Significance
Despite being discovered a long time ago, the functional properties of VEGF-B remain poorly understood. However, several clinical treatments use drugs that target VEGF-B and other VEGF family members. It is therefore crucial to gain deeper insights into the function of VEGF-B and the underlying mechanisms. Here, we found that VEGF-B has potent antioxidative functions, making it a VEGF family member to show such a property. We further identified a critical downstream effector of VEGF-B, Gpx1, through which it protects against retinal degeneration. In addition, being an otherwise “inert” molecule, as shown by previous studies, makes VEGF-B a promising molecule for clinical applications. Our findings suggest that VEGF-B could be a potent therapeutic agent against oxidative stress-related diseases.
Keywords: VEGF-B, antioxidant, oxidative stress, Gpx1, retinal degeneration
Abstract
VEGF-B was discovered a long time ago. However, unlike VEGF-A, whose function has been extensively studied, the function of VEGF-B and the mechanisms involved still remain poorly understood. Notwithstanding, drugs that inhibit VEGF-B and other VEGF family members have been used to treat patients with neovascular diseases. It is therefore critical to have a better understanding of VEGF-B function and the underlying mechanisms. Here, using comprehensive methods and models, we have identified VEGF-B as a potent antioxidant. Loss of Vegf-b by gene deletion leads to retinal degeneration in mice, and treatment with VEGF-B rescues retinal cells from death in a retinitis pigmentosa model. Mechanistically, we demonstrate that VEGF-B up-regulates numerous key antioxidative genes, particularly, Gpx1. Loss of Gpx1 activity largely diminished the antioxidative effect of VEGF-B, demonstrating that Gpx1 is at least one of the critical downstream effectors of VEGF-B. In addition, we found that the antioxidant function of VEGF-B is mediated mainly by VEGFR1. Given that oxidative stress is a crucial factor in numerous human diseases, VEGF-B may have therapeutic value for the treatment of such diseases.
VEGF-B was discovered in 1996 as a homolog of VEGF-A (1). VEGF-B binds to VEGFR1 and NP1 (2, 3), and is abundantly expressed in most tissues and organs (4–8). Unlike VEGF-A, whose function has been extensively studied, the function of VEGF-B and the mechanisms involved have not been well understood and remain debatable. Studies have shown that VEGF-B does not induce neovessel growth or blood vessel permeability under most conditions (7, 9–12). VEGF-B has also been shown to be a potent inhibitor of apoptosis by suppressing the BH3-only protein genes (7). In addition, under conditions of tissue/vessel injury, VEGF-B has been shown to act as a critical survival factor that protects cells from death (6–9, 13, 14). Under normal conditions, VEGF-B appears to be “inert” with no obvious function (9, 15, 16). More recently, VEGF-B has been reported to play a role in diabetes. However, different studies have reported diverse findings (17–19). Despite the poor understanding of VEGF-B’s function and the mechanisms involved, drugs that can inhibit VEGF-B together with other VEGF family members have been extensively used to treat patients with neovascular diseases and cancer (20, 21). It is therefore essential to have a better understanding of the function of VEGF-B and the underlying mechanisms to be better able to gauge its clinical implications.
Retinitis pigmentosa (RP) is a heterogeneous retinal dystrophy characterized by the progressive loss of photoreceptors followed by retinal degeneration (22). RP is the leading cause of blindness in inherited retinal degenerative diseases. Retinal photoreceptors are metabolically highly active and therefore extremely susceptible to oxidative stress (22). A large number of genes and mutations have been implicated in RP. Therefore, correcting the defective genes/mutations represents an overwhelming challenge. Currently, available therapies for RP include vitamin supplements and protection from sunlight (22). However, such treatments can neither stop the progress of the disease nor restore vision. Therefore, new and better therapies are needed. Since VEGF-B has been shown to be a potent survival factor with minimal side effects, we hypothesize that VEGF-B may be useful in rescuing retinal degeneration in RP. However, no study has tested this hypothesis thus far.
Oxidative stress is a key factor in numerous human diseases and causes progressive damage to cells and tissues. Neuronal cells are particularly vulnerable to oxidative stress due to their very high oxygen consumption and relatively weak antioxidant defense system. Therefore, it is anticipated that antioxidants that can reduce oxidative stress may have therapeutic value against degenerative diseases. Glutathione peroxidase-1 (GPX-1) is a ubiquitous and key intracellular antioxidant that can enzymatically reduce hydrogen peroxide to prevent its harmful effects (23). By limiting hydrogen peroxide accumulation, GPX-1 can also regulate signal transduction, mitochondrial function, and thiol redox balance. Due to its potent antioxidative effects, GPX-1 has been shown to play important roles in numerous human diseases, such as tissue degeneration, cancer, and cardiovascular disorders (23).
Here, we report our finding that VEGF-B is a critical regulator of the antioxidant pathway and acts by up-regulating many key antioxidative genes, particularly, GPX1. Indeed, loss of VEGF-B led to retinal degeneration, and VEGF-B treatment rescued retinal cells from death in a retinitis pigmentation model. We further show that the antioxidant function of VEGF-B is mainly mediated by VEGFR1, since a neutralizing antibody (nAb) against Vegfr-1 largely abolished the effect of VEGF-B. Since oxidative stress is a key factor in numerous human diseases, VEGF-B with its potent antioxidative function may have therapeutic value in the treatment of such diseases.
Results
Genetic Deletion of Vegf-B Leads to Retinal Degeneration.
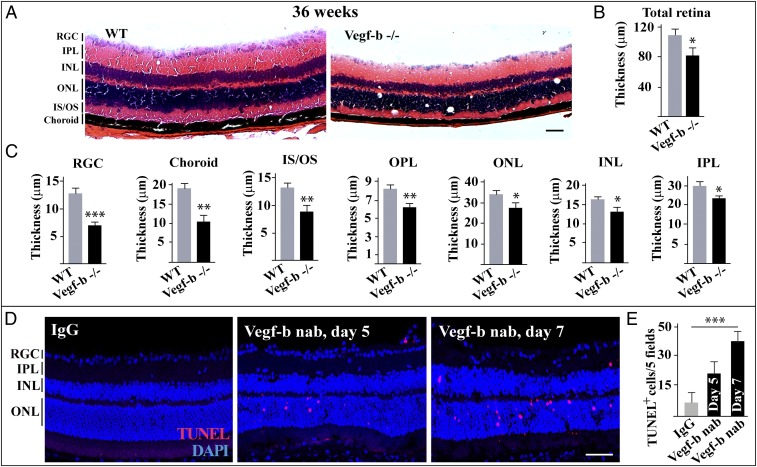
VEGF-B is highly expressed in the retina (7, 24). However, it remains unknown whether VEGF-B plays a role in the retinas of aging mice. To explore this, we utilized Vegf-b–deficient mice and investigated the morphology of the retinas. We found that, at 36 wk of age, the thickness of the retinas of Vegf-b−/− mice was significantly reduced (Fig. 1 A and B). Thinning was observed in nearly all retinal layers, including the retinal ganglion cell (RGC) layer, choroid, inner segment/outer segment (IS/OS), outer plexiform layer (OPL), outer nuclear layer (ONL), inner nuclear layer (INL), and inner plexiform layer (IPL) (Fig. 1C). In addition, the thinning of the retinas was also found in 16-wk-old Vegf-b–deficient mice, albeit to a lesser degree (SI Appendix, Fig. S1 A–C). These data suggest that Vegf-b deficiency leads to retinal degeneration in mice.
Fig. 1.
Genetic deletion of Vegf-b leads to retinal degeneration. (A–C) H&E staining shows that the thickness of the retinal layers of 36-wk-old Vegf-b−/− mice was significantly reduced, including the retinal ganglion cell layer (RGC), choroid, inner segment/outer segment (IS/OS), outer plexiform layer (OPL), outer nuclear layer (ONL), inner nuclear layer (INL), and inner plexiform layer (IPL) (n = 8; ***P < 0.001, **P < 0.01, *P < 0.05). (D and E) TUNEL staining shows that intravitreal injection of Vegf-b nAb into the vitreous of normal mice led to retinal apoptosis 1 wk after injection (n = 8; ***P < 0.001). (Scale bar: 50 µm.)
Blocking Vegf-B by nAb Leads to Apoptosis of Retinal Cells.
To verify the above findings, we utilized yet another loss-of-function approach using Vegf-b nAb. We found by TUNEL staining that intravitreal injection of Vegf-b nAb caused retinal apoptosis in normal mice at different time points (Fig. 1 D and E), demonstrating that VEGF-B is essential for retinal cell survival. Thus, loss of VEGF-B activity can result in loss of retinal cells.
VEGF-B Treatment Rescues Retinal Degeneration.
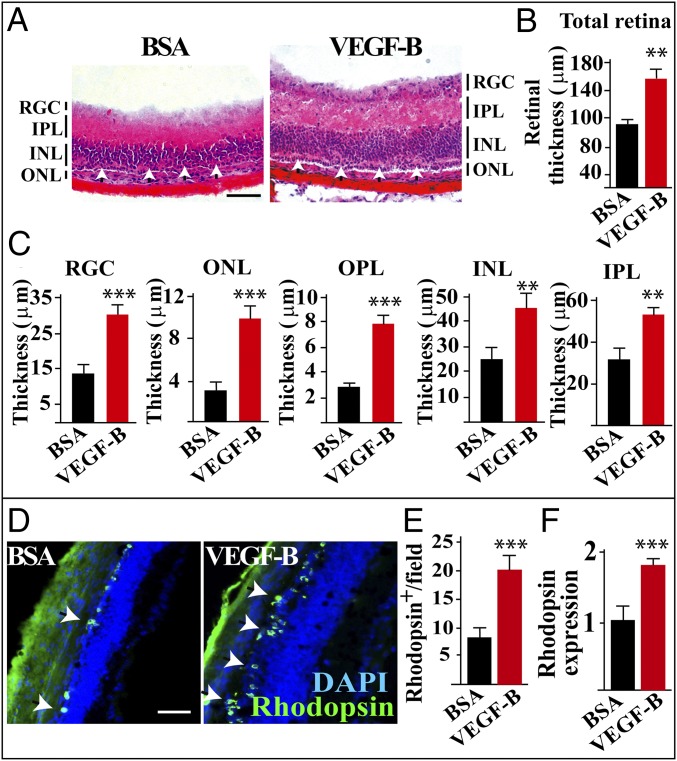
We next investigated whether VEGF-B treatment could rescue retinal degeneration. For this purpose, we used rd1 mice, in which retinal degeneration occurs at about postnatal day 12 (P12) and completes at P26 (25, 26). We found that BSA-treated retinas appeared to be severely degenerated and were very thin with almost complete loss of the ONL (Fig. 2A, arrows in the Left). However, in the VEGF-B–treated mice, the retinas were protected from degeneration, and these mice had significantly thicker retinal layers (Fig. 2 A–C), including the RGC layer, ONL, OPL, INL, and IPL. Consistently, immunofluorescence staining revealed more rhodopsin+ rods (Fig. 2 D and E) and peanut agglutinin (PNA) staining revealed more PNA+ cones (SI Appendix, Fig. S2 A and B) in the VEGF-B–treated retinas. Real-time PCR also confirmed the increased amount of rhodopsin transcripts in the VEGF-B–treated retinas (Fig. 2F). Furthermore, we found that, while VEGF-B treatment increased retinal thickness in rd1 mice, PlGF, another VEGF member, did not show such an effect (SI Appendix, Fig. S2 C and D), demonstrating that the effect of VEGF-B was specific. Thus, VEGF-B treatment is sufficient to rescue retinas from degeneration in rd1 mice.
Fig. 2.
VEGF-B treatment rescues retinal degeneration in rd1 mice. (A–C) H&E staining shows that, in rd1 mice, intravitreous injection of VEGF-B at P11 significantly increased the thickness of the retinal layers at P26 (n = 8; ***P < 0.001, **P < 0.01), including the inner nuclear layer (INL), inner plexiform layer (IPL), outer nuclear layer (ONL), outer plexiform layer (OPL), and retinal ganglion cell layer (RGCL). In contrast to the severely degenerated ONL of the BSA-treated retinas, the ONL of the VEGF-B–treated retinas was thicker with several rows of nuclei (A, arrowheads). (D–F) Immunofluorescence staining shows more rhodopsin+ rods in the VEGF-B–treated retinas (D and E) (n = 8; ***P < 0.001). The increased amount of rhodopsin transcripts was also confirmed by real-time PCR (F) (n = 8; ***P < 0.001). (Scale bar: 50 µm.)
VEGF-B Up- and Down-Regulates the Expression of Critical Antioxidative and Prooxidative Genes, Respectively.
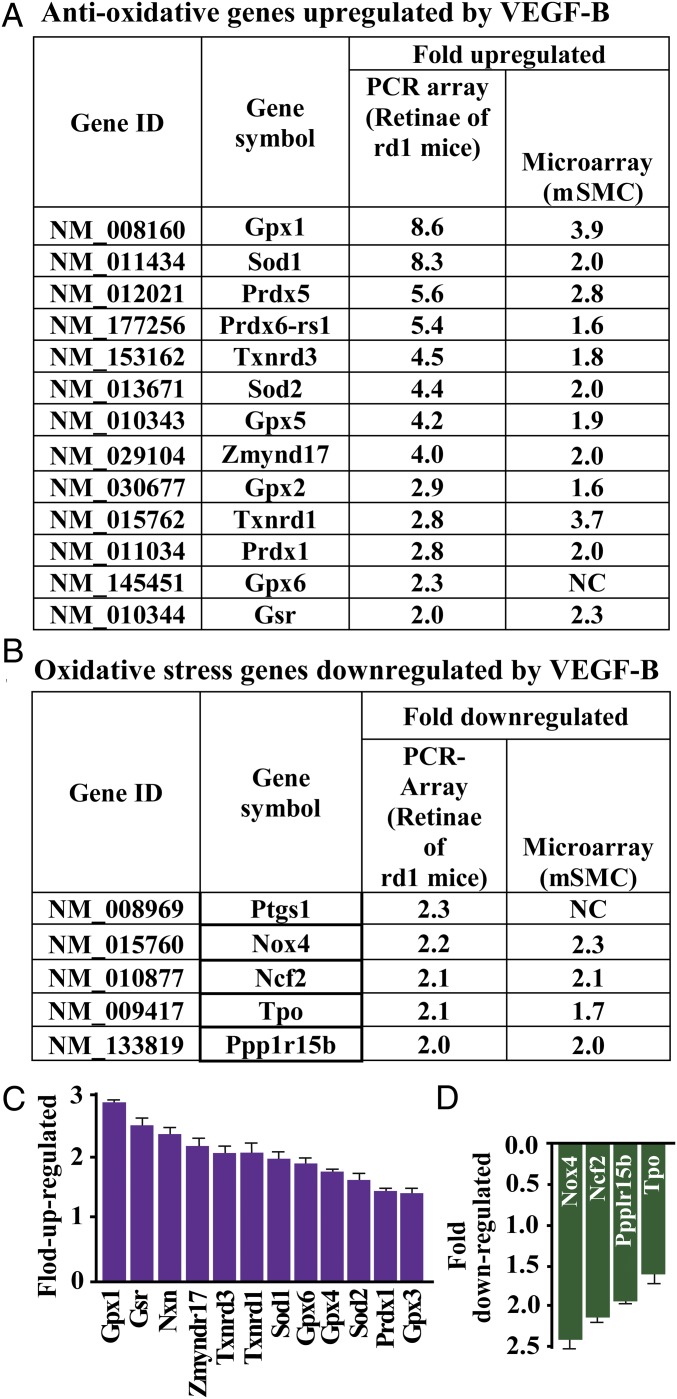
We subsequently explored the genes regulated by VEGF-B. Using high-throughput PCR array assays, we found that VEGF-B treatment markedly up-regulated numerous critical antioxidative genes in the retinas of rd1 mice, including Gpx1, Sod1, Prdx5, Prdx6-rs1, Txnrd3, Sod2, and Gpx5 (Fig. 3A). In addition, VEGF-B also down-regulated many oxidative stress genes, such as Ptgs1, Nox4, and Ncf2 (Fig. 3B). These findings were confirmed by microarray assay, which revealed that VEGF-B treatment consistently up- and down-regulated many antioxidative and oxidative genes, respectively, in primary mouse aortic artery smooth muscle cells (mSMCs) (Fig. 3 A and B). Importantly, the up- and down-regulation of these genes by VEGF-B was confirmed by real-time PCR in the VEGF-B–treated retinas of rd1 mice (Fig. 3 C and D). Thus, VEGF-B was found to function as a critical regulator of important antioxidative genes.
Fig. 3.
VEGF-B up- and down-regulates the expression of antioxidative and prooxidative genes. (A) Using a high-throughput PCR array assay, we found that VEGF-B treatment markedly up-regulated the expression of many critical antioxidative genes in the retinas, including Gpx1, Sod1, Prdx5, Prdx6-rs1, Txnrd3, Sod2, and Gpx5. This finding was also confirmed by a microarray assay using primary mSMCs. (B) A high-throughput PCR array assay shows that VEGF-B down-regulated the expression of many oxidative stress genes, such as Ptgs1, Nox4, and Ncf2. This finding was also confirmed by a microarray assay using mSMCs. (C and D) In VEGF-B–treated retinas of rd1 mice, up- and down-regulation of the antioxidative and prooxidative genes by VEGF-B, respectively, was confirmed by real-time PCR (n = 8).
Gpx1 Is Critical for the Regulatory Effect of VEGF-B on Antioxidative and Prooxidative Genes.
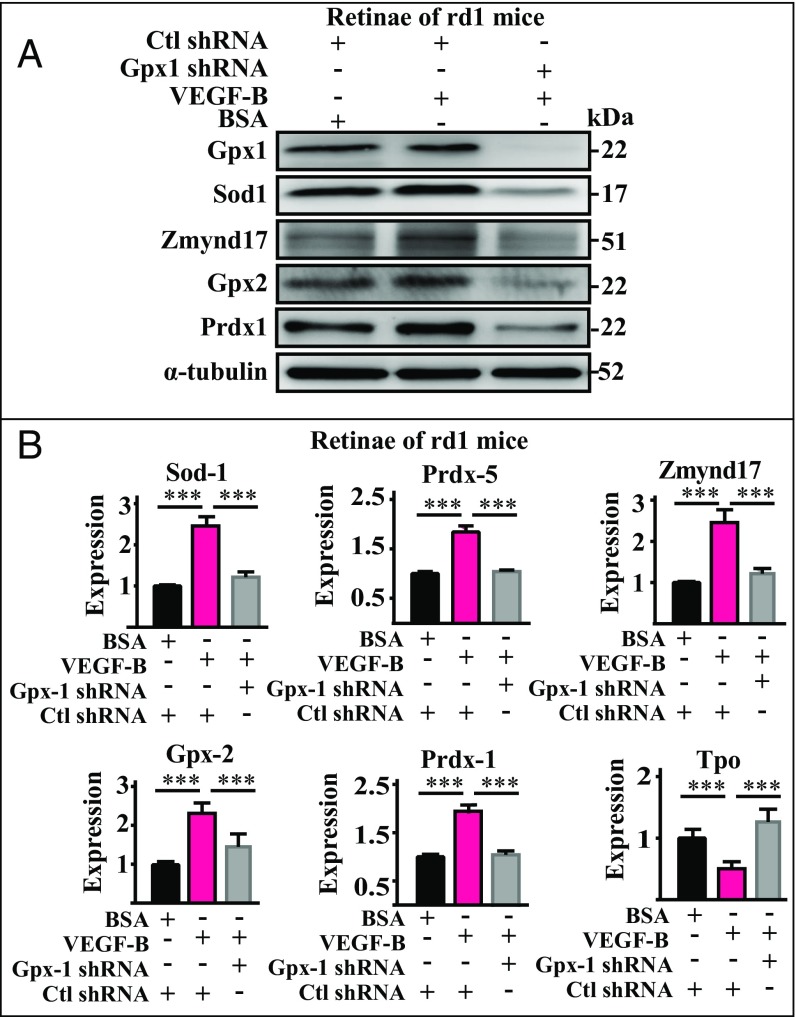
GPX1 is a key intracellular antioxidant enzyme and a gatekeeper in inhibiting reactive oxygen species (ROS) (23). It has been shown that, in rd1 mouse retinas, Gpx1 level decreases with increased oxidative stress (27). Since Gpx1 was most prominently up-regulated by VEGF-B (Fig. 3 A and C), we hypothesized that it might play an important role in mediating the rescue effect of VEGF-B. To test this, we knocked down Gpx1 in the eyes of rd1 mice by intravitreous injection of shRNA and investigated the effect of VEGF-B on the expression of the antioxidative and prooxidative genes. Successful knockdown of Gpx1 was confirmed by Western blot (Fig. 4A). We found that, while VEGF-B treatment increased the protein levels of Gpx1, Sod1, Zmynd17, Gpx2, and Prdx1 (Fig. 4A, Middle) in the retinas of rd1 mice, this effect was abolished by the knockdown of Gpx1 (Fig. 4B). This result was further confirmed at the mRNA level by real-time PCR, which revealed that Gpx1 knockdown largely reduced the VEGF-B–induced expression of Sod1, Prdx5, Zmynd17, Gpx2, and Prdx1 and VEGF-B’s inhibitory effect on Tpo expression (Fig. 4B). In addition, similar results were also obtained in the choroids of the rd1 mice (SI Appendix, Fig. S3). Together, these data indicate that Gpx1 is at least one of the critical effectors for VEGF-B–induced expression of antioxidative and prooxidative genes.
Fig. 4.
Gpx1 is required for the regulatory effect of VEGF-B on the expression of antioxidative and prooxidative genes. (A) Western blot confirms knockdown of Gpx1 by shRNA in the eyes of rd1 mice after intravitreous shRNA injection. While VEGF-B treatment increased the protein levels of Gpx1, Sod1, Zmynd17, Gpx2, and Prdx1 in the retinas of rd1 mice, Gpx1 knockdown completely abolished the effect of VEGF-B. (B) Real-time PCR also shows that Gpx1 knockdown diminished the up-regulatory effect of VEGF-B on the expression of Sod1, Prdx5, Zmynd17, Gpx2, and Prdx1, and the inhibitory effect on TOP expression (n = 8; ***P < 0.001).
Gpx1 Mediates the Antioxidative Function of VEGF-B.
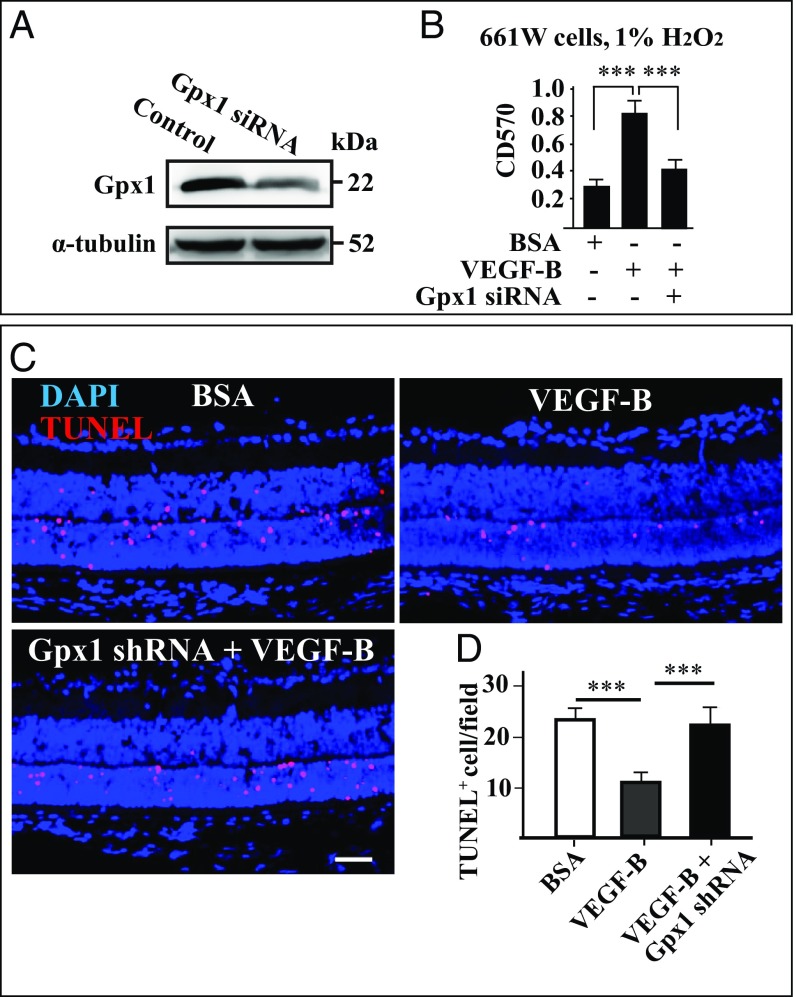
We next tested at a functional level whether Gpx1 is required for the antioxidative effect of VEGF-B both in vitro and in vivo. We knocked down Gpx1 using siRNA in 661W cells, a mouse cone photoreceptor cell line, and the result was confirmed by Western blot (Fig. 5A). An MTT assay revealed that while VEGF-B treatment markedly increased the survival of the 661W cells under H2O2-induced oxidative stress, the effect of VEGF-B was significantly reduced by Gpx1 knockdown (Fig. 5B). Moreover, in rd1 retinas in vivo, TUNEL staining revealed that loss of Gpx1 by shRNA treatment largely abolished the protective effect of VEGF-B on cellular apoptosis in the retinas (Fig. 5 C and D). Consistently, we found that, in rd1 mice, Gpx1 expression was decreased after retinal degeneration together with some other antioxidative genes (SI Appendix, Fig. S4A). Together, these data show that Gpx1 is critical for the antioxidative function of VEGF-B.
Fig. 5.
Gpx1 mediates the antioxidative function of VEGF-B. (A) Western blot shows that Gpx1 was knocked down after siRNA treatment in 661W cells, a mouse cone photoreceptor cell line. (B) MTT assay shows that Gpx1 knockdown abolished the protective effect of VEGF-B on 661W cells under H2O2-induced oxidative stress (n = 6; ***P < 0.001). (C and D) TUNEL staining shows that, in rd1 retinas, while intravitreal injection of VEGF-B decreased cellular apoptosis, loss of Gpx1 by shRNA treatment abolished the effect of VEGF-B (n = 8; ***P < 0.001). (Scale bar: 50 µm.)
The Antioxidative Effect of VEGF-B Is Exerted Mainly via VEGFR1.
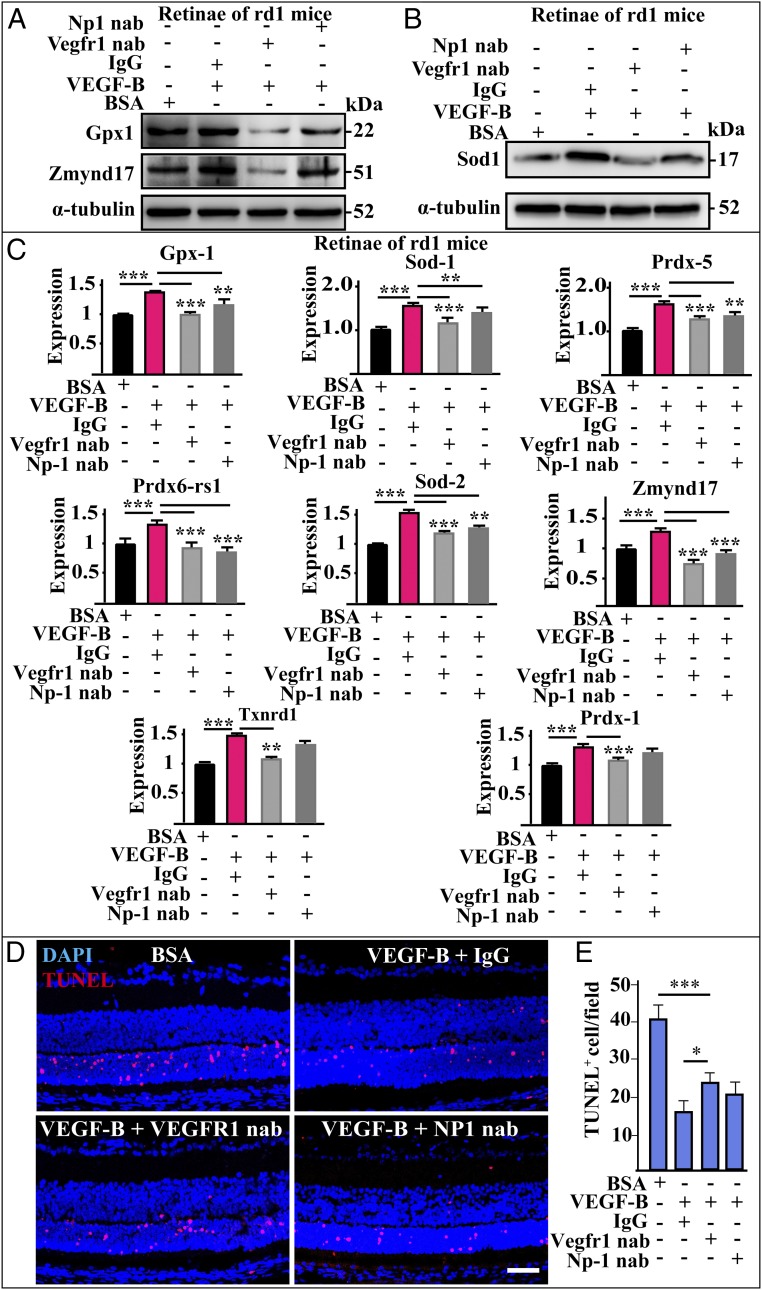
VEGF-B is known to bind VEGFR1 (2) and NP1 (3). We therefore investigated whether these receptors play a role in the antioxidative effect of VEGF-B by utilizing nAbs against them. Western blot revealed that, in the retinas of rd1 mice, coinjection of Vegfr-1 nAb completely abolished the VEGF-B–induced up-regulation of Gpx1, Zmynd17, and Sod1 (Fig. 6 A and B), whereas Np1 nAb displayed little effect (Fig. 6A), suggesting that Vegfr-1 is the major receptor mediating the antioxidative effect of VEGF-B. Indeed, this notion was further supported by real-time PCR, which revealed that coadministration of Vegfr-1 nAb completely abolished the effect of VEGF-B, while Np1 nAb only, in some cases, partially diminished the effect of VEGF-B (Fig. 6C). In the choroids, both Vegfr-1 and Np1 nAbs could abolish the effect of VEGF-B (SI Appendix, Fig. S5). Importantly, in vivo experiments and TUNEL staining also revealed that coinjection of Vegfr-1 nAb decreased the protective effect of VEGF-B against retinal degeneration in the eyes of rd1 mice, while Np1 nAb showed no significant effect (Fig. 6 D and E).
Fig. 6.
The antioxidative effect of VEGF-B is mainly fulfilled via VEGFR1. (A and B) Western blot shows that, in the retinas of rd1 mice, coinjection of Vegfr-1 nAb completely abolished the up-regulatory effect of VEGF-B on the expressions of Gpx1, Zmynd17, and Sod1 (n = 6), whereas Np1 nAb displayer a weaker effect. (C) Real-time PCR results show that coadministration of Vegfr-1 nAb completely abolished the up-regulatory effect of VEGF-B on the expression of many antioxidative genes, while Np1 nAb only partially diminished the effect of VEGF-B. ***P < 0.001, **P < 0.01. (D and E) TUNEL staining shows that, in vivo, in the eyes of rd1 mice with retinal degeneration, coinjection of Vegfr-1 nAb to a certain extent diminished the protective effect of VEGF-B, while Np1 nAb showed no significant effect (n = 8; ***P < 0.001, *P < 0.05). (Scale bar: 50 µm.)
Discussion
In this study, we have identified a function of VEGF-B as a potent regulator of the antioxidant pathway. We found that VEGF-B exerts this function by up-regulating Gpx1 and other antioxidative genes. Indeed, loss of Vegf-b function by gene deletion led to retinal degeneration in mice, and VEGF-B treatment rescued retinal degeneration in a RP disease model. We further reveal that Gpx1 and Vegfr-1 are critical in mediating the antioxidative function of VEGF-B, since loss of Gpx1 or Vegfr-1 largely diminished the effect of VEGF-B in vitro and in vivo. Given that oxidative stress is critically involved in numerous human diseases, VEGF-B may have therapeutic value in treating such diseases by enhancing the defense mechanism against oxidation.
GPX1 is a gatekeeper in counteracting ROS and a major intracellular antioxidant enzyme. It is also the most abundant member of the glutathione peroxidase family. GPX1 catalyzes the reduction of organic hydroperoxides and hydrogen peroxide to protect cells and tissues from oxidative damage. GPX1 expression is up-regulated under pathological conditions, such as in hypoxic retinas (28) and in retinal pigment epithelial cells under oxidative stress (29). It has been shown that, in rd1 mouse retinas, Gpx1 level is decreased after retinal degeneration with increased oxidative stress (27). Loss of Gpx1 exacerbates retinal neovascularization in mice (30). In this study, we found that VEGF-B treatment in rd1 mice significantly up-regulated many antioxidant defense-related genes, with Gpx1 being most prominent. Importantly, loss of Gpx1 by shRNA knockdown largely diminished the protective effect of VEGF-B both in vitro and in vivo. Our data thus show that Gpx1 is at least one of many molecules that are critical for the antioxidative function VEGF-B.
The retina has the highest metabolic rate among different human tissues. Particularly, the retinal photoreceptors have extremely high oxygen consumption. In RP, the gradual death of the rod photoreceptors decreases oxygen consumption of the retina markedly and results in a higher retinal oxygen level. Consequently, this causes oxidative damage to the retina. Indeed, studies have shown that oxidative stress in the degenerating retinas is considerably higher than that of normal retinas. Apart from retinal degeneration, oxidative damage is also a key pathology of many other diseases, such as age-related macular degeneration, glaucoma, diabetic retinopathy (31), retinopathy of prematurity (30), dry eye syndrome (32), keratitis (33), and retinopathy after radiotherapy (34). Different antioxidants have been used in the clinic to treat patients with degenerative diseases (35). However, such treatment cannot stop the progression of the diseases into advanced stages. Therefore, new and better therapies are urgently needed. Since VEGF-B displays a strong antioxidative effect, it may be a promising drug candidate for the treatment of diseases involving oxidative damage. Apart from the antioxidative effect of VEGF-B, we have previously also shown that VEGF-B is a potent inhibitor of apoptosis by suppressing the expression of the BH3 protein family genes (7). Thus, VEGF-B could exert multiple beneficial effects through different mechanisms for the treatment of degenerative diseases. Noteworthy, the advantage of VEGF-B as a therapeutic molecule is further highlighted by its unique property of being inert under normal conditions with no obvious effect (4, 7, 9, 15, 16).
In summary, in this study, we show that VEGF-B is a critical endogenous antioxidant that induces the expression of numerous key antioxidative genes to mount an antioxidant defense mechanism. Given its unique safety profile and minimal side effects, it is envisioned that modulating VEGF-B activity may be highly useful in the treatment of human diseases involving oxidative stress.
Materials and Methods
All animal experiments were performed according to the Association for Research in Vision and Ophthalmology Statement for the Use of Animals and were approved by the Animal Care and Use Committee at the Zhongshan Ophthalmic Center, Sun Yat-sen University. Littermates from mice on C57BL/6 background for more than six generations were used for the experiments. The rd1/rd1 (FVB/NJ) mice were used as a model for RP to analyze retinal degeneration. The high-throughput mouse RT2profiler PCR array (SuperArray) was used to investigate the expression of 84 antioxidative and oxidative genes according to the manufacturer’s protocol with five housekeeping genes as controls. More details of materials and methods are provided in SI Appendix, Materials and Methods.
Supplementary Material
Acknowledgments
This work was supported by the State Key Laboratory of Ophthalmology at the Zhongshan Ophthalmic Center, Sun Yat-sen University, Guangzhou, China; a Key Program of the National Natural Science Foundation of China (NSFC) (81330021) (to X. Li); NSFC Grant 81670855 (to X. Li); NSFC–Swedish Research Foundation International Collaboration Grant 81611130082 (to X. Li); a Guangdong Province Leading Expert Program grant (to X. Li); and NSFC Grants 81525006 and 81730025 (to C.Z.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1801379115/-/DCSupplemental.
References
- 1.Olofsson B, et al. Vascular endothelial growth factor B, a novel growth factor for endothelial cells. Proc Natl Acad Sci USA. 1996;93:2576–2581. doi: 10.1073/pnas.93.6.2576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Olofsson B, et al. Vascular endothelial growth factor B (VEGF-B) binds to VEGF receptor-1 and regulates plasminogen activator activity in endothelial cells. Proc Natl Acad Sci USA. 1998;95:11709–11714. doi: 10.1073/pnas.95.20.11709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Makinen T, et al. Differential binding of vascular endothelial growth factor B splice and proteolytic isoforms to neuropilin-1. J Biol Chem. 1999;274:21217–21222. doi: 10.1074/jbc.274.30.21217. [DOI] [PubMed] [Google Scholar]
- 4.Li X, Eriksson U. Novel VEGF family members: VEGF-B, VEGF-C and VEGF-D. Int J Biochem Cell Biol. 2001;33:421–426. doi: 10.1016/s1357-2725(01)00027-9. [DOI] [PubMed] [Google Scholar]
- 5.Li X, Aase K, Li H, von Euler G, Eriksson U. Isoform-specific expression of VEGF-B in normal tissues and tumors. Growth Factors. 2001;19:49–59. doi: 10.3109/08977190109001075. [DOI] [PubMed] [Google Scholar]
- 6.Sun Y, et al. Increased severity of cerebral ischemic injury in vascular endothelial growth factor-B-deficient mice. J Cereb Blood Flow Metab. 2004;24:1146–1152. doi: 10.1097/01.WCB.0000134477.38980.38. [DOI] [PubMed] [Google Scholar]
- 7.Li Y, et al. VEGF-B inhibits apoptosis via VEGFR-1-mediated suppression of the expression of BH3-only protein genes in mice and rats. J Clin Invest. 2008;118:913–923. doi: 10.1172/JCI33673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Poesen K, et al. Novel role for vascular endothelial growth factor (VEGF) receptor-1 and its ligand VEGF-B in motor neuron degeneration. J Neurosci. 2008;28:10451–10459. doi: 10.1523/JNEUROSCI.1092-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li X, et al. Reevaluation of the role of VEGF-B suggests a restricted role in the revascularization of the ischemic myocardium. Arterioscler Thromb Vasc Biol. 2008;28:1614–1620. doi: 10.1161/ATVBAHA.107.158725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brkovic A, Sirois MG. Vascular permeability induced by VEGF family members in vivo: Role of endogenous PAF and NO synthesis. J Cell Biochem. 2007;100:727–737. doi: 10.1002/jcb.21124. [DOI] [PubMed] [Google Scholar]
- 11.Karpanen T, et al. Overexpression of vascular endothelial growth factor-B in mouse heart alters cardiac lipid metabolism and induces myocardial hypertrophy. Circ Res. 2008;103:1018–1026. doi: 10.1161/CIRCRESAHA.108.178459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mould AW, et al. Transgenic overexpression of vascular endothelial growth factor-B isoforms by endothelial cells potentiates postnatal vessel growth in vivo and in vitro. Circ Res. 2005;97:e60–e70. doi: 10.1161/01.RES.0000182631.33638.77. [DOI] [PubMed] [Google Scholar]
- 13.Yue X, et al. Comparative study of the neurotrophic effects elicited by VEGF-B and GDNF in preclinical in vivo models of Parkinson’s disease. Neuroscience. 2014;258:385–400. doi: 10.1016/j.neuroscience.2013.11.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guaiquil VH, et al. VEGF-B selectively regenerates injured peripheral neurons and restores sensory and trophic functions. Proc Natl Acad Sci USA. 2014;111:17272–17277. doi: 10.1073/pnas.1407227111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bellomo D, et al. Mice lacking the vascular endothelial growth factor-B gene (Vegfb) have smaller hearts, dysfunctional coronary vasculature, and impaired recovery from cardiac ischemia. Circ Res. 2000;86:E29–E35. doi: 10.1161/01.res.86.2.e29. [DOI] [PubMed] [Google Scholar]
- 16.Aase K, et al. Vascular endothelial growth factor-B-deficient mice display an atrial conduction defect. Circulation. 2001;104:358–364. doi: 10.1161/01.cir.104.3.358. [DOI] [PubMed] [Google Scholar]
- 17.Hagberg CE, et al. Targeting VEGF-B as a novel treatment for insulin resistance and type 2 diabetes. Nature. 2012;490:426–430. doi: 10.1038/nature11464. [DOI] [PubMed] [Google Scholar]
- 18.Dijkstra MH, et al. Lack of cardiac and high-fat diet induced metabolic phenotypes in two independent strains of Vegf-b knockout mice. Sci Rep. 2014;4:6238. doi: 10.1038/srep06238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Robciuc MR, et al. VEGFB/VEGFR1-induced expansion of adipose vasculature counteracts obesity and related metabolic complications. Cell Metab. 2016;23:712–724. doi: 10.1016/j.cmet.2016.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ricci V, Ronzoni M, Fabozzi T. Aflibercept a new target therapy in cancer treatment: A review. Crit Rev Oncol Hematol. 2015;96:569–576. doi: 10.1016/j.critrevonc.2015.07.001. [DOI] [PubMed] [Google Scholar]
- 21.Parvin P, Zola M, Dirani A, Ambresin A, Mantel I. Two-year outcome of an observe-and-plan regimen for neovascular age-related macular degeneration treated with Aflibercept. Graefes Arch Clin Exp Ophthalmol. 2017;255:2127–2134. doi: 10.1007/s00417-017-3762-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hartong DT, Berson EL, Dryja TP. Retinitis pigmentosa. Lancet. 2006;368:1795–1809. doi: 10.1016/S0140-6736(06)69740-7. [DOI] [PubMed] [Google Scholar]
- 23.Lubos E, Loscalzo J, Handy DE. Glutathione peroxidase-1 in health and disease: From molecular mechanisms to therapeutic opportunities. Antioxid Redox Signal. 2011;15:1957–1997. doi: 10.1089/ars.2010.3586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang F, et al. VEGF-B is dispensable for blood vessel growth but critical for their survival, and VEGF-B targeting inhibits pathological angiogenesis. Proc Natl Acad Sci USA. 2009;106:6152–6157. doi: 10.1073/pnas.0813061106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Farber DB. From mice to men: The cyclic GMP phosphodiesterase gene in vision and disease. The Proctor lecture. Invest Ophthalmol Vis Sci. 1995;36:263–275. [PubMed] [Google Scholar]
- 26.Lahdenranta J, et al. An anti-angiogenic state in mice and humans with retinal photoreceptor cell degeneration. Proc Natl Acad Sci USA. 2001;98:10368–10373. doi: 10.1073/pnas.181329198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ahuja-Jensen P, et al. Low glutathione peroxidase in rd1 mouse retina increases oxidative stress and proteases. Neuroreport. 2007;18:797–801. doi: 10.1097/WNR.0b013e3280c1e344. [DOI] [PubMed] [Google Scholar]
- 28.Schultz R, Witte OW, Schmeer C. Increased frataxin levels protect retinal ganglion cells after acute ischemia/reperfusion in the mouse retina in vivo. Invest Ophthalmol Vis Sci. 2016;57:4115–4124. doi: 10.1167/iovs.16-19260. [DOI] [PubMed] [Google Scholar]
- 29.Tokarz P, Kaarniranta K, Blasiak J. Inhibition of DNA methyltransferase or histone deacetylase protects retinal pigment epithelial cells from DNA damage induced by oxidative stress by the stimulation of antioxidant enzymes. Eur J Pharmacol. 2016;776:167–175. doi: 10.1016/j.ejphar.2016.02.049. [DOI] [PubMed] [Google Scholar]
- 30.Tan SM, Stefanovic N, Tan G, Wilkinson-Berka JL, de Haan JB. Lack of the antioxidant glutathione peroxidase-1 (GPx1) exacerbates retinopathy of prematurity in mice. Invest Ophthalmol Vis Sci. 2013;54:555–562. doi: 10.1167/iovs.12-10685. [DOI] [PubMed] [Google Scholar]
- 31.Dehdashtian E, et al. Diabetic retinopathy pathogenesis and the ameliorating effects of melatonin; involvement of autophagy, inflammation and oxidative stress. Life Sci. 2018;193:20–33. doi: 10.1016/j.lfs.2017.12.001. [DOI] [PubMed] [Google Scholar]
- 32.Zernii EY, et al. Mitochondria-targeted antioxidant SkQ1 prevents anesthesia-induced dry eye syndrome. Oxid Med Cell Longev. 2017;2017:9281519. doi: 10.1155/2017/9281519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ruban VV, Archana PT, Sundararajan M, Geraldine P, Thomas PA. Inflammation and oxidative stress in corneal tissue in experimental keratitis due to Fusarium solani: Amelioration following topical therapy with voriconazole and epigallocatechin gallate. Mycoses. 2018;61:159–171. doi: 10.1111/myc.12718. [DOI] [PubMed] [Google Scholar]
- 34.Özer MA, et al. Effects of molsidomine on retinopathy and oxidative stress induced by radiotheraphy in rat eyes. Curr Eye Res. 2017;42:803–809. doi: 10.1080/02713683.2016.1238943. [DOI] [PubMed] [Google Scholar]
- 35.Evans JR, Lawrenson JG. Antioxidant vitamin and mineral supplements for preventing age-related macular degeneration. Cochrane Database Syst Rev. 2017;7:CD000253. doi: 10.1002/14651858.CD000253.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.