Abstract
23-Hydroxybetulinic acid (23-HBA) is a complex lupane triterpenoid, which has attracted increasing attention as an anticancer agent. However, its detailed mechanism of anticancer action remains elusive so far. To reveal its anticancer mode of action, a series of fluorescent 23-HBA derivatives conjugated with coumarin dyes were designed, synthesized, and evaluated for their antiproliferative activities. Subcellular localization and uptake profile studies of representative fluorescent 23-HBA probe 26c were performed in B16F10 cells, and the results suggested that probe 26c was rapidly taken up into B10F10 cells in a dose-dependent manner and mitochondrion was the main site of its accumulation. Further mode of action studies implied that the mitochondrial pathway was involved in 23-HBA-mediated apoptosis. Together, our results provided new clues for revealing the molecular mechanism of natural product 23-HBA for its further development into an antitumor agent.
Keywords: Betulinic acid, Fluorescent probe, Antitumor, Mechanism, Mitochondrion
Natural products represent an enormous source of pharmacologically active compounds and are often used as the starting point in modern drug discovery.1 23-Hydroxybetulinic acid (23-HBA, 1, Figure 1) is a natural lupane-type pentacyclic triterpene isolated from the root of the traditional Chinese herb Pulsatilla chinensis.2 In recent years, 23-HBA has been attracting considerable attention due to its remarkable antineoplastic activities.3−6 Several reports have indicated that the anticancer mechanisms of 23-HBA or its derivatives may be associated with depolarization of the mitochondrial membrane potential (MMP) and subsequent cell apoptosis.7,8 However, the detailed molecular mechanism and targets underlying the antitumor activities of 23-HBA are still not fully understood.
Figure 1.
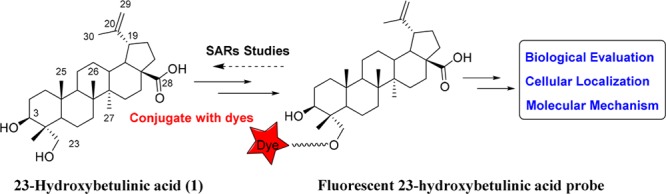
Overall strategy of fluorescent 23-HBA probe for subcellular localization and mechanism studies.
In recent years, much progress has been made in establishing methods for the identification of the cellular targets or mode of action (MOA) of small molecules,9 but current approaches mostly require lysis or rely on indirect assays. Ideal strategies that are capable of sensitive detection of biological processes in living systems (e.g., live cells, tissues, or animals) are required for the MOA studies and target proteins identification.10
Fluorescence-based cell-imaging strategies are highly useful in monitoring biological processes in living cells owing to their practicality and high sensitivity.11,12 In recent years, fluorescently labeled probes of bioactive small molecules have become powerful tools to explore biological phenomena and provide insights into the mechanism of actions of these small molecules.13−16 With the help of fluorescently labeled probes, this approach makes it applicable in imaging of drug uptake and subcellular distribution.17 Besides, it can make the drug–target complex visible by using in-cell imaging, or in-gel fluorescence scanning after the sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) separation.18
In our previous studies, we have prepared hundreds of derivatives of 23-HBA to develop novel potential anticancer therapeutics.3−6 However, the detailed anticancer mechanism, mode of action, and intracellular target of 23-HBA remain elusive. To better understand the biological mechanism and illuminate the mode of action of 23-HBA, Soural et al. employed a solid-phase synthetic approach to prepare biotinylated 23-HBA;19 however, no fluorescent 23-HBA probes have been reported so far to the best of our knowledge.
As our interest continues for developing novel fluorescent probes of natural products for mechanism of action studies,14 we herein report the synthesis, biological activities, and labeling performance of fluorescent 23-HBA probes. We expect that the novel fluorescent probes could provide an insight into understanding the subcellular localization and anticancer mechanism of 23-HBA and ultimately accelerate its development as an antitumor agent.
Various conjugation strategies have been developed to construct fluorescent probes of natural products, and the most practical method is attaching a small-molecule fluorescent probe to the tolerated position of natural products with appropriate linkers.20,21 In this work, coumarin was selected as the fluorescent probe due to its water-solubility, small size, photostability, and nontoxicity.22,23 Various linkers were employed because linker type and length may often affect the antiproliferative activities of probes and their interactions with target proteins. And above all, fluorescent probes must be attached to the compounds at a position that does not interfere with target protein binding.24 We recently synthesized hundreds of derivatives of 23-HBA and found that the C-23 hydroxy on 23-HBA can be conjugated with a bulky group without affecting its ability to inhibit cancer cell growth and therefore could be used to attach fluorescent probes.
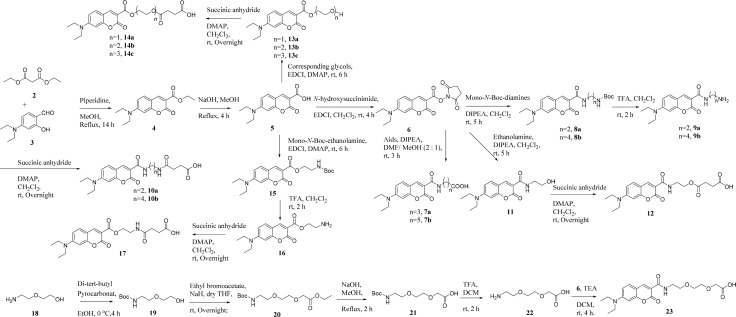
Fluorescent N,N-diethyl-7-aminocoumarin dyes were synthesized according to the route depicted in Scheme 1. Briefly, 4-(diethylamino)salicylaldehyde was reacted with diethyl malonate in the presence of catalytic piperidine to yield coumarin-3-carboxylic acid ethyl ester (4). The ester was then hydrolyzed with sodium hydroxide to obtain acid 5. Compound 6 was prepared by the reaction of 5 with N-hydroxysuccinimide in CH2Cl2. Derivatives 7a–b were synthesized by the reaction of compound 6 with corresponding amino acids while 8a–b were prepared from the corresponding mono-N-boc-diamines. Following deprotection of amino group with trifluoroacetic acid afforded 9a–b. Treated with succinic anhydride in the presence of DMAP, compounds 9a–b were converted to acids 10a–b. On the other hand, compound 5 afforded alcohols 13a–c upon reaction with corresponding glycols, which were reacted with succinic anhydride to yield subsequent compounds 14a–c.
Scheme 1. Synthesis of N, N-Diethyl-7-aminocoumarin Derivatives.
Compounds 12 and 17 were synthesized by reacting with corresponding ethanolamines following the steps described above. In addition, in order to understand the impact of water-soluble ethylene glycol linkers on antiproliferative activities of target compounds, 23 was synthesized from diglycolamine.
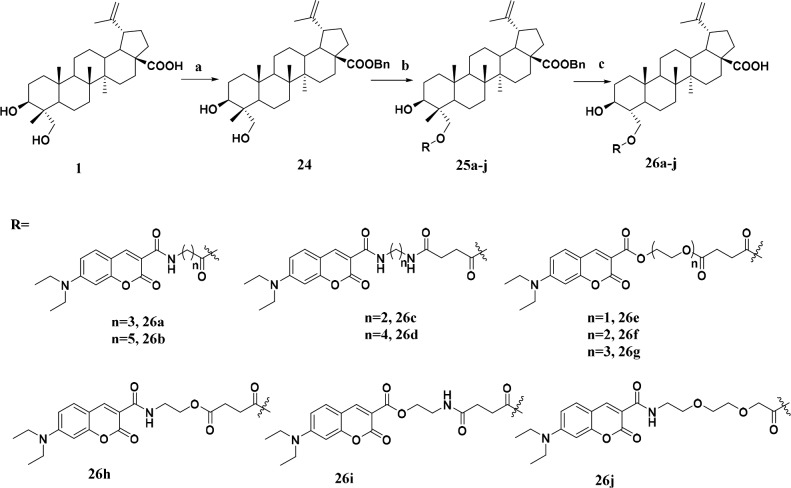
23-HBA was treated with BnBr in DMF to yield 28-benzyl-23-hydroxybetulinic ester (24, Scheme 2). Compounds 7a–b were converted to acyl chloride and then reacted with ester 24 using DMF as a catalyst in dry CH2Cl2 to give compounds 25a–b. Target compounds 26a–b were obtained by debenzylation of ester 25a–b with 10% Pd/C as catalyst under atmospheric pressure of hydrogen. Compounds 26c–j were prepared in a route similar to that for 26a–b.
Scheme 2. Synthesis of Fluorescent 23-HBA Probes 26a–j.
Reagents and conditions: (a) BnBr, K2CO3, DMF, rt, 12 h; (b) Corresponding coumarin acids, oxalyl chloride, triethylamine, cat. DMF, CH2Cl2, rt, 0.5 h; (c) H2, 10% Pd/C, THF/MeOH, 50 °C, 1 h.
Ideally, the designed natural product fluorescent probes must retain the activity of this natural product. The in vitro antiproliferative activities of newly synthesized fluorescent 23-HBA probes were evaluated on two cancer cell lines (B16F10 mice skin melanoma cells, MCF-7 human breast cancer cells) and one normal cell line (Helf human embryonic lung fibroblast cells) by MTT assay. The abilities of these new analogues to inhibit the growth of cells were summarized in Table 1. Most of the derivatives exhibited stronger antiproliferative activities than parent compound 23-HBA against three cell lines, especially on mice skin melanoma B16F10 cells. The antiproliferative activities of 26a–i showed that linkers with appropriate length and the introduction of amido groups were favorable to their anticancer activities (Table 1). The most potent derivatives 26c and 26d with lower IC50 values on two tested tumor cell lines had diamine linkers while compounds 26e–g with ester linkers only had moderate antiproliferative activities ranging from 11.78 to 35.52 μM. When compared with 26a–b, enhanced activity of 26j indicated that the introduction of water-soluble ethylene glycol linkers was beneficial to its antiproliferative activity. It should be noted that coumarin dye (10a) exhibited no obvious toxicity against cancer cell lines. Given the potent antiproliferative activity of compound 26c, it was finally selected as the candidate for further studies.
Table 1. Antiproliferative Activities of Fluorescent 23-HBA Probes against Different Cell Lines.
| Cell lines (IC50, μM)a |
|||
|---|---|---|---|
| Compounds | B16F10 | MCF-7 | Helf |
| 23-HBA | 24.46 ± 2.11 | 20.83 ± 1.46 | 31.49 ± 1.95 |
| 26a | 11.27 ± 0.92 | 18.06 ± 1.07 | 27.76 ± 0.88 |
| 26b | 12.27 ± 0.78 | 37.77 ± 1.66 | 79.65 ± 3.20 |
| 26c | 4.07 ± 0.05 | 6.26 ± 0.27 | 11.70 ± 0.85 |
| 26d | 7.77 ± 0.33 | 9.70 ± 0.49 | 17.58 ± 1.21 |
| 26e | 11.78 ± 0.56 | 19.67 ± 2.43 | 23.81 ± 2.08 |
| 26f | 35.51 ± 3.11 | 30.52 ± 3.04 | 21.40 ± 1.75 |
| 26g | 29.99 ± 3.44 | 24.23 ± 2.51 | 19.38 ± 1.38 |
| 26h | 10.53 ± 0.82 | 18.13 ± 1.72 | 25.12 ± 4.27 |
| 26i | 11.50 ± 1.81 | 19.90 ± 2.06 | 24.03 ± 1.53 |
| 26j | 5.25 ± 0.93 | 8.16 ± 0.12 | 10.85 ± 1.05 |
| 10a | >100 | ||
| ADMb | 0.19 ± 0.03 | 0.15 ± 0.02 | 0.21 ± 0.02 |
Each data point represents mean ± SD from three independent experiments.
ADM: Doxorubicin.
The fluorescent properties of compound 26c including the electronic excitation and emission spectra were evaluated first. The results showed that probe 26c exhibited significant differences between maximum excitation (λexc 415 nm) and emission (λem 475 nm) wavelength, namely Stokes shift, which was suitable for the imaging studies (Supporting Information, Figure S1). Then, the cell labeling property of the probe 26c was investigated by incubating B16F10 cells with various concentrations of 26c in a time dependent manner. The results showed that probe 26c was taken up into B16F10 cells quickly, and distinguishable fluorescence could be detected as early as 15 min after incubation with 10 μM 26c (Figure S2–5). To understand the detailed cellular uptake profile of compound 26c in live B16F10 cells, quantitative research was carried out using flow cytometry. The results showed that after incubation with 10 μM 26c, the fluorescent intensity increased quickly at the beginning (0–15 min). Then, a slow growth in fluorescent intensity was observed during the following period of time (15–120 min). Subsequently, the fluorescent intensity grew rapidly (Figure S6). Interestingly, cells stained with naked coumarin dye (10a) were used as a control and the results showed that no obvious fluorescence could be observed after 1 h staining with up to 50 μM of 10a (Figure S7). Considering the poor cell permeability and cytotoxicity of naked coumarin dye (10a), the above results implied the good cell permeability of 23-HBA.
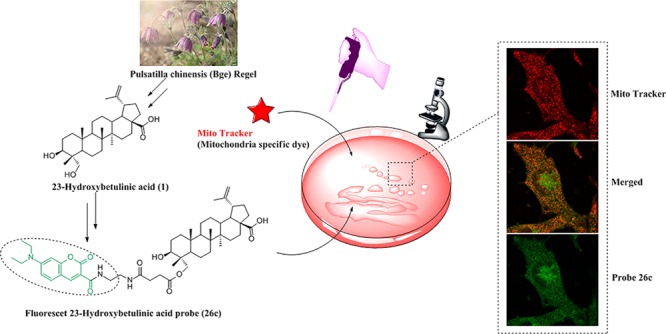
The search for cellular targets may well be regarded as a quest for a needle in a giant haystack; the traditional affinity-based proteomics (“pulldown”) method often suffers from nonspecific binders.25 To minimize unspecific binding in affinity-based approaches, lysate prefractionation may be considered. Use of a particular subcellular fraction will reduce the sample complexity and enrich the target protein. A prerequisite to the fractionation is the knowledge about subcellular localization of the binding proteins which often can be obtained through colocalization study using fluorescently labeled compound. To understand the subcellular localization of 23-HBA in living cells, probe 26c and commercially available MitoTracker (Mitochondria specific dye) were employed for colocalization studies first. Taking into account the result of qualitative and quantitative research on cellular uptake, the minimum concentration of compound 26c was optimized to 4 μM and the most appropriate incubation time was 1 h. Cells were imaged with confocal laser scanning microscopy. The location of probe 26c was viewed as green fluorescent spots upon excitation at 415 nm (Figure 2, left column, green), and the MitoTracker emitted red fluorescence at 579 nm (Figure 2, right column, red). Overlapping the fluorescent images of probe 26c and MitoTracker showed an extensive orange image in the same area (Figure 2, middle column, orange), revealing the colocalization of these two probes. This observation provided compelling evidence for the conclusion that mitochondrion is the main accumulation site of 23-HBA, which was consistent with reports revealing that 23-HBA’s antitumor mechanism is partially involved in effecting the mitochondrial pathway. On the other hand, some nonmitochondrial regions in cells (near the nucleus) where 26c is also shown to accumulate are observed in Figure 2a, indicating the possibility of other proteins targeted by 23-HBA. To explain the specificity of the probe for mitochondria, comparative studies with lysosome and nucleus specific dyes are performed and results are shown in Figure 3. Overlapping of the fluorescent images arising from 26c and LysoTracker Red (lysosome specific dye) or DAPI (nucleus specific dye) did not show obvious colocalization. As a result, fluorescent 23-HBA probes provided direct visual evidence that 23-HBA colocalized mainly with mitochondrion in a living cell system and following target identification performed with mitochondrion should be a priority.
Figure 2.
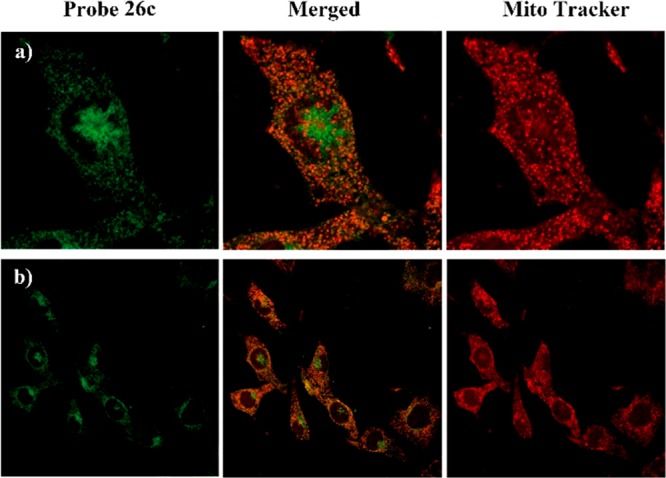
Subcellular colocalization of probe 26c with MitoTracker (mitochondria specific dye) in B16F10 cells. Images were collected using confocal microscopy with excitation of probe 26c at λex= 415 nm and emission at λem= 475 nm, MitroTracker at λex= 579 nm and emission at λem= 600 nm. Images are provided with amplification factor (a) enlarged view of representative cells, 60×, (b) 40×.
Figure 3.
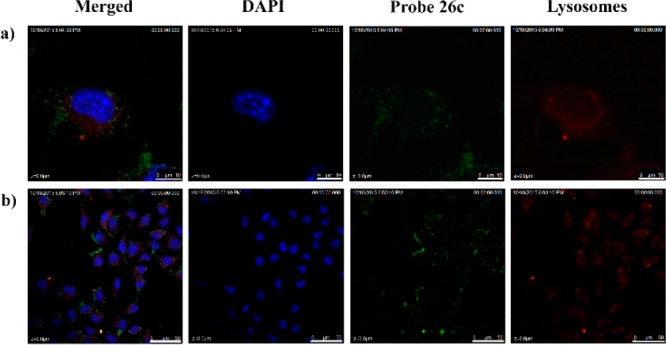
B16F10 cells were costained with 26c (4 μM) and LysoTracker Red and then analyzed under a confocal microscope. Nuclei were stained with DAPI. Blue: Nucleus; red: LysoTracker Red,; green: 26c. Micron bar: (a) 10 μm; (b) 50 μm.
Mitochondrion plays an essential role in the intrinsic pathway of apoptosis. Thus, a commercially available fluorescent probe JC-1 was used to measure the MMP of B16F10 cells affected by 23-HBA and 26c using flow cytometry. As shown in Figure 4, B16F10 cells were incubated with different concentrations of 23-HBA (0, 7.5, 15, and 30 μM) for 48 h prior to staining with the JC-1. The number of cells with collapsed MMPs in different groups were determined by flow cytometry analysis, yielding 1.79%, 10.57%, 22.85%, and 42.87% apoptotic cells, respectively. Similar decreased MMPs were also observed on B16F10 cells treated with 0, 1.5, 3.0, and 6.0 μM 26c, this result suggests that fluorescent conjugated 23-HBA intracellularly behaves similarly as the starting material 23-HBA.
Figure 4.
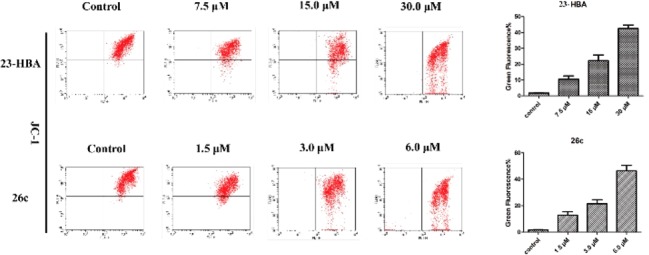
Effect of compounds 23-HBA and 26c on the mitochondrial membrane potentials of B16F10 cells. Cells were incubated with the indicated concentrations of compounds for 48 h prior to staining with JC-1.
The above results showed that 23-HBA-induced apoptosis was closely related to the mitochondrial pathway. The Bcl-2 family including pro-apoptotic proteins (e.g., Bax, Bad) and antiapoptotic proteins (e.g., Bcl-2, Bcl-xL) has been identified as essential proteins in controlling the mitochondrial pathway. To confirm whether these proteins are involved in apoptosis induced by 23-HBA, the expression of Bax, Bcl-2, cytochrome c, caspase 3, and caspase 9 was examined by Western Blot analysis. As shown in Figure 5, the expression of Bax, cytochrome c, caspase 3, and caspase 9 began to increase after the treatment of 23-HBA or 26c for 48 h, while the Bcl-2 expression decreased markedly. The above results suggested that the mitochondrial death pathway was involved in 23-HBA-induced apoptosis.
Figure 5.
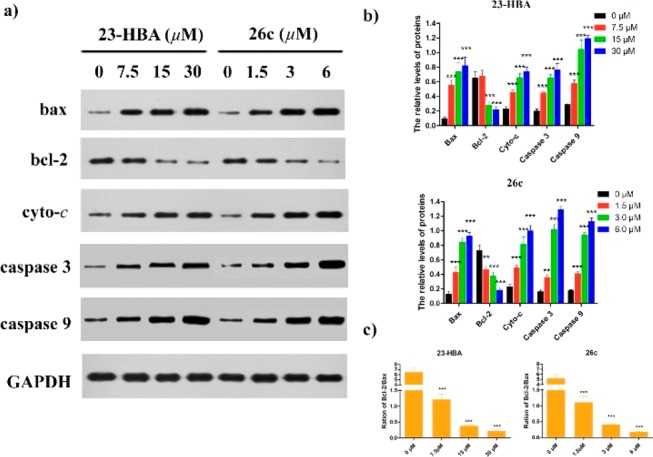
Western Blotting analysis of compounds 23-HBA and 26c on the expression of apoptosis-related proteins bax, bcl-2, cytochrome c, caspase 3, and caspase 9. GAPDH was used as the loading control. (a) B16F10 cells were treated with 23-HBA of various concentrations (0, 7.5, 15, and 30 μM) and 26c (0, 1.5, 3.0, and 6.0 μM). (b) The density ratio of proteins to GAPDH was shown as relative expression, data was represented as mean ± SD of three independent experiments. (c) The density ratio between Bcl-2 and Bax. **P < 0.01, ***P < 0.001, control compared with treated cells.
In summary, in order to reveal the mechanism of anticancer action of 23-HBA, a series of fluorescent probes of 23-HBA were designed and synthesized by connecting the 23-hydroxyl group of 23-HBA with coumarin dye through various linkers. The pharmacological activity, uptake profile, and subcellular localization of fluorescent 23-HBA probes were investigated in living cells. Qualitative and quantitative research on cellular uptake indicated that probe 26c was rapidly taken up by B16F10 cells in a dose-dependent manner. Importantly, colocalization studies with organelle specific dyes revealed that mitochondrion is the main site of its accumulation, and subsequent mode of action studies implied that the mitochondrial pathway was involved in the 23-HBA-mediated apoptosis. Overall, our findings indicate that the novel fluorescent probes of 23-HBA could be used for live cell imaging and further to elucidate the mechanisms of anticancer action of 23-HBA.
Acknowledgments
This work is supported by the National Natural Science Foundation of China (No. 81673306, 81703348) and Newton Fund (No. 201603780078).
Glossary
Abbreviations
- 23-HBA
23-hydroxybetulinic acid
- MOA
mode of action
- SDS-PAGE
sodium dodecyl sulfate-polyacrylamide gel electrophoresis
- EDCI
1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride
- DMAP
4-dimethylaminopyridine
- DMF
N, N-dimethylformamide
- THF
tetrahydrofuran
- MMP
mitochondrial membrane potential
- JC-1
5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethyl-imidacarbocyanine iodide
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acsmedchemlett.8b00321.
Experimental protocols and NMR spectra. (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Li G.; Lou H.-X. Strategies to diversify natural products for drug discovery. Med. Res. Rev. 2018, 38, 1255–1294. 10.1002/med.21474. [DOI] [PubMed] [Google Scholar]
- Ye W.-C.; Ji N.-N.; Zhao S.-X.; Liu J.-H.; Ye T.; McKervey M. A.; Stevenson P. Triterpenoids from Pulsatilla chinensis. Phytochemistry 1996, 42, 799–802. 10.1016/0031-9422(96)00043-X. [DOI] [PubMed] [Google Scholar]
- Zhu P. Q.; Bi Y.; Xu J. Y.; Li Z.; Liu J.; Zhang L. Y.; Ye W. C.; Wu X. M. Terpenoids. III: Synthesis and biological evaluation of 23-hydroxybetulinic acid derivatives as novel inhibitors of glycogen phosphorylase. Bioorg. Med. Chem. Lett. 2009, 19, 6966–6969. 10.1016/j.bmcl.2009.10.055. [DOI] [PubMed] [Google Scholar]
- Zhang H. Y.; Zhu P. Q.; Liu J.; Lin Y.; Yao H. Q.; Jiang J. Y.; Ye W. C.; Wu X. M.; Xu J. Y. Synthesis, in vitro and in vivo antitumor activity of pyrazole-fused 23-hydroxybetulinic acid derivatives. Bioorg. Med. Chem. Lett. 2015, 25, 728–732. 10.1016/j.bmcl.2014.11.058. [DOI] [PubMed] [Google Scholar]
- Zhang H. Y.; Wang Y. W.; Zhu P. Q.; Liu J.; Xu S. T.; Yao H. Q.; Jiang J. Y.; Ye W. C.; Wu X. M.; Xu J. Y. Design, synthesis and antitumor activity of triterpenoid pyrazine derivatives from 23-hydroxybetulinic acid. Eur. J. Med. Chem. 2015, 97, 235–244. 10.1016/j.ejmech.2015.04.057. [DOI] [PubMed] [Google Scholar]
- Zhang H. Y.; Zhu P. Q.; Liu J.; Yang X.; Xu S. T.; Yao H. Q.; Jiang J. Y.; Ye W. C.; Wu X. M.; Xu J. Y. Synthesis and antitumor activity of novel 3-oxo-23-hydroxybetulinic acid derivatives. Eur. J. Med. Chem. 2014, 87, 159–167. 10.1016/j.ejmech.2014.09.058. [DOI] [PubMed] [Google Scholar]
- Liu M.; Zhao X. Z.; Xiao L.; Liu G.; Liu H. Z.; Wang X. Y.; Feng X.; Lin X. K. Cytotoxicity of the compounds isolated from Pulsatilla chinensis saponins and apoptosis induced by 23-hydroxybetulinic acid. Pharm. Biol. 2015, 53, 1–9. 10.3109/13880209.2014.907323. [DOI] [PubMed] [Google Scholar]
- Yao N.; Li Y. J.; Zhang D. M.; Liu D. L.; Tang M. K.; Yiu A.; Li Y.; Chen W. M.; Lan P.; Yao Z.; Chen Z. S.; Ye W. C. B4G2 induces mitochondrial apoptosis by the ROS-Mediated opening of Ca2+-dependent permeability transition pores. Cell. Physiol. Biochem. 2015, 37, 838–852. 10.1159/000430212. [DOI] [PubMed] [Google Scholar]
- Pan S. J.; Zhang H. L.; Wang C. Y.; Yao S. C.; Yao S. Q. Target identification of natural products and bioactive compounds using affinity-based probes. Nat. Prod. Rep. 2016, 33, 612–620. 10.1039/C5NP00101C. [DOI] [PubMed] [Google Scholar]
- Fetz V.; Prochnow H.; Brönstrup M.; Sasse F. Target identification by image analysis. Nat. Prod. Rep. 2016, 33, 655–667. 10.1039/C5NP00113G. [DOI] [PubMed] [Google Scholar]
- Umezawa K.; Yoshida M.; Kamiya M.; Yamasoba T.; Urano Y. Rational design of reversible fluorescent probes for live-cell imaging and quantification of fast glutathione dynamics. Nat. Chem. 2017, 9, 279–286. 10.1038/nchem.2648. [DOI] [PubMed] [Google Scholar]
- Liu Z.; Zhou X.; Miao Y.; Hu Y.; Kwon N.; Wu X.; Yoon J. A. Reversible fluorescent probe for real-time quantitative monitoring of cellular glutathione. Angew. Chem., Int. Ed. 2017, 56, 5812–5816. 10.1002/anie.201702114. [DOI] [PubMed] [Google Scholar]
- DeGuire S. M.; Earl D. C.; Du Y.; Crews B. A.; Jacobs A. T.; Ustione A.; Daniel C.; Chong K. M.; Marnett L. J.; Piston D. W.; Bachmann B. O.; Sulikowshi G. A. Fluorescent probes of the apoptolidins and their utility in cellular localization studies. Angew. Chem., Int. Ed. 2015, 54, 961–964. 10.1002/anie.201408906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu S. T.; Luo S. S.; Yao H.; Cai H.; Miao X. M.; Wu F.; Yang D. H.; Wu X. M.; Xie W. J.; Yao H. Q.; Chen Z. S.; Xu J. Y. Probing the anticancer action of oridonin with fluorescent analogues: visualizing subcellular localization to mitochondria. J. Med. Chem. 2016, 59, 5022–5034. 10.1021/acs.jmedchem.6b00408. [DOI] [PubMed] [Google Scholar]
- Xu W.; Zeng Z.; Jiang J.-H.; Chang Y.-T.; Yuan L. Discerning the chemistry in individual organelles with small-molecule fluorescent probes. Angew. Chem., Int. Ed. 2016, 55, 13658–13699. 10.1002/anie.201510721. [DOI] [PubMed] [Google Scholar]
- Zhang X.; Ba Q.; Gu Z. N.; Guo D. L.; Zhou Y.; Xu Y. G.; Wang H.; Ye D. J.; Liu H. Fluorescent coumarin-artemisinin conjugates as mitochondria-targeting theranostic probes for enhanced anticancer activities. Chem. - Eur. J. 2015, 21, 17415–17421. 10.1002/chem.201502543. [DOI] [PubMed] [Google Scholar]
- Wu J.; Shen Q.; Wang Y.; Zhao D.; Peng C.; Li J.-X. Fluorescent probes for subcellular localization during osteoclast formation. ACS Med. Chem. Lett. 2014, 5, 911–914. 10.1021/ml500181e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi M.; Kawamura A.; Kato N.; Nishi T.; Hamachi I.; Ohkanda J. Phosphopeptide-dependent labeling of 14–3-3ζ proteins by fusicoccin-based fluorescent probes. Angew. Chem., Int. Ed. 2012, 51, 509–512. 10.1002/anie.201106995. [DOI] [PubMed] [Google Scholar]
- Soural M.; Hodon J.; Dickinson N. J.; Sidova V.; Gurska S.; Dzubak P.; Hajduch M.; Sarek J.; Urban M. Preparation of conjugates of cytotoxic lupane triterpenes with biotin. Bioconjugate Chem. 2015, 26, 2563–2570. 10.1021/acs.bioconjchem.5b00567. [DOI] [PubMed] [Google Scholar]
- Jurášek M.; Rimpelová S.; Kmoníčková E.; Drašar P.; Ruml T. Tailor-made fluorescent trilobolide to study its biological relevance. J. Med. Chem. 2014, 57, 7947–7954. 10.1021/jm500690j. [DOI] [PubMed] [Google Scholar]
- Pal A.; Ganguly A.; Chowdhuri S.; Yousuf M.; Ghosh A.; Barui A. K.; Kotcherlakota R.; Adhikari S.; Banerjee R. Bis-arylidene oxindole-betulinic acid conjugate: a fluorescent cancer cell detector with potent anticancer activity. ACS Med. Chem. Lett. 2015, 6, 612–616. 10.1021/acsmedchemlett.5b00095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hori Y.; Norinobu T.; Sato M.; Arita K.; Shirakawa M.; Kikuchi K. Development of fluorogenic probes for quick no-wash live-cell imaging of intracellular proteins. J. Am. Chem. Soc. 2013, 135, 12360–12365. 10.1021/ja405745v. [DOI] [PubMed] [Google Scholar]
- Rong L.; Liu L. H.; Chen S.; Cheng H.; Chen C. S.; Li Z. Y.; Qin S. Y.; Zhang X. Z. A coumarin derivative as a fluorogenic glycoproteomic probe for biological imaging. Chem. Commun. 2014, 50, 667–669. 10.1039/C3CC47323F. [DOI] [PubMed] [Google Scholar]
- Kanoh N. Photo-cross-linked small-molecule affinity martix as a tool for target identification of bioactive small molecules. Nat. Prod. Rep. 2016, 33, 709–718. 10.1039/C5NP00117J. [DOI] [PubMed] [Google Scholar]
- Ziegler S.; Pries V.; Hedberg C.; Waldmann H. Target identification for small bioactive molecules finding the needle in the haystack. Angew. Chem., Int. Ed. 2013, 52, 2744–2792. 10.1002/anie.201208749. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.