371/journal.pntd.0003573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gondwe EN, et al. Importance of antibody and complement for oxidative burst and killing of invasive nontyphoidal Salmonella by blood cells in Africans. Proc Natl Acad Sci USA. 2010;107:3070–3075. doi: 10.1073/pnas.0910497107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rosenqvist E, et al. Effect of aluminium hydroxide and meningococcal serogroup C capsular polysaccharide on the immunogenicity and reactogenicity of a group B Neisseria meningitidis outer membrane vesicle vaccine. Dev Biol Stand. 1998;92:323–333. [PubMed] [Google Scholar]
- 36.MacLennan CA. Vaccines for low-income countries. Semin Immunol. 2013;25:114–123. doi: 10.1016/j.smim.2013.05.004. [DOI] [PubMed] [Google Scholar]
- 37.MacLennan CA, Saul A. Vaccines against poverty. Proc Natl Acad Sci USA. 2014;111:12307–12312. doi: 10.1073/pnas.1400473111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.De Benedetto G, Cescutti P, Giannelli C, Rizzo R, Micoli F. Multiple techniques for size determination of generalized modules for membrane antigens from Salmonella Typhimurium and Salmonella Enteritidis. ACS Omega. 2017;2:8282–8289. doi: 10.1021/acsomega.7b01173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Micoli F, et al. A scalable method for O-antigen purification applied to various Salmonella serovars. Anal Biochem. 2013;434:136–145. doi: 10.1016/j.ab.2012.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Micoli F, et al. O:2-CRM(197) conjugates against Salmonella Paratyphi A. PLoS One. 2012;7:e47039. doi: 10.1371/journal.pone.0047039. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.