Abstract
Background:
The structure and function of the anterolateral aspect of the knee have been significantly debated, with renewed interest in this topic since the description of the anterolateral ligament (ALL).
Purpose:
To define and describe the distinct structures of the lateral knee and to correlate the macroscopic and histologic anatomic features.
Study Design:
Descriptive laboratory study.
Methods:
Twelve fresh-frozen human cadavers were used for anatomic analysis. In the left knee, a layer-by-layer dissection and macroscopic analysis were performed. In the right knee, an en bloc specimen was obtained encompassing an area from the Gerdy tubercle to the posterior fibular head and extending proximally from the anterior aspect to the posterior aspect of the lateral femoral epicondyle. The en bloc resection was then frozen, sliced at the level of the joint line, and reviewed by a musculoskeletal pathologist.
Results:
Macroscopically, the lateral knee has 4 main layers overlying the capsule of the knee: the aponeurotic layer, the superficial layer including the iliotibial band (ITB), the deep fascial layer, and the ALL. Histologically, 8 of 12 specimens demonstrated 4 consistent, distinct structures: the ITB, the ALL, the lateral collateral ligament, and the meniscus.
Conclusion:
The lateral knee has a complex orientation of layers and fibers. The ALL is a distinct structure from the ITB and is synonymous to the previously described capsulo-osseous layer of the ITB.
Clinical Relevance:
Increasingly, lateral extra-articular procedures are performed at the time of anterior cruciate ligament reconstruction. Understanding the anatomic features of the anterolateral aspect of the knee is necessary to understand the biomechanics and function of the structures present and allows surgeons to attempt to replicate those anatomic characteristics when performing extra-articular reconstruction.
Keywords: knee anterolateral ligament, anatomic features of the lateral knee, histology
Several recent studies have reported that the anterolateral structures of the knee have an important biomechanical role in a knee with anterior cruciate ligament (ACL) injury with respect to restoring normal kinematics,19 reducing the rate of persistent anterolateral rotatory instability,34,40 and reducing graft rupture rates.44 Despite the demonstrable biomechanical function of the anterolateral structures of the knee, the precise anatomic characteristics remain a controversial topic; some authors have questioned whether the anterolateral ligament (ALL) is “fact or fiction,”29 whereas others have stated that the ALL is simply a capsular thickening.6,7,45
Smeets et al38 demonstrated that the ALL is a discrete structure and is similar in tensile and histological properties to the inferior glenohumeral ligament. However, the controversy persists given that in recent reports, the rate of identification of the ALL as a discrete ligament, defined as a collagenous structure connecting 2 osseous structures, has varied from 0% to 100%.12,16,20,36,45,51 Some authors have suggested that part of this discrepancy is due to the variation in dissection protocols and lack of a standardized dissection technique.10,44
Significant debate remains regarding the existence of the ALL, the presence of a distinct ligamentous structure, and the nomenclature used to describe the structures of the lateral aspect of the knee.14,23,32,37 The purpose of this study was to define the anatomic structures of the lateral side of the knee through use of both macroscopic and histologic analysis of an en bloc resection.
Methods
The lateral side of the knee was examined in 12 paired fresh-frozen specimens provided for study by the donation program of Kansas City University. The left knee of each fresh-frozen specimen was used for a layer-by-layer anatomic analysis performed by the lead author (M.D.) and coauthor (C.S.) together. An en bloc resection of the lateral side of the knee was then performed on the right knee of each specimen. Knees with previous total knee arthroplasty, below-knee amputation, evidence of ACL injury or ACL reconstruction, or evidence of prior surgery affecting the lateral aspect of the knee were excluded from analysis. This left 7 male cadavers (14 knees) and 5 female cadavers (10 knees) available for dissection (median age at death, 76.5 years; range, 55-95 years). The average weight was 162.4 lb (range, 105-260 lb). These data were self-reported at the time of volunteering to the Gift Body program at Kansas City University.
Macroscopic Analysis
A layer-by-layer dissection was performed by use of a 10-blade scalpel consistent with the technique described by Terry et al.46 The skin was removed circumferentially, and underlying adipose tissue was removed. Careful dissection and documentation of each layer anterior of the lateral collateral ligament were then performed until the joint was fully exposed.
En Bloc Resection
The superior and anterior joint line aspects of the resection were marked with No. 0 silk and No. 0 PDS (Ethicon), respectively. An en bloc resection of the lateral side of the knee was performed (M.D., B.W.) with a 10-blade scalpel (Figure 1).
Figure 1.
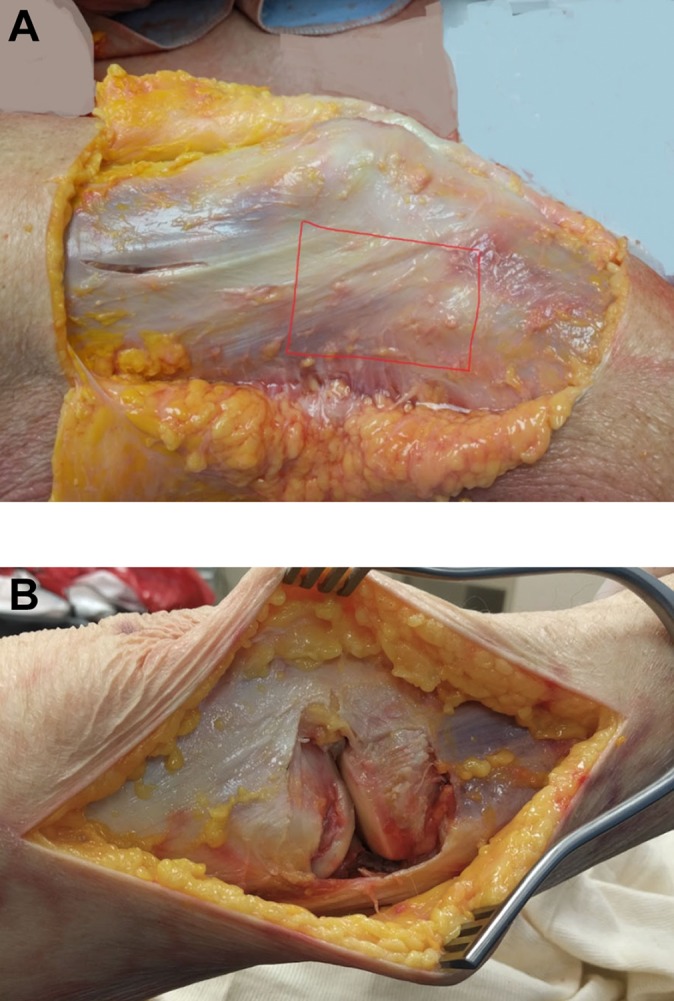
(A) The lateral side of the right knee was exposed after the skin and subcutaneous tissue were removed. The area outlined in red was used as the border for en bloc resection. (B) View of the lateral knee after a full-thickness, en bloc resection of the lateral knee.
All soft tissues were excised en bloc from their osseous structures. The posterior margin of the en bloc resection was the lateral collateral ligament (LCL), and the anterior margin was the patellar tendon. Superiorly, the margins included all tissue inferior to the level of the lateral epicondyle, and the margins inferiorly were defined by the attachment site of all lateral structures. The specimens were then frozen under suture tension to ensure accuracy of slicing. Frozen en bloc sections were then sliced horizontally at the superior margin of the meniscus of the joint line, along the No. 0 PDS suture into 2.5 cm–long portions (Figure 2).
Figure 2.
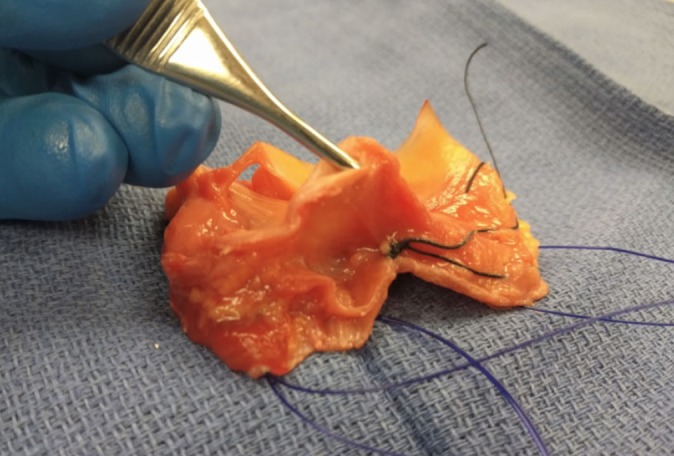
No. 0 PDS sutures were used to mark the joint line of the en bloc resection of the lateral knee. Frozen en bloc sections were then sliced horizontally at the joint line along the No. 0 PDS suture.
These specimens were then placed into tissue cassettes and thawed to room temperature. Each cassette was placed in 10% neutral buffered formalin fixative and submitted for routine tissue processing. Sections were cut at 5 μm and stained with hematoxylin and eosin. Slides were then reviewed by a board-certified pathologist (J.D.).
Results
Macroscopic Dissection
The layer-by-layer dissection and reflection of the lateral knee resulted in 4 distinct layers anterior to the lateral collateral ligament.
Layer 1: Aponeurotic Layer
After complete removal of subcutaneous fat, the initial layer encountered is the aponeurotic layer.49 This layer completely engulfs the lateral aspect of the knee and continues over the anterior and posterior aspects of the knee (Figure 3).
Figure 3.
The superficial layer of the lateral knee is the aponeurotic layer.
Layer 2: The Superficial Layer
The next layer consists of the biceps femoris (BF) and a complex interconnection of structures coming from the iliotibial tract (ITT), including the iliotibial band (ITB), the iliopatellar band (IPB), and the lateral patellotibial ligament (LPTL) (Figure 4). The ITT proximally is uniform and is associated with the underlying vastus lateralis muscle belly. As the ITT courses distally, it becomes thin and broad and diverges into 2 main tracts that are still continuous with one another: the ITB and the IPB. The fibers of the IPB are broad and have an insertion in close proximity to the vastus lateralis insertion on the patella, and others course distally to the patellar tendon. The fibers of the ITB course distally and attach directly onto the Gerdy tubercle. The LPTL courses from the patellar insertion of the IPB distally to the fibers connecting the ITB to the Gerdy tubercle. Posteriorly, the BF attaches to the fibular head, and reflection exposes the LCL.
Figure 4.
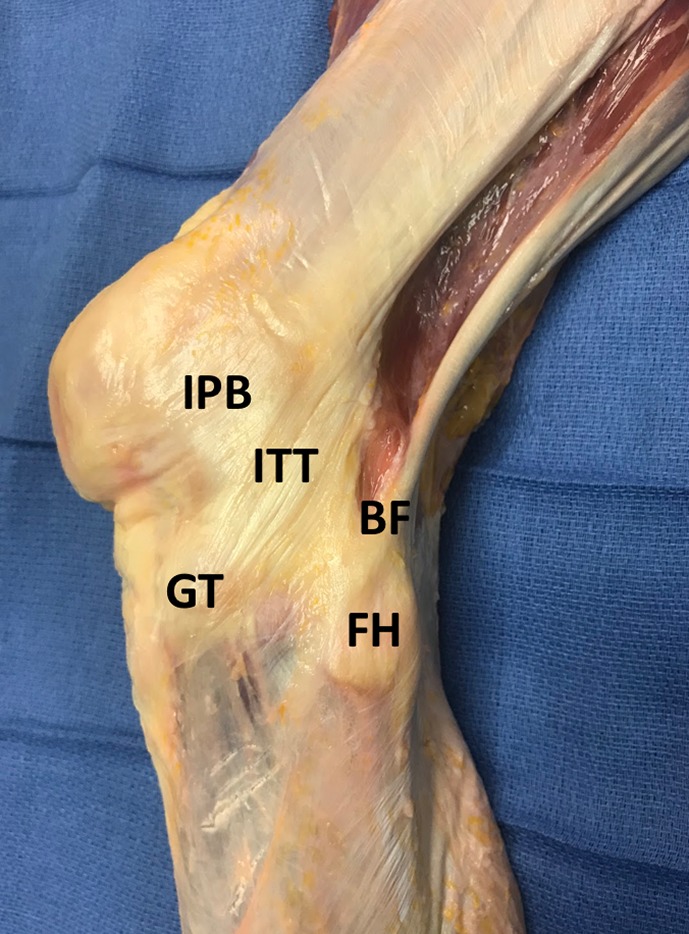
After removal of the aponeurotic layer of the lateral knee, the subsequent layer consists of the biceps femoris (BF) and a complex interconnection of structures coming from the iliotibial tract (ITT) including the iliotibial band, iliopatellar band (IPB), and lateral patellotibial ligament. FH, fibular head; GT, Gerdy tubercle.
Layer 3: Deep Fascia
After reflection of the superficial layers, complete exposure to another layer of tissue is seen (Figure 5). This tissue is a loose fascia that is partially exposed prior to reflection of the ITB and BF and is located between these 2 superficial structures distally toward the tibia. After reflection of the ITB and BF, this layer can be completely visualized. The tissue is loose and multidirectional, with intermittent adipose tissue present. It courses proximally both anteriorly and posteriorly, with some fibers wrapping around the lateral gastrocnemius and short head of the BF. Distally, the layer is continuous with the fascial layers of the anterior compartment of the leg.
Figure 5.
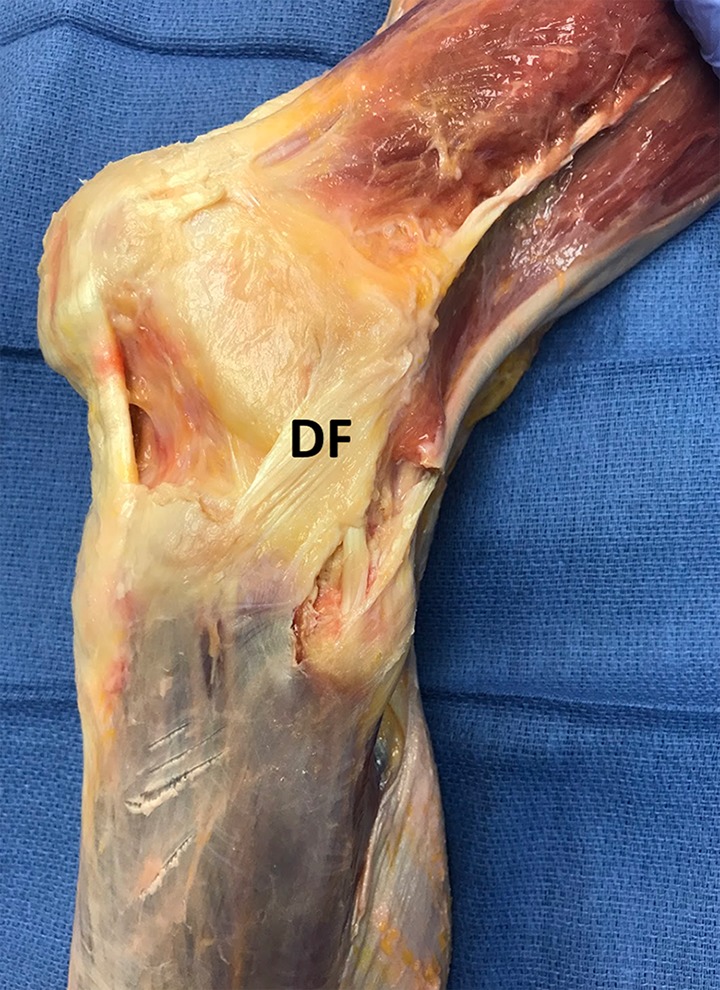
The third layer of the lateral knee demonstrates a deep fascia (DF) with loose association to surrounding structures and layered with intermittent adipose tissue.
Layer 4: ALL/Deep Capsulo-osseous Layer of the ITB
After reflection of the loose connective tissue in layer 3, a structure (previously described variably as the ALL8–10,12 or deep capsulo-osseous layer of the ITB47), in which the great majority of fibers attach slightly posterior and proximal to the lateral epicondyle, courses distally in a fanlike fashion and attaches directly upon the tibia between the Gerdy tubercle and the fibular head (Figure 6). This structure is also distinct from the capsule posteriorly and is differentiated from the capsule on its anterior border. Differentiation between layer 4 and the underlying capsule was inconsistent. Anteriorly, the fibers attach slightly posterior to the Gerdy tubercle and just distal to the joint line. Posteriorly, the fibers fan out slightly and attach just anterior to the fibular head, slightly more distal to the joint line compared with the anterior attachment. Distally, some fasciae cover the fibular head adjacent to, yet distinct from, this structure. This layer appears distinct from the capsule posteriorly, anteriorly, and superiorly; however, it appears closely adhered to the lateral meniscus body as it courses distally.
Figure 6.
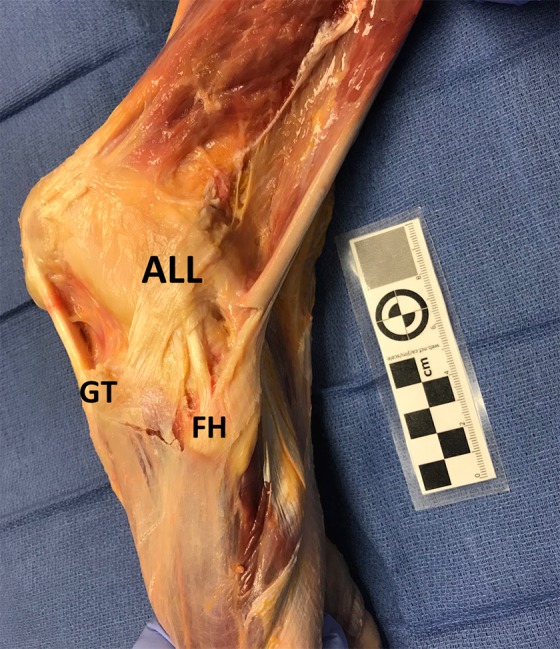
The fourth layer of the lateral knee shows a structure that attaches slightly posterior and proximal to the lateral epicondyle, courses distally in a fanlike fashion, and attaches directly upon the tibia between the Gerdy tubercle (GT) and the fibular head (FH). This structure has previously been described as the deep capsulo-osseous layer of the iliotibial band and as the anterolateral ligament (ALL).
En Bloc Histologic Analysis
Eight of 12 specimens revealed similar organization and orientation of 4 distinct, organized structures, albeit at slightly different levels. These structures were surrounded by unorganized fibers and were distinct from the surrounding tissue and each other. Based on the specimen orientation, these structures were labeled as follows: ITB, ALL/deep capsulo-osseous layer of ITB, meniscus, and LCL (Figure 7). The remaining 4 specimens were missing 1 of these 4 components in the slide. Two specimens demonstrated meniscus tissue only, and 2 specimens demonstrated 3 of the 4 components, missing either the ITB, ALL, or LCL.
Figure 7.
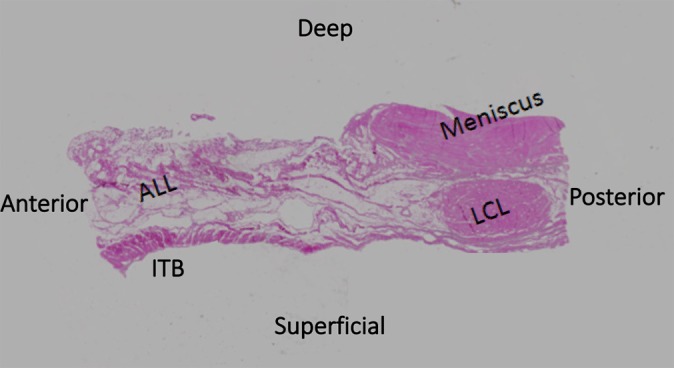
Hematoxylin and eosin stain: 40× magnification demonstrating the 4 identifiable, separate structures of the lateral knee: the iliotibial band (ITB), the anterolateral ligament (ALL), the lateral collateral ligament (LCL), and the meniscus.
Discussion
The results of this study demonstrate complex anatomic relationships between surrounding structures on the lateral side of the knee. The main finding was verification of the presence of the ALL as a discrete structure with a femoral origin near the lateral epicondyle and a tibial insertion posterior to the Gerdy tubercle. Given its bony origin and insertion, this tissue is by definition a ligamentous structure. The ALL was a histologically distinct ligamentous tissue, and although it was in close proximity to the ITB, it was separated by the loose connective tissue of layer 3 and located deep and slightly posterior to layer 2. In most specimens, the ALL was also distinct from the underlying capsule and, through careful dissection, could be isolated from the capsular layer of the knee. However, based on our findings, we cannot definitively say that the ALL and underlying capsule are distinct layers.
Our findings also demonstrate a wide connective matrix of the ITB, as it gives rise to connections to the patella and patellar tendon, previously referred to as the IPB. Structures previously referred to as the deep ITT layer, also called Kaplan fibers,24,47 attached to the lateral intermuscular septum and the lateral retinaculum8 but appeared macroscopically as part of the ITB and as part of the deep fascia of layer 3. One distinct difference between the ITB and the ALL concerned the tibial attachments. The ITB tibial attachment was limited to the Gerdy tubercle, whereas the ALL attached immediately posterior to the Gerdy tubercle in a fanlike fashion on the tibia.
The lateral structures of the knee have been the topic of much discussion since the publication by Claes et al8 describing the ALL. Some authors suggest that the ALL is a capsular thickening,11,20,25 whereas others define it as a discrete structure with a bony origin and insertion.9,10,17,26,29,34,50 Other authors who conducted layer-by-layer dissections noted the presence of a structure with significant similarities in appearance as the ALL but referred to the structure as the deep capsulo-osseous layer of the ITB.18,46 In agreement with previous authors, we believe that the terms capsulo-osseous layer of the ITB and ALL are synonymous.11 The ALL as described in this study shares the same characteristics as the description of the capsulo-osseous layer of the ITB by Terry et al46 with 1 major difference: The great majority of the proximal fibers of the ALL demonstrated a distinct bony attachment to the femur and a minority of fibers coursing proximally and posteriorly continuous with the fascia of the gastrocnemius and posterior musculature. To promote clarity regarding the precise description of this structure, we propose that the term ALL be used preferentially. This is proposed on the basis that the ALL is macroscopically and histologically discrete from the ITB, being separated from it by loose connective tissue. Furthermore, the ALL has a bony attachment to the area near the lateral epicondyle of the femur (rather than a more proximal attachment, as suggested by the term deep capsulo-osseous layer of the ITB) and the tibia, as described in the current study and by previous authors.8,9,11,26,50
Many reasons have led to the significant discrepancies in anatomic descriptions of the lateral knee and subsequent clinical impact. Ingham et al,20 in an anatomic study of 24 animal species, were unable to find an ALL in any of the 58 specimens (including humans), but a dissection protocol was not clearly described. In contrast, Helito et al14 demonstrated the ALL in all knees in fetal cadaveric human specimens, and Brockmeyer et al,3 using standardized dissection protocols, identified the ALL in all adult knees.8,10
The anterolateral knee structures are in close proximity to each other, and previous authors have noted the difficulty in dissecting embalmed specimens.9,35 We believe that dissections using fresh or fresh-frozen specimens are the only way to appreciate and describe these structures accurately. Further, meticulous dissection is required to fully differentiate these structures, as the distance between them is quite minimal. Finally, initial anatomic descriptions22,39,47 of the lateral knee, combined with recent publications investigating the ALL,9,26,29,35,50 have led to significant confusion regarding nomenclature and the description of similar structures. Consistent nomenclature for the structures of the lateral knee is essential for effective research on the biomechanics and clinical influence of these structures on knee function and stability.
The lack of consistent identification and nomenclature of the lateral structures of the knee has led to significant debate regarding the role of the ALL in knee stability. The ALL has been identified as a contributor to rotational stability of the knee, as referenced by multiple authors.1,2,19,27,28,33,42 Some authors have demonstrated that a lesion of the ALL is required for the presence of a pivot shift,1,42 whereas others have demonstrated significant changes in the rotation and translation of the tibia in a pivot-shift maneuver with an ALL lesion present.27,28 Helito et al15 reported the mean maximum strength of the ALL as 204.8 ± 114.9 N, stiffness as 41.9 ± 25.7 N/mm, and deformation as 10.3 ± 3.5 mm. Kennedy et al25 demonstrated similar findings, with a mean maximum load of the ALL of 175 N and stiffness of 20 N/mm. Other authors have not found a significant contribution of the ALL to knee stability.24,31,50
Given that isolated ACL reconstruction has drawbacks, such as graft rupture, persistent rotatory instability, and low rates of return to sport,4,5,13,21,30,47,52 some authors have investigated ALL reconstruction as a clinical treatment option.43 Although early results have demonstrated promise,41,48 more work is needed to identify the role of surgical treatment of the anterolateral knee.
The current study had limitations, given that it was a cadaveric study with a limited number of samples. The histologic samples contained some artifacts that are believed to be due to the freezing and processing of the en bloc sections. These artifacts mainly consisted of fragmentation of some of the sections but did not appear to affect our results histologically. Specific tests were not performed to histologically classify the structure of the ALL as a ligament, and future studies might better describe this structure histologically. Another limitation was that we looked at the cross-sectional histologic samples at only 1 level, and our findings may have been different at other levels. The potential for performance bias exists in the dissection of these specimens, as 2 authors were responsible for the layer-by-layer dissection and independent observers were not present during the dissection.
Conclusion
The anterolateral aspect of the knee has a complex orientation of layers and fibers. The ALL is a distinct structure from the superficial ITB and is synonymous with the previously described capsulo-osseous layer of the ITB.
Footnotes
One or more of the authors has declared the following potential conflict of interest or source of funding: M.D. has received educational support from Arthrex, Zimmer, Titan Surgical Group, Smith & Nephew, DePuy Synthes, and Apollo Surgical Group. E.M., A.S., and B.S.-C. are paid consultants for Arthrex. AOSSM checks author disclosures against the Open Payments Database (OPD). AOSSM has not conducted an independent investigation on the OPD and disclaims any liability or responsibility relating thereto.
Ethical approval was not sought for the present study.
References
- 1. Bonanzinga T, Signorelli C, Grassi A, et al. Kinematics of ACL and anterolateral ligament, part I: combined lesion. Knee Surg Sports Traumatol Arthrosc. 2017;25(4):1055–1061. [DOI] [PubMed] [Google Scholar]
- 2. Bonanzinga T, Signorelli C, Grassi A, et al. Kinematics of ACL and anterolateral ligament, part II: anterolateral and anterior cruciate ligament reconstruction. Knee Surg Sports Traumatol Arthrosc. 2017;25(4):1062–1067. [DOI] [PubMed] [Google Scholar]
- 3. Brockmeyer M, Höfer D, Schäfer K, et al. The anterolateral ligament (ALL) of the knee—part of the iliotibial tract or a truly separate structure? Ann Anat. 2017;212:1–3. [DOI] [PubMed] [Google Scholar]
- 4. Brophy RH, Schmitz L, Wright RW, et al. Return to play and future ACL injury risk after ACL reconstruction in soccer athletes from the Multicenter Orthopaedic Outcomes Network (MOON) group. Am J Sports Med. 2012;40:2517–2522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Brusalis CM, Lakomkin N, Suryavanshi JR, et al. Clinical outcome reporting in youth ACL literature is widely variable. Orthop J Sports Med. 2017;5(8):2325967117724431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Campos JC, Chung CB, Lektrakul N, et al. Pathogenesis of the Segond fracture: anatomic and MR imaging evidence of an iliotibial tract or anterior oblique band avulsion. Radiology. 2001;219(2):381–386. [DOI] [PubMed] [Google Scholar]
- 7. Caterine S, Litchfield R, Johnson M, Chronik B, Getgood A. A cadaveric study of the anterolateral ligament: re-introducing the lateral capsular ligament. Knee Surg Sports Traumatol Arthrosc. 2015;23(11):3186–3195. [DOI] [PubMed] [Google Scholar]
- 8. Claes S, Vereecke E, Maes M, Victor J, Verdonk P, Bellemans J. Anatomy of the anterolateral ligament of the knee. J Anat. 2013;223(4):321–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Dodds AL, Halewood C, Gupte CM, Williams A, Amis AA. The anterolateral ligament: anatomy, length changes and association with the Segond fracture. Bone Joint J. 2014;96(3):325–331. [DOI] [PubMed] [Google Scholar]
- 10. Daggett M, Busch K, Sonnery-Cottet B. Surgical dissection of the anterolateral ligament. Arthrosc Tech. 2016;5(1):e185–e188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Daggett M, Claes S, Helito CP, et al. The role of the anterolateral structures and the ACL in controlling laxity of the intact and ACL-deficient knee: letter to the editor. Am J Sports Med. 2016;44(4):NP14–NP15. [DOI] [PubMed] [Google Scholar]
- 12. Daggett M, Ockuly AC, Cullen M, et al. Femoral origin of the anterolateral ligament: an anatomic analysis. Arthroscopy. 2016;32(5):835–841. [DOI] [PubMed] [Google Scholar]
- 13. Feucht MJ, Zens M. The anterolateral ligament of the knee: anatomy, biomechanics, and clinical implications. Curr Orthop Pract. 2016;27:247–253. [Google Scholar]
- 14. Helito CP, Amaral C, Nakamichi Y, et al. Why do authors differ with regard to the femoral and meniscal anatomic parameters of the knee anterolateral ligament? Dissection by layers and a description of its superficial and deep layers. Orthop J Sports Med. 2016;4(12):2325967116675604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Helito CP, Bonadio MB, Rozas JS, et al. Biomechanical study of strength and stiffness of the knee anterolateral ligament. BMC Musculoskelet Disord. 2016;17:193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Helito CP, Demange MK, Bonadio MB, et al. Anatomy and histology of the knee anterolateral ligament. Orthop J Sports Med. 2013;1(7):2325967113513546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Helito CP, do Prado Torres JA, Bonadio MB, et al. Anterolateral ligament of the fetal knee: an anatomic and histological study. Am J Sports Med. 2017;45(1):91–96. [DOI] [PubMed] [Google Scholar]
- 18. Herbst E, Albers M, Burnham JM, et al. The anterolateral complex of the knee: a pictorial essay. Knee Surg Sports Traumatol Arthrosc. 2017;25(4):1009–1014. [DOI] [PubMed] [Google Scholar]
- 19. Inderhaug E, Stephen JM, Williams A, Amis AA. Biomechanical comparison of anterolateral procedures combined with anterior cruciate ligament reconstruction. Am J Sports Med. 2017;45(2):347–354. [DOI] [PubMed] [Google Scholar]
- 20. Ingham SJM, de Carvalho RT, Martins CAQ, et al. Anterolateral ligament anatomy: a comparative anatomical study. Knee Surg Sports Traumatol Arthrosc. 2017;25(4):1048–1054. [DOI] [PubMed] [Google Scholar]
- 21. Kamath GV, Murphy T, Creighton RA, Viradia N, Taft TN, Spang JT. Anterior cruciate ligament injury, return to play, and reinjury in the elite collegiate athlete: analysis of an NCAA Division I cohort. Am J Sports Med. 2014;42:1638–1643. [DOI] [PubMed] [Google Scholar]
- 22. Kaplan EB. The iliotibial tract; clinical and morphological significance. J Bone Joint Surg Am. 1958;40(4):817–832. [PubMed] [Google Scholar]
- 23. Keizer MNJ, Hoogeslag RAG, van Raay JJAM, Otten E, Brouwer RW. Superior return to sports rate after patellar tendon autograft over patellar tendon allograft in revision anterior cruciate ligament reconstruction. Knee Surg Sports Traumatol Arthrosc. 2018;26(2):574–581. [DOI] [PubMed] [Google Scholar]
- 24. Kittl C, El-Daou H, Athwal KK, et al. The role of the anterolateral structures and the ACL in controlling laxity of the intact and ACL-deficient knee: response. Am J Sports Med. 2016;44(4):NP15–NP18. [DOI] [PubMed] [Google Scholar]
- 25. Kennedy MI, Claes S, Fuso FA, et al. The anterolateral ligament: an anatomic, radiographic, and biomechanical analysis. Am J Sports Med. 2015;43(7):1606–1615. [DOI] [PubMed] [Google Scholar]
- 26. Lutz C, Sonnery-Cottet B, Niglis L, Freychet B, Clavert P, Imbert P. Behavior of the anterolateral structures of the knee during internal rotation. Orthop Traumatol Surg Res. 2015;101(5):523–528. [DOI] [PubMed] [Google Scholar]
- 27. Monaco E, Fabbri M, Mazza D, et al. The effect of sequential tearing of the anterior cruciate and anterolateral ligament on anterior translation and the pivot shift phenomenon: a cadaveric study using navigation. Arthroscopy. 2018;34(4):1009–1014. [DOI] [PubMed] [Google Scholar]
- 28. Monaco E, Ferretti A, Labianca L, et al. Navigated knee kinematics after cutting of the ACL and its secondary restraint. Knee Surg Sports Traumatol Arthrosc. 2012;20(5):870–877. [DOI] [PubMed] [Google Scholar]
- 29. Musahl V, Rahnemai-Azar AA, van Eck CF, Guenther D, Fu FH. Anterolateral ligament of the knee, fact or fiction? Knee Surg Sports Traumatol Arthrosc. 2016;24:2–3. [DOI] [PubMed] [Google Scholar]
- 30. Nedeff DD, Bach BR. Arthroscopic anterior cruciate ligament reconstruction using patellar tendon autografts. Orthopedics. 2002;25:343–357. [DOI] [PubMed] [Google Scholar]
- 31. Noyes FR, Huser LE, Levy MS. Rotational knee instability in ACL-deficient knees: role of the anterolateral ligament and iliotibial band as defined by tibiofemoral compartment translations and rotations. J Bone Joint Surg Am. 2017;99(4):305–314. [DOI] [PubMed] [Google Scholar]
- 32. Rahnemai-Azar A, Miller R, Guenther D, et al. Structural properties of the anterolateral capsule and iliotibial band of the knee. Am J Sports Med. 2016;44(4):892–897. [DOI] [PubMed] [Google Scholar]
- 33. Rasmussen MT, Nitri M, Williams BT, et al. An in vitro robotic assessment of the anterolateral ligament, part 1: secondary role of the anterolateral ligament in the setting of an anterior cruciate ligament injury. Am J Sports Med. 2016;44(3):585–592. [DOI] [PubMed] [Google Scholar]
- 34. Rezende FC, de Moraes VY, Martimbianco ALC, Luzo MV, da Silveira Franciozi CE, Belloti JC. Does combined intra- and extraarticular ACL reconstruction improve function and stability? A meta-analysis. Clin Orthop Relat Res. 2015;473:2609–2618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Saiegh YA, Suero EM, Guenther D, et al. Sectioning the anterolateral ligament did not increase tibiofemoral translation or rotation in an ACL-deficient cadaveric model. Knee Surg Sports Traumatol Arthrosc. 2017;25(4):1086–1092. [DOI] [PubMed] [Google Scholar]
- 36. Shea KG, Polousky JD, Jacobs JC, Jr, Yen YM, Ganley TJ. The anterolateral ligament of the knee: an inconsistent finding in pediatric cadaveric specimens. J Pediatr Orthop. 2016;36(5):e51–e54. [DOI] [PubMed] [Google Scholar]
- 37. Shah R, Singh R, Dugdale C, Geutjens G. Does additional reconstruction of the anterolateral ligament during a primary anterior cruciate ligament reconstruction affect tibial rotational laxity—a case series. Ann Med Surg (Lond). 2017;19:7–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Smeets K, Slane J, Scheys L, Forsyth R, Claes S, Bellemans J. The anterolateral ligament has similar biomechanical and histologic properties to the inferior glenohumeral ligament. Arthroscopy. 2017;33(5):1028–1035. [DOI] [PubMed] [Google Scholar]
- 39. Segond P. Recherches Cliniques et Experimentales sur les Epanchements Sanguins du 450 Genou par Entorse. Paris: National Library of France; 1879:1–85. [Google Scholar]
- 40. Song G-Y, Hong L, Zhang H, Zhang J, Li Y, Feng H. Clinical outcomes of combined lateral extra-articular tenodesis and intra-articular anterior cruciate ligament reconstruction in addressing high-grade pivot-shift phenomenon. Arthroscopy. 2016;32(5):898–905. [DOI] [PubMed] [Google Scholar]
- 41. Sonnery-Cottet B, Barbosa NC, Vieira TD, Saithna A. Clinical outcomes of extra-articular tenodesis/anterolateral reconstruction in the ACL injured knee. Knee Surg Sports Traumatol Arthrosc. 2018;26(2):596–604. [DOI] [PubMed] [Google Scholar]
- 42. Sonnery-Cottet B, Lutz C, Daggett M, et al. The involvement of the anterolateral ligament in rotational control of the knee. Am J Sports Med. 2016;44(5):1209–1214. [DOI] [PubMed] [Google Scholar]
- 43. Sonnery-Cottet B, Saithna A, Cavalier M, et al. Anterolateral ligament reconstruction is associated with significantly reduced ACL graft rupture rates at a minimum follow-up of 2 years: a prospective comparative study of 502 patients from the SANTI Study Group. Am J Sports Med. 2017;45(7):1547–1557. [DOI] [PubMed] [Google Scholar]
- 44. Sonnery-Cottet B, Saithna A, Helito C, Daggett M, Thaunat M. Regarding “anterolateral ligament of the knee, fact or fiction?” Arthroscopy. 2016;32(9):1740–1741. [DOI] [PubMed] [Google Scholar]
- 45. Stijak L, Bumbaširević M, Radonjić V, et al. Anatomic description of the anterolateral ligament of the knee. Knee Surg Sports Traumatol Arthrosc. 2016;24(7):2083–2088. [DOI] [PubMed] [Google Scholar]
- 46. Terry GC, Hughston JC, Norwood LA. The anatomy of the iliopatellar band and iliotibial tract. Am J Sports Med. 1986;14(1):39–45. [DOI] [PubMed] [Google Scholar]
- 47. Terry GC, Norwood LA, Hughston JC, Caldwell KM. How iliotibial tract injuries of the knee combine with acute anterior cruciate ligament tears to influence abnormal anterior tibial displacement. Am J Sports Med 1993;21:55–60. [DOI] [PubMed] [Google Scholar]
- 48. Thaunat M, Clowez G, Saithna A, et al. Reoperation rates after combined anterior cruciate ligament and anterolateral ligament reconstruction: a series of 548 patients from the SANTI Study Group with a minimum follow-up of 2 years. Am J Sports Med. 2017;45(11):2569–2577. [DOI] [PubMed] [Google Scholar]
- 49. Thein R, Boorman-Padgett J, Stone K, Wickiewicz TL, Imhauser CW, Pearle AD. Biomechanical assessment of the anterolateral ligament of the knee: a secondary restraint in simulated tests of the pivot shift and of anterior stability. J Bone Joint Surg Am. 2016;98(11):937–943. [DOI] [PubMed] [Google Scholar]
- 50. Vincent JP, Magnussen RA, Gezmez F, et al. The anterolateral ligament of the human knee: an anatomic and histologic study. Knee Surg Sports Traumatol Arthrosc. 2012;20(1):147–152. [DOI] [PubMed] [Google Scholar]
- 51. Watanabe J, Suzuki D, Mizoguchi S, Yoshida S, Fujimiya M. The anterolateral ligament in a Japanese population: study on prevalence and morphology. J Orthop Sci. 2016;21(5):647–651. [DOI] [PubMed] [Google Scholar]
- 52. Zaffagnini S, Urrizola F. Residual rotatory laxity after anterior cruciate ligament reconstruction: how do we diagnose it? Curr Orthop Pract. 2016;27:241–246. [Google Scholar]


