Abstract
Advances in immunotherapy have led to radical improvements in outcomes, including overall survival, such as in non-small cell lung cancer (NSCLC) patients with metastatic disease treated with immune checkpoint inhibitors. More recently, promising results have been obtained in earlier disease settings, and combinations with other therapies are being actively investigated. Durvalumab, a monoclonal antibody directed against the programmed death ligand 1, has demonstrated significant activity in NSCLC, including increased progression-free survival rates after chemoradiation for unresectable stage III disease, with a favourable safety profile. Clinical trials, including phase III studies, are ongoing as monotherapy and in combination with chemotherapy, radiotherapy and other immunotherapies, such as the anti-cytotoxic T-lymphocyte antigen 4 drug tremelimumab, in diverse stages of the disease.
Keywords: checkpoint inhibitors, immunotherapy, lung cancer, PD-L1 expression testing, predictive biomarker, radiotherapy, tumour mutational burden
Introduction
Non-small cell lung cancer (NSCLC) is one of the most common neoplasms in developed countries, and the leading cause of cancer mortality worldwide.1 Early stages (I–II) account for approximately 20% of lung cancer diagnoses, with 5-year overall survival (OS) rates of 40–70% following standard surgical treatment (lobectomy with systemic lymph node resection) or stereotactic body radiation therapy (SBRT). Locally advanced tumours are frequently treated with multidisciplinary approaches, including chemotherapy and radiotherapy or surgery, and expected survival at 5 years is in the range of 15–30%. In the advanced disease setting, the 5-year OS rate with standard treatment based on platinum-doublet chemotherapy is below 5%. Recently, however, immune checkpoint inhibitors (ICIs), monoclonal antibodies directed to PD-1/PD-L1 (programmed death 1/programmed death ligand 1) and to a lesser extent to CTLA-4 (cytotoxic T-lymphocyte antigen 4), have led to a revolution in the treatment of advanced stages of NSCLC, offering durable responses in around 15–20% of patients and a significant impact on survival.2–4 Indeed, several phase III clinical trials have demonstrated the superiority of ICIs, with a favourable safety profile compared with chemotherapy for advanced NSCLC, leading to its approval for use in different settings. Pembrolizumab (Keytruda®), a PD-1 inhibitor, can be administered to previously treated patients with advanced NSCLC and PD-L1 expression in ⩾1% of tumour cells,5 and to treatment-naïve patients with PD-L1 expression in ⩾50% of tumour cells.6 Nivolumab (Opdivo®), another PD-1 inhibitor, and atezolizumab (Tecentriq®), a PD-L1 inhibitor, have been approved for previously treated advanced NSCLC regardless of PD-L1 expression,7,8 while durvalumab (MEDI4637), another PD-L1 inhibitor, has demonstrated durable progression-free survival (PFS) rates in patients with stage III NSCLC after concurrent treatment with chemoradiotherapy.9
An unmet need is to reliably identify which patients are going to benefit from ICI treatment. PD-L1 expression by immunohistochemistry (IHC) in tumour tissue has been described as a predictive biomarker of benefit with this class of agents;10 however, relevant responses have also been seen in some patients with PD-L1-negative tumours. Other potential predictive tools may include the immune microenvironment profile, the tumour mutational burden (TMB), specific genomic aberrations underlying the tumour, and the immune gene expression profile. This demonstrates the need for finding validated biomarkers of immune response applicable in the clinical practice.
Durvalumab is a monoclonal immunoglobulin (Ig)G1 κ antibody directed against the PD-L1, whose immunomodulatory effect has been studied in advanced stages of NSCLC, demonstrating activity and an adequate safety profile.11–13 In this review we will discuss the development, efficacy and safety of durvalumab, ongoing studies and future directions in NSCLC.
Mechanism of action of ICIs
Developments within the immunotherapeutic field commenced more than 120 years ago with contributions by scientists such as Paul Ehrlich or Burnett and Thomas. These pioneers laid the foundations of the cancer immunosurveillance hypothesis, which implies that innate and adaptive immunity are responsible for preventing cancer development in immunocompetent hosts.14 Cancer cells express neoantigens on their surface that are often the products of mutated cellular genes or aberrantly expressed normal genes, alerting the immune system of the presence of abnormal or ‘nonself’ cells. The release of proinflammatory cytokines such as interferon (IFN)-γ or tumour necrosis factor (TNF)-α is key for an effective immune response, but tumour cells can also modulate immunogenicity by other mechanisms. These include: the secretion of immunosuppressive factors such as vascular endothelial growth factor, transforming growth factor (TGF)-b or indoleamine 2,3-dioxygenase (IDO); recruiting regulatory immune cells (Tregs) that function as the effectors of immunosuppression; down-regulating cell surface major histocompatibility complex (MHC) class I molecules; or expressing negative co-stimulatory molecules (CTLA-4, PD-1, PD-L1). The duality between host-protective and tumour-promoting actions represents the immunoediting process, whose function is to achieve an equilibrium that avoids the escape of developing tumour cells from the immune system.15
Numerous immunotherapies have been explored to reverse cancer outgrowth, including vaccines, cytokines, genetically-engineered tumour-specific lymphocytes, the therapeutic administration of monoclonal antibodies, and many other approaches. At present, the most advanced and successful therapies in clinical practice have been the ICIs, which are involved in the regulation of T-cell responses.3,16 Among them, the most frequently used are antibodies against PD-1, PD-L1 and PD-L2, and CTLA4, but other molecules such as T-cell immunoglobulin and mucin domain 3 (TIM3), OX40, B- and T-lymphocyte attenuator or lymphocyte-activation gene 3 (LAG3) have been recently identified as potential targets for cancer immunotherapy.17
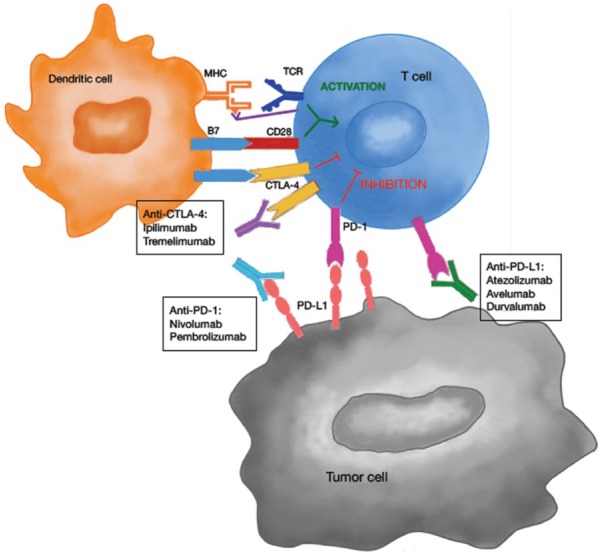
The process by which T-cells can recognize and then kill tumour cells involves multiple steps.3,18 It starts with the presentation of antigenic tumour peptides through MHC on antigen-presenting cells (APCs), with the subsequent expression of B7-1 (CD80) and B7-2 (CD86) molecules on their surface. Thereafter, APCs migrate to lymph nodes, where they interact with T-cells. In the ‘two signal’ antigen-specific model of T-cell activation, both T-cell receptor (TCR) engagement (signal 1) and a co-stimulatory signal, B7-CD28 (signal 2), are required (Figure 1). CTLA-4 is up-regulated shortly after T-cell activation, and through a series of mechanisms negatively regulates T-cells. Anti-CTLA-4 antibodies, such as ipilimumab and tremelimumab, block this inhibitory signal and thereby enhance antitumour activity by their interaction with CD80 and CD86. In the effector phase, once the T-cells have been activated, they can subsequently recognize and neutralize tumour cells. PD-1 is expressed by T-cells after antigen exposure, and its interaction with PD-L1 and PD-L2, which can be expressed by tumour cells and tumour stroma as well as in APCs, results in the negative regulation of T-cells in the tumour microenvironment, leading to T-cell exhaustion or anergy. Blockade with antibodies to PD-1 or PD-L1 (e.g. nivolumab and pembrolizumab or durvalumab and atezolizumab) results in the activation of T-cells with cancer specificity. To date, there is no compelling evidence indicating that antibodies against PD-1, which block binding to both PD-L1 and PD-L2, have differential immune and clinical effects of relevance as compared with antibodies against PD-L1.19
Figure 1.
Targeting the CTLA-4 and PD-(L)1 pathways with immune checkpoint inhibitors. Mechanism of action: T-cell activation requires a ‘two signal’ mechanism: the recognition and engagement of the TCR with antigenic tumour peptides on the MHC presented by the APCs, and a co-stimulatory signal via B7 and CD28 binding. CTLA-4 acts as a negative receptor that inhibits the activation of T-cells regulating the immune response. By blocking this receptor with drugs such as ipilimumab and tremelimumab, antitumour activity is enhanced. In the effector phase, once the T-cells have been activated, binding of the PD-1 receptor with its ligand (PD-L1 or PD-L2), expressed on tumour cells, results in the inhibition of T-cells in the tumour microenvironment. By blocking this receptor with drugs such as nivolumab, pembrolizumab, atezolizumab or durvalumab, T-cells can be activated against cancer cells.
APC, antigen-presenting cell; CTLA-4, cytotoxic T-lymphocyte antigen 4; MHC, major histocompatibility complex; PD-L1, programmed death ligand 1; TCR, T-cell receptor.
Checkpoint inhibitors in NSCLC: comparisons and overall results
Despite significant efforts being made to target immune checkpoints, it was not until 2011 that the United States Food and Drug Administration (US FDA) first approved an antibody directed against the CTLA-4 receptor (ipilimumab) for the treatment of advanced melanoma.20 In advanced NSCLC CTLA-4 antibodies have shown minimal antitumour activity as monotherapy, but agents targeting the PD-1/PD-L1 axis alone, in combination with anti-CTLA4 or with chemotherapy have transformed the management of NSCLC and emerged as a new standard of care for treatment-naïve and previously treated NSCLC patients with advanced disease (Table 1).
Table 1.
Principal clinical trials with immune checkpoint inhibitors targeting the PD-(L)1 pathway in monotherapy.
|
Pembrolizumab
|
Nivolumab
|
Atezolizumab
|
Durvalumab
|
Avelumab
|
|||||
|---|---|---|---|---|---|---|---|---|---|
| Keynote 0246 | Keynote 0105 | CheckMate 02623 | CheckMate 01721 | CheckMate 05722 | Birch (Cohort 1)24 | Oak7 | Study 1108 (Expansion cohort)11 | Javelin Solid Tumor25 (Expansion cohort) | |
| Phase | III | II/III | III | III | III | II | III | I/II | Ib |
| N (number of patients) | 305 (1:1) | 1034 (1:1:1) 345 pembrolizumab 2 mg/kg |
423 (1:1) | 272 (1:1) | 582 (1:1) | 659 139 1L (cohort 1) |
1225 (1:1) | 304 | 184 |
| Histology | All comers | All comers | All comers | Squamous cell | Nonsquamous cell | All comers | All comers | All comers | All comers |
| Line | 1 L | 2 L | 1 L | 2 L | 2 L | 1 L | 2 L | 1-2-3 L | 2 L |
| PD-L1 | >50% | >1% | >5% | – | – | >5% | – | >25% | – |
| Doses | 200 mg (flat dose) Q3w | 2 mg/kg 10 mg/kg Q3w |
3 mg/kg Q2w | 3 mg/kg Q2w | 3 mg/kg Q2w | 1200 mg flat dose Q3w | 1200 mg flat dose Q3w | 10 mg/kg | 10 mg/kg Q2w |
| ORR (ITT) | 44.8% (95% CI 36.8–56) | 18% (95% CI 14.1–22.5) | 26% (95% CI 20–33) | 20% (95% CI 14–28) | 19% (95% CI 15–24) | 25% (95% CI 18–33) | 14% (95% CI 11–17) | All: 25% (95% CI 18–32) 1 L: 29% (95% CI 17–43) 2L: 26% (95% CI 14–41) ⩾3 L 22% (95% CI 12–35) |
12% (95% CI 8–18%) |
| PFS | 10.3 (95% CI 6.7–NR) | 3.9 (95% CI 3.1–4.1) | 4.2 (95% CI 3–5.6) | 3.5 (95% CI 2.1–4.9) | 2.3 (95% CI 2.2–3.3) | 5.4 (95% 3–6.9) | 2.8 (95% CI 2.6–3) | NA | 2.9 (95% CI 2.1–3.4) |
| OS (ITT) | NR | 10.4 (95% CI 9.4–11.9) | 14.4 (95% CI 11.7–17.1) | 9.2 (95% CI 7.3–13.3) | 12.2 (95% CI 9.7–15) | 23.5 (95% CI 18.1–NE) | 13.8 (95% CI 11.8–15.7) | 1 L: No mature 2 L: 17.8 (95% CI 7.9–22.4) 3 L: 13 (95% CI 6–NA) |
8.4 (95% CI 7.3–10.6) |
| Duration of response | NR (range 1.9–14.5) | 2.0 (0.66–20.3) | 12.1 (95% CI 1.7–19.4) | 25.2 (95% CI 9.8–30.4) | 17.2 (95% CI 8.4–NR) | 9.8 (95% CI 5.6–NE) | 16.3 (95% CI 10–NE) | NA | NA |
| AEs G3/4 | 26.6% (G5 included) | 43% (G5 included) | 18% | 7% | 10% | 41% (G5 included) | 15% | 10 % | 13% |
1L, first line; 2L, second line; 3L, third line; AEs, adverse events; CI, confidence interval; ITT, intention to treat; NE, nonevaluable; NA, Not Available; NR, not reached; ORR, overall response rate; OS, overall survival; PFS, progression-free survival.
Robust evidence of the clinical efficacy of ICIs in advanced NSCLC came from four phase III trials comparing docetaxel with PD-1 inhibitors (pembrolizumab or nivolumab) or anti-PD-L1 agents (atezolizumab) in the second-line setting.5,7,21,22 All of the trials showed improved OS over chemotherapy and confirmed their indication in this context. Consistently across trials and agents, the magnitude of the benefit observed was proportional to the level of PD-L1 expression in the tumour.23 Furthermore, all anti-PD-1/PD-L1 agents have shown efficacy when used as first-line treatment,23–27 although nivolumab failed to improve PFS or OS over chemotherapy in patients expressing PD-L1 in ⩾5% of tumour cells (primary endpoints).24 On the other hand, pembrolizumab resulted in improved PFS and OS compared with platinum-based chemotherapy in treatment-naïve patients with advanced NSCLC exhibiting PD-L1 expression on ⩾50% and ⩾1% of tumour cells.6,23
Another clinical approach gaining interest is the combinations of ICIs with chemotherapy or other immune-based therapies.28–34 The latest findings report a benefit in efficacy for the new agents added to chemotherapy compared with chemotherapy alone in the first-line setting irrespective of the level of PD-L1 expression. In the Keynote-189 phase III clinical trial, pembrolizumab combined with pemetrexed plus cisplatin or carboplatin showed improved outcomes compared with standard of care chemotherapy in nonsquamous NSCLC, with hazard ratios (HRs) of 0.52 (0.43–0.64) for PFS and 0.49 (0.38–0.64) for OS. For squamous histology, in the Keynote 407 clinical trial, pembrolizumab combined with carboplatin plus nab-paclitaxel/paclitaxel also showed durable responses and improved OS and PFS.32 Nivolumab plus standard of care chemotherapy is also being explored in a cohort of the CheckMate 227 trial, with a PFS benefit for the combination compared with chemotherapy alone in the population with PD-L1 expression <1%, and OS results yet to be reported.33 At the first part of this trial the authors compared nivolumab plus ipilimumab versus chemotherapy, confirming a longer PFS for the first arm in the subset of tumours with high mutational burden (at least 10 mutations per megabase). Of note, the latter did not correlate with PD-L1 expression, suggesting it may be a useful complementary biomarker for ICIs in the clinic. In addition, the ongoing phase III trial Impower150 combining carboplatin, paclitaxel and bevacizumab with or without atezolizumab reported improved PFS and OS for the four drugs regardless of the PD-L1 and epidermal growth factor receptor (EGFR) status, a population that had previously had limited therapeutic benefit with checkpoint-inhibitor monotherapy.34
Regarding safety, as expected for all these combinations, chemotherapy plus ICI or anti-PD-(L)1 plus anti-CTLA-4 drugs, the rate of treatment-related adverse events (TRAEs) was slightly increased, with grade 3–4 adverse events reported in 67.2% of patients in the combination arm of the Keynote 189 trial. In total, 13.8% of patients discontinued treatment because of adverse events in this trial, data that are consistent within the rest of the mentioned clinical trials. Nevertheless, a better understanding of the mechanisms of toxicity will lead to better management of immune-related adverse events.
Clinical development of durvalumab
PD-L1 is a type I transmembrane protein from the B7 family that binds to either the PD-1 receptor, expressed on activated T-cells, or to CD80, expressed on both APCs and activated T-cells. As mentioned above, both interactions release an inhibitory signal that blocks T-cell activation and the effector phase. PD-L1 expression has been shown to correlate inversely with the clinical outcomes of some malignancies such as NSCLC, renal cell carcinoma, pancreatic and ovarian cancer.35 Based on these findings, durvalumab (MEDI4736), a high-affinity human IgG1-1κ monoclonal antibody that blocks PD-L1 binding to PD-1 and CD-80, was developed. Durvalumab binds with a high affinity to PD-L1 but not to PD-L2, helping T-cells to recognize and neutralize tumour cells and potentially reduce the risk of inflammation in normal lung tissue. It has been specifically engineered to disable cytotoxic effector functions, such as antibody-dependent cell-mediated cytotoxicity and complement-dependent cytotoxicity against cells expressing PD-L1.36,37
Durvalumab used as monotherapy in NSCLC: advanced disease
Durvalumab was first evaluated as monotherapy in an ongoing phase I/II clinical trial (Study 1108, ClinicalTrials.gov identifier: NCT01693562) in patients with advanced solid tumours, including patients with refractory and treatment-naïve NSCLC.11,38 After a recommended dose of durvalumab 10 mg/kg every 2 weeks was established, several expansion cohorts were implemented. Initially patients were included regardless of the level of tumour PD-L1 expression, but later they were required to have high tumour cell PD-L1 expression, defined as ⩾25% of tumour cells staining for PD-L1 at any intensity using a validated immunohistochemical assay [VENTANA IHC PD-L1 (SP263) assay, Ventana Medical Systems, Inc.]. Objective response rates (ORRs) were variable in the different cohorts, observing the highest treatment benefit in treatment-naïve patients with high PD-L1 expressing tumours.27 TRAEs of any grade and of grade 3/4 were seen in 57.2% and 10.2% respectively, of durvalumab-treated patients. Durvalumab-related adverse events resulting in treatment discontinuation were reported for 17 patients (5.6%), and 1 patient died of pneumonia. The most frequent adverse events were fatigue (17.4%), decreased appetite (9.2%) and diarrhoea (8.9%).
Between Feb 2014 and Dec 2015, 444 patients were enrolled in the phase II, open-label, single-arm ATLANTIC trial (ClinicalTrials.gov identifier: NCT02087423). Eligible patients had advanced NSCLC with disease progression following at least two previous systemic regimens, including platinum-based chemotherapy and tyrosine kinase inhibitor (TKI) therapy if indicated. They received durvalumab 10 mg/kg every 2 weeks for up to 12 months in three different cohorts defined by EGFR/ALK (anaplastic lymphoma kinase) status [wild type (wt) or mutated (mut)] and tumour expression of PD-L1 (Table 2).
Table 2.
Cohorts included in the ATLANTIC clinical trial.
| Cohort | EGFR/ALK status | PD-L1 expression | Number of patients |
|---|---|---|---|
| 1 | Mutated/positive | <25% or ⩾25% | 111 |
| 2 | Wild type/wild type | <25% or ⩾25% | 265 |
| 3 | Mutated/positive | ⩾90% | 68 |
ALK, anaplastic lymphoma kinase; EGFR, epidermal growth factor receptor; PD-L1, programmed death ligand 1.
A final analysis of the primary efficacy endpoint was recently published,12 showing results comparable with those of the initial phase I/II trial: durvalumab was active and induced durable responses in a proportion of heavily pretreated patients. EGFRmut/ALK-positive NSCLC patients showed lower responses than those with EGFRwt/ALKwt NSCLC, whereas higher PD-L1 expression increased the response both in patients with EGFRmut/ALK-positive NSCLC [ORR 12.2%, 95% confidence interval (CI): 5.7–21.8, cohort 1] and those with EGFRwt/ALKwt NSCLC (ORR 16.4%, 95% CI: 10.8–23.5, cohort 2). Nevertheless, the highest proportion of patients achieving an objective and durable response (30.9%, 95% CI: 20.2–43.3) was in cohort 3 (EGFRwt/ALKwt, ⩾90% of tumour cells expressing PD-L1). Similar results were seen in relation to PFS and median OS, with higher median OS (11–13 months) seen in patients with at least 25% of tumour cells expressing PD-L1 irrespective of EGFR or ALK status. Of note, OS data for patients with less than 25% of tumour cells expressing PD-L1 (9–10 months) were better than previously reported.11,27
The toxicity profile was consistent with that of other PD(L)-1 inhibitors.39 TRAEs occurred in 256 (58%) of 444 patients, the most common being fatigue (50; 11%), hypothyroidism (36; 8%), asthaenia (31; 7%), nausea (28; 6%), and diarrhoea (27; 6%). Grade 3/4 TRAEs occurred in 40 (9%) of 444 patients: 6 (5%) of 111 patients in cohort 1, 22 (8%) of 265 patients in cohort 2, and 12 (18%) of 68 patients in cohort 3. The higher rate of adverse events in cohort 3 could be explained by the longer exposure of this subgroup to the drug. Interestingly, concerning adverse events of special interest, the incidence of pneumonitis was lower in cohort 1 (1.8%) than in cohorts 2 (2.6%) and 3 (4.4%), even though the first cohort had already received a TKI at some point in their disease.
Based on the results of these phase I and II trials, an ongoing randomized phase III clinical trial is now exploring the efficacy and safety of durvalumab versus standard of care chemotherapy as first-line treatment for patients with advanced EGFRwt/ALKwt NSCLC and a high PD-L1 expression in tumour cells (⩾25%; PEARL; ClinicalTrials.gov identifier: NCT03003962).
In an effort to explore the efficacy and safety of anti-PD(L)-1 treatment in subgroups with specific clinical characteristics that are normally under-represented in clinical trials, the following studies have been designed with durvalumab. The DURATION trial (ClinicalTrials.gov identifier: NCT03345810) evaluates the safety and tolerability of durvalumab after two cycles of standard of care mono- or combination chemotherapy in comparison with standard of care chemotherapy in frail/elderly patients (>70 years). Another phase II study is evaluating the efficacy and tolerability of durvalumab administered to patients with refractory/recurrent brain metastasis and NSCLC or other solid tumours (ClinicalTrials.gov identifier: NCT02669914).
As there is currently no established treatment for patients who have already received ICIs and platinum-doublet therapies, novel drugs are urgently needed. For this purpose, HUDSON (ClinicalTrials.gov identifier: NCT03334617) is an ongoing phase II clinical trial with an umbrella design for patients with metastatic NSCLC who have progressed on an anti-PD-1/PD-L1 containing therapy.
Durvalumab in unresectable stage III NSCLC: the PACIFIC clinical trial
Substantial data from preclinical studies have shown that irradiation of a tumour provokes DNA damage and cell death, but also enhances the formation of tumour-associated neoantigens and damage-associated molecular patterns, that can induce an immunogenic response and promote the activation of cytotoxic T-cells.40 By inducing a systemic increase in antigen recognition, radiation may also induce the T-cell-mediated inhibition of untreated distant tumours (the abscopal effect). It has been demonstrated that radiotherapy in mice induces an increase in PD-L1 levels in the tumour, with a consequent increased tumour sensitivity to a PD-L1 inhibitor added to the effects of the irradiation.41–43
Several case reports and retrospective data from patients treated with ICIs and radiotherapy have reported improved outcomes with the radiotherapy plus PD-1 or PD-L1 inhibitors.44 A secondary analysis of the phase I Keynote 001 clinical trial of pembrolizumab in NSCLC patients45 showed improved PFS and OS in patients that had previously received radiotherapy compared with those that did not (4.4 versus 2.1 months and 10.7 versus 5.3 months, respectively). Although pulmonary toxicity was higher in the group that had already been treated with radiotherapy, it was mainly grade 1 and 2, without any difference in grade 3 and 4 pulmonary toxicities.
With these premises, a randomized, double-blind, placebo-controlled, phase III study of patients with stage III locally advanced unresectable NSCLC who had not progressed following definitive platinum-based chemoradiation (⩾2 cycles), was designed to explore the benefit of durvalumab after standard treatment (PACIFIC; ClinicalTrials.gov identifier: NCT02125461). The first planned interim analysis showed that PFS was significantly increased in the durvalumab arm (16.8 months, 95% CI: 13.0–18.1) compared with placebo (5.6 months, 95% CI: 4.6–7.8), with a stratified HR for progression or death of 0.52 (95% CI: 0.42–0.65, p < 0.0001) and a similar toxicity profile in both arms.9 After a follow up of 14 months, the rate of distant metastasis, including brain metastasis, was lower in the experimental arm (5.5% versus 11%) and all responders maintained a partial or complete response 6 months after having discontinued durvalumab. The PFS benefit with durvalumab was observed in all prespecified groups and was irrespective of PD-L1 expression. Of note, all biopsies were taken before patients underwent chemoradiotherapy, which does not assess the real status of PD-L1 just before starting durvalumab as this could have changed. The identification of a serum biomarker would be ideal in this situation to avoid re-biopsying these patients and potentially increasing the risk of complications. TMB has been described as a reliable tissue predictive biomarker for ICI response, and its assessment in blood (TMBb) could be explored in upcoming clinical trials.29
The toxicity profile of durvalumab was consistent with prior data in metastatic disease, including the immunological side effects. As expected, the incidence of pneumonitis was somehow increased in the durvalumab-treated group (33.9% versus 24.8%), that was mostly grade 1 and 2, and the incidence of more severe cases was low (3.4%) and comparable to that in the control arm (2.6%).
While mature OS results are awaited to confirm the benefit of including durvalumab in the treatment of this population after standard treatment, other unanswered questions will need to be addressed in upcoming clinical trials. First of all, the timing and duration of immunotherapy seems to have played a role in the PACIFIC trial, taking into account that patients who received durvalumab fewer than 14 days after chemoradiation achieved better outcomes (HR of 0.39, 95% CI 0.26–0.58) than those patients treated later (HR 0.63, 95% CI 0.49–0.80). Secondly, it is not known if there is an optimal prior chemoradiation regimen (concurrent versus sequential) or whether chemotherapy could be ideally administered in combination with ICIs, in light of the impact of the combination therapy in recent trials.28–34
Durvalumab in early-stage NSCLC
Early stages (IB–III) of NSCLC have a 5-year OS rate of 25–70% following standard surgical treatment (lobectomy/pneumonectomy with systemic lymph node resection) or SBRT in the earliest stages. Neoadjuvant treatment based on platinum-doublet chemotherapy has been shown to result in an absolute increase in OS of 5% at 5 years, with comparable results to adjuvant treatment. However, >50% of early-stage patients will still die of lung cancer, meaning that novel and more effective strategies are therefore needed to improve outcomes in this group.
Several ongoing clinical trials are exploring durvalumab in the neoadjuvant and adjuvant setting (ClinicalTrials.gov identifiers: NCT 03030131, NCT02572843, NCT03130764). One randomized phase III, double-blind, placebo-controlled clinical trial (ClinicalTrials.gov identifier: NCT02273375), has planned for 1360 patients with completely resected NSCLC (stages IB–IIIA) to be enrolled and randomized to receive durvalumab 10 mg/kg every 2 weeks versus placebo every 2 weeks for up to 12 months after surgery and standard of care platinum-based chemotherapy. In another, phase II trial (ClinicalTrials.gov identifier: NCT03446547), durvalumab will be administered after SBRT as adjuvant treatment for stage I NSCLC patients that are medically inoperable or have refused surgery.
Combination therapy: durvalumab plus tremelimumab
Despite the clinical benefit shown in patients treated with drugs that block the PD-1/PD-L1 pathway, less than one fifth of all patients will experience durable responses. As such, strategies are needed that enhance T-cell activity to improve outcomes. Preclinical data suggest that combinations of CTLA-4 and PD-(L)1 inhibitors have a synergistic effect via different mechanisms of action. That is, by blocking CTLA-4, CD28 can bind to CD80/CD86 and thereby enhance the co-stimulatory signal necessary for robust T-cell activation and effector function, while PD-1 inhibition improves T-cell activation and cytotoxic activity on activated and exhausted lymphocytes.46 Although toxicities appear to occur more frequently with such combinations, the most significant toxicities are immune-related adverse effects, which can be severe but are largely manageable with immunosuppressants.
Greater clinical activity in humans has been shown with the inhibition of multiple immune checkpoints rather than of one checkpoint in melanoma and other tumour types, including NSCLC.47,48 The phase I CheckMate 012 clinical trial combined nivolumab plus ipilimumab as first-line treatment for advanced NSCLC in a multiple arm design comparing different dosage regimens. Although high toxicity rates were seen in several cohorts, leading to treatment discontinuation, nivolumab 3 mg/kg every 2 weeks plus ipilimumab 1 mg/kg every 6 or 12 weeks appeared to be more tolerable and had promising antitumour activity (ORR 38–47%). This regimen was chosen for the phase III CheckMate 227 clinical trial, that showed improved PFS in patients with a high TMB.29
Tremelimumab is a selective fully humanized IgG2 monoclonal antibody inhibitor of CTLA-4 that promotes activation of cytotoxic T-cells in an early stage of the immune response. Preclinical data indicate that targeting PD-L1/PD-1 and CTLA-4 pathways could have additive or synergistic effects.49
The first randomized open-label, multicentre, phase Ib clinical trial of durvalumab and tremelimumab (Study 006; ClinicalTrials.gov identifier: NCT02000947) showed that durvalumab 20 mg/kg plus tremelimumab 1 mg/kg every 4 weeks had a manageable tolerability profile, with antitumour activity irrespective of PD-L1 status; this was selected as the dose for the expansion phase and phase III studies, which are now ongoing.13 The most frequent adverse events were consistent with the known toxicity profiles of durvalumab and tremelimumab, and comparable to those of the nivolumab and ipilimumab combinations.47,48 Grade 3 or 4 drug-related adverse events were reported in 42% of the overall population, and in 17% of the cohort receiving durvalumab 20 mg/kg and tremelimumab 1 mg/kg every 4 weeks; the most common of these events were diarrhoea (11; 11%), colitis (9; 9%) and increased lipase (8; 8%).
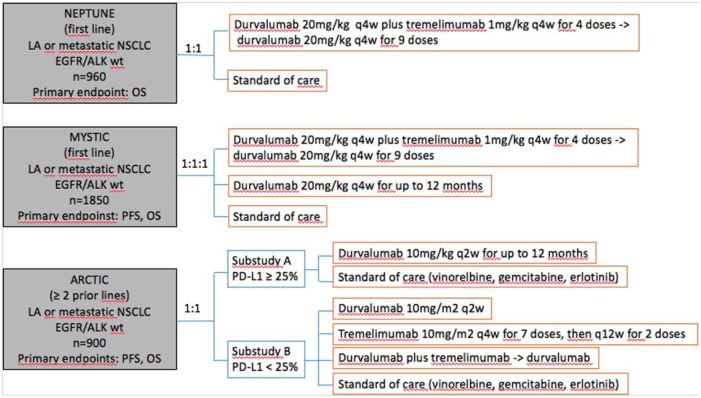
At present, there are three ongoing phase III clinical trials assessing the efficacy of durvalumab in combination with tremelimumab in different NSCLC populations (Figure 2).50–52 Of all of them, researchers of the MYSTIC clinical trial informed last year that tremelimumab plus durvalumab did not meet one of its primary endpoints (PFS) compared with standard chemotherapy.
Figure 2.
Ongoing phase III clinical trials combining durvalumab with tremelimumab in different NSCLC populations.
LA, locally advanced; OS, overall survival; PFS, progression-free survival; q2w, every 2 weeks; q4w, every 4 weeks; q12w, every 12 weeks; wt, wild type.
Combination therapy: durvalumab with chemotherapy and other drugs
The dual role of cytotoxicity and immune system activation played by chemotherapy has provided a biological rationale for the development of combinations with immunotherapy. Following the results of the clinical trials combining an anti-PD-1 with chemotherapy in advanced NSCLC in the first-line setting28,29 and an anti-PD-L1 with chemotherapy and bevacizumab,34 several studies are currently exploring the combination of an ICI with chemotherapy to improve effectiveness.53 The Canadian Cancer Trials Group is conducting a phase Ib trial (INC.226 study, ClinicalTrials.gov identifier: NCT02537418) to evaluate the safety and efficacy of platinum-based chemotherapy plus durvalumab with or without tremelimumab in solid malignancies in a PD-L1 un-selected population. They reported that durvalumab 15 mg/kg every 3 weeks and tremelimumab 1 mg/kg (multiple doses q6w) or 3 mg/kg (3 doses q6w) can be safely combined with full doses of platinum-doublet chemotherapy.54 In 17 of the 24 patients with advanced nonsquamous NSCLC, an objective response rate of 52.9% (95% CI: 28–77%) was seen. Most of the drug-related adverse events were grade 1 or 2, thus enabling the dose for the planned phase II and III studies of the quadruplet treatment to be established.
One of the largest phase III studies recruiting patients in this scenario is the POSEIDON clinical trial (ClinicalTrials.gov identifier: NCT03164616), which investigates the efficacy and safety of durvalumab plus tremelimumab with platinum-based chemotherapy versus durvalumab with platinum-based chemotherapy versus platinum-based chemotherapy alone in NSCLC patients with confirmed tumour PD-L1 status prior to randomization. Other combinations of chemotherapy with durvalumab are being explored in a phase II trial with nab-paclitaxel and durvalumab in previously treated NSCLC patients (ClinicalTrials.gov identifier: NCT02250326).
Patients with advanced NSCLC harbouring EGFR mutations and ALK rearrangements have been frequently excluded from clinical trials of ICIs, and the small number of patients included in these trials does not allow us to determine whether this population would benefit from these treatments.55,56 Nevertheless, recent data from the Impower 150 clinical trial, suggest that the combination of chemotherapy and an antiPDL1 can be effective in the EGFRmut population.34 Regarding durvalumab, data from cohort 1 of the ATLANTIC clinical trial showed promising activity of this drug, particularly in patients with EGFRmut and high PD-L1 expression in tumour cells.12
Numerous studies have been designed to evaluate the efficacy of combined treatment of a TKI with ICIs, where high response rates have been reported, although accompanied by higher toxicity.57,58 Among these, two clinical trials have evaluated the safety profile of the combination of a TKI with durvalumab.59,60 The first is a phase I study that combines gefitinib 250 mg once daily with durvalumab 10 mg/kg every 2 weeks (ClinicalTrials.gov identifier: NCT02088112); there, an ORR of 80% in 20 treatment-naïve EGFRmut NSCLC patients was reported. TRAEs consisted of diarrhoea (80%), increased ALT/AST (55%) and rash (60%), leading to discontinuation in four patients (three due to increased ALT/AST and one due to pneumonitis). TATTON (ClinicalTrials.gov identifier: NCT02143466), is a multi-arm phase Ib study of osimertinib in combination with different novel drugs in patients with advanced NSCLC and EGFRmut. After a dose escalation phase of osimertinib 80 mg once daily with durvalumab 10 mg/kg every 2 weeks, 11 treatment-naïve EGFRmut NSCLC patients were included in the expansion phase, reaching an ORR of 80%. Despite this outcome, interstitial lung disease was reported in 7 out of the 11 patients (64%; including 3 grade 3/4 cases) and diarrhoea in 6 patients (55%), which led to a premature cessation of this arm of the study due to pulmonary toxicity.
Cases of ALK-rearranged NSCLC in these clinical trials were seen less frequently than EGFRmut, so little evidence has been obtained concerning the efficacy of immunotherapy in this population.61 To date, a phase I/II study of the safety and tolerability of nivolumab plus crizotinib for the first-line treatment of ALK-positive advanced NSCLC had to be terminated prematurely due to cases of severe hepatic toxicity.62 An ongoing phase I/II clinical trial is evaluating the efficacy of a new ALK TKI (ensartinib) with durvalumab in ALK-positive advanced NSCLC patients (ClinicalTrials.gov identifier: NCT02898116).
Combination therapy: durvalumab with radiotherapy
The impressive results of the PACIFIC clinical trial9 have laid the foundations for several clinical trials combining radiotherapy with durvalumab, including a Pacific-2 study where a concomitant chemoradiation plus durvalumab arm will be studied. The benefit of durvalumab with or without tremelimumab administered every 4 weeks for two doses concurrently with standard thoracic radiation (RT) (45 Gy in 25 fractions) as neoadjuvant immunoradiation, is now being evaluated in patients with stage IIIa NSCLC (ClinicalTrials.gov identifier: NCT03237377). Also in the neoadjuvant setting, a study to assess the efficacy and tolerability of durvalumab with or without SBRT in patients with stage I–IIIa NSCLC is underway (ClinicalTrials.gov identifier: NCT02904954). Based on the abscopal effect hypothesis, several ongoing clinical trials for patients with oligometastatic and metastatic disease are evaluating the clinical benefit and safety of durvalumab with or without tremelimumab combined with different doses and schedules of radiotherapy (SBRT, high- or low-dose radiation therapy or hypofractionated radiotherapy).
Biomarkers: PD-L1 expression and beyond
One of the most significant challenges confronting immunotherapy is the identification of biomarkers that allow a more personalized medicine to be offered to each patient, thereby avoiding unnecessary exposure to potential toxicities and providing the greatest effectiveness, both clinically and financially.
The expression of PD-L1 in tumour tissue serves as one of the most studied biomarkers for ICIs. This expression correlates with a poor prognosis in many solid tumours, but a directly proportional relationship between PD-L1 expression and the efficacy of inhibitors of the PD-1/PD-L1 pathway has been shown in some clinical trials, including NSCLC.19,63 Nevertheless, when adding chemotherapy to immunotherapy or combining two different ICIs, the benefit in efficacy of these combinations was reproduced in every subgroup of patients regardless of PD-L1 expression in tumour cells, although the magnitude of the benefit seems to be still largely related PD-L1 expression. On the other hand, as different drugs have been approved, they have been accompanied by different IHC assays for PD-L1 expression, each designed by different companies and each using a different cut-off for the expression of this biomarker. The use of PD-L1 IHC as a predictive biomarker is confounded by multiple unresolved issues: antibodies with variable detection, differing IHC cut-offs, tissue preparation, processing variability, primary versus metastatic biopsies, oncogenic versus induced PD-L1 expression, and staining of tumour versus immune cells. This makes it difficult to interchange the current assays and cut-offs in each centre for the different drugs available and therefore may lead to a misclassification of the PD-L1 status for some patients.64–66
The BLUEPRINT project sought to compare in different samples of NSCLC tumour tissue some of the currently available IHC assays in an effort to clarify concordance levels for each one.67 In the phase I of that study, investigators concluded that three of the four assays (22C3, 28-8 and SP263) were closely aligned on tumour cell staining whereas the forth (SP142) showed a consistently lower number of tumour cells being stained. Despite these results, replacement of the validated cut-off for each assay with any other cut-off reduces the overall agreement compared with the reference standard. It would be interesting, perhaps in the postmarket setting, to study the correlation between the PD-L1 staining and different cut-offs with outcomes for the different anti-PD-(L)1 agents in the NSCLC population. The PD-L1 IHC assays also help to detect PD-L1 expression in immune cells, which has also been described as a predictive biomarker of response to ICIs. In the above-mentioned study, all of the assays demonstrated immune cell staining, but with greater variability than with tumour cell staining.
Taking into account the results of the CheckMate 227 and Keynote-189 trials, which show that some patients with PD-L1-negative tumours have durable responses to ICIs,28,29 a major need is to define other predictive biomarkers. The MSKCC group and others have demonstrated that a greater somatic mutation burden is associated with a higher likelihood of response to immunotherapy in several tumour types, including melanoma, bladder cancer, NSCLC and mismatch repair-deficient tumours.68 Recent analyses from the CheckMate 568 trial, a phase II trial of nivolumab plus ipilimumab in NSCLC, identified a TMB of at least 10 mutations per megabase (determined in targeted next-generation sequencing panels) as an effective cut-off for selecting patients most likely to exhibit a response.69 These results were confirmed in the CheckMate 227 trial irrespective of the tumour PD-L1 expression level, thus establishing the importance of TMB as a biomarker that may be relevant across tumour types. This is also being investigated in blood (bTMB).70
Conclusions: clinical potential and future directions
The immunotherapy era has broadened treatment options for patients with advanced NSCLC, thereby improving survival outcomes with potentially less toxicity. One of the latest strategies in NSCLC clinical trials, combining an anti-PD-(L)1 with chemotherapy, has shown durable responses and significant improvement of PFS and OS across all categories of PD-L1 expression in tumour cells.
In light of the recently reported relationship between TMB and response to immunotherapy and given that mutant EGFR and PD-L1-negative patients can also benefit from treatment with durvalumab and other immunotherapies, the search of a reliable predictive biomarker is one of the most important issues that need to be assessed. On the other hand, it is vitally important to understand the biology behind tumours of nonresponders as well as reasons for disease progression in initial responders.
Durvalumab has shown durable responses and clinical benefit, particularly in advanced NSCLC patients with PD-L1 expression in ⩾25% of tumour cells, but also in patients not selected on the basis of their PD-L1 status. The most robust data until now have been shown in stage III NSCLC patients after chemoradiation, where, PFS was significantly longer with durvalumab (16.8 months) than with placebo (5.6 months). Moreover, the median time to distant metastasis or death was also increased in the experimental arm. The risk of pneumonitis, one of the expected risks of the proposed treatment sequence, was increased but only of low grade and was easily manageable in most instances.
There are currently numerous ongoing clinical trials with durvalumab as monotherapy and in combination with other agents, being tremelimumab one of the most promising partners for durvalumab. Results from two phase III trials in the first-line setting (NEPTUNE and MYSTIC) are awaited to try to assess its efficacy compared with standard of care chemotherapy.
Other areas of interest include the potential role of retreating patients with these agents after progression or TRAEs, the timing and duration of treatment, or the possibility of treating patients with chemotherapy or radiation therapy that could theoretically alter PD-1/PD-L1 expression levels in tumours. All ongoing clinical trials with durvalumab and other ICIs will help in the evaluation of their perceived benefits in NSCLC patients.
Footnotes
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest statement: The authors declare that there is no conflict of interest.
ORCID iD: Nerea Muñoz-Unceta  https://orcid.org/0000-0003-4962-3949
https://orcid.org/0000-0003-4962-3949
Contributor Information
Nerea Muñoz-Unceta, Medical Oncology Department, Hospital Universitario 12 de Octubre and Instituto de Investigación i+12, Madrid, Spain.
Isabel Burgueño, Medical Oncology Department, Hospital Universitario 12 de Octubre and Instituto de Investigación i+12, Madrid, Spain.
Elizabeth Jiménez, Medical Oncology Department, Hospital Universitario 12 de Octubre and Instituto de Investigación i+12, Madrid, Spain.
Luis Paz-Ares, Servicio de Oncología Médica, Hospital Universitario 12 de Octubre, Av. de Córdoba km 5,4, 28041 Madrid, Spain.
References
- 1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin 2017; 67: 7–30. [DOI] [PubMed] [Google Scholar]
- 2. Hirsch FR, Scagliotti GV, Mulshine JL, et al. Lung cancer: current therapies and new targeted treatments. Lancet 2017; 389: 299–311. [DOI] [PubMed] [Google Scholar]
- 3. Kyi C, Postow MA. Checkpoint blocking antibodies in cancer immunotherapy. FEBS Lett 2014; 588: 368–376. [DOI] [PubMed] [Google Scholar]
- 4. Remon J, Vilariño N, Reguart N. Immune checkpoint inhibitors in non-small cell lung cancer (NSCLC): approaches on special subgroups and unresolved burning questions. Cancer Treat Rev 2018; 64: 21–29. [DOI] [PubMed] [Google Scholar]
- 5. Herbst RS, Baas P, Kim DW, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non- small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet 2016; 387: 1540–1550. [DOI] [PubMed] [Google Scholar]
- 6. Reck M, Rodríguez-Abreu D, Robinson AG, et al. Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N Engl J Med 2016; 375: 1823–1833. [DOI] [PubMed] [Google Scholar]
- 7. Rittmeyer A, Barlesi F, Waterkamp D, et al. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial. Lancet 2017; 389: 255–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Vokes EE, Ready N, Felip E, et al. Nivolumab versus docetaxel in previously treated advanced non-small cell lung cancer (CheckMate 017 and CheckMate 057): 3-year update and outcomes in patients with liver metastases. Ann Oncol. 2018; 29: 959–965. [DOI] [PubMed] [Google Scholar]
- 9. Antonia SJ, Villegas A, Daniel D, et al. Durvalumab after chemoradiotherapy in stage III non-small-cell lung cancer. N Engl J Med 2017; 377: 1919–1929. [DOI] [PubMed] [Google Scholar]
- 10. Patel SP, Kurzrock R. PD-L1 IHC as a predictive biomarker in cancer immunotherapy. Mol Cancer Ther 2015; 14: 847–856. [DOI] [PubMed] [Google Scholar]
- 11. Antonia SJ, Brahmer JR, Khleif S, et al. Phase 1/2 study of the safety and clinical activity of durvalumab in patients with non-small cell lung cancer (NSCLC). Ann Oncol 2016; 27(Suppl. 6): 1216PD. [Google Scholar]
- 12. Garassino MC, Cho BC, Kim JH, et al. Durvalumab as third-line or later treatment for advanced non-small-cell lung cancer (ATLANTIC): an open-label, single-arm, phase 2 study. Lancet Oncol 2018; 19: 521–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Antonia S, Goldberg SB, Balmanoukian A, et al. Safety and antitumour activity of durvalumab plus tremelimumab in non-small-cell lung cancer: a multicentre, phase 1b study. Lancet Oncol 2016; 17: 299–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Burnet FM. The concept of immunological surveillance. Prog Exp Tumor Res 1970; 13: 1–27. [DOI] [PubMed] [Google Scholar]
- 15. Schreiber RD, Old LJ, Smyth MJ. Cancer immunoediting: integrating immunity’s roles in cancer suppression and promotion. Science 2011; 331: 1565–1570. [DOI] [PubMed] [Google Scholar]
- 16. Pennock GK, Chow LQ. The evolving role of immune checkpoint inhibitors in cancer treatment. Oncologist 2015; 20: 812–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer 2012; 12: 252–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Seetharamu N, Burdman DR, Sullivan KM. Immune checkpoint inhibitors in lung cancer: present, past and future. Future Oncol 2016; 12: 1151–1163. [DOI] [PubMed] [Google Scholar]
- 19. Yearley JH, Gibson C, Yu N, et al. PD-L2 expression in human tumors: relevance to anti-PD-1 therapy in cancer. Clin Cancer Res 2017; 23: 3158–3167. [DOI] [PubMed] [Google Scholar]
- 20. Hodi FS, O’Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med 2010; 363: 711–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med 2015; 373: 123–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med 2015; 373: 1627–1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lopes G, Wu Y-L, Kudaba I, et al. Pembrolizumab (pembro) versus platinum-based chemotherapy (chemo) as first-line therapy for advanced/metastatic NSCLC with a PD-L1 tumor proportion score (TPS) ⩾1%: open-label, phase 3 KEYNOTE-042 study. J Clin Oncol 2018; 36(Suppl. 18): LBA4–LBA4. [Google Scholar]
- 24. Carbone DP, Reck M, Paz-Ares L, et al. First-line nivolumab in stage IV or recurrent non-small-cell lung cancer. N Engl J Med 2017; 376: 2415–2426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Peters S, Gettinger S, Johnson ML. Phase II trial of atezolizumab as first-line or subsequent therapy for patients with programmed death-ligand 1-selected advanced non-small-cell lung cancer (BIRCH). J Clin Oncol 2017; 35: 2781–2789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Gulley JL, Rajan A, Spigel DR, et al. Avelumab for patients with previously treated metastatic or recurrent non-small-cell lung cancer (JAVELIN Solid Tumor): dose-expansion cohort of a multicentre, open-label, phase 1b trial. Lancet Oncol 2017; 18: 599–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hellmann MD, Antonia SJ, Balmanoukian AS, et al. Updated overall survival and safety profile of durvalumab monotherapy in advanced NSCLC. J Clin Oncol 2018; 36(Suppl. 5): 169–169. [Google Scholar]
- 28. Gandhi L, Rodríguez-Abreu D, Gadgeel S, et al. Pembrolizumab plus chemotherapy in metastatic non-small-cell lung cancer. N Engl J Med 2018; 378: 2078–2092. [DOI] [PubMed] [Google Scholar]
- 29. Hellmann MD, Ciuleanu TE, Pluzanski A, et al. Nivolumab plus ipilimumab in lung cancer with a high tumor mutational burden. N Engl J Med 2018; 378: 2093–2104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Langer CJ, Gadgeel SM, Borghaei H, et al. Carboplatin and pemetrexed with or without pembrolizumab for advanced, non-squamous non-small-cell lung cancer: a randomised, phase 2 cohort of the open-label KEYNOTE-021 study. Lancet Oncol 2016; 17: 1497–1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Rizvi NA, Hellmann MD, Brahmer JR, et al. Nivolumab in combination with platinum-based doublet chemotherapy for first-line treatment of advanced non-small-cell lung cancer. J Clin Oncol 2016; 34: 2969–2979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Paz-Ares LG, Luft A, Tafreshi A, et al. Phase 3 study of carboplatin-paclitaxel/nab-paclitaxel (Chemo) with or without pembrolizumab (Pembro) for patients (Pts) with metastatic squamous (Sq) non-small cell lung cancer (NSCLC). J Clin Oncol 2018; 36(Suppl. 15): 105–105. [Google Scholar]
- 33. Borghaei H, Hellmann MD, Paz-Ares LG, et al. Nivolumab (Nivo) + platinum-doublet chemotherapy (Chemo) vs chemo as first-line (1L) treatment (Tx) for advanced non-small cell lung cancer (NSCLC) with <1% tumor PD-L1 expression: results from CheckMate 227. J Clin Oncol 2018; 36(Suppl. 15): 9001–9001. [Google Scholar]
- 34. Socinski MA, Jotte RM, Cappuzzo F, et al. Atezolizumab for first-line treatment of metastatic nonsquamous NSCLC. N Engl J Med. 2018; 378: 2288–2301. [DOI] [PubMed] [Google Scholar]
- 35. Mu CY, Huang JA, Chen Y, et al. High expression of PD-L1 in lung cancer may contribute to poor prognosis and tumor cells immune escape through suppressing tumor infiltrating dendritic cells maturation. Med Oncol 2011; 28: 682–688. [DOI] [PubMed] [Google Scholar]
- 36. Stewart R, Morrow M, Hammond SA, et al. Identification and characterization of MEDI4736, an antagonistic anti-PD-L1 monoclonal antibody. Cancer Immunol Res 2015; 3: 1052–1062. [DOI] [PubMed] [Google Scholar]
- 37. Ibrahim R, Stewart R, Shalabi A. PD-L1 blockade for cancer treatment: MEDI4736. Semin Oncol 2015; 42: 474–483. [DOI] [PubMed] [Google Scholar]
- 38. Segal NH, Hamid O, Hwu W, et al. A phase I multi-arm dose-expansion study of the anti-programmed cell death-ligand-1 (PD-L1) antibody MEDI4736: preliminary data. Ann Oncol 2014; 25(Suppl. 4): iv361–iv372 (abstract 1058PD ESMO 2014). [Google Scholar]
- 39. Pillai RN, Behera M, Owonikoko TK, et al. Comparison of the toxicity profile of PD-1 versus PD-L1 inhibitors in non-small cell lung cancer: a systematic analysis of the literature. Cancer 2018; 124: 271–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Wang Y, Deng W, Li N, et al. Combining immunotherapy and radiotherapy for cancer treatment: current challenges and future directions. Front Pharmacol 2018; 9: 185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Dovedi SJ, Adlard AL, Lipowska-Bhalla G, et al. Acquired resistance to fractionated radiotherapy can be overcome by concurrent. Cancer Res. 2014; 74: 5458–5468. [DOI] [PubMed] [Google Scholar]
- 42. Derer A, Spiljar M, Baumler M, et al. Chemoradiation increases PD-L1 expression in certain melanoma and glioblastoma cells. Front Immunol 2016; 7: 610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Hecht M, Buttner-Herold M, Erlenbach-Wunsch K, et al. PD-L1 is upregulated by radiochemotherapy in rectal adenocarcinoma patients and associated with a favourable prognosis. Eur J Cancer 2016; 65: 52–60. [DOI] [PubMed] [Google Scholar]
- 44. Levy A, Massard C, Soria JC, et al. Concurrent irradiation with the anti-programmed cell death ligand-1 immune checkpoint blocker durvalumab: single centre subset analysis from a phase 1/2 trial. Eur J Cancer 2016; 68: 156–162. [DOI] [PubMed] [Google Scholar]
- 45. Shaverdian N, Lisberg AE, Bornazyan K, et al. Previous radiotherapy and the clinical activity and toxicity of pembrolizumab in the treatment of non-small-cell lung cancer: a secondary analysis of the KEYNOTE-001 phase 1 trial. Lancet Oncol 2017; 18: 895–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Mahoney KM, Rennert PD, Freeman GJ. Combination cancer immunotherapy and new immunomodulatory targets. Nat Rev Drug Discov 2015; 14: 561–584. [DOI] [PubMed] [Google Scholar]
- 47. Larkin J, Chiarion-Sileni V, Gonzalez R, et al. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med 2015; 373: 23–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Hellmann MD, Rizvi NA, Goldman JW, et al. Nivolumab plus ipilimumab as first-line treatment for advanced non-small-cell lung cancer (CheckMate 012): results of an open-label, phase 1, multicohort study. Lancet Oncol 2017; 18: 31–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Stewart R, Mullins S, Watkins A, et al. Preclinical modeling of immune checkpoint blockade (P2012). J Immunol 2013; 190(Suppl): abstract 214.7. [Google Scholar]
- 50. Planchard D, Yokoi T, McCleod MJ, et al. A phase III study of durvalumab (MEDI4736) with or without tremelimumab for previously treated patients with advanced NSCLC: rationale and protocol design of the ARCTIC study. Clin Lung Cancer 2016; 17: 232–236. [DOI] [PubMed] [Google Scholar]
- 51. Peters S, Antonia S, Goldberg SB, et al. 191TiP: MYSTIC: a global, phase 3 study of durvalumab (MEDI4736) plus tremelimumab combination therapy or durvalumab monotherapy versus platinum-based chemotherapy (CT) in the first-line treatment of patients (pts) with advanced stage IV NSCLC. J Thorac Oncol 2016; 11(Suppl. 4): S139–S140. [Google Scholar]
- 52. Mok T, Schmid P, Arén O, et al. 192TiP: NEPTUNE: a global, phase 3 study of durvalumab (MEDI4736) plus tremelimumab combination therapy versus standard of care (SoC) platinum-based chemotherapy in the first-line treatment of patients (pts) with advanced or metastatic NSCLC. J Thorac Oncol 2016; 11(Suppl. 4): S140–S141. [Google Scholar]
- 53. Harris SJ, Brown J, Lopez J, et al. Immuno-oncology combinations: raising the tail of the survival curve. Cancer Biol Med 2016; 13: 171–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Juergens R, Hao D, Laurie S, et al. MA09.03 Cisplatin/pemetrexed + durvalumab +/− tremelimumab in pts with advanced non-squamous NSCLC: a CCTG phase IB study - IND.226. J Thorac Oncol 2017; 12(Suppl. 1): S392–S393. [Google Scholar]
- 55. Soo RA, Lim SM, Syn NL, et al. Immune checkpoint inhibitors in epidermal growth factor receptor mutant non-small cell lung cancer: current controversies and future directions. Lung Cancer 2018; 115: 12–20. [DOI] [PubMed] [Google Scholar]
- 56. Lee CK, Man J, Lord S, et al. Checkpoint inhibitors in metastatic EGFR-mutated non-small cell lung cancer - a meta-analysis. J Thorac Oncol 2017; 12: 403–407. [DOI] [PubMed] [Google Scholar]
- 57. Ma BBY, Rudin CM, Cervantes A, et al. Preliminary safety and clinical activity of erlotinib plus atezolizumab from a phase 1b study in advanced NSCLC. Ann Oncol 2016; 27(Suppl. 9): ix139–ix156. [Google Scholar]
- 58. Rizvi NA, Chow LQM, Borghaei H, et al. Safety and response with nivolumab (anti-PD-1; BMS-936558, ONO-4538) plus erlotinib in patients (pts) with epidermal growth factor receptor mutant (EGFR MT) advanced NSCLC. J Clin Oncol 2014; 32(Suppl. 5): abstract 8022. [Google Scholar]
- 59. Gibbons DL, Chow LQM, Kim DW, et al. Efficacy, safety and tolerability of MED (durvalumab) a human IgG1 anti-programmed cell death-ligand-1 antibody, combined with gefitinib: a phase I expansion in TKI-naive patients with EGFR mutant NSCLC. J Thorac Oncol 2016; 11(Suppl. 4): S79. [Google Scholar]
- 60. Ahn MJ, Yang J, Yu H, et al. Osimertinib combined with durvalumab in EGFR-mutant non-small cell lung cancer: results from the TATTON phase Ib trial. J Thorac Oncol 2016; 11(Suppl. 4): S115. [Google Scholar]
- 61. Bylicki O, Paleiron N, Margery J, et al. Targeting the PD-1/PD-L1 immune checkpoint in EGFR-mutated or ALK-translocated non-small-cell lung cancer. Target Oncol 2017; 12: 563–569. [DOI] [PubMed] [Google Scholar]
- 62. Spigel DR, Reynolds C, Waterhouse D, et al. Phase 1/2 study of the safety and tolerability of nivolumab plus crizotinib for the first-line treatment of ALK translocation-positive advanced non-small cell lung cancer (CheckMate 370). J Thorac Oncol 2018; 13: 682–688. [DOI] [PubMed] [Google Scholar]
- 63. Brody R, Zhang Y, Ballas M, et al. PD-L1 expression in advanced NSCLC: insights into risk stratification and treatment selection from a systematic literature review. Lung Cancer 2017; 112: 200–215. [DOI] [PubMed] [Google Scholar]
- 64. Soo RA, Yun Lim JS, Asuncion BR, et al. Determinants of variability of five programmed death ligand-1 immunohistochemistry assays in non-small cell lung cancer samples. Oncotarget 2018; 9: 6841–6851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Rimm DL, Han G, Taube JM, et al. A Prospective, multi-institutional assessment of four assays for PD-L1 expression in NSCLC by immunohistochemistry. JAMA Oncol 2017; 3: 1051–1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Diggs LP, Hsueh EC. Utility of PD-L1 immunohistochemistry assays for predicting PD-1/PD-L1 inhibitor response. Biomark Res 2017; 5: 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Hirsch FR, McElhinny A, Stanforth D, et al. PD-L1 Immunohistochemistry assays for lung cancer: results from phase 1 of the blueprint PD-L1 IHC assay comparison project. J Thorac Oncol 2017; 12: 208–222. [DOI] [PubMed] [Google Scholar]
- 68. Rizvi NA, Hellmann MD, Snyder A, et al. Cancer immunology. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science 2015; 348: 124–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Ramalingam SS, Hellmann MD, Awad MM, et al. Tumor mutation burden (TMB) as a biomarker for clinical benefit from dual immune checkpoint blockade with nivolumab (nivo) + ipilimumab (ipi) in first-line (1L) non-small cell lung cancer (NSCLC): identification of TMB cutoff from CheckMate 568. Presented at the American Association for Cancer Research 2018 Annual Meeting, 16 April 2018, Chicago. [Google Scholar]
- 70. Gandara DR, Kowanetz M, Mok T, et al. Blood-based biomarkers for cancer immunotherapy: Tumor mutational burden in blood (bTMB) is associated with improved atezolizumab (atezo) efficacy in 2L+ NSCLC (POPLAR and OAK). Ann Oncol 2017; 28(Suppl. 5): v460–v496. [Google Scholar]