Summary
Trypanosoma cruzi, the aetiological agent of Chagas disease, has a highly efficient detoxification system to deal with the oxidative burst imposed by its host. One of the antioxidant enzymes involved is the cytosolic tryparedoxin peroxidase (c‐TXNPx), which catalyses the reduction to hydrogen peroxide, small‐chain organic hydroperoxides and peroxynitrite. This enzyme is present in all parasite stages, and its overexpression renders parasites more resistant to the oxidative defences of macrophages, favouring parasite survival. This work addressed the study of the specific humoral and cellular immune response triggered by c‐TXNPx in human natural infection. Thus, sera and peripheral blood mononuclear cells (PBMC) were collected from chronically infected asymptomatic and cardiac patients, and non‐infected individuals. Results showed that levels of IgG antibodies against c‐TXNPx were low in sera from individuals across all groups. B‐cell epitope prediction limited immunogenicity to a few, small regions on the c‐TXNPx sequence. At a cellular level, PBMC from asymptomatic and cardiac patients proliferated and secreted interferon‐γ after c‐TXNPx stimulation, compared with mock control. However, only proliferation was higher in asymptomatic patients compared with cardiac and non‐infected individuals. Furthermore, asymptomatic patients showed an enhanced frequency of CD19+ CD69+ cells upon exposure to c‐TXNPx. Overall, our results show that c‐TXNPx fails to induce a strong immune response in natural infection, being measurable only in those patients without any clinical symptoms. The low impact of c‐TXNPx in the human immune response could be strategic for parasite survival, as it keeps this crucial antioxidant enzyme activity safe from the mechanisms of adaptive immune response.
Keywords: B‐cell epitope prediction, chronic Chagas disease, peroxiredoxin, T‐cell and B‐cell response
Abbreviations
- AS
asymptomatic patient
- CCC
Chagasic cardiac patient
- c‐TXNPx
cytosolic tryparedoxin peroxidase
- ELISA
enzyme‐linked immunosorbent assay
- GM‐CSF
granulocyte–macrophage colony‐stimulating factor
- IC50
50% of the maximum response
- IFN
interferon
- IgG
immunoglobulin G
- IL
interleukin
- LMM
linear mixed‐effects models
- m‐TXNPx
mitochondrial tryparedoxin peroxidase
- NI
non‐infected individual
- PBMC
peripheral blood mononuclear cells
- PBS
phosphate‐buffered saline
- Prx
peroxiredoxins
- Th
helper CD4+ T lymphocyte
- TLR
Toll‐like receptor
- TNF
tumour necrosis factor
Introduction
The success of Trypanosoma cruzi infection lies mainly in its ability to resist the oxidative attack imposed by the host's immune cells.1, 2 Among the parasite's antioxidant defences, one of the most relevant pathways relies on the trypanothione metabolism enzymes, the tryparedoxin peroxidases (TXNPx).1, 3, 4 As they have no homologue in the mammal host, these enzymes constitute an attractive candidate as a therapeutic target. TXNPx belong to the family of typical 2‐Cys peroxiredoxins (Prx) that use tryparedoxin as an electron donor to detoxify endogenous peroxynitrites and macrophage‐produced molecules, hydrogen peroxide and short‐chain organic hydroperoxides.4 Structurally, TXNPx is a decamer organized as a pentamer of symmetric dimers, with an apparent molecular weight of 166 kDa that can be found in the cytosol (c‐TXNPx) and in the mitochondria (m‐TXNPx) in the three life‐cycle stages of the parasite.5, 6, 7, 8 It was demonstrated that c‐TXNPx but not m‐TXNPx is released into the extracellular medium, and it could help the parasite to counteract the oxygen and nitrogen reactive species produced by the host.9
Several studies have described the role of T. cruzi TXNPx as virulence factors.10 It is known that overexpression of both the cytosolic and mitochondrial forms significantly increases the detoxification of reactive oxygen species, which leads to an increase in parasitaemia together with a greater number of inflammatory infiltrates in skeletal muscle and heart.11 Data from proteomic and biochemical analysis of the parasite in different stages of its life cycle show that the expression of both isotypes, among other enzymes of the antioxidant network, is increased in metacyclic trypomastigotes compared with the epimastigotes, which reinforces the role of this enzyme in the survival of T. cruzi in the mammalian host.12, 13, 14 Even more, both TXNPx forms in trypomastigotes, and only c‐TXNPx in epimastigotes, display major abundance in those parasite strains that are more infective and virulent.14, 15 All of these findings highlight the importance of these enzymes in the parasite's ability to successfully infect host cells.
However, the functions of peroxiredoxins are not limited to their antioxidant activity.16 Recent studies show that these enzymes are secreted by tumour and/or infected cells and their interactions with different receptors, such as Toll‐like receptor 2 (TLR2) and TLR4 present on the surface of host cells, modulate inflammation, immunity and tissue‐repairing reactions.16, 17 With regard to parasitic infections, the Prx of the helminth Fasciola hepatica, a multicellular parasite responsible for chronic and persistent infections, acts on host macrophages by stimulating the secretion of cytokines that suppress the T helper type 1 (Th1) lymphocyte phenotype, without altering the differentiation of naive T lymphocytes to Th2 phenotype.18, 19 This effect of Prx is independent of its peroxidase activity.19 On the other hand, the 2‐Cys peroxiredoxin (PfTPx‐1) of the protozoa Plasmodium falciparum, the causative agent of malaria, induces the secretion of tumour necrosis factor, through binding to TLR4 and via the activation of the transcription factor nuclear factor‐κB.20 Hence, parasite Prx seems to act in harmony with the interplay imposed by each particular microorganism: the Plasmodium Prx biases the response towards an inflammatory Th1 phenotype, whereas the Fasciola Prx bends the balance towards a Th2 response with production of interleukin‐4 (IL‐4), IL‐10 and prostaglandin E2.21
In line with this, another example of Prx that favour parasite survival by modulating the host's immune response are those from Toxoplasma gondii and Leishmania infantum.22, 23 The Prx of Toxoplasma gondii modulates the function of the host macrophages by inhibiting the production of IL‐1β and favouring the secretion of IL‐10.22 On the other hand, the Prx of Leishmania when secreted/excreted into the extracellular medium acts as an activator of B lymphocytes by a T‐cell‐independent mechanism, producing not only cell proliferation but also IL‐10 secretion, which suggests that this protein may play a role of importance in the progression of the disease.23
Having in mind that T. cruzi, through c‐TXNPx, could modulate host immune response, this study sought to analyse the immune response developed in the context of parasite natural infection. For this purpose, we assessed specific antibodies, and B‐cell and T‐cell response using peripheral blood mononuclear cells (PBMC) from a cohort of patients with chronic Chagas disease, asymptomatic and with cardiac alterations, and from non‐infected individuals as the control group.
Materials and methods
Study population
The study population consisted of 32 patients (15 asymptomatic and 17 cardiac) with positive serology for Chagas disease, determined by two or more tests [indirect immunofluorescence, enzyme‐linked immunosorbent assay (ELISA), indirect haemagglutination], and 18 non‐T. cruzi‐infected individuals with negative serological tests for Chagas disease (control group). All groups of individuals were matched for age and gender (Table 1). The exclusion criteria included no record of treatment with benznidazole or nifurtimox, the presence of systemic arterial hypertension, diabetes mellitus, thyroid dysfunction, renal insufficiency, chronic obstructive pulmonary disease, hydroelectrolytic disorders, alcoholism, history suggesting coronary artery obstruction and rheumatic disease, and the impossibility of undergoing the examinations. The patients in the chronic phase of the infection underwent a complete clinical and cardiological examination and were stratified as asymptomatic (without demonstrable pathology) or cardiac patients according to a modified Kuschnir classification.24
Table 1.
Demographic and clinical characteristics of the study population
| Non‐infected (NI) | Chronic Chagas patients | ||
|---|---|---|---|
| Asymptomatic (AS) | Cardiac (CCC) | ||
| Number of individuals (n) | 18 | 15 | 17 |
| Gender (female/male) | 10/8 | 8/7 | 9/8 |
| Age (range) | 46 (30–60) | 60 (56–70) | 54 (42–67) |
| Kuschnir (grade 1‐2‐3) | 0‐0‐0 | 0‐0‐0 | 12‐1‐1 |
| Range of anti‐Trypanosoma cruzi antibody titrea | 10–60 | 169–99 407 | 1000–29 975 |
Titre of antibodies against T. cruzi was determined as IC50 sera dilution factor.
The research protocols followed the tenets of the Declaration of Helsinki and were approved by the Medical Ethics Committee of the National Institute of Parasitology ‘Dr. Mario Fatala Chabén’. All enrolled patients gave written informed consent, according to the guidelines of the ethics committee, before blood collection and after the nature of the study was explained.
Recombinant c‐TXNPx expression and purification
The c‐TXNPx was expressed and purified as previously described with modifications.7 ClearColi bacterial strain, expressing a human non‐endotoxic form of lipopolysaccharide,25 was grown at 30° in LB‐Miller medium. Expression of recombinant TcH6TXNPx was induced with 0·5 mm isopropyl‐β‐d‐thiogalactopyranoside when the culture reached an absorbance at 600 nm of 0·6. The purification was performed in a 5‐ml HiTrap affinity column (Amersham, Buckinghamshire, UK) charged with Ni2+ and equilibrated with binding buffer (50 mm sodium phosphate, pH 7·6, containing 20 mm imidazole, 500 mm NaCl) at a flow rate of 3 ml/min. The His‐tagged c‐TXNPx was eluted in 50 mm sodium phosphate, pH 7·6, containing 400 mm imidazole, 500 mm NaCl. Mock control was produced from the extract of bacteria transformed with the same vector used for recombinant c‐TXNPx production (without the construct) submitted to the same purification process. Residual endotoxins were removed from protein samples using the Thermo Scientific Pierce High Capacity Endotoxin Removal Resin (Thermo Scientific Pierce, Waltham, MA, USA).
Parasite lysate
Whole antigenic lysate from T. cruzi epimastigote was prepared from axenic cultures (CL Brener strain) in liver infusion tryptose medium as previously described.26 After lysis, the suspension was filter‐sterilized through a 0·2‐μm pore size membrane, split into aliquots and stored at −80° until use.
PBMC and serum isolation
The PBMC were isolated from whole blood by Ficoll‐Hypaque density gradient centrifugation (GE Healthcare Bio‐Sciences AB, Uppsala, Sweden) according to manufacturer‐provided instructions, within 4 hr after collection in EDTA‐anticoagulated tubes. Isolated PBMC were resuspended in fetal bovine serum (Natocor, Córdoba, Argentina) containing 10% dimethylsulphoxide and cryopreserved in liquid nitrogen until used. An aliquot of whole blood (4 ml) from each participant was separated and centrifuged at 400 g to obtain the serum, which was stored at −20° until use.
Anti‐T. cruzi and anti‐c‐TXNPx antibody titre
Anti‐T. cruzi and anti‐c‐TXNPx immunoglobulin G (IgG) titres were determined by ELISA, as previously described,27 in sera from Chagas patients (15 asymptomatic and 17 cardiac) and 15 non‐infected individuals. Briefly, 20 μg/ml of T. cruzi lysate or 5 μg/ml of recombinant c‐TXNPx was coated in a 96‐well flat‐bottom plate overnight at 4° in 0·05 m bicarbonate–carbonate buffer (pH 9·6). Coated wells were washed three times with 0·05% Tween‐20 in phosphate‐buffered saline (PBS‐T) and blocked for 1 hr with 5% skimmed milk in PBS‐T at 37°. After three more washes in PBS‐T, serial dilutions of sera from each participant were incubated in 1% skimmed milk in PBS‐T at 37° for 2 hr. All dilutions were tested in duplicate. Bound IgG was detected with peroxidase‐labelled rabbit anti‐human IgG at 37° for 1 hr (Sigma, St Louis, MO, USA.). Enzyme activity was revealed with 3,3′,5,5′‐Tetramethylbenzidine (TMB), and optical density was measured at 450 nm wavelength with an Automated Plate Reader (Molecular Devices, San Jose, CA, USA.). Antibody titre from each individual was determined as the serum dilution factor that corresponds to 50% of the maximum response (IC50) calculated by non‐linear, dose–response regression analysis using graphpad prism software (GraphPad Software, La Jolla, CA, USA).
In silico c‐TXNPx B‐cell epitope prediction
The web server bepipred‐2.0 28 was used to predict linear B‐cell epitopes within c‐TXNPx structure from protein data base (accession number: 4LLR). This software analyses each amino acid separately and assigns it a score value. Higher scores indicate higher probability for the existing epitope. A threshold score value of 0·53 was selected to define epitopes. On the other hand, discotope 2.029 was used to predict conformational B‐cell epitopes. A score threshold of −3·7 (corresponding to a sensitivity of 0·47 and a specificity of 0·75, default setting) was defined for conformational epitope prediction. For both tools, the representative unique chain of c‐TXNPx structure (which has a total of 10 chains) was used. Predicted linear and conformational epitopes and overlap regions were plotted in pyMOL software (DeLano Scientific LLC. Portland, OR, USA).
PBMC stimulation and culture
Number and viability of PBMC were determined by Trypan blue exclusion staining in a Neubauer counting chamber. Cell suspensions were seeded in 96‐well U‐bottom plates at a density of 2 × 105 cells/well in 200 μl of RPMI medium containing 100 U/ml penicillin, 100 μg/ml streptomycin, 2 mm l‐glutamine and 5% heat‐inactivated male AB Rhesus‐positive human serum (HS; Sigma, St Louis, MO, USA). Cells were stimulated in triplicates with recombinant c‐TXNPx (10 μg/ml) or no antigen (culture medium alone). ‘Mock’ stimulations were included to control the background response potentially induced by contaminants from the recombinant protein production process. Non‐specific stimulation with 1 μg/ml phytohaemagglutinin (Sigma, St Louis, MO, USA) and specific stimulation with whole T. cruzi epimastigote lysate (10 μg/ml) were used as positive control conditions. After 5 days of incubation, the PBMC response was evaluated as proliferation, cytokine secretion and expression of cell surface activation markers.
Proliferation assay
Culture supernatants were collected and stored at −20° until cytokine secretion assessment. Subtracted medium was replaced with complete RPMI medium, and cells were pulsed for 18 hr with 0·5 μCi/well [methyl‐3H]‐thymidine (Perkin Elmer, Waltham, MA, USA) after which they were harvested on glass‐fibre filters. Proliferation was measured as incorporated radioactivity, assessed by liquid scintillation counting. Results were expressed as Stimulation Index obtained as the mean counts per minute of stimulated cultures divided by the mean counts per minute of unstimulated cultures (culture medium only).
Cytokine quantification
Interferon‐γ (IFN‐γ), granulocyte–macrophage colony‐stimulating factor (GM‐CSF) and IL‐10 secretion were measured in culture supernatants by ELISA (OptEIA™ Human IFN‐γ and Human GM‐CSF ELISA sets; BD Pharmingen, San Diego, CA, USA; Human IL‐10 ELISA Ready‐SET‐Go!®; eBiosciences, San Diego, CA, USA) according to the manufacturer′s instructions. Results were expressed as Stimulation Index obtained as the mean pg/ml of cytokine of stimulated cultures divided by the mean pg/ml of cytokine of unstimulated cultures. In cases for which the level of secreted IFN‐γ was undetectable, the obtained value from the stimulated cultures was divided by the lowest detectable value (2·3 pg/ml).
Assessment of PBMC activation markers
Frequency of positive cells for HLA‐DR and CD69 activation markers on T (CD3+ CD4+ and CD3+ CD8+) and B (CD3− CD19+) cells stimulated with T. cruzi lysate, c‐TXNPx or mock was evaluated by flow cytometry. One million cells/well were seeded in a 48‐well flat‐bottom plate in complete RPMI medium and stimulated with the antigens mentioned above. After the incubation period, cells were harvested, resuspended in ice‐cold PBS and stained for 30 min at room temperature with the fluorescence‐labelled monoclonal antibodies shown in Table 2. Cells were then washed once with PBS, fixed in 200 μl of 1% paraformaldehyde in PBS and kept at 4° until analysed by flow cytometry. Cells stained with fluorescence‐labelled isotype control antibodies were included in all experiments. One hundred thousand events within lymphocyte population were acquired using a FACSCanto II flow cytometer (Becton Dickinson, Franklin Lakes, NJ, USA). Data analyses were carried out with flowjo Software (TreeStar Inc., Ashland, OR, USA); the gate pathway used to determine the activation marker expression is shown in the Supplementary material (Fig. S1). Frequency of CD4+ HLA‐DR+, CD8+ HLA‐DR+, CD19+ HLA‐DR+, CD4+ CD69+, CD8+ CD69+ and CD19+ CD69+ cells was analysed. Results were expressed as fold change, which was calculated by dividing the frequency of each subpopulation of interest in stimulated cultures by the frequency of each subpopulation of interest in unstimulated cultures (culture medium only).
Table 2.
Fluorescence‐labelled antibodies and isotype controls used in FACS experiments
| Antibody | Isotype control |
|---|---|
| FITC‐conjugated anti‐CD3 (BD Biosciences, San Diego, CA) | FITC‐mouse IgG1 κ (BD Biosciences, San Diego, CA) |
| PE‐Cy7‐conjugated anti‐CD4 (BioLegend, San Diego, CA) | PE‐Cy7‐Rat IgG 2b κ (BioLegend, San Diego, CA) |
| PE‐Cy5‐conjugated anti‐CD8 (BD Biosciences, San Diego, CA) | FITC‐mouse IgG1 κ (BD Biosciences, San Diego, CA) |
| PE‐Cy5‐conjugated anti‐CD19 (BD Biosciences, San Diego, CA) | PECy5‐mouse IgG1 κ (BD Biosciences, San Diego, CA) |
| PE‐conjugated anti‐HLA‐DR (BD Biosciences, San Diego, CA) | PE‐mouse IgG2a κ (BD Biosciences, San Diego, CA) |
| BV510‐conjugated anti‐CD69 (BioLegend, San Diego, CA) | BV510‐mouse IgG1 κ (BioLegend, San Diego, CA) |
Abbreviations: BV510, brilliant violet 510; Cy5, cyanine 5; FITC, fluorescein isothiocyanate; PE, phycoerythrin.
All antibodies were validated and titrated using biological and/or isotype control.
Statistical analysis
Normality and homoscedasticity of the data were evaluated using the Shapiro–Wilk normality test and Bartlett test, respectively. Data from proliferation and cytokine secretion were transformed by log10 to obtain a Gaussian distribution of errors and homoscedasticity. Linear mixed‐effects models (LMM) fitted by maximum likelihood and Tukey honest significant difference for post hoc comparisons were applied to analyse differences in PBMC proliferation, cytokine secretion and activation marker expression. LMM were performed using group (asymptomatic, cardiac, non‐infected) and stimulation condition as fixed factors and patient as a random factor. LMM models were fitted in R3.2.1,30 using the function lmer of the R‐package lme4.31 The average of antibody titre between groups was compared using the non‐parametric Kruskal–Wallis test. A P‐value of < 0·05 was considered statistically significant, and the confidence interval (CI) was 95%.
Results
Humoral immune response triggered by c‐TXNPx in patients with chronic Chagas disease
With the aim of determining the presence of a specific humoral response triggered by c‐TXNPx in patients with chronic Chagas disease, we assessed total anti‐c‐TXNPx IgG in sera from asymptomatic (AS) and cardiac (CCC) Chagas patients, and non‐infected individuals (NI) by ELISA. Total IgG antibody response against T. cruzi lysate in the study population was also measured (range of anti‐T. cruzi antibody titre in each group is shown in Table 1). Results showed that total anti‐c‐TXNPx IgG titre ranged from 10 to 999 in asymptomatic, from 9 to 973 in cardiac patients and from 10 to 542 in non‐infected individuals. Although the titres were low across the study population and no statistically significant differences were found between groups, the median of anti‐c‐TXNPx antibody titre in chronic Chagas patients was slightly higher (median AS: 181·5; median CCC: 225) than in non‐infected individuals (group median: 98), (Fig. 1a). As expected, total anti‐T. cruzi IgG titres in the asymptomatic and cardiac groups of patients were significantly higher than those from the non‐infected group (Fig. 1b and Table 1). The non‐parametric Spearman test revealed a positive correlation between antibody titres against T. cruzi and c‐TXNPx in asymptomatic patients (r = 0·830, P = 0·0004; see Supplementary material, Fig. S2a), whereas no correlation was found between these responses in patients with the cardiac form of chronic Chagas disease (r = 0·125, P = 0·6322; see Supplementary material, Fig. S2b). Overall, these results indicate that c‐TXNPx induces a poor humoral IgG response, as the titre of antibodies produced against this protein does not differ between chronic Chagas patients and the non‐infected control group. Furthermore, anti‐c‐TXNPx IgG levels represent a very small fraction of the total anti‐T. cruzi lysate IgG.
Figure 1.
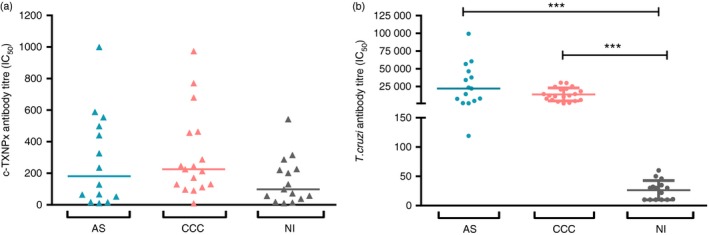
Humoral response against cytosolic tryparedoxin peroxidase (c‐TXNPx) in chronic Chagas disease patients. Anti‐c‐TXNPx (a) and anti‐Trypanosoma cruzi (b) antibody titration was carried out in sera from patients with chronic asymptomatic (AS; n = 14) and chronic cardiac (CCC; n = 17) Chagas disease, and non‐infected individuals (NI; n = 15) by ELISA. The titre of antibodies from each individual was expressed as the sera dilution factor that corresponds to the IC 50 value of the adjusted curve, which was calculated by non‐linear regression analysis. Each dot corresponds to the value from a single individual, and the horizontal line shows the median value for each group. Statistical analysis was performed using the non‐parametric Kruskal–Wallis test. Statistically significant differences are indicated (***P < 0·001).
In silico B‐cell epitope prediction within c‐TXNPx protein sequence
bepipred software was used to predict linear B‐cell epitopes within c‐TXNPx protein sequence. Figure 2(a) shows the score for each amino acid in the c‐TXNPx sequence (considering only one representative chain of the decamer), and the threshold score value is indicated by a horizontal line. According to this threshold value, the software predicted seven probable epitopes, which are ranked by mean residue score in the Supplementary material (Table S1). Predicted linear epitopes contain 4–13 amino acids and show low score values compared with other immunogenic proteins in T. cruzi.32
Figure 2.
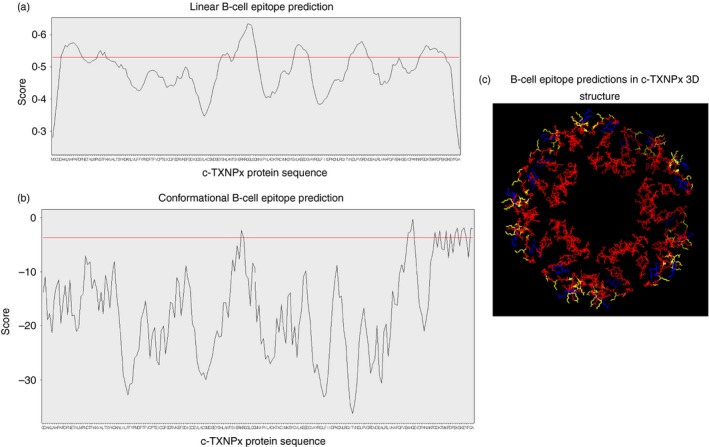
B‐cell epitope prediction within cytosolic tryparedoxin peroxidase (c‐TXNPx) protein sequence. (a) BepiPred linear B‐cell epitope prediction. The x‐axis shows the amino acid position in c‐TXNPx sequence, and the y‐axis shows the score value for each residue. The horizontal red line indicates the threshold score value of 0·53, from which positive epitopes are considered. (b) DiscoTope conformational B‐cell epitope prediction. The x‐axis shows the amino acid position in c‐TXNPx sequence, and the y‐axis shows the score value for each residue. The horizontal red line indicates the threshold value of −3·7 (default setting), from which positive epitopes are considered. (c) Graphical three‐dimensional representation of predicted epitopes within c‐TXNPx structure. Linear (red) and conformational (blue) epitopes and regions of overlapping (yellow) are specified.
discotope prediction showed 144 conformational B‐cell epitope residues of 1940 total residues (Fig. 2b; see Supplementary material, Table S2). These residues interact as a consequence of c‐TXNPx folding and form conformational epitopes. Figure 2(a,b) shows linear and conformational residue scores, and threshold values are indicated by a horizontal line, delimiting probable epitopes.
We found some specific regions of overlap between linear and conformational epitopes (Fig. 2c; yellow labelled residues).
These data suggest that linear and conformational B‐cell epitopes on c‐TXNPx are limited to a few, relatively small regions on the sequence of c‐TXNPx.
Cellular immune response triggered by c‐TXNPx in patients with chronic Chagas disease
We next sought to establish whether c‐TXNPx can trigger a cellular adaptive immune response in patients with chronic Chagas disease and whether there is a difference in the response against c‐TXNPx between patients with the asymptomatic or cardiac form of chronic Chagas disease. Hence, we evaluated the specific proliferative response and cytokine secretion of PBMC from patients of each group and compared this response with that of PBMC from non‐infected individuals. First, different c‐TXNPx concentrations (1 and 10 μg/ml) and incubation periods (3 days or 5 days) were tested using samples from six chronic Chagas patients and three non‐infected donors, to optimize the in vitro stimulation conditions. These preliminary results showed that 10 μg/ml of c‐TXNPx and 5 days of incubation with the protein were optimal to trigger proliferative responses and cytokine secretion, and these conditions were therefore used in the following experiments.
After a 5‐day incubation period, PBMC from all participants proliferated in response to phytohaemagglutinin and responses were not significantly different between groups (data not shown), indicating that physiological state and proliferative capacity of all samples remained intact during the experimental procedure. Results showed that c‐TXNPx stimulation induced proliferation in asymptomatic patients and cardiac patients compared with mock but not in non‐infected donors (Fig. 3a; and see Supplementary material, Fig. S3a–c). Similarly, levels of IFN‐γ were increased upon stimulation with c‐TXNPx compared with mock in asymptomatic and cardiac patients but not in non‐infected donors (Fig. 3b; and see Supplementary material, Fig. S4a–c). GM‐CSF and IL‐10 did not change in any of the groups evaluated upon stimulation with c‐TXNPx (data not shown).
Figure 3.
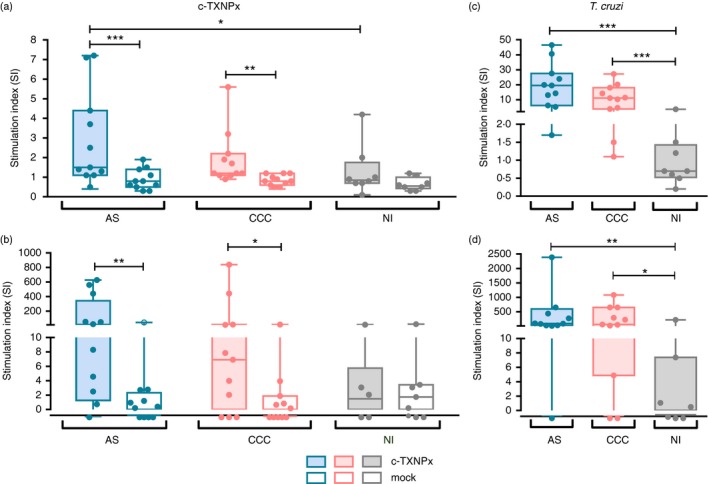
Cellular response of peripheral blood mononuclear cells (PBMC) from chronic Chagas disease patients triggered by cytosolic tryparedoxin peroxidase (c‐TXNPx): 2 × 105 PBMC/well from asymptomatic (AS; n = 11) and cardiac (CCC; n = 11) Chagas patients and non‐infected donors (NI; n = 8) were incubated with Trypanosoma cruzi lysate, recombinant c‐TXNPx and no‐antigen (culture medium only) or mock as controls for 5 days in complete RPMI medium. Each condition was performed in triplicate. (a, c) Cells were pulsed with [3H]thymidine for the last 18 hr of incubation, and PBMC proliferation was determined by [3H]thymidine uptake. (b, d) Culture supernatants were collected after 5 days of stimulation with c‐TXNPx, mock or T. cruzi lysate, and interferon‐γ (IFN‐γ) secretion was quantified by ELISA. Box and whisker plots (min to max) show the stimulation index (SI), calculated for each readout as the mean value for triplicate stimulated cultures of each individual divided by the mean value of triplicate non‐stimulated cultures (medium only). Each dot represents data from a single individual. Statistical analysis was performed using linear mixed‐effects models fitted by maximum likelihood and Tukey honest significant difference for post hoc comparisons. Statistically significant differences between groups are indicated (***P < 0·001, **P < 0·01, *P < 0·05).
When comparing the response triggered by c‐TXNPx among the groups of patients, results showed that c‐TXNPx‐induced proliferation was higher in asymptomatic patients than in non‐infected participants (Fig. 3a). On the other hand, no increase in proliferation upon exposure to c‐TXNPx was observed in cardiac patients compared with non‐infected participants and between asymptomatic and cardiac patients. However, although not statistically different, there was a tendency for higher levels of IFN‐γ in response to stimulation with c‐TXNPx in Chagas patients compared with the non‐infected group (Fig. 3b).
Furthermore, as seen in previously reported results 26, 33, PBMC from asymptomatic and cardiac Chagas patients proliferated (Fig. 3c; and see Supplementary material, Fig. S3d–f) and secreted IFN‐γ (Fig. 3d; and see Supplementary material, Fig. S4d–f) in response to T. cruzi lysate, but none of these responses were observed in PBMC from non‐infected individuals. Nevertheless, only GM‐CSF, but not IL‐10, secretion was increased in both groups of Chagas patients compared with the non‐infected group (see Supplementary material, Fig. S4g–i and j–l, respectively).
Finally, we were interested in determining the relation between cellular response to T. cruzi lysate and c‐TXNPx. A positive correlation between proliferative response to T. cruzi and c‐TXNPx was shown in asymptomatic and cardiac patients (AS: r = 0·81, P = 0·0039; CCC: r = 0·91, P = 0·001, non‐parametric Spearman test; see Supplementary material, Fig. S5a,b, respectively). No correlation was found in IFN‐γ secretion levels in response to these antigenic challenges (AS r = 0·39, P = 0·2632; CCC: r = 0·49, P = 0·1769; see Supplementary material, Fig. S5c,d).
Expression of activation markers on PBMC stimulated with c‐TXNPx
To characterize the phenotype of stimulated cells upon a 5‐day incubation with the antigens presented above, cells from asymptomatic or cardiac patients and non‐infected individuals were stained with antibodies against population (CD3, CD4, CD8 and CD19) and activation (HLA‐DR and CD69) markers before being acquired in a flow cytometer.
Interestingly, the frequency of CD19+ CD69+ cells was higher in asymptomatic patients stimulated with c‐TXNPx compared with the mock stimulus (Fig. 4a). However, no changes in CD69 expression on B cells were observed in cardiac patients and non‐infected individuals. In addition, the frequency of CD19+ CD69+ cells stimulated with c‐TXNPx in asymptomatic patients was not different from that of non‐infected subjects (Fig. 4a).
Figure 4.
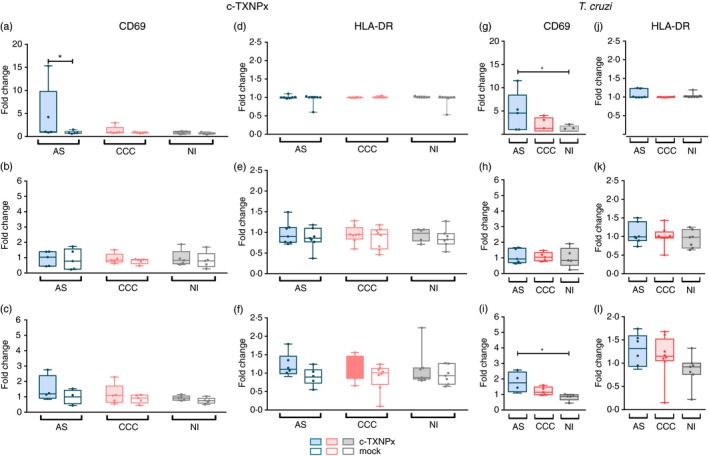
Expression of CD69 and HLA‐DR activation marker on CD19+, CD4+ and CD8+ lymphocytes from patients with chronic Chagas disease stimulated with cytosolic tryparedoxin peroxidase (c‐TXNPx). Peripheral blood mononculear cells (PBMC) (1 × 106 cells/well) from asymptomatic (AS; n = 5) and cardiac (CCC; n = 5) Chagas patients and non‐infected donors (NI; n = 7) were incubated with Trypanosoma cruzi lysate, recombinant c‐TXNPx and no‐antigen (culture medium only) or mock as controls for 5 days in complete RPMI medium in a 48‐well plate. After the incubation period, cells were harvested, washed with phosphate‐buffered saline and stained with fluorescence‐labelled monoclonal antibodies as detailed in Table 2. One hundred thousand events within lymphocyte population were acquired in a FACSCanto II Flow cytometer. Dead cells were excluded by forward‐ versus side‐scatter (FSC/SSC) gating. Doublets were excluded by FSC‐A versus FSC‐H. Singlet cells were then determined according to the gating strategy detailed in the Supplementary material (Fig. S1). Fold change of CD19+ CD69+ (a and g, CD4+ CD69+ (b and h), CD8+ CD69+ (c and i), CD19+ HLA‐DR + (d and j), CD4+ HLA‐DR + (e and k), CD8+ HLA‐DR + (f and l) upon stimulation with c‐TXNPx or mock and T. cruzi lysate, respectively. Statistical analysis was performed using linear mixed‐effects models fitted by maximum likelihood and Tukey honest significant difference for post hoc comparisons. Statistically significant differences between groups are indicated (*P < 0·05).
On the other hand, CD69 expression was similar in T‐cell populations (CD3+ CD4+ and CD3+ CD8+) from all groups of individuals upon exposure to c‐TXNPx (Fig. 4b,c), suggesting that this protein does not induce T‐cell activation. Frequency of HLA‐DR+ on T and B cells did not change upon stimulation with c‐TXNPx in any of the groups evaluated (Fig. 4d–f).
Furthermore, T. cruzi stimulation increased the frequency of CD8+ CD69+ T cells, CD19+ CD69+ B cells in asymptomatic patients compared with non‐infected individuals (Fig. 4g,i). No changes in the frequency of CD4+ CD69+ were observed (Fig. 4h). HLA‐DR activation marker expression on T and B cells was not different between groups in T. cruzi‐stimulated cultures (Fig. 4j–l).
Discussion
During cell infection, T. cruzi is exposed to reactive oxygen and nitrogen species as a result of the oxidative attack deployed by the host innate immune response.1 The relevance of T. cruzi TXNPx for the infection process, drug resistance, and for parasite viability, virulence and growth has been extensively studied.3, 6, 14, 34, 35 All these functions have been related to these enzymes’ detoxifying capability to cope with oxidative stress. The fact that c‐TXNPx is secreted into the extracellular medium suggests that, like other parasite peroxiredoxins,23 it could have a role in the interplay between the parasite and the host immune response.
In this sense, this work attempted to address c‐TXNPx functions on the host immune system in the context of natural human infection, focusing on the analysis of the specific antibody response as well as T‐cell and B‐cell activation upon in vitro c‐TXNPx exposure of PBMC from patients with chronic Chagas disease.
To evaluate the presence of a specific humoral response triggered by c‐TXNPx in patients with the different clinical forms of Chagas disease, we determined the titre of antibodies against this protein in sera from asymptomatic and cardiac patients and non‐infected individuals. Results showed that the antibody response developed against c‐TXNPx in patients with chronic Chagas is mild, and no significant differences were observed in comparison with the control group. In addition, B‐cell epitope predictions showed that probable linear and conformational epitopes in c‐TXNPx are scarce and most of them are in structural regions not exposed to solvent, indicating that they would be not likely to interact with the B‐cell receptor, the initial event that triggers B‐cell activation and specific antibody response. The low antibody production observed in chronic patients, together with the results from B‐cell epitope predictions, suggests that c‐TXNPx is not a good target for the development of a relevant humoral response in the context of a natural infection with T. cruzi. Another reason that could explain the poor antibody response observed is the relatively small proportion of c‐TXNPx within total T. cruzi proteins, compared with another peroxiredoxin in trypanosomatids.6 Furthermore, in agreement with this, our results show that anti‐c‐TXNPx IgG represents a small fraction of total anti‐T. cruzi IgG, which may lead to a lesser immune activation by c‐TXNPx relative to that generated by other, more abundant, proteins, as anti‐c‐TXNPx antibodies are diluted in the whole humoral anti‐T. cruzi response.
By focusing on the cellular arm of the immune response, the majority of recombinant T. cruzi proteins favour a Th1‐type cytokine profile, which is in line with the typical pattern of inflammatory response described for the parasite lysate in cardiac patients.36, 37, 38, 39, 40, 41 However, T. cruzi ribosomal P proteins trigger the release of IL‐10, tumour necrosis factor‐α and GM‐CSF, a mixed cytokine prolife with immunoregulatory and pro‐inflammatory potential.26 Interestingly, this study also demonstrated that GM‐CSF is also secreted at high levels by PBMC, mostly CD4+ and CD8+ T cells from cardiac patients upon stimulation with T. cruzi lysate.26 Here, we analysed the specific cellular response against c‐TXNPx in patients with chronic Chagas disease by proliferation and secretion of IFN‐γ, GM‐CSF and IL‐10. Results showed that c‐TXNPx induced proliferation and IFN‐γ, but not GM‐CSF and IL‐10 secretion, in both asymptomatic and cardiac patients, in comparison with non‐infected individuals. However, the c‐TXNPx response was remarkably low compared with that triggered by T. cruzi lysate. In addition, unlike our previous results,26 T. cruzi lysate failed to provoke IL‐10 production. However, this discrepancy might be explained by the different sensitivity of the assay used to detect cytokines in the cell supernatant in each piece of research, ELISA or multiplex technology.42
Proliferation, but not cytokine secretion, was higher in PBMC samples from asymptomatic patients than in those from cardiac Chagas patients and non‐infected individuals upon its stimulation with c‐TXNPx, suggesting a differential cellular immune response developed against this enzyme in the different clinical forms of chronic Chagas disease. Although it is known that proliferation and the secretion of cytokines as IFN‐γ are not tethered to each other after antigen challenge,43 it can be speculated that naive or even central memory cells are being activated by c‐TXNPx in asymptomatic patients. When stimulated in vitro, cells from these subsets show strong proliferation but a low‐activation onset measured as cytokine production.44 It is tempting to discard the naive population in the response raised against c‐TXNPx in asymptomatic patients, given the lack of proliferation detected in PBMC from non‐infected individuals. In fact, an augmentation in central memory T cells was detected within the CD4+ memory T‐cell population from asymptomatic patients not only ex vivo but also upon T. cruzi lysate stimulation.45 However, it is important to mention that a switch in memory compartment towards central but not effector T cells is not always observed in recall responses after in vitro stimulation with T. cruzi proteins.46
The analysis of specific activation marker expression (HLA‐DR and CD69) on T and B cells revealed that c‐TXNPx stimulation activated CD69 expression on the surface of B (CD19+) cells from asymptomatic patients in concordance with the proliferation increment mentioned above. The lack of proliferation in response to c‐TXNPx in cardiac patients was associated with the absence of an enhanced CD69 expression on lymphocyte populations. Given that the humoral response raised against c‐TXNPx was not significant in patients with chronic Chagas disease, but a specific early activation of B cells was observed, we hypothesize that c‐TXNPx may activate B cells to modulate the immune response, but not to exert humoral effector functions. In addition, the fact that CD69 is an early B‐cell activation marker47, 48 suggests that c‐TXNPx might mount a response that does not progress in the course of the natural infection, allowing parasite survival. Following this idea, studies in Prx1, the major protein in the mammalian peroxiredoxin family, revealed that tumour cell‐released Prx1 interacts with TLR4, which triggers nuclear factor‐κB activation and stimulates inflammatory cytokine secretion from macrophages and dendritic cells.16, 17 The consequence of the release of Prx1 into the tumour microenvironment is unknown, but these authors suggest that it could contribute to chronic inflammation, leading to tumour growth and immune evasion.17
Finally, our results are in agreement with studies performed in animal models showing that Prx expressed by protozoa and helminths modulates the immune response for their persistence and progression in their host.18, 19, 20 However, these proteins tested as vaccine candidates, adjuvanted with different TLR activators, induce a strong immune response and stimulate protection against infection in mice.49, 50 Indeed, Toxoplasma gondii peroxiredoxin 1 (TgPrx1) prompts the secretion of the anti‐inflammatory cytokine IL‐10 by macrophages of infected mice, but as an immunogen TgPrx1 turns into a potent stimulator of macrophages and spleen cells for production of IL‐12 and IFN‐γ, respectively. Mice immunized with TgPrx1 exhibited certain resistance to Toxoplasma gondii infection.50 In a similar fashion, Prx from Leishmania donovani stimulates IL‐10 secretion by B cells in infected mice, but it induced a strong immunological response with a Th1 phenotype when co‐administered with different adjuvants.51 Recently, Bontempi et al.52 demonstrated that c‐TXNPx, named as CPX, emulsified with the new‐generation adjuvant ISCOMATRIX™ (IMX), prompts production of a high level of IgG antibodies, mainly IgG2a over IgG1, and delayed‐type hypersensitivity responses, which is consistent with a Th1‐biased profile. In a challenge experiment with T. cruzi trypomastigotes (Tulahuen strain), mice immunized with CPX‐IMX extended their survival time in comparison with the control groups (PBS or CPX alone).52
Overall, our presented results showed that c‐TXNPx stimulate only cellular, not humoral, immune responses during natural T. cruzi infection and induce a weak proliferative response in asymptomatic patients that can be recalled in vitro. This restrained response might help the parasite evade the human immune system, as it avoids a specific response towards an enzyme critical for its survival and therefore for the establishment of chronic infection.
Disclosures
The authors declare no conflict of interests.
Supporting information
Figure S1. Gating strategy used to determine HLA‐DR and CD69 expression on T cells (CD3+ CD4+ or CD3+ CD8+) and B cells (CD3− CD19+).
Figure S2. Relation between humoral response against cytosolic tryparedoxin peroxidase (c‐TXNPx) and Trypanosoma cruzi in asymptomatic (a) and cardiac patients (b).
Figure S3. Proliferation of peripheral blood mononuclear cells (PBMC) stimulated with cytosolic tryparedoxin peroxidase (c‐TXNPx) (circles) or mock (squares) and Trypanosoma cruzi lysate (Tc; circles) or no‐antigen (NA; squares) in asymptomatic (n = 11, blue symbols; a,d), or cardiac (n = 11, red symbols; b,e) patients and non‐infected individuals (n = 8, grey symbols; c,f) assessed by [3H]thymidine uptake after 5 days incubation period for each condition.
Figure S4. Interferon‐γ (IFN‐γ) (a–f), granulocyte–macrophage colony‐stimulating factor (GM‐CSF) (g–i) and interleukin‐10 (IL‐10) (j–l) secretion in supernatants of culture upon 5 days stimulation with cytosolic tryparedoxin peroxidase (c‐TXNPx) (circles) or mock (squares) and Trypanosoma cruzi lysate (Tc; circles) or no‐antigen (NA; squares) in asymptomatic (n = 11; blue symbols) or cardiac (n = 11; red symbols) patients and non‐infected individuals (n = 8, grey symbols).
Figure S5. Relation between cellular response against cytosolic tryparedoxin peroxidase (c‐TXNPx) and Trypanosoma cruzi. Correlation analysis between proliferation or interferon‐γ (IFN‐γ) secretion, respectively upon c‐TXNPx and T. cruzi in asymptomatic (a and c) and cardiac (b and d) patients.
Table S1 Linear B cell c‐TXNPx epitopes predicted by Bepipred 2.0.
Table S2 Conformational B‐cell epitope prediction performed by Discotope 2.0.
Acknowledgements
This work was supported by the Consejo Nacional de Investigaciones Científicas y Tecnológicas (CONICET; Grant number 112‐200801‐02915) and by the Agencia Nacional de Promoción Científica y Tecnológica (ANPCyT; Grant number 2014‐1026) for KAG and by Fundación AJ. ROEMMERS for GRA. We thank all the patients and non‐infected individuals who participated in this study. We are also grateful to the staff of the Instituto Nacional de Parasitología ‘Doctor Mario Fatala Chabén’, Buenos Aires, Argentina, Violeta Chiauzzi from Instituto de Biología y Medicina Experimental (IBYME‐CONICET) and Sofía Micolini from Instituto de Ingeniería Genética y Biología Molecular ‘Prof. Héctor N. Torres’ (INGEBI‐CONICET) for technical assistance in blood sample collection. We express our gratitude to Edward A. Valera‐Vera from Laboratorio de Parasitología Molecular, Instituto de Investigaciones Alfredo Lanari for his contribution with regard to the implementation of the pyMOL software and to Micaela Ossowski from Instituto de Ingeniería Genética y Biología Molecular (INGEBI‐CONICET) for her graphic art.
Contributor Information
Magalí C. Girard, Email: mgirard@dna.uba.ar
Karina A. Gómez, Email: gomez@dna.uba.ar.
References
- 1. Cardoso MS, Reis‐Cunha JL, Bartholomeu DC. Evasion of the immune response by Trypanosoma cruzi during acute infection. Front Immunol 2015; 6:659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Machado FS, Dutra WO, Esper L, Gollob KJ, Teixeira MM, Factor SM et al Current understanding of immunity to Trypanosoma cruzi infection and pathogenesis of Chagas disease. Semin Immunopathol 2012; 34:753–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Trujillo M, Budde H, Piñeyro MD, Stehr M, Robello C, Flohé L et al Trypanosoma brucei and Trypanosoma cruzi tryparedoxin peroxidases catalytically detoxify peroxynitrite via oxidation of fast reacting thiols. J Biol Chem 2004; 279:34175–82. [DOI] [PubMed] [Google Scholar]
- 4. Piacenza L, Peluffo G, Alvarez MN, Kelly JM, Wilkinson SR, Radi R. Peroxiredoxins play a major role in protecting Trypanosoma cruzi against macrophage‐and endogenously‐derived peroxynitrite. Biochem J 2008; 410:359–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wilkinson SR, Temperton NJ, Mondragon A, Kelly JM. Distinct mitochondrial and cytosolic enzymes mediate trypanothione‐dependent peroxide metabolism in Trypanosoma cruzi . J Biol Chem 2000; 275:8220–5. [DOI] [PubMed] [Google Scholar]
- 6. Piñeyro MD, Parodi‐Talice A, Arcari T, Robello C. Peroxiredoxins from Trypanosoma cruzi: virulence factors and drug targets for treatment of Chagas disease? Gene 2008; 408:45–50. [DOI] [PubMed] [Google Scholar]
- 7. Piñeyro MD, Arcari T, Robello C, Radi R, Trujillo M. Tryparedoxin peroxidases from Trypanosoma cruzi: high efficiency in the catalytic elimination of hydrogen peroxide and peroxynitrite. Arch Biochem Biophys 2011; 507:287–95. [DOI] [PubMed] [Google Scholar]
- 8. MaD Piñeyro, Pizarro JC, Lema F, Pritsch O, Cayota A, Bentley GA et al Crystal structure of the tryparedoxin peroxidase from the human parasite Trypanosoma cruzi . J Struct Biol 2005; 150:11–22. [DOI] [PubMed] [Google Scholar]
- 9. Gadelha F, Gonçalves C, Mattos E, Alves M, Pineyro M, Robello C et al Release of the cytosolic tryparedoxin peroxidase into the incubation medium and a different profile of cytosolic and mitochondrial peroxiredoxin expression in H2O2‐treated Trypanosoma cruzi tissue culture‐derived trypomastigotes. Exp Parasitol 2013; 133:287–93. [DOI] [PubMed] [Google Scholar]
- 10. Piacenza L, Peluffo G, Alvarez MN, Martínez A, Radi R. Trypanosoma cruzi antioxidant enzymes as virulence factors in Chagas disease. Antioxid Redox Signal 2013; 19:723–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Alvarez MN, Peluffo G, Piacenza L, Radi R. Intraphagosomal peroxynitrite as a macrophage‐derived cytotoxin against internalized Trypanosoma cruzi consequences for oxidative killing and role of microbial peroxiredoxins in infectivity. J Biol Chem 2011; 286:6627–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Atwood J, Weatherly D, Minning T, Bundy B, Cavola C, Opperdoes F et al The Trypanosoma cruzi proteome. Science 2005; 309:473–6. [DOI] [PubMed] [Google Scholar]
- 13. Parodi‐Talice A, Monteiro‐Goes V, Arrambide N, Avila A, Duran R, Correa A et al Proteomic analysis of metacyclic trypomastigotes undergoing Trypanosoma cruzi metacyclogenesis. J Mass Spectrom 2007; 42:1422–32. [DOI] [PubMed] [Google Scholar]
- 14. Piacenza L, Alvarez MN, Peluffo G, Radi R. Fighting the oxidative assault: the Trypanosoma cruzi journey to infection. Curr Opin Microbiol 2009; 12:415–21. [DOI] [PubMed] [Google Scholar]
- 15. Zago MP, Hosakote YM, S‐j Koo, Dhiman M, Piñeyro MD, Parodi‐Talice A et al TcI isolates of Trypanosoma cruzi exploit the antioxidant network for enhanced intracellular survival in macrophages and virulence in mice. Infect Immun 2016; 84:1842–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ishii T, Warabi E, Yanagawa T. Novel roles of peroxiredoxins in inflammation, cancer and innate immunity. J Clin Biochem Nutr 2012; 50:91–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Riddell JR, Wang X‐Y, Minderman H, Gollnick SO. Peroxiredoxin 1 stimulates secretion of proinflammatory cytokines by binding to TLR4. J Immunol 2010; 184:1022–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Donnelly S, O'Neill SM, Sekiya M, Mulcahy G, Dalton JP. Thioredoxin peroxidase secreted by Fasciola hepatica induces the alternative activation of macrophages. Infect Immun 2005; 73:166–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Donnelly S, Dalton JP, Loukas A. Proteases in Helminth‐and Allergen‐Induced Inflammatory Responses. Parasites and Allergy. 90. Basel, Switzerland: Karger Publishers, 2006: 45–64. [DOI] [PubMed] [Google Scholar]
- 20. Furuta T, Imajo‐Ohmi S, Fukuda H, Kano S, Miyake K, Watanabe N. Mast cell‐mediated immune responses through IgE antibody and Toll‐like receptor 4 by malarial peroxiredoxin. Eur J Immunol 2008; 38:1341–50. [DOI] [PubMed] [Google Scholar]
- 21. Knoops B, Argyropoulou V, Becker S, Ferté L, Kuznetsova O. Multiple roles of peroxiredoxins in inflammation. Mol Cells 2016; 39:60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Marshall ES, Elshekiha HM, Hakimi M‐A, Flynn RJ. Toxoplasma gondii peroxiredoxin promotes altered macrophage function, caspase‐1‐dependent IL‐1β secretion enhances parasite replication. Vet Res 2011; 42:80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Menezes Cabral S, Leal Silvestre R, Moreira Santarém N, Costa Tavares J, Franco Silva A, Cordeiro‐da‐Silva A. A Leishmania infantum cytosolic tryparedoxin activates B cells to secrete interleukin‐10 and specific immunoglobulin. Immunology 2008; 123:555–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bern C, Montgomery SP, Herwaldt BL, Rassi A, Marin‐Neto JA, Dantas RO et al Evaluation and treatment of Chagas disease in the United States: a systematic review. JAMA 2007; 298:2171–81. [DOI] [PubMed] [Google Scholar]
- 25. Mamat U, Wilke K, Bramhill D, Schromm AB, Lindner B, Kohl TA et al Detoxifying Escherichia coli for endotoxin‐free production of recombinant proteins. Microb Cell Fact 2015; 14:57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Longhi SA, Atienza A, Prados GP, Buying A, Balouz V, Buscaglia CA et al Cytokine production but lack of proliferation in peripheral blood mononuclear cells from chronic Chagas’ disease cardiomyopathy patients in response to T. cruzi ribosomal P proteins. PLoS Negl Trop Dis 2014; 8:e2906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Levy GV, Tasso LM, Longhi SA, Rivello HG, Kytö V, Saukko P et al Antibodies against the Trypanosoma cruzi ribosomal P proteins induce apoptosis in HL‐1 cardiac cells. Int J Parasitol 2011; 41:635–44. [DOI] [PubMed] [Google Scholar]
- 28. Larsen JEP, Lund O, Nielsen M. Improved method for predicting linear B‐cell epitopes. Immunome Res 2006; 2:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Haste Andersen P, Nielsen M, Lund O. Prediction of residues in discontinuous B‐cell epitopes using protein 3D structures. Protein Sci 2006; 15:2558–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Team RC . R: A language and environment for statistical computing, 2013.
- 31. Bates DM. lme4: Mixed‐effects modeling with R, 2010.
- 32. de Oliveira Mendes TA, Cunha JLR, de Almeida Lourdes R, Luiz GFR, Lemos LD, dos Santos ARR et al Identification of strain‐specific B‐cell epitopes in Trypanosoma cruzi using genome‐scale epitope prediction and high‐throughput immunoscreening with peptide arrays. PLoS Negl Trop Dis 2013; 7:e2524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Acevedo GR, Longhi SA, Bunying A, Sabri N, Atienza A, Zago MP et al Methodological approach to the ex vivo expansion and detection of T. cruzi‐specific T cells from chronic Chagas disease patients. PLoS ONE 2017; 12:e0178380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Nogueira FB, Ruiz JC, Robello C, Romanha AJ, Murta SM. Molecular characterization of cytosolic and mitochondrial tryparedoxin peroxidase in Trypanosoma cruzi populations susceptible and resistant to benznidazole. Parasitol Res 2009; 104:835–44. [DOI] [PubMed] [Google Scholar]
- 35. Wilkinson SR, Kelly JM. The role of glutathione peroxidases in trypanosomatids. Biol Chem 2003; 384:517–25. [DOI] [PubMed] [Google Scholar]
- 36. Abel LC, Rizzo LV, Ianni B, Albuquerque F, Bacal F, Carrara D et al Chronic Chagas’ disease cardiomyopathy patients display an increased IFN‐γ response to Trypanosoma cruzi infection. J Autoimmun 2001; 17:99–107. [DOI] [PubMed] [Google Scholar]
- 37. Ribeirao M, Lücia V, Ferguson PC, Rénia L, Fragata Filho AA, Schenkman S. Chagasic patients develop a type 1 immune response to Trypanosoma cruzi trans‐sialidase. Parasite Immunol 2000; 22:49–53. [DOI] [PubMed] [Google Scholar]
- 38. Michailowsky V, Luhrs K, Rocha MOC, Fouts D, Gazzinelli RT, Manning JE. Humoral and cellular immune responses to Trypanosoma cruzi‐derived paraflagellar rod proteins in patients with Chagas’ disease. Infect Immun 2003; 71:3165–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Lorena V, Lorena I, Braz S, Melo A, Melo M, Melo M et al Cytokine levels in serious cardiopathy of Chagas disease after in vitro stimulation with recombinant antigens from Trypanosoma cruzi . Scand J Immunol 2010; 72:529–39. [DOI] [PubMed] [Google Scholar]
- 40. de Melo AS, de Lorena VMB, de Moura Braz SC, Docena C, de Miranda Gomes Y. IL‐10 and IFN‐γ gene expression in chronic Chagas disease patients after in vitro stimulation with recombinant antigens of Trypanosoma cruzi . Cytokine 2012; 58:207–12. [DOI] [PubMed] [Google Scholar]
- 41. Morán‐Utrera Y, López‐Monteon A, Rosales‐Encina JL, Méndez‐Bolaina E, Ramos‐Ligonio A. Trypanosoma cruzi SSP4 amastigote protein induces expression of immunoregulatory and immunosuppressive molecules in peripheral blood mononuclear cells. J Trop Med 2012; 2012:829139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Leng SX, McElhaney JE, Walston JD, Xie D, Fedarko NS, Kuchel GA. ELISA and multiplex technologies for cytokine measurement in inflammation and aging research. J Gerontol A Biol Sci Med Sci 2008; 63:879–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Anthony DD, Milkovich KA, Zhang W, Rodriguez B, Yonkers NL, Tary‐Lehmann M et al Dissecting the T cell response: proliferation assays vs. cytokine signatures by ELISPOT. Cells 2012; 1:127–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Sallusto F, Geginat J, Lanzavecchia A. Central memory and effector memory T cell subsets: function, generation, and maintenance. Annu Rev Immunol 2004; 22:745–63. [DOI] [PubMed] [Google Scholar]
- 45. Fiuza JA, Fujiwara RT, Gomes JAS, das Costa Rocha MO, Chaves AT, de Araújo FF et al Profile of central and effector memory T cells in the progression of chronic human Chagas disease. PLoS Negl Trop Dis 2009; 3:e512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Villanueva‐Lizama LE, Cruz‐Chan JV, Aguilar‐Cetina AdC, Herrera‐Sanchez LF, Rodriguez‐Perez JM, Rosado‐Vallado ME et al Trypanosoma cruzi vaccine candidate antigens Tc24 and TSA‐1 recall memory immune response associated with HLA‐A and‐B supertypes in Chagasic chronic patients from Mexico. PLoS Negl Trop Dis 2018; 12:e0006240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Bankovich AJ, Shiow LR, Cyster JG. CD69 suppresses sphingosine 1‐phosophate receptor‐1 (S1P1) function through interaction with membrane helix 4. J Biol Chem 2010; 285:22328–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. González‐Amaro R, Cortés JR, Sánchez‐Madrid F, Martín P. Is CD69 an effective brake to control inflammatory diseases? Trends Mol Med 2013; 19:625–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Campos‐Neto A, Porrozzi R, Greeson K, Coler RN, Webb JR, Seiky YA et al Protection against cutaneous leishmaniasis induced by recombinant antigens in murine and nonhuman primate models of the human disease. Infect Immun 2001; 69:4103–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Fereig RM, Kuroda Y, Terkawi MA, Mahmoud ME, Nishikawa Y. Immunization with Toxoplasma gondii peroxiredoxin 1 induces protective immunity against toxoplasmosis in mice. PLoS ONE 2017; 12:e0176324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Daifalla NS, Bayih AG, Gedamu L. Differential immune response against recombinant Leishmania donovani peroxidoxin 1 and peroxidoxin 2 proteins in BALB/c mice. J Immunol Res 2015; 2015:348401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Bontempi I, Fleitas P, Poato A, Vicco M, Rodeles L, Prochetto E et al Trans‐sialidase overcomes many antigens to be used as a vaccine candidate against Trypanosoma cruzi . Immunotherapy 2017; 9:555–65. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Gating strategy used to determine HLA‐DR and CD69 expression on T cells (CD3+ CD4+ or CD3+ CD8+) and B cells (CD3− CD19+).
Figure S2. Relation between humoral response against cytosolic tryparedoxin peroxidase (c‐TXNPx) and Trypanosoma cruzi in asymptomatic (a) and cardiac patients (b).
Figure S3. Proliferation of peripheral blood mononuclear cells (PBMC) stimulated with cytosolic tryparedoxin peroxidase (c‐TXNPx) (circles) or mock (squares) and Trypanosoma cruzi lysate (Tc; circles) or no‐antigen (NA; squares) in asymptomatic (n = 11, blue symbols; a,d), or cardiac (n = 11, red symbols; b,e) patients and non‐infected individuals (n = 8, grey symbols; c,f) assessed by [3H]thymidine uptake after 5 days incubation period for each condition.
Figure S4. Interferon‐γ (IFN‐γ) (a–f), granulocyte–macrophage colony‐stimulating factor (GM‐CSF) (g–i) and interleukin‐10 (IL‐10) (j–l) secretion in supernatants of culture upon 5 days stimulation with cytosolic tryparedoxin peroxidase (c‐TXNPx) (circles) or mock (squares) and Trypanosoma cruzi lysate (Tc; circles) or no‐antigen (NA; squares) in asymptomatic (n = 11; blue symbols) or cardiac (n = 11; red symbols) patients and non‐infected individuals (n = 8, grey symbols).
Figure S5. Relation between cellular response against cytosolic tryparedoxin peroxidase (c‐TXNPx) and Trypanosoma cruzi. Correlation analysis between proliferation or interferon‐γ (IFN‐γ) secretion, respectively upon c‐TXNPx and T. cruzi in asymptomatic (a and c) and cardiac (b and d) patients.
Table S1 Linear B cell c‐TXNPx epitopes predicted by Bepipred 2.0.
Table S2 Conformational B‐cell epitope prediction performed by Discotope 2.0.


