Abstract
Concurrent multiple tumors developing in the spinal cord are rare, except for in genetic disorders, such as neurofibromatosis and von Hippel-Lindau disease. Furthermore, concurrent tumors arising in the same spinal level with discrete histopathology are much rarer. We report two such cases. Case 1: A 53-year-old man presented with intracranial hemorrhage that manifested as disturbed consciousness and right hemiparesis. Magnetic resonance (MR) angiography demonstrated severe stenosis of the terminal portion of the bilateral internal carotid arteries, implying Moyamoya disease. Cranial MR images showed a hematoma in the left basal ganglia perforating into the lateral ventricle, which was incidentally detected as a spinal tumor compressing the cervical cord at the C2 level. After conservative management for cerebral hemorrhage, the patient underwent total removal of the spinal tumor. Surgical findings showed that the tumor consisted of extra- and intradural components. Histopathological findings showed that the extra- and intradural components were schwannoma and meningioma, respectively. Case 2: A 70-year-old man presented with progressive left hemiparesis and numbness in both lower extremities. Craniocervical MR images demonstrated a paraspinal tumor compressing the spinal cord at C2 level. Surgical findings disclosed that the tumor consisted of major extradural- and minor intradural components. Histopathological study showed that these components had discrete histological findings: extradural lesion was schwannoma and intradural lesion was meningioma. Concurrent tumors with discrete histopathology should be considered in tumors with extra- and intradural components, particularly, when they are located in the high cervical spine.
Keywords: concurrent tumor, extradural, intradural, meningioma, schwannoma
Introduction
Multiple primary spinal tumors are rare, except for in genetic disorders, such as neurofibromatosis and von Hippel-Lindau disease.1–4) Moreover, multiple tumors with discrete histological types arising in the same spinal level are significantly rarer, with only six cases reported to date.1–6) In this study, we report two rare cases of concurrent tumors consisting of meningioma and schwannoma arising in the C2 level of the spine.
Case Report
Case 1
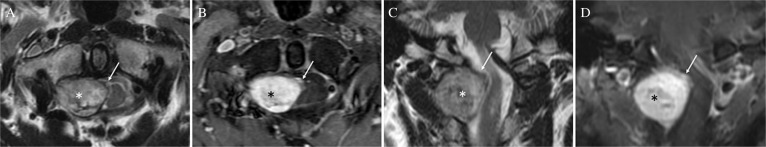
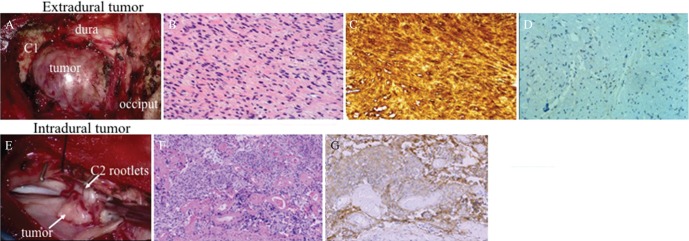
A 53-year-old man presented with intracerebral hemorrhage that manifested as disturbed consciousness and right hemiparesis. Magnetic resonance (MR) images of the brain showed a hematoma in the left basal ganglia perforating into the lateral ventricle, and a spinal tumor at the C2 level was incidentally found. MR angiography showed severe stenosis of the terminal portion of the bilateral internal cerebral arteries, implying moyamoya disease. Conservative treatment resulted in good symptom recovery, except for residual mild cognitive disorder. A coronal section of T2-weighted MR images of the cervical spine showed distortion of the cervical cord with intramedullary signal change. Axial T2-weighted MR images suggested the tumor has two components: extradural round-shaped mass and intradural crescent-shaped extramedullary mass. The extradural mass showed mixed signal intensity, while the intradural lesion was depicted as low signal intensity on T2-weighted MR images (Figs. 1A and 1B). On T1-weighted MR images after gadolinium-diethylenetriamine pentaacetic acid (gadolinium-DTPA) administration, both portions were heterogeneously enhanced (Figs. 1C and 1D). Surgery was performed for the asymptomatic spinal lesion following the patient’s request. The patient was placed under general anesthesia and in the prone position. The tumor was removed through C1–C2 laminectomy. Surgical findings revealed that the extradural component was derived from the right C2 nerve root, and the intradural tumor was attached to the dura and involved several C2 rootlets. We performed subcapsular removal of the extradural tumor. Meanwhile, the intradural tumor was detached from the dura and totally removed. Intraoperative findings showed that the dura mater completely separated the extra- and intratumors (Figs. 2A and 2E). Histopathologically, the extradural tumor showed two patterns of cellular arrangements: (1) compactly arranged proliferation of spindle cells with occasional nuclear palisading and (2) loosely arranged cells with round to oval hyperchromatic nuclei and moderate amount of eosinophilic cytoplasm. The tumor cells were diffusely positive for S-100 protein and negative for epithelial membrane antigen (EMA). Vascular endothelial growth factor (VEGF) was weakly and focally expressed around the vessels. These findings were consistent with the histological characteristics of schwannoma (Figs. 2B–2D). Meanwhile, the intradural tumor consisted of the nest of meningothelial cells and psammoma bodies. Immunohistochemically, the tumor cells were positive for EMA and non-reactive for S-100 protein. These histological features were consistent with that of meningioma (Figs. 2F and 2G). Intraoperative and histopathological findings demonstrated that the patient had concurrently two histologically distinct tumors at the same spinal level. Postoperative MR images showed sufficient decompression of the cervical spinal cord. The patient was discharged from the hospital without additional neurological symptoms.
Fig. 1.
Magnetic resonance (MR) images of the cervical spine showing extra- (*) and intradural extramedullary mass (white arrow). (A) Axial T2-weighted MR image depicting the extradural mass as mixed signal intensity and the intradural lesion as low signal intensity. (B) Coronal view of T2-weighted MR image showing distortion of the cervical cord with intramedullary signal change. (C) and (D) Axial (C) and coronal (D) view of postcontrast T1-weighted MR revealed that both portions were heterogeneously enhanced after Gd-DTPA administration.
Fig. 2.
(A) Intraoperative photograph showing the extradural tumor compressing the dural theca between the occiput and C1. (B) Histopathological examination of the extradural tumor via H&E staining showed compactly arranged proliferation of spindle cells with occasional nuclear palisading and loosely arranged cells with round to oval hyperchromatic nuclei. (C) Immunohistochemical study showed that the tumor cells were diffusely positive for S-100 protein. These findings were consistent with histological characteristics of schwannoma. (D) Vascular endothelial growth factor (VEGF) was weakly and focally expressed around the vessels. (E) Intraoperative image showed that the intradural tumor was attached to the dura and involved several C2 rootlets. (F) Histopathological examination of the intradural tumor via H&E staining showed that it consists of the nest of meningothelial cells and psammoma bodies. (G) Immunohistochemically, the tumor cells were positive for epithelial membrane antigen. These histological features were in accordance with the histopathological characteristic of meningioma.
Case 2
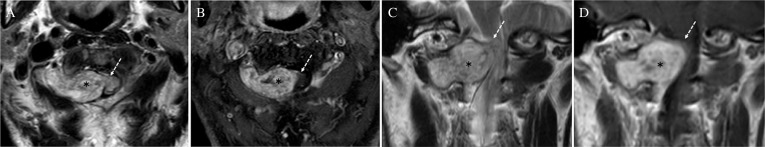
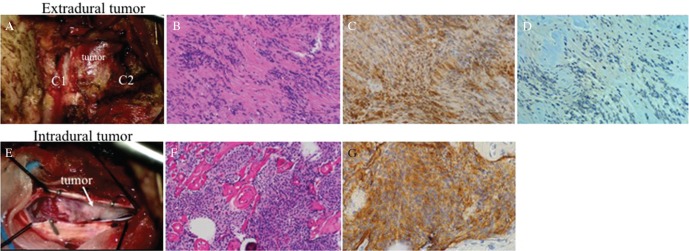
A 70-year-old man presented with myelopathy manifested as progressive left hemi-weakness and numbness in both lower limbs. Craniocervical MR images demonstrated a paraspinal tumor compressing the spinal cord at C2 level. Axial T2-weighted MR images suggested that the tumor consisted of two components: extradural dumbbell-shaped part and intradural crescent part. Both parts showed similar signal intensity: mixed signal intensity on T2-weighted MR images and heterogeneous high signal intensity with post-contrast T1-weighted images (Figs. 3A–3D). Surgical resection via right-sided suboccipital craniectomy combined with helmilaminectomy of C1 to C2 revealed that the extradural component was derived from C2 nerve root, and the intradural tumor was attached to the ventral dura matter (Figs. 4A and 4E). Gross total resection of the tumor achieved effective decompression of the spinal cord. Histopathological examination showed that extradural component was schwannoma and intradural lesion being meningioma (Figs. 4B–D, F, and G). Immunohistochemically, schwannoma cells were positive for S-100 protein and negative for EMA. Expression of VEGF was focally seen around the vessels in schwannoma component (Figs. 4C and 4D). Meningioma cells were immunoreactive for EMA and focally reactive for S-100 protein (Fig. 4G). Postoperative MR images confirmed decompression of the spinal cord. The patient showed good recovery and was discharged from the hospital.
Fig. 3.
Magnetic resonance (MR) images of the cervical spine showing extra- (*) and intradural extramedullary mass (white dashed arrow). (A) Axial T2-weighted MR image depicting the extradural- and the intradural mass lesions with mixed signal intensity. (B) Coronal view of T2-weighted MR image showing the tumor (*) compressing the cervical cord. (C) and (D) Axial (C) and coronal (D) view of postcontrast T1-weighted MR images revealed that extra- and intra-dural mass lesions were heterogeneously enhanced after Gd-DTPA administration.
Fig. 4.
(A) Intraoperative photograph showing the extradural tumor developing outside of the laminae between C1 and C2. (B) Histopathological examination of the extradural tumorstaining showed proliferation of the bipolar spindle cells arranged dominantly in compact Antoni A and also loose Antoni B with round to oval hyperchromatic nuclei and moderate amount of eosinophilic cytoplasm (H&E staining). These findings suggested schwannoma. (C) The tumor cells were immunoreactive for S-100 protein. These findings were consistent with histological characteristics of schwannoma. (D) VEGF was weakly and focally expressed around the vessels. (E) Intraoperative image showed that the intradural tumor was small and attached to the dura. (F) Histopathological examination of the intradural tumor staining showed that it consists of the nest of meningothelial cells and psammoma bodies (H&E). These findings were consistent with the characteristics of meningioma. (G) Immunohistochemically, the tumor cells were positive for epithelial membrane antigen. This finding supports this lesion being meningioma.
Discussion
Schwannomas and meningiomas are representative spinal tumors, comprising 30% and 25% of all spinal cord tumors, respectively.3–5) Approximately, 75% of schwannomas arise in the intradural extramedullary region. A total of 15% of the lesions are found exclusively in the extradural area, and the rest have both intra- and extradural components.7) Regarding tumor origin, 70% of schwannomas are derived from the sensory nerve rootlets, 20% from the motor nerve rootlets, and the rest are derived from both sensory and motor rootlets.7–8) Schwannomas are more frequently found in the high cervical region, i.e., C1–C3 levels, than in other spinal levels. Among the tumors arising in the high cervical spine, those arising from the C2 nerve root are the most common, comprising 15% of all spinal schwannomas.7) Meanwhile, spinal meningiomas account for 12% of all meningiomas and 25% of all spinal cord tumors. Approximately, 80% of meningiomas are found in the thoracic spine and 15% are in the cervical spine; meanwhile, tumors rarely develop in the lumbosacral region.4,9)
Differential diagnosis of schwannoma and meningioma based on MR images has been long debated. Schwannomas are typically well-circumscribed tumors derived from the peripheral nerve.3) Approximately 10–15% of schwannomas are dumbbell shaped. Schwannomas are usually depicted as iso- to hypo signal intensity on T1-weighted MR images and hyper- or mixed-signal intensity on T2-weighted MR images.1,3,10) Meanwhile, in contrast-enhanced MR images (T1-CE), the tumor is well enhanced but often shows ring-like or irregular enhancement depending on the degenerative changes within the tumor.1,10–12) By contrast, meningiomas tend to be small, single tumors and are typically found in the intradural extramedullary region.1,3) Approximately 10% of spinal meningiomas coexist with other tumors in the intra- and extradural location, and a few cases located in the extradural space have been reported as well. Meningiomas appear as iso- to slight hypo-signal intensity on T1-weighted MR images. Meanwhile, T2-weighted MR images depict the tumor as iso- to slight hyper-signal intensity mass lesion.1,3) The tumor is homogeneously enhanced on postcontrast T1-weighted MR images and may be accompanied with dural tail sign.10,12) Accordingly, signal intensity on T2-weighted MR images and enhancement pattern on postcontrast T1-weighted MR images might be helpful to differentiate these tumors. The spatial relation between the tumor and the spinal cord might be also helpful for imaging diagnosis. Meningiomas often arise posterolateral to the spinal cord in the thoracic spine and anterolateral to the cervical cord. By contrast, schwannomas generally originate from the dorsal rootlets and are usually found in the dorsal or dorsolateral side to the spinal cord.13) In the present case, T2-weighted MR images depicted the extradural part as mixed signal intensity and the intradural component as low signal intensity. Retrospectively, these differences in the signal intensity suggested that the extra- and intradural components had discrete histology. However, we could not anticipate this rare instance pre- and intraoperatively.
Multiple primary spinal cord tumors are rare, and only 1.2–9.5% of such tumors arise in patients with neurofibromatosis.2) Excluding the cases with neurofibromatosis, only six cases of concurrent spinal cord tumors with different histology in the same spinal level have been reported in the literature. In this paper, we report the additional two cases of such rare instance (Table 1). The patients’ mean age was 59.4 years, and no apparent preponderance in sex was noted. All spinal tumors were distributed in the cervical spine and seven out of the eight lesions were found above the C3 level.1–6) Extradural components consisted of seven schwannomas and single neurofibroma. Notably, all intradural tumors were meningiomas.
Table 1.
Reported cases of concurrent spinal tumors without neurofibromatosis/von Hippel Lindau disease in the same spinal level
| Author | Year | Age/sex | Spinal level | Tumor histology | |
|---|---|---|---|---|---|
| Extradural lesion | Intradural lesion | ||||
| Hokari et al.2) | 2002 | 59/F | C2 | Neurofibroma | Meningioma |
| Ogihara et al.6) | 2003 | 54/F | C5 | Schwannoma | Meningioma |
| Nakamizo et al.3) | 2011 | 49/M | C2 | Schwannoma | Meningioma |
| Chen et al.1) | 2013 | 72/F | C3–C4 | Schwannoma | Meningioma |
| Oichi et al.5) | 2015 | 64/M | C2 | Schwannoma | Meningioma |
| Liebelt et al.4) | 2016 | 58/M | CCJ | Schwannoma | Meningioma |
| Present case 1 | 2017 | 49/M | C2 | Schwannoma | Meningioma |
| Present case 2 | 2017 | 70/M | C2 | Schwannoma | Meningioma |
C: cervical spine, CCJ: craniocervical junction, F: female, M: male.
The pathophysiology of different tumors concurrently arising in the same spinal level has remained unclear. Three possible theories have been formulated to explain their occurrence.1–6) The first theory is that de novo tumor might be induced by microenvironmental factors influenced by the preexisting tumor.2,3,5) As a possible microenvironmental factor, we examined the expression level of VEGF, which is known to be related to angiogenesis and tumor progression.14,15) Because the expression of VEGF was limited just around the vessels in both schwannomas, the role of VEGF in concurrent tumors was inconclusive in this report. Further evaluation of the expression of tumorigenesis-related cytokines might play a role in elucidating the pathophysiology of concurrent tumors. The difference of the volume between two components may be partially explained by the first theory. According to the literature, all intradural meningiomas were significantly smaller than extradural schwannoma/neurofibromas.1–6) This fact seems to support the hypothesis that preexisting large schwannoma/neurofibroma induces small de novo meningioma. The second theory is explained from the embryological perspective. Common mesenchymal progenitor cells may separately differentiate into schwannoma cells and meningioma cells in the same spinal level.2–5) The third hypothesis is that this rare entity develops only due to incidence.2,3,5) Schwannomas and meningiomas are two of the most common tumors developing in the spinal cord, comprising 55% of all extramedullary tumors.16) They may accidentally arise at the same spine level in very rare occasions.2,3,5)
In the present two cases, our preoperative diagnosis was dumbbell-shaped schwannoma with a dominantextradural component. However, histological examination showed the presence of discrete tumors at the same spinal level. Such tumors might have been undetected in the past cases of dumbbell-type schwannomas probably because the intradural meningiomas are usually small lesions compared to the extradural schwannomas. Chen et al. have advocated the necessity to open the dural sac and explore intradural space because of the unexpected lesion.1) The possibility of concurrent multiple tumors, particularly in tumors with extra- and intradural components in high cervical spine, should always be considered.
Footnotes
Conflicts of Interest Disclosure
The author has no conflicts of interest regarding this manuscript and has registered online self-reported COI Disclosure Statement Forms through the website for the Japan Neurosurgical Society (JNS) members.
References
- 1).Chen KY, Wu JC, Lin SC, Haung WC, Cheng H: Coexistence of neurofibroma and meningioma at exactly the same level of the cervical spine. J Chi Med Assoc 77: 594–597, 2014 [DOI] [PubMed] [Google Scholar]
- 2).Hokari M, Hida K, Ishii N, Seki T, Iwasaki Y, Nakamura N: Associated meningioma and neurofibroma at the same cervical level without clinical signs of neurofibromatosis: case report. No Shinkei Geka 30: 953–957, 2002. (Japanese) [PubMed] [Google Scholar]
- 3).Nakamizo A, Suzuki SO, Shimogawa T, et al. : Concurrent spinal nerve root schwannoma and meningioma mimicking single-component schwannoma. Neuropathology 32: 190–195, 2012 [DOI] [PubMed] [Google Scholar]
- 4).Liebelt BD, Haider AS, Steele WJ, Krishna C, Blacklock JB: Spinal schwannoma and meningioma mimicking a single mass at the craniocervical junction subsequent to remote radiation therapy for acne vulgaris. World Neurosurg 93: 484.e13–e16, 2016 [DOI] [PubMed] [Google Scholar]
- 5).Oichi T, Chikuda H, Morikawa T, et al. : Concurrent spinal schwannoma and meningioma mimicking a single cervical dumbbell-shaped tumor: case report. J Neurosurg Spine 23: 784–787, 2015 [DOI] [PubMed] [Google Scholar]
- 6).Ogihara S, Seichi A, Iwasaki M, et al. : Concurrent spinal schwannomas and meningiomas. Case illustration. J Neurosurg 98: 300, 2003 [DOI] [PubMed] [Google Scholar]
- 7).Chowdhury FH, Haque MR, Sarker MH: High cervical spinal schwannoma; microneurosurgical management: an experience of 15 cases. Acta Neurol Taiwan 22: 59–66, 2013 [PubMed] [Google Scholar]
- 8).Kyoshima K, Uehara T, Koyama J, Idomori K, Yomo S: Dumbbell C2 schwannomas involving both sensory and motor rootlets: report of two cases. Neurosurgery 53: 436–439; discussion 439–440, 2003 [DOI] [PubMed] [Google Scholar]
- 9).Yoon SH, Chung CK, Jahng TA: Surgical outcome of spinal canal meningiomas. J Korean Neurosurg Soc 42: 300–304, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10).Liu WC, Choi G, Lee SH, et al. : Radiological findings of spinal schwannomas and meningiomas: focus on discrimination of two disease entities. Eur Radiol 19: 2707–2715, 2009 [DOI] [PubMed] [Google Scholar]
- 11).De Verdelhan O, Haegelen C, Carsin-Nicol B, et al. : MR imaging features of spinal schwannomas and meningiomas. J Neuroradiol 32: 42–49, 2005 [DOI] [PubMed] [Google Scholar]
- 12).Wein S, Gaillard F: Intradural spinal tumors and their mimics: a review of radiographic features. Postgrad Med J 89: 457–469, 2013 [DOI] [PubMed] [Google Scholar]
- 13).Yamaguchi S, Takeda M, Takahashi T, et al. : Ginkgo leaf sign: a highly predictive imaging feature of spinal meningioma. J Neurosurg Spine 23: 642–646, 2015 [DOI] [PubMed] [Google Scholar]
- 14).Zhang N, Chen J, Ferraro GB, et al. : Anti-VEGF treatment improves neurological function in tumors of the nervous system. Exp Neurol 299: 326–333, 2018 [DOI] [PubMed] [Google Scholar]
- 15).Gao X, Zhao Y, Stemmer-Rachamimov AO, et al. : Anti-VEGF treatment improves neurological function and augments radiation response in NF2 schwannoma model. Proc Natl Acad Sci USA 112: 14676–14681, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16).Abul-Kasim K, Thurnher MM, McKeever P, Sundgren PC: Intradural spinal tumors: current classification and MRI features. Neuroradiology 50: 301–314, 2008 [DOI] [PubMed] [Google Scholar]