Abstract
A novel series of of 4-[(3-phenyl-4-aryl-2,3-dihydro-1,3-thiazol-2-ylidene)amino]benzene-1-sulfonamides (EMAC10111a–g) was synthesized and assayed toward both human carbonic anhydrase isozymes I, II, IX, and XII and cyclooxygenase isoforms. The majority of these derivatives preferentially inhibit hCA isoforms II and XII and hCOX-2 isozyme, indicating that 2,3,4-trisubstituted 2,3-dihydrothiazoles are a promising scaffold for the inhibition of hCA isozymes and of hCOX-2 enzyme. The nature of the substituent at the dihydrothiazole ring position 4 influenced the activity and selectivity toward both enzyme families. EMAC10111g resulted as the best performing compound toward both enzyme families and exhibited preferential activity toward hCA XII and hCOX-2 isozymes.
Keywords: Dual inhibitors, hCA XII, hCA II, hCOX-2, tumor, molecular docking
The potential of human carbonic anhydrases (hCAs) and human cyclooxygenase (hCOX) dual inhibitors for the treatment of cancer is an attractive yet challenging goal in the field of medicinal chemistry. CAs are a class of well-studied metalloenzymes that are widely distributed in all living organisms.1−4 These enzymes are encoded by seven different gene families, αCA, βCA, γCA, δCA, ζCA, ηCA, and θCA.5−8 Sixteen αCA isozymes have been identified in humans so far, each differing for cellular localization and tissue distribution.9 Thus, cytosolic forms (hCA I–III, VII), membrane-bound (hCA IV, IX, XII, and XIV), mitochondrial form (hCA V), and secreted (hCA VI) isozymes can be distinguished.9 The role of hCAs in the regulation of hypoxic-tumors pH has been extensively reported,4,10−19 and isoforms II, IX, and XII are validated targets for cancer therapy.20−22 On the other hand, the relevant role of hCOX 1 and 2 in different tumors has been outlined.23−26 Celecoxib, a selective hCOX-2 inhibitor, has been approved by the FDA for adjuvant treatment of patients with familial adenomatous polyposis.27 Furthermore, the role of Aspirin as an adjuvant agent in the prevention and treatment of several solid tumors has been reported.28−30 Furthermore, a COX-dependent evasion of immunity has been observed in tumors.31 Therefore, hCOX inhibitors could be considered as adjuvant agents for the therapy of different cancers. On the basis of the above, the identification of dual inhibitors of hCOX and hCA might lead to highly efficient anticancer agents, capable of simultaneously interacting with two different tumor metabolic pathways. Such compounds are intrinsically advantageous, as they are less prone to induce drug resistance and drug–drug interactions and, not last, they might increase drug-compliance. A key step for the design of dual inhibitors is represented by the identification of the common pharmacophoric features between hCAs inhibitors (hCAIs) and hCOX inhibitors (hCOXIs), particularly hCOX-2Is.
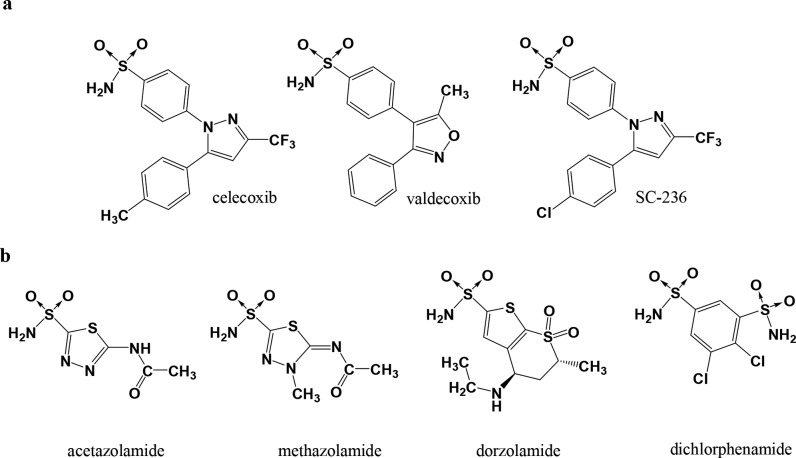
In this respect, it could be evident that the sulfonamide moiety, although with different specific roles,32−34 is a common chemical feature of several inhibitors of hCAs and hCOX-2 (Figure 1).
Figure 1.
Structurally representative sulfonamide-based (a) COX-2 selective and (b) hCA inhibitors.
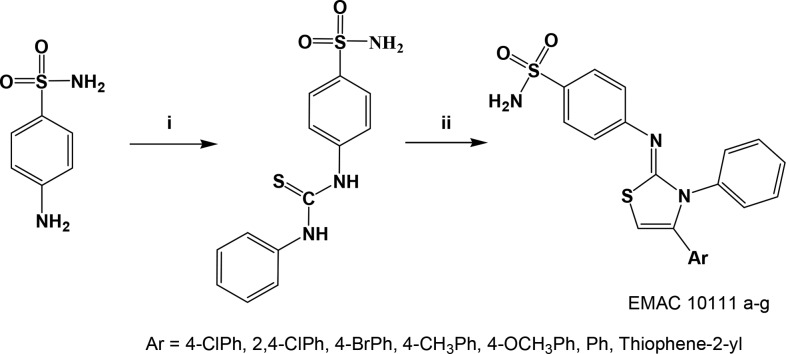
The sulfonamide moiety is widely represented within the hCAIs.33,35,36 Moreover it is a synthetically accessible and versatile scaffold that can be appropriately decorated to achieve isozyme selectivity.21,35,37−41 In the case of hCOX-2Is a benzene-sulfonamide or a benzene-sulfonyl methyl moiety is present in most of the active compounds in order to occupy a hydrophilic pocket that is made accessible mainly by the substitution of the hCOX-1 Ile523 residue by a smaller valine in hCOX-2. The substitution of the residues Ile434 and His513 of COX-1 with valine and arginine in hCOX-2, respectively, further contributes to enlarge the hydrophilic pocket and to differentiate the two isozyme inhibitors sensitivity.32 Prompted by these considerations and pursuing our studies on hCAIs,35,42−44 we have designed and synthesized a small library of 4-[(3-phenyl-4-aryl-2,3-dihydro-1,3-thiazol-2-ylidene)amino]benzene-1-sulfonamides (EMAC10111a–g) and evaluated their activity against the hCA I, II, IX, and XII isozymes as well as their inhibition activity toward hCOX 1 and 2 isoforms. These compounds represent a new example of hybrid structures with CA inhibitory activity.45,46The synthetic pathway toward compounds EMAC10111a–g consists of the reaction of equimolar amounts of 4-amidobenzensulfonamide with phenyl-isothiocyanate in refluxing 2-propanol (Scheme 1). The obtained 1-phenyl-3-(4-sulphamoylphenyl)thiourea was further reacted with the appropriate α-haloketone to give the desired compounds in good yields.
Scheme 1. Synthetic Pathway to Compounds EMAC10111a–g.
Reagents and conditions: (i) phenyl-isothiocyanate, 2-propanol, reflux; (ii), α-haloketone, RT/50 °C, 1–2 h.
Compounds EMAC10111a–g were characterized by means of analytical and spectroscopic methods (Figures S2–22 and Table S1–2) and then submitted for biological evaluation toward hCA isoforms I, II, IX, and XII and hCOX isozymes 1 and 2 (Table 1). Acetazolamide (AAZ) was chosen as reference compound for hCA activity while indomethacin, diclofenac, FR122047, nimesulide, and DuP 697 were selected as reference compounds for hCOX activity.
Table 1. Inhibition Data toward hCA I, II, IX, XII, and hCOX 1 and 2 Isozymes of Compounds EMAC10111a–g.
|
Ki(μM) |
IC50 (μM) |
||||||
|---|---|---|---|---|---|---|---|
| Compound EMAC | R | hCA I | hCA II | hCA IX | hCA XII | hCOX-1 | hCOX-2 |
| 10111a | 4-Cl | 0.49 | 0.28 | 1.25 | 0.65 | * | 16.21 |
| 10111b | 2,4-Cl | 0.75 | 0.053 | 2.78 | 0.34 | * | 18.32 |
| 10111c | 4-Br | 3.46 | 0.32 | 2.44 | 0.80 | * | 19.73 |
| 10111d | 4-CH3 | 0.96 | 0.26 | 2.26 | 0.78 | * | * |
| 10111le | 4-OCH3 | 3.37 | 0.28 | 2.25 | 0.84 | * | 21.78 |
| 10111f | H | 8.02 | 3.10 | 3.54 | 0.30 | * | * |
| 10111g | // | 9.07 | 0.86 | 3.43 | 0.06 | * | 12.61 |
| Reference compounds | |||||||
| AAZ | / | 0.25 | 0.01 | 0.02 | 0.006 | / | / |
| Indomethacin | / | / | / | / | / | 12.16 | 35.20 |
| Diclofenac | / | / | / | / | / | 18.23 | 23.62 |
| FR122047 | / | / | / | / | / | 0.09 | ** |
| Nimesulide | / | / | / | / | / | *** | 23.14 |
| DuP 697 | / | / | / | / | / | 22.61 | 0.12 |
Inactive at 25 μM (highest concentration tested). At higher concentrations, the compounds precipitate.
Inactive at 100 μM (highest concentration tested). At higher concentrations, the compound precipitates.
Inactive at 500 μM (highest concentration tested). At higher concentrations, the compound precipitates.
With respect to the hCA inhibition, the majority of EMAC derivatives exhibited a preferential activity toward the isoforms hCA II and hCA XII. Interestingly compound EMAC10111b, bearing a 2,4-dichlorophenyl moiety in the position 4 of the dihydrothiazole ring, exhibited the highest activity toward hCA II with a Ki value equal to 0.053 μM. All the other compounds, with the exception of EMAC10111f, resulted as almost equipotent in the inhibition of hCA II with Ki values ranging from 0.28 to 0.86 μM.
Compounds EMAC10111a, b, and d were the most potent for the inhibition of hCA I isoform. EMAC10111a was the most active toward hCA IX, but, on the other hand, it was demonstrated to be one of the less selective derivatives toward a specific hCA isoform, within the studied compounds. With respect to the hCA XII isoform, compound EMAC10111g, bearing a thiophene substituent in the position 4 of the dihydrothiazole ring, was identified as the most potent and selective inhibitor, with a Ki value of 0.06 μM and with a selective index (Ki hCA II/Ki hCA XII) higher than 10 fold.
When tested toward the two isoforms of hCOX, none of the new compounds exhibited activity on the hCOX-1 isozyme up to the concentration of 25 μM. Unfortunately, at higher concentrations the compounds precipitated from the test solution. On the contrary, all compounds, except for EMAC10111d and EMAC10111f, were active toward the COX-2 at concentrations comparable with those of the reference inhibitors indomethacin, diclofenac, and nimesulide.
Among the new derivatives, EMAC10111g resulted as the most potent COX-2 inhibitor, with an IC50 equal to 12.61 μM. Interestingly, EMAC10111g was the most selective inhibitor of hCA XII indicating that the presence of the thiophene group in the position 4 of the dihydrothiazole could be optimal for the interaction with both COX-2 and hCA XII. To achieve a better understanding on the recognition of EMAC10111g by both targets and to obtain useful information to further develop such compounds as tumor hCAs and COX-2 dual inhibitors, molecular modeling studies were performed. Due to the presence of the double bond between the amino benzene sulfonamide and the dihydrothiazole moieties, merging known theoretical approaches,47,48 the population of “E” and “Z” isomers was preliminarily investigated. Conformational search (Supporting Information) was carried out on both EMAC10111g isomers. The internal energy of each generated conformer was considered in a Boltzmann analysis computed at 300 K. Interestingly theoretical results indicated the “Z” isomer population of about 100%, basically discarding the presence of “E” isomer. As a consequence, further molecular modeling was carried out taking into account the (Z)-EMAC10111g configuration only.
Docking protocols were first validated with self- and cross-docking experiments. In particular, the validation highlighted the ability of all settings protocols to reproduce, with acceptable RMSD, the experimental binding mode of most ligands.
Furthermore, all methods clearly confirmed that highly selective hCOX-2Is cannot be docked inside hCOX-1.
Therefore, not surprisingly, we observed that (Z)-EMAC10111g was not able to recognize the hCOX-1 active site, thus corroborating biological results. On the contrary, the putative binding mode depicted in Figure 2 shows that the compound can be accommodated into the hCOX-2 pocket. The theoretical complex was stabilized by hydrophobic interactions with several residues such as Val89, Pro86, Leu123, Tyr115, Ala527, Val116, Tyr355, Leu531, Leu83, and Pro84. Furthermore, the sulfonamide moiety was involved in a hydrogen bond with Leu82. Finally, the aromatic moieties interacted with Lys83 and Arg120, which act as a channel gate that opens the hCOX active site.49
Figure 2.

3D representation of the putative binding mode obtained by docking experiment of (Z)-EMAC10111g into hCOX-2 and 2D representation of the complex stabilizing interactions with the binding site residues.
The hCA isoforms docking simulations were performed with a previously applied protocol.43,44 We improved cross docking validation and compared the previously utilized crystal structures with the newest and with better resolution pdb entries. While in the case of hCA XII and hCA IX we did not change the receptor, when hCA II was investigated, we considered both the previously applied 3F8E50 and the 3K34 crystal (resolution 0.9 Å),51 where the His64 shows an alternate conformation compared to the previously considered 3F8E. The new receptor model improved cross-docking RMSD results. Concerning the validation, it was observed that the docking program was able to reproduce the binding mode of the Zn2+ interacting portion, with both the ion and the other catalytic site residues. On the contrary, different binding conformations, due to the absence of anchoring residues, were observed when the solvent accessible ligand portion was docked. Furthermore, in our theoretical protocol, we considered the receptor without waters and other agents, such as glycerol and ethylene glycol, used for crystallization. Considering that these latter compounds often occupy the entrance cavity in the crystals, more space was available to accommodate the cap of docked hCAIs. The binding mode of the most promising compound (Z)-EMAC10111g in all isoforms showed how the compound was able to reach the catalytic site with the benzene sulfonamide group. Furthermore, despite the bulky cap exposed to solvent, the “Z” configuration allowed for a Y shaped geometry of the three aromatic groups that can, therefore, be accommodated in hCA II, IX, and XII. However, when docked in hCA II, the cap aromatic rings are both pushed toward one side (Figure 3c). The reason was mainly addressed to the presence of Phe131, which is replaced by an Ala by a Val in hCAXII and hCAIX respectively.
Figure 3.
3D representation of the putative binding mode obtained by docking experiment of (Z)-EMAC10111g into (a) hCA XII, (b) hCA IX, and (c) hCA II. Below each is depicted the relative 2D representation of the complexes stabilizing interactions with the residues of the binding site.
Our data indicated that 4-[(3-phenyl-4-aryl-2,3-dihydro-1,3-thiazol-2-ylidene)amino]benzene-1-sulfonamide derivatives could be considered as a promising scaffold for the dual inhibition of hCA and hCOX-2 enzymes. The information on the putative binding modes in both targets and relative isoforms of the newly synthesized inhibitors encourage us to further investigate and optimize these derivatives in order to improve their activity and selectivity.
Acknowledgments
The authors wish to acknowledge the “Ufficio Valorizzazione dei Risultati della Ricerca” of Sardegna Ricerche Technological Park, Pula (CA), Italy. The authors also thank the COST action CA15135 (Multitarget Paradigm for Innovative Ligand Identification in the Drug Discovery Process MuTaLig) for support.
Glossary
Abbreviations
- hCOX
human cyclooxygenase
- hCA
human carbonic anhydrase
- hCOXIs
human cyclooxygenase inhibitors
- hCAIs
human carbonic anhydrase inhibitors
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acsmedchemlett.8b00352.
Experimental procedures and compounds’ characterization (PDF)
Author Contributions
○ R.M. and S.D. contributed equally. The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript.
The authors declare no competing financial interest.
Supplementary Material
References
- De Monte C.; Carradori S.; Gentili A.; Mollica A.; Trisciuoglio D.; Supuran C. T. Dual Cyclooxygenase and Carbonic Anhydrase Inhibition by Nonsteroidal Anti-Inflammatory Drugs for the Treatment of Cancer. Curr. Med. Chem. 2015, 22 (24), 2812–2818. 10.2174/0929867322666150716113501. [DOI] [PubMed] [Google Scholar]
- Carbonic Anhydrase/COX-2 Inhibitors in the Treatment of Various Diseases. Expert Opin. Ther. Pat. 2004, 14 ( (12), ), 1803–1806. 10.1517/13543776.14.12.1803 [DOI] [Google Scholar]
- Supuran C. T. COX-2 Selective Inhibitors, Carbonic Anhydrase Inhibition and Anticancer Properties of Sulfonamides Belonging to this Class of Pharmacological Agents. Mini-Rev. Med. Chem. 2004, 4, 625–632. 10.2174/1389557043403792. [DOI] [PubMed] [Google Scholar]
- Guler O. O.; De Simone G.; Supuran C. T. Drug Design Studies of the Novel Antitumor Targets Carbonic Anhydrase IX and XII. Curr. Med. Chem. 2010, 17 (15), 1516–1526. 10.2174/092986710790979999. [DOI] [PubMed] [Google Scholar]
- Hewett-Emmett D.Evolution and Distribution of the Carbonic Anhydrase Gene Families. EXS 2000, 90 (Carbonic Anhydrases), 29–76. 10.1007/978-3-0348-8446-4_3 [DOI] [PubMed] [Google Scholar]
- Hewett-Emmett D.; Tashian R. E. Functional Diversity, Conservation, and Convergence in the Evolution of the α-, β-, and γ-Carbonic Anhydrase Gene Families. Mol. Phylogenet. Evol. 1996, 5 (1), 50–77. 10.1006/mpev.1996.0006. [DOI] [PubMed] [Google Scholar]
- Imtaiyaz Hassan M.; Shajee B.; Waheed A.; Ahmad F.; Sly W. S. Structure, Function and Applications of Carbonic Anhydrase Isozymes. Bioorg. Med. Chem. 2013, 21 (6), 1570–1582. 10.1016/j.bmc.2012.04.044. [DOI] [PubMed] [Google Scholar]
- Supuran C. T. Carbonic Anhydrases: Novel Therapeutic Applications for Inhibitors and Activators. Nat. Rev. Drug Discovery 2008, 7 (2), 168–181. 10.1038/nrd2467. [DOI] [PubMed] [Google Scholar]
- Supuran C. T. In Carbonic Anhydrases as Drug Targets: General Presentation; John Wiley & Sons, Inc.: 2009; pp 15–38. [Google Scholar]
- Abbate F. Carbonic Anhydrase Inhibitors: E7070, A Sulfonamide Anticancer Agent, Potently Inhibits Cytosolic Isozymes I and II, and Transmembrane, Tumor-Associated Isozyme IX. Bioorg. Med. Chem. Lett. 2004, 14, 217–223. 10.1016/j.bmcl.2003.09.062. [DOI] [PubMed] [Google Scholar]
- Dorai T. Role of Carbonic Anhydrases in the Progression of Renal Cell Carcinoma Subtypes: Proposal of a Unified Hypothesis. Cancer Invest. 2006, 24, 754–779. 10.1080/07357900601062321. [DOI] [PubMed] [Google Scholar]
- Garaj V. Carbonic Anhydrase Inhibitors: Synthesis And Inhibition of Cytosolic/Tumor-Associated Carbonic Anhydrase Isozymes I, II, and IX with Sulfonamides Incorporating 1,2,4-Triazine Moieties. Bioorg. Med. Chem. Lett. 2004, 14, 5427–5433. 10.1016/j.bmcl.2004.07.087. [DOI] [PubMed] [Google Scholar]
- Ilies M. A. Carbonic Anhydrase Inhibitors. Inhibition of Tumor-Associated Isozyme IX by Halogenosulfanilamide and Halogenophenylaminobenzolamide Derivatives. J. Med. Chem. 2003, 46, 2187–2196. 10.1021/jm021123s. [DOI] [PubMed] [Google Scholar]
- Zhou Y.; Mokhtari R. B.; Pan J.; Cutz E.; Yeger H. Carbonic Anhydrase II Mediates Malignant Behavior of Pulmonary Neuroendocrine Tumors. Am. J. Respir. Cell Mol. Biol. 2015, 52 (2), 183–92. 10.1165/rcmb.2014-0054OC. [DOI] [PubMed] [Google Scholar]
- Perez-Sayans M.; Garcia-Garcia A.; Scozzafava A.; Supuran C. T. Inhibition of V-ATPase and Carbonic Anhydrases as Interference Strategy with Tumor Acidification Processes. Curr. Pharm. Des. 2012, 18 (10), 1407–1413. 10.2174/138161212799504876. [DOI] [PubMed] [Google Scholar]
- Swietach P.; Hulikova A.; Vaughan-Jones R. D.; Harris A. L. New Insights into the Physiological Role of Carbonic Anhydrase IX in Tumor pH Regulation. Oncogene 2010, 29 (50), 6509–6521. 10.1038/onc.2010.455. [DOI] [PubMed] [Google Scholar]
- Supuran C. T.; Winum J.-Y. Carbonic Anhydrase IX Inhibitors in Cancer Therapy: an Update. Future Med. Chem. 2015, 7 (11), 1407–1414. 10.4155/fmc.15.71. [DOI] [PubMed] [Google Scholar]
- Svastova E. Hypoxia Activates the Capacity of Tumor-Associated Carbonic Anhydrase IX To Acidify Extracellular pH. FEBS Lett. 2004, 577, 439–445. 10.1016/j.febslet.2004.10.043. [DOI] [PubMed] [Google Scholar]
- Semenza G. L. Hypoxia and Cancer. Cancer Metastasis Rev. 2007, 26, 223–224. 10.1007/s10555-007-9058-y. [DOI] [PubMed] [Google Scholar]
- Alafeefy A. M.; Ahmad R.; Abdulla M.; Eldehna W. M.; Al-Tamimi A.-M. S.; Abdel-Aziz H. A.; Al-Obaid O.; Carta F.; Al-Kahtani A. A.; Supuran C. T. Development of Certain New 2-Substituted-Quinazolin-4-Yl-Aminobenzenesulfonamide as Potential Antitumor Agents. Eur. J. Med. Chem. 2016, 109, 247–253. 10.1016/j.ejmech.2016.01.001. [DOI] [PubMed] [Google Scholar]
- Ibrahim H. S.; Abou-Seri S. M.; Tanc M.; Elaasser M. M.; Abdel-Aziz H. A.; Supuran C. T. Isatin-Pyrazole Benzenesulfonamide Hybrids Potently Inhibit Tumor-Associated Carbonic Anhydrase Isoforms IX and XII. Eur. J. Med. Chem. 2015, 103, 583–593. 10.1016/j.ejmech.2015.09.021. [DOI] [PubMed] [Google Scholar]
- Supuran C. T. Inhibition of Carbonic Anhydrase IX as a Novel Anticancer Mechanism. World J. Clin. Oncol. 2012, 3 (7), 98–103. 10.5306/wjco.v3.i7.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grondin V.; Seksik P.; Dumont S.; Thomas G.; Trugnan G.; Flejou J. F.; Masliah J.; Wendum D.; Bachelet M. Regulation of Colon Cancer Cell Proliferation and Migration by MD-2 Activity. Innate Immun. 2011, 17 (4), 414–422. 10.1177/1753425910375583. [DOI] [PubMed] [Google Scholar]
- Antunes A. Jr.; Andrade L. A. L. A.; Pinto G. A.; Leao R.; Pinto-Neto A. M.; Costa-Paiva L. Is the immunohistochemical Expression of Proliferation (Ki-67) and Apoptosis (Bcl-2) Markers and Cyclooxigenase-2 (COX-2) Related to Carcinogenesis in Postmenopausal Endometrial Polyps?. Anal. Quant. Cytopathol. Histpathol. 2012, 34 (5), 264–72. [PubMed] [Google Scholar]
- Lu X.-Y.; Wang Z.-C.; Wei T.; Yan X.-Q.; Wang P.-F.; Zhu H.-L. Design, Synthesis and Evaluation of Benzenesulfonamide-Substituted 1,5-Diarylpyrazoles Containing Phenylacetohydrazide Derivatives as COX-1/COX-2 Agents Against Solid Tumors. RSC Adv. 2016, 6 (27), 22917–22935. 10.1039/C6RA02168A. [DOI] [Google Scholar]
- Li M.; Wu X.; Xu X. C. Induction of Apoptosis by Cyclo-Oxygenase-2 Inhibitor NS398 Through a Cytochrome C-Dependent Pathway in Esophageal Cancer Cells. Int. J. Cancer 2001, 93 (2), 218–23. 10.1002/ijc.1322. [DOI] [PubMed] [Google Scholar]
- Grösch S.; Maier T. J.; Schiffmann S.; Geisslinger G. Cyclooxygenase-2 (COX-2)–Independent Anticarcinogenic Effects of Selective COX-2 Inhibitors. J. Natl. Cancer Inst. 2006, 98 (11), 736–747. 10.1093/jnci/djj206. [DOI] [PubMed] [Google Scholar]
- Giampieri R.; Restivo A.; Pusceddu V.; Del Prete M.; Maccaroni E.; Bittoni A.; Faloppi L.; Andrikou K.; Bianconi M.; Cabras F.; Berardi R.; Zorcolo L.; Scintu F.; Cascinu S.; Scartozzi M. The Role of Aspirin as Antitumoral Agent for Heavily Pretreated Patients With Metastatic Colorectal Cancer Receiving Capecitabine Monotherapy. Clin. Colorectal Cancer 2017, 16 (1), 38–43. 10.1016/j.clcc.2016.07.011. [DOI] [PubMed] [Google Scholar]
- Qiao Y.; Yang T.; Gan Y.; Li W.; Wang C.; Gong Y.; Lu Z. Associations Between Aspirin Use and the Risk of Cancers: A Meta-Analysis of Observational Studies. BMC Cancer 2018, 18 (1), 288. 10.1186/s12885-018-4156-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye X. F.; Wang J.; Shi W. T.; He J. Relationship Between Aspirin Use After Diagnosis of Colorectal Cancer and Patient Survival: A Meta-Analysis of Observational Studies. Br. J. Cancer 2014, 111 (11), 2172–9. 10.1038/bjc.2014.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zelenay S.; van der Veen A. G.; Bottcher J. P.; Snelgrove K. J.; Rogers N.; Acton S. E.; Chakravarty P.; Girotti M. R.; Marais R.; Quezada S. A.; Sahai E.; Reis e Sousa C. Cyclooxygenase-Dependent Tumor Growth through Evasion of Immunity. Cell 2015, 162 (6), 1257–70. 10.1016/j.cell.2015.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurumbail R. G.; Stevens A. M.; Gierse J. K.; McDonald J. J.; Stegeman R. A.; Pak J. Y.; Gildehaus D.; iyashiro J. M.; Penning T. D.; Seibert K.; Isakson P. C.; Stallings W. C. Structural Basis for Selective Inhibition of Cyclooxygenase-2 by Anti-Inflammatory Agents. Nature 1996, 384, 644. 10.1038/384644a0. [DOI] [PubMed] [Google Scholar]
- Supuran C. T., Winum J.-Y., Eds. Drug Design of Zinc-Enzyme Inhibitors Functional, Structural, and Disease Applications; John Wiley & Sons, Inc.: 2009; p 1022 pp. [Google Scholar]
- Alterio V.; Di Fiore A.; D’Ambrosio K.; Supuran C. T.; De Simone G. Multiple Binding Modes of Inhibitors to Carbonic Anhydrases: How to Design Specific Drugs Targeting 15 Different Isoforms?. Chem. Rev. 2012, 112 (8), 4421–68. 10.1021/cr200176r. [DOI] [PubMed] [Google Scholar]
- Meleddu R.; Maccioni E.; Distinto S.; Bianco G.; Melis C.; Alcaro S.; Cottiglia F.; Ceruso M.; Supuran C. T. New 4-[(3-Cyclohexyl-4-Aryl-2,3-Dihydro-1,3-Thiazol-2-Ylidene)Amino]Benzene-1-Sulfonamides, Synthesis and Inhibitory Activity Toward Carbonic Anhydrase I, II, IX, XII. Bioorg. Med. Chem. Lett. 2015, 25 (16), 3281–4. 10.1016/j.bmcl.2015.05.076. [DOI] [PubMed] [Google Scholar]
- Ceruso M.; Bragagni M.; AlOthman Z.; Osman S. M.; Supuran C. T. New Series of Sulfonamides Containing Amino Acid Moiety Act as Effective and Selective Inhibitors of Tumor-Associated Carbonic Anhydrase XII. J. Enzyme Inhib. Med. Chem. 2015, 30 (3), 430–434. 10.3109/14756366.2014.942659. [DOI] [PubMed] [Google Scholar]
- Eldehna W. M.; Fares M.; Ceruso M.; Ghabbour H. A.; Abou-Seri S. M.; Abdel-Aziz H. A.; Abou El Ella D. A.; Supuran C. T. Amido/Ureidosubstituted Benzenesulfonamides-Isatin Conjugates as Low Nanomolar/Subnanomolar Inhibitors of the Tumor-Associated Carbonic Anhydrase Isoform XII. Eur. J. Med. Chem. 2016, 110, 259–266. 10.1016/j.ejmech.2016.01.030. [DOI] [PubMed] [Google Scholar]
- Guzel-Akdemir O.; Akdemir A.; Karali N.; Supuran C. T. Discovery of Novel Isatin-Based Sulfonamides with Potent and Selective Inhibition of the Tumor-Associated Carbonic Anhydrase Isoforms IX and XII. Org. Biomol. Chem. 2015, 13 (23), 6493–6499. 10.1039/C5OB00688K. [DOI] [PubMed] [Google Scholar]
- Grandane A.; Tanc M.; Di Cesare Mannelli L.; Carta F.; Ghelardini C.; Zalubovskis R.; Supuran C. T. 6-Substituted Sulfocoumarins Are Selective Carbonic Anhydrase IX and XII Inhibitors with Significant Cytotoxicity against Colorectal Cancer Cells. J. Med. Chem. 2015, 58 (9), 3975–3983. 10.1021/acs.jmedchem.5b00523. [DOI] [PubMed] [Google Scholar]
- Bozdag M.; Ferraroni M.; Nuti E.; Vullo D.; Rossello A.; Carta F.; Scozzafava A.; Supuran C. T. Combining the Tail and the Ring Approaches for Obtaining Potent and Isoform-Selective Carbonic Anhydrase Inhibitors: Solution and X-Ray Crystallographic Studies. Bioorg. Med. Chem. 2014, 22 (1), 334–340. 10.1016/j.bmc.2013.11.016. [DOI] [PubMed] [Google Scholar]
- Pala N.; Micheletto L.; Sechi M.; Aggarwal M.; Carta F.; McKenna R.; Supuran C. T. Carbonic Anhydrase Inhibition with Benzenesulfonamides and Tetrafluorobenzenesulfonamides Obtained via Click Chemistry. ACS Med. Chem. Lett. 2014, 5 (8), 927–930. 10.1021/ml500196t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melis C.; Meleddu R.; Angeli A.; Distinto S.; Bianco G.; Capasso C.; Cottiglia F.; Angius R.; Supuran C. T.; Maccioni E. Isatin: a Privileged Scaffold for the Design of Carbonic Anhydrase Inhibitors. J. Enzyme Inhib. Med. Chem. 2017, 32 (1), 68–73. 10.1080/14756366.2016.1235042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianco G.; Meleddu R.; Distinto S.; Cottiglia F.; Gaspari M.; Melis C.; Corona A.; Angius R.; Angeli A.; Taverna D.; Alcaro S.; Leitans J.; Kazaks A.; Tars K.; Supuran C. T.; Maccioni E. N-Acylbenzenesulfonamide Dihydro-1,3,4-Oxadiazole Hybrids: Seeking Selectivity Toward Carbonic Anhydrase Isoforms. ACS Med. Chem. Lett. 2017, 8 (8), 792–796. 10.1021/acsmedchemlett.7b00205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melis C.; Distinto S.; Bianco G.; Meleddu R.; Cottiglia F.; Fois B.; Taverna D.; Angius R.; Alcaro S.; Ortuso F.; Gaspari M.; Angeli A.; Del Prete S.; Capasso C.; Supuran C. T.; Maccioni E.. Targeting Tumor Associated Carbonic Anhydrases IX and XII: Highly Isozyme Selective Coumarin and Psoralen Inhibitors. ACS Med. Chem. Lett. 2018, 9, 725. 10.1021/acsmedchemlett.8b00170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bua S.; Di Cesare Mannelli L.; Vullo D.; Ghelardini C.; Bartolucci G.; Scozzafava A.; Supuran C. T.; Carta F. Design and Synthesis of Novel Nonsteroidal Anti-Inflammatory Drugs and Carbonic Anhydrase Inhibitors Hybrids (NSAIDs–CAIs) for the Treatment of Rheumatoid Arthritis. J. Med. Chem. 2017, 60 (3), 1159–1170. 10.1021/acs.jmedchem.6b01607. [DOI] [PubMed] [Google Scholar]
- Mollica A.; Costante R.; Akdemir A.; Carradori S.; Stefanucci A.; Macedonio G.; Ceruso M.; Supuran C. T. Exploring New Probenecid-Based Carbonic Anhydrase Inhibitors: Synthesis, Biological Evaluation and Docking Studies. Bioorg. Med. Chem. 2015, 23 (17), 5311–5318. 10.1016/j.bmc.2015.07.066. [DOI] [PubMed] [Google Scholar]
- Chimenti F.; Maccioni E.; Secci D.; Bolasco A.; Chimenti P.; Granese A.; Befani O.; Turini P.; Alcaro S.; Ortuso F.; Cardia M. C.; Distinto S. Selective Inhibitory Activity against MAO and Molecular Modeling Studies of 2-Thiazolylhydrazone Derivatives. J. Med. Chem. 2007, 50 (4), 707–712. 10.1021/jm060869d. [DOI] [PubMed] [Google Scholar]
- Carradori S.; Ortuso F.; Petzer A.; Bagetta D.; De Monte C.; Secci D.; De Vita D.; Guglielmi P.; Zengin G.; Aktumsek A.; Alcaro S.; Petzer J. P. Design, Synthesis and Biochemical Evaluation of Novel Multi-Target Inhibitors as Potential Anti-Parkinson Agents. Eur. J. Med. Chem. 2018, 143, 1543–1552. 10.1016/j.ejmech.2017.10.050. [DOI] [PubMed] [Google Scholar]
- Zarghi A.; Arfaei S. Selective COX-2 Inhibitors: A Review of Their Structure-Activity Relationships. Iran. J. Pharm. Res. 2011, 10 (4), 655–683. [PMC free article] [PubMed] [Google Scholar]
- Maresca A.; Temperini C.; Vu H.; Pham N. B.; Poulsen S.-A.; Scozzafava A.; Quinn R. J.; Supuran C. T. Non-Zinc Mediated Inhibition of Carbonic Anhydrases: Coumarins Are a New Class of Suicide Inhibitors. J. Am. Chem. Soc. 2009, 131 (8), 3057–3062. 10.1021/ja809683v. [DOI] [PubMed] [Google Scholar]
- Behnke C. A.; Le Trong I.; Godden J. W.; Merritt E. A.; Teller D. C.; Bajorath J.; Stenkamp R. E. Atomic Resolution Studies of Carbonic Anhydrase II. Acta Crystallogr., Sect. D: Biol. Crystallogr. 2010, 66 (Pt 5), 616–27. 10.1107/S0907444910006554. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.