Significance
Perceptual learning is a form of memory involving improvement in the ability to detect or discriminate between sensory stimuli. Encoding learned information requires plasticity of cortical circuits. Physiological studies have suggested that even the earliest stages in sensory cortical processing show functional changes associated with perceptual improvements. Previously, we have shown experience-dependent change in cortical circuits associated with retinal lesions that can account for functional adaptation to CNS lesions. Here, we have studied whether these changes are also associated with normal visual experience, specifically with perceptual learning. We found that during the course of perceptual learning axon collaterals in primary visual cortex (V1) undergo sprouting and pruning, suggesting how V1 is engaged in encoding learned information.
Keywords: cortical plasticity, adult plasticity, horizontal connections, axon sprouting, axon pruning
Abstract
Perceptual learning is associated with changes in the functional properties of neurons even in primary sensory areas. In macaque monkeys trained to perform a contour detection task, we have observed changes in contour-related facilitation of neuronal responses in primary visual cortex that track their improvement in performance on a contour detection task. We have previously explored the anatomical substrate of experience-dependent changes in the visual cortex based on a retinal lesion model, where we find sprouting and pruning of the axon collaterals in the cortical lesion projection zone. Here, we attempted to determine whether similar changes occur under normal visual experience, such as that associated with perceptual learning. We labeled the long-range horizontal connections in visual cortex by virally mediated transfer of genes expressing fluorescent probes, which enabled us to do longitudinal two-photon imaging of axonal arbors over the period during which animals improve in contour detection performance. We found that there are substantial changes in the axonal arbors of neurons in cortical regions representing the trained part of the visual field, with sprouting of new axon collaterals and pruning of preexisting axon collaterals. Our findings indicate that changes in the structure of axonal arbors are part of the circuit-level mechanism of perceptual learning, and further support the idea that the learned information is encoded at least in part in primary visual cortex.
Experience-dependent plasticity is a ubiquitous property of the cerebral cortex. In the visual system, it extends from the primary visual cortex (V1) to higher-order cortical areas. Various forms of experiential manipulation, including retinal lesions and perceptual learning, have been shown to alter the functional properties of visual cortical neurons, which can involve shifting receptive field (RF) position and altered tuning to trained stimulus attributes. Perceptual learning has been demonstrated in a contour detection task, where a contour composed of a series of collinear line segments is embedded in a background of randomly oriented and positioned line segments. With practice, subjects can detect contours containing fewer lines. Here, we use this experimental model of perceptual learning combined with two-photon imaging in monkeys to determine the nature of the circuit alterations occurring during the course of learning.
Although some cortical connections are fixed after a critical period in early postnatal life, other connections are mutable throughout life. In particular, the axon collaterals of long-range horizontal connections formed by cortical pyramidal cells have been shown to undergo sprouting and pruning following retinal lesions in primates. In the absence of manipulation of experience the axonal arbors of superficial layer pyramidal cells are stable—there is no addition or pruning of axon collaterals, although there is a high rate of turnover of axonal boutons (1). Immediately following retinal lesions, however, one sees a parallel process of exuberant axonal sprouting and pruning, with a net enrichment of the axonal arbors in the lesion projection zone (LPZ) within V1 (2, 3). This process begins immediately after making the lesion and extends for months thereafter. The resultant increase in density of the horizontal connections projecting into the LPZ can account for the remapping of visual topography and shifting RFs. A very similar process is associated with remapping of the somatosensory barrel cortex in the mouse following whisker plucking (4, 5).
The learning-associated changes in neuronal tuning observed in V1 and the capability of the horizontal connections to change with experience begs the question as to whether the kinds of axonal changes seen following retinal lesions can also explain the functional alterations occurring during perceptual learning. Although Hebbian plasticity posits that experience-dependent learning involves changes in the strength of existing synapses, the large-scale morphological changes in axon collateral arbors in V1 of the primate seen following retinal lesions or in mouse barrel cortex whisker plucking raises the possibility that experience leads to a more substantial rewiring of cortical circuits.
In this study, we use a model of perceptual learning involving a contour detection task, which entails determining the presence of a contour composed of a series of collinear line segments embedded in a complex background of randomly oriented and positioned line segments. A contour composed of many line segments is perceptually salient, popping out of the random background. With practice, one can detect contours made of fewer line elements, or line elements spaced farther apart (6–8). The shifting threshold, measured as the number of line segments required for detection of the embedded contour, provides a quantitative measure of the degree of perceptual learning the animal experiences. Because of the topographical extent and columnar specificity of the long-range horizontal connections in cortex, we have focused our analysis on the potential role of these connections in perceptual learning of contour detection. We can compare the axonal changes occurring during the learning period by combining viral-based labeling of the horizontal connections with longitudinal in vivo two-photon imaging of the axons within the cortical region representing the training stimulus. In this study, we find substantial axonal changes, including collateral sprouting and pruning, in V1 of primates during the course of perceptual learning.
Results
To maximize the amount of perceptual learning occurring during the period available for imaging, we take advantage of the specificity of perceptual learning for visuotopic location. This affords us with the time required for operational learning—getting the animal to understand the task and to respond appropriately to the stimulus configuration—without inducing perceptual learning changes at the location to be later imaged. We therefore train the animal on the task in the upper visual hemifield, then shift the task to the lower visual field, the area represented by the cortical region under the craniotomy, after we do baseline imaging of the horizontal connections in V1. The connections are labeled by injecting the region to be imaged, over several sites, with an adeno-associated virus (AAV) vector containing the gene encoding the eGFP or RFP proteins (the injections are done several months before the start of imaging to allow sufficient time for gene expression). The animal is presented with two patches of randomly oriented and positioned lines, one patch containing a contour with a variable number of collinear line segments (Fig. 1). The patches are then extinguished and the animal is shown two saccade targets, where a correct response is a saccade toward the spot in the location of the previously presented patch containing the embedded contour.
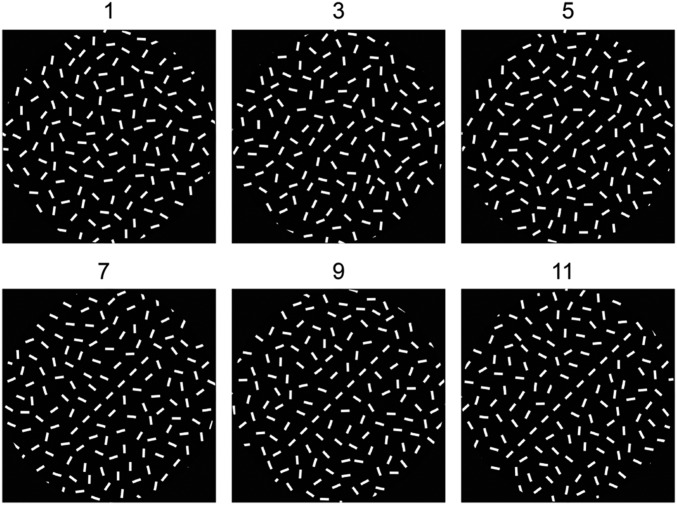
Fig. 1.
Stimuli used for contour detection. Increasing the number of line segments from 1 to 11 increases the perceptual saliency of the embedded contour. With training, shorter contours, made of fewer line segments, can be detected.
Here, we present psychophysical and imaging data from two animals. Both experienced a change in the behavioral threshold over the training period, being able to detect embedded contours composed of progressively fewer line segments or with increasing background brightness. One animal, M, had been extensively trained in the first visual field position and could detect embedded contours comprised of three-line segments at ∼75% correct performance. After obtaining a baseline image of the horizontal axons in V1, the stimulus was shifted to the position represented by the imaged area of cortex, where initially there was a sharp decrement in performance compared with the initial position, requiring a nine-line segment contour to be salient. Then, over the next several weeks, the psychometric curve shifted up and to the left, with performance approximating that seen at the initially trained position (Fig. 2). The second animal, C, showed improvement in detecting the contour at progressively higher background luminance, over the period of imaging (see Fig. 5).
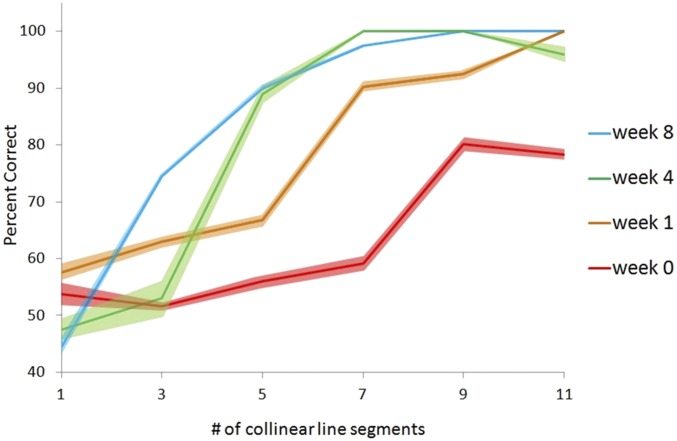
Fig. 2.
Performance of monkey M on the contour detection task, showing a marked improvement during the training period, where M was able to detect embedded contours composed of fewer line segments with practice. At the outset of training, M could detect contours made of nine collinear line segments, then seven after a week of training, five by 4 wk, and three by the end of the training period. Baseline imaging (Figs. 3 and 4) was done before the week 0 psychometric curve, and the second and third reconstructions were taken from imaging sessions between week 4 and week 8. Dark colors indicate mean performance, and flanking lighter colors indicate SE.
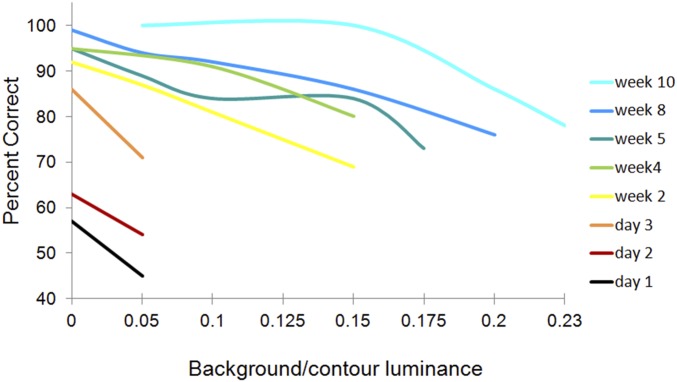
Fig. 5.
Performance of monkey C on contour detection task. Here, luminance of the background elements was changed relative to the contour. At the outset of training, the animal could not perform the task at low background levels but showed progressive improvement with increase of background luminance over the subsequent weeks.
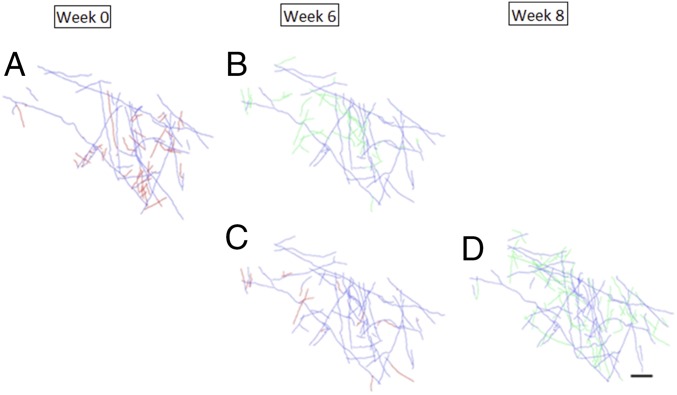
During the period of training, we imaged the horizontally projecting axonal arbors in the superficial layers of V1, at the cortical representation of visuotopic locations corresponding to the position of the trained stimulus. We reconstructed the axons labeled in the opercular (surface) cortex of area V1 of monkey M at three time points before and during the training. The axons were labeled several months before the start of imaging, by injection of AAV.eGFP or AAV.RFP, to allow sufficient time for expression of the fluorescent label. Injections were made at several sites to optimize our chances of finding an area providing clear imaging over as many time points as possible. Here, by selecting sites for imaging relatively far from the injection sites (∼1 mm), we were able to focus on the horizontally projecting axon collaterals of pyramidal cells. An example of an imaged axon cluster separated by several weeks, during which monkey M underwent substantial perceptual learning, is shown in Fig. 3. A number of axon collaterals were added over this period. A larger region was reconstructed by tracing the axon collaterals over a tiled series of z stacks, extending over a region of 2.6 mm2 for monkey M and 0.7 mm2 for monkey C at cortical depths ranging from 0 to 300 µm. The reconstructions at the compared time points were made over the same depth ranges. In the upper part of Fig. 4, we compare, for monkey M, the pattern of axonal arbors at the pretraining baseline image (Fig. 4A) and after 6 wk of training (Fig. 4B). The pretraining imaging time point was collected in the week before the week 0 psychometric curve, and the second imaging time point occurred between the week 4 and week 8 psychometric curves. Axon collaterals that were pruned are shown in red, newly sprouted axons are shown in green, and axons that were stable between the two time points are shown in blue. Here, substantial changes were seen in the imaged region over the training period. Continued training was associated with further pruning and axonal outgrowth, as seen in the pair of images in the bottom part of the Fig. 4 C and D, with the third reconstruction (Fig. 4D) made from imaging done around the week 8 psychometric curve.
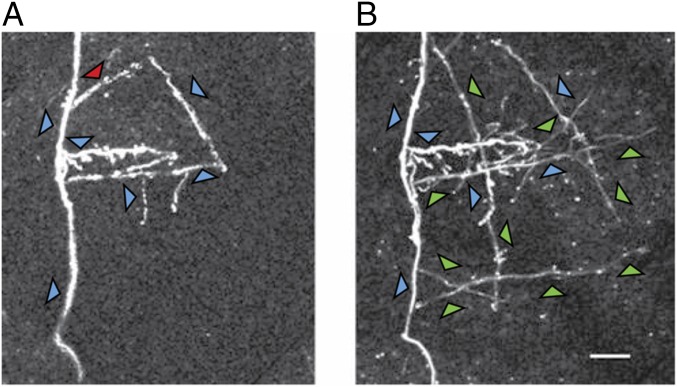
Fig. 3.
Two-photon image of a selected region of cortex taken at the beginning of training (A) and the same region imaged 8 wk later during the course of training (B). Between the two time points, several axon collaterals have sprouted. Axons that were stable over the time period are indicated by blue arrows, a pruned axon collateral is indicated by the red arrow, and newly sprouted axons are indicated by the green arrows.
Fig. 4.
Reconstruction of axons of monkey M imaged at three time points before training (week 1) and over the course of perceptual learning. The top row compares axons imaged at week 0 (A) and week 6 (B), and the bottom row compares axons imaged at week 6 (C) and week 8 (D). Axons that were stable between these pairs of imaging sessions are shown in blue, pruned axons are shown in red, and newly sprouted axons are shown in green. (Scale bar: 200 μm.)
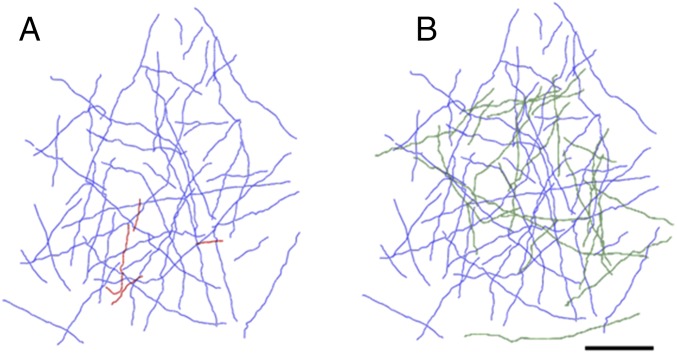
For the second monkey C, we did a similar reconstruction, comparing two time points during training. Over the time points represented in the axonal reconstructions, the monkey improved in his performance in the task during the early period of training, represented as the change observed between day 3 and week 4 of training (Fig. 5). The baseline imaging session, and the associated axonal reconstruction was done during week 0, and the second time point was between week 2 and 4, after the monkey showed a measure of perceptual learning. Between these two time points, as with monkey M, there was outgrowth of new axon collaterals, and pruning of a few axon collaterals in the imaged region (Fig. 6 A and B).
Fig. 6.
Reconstruction of axons of monkey C imaged at the outset of training (A) between weeks 2 and 4 of training (B). (Scale bar: 200 μm.)
Overall, both animals showed substantial changes in the axonal arbors of horizontal connections, with the two animals showing varying degrees of sprouting and pruning and monkey M showing different rates of sprouting and pruning over the course of training (Table 1). The area used for comparison between week 0 and week 6 was slightly different from that used for comparing week 6 and week 8. Although most of the improvement in performance occurred between the initial two time points, there was a continuing substantial change in axonal arbors between the second and third time points, when much less improvement occurred. Monkey C also showed substantial sprouting of new axon collaterals, with less pruning. In general, it appeared that there was more sprouting than pruning, and the changes represented a substantial fraction of the preexisting plexus of axon collaterals.
Table 1.
Percentage of baseline in axon length relative to baseline across imaging sessions for the two monkeys
| Imaging session comparison | Axon length, μm | % of Baseline | |
| Monkey M: comparison, week 0 to week 6 | |||
| Total baseline axon length | 21,266 | ||
| Added | 7,007.7 | 32.95 | |
| Retracted | 5,540.5 | 26.05 | |
| Stable | 15,725.5 | 73.95 | |
| Monkey M: comparison, week 6 to week 8 | |||
| Total week 6 axon length | 24,873 | ||
| Added | 15,516 | 62.38 | |
| Retracted | 2,313 | 9.30 | |
| Stable | 22,560 | 90.70 | |
| Monkey C: comparison, week 0 to week 4 | |||
| Total baseline axon length | 15,089 | ||
| Added | 7,330.1 | 48.58 | |
| Retracted | 633.1081 | 4.20 | |
| Stable | 14,455.8919 | 95.80 |
Another measure of axonal dynamics is the turnover of axonal branches, which provides a further comparison with our earlier measurements in animals not undergoing perceptual learning. Here, we found in both of the trained animals that a large proportion of the axon collaterals in the imaged area underwent sprouting and pruning over the time course of perceptual learning (Table 2). This contrasts markedly with the untrained animals, where no new branches were observed, nor were any of the initially imaged branches pruned.
Table 2.
Percentage of baseline of axon branches across imaging sessions during the course of perceptual learning
| Imaging session comparison | Branch points | % of Baseline |
| Monkey M: branch point comparison, week 0 to week 6 | ||
| Baseline branch points | 126 | |
| Added | 34 | 26.98 |
| Retracted | 42 | 33.33 |
| Stable | 84 | 66.67 |
| Monkey M: branch point comparison, week 6 to week 8 | ||
| Added | 59 | 77.63 |
| Retracted | 11 | 14.47 |
| Stable | 65 | 85.52 |
| Monkey C: branch point comparison, week 0 to week 4 | ||
| Baseline branch points | 63 | |
| Added | 25 | 39.68 |
| Retracted | 4 | 6.35 |
| Stable | 59 | 93.65 |
Discussion
In this study, to maximize the period of time available for imaging during the course of perceptual learning, we use our previous study (1) as a control. In that study, the methods, in terms of the surgical procedures, viral vectors, injections, and patterns of labeling were equivalent to those applied here. The differences between the earlier controls and the results seen here were striking. In the absence of perceptual learning, while there was a steady-state turnover of axonal boutons at the rate of 7% per week, there was no change in the branching patterns of axons, and the “additions or subtractions of axonal collaterals longer than 11 µm were not observed” among ∼6 mm of axons reconstructed in 16 locations from two animals across time points (1). In marked distinction to this, here we saw substantial changes in the branching pattern in the area of V1 representing the trained stimulus, with both growth and elimination of extended axon collaterals (Table 2). As with boutons en passant, boutons terminaux, along with their small (<10-µm) side branches, did appear and disappear both in the control and perceptual learning groups.
Plasticity is a ubiquitous property of the cerebral cortex. We know that, without manipulation of visual experience, the horizontally projecting axons of visual cortical pyramidal cells are stable (1). The plasticity seen under these conditions involves turnover of axonal boutons, which occurs at a rate of 7% per week or modest changes to existing axonal branches (9) along with turnover of synaptic spines (10). However, with alteration of experience, one can see rapid and long-term changes in axonal arbors. Although certain cortical connections, most notably thalamocortical projections, have a limited period of plasticity in postnatal life, the critical period, other connections retain the capacity for change throughout life. We have previously used a retinal lesion model in the monkey to induce plasticity of visual cortical connections: focal binocular retinal lesions lead to remapping of the retinotopic organization of V1, with neurons in the LPZ shifting RFs to positions outside the retinal scotoma (11–25). The circuitry underlying this functional change involves exuberant sprouting (along with a measure of pruning) of the long-range horizontal connections, projecting from normal cortex into the LPZ, and of a reciprocal elaboration of inhibitory connections originating from the LPZ (2, 3, 16, 26). The pattern of axonal turnover associated with perceptual learning is reminiscent of that observed following retinal lesions, where, although there is a net increase in the horizontal projections into the LPZ, it is striking that the axonal changes involve substantial pruning as well as sprouting. Analogous changes in cortical circuitry are seen in the mouse barrel cortex following whisker plucking (4, 5). That the adult cortex has this capacity for changing axonal arbors raised the possibility that it could be used not just for functional recovery following CNS lesions but to mediate the functional changes associated with normal experience-dependent change, notably perceptual learning. Our observations here support this idea. Axons in the part of area V1 representing the trained part of the visual field elaborated new collaterals and pruned some of the preexisting collaterals. Horizontal connections are a fundamental component of cortical circuitry and normally contribute to inputs arising from outside the RF (as defined by simple stimuli), providing contextual influences mediating a diversity of functions, including contour integration, and encoding the Gestalt rules of perceptual grouping (7, 8, 27–32). While the sprouting of horizontal connections into the LPZ following retinal lesions can account for the shifting RFs of LPZ neurons, changes of the horizontal connections during the course of perceptual learning of contour saliency may facilitate contour integration. Longitudinal in vivo two-photon imaging affords us the ability to track the axonal changes occurring during the course of perceptual learning, and importantly we have shown that not only the formation of new axon collaterals but the pruning of preexisting collaterals is part of this process.
It is important to distinguish operational learning from perceptual learning. Operational learning involves getting the animal to understand the nature of the task to be performed, to do fixation during stimulus presentation, and to make a saccade in response to his judgment about the location of the embedded contour. Perceptual learning can also occur during the period of operational training, although it is difficult to assign a precise time when it starts. Once the animal has learned the task, however, he is readily able to shift to a new visual field position, and one can clearly identify the period during which learning occurs by the shifting threshold in task performance. The current experimental design allowed us to establish the onset of perceptual learning following the initial baseline imaging session, limiting the amount of operational learning required.
Changes at the dendritic level have been associated with various models of learning. Increased dendritic spine density in mouse barrel cortex accompanies learning on a whisker-based object localization task (33). Motor skill learning in the mouse has been associated with turnover and stabilization of dendritic spines and changes in the axonal boutons of inhibitory neurons in motor cortex (34). Shrinkage of the potentiated spines disrupts the acquired skills (35). Various training paradigms have been employed to induce motor cortex plasticity, including skill learning on a rotorod task and improvement on producing more reproducible movements in a lever press task. Fear conditioning based on auditory cues produces a transient increase in spine formation in the auditory cortex (36). Here, we find more extensive changes involving the axonal arbors of long-range horizontal connections. These findings mirror the similarly extensive level of axonal plasticity seen following the more dramatic experimental manipulations of retinal lesions in monkeys and whisker plucking in mice. One might speculate that similar extensive changes in long-range horizontal connections would occur in the motor cortex to the extent that the motor task engages the horizontal connections. The dynamics of the dendritic changes reported earlier and the axonal changes seen in the current study are notable, not just for the formation of new spines or axon collaterals but for the elimination of preexisting connections. The parallel processes of pruning and sprouting may indicate that plasticity is under homeostatic control, although whether given sufficient time pruning fully counterbalances the outgrowth requires imaging over a more extended period. Still, it is of interest to note that monkey M continued to show substantial sprouting even when the rate of learning had considerably slowed. This may reflect a period of consolidation of circuit changes that follows but is due to an earlier period of learning.
The availability of a morphological signature of perceptual learning, the axonal changes reported here, has implications for where along the visual pathway the learned information is represented. For many discrimination tasks, training induces changes in the tuning of V1 neurons to stimulus-relevant attributes (8, 37–41), and learning-dependent changes have been seen in primary somatosensory and auditory cortex (42–44). In the contour detection task, one sees parallel shifts in the psychometric curve and the neurometric curve of V1 responses, pointing toward changes in contour representation at the sensory side (8). Here, we imaged the horizontal connections in V1 as a likely candidate for carrying the contextual information involved in the contour detection task. Other studies have provided evidence for the involvement of early visual areas in perceptual learning. Based on magnetic resonance spectroscopy, changes in the balance of glutamate and GABA levels have been associated with different stages of perceptual learning in early visual cortical areas (45). fMRI neurofeedback from V1/V2 classifiers can induce visual associative learning (46). However, other studies have attributed perceptual learning to the areas involved in the readout of sensory information and decision making (47). It has been argued that the changes in neuronal tuning that correlate with perceptual learning could be due to transient attentional influences, but the structural changes seen in the current study point to long-term influences. The involvement of early and late stages in the visual pathway are not mutually exclusive mechanisms—learning may entail a distributed representation across multiple areas. That perceptual learning is associated with changes in V1 circuitry lends further support to the idea that the contour-related responses are due at least in part to local V1 circuits, and that changes in the contour-related responses occurring during the course of perceptual learning involve modification of V1 circuitry.
Beyond the role of intrinsic cortical connections in mediating the attributes of the trained task, we know there is a further involvement of top-down influences in exhibiting the learned properties (8, 31, 39, 48). The contour-related responses in V1 are much stronger when the animal performs the contour detection task, suggesting that feedback to V1, conveying information about the nature of the perceptual task, interacts with the intrinsic connections and influences their functional connectivity. This is supported by measures of coherent activity between cortical sites recorded by electrode arrays (31), by biologically based computational models (30), and by conditional Granger causality analysis, showing that lateral interactions within V1 are dependent on input from V4 (49). This has led us to propose that perceptual learning is not purely a matter of a homosynaptic, Hebbian process but involves setting up an interaction, or “addressing,” between feedback and intrinsic cortical connections. One might speculate that the axonal changes we see here may serve to establish the proper mapping between feedback and intrinsic connections to enable neurons to gate inputs that are relevant to the current task. It remains to be seen whether the feedback to V1 undergoes a similar sculpting of axonal arbors as seen here for the intrinsic horizontal connections, and the relative roles of changes in the strength of cortical connections versus setting up the appropriate interactions between neuronal inputs, either of which may be mediated by the changes seen here.
Experimental Procedures
Animal Surgery.
Ethical approval for experiments was granted by the Institutional Animal Care and Use Committee of The Rockefeller University. Surgical procedures and training in adult Macaca mulatta were done in accordance with institutional and federal guidelines for the treatment of animals. Implantations and viral injections were done under anesthesia. Procedures involving exposing the cortex were preceded with daily treatment with dexamethasone (0.1–0.25 mg/kg, i.m.), starting 3 d before the procedure and gradually decreased over the course of the subsequent 10 d, to prevent cerebral edema. Following initial induction of anesthesia with ketamine (10 mg/kg, i.m.) and dexdomitor (5–20 mcg/kg, i.m.), a venous cannula was inserted, and the animal was intubated with an endotracheal tube. Anesthesia was maintained with isoflurane (0.5–1.5%) and dexdomitor (1–4 mcg⋅kg−1⋅h−1). Postsurgical medication included antibiotics and analgesics. The initial surgical procedure involved implanting a titanium head post, and subsequent procedures involved viral injections, chamber implantation, and imaging under anesthesia.
Visual Stimuli and Behavior.
Visual stimuli were generated using the same approach as in our previous electrophysiological study (8). Two animals were used in this study, both living in the same environment and trained and tested under similar conditions. The animals were presented with two patches of randomly oriented lines, with one containing an embedded contour consisting of 1, 3, 5, 7, 9, or 11 collinear lines embedded in the center (Fig. 1). The other patch had no embedded contour. The two patches, one with and one without a contour, were presented on either side of a fixation spot. A trial began with presentation of a 0.08° fixation point, which was displayed at the CRT center. The animal had to maintain fixation during the course of each trial, fixating within an invisible circular window of 0.5° in radius around the fixation point, covertly attending to the stimulus patches. Eye positions were sampled at 30 Hz by an infrared tracking system (50). After the animal maintained fixation for 191 ms, the visual stimulus patterns were presented for 600 ms. At the end of the trial, the patches were turned off and the animal was presented with two saccade targets, whereupon it made a saccade to the target in the hemifield containing the patch with the embedded contour. Two stages of training and perceptual learning were conducted. First, the background elements were presented at lower contrast than those making up the contour, and over successive days the background contrast was increased to eventually match that of the contour. Over time, the animals were able to detect the contour in the presence of higher-contrast backgrounds. Then, the length of the contour, that is, the number of line segments of which the contour was composed, was varied. From trial to trial, the embedded contour was randomly made of 1, 3, 5, 7, 9, or 11 line segments. The one-line segment condition was equivalent to the patch with no embedded contour. Over time, the animals were able to detect contours made of fewer line segments. Performance in the task was expressed as a function of the background/foreground contrast ratio in the first stage of training, and as a function of the number of line segments in the second stage of learning. The imaging experiments were done in monkey M in the second phase of training, and in monkey C in the first.
Imaging.
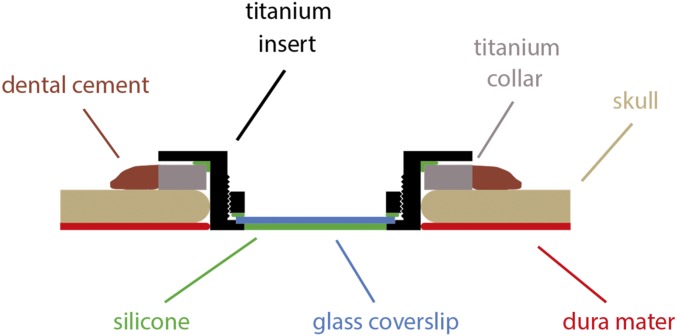
Approximately 2 mo before baseline imaging, we made a craniotomy over the opercular cortex, exposing a region of V1 posterior to the lunate sulcus; a cut was made in the dura and reflected to expose the cortical surface. The site of the craniotomy was selected, based on skull coordinates, to expose a cortical region representing a known visuotopic location (based on previous studies) and corresponding to the location of the trained stimulus. We made a series of injections of AAV2/1.CMV.eGFP and AAV2/1.CBA.TurboRFP, at multiple depths, placed with ∼3-mm separation, to label axonal arbors for subsequent imaging (2). After the injections were completed, a piece of artificial dura was placed on the cortical surface and the dura flap was sewn back in place. To prepare for imaging, we made a circular craniotomy, 24 mm in diameter, and implanted a custom-designed chamber on the skull surrounding the craniotomy (Fig. 7). The dura was resected to expose the entire area of cortex under the craniotomy. The imaging chamber consisted of a threaded titanium collar that was placed on the skull surface and cemented in place by dental acrylic (Palacos) that covered a series of titanium screws that were screwed into the skull. We lowered a glass-bottomed titanium insert, fitted with a silicone O-ring, into the collar until it touched the cortical surface, and screwed it into place. Several of these inserts were made so that we would be provided with one that accommodated the depth of the cortical surface below the collar, enabling the insert to lightly touch the cortical surface. The resulting water-tight chamber minimized artifacts from cortical movement when imaging.
Fig. 7.
Sketch of imaging chamber. A titanium collar (gray) is implanted and sealed with dental cement (dark brown). A titanium insert (black), sealed with a silicone ring (green), is inserted into the collar, replacing both the skull (light brown) and the dura mater (red). A glass coverslip (blue) is held in place by a threaded titanium ring (black) and sealed with silicone (green).
The cortex under the chamber was imaged with a custom-built two-photon microscope fitted with a Nikon 16× objective. Two-photon excitation (88–890 nm) was provided by a mode-locked Ti:sapphire laser pumped by a 10-W frequency-doubled Nd:vanadate laser (Tsunami/Millenia system; Spectra Physics). Imaging of eGFP used a filter set with peak of 525 nm and bandwidth of 50 nm.
Analysis.
The reconstruction of axonal arbors was done with the use of ImageJ, in order to view image stacks off-line. Images were deconvolved using Huygens deconvolution software (Scientific Volume Imaging). Neuromantic (version 1.7.5; https://groups.google.com/forum/#!topic/neuromanticusergroup/1u2YSpiXgLY) was used to reconstruct axonal arbors of corresponding areas. Image stacks were imported into Neuromantic, and overlapping regions were aligned from one stack to another using vasculature and fluorescently labeled neurites. Corresponding areas were reconstructed using landmarks such as vasculature, and imaging sessions were compared at the same depths in both imaging sessions. Following reconstruction of the area, the two time points were then reassessed axon by axon to ensure that all axons were accounted for. Voxel size was corrected for by using a custom Matlab script (Mathworks) before axonal length was determined. Changes in axonal morphology were determined by visualizing stacks and reconstructions from two imaging sessions on two open Neuromantic windows and comparing the morphology throughout the reconstructed stack. Axons and/or portions of axons were then encoded as added, stable, or pruned by changing the structure identifier of the swc file generated by Neuromantic. We arbitrarily used structure identifier type 3 for stable axons, type 4 for retracted axons, and type 8 for added axons. Axonal lengths for added, retracted, and stable axons were determined by importing the finalized swc files into Matlab and analyzing the data using a custom Matlab script.
Acknowledgments
This work was supported by NIH National Eye Institute Grant R01 EY007968.
Footnotes
The authors declare no conflict of interest.
References
- 1.Stettler DD, Yamahachi H, Li W, Denk W, Gilbert CD. Axons and synaptic boutons are highly dynamic in adult visual cortex. Neuron. 2006;49:877–887. doi: 10.1016/j.neuron.2006.02.018. [DOI] [PubMed] [Google Scholar]
- 2.Yamahachi H, Marik SA, McManus JNJ, Denk W, Gilbert CD. Rapid axonal sprouting and pruning accompany functional reorganization in primary visual cortex. Neuron. 2009;64:719–729. doi: 10.1016/j.neuron.2009.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Marik SA, Yamahachi H, Meyer zum Alten Borgloh S, Gilbert CD. Large-scale axonal reorganization of inhibitory neurons following retinal lesions. J Neurosci. 2014;34:1625–1632. doi: 10.1523/JNEUROSCI.4345-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kossut M, Juliano SL. Anatomical correlates of representational map reorganization induced by partial vibrissectomy in the barrel cortex of adult mice. Neuroscience. 1999;92:807–817. doi: 10.1016/s0306-4522(98)00722-2. [DOI] [PubMed] [Google Scholar]
- 5.Marik SA, Yamahachi H, McManus JN, Szabo G, Gilbert CD. Axonal dynamics of excitatory and inhibitory neurons in somatosensory cortex. PLoS Biol. 2010;8:e1000395. doi: 10.1371/journal.pbio.1000395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li W, Gilbert CD. Global contour saliency and local colinear interactions. J Neurophysiol. 2002;88:2846–2856. doi: 10.1152/jn.00289.2002. [DOI] [PubMed] [Google Scholar]
- 7.Li W, Piëch V, Gilbert CD. Contour saliency in primary visual cortex. Neuron. 2006;50:951–962. doi: 10.1016/j.neuron.2006.04.035. [DOI] [PubMed] [Google Scholar]
- 8.Li W, Piëch V, Gilbert CD. Learning to link visual contours. Neuron. 2008;57:442–451. doi: 10.1016/j.neuron.2007.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.De Paola V, et al. Cell type-specific structural plasticity of axonal branches and boutons in the adult neocortex. Neuron. 2006;49:861–875. doi: 10.1016/j.neuron.2006.02.017. [DOI] [PubMed] [Google Scholar]
- 10.Trachtenberg JT, et al. Long-term in vivo imaging of experience-dependent synaptic plasticity in adult cortex. Nature. 2002;420:788–794. doi: 10.1038/nature01273. [DOI] [PubMed] [Google Scholar]
- 11.Gilbert CD, Hirsch JA, Wiesel TN. Lateral interactions in visual cortex. Cold Spring Harb Symp Quant Biol. 1990;55:663–677. doi: 10.1101/sqb.1990.055.01.063. [DOI] [PubMed] [Google Scholar]
- 12.Kaas JH, et al. Reorganization of retinotopic cortical maps in adult mammals after lesions of the retina. Science. 1990;248:229–231. doi: 10.1126/science.2326637. [DOI] [PubMed] [Google Scholar]
- 13.Heinen SJ, Skavenski AA. Recovery of visual responses in foveal V1 neurons following bilateral foveal lesions in adult monkey. Exp Brain Res. 1991;83:670–674. doi: 10.1007/BF00229845. [DOI] [PubMed] [Google Scholar]
- 14.Chino YM, Kaas JH, Smith EL, 3rd, Langston AL, Cheng H. Rapid reorganization of cortical maps in adult cats following restricted deafferentation in retina. Vision Res. 1992;32:789–796. doi: 10.1016/0042-6989(92)90021-a. [DOI] [PubMed] [Google Scholar]
- 15.Gilbert CD, Wiesel TN. Receptive field dynamics in adult primary visual cortex. Nature. 1992;356:150–152. doi: 10.1038/356150a0. [DOI] [PubMed] [Google Scholar]
- 16.Darian-Smith C, Gilbert CD. Axonal sprouting accompanies functional reorganization in adult cat striate cortex. Nature. 1994;368:737–740. doi: 10.1038/368737a0. [DOI] [PubMed] [Google Scholar]
- 17.Chino YM, Smith EL, 3rd, Kaas JH, Sasaki Y, Cheng H. Receptive-field properties of deafferentated visual cortical neurons after topographic map reorganization in adult cats. J Neurosci. 1995;15:2417–2433. doi: 10.1523/JNEUROSCI.15-03-02417.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Darian-Smith C, Gilbert CD. Topographic reorganization in the striate cortex of the adult cat and monkey is cortically mediated. J Neurosci. 1995;15:1631–1647. doi: 10.1523/JNEUROSCI.15-03-01631.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Das A, Gilbert CD. Long-range horizontal connections and their role in cortical reorganization revealed by optical recording of cat primary visual cortex. Nature. 1995;375:780–784. doi: 10.1038/375780a0. [DOI] [PubMed] [Google Scholar]
- 20.Calford MB, et al. Plasticity in adult cat visual cortex (area 17) following circumscribed monocular lesions of all retinal layers. J Physiol. 2000;524:587–602. doi: 10.1111/j.1469-7793.2000.t01-1-00587.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Giannikopoulos DV, Eysel UT. Dynamics and specificity of cortical map reorganization after retinal lesions. Proc Natl Acad Sci USA. 2006;103:10805–10810. doi: 10.1073/pnas.0604539103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Keck T, et al. Massive restructuring of neuronal circuits during functional reorganization of adult visual cortex. Nat Neurosci. 2008;11:1162–1167. doi: 10.1038/nn.2181. [DOI] [PubMed] [Google Scholar]
- 23.Dilks DD, Baker CI, Peli E, Kanwisher N. Reorganization of visual processing in macular degeneration is not specific to the “preferred retinal locus.”. J Neurosci. 2009;29:2768–2773. doi: 10.1523/JNEUROSCI.5258-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Abe H, et al. Adult cortical plasticity studied with chronically implanted electrode arrays. J Neurosci. 2015;35:2778–2790. doi: 10.1523/JNEUROSCI.3579-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Palagina G, Eysel UT, Jancke D. Strengthening of lateral activation in adult rat visual cortex after retinal lesions captured with voltage-sensitive dye imaging in vivo. Proc Natl Acad Sci USA. 2009;106:8743–8747. doi: 10.1073/pnas.0900068106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Florence SL, Taub HB, Kaas JH. Large-scale sprouting of cortical connections after peripheral injury in adult macaque monkeys. Science. 1998;282:1117–1121. doi: 10.1126/science.282.5391.1117. [DOI] [PubMed] [Google Scholar]
- 27.Stettler DD, Das A, Bennett J, Gilbert CD. Lateral connectivity and contextual interactions in macaque primary visual cortex. Neuron. 2002;36:739–750. doi: 10.1016/s0896-6273(02)01029-2. [DOI] [PubMed] [Google Scholar]
- 28.Sigman M, Cecchi GA, Gilbert CD, Magnasco MO. On a common circle: Natural scenes and Gestalt rules. Proc Natl Acad Sci USA. 2001;98:1935–1940. doi: 10.1073/pnas.031571498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li RW, Levi DM, Klein SA. Perceptual learning improves efficiency by re-tuning the decision “template” for position discrimination. Nat Neurosci. 2004;7:178–183. doi: 10.1038/nn1183. [DOI] [PubMed] [Google Scholar]
- 30.Piëch V, Li W, Reeke GN, Gilbert CD. Network model of top-down influences on local gain and contextual interactions in visual cortex. Proc Natl Acad Sci USA. 2013;110:E4108–E4117. doi: 10.1073/pnas.1317019110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ramalingam N, McManus JNJ, Li W, Gilbert CD. Top-down modulation of lateral interactions in visual cortex. J Neurosci. 2013;33:1773–1789. doi: 10.1523/JNEUROSCI.3825-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen M, et al. Incremental integration of global contours through interplay between visual cortical areas. Neuron. 2014;82:682–694. doi: 10.1016/j.neuron.2014.03.023. [DOI] [PubMed] [Google Scholar]
- 33.Kuhlman SJ, O’Connor DH, Fox K, Svoboda K. Structural plasticity within the barrel cortex during initial phases of whisker-dependent learning. J Neurosci. 2014;34:6078–6083. doi: 10.1523/JNEUROSCI.4919-12.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xu T, et al. Rapid formation and selective stabilization of synapses for enduring motor memories. Nature. 2009;462:915–919. doi: 10.1038/nature08389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hayashi-Takagi A, et al. Labelling and optical erasure of synaptic memory traces in the motor cortex. Nature. 2015;525:333–338. doi: 10.1038/nature15257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Moczulska KE, et al. Dynamics of dendritic spines in the mouse auditory cortex during memory formation and memory recall. Proc Natl Acad Sci USA. 2013;110:18315–18320. doi: 10.1073/pnas.1312508110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Crist RE, Li W, Gilbert CD. Learning to see: Experience and attention in primary visual cortex. Nat Neurosci. 2001;4:519–525. doi: 10.1038/87470. [DOI] [PubMed] [Google Scholar]
- 38.Schoups A, Vogels R, Qian N, Orban G. Practising orientation identification improves orientation coding in V1 neurons. Nature. 2001;412:549–553. doi: 10.1038/35087601. [DOI] [PubMed] [Google Scholar]
- 39.Li W, Piëch V, Gilbert CD. Perceptual learning and top-down influences in primary visual cortex. Nat Neurosci. 2004;7:651–657. doi: 10.1038/nn1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sigman M, et al. Top-down reorganization of activity in the visual pathway after learning a shape identification task. Neuron. 2005;46:823–835. doi: 10.1016/j.neuron.2005.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Poort J, et al. Learning enhances sensory and multiple non-sensory representations in primary visual cortex. Neuron. 2015;86:1478–1490. doi: 10.1016/j.neuron.2015.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Recanzone GH, Merzenich MM, Jenkins WM. Frequency discrimination training engaging a restricted skin surface results in an emergence of a cutaneous response zone in cortical area 3a. J Neurophysiol. 1992;67:1057–1070. doi: 10.1152/jn.1992.67.5.1057. [DOI] [PubMed] [Google Scholar]
- 43.Recanzone GH, Merzenich MM, Jenkins WM, Grajski KA, Dinse HR. Topographic reorganization of the hand representation in cortical area 3b owl monkeys trained in a frequency-discrimination task. J Neurophysiol. 1992;67:1031–1056. doi: 10.1152/jn.1992.67.5.1031. [DOI] [PubMed] [Google Scholar]
- 44.Recanzone GH, Schreiner CE, Merzenich MM. Plasticity in the frequency representation of primary auditory cortex following discrimination training in adult owl monkeys. J Neurosci. 1993;13:87–103. doi: 10.1523/JNEUROSCI.13-01-00087.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shibata K, et al. Overlearning hyperstabilizes a skill by rapidly making neurochemical processing inhibitory-dominant. Nat Neurosci. 2017;20:470–475. doi: 10.1038/nn.4490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Amano K, Shibata K, Kawato M, Sasaki Y, Watanabe T. Learning to associate orientation with color in early visual areas by associative decoded fMRI neurofeedback. Curr Biol. 2016;26:1861–1866. doi: 10.1016/j.cub.2016.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Law C-T, Gold JI. Neural correlates of perceptual learning in a sensory-motor, but not a sensory, cortical area. Nat Neurosci. 2008;11:505–513. doi: 10.1038/nn2070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.McManus JN, Li W, Gilbert CD. Adaptive shape processing in primary visual cortex. Proc Natl Acad Sci USA. 2011;108:9739–9746. doi: 10.1073/pnas.1105855108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Liang H, et al. Interactions between feedback and lateral connections in the primary visual cortex. Proc Natl Acad Sci USA. 2017;114:8637–8642. doi: 10.1073/pnas.1706183114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Matsuda K, Nagami T, Kawano K, Yamane S. A new system for measuring eye position on a personal computer. Soc Neurosci Abstr. 2000;26:744.742. [Google Scholar]