Abstract
Background:
Antipsychotic-induced metabolic side effects are major concerns in psychopharmacology and clinical psychiatry. Their pathogenetic mechanisms are still not elucidated.
Methods:
Herein, we review the impact of neurotransmitters on metabolic regulation, providing insights into antipsychotic-induced metabolic side effects.
Results:
Antipsychotic drugs seem to interfere with feeding behaviors and energy balance, processes that control metabolic regulation. Reward and energy balance centers in central nervous system constitute the cen-tral level of metabolic regulation. The peripheral level consists of skeletal muscles, the liver, the pancreas, the adipose tissue and neuroendocrine connections. Neurotransmitter receptors have crucial roles in metabolic regulation and they are also tar-gets of antipsychotic drugs. Interaction of antipsychotics with neurotransmitters could have both protective and harmful ef-fects on metabolism.
Conclusion:
Emerging evidence suggests that antipsychotics have different liabilities to induce obesity, diabetes and dyslipidemia. However this diversity cannot be explained merely by drugs’pharmacodynamic profiles, highlighting the need for further research
Keywords: Receptor-binding profiles, antipsychotics, metabolic side effects, neurotransmitters, obesity, diabetes, metabolic regulation, feeding behavior
1. INTRODUCTION
Schizophrenia and most neuropsychiatric disorders have reciprocal connections with metabolic disturbances [1, 2]. Obesity, diabetes and metabolic syndrome are common comorbidities in patients with schizophrenia, especially on treatment with antipsychotics [3, 4]. They are more prominent in young, lean, female, drug-naïve patients and have been linked to health inequalities, polypharmacy, smoking, dietary habits, lack of exercise and illness characteristics [5]. Besides the direct connection between brain disorders and metabolic dysfunction, antipsychotic drugs can impair metabolic regulation per se [2]. Antipsychotics are strongly associated with the core components of metabolic syndrome (weight gain, glucose intolerance and dyslipidemia) and to a lesser degree with hypertension [4, 6], which if taken together can lead to therapy discontinuation, low self-esteem and cardiovascular disease [7].
Herein, we will review the current knowledge on central and peripheral mechanisms of antipsychotic-induced metabolic dysfunction (AIMD), based on drug binding profile.
1.1. Search Strategy
A literature search was conducted, using the Medline and Scopus electronic databases, including search terms and keywords for metabolic side effects (weight gain, obesity, food intake, diabetes, glucose intolerance, insulin resistance, insulin secretion, metabolic syndrome) combined with the keyword “antipsychotic” and search terms for the receptors (receptor, dopamine, serotonin, histamine, acetylcholine, muscarinic, epinephrine, norepinephrine, adrenergic). The search covered all peer-reviewed publications in English from January 2000 to December 2016. Additional papers were identified via citations in other reviewed papers. The binding profile of antipsychotic drugs was retrieved from PDSP and iPHACE databases along with relevant papers. Subsequently, papers that associate aspects of metabolic side effects with binding profiles were included.
2. METABOLIC REGULATION AND ANTIPSYCHOTIC-INDUCED METABOLIC DYSFUNCTIONS
2.1. Regulation of Energy Balance and Metabolism
Feeding behavior and metabolic regulation are closely and highly regulated systems. The metabolic system is regulated at two different levels involving the central and the peripheral system. The central nervous system regulates metabolism through two different axes. The first axis originates mainly in hypothalamic centers. Satiety centers (e.g. POMC-melanocortin pathway) and hunger centers (e.g. NPY/AgRP pathway) are opposing in order to regulate energy balance. They have connections with higher-order brain regions, nuclei of the autonomous system and the reward system [8]. The second axis consists of the reward system that regulates food craving and pleasure of feeding. The ventral tegmental area, limbic cortex and striatum are core components of the reward system [9]. Feeding behavior, motivation and metabolism are regulated by direct and indirect connections between those axes [10]. The peripheral system consists of the gastrointestinal system, the liver, the pancreas, the adipose tissue (white and brown), the skeletal muscles, the immune system and the autonomous nervous system (ANS). The central and peripheral systems are closely interconnected via a wide array of neural and endocrine communications. Evolution biases metabolic homeostasis to an anabolic equilibrium point [11], a trend that can be potentiated by antipsychotic drugs.
2.2. Antipsychotic Drug Induced Metabolic Dysfunctions
Almost all antipsychotic drugs, typical and atypical, cause metabolic side effects [12] with quantitative and qualitative differences [13]. Meta-analyses and head-to-head comparisons of antipsychotic drugs have provided insights into the differential risk of weight gain induction and glucose and lipid handling disruption [4, 12, 14-18] (see ‘risk’ section in Table 1). Brexpiprazole and cariprazine are suggested to induce mild weight gain, but current experience with these drugs is limited [19, 20]. A recent meta-analysis classified antipsychotics based on their liability to cause metabolic syndrome according to the following order: clozapine >olanzapine ≥quetiapine =risperidone =typical antipsychotics =amisulpride ≥aripiprazole > placebo [6]. Despite this current evidence cannot elucidate the magnitude of the association between antipsychotics and diabetes/dyslipidemia [18].
Table 1.
Receptor-binding profile and metabolic risk of antipsychotic drugs.
| RECEPTOR BINDING PROFILE | RISK | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| D1 | D2 | D3 | D4 | H1 | H2 | H3 | 5-HT1A | 5-HT1B | 5-HT2A | 5-HT2B | 5-HT2C | 5-HT6 | 5-HT7 | M1 | M3 | α1 | α2A | α2B | α2C | Transporter | Weight Gain | Glucose Abn | Lipid Abn | |
| Olanzapine | ++ | ++ | ++ | ++ | +++ | ++ | + | + | +++ | ++ | ++ | +++ | ++ | ++ | ++ | ++ | + | ++ | ++ | ++++ | ++ | ++ | ||
| Zotepine | ++ | +++ | +++ | + | +++ | + | + | ++ | +++ | +++ | +++ | ++ | + | + | +++ | + | +++ | ++ | SERT, NET | +++/++++ | (LD) | (LD) | ||
| Clozapine | + | + | + | ++ | +++ | + | + | + | ++ | +++ | ++ | ++ | ++ | +++ | ++ | +++ | ++ | ++ | ++ | +++/++++ | ++ | ++ | ||
| Chlorpromazine | ++ | +++ | +++ | +++ | +++ | + | + | +++ | +++ | ++ | +++ | +++ | ++ | ++ | +++ | + | ++ | ++ | +++/++++ | +/++ | +/++ | |||
| Sertindole | +++ | +++ | +++ | + | + | ++ | ++++ | +++ | ++ | +++ | + | + | + | +++/++++ | +/++ | +/++ | ||||||||
| Iloperidone | + | +++ | +++ | ++ | + | ++ | ++ | +++ | ++ | + | + | +++ | + | + | ++ | +++/++++ | +/++ | +/++ | ||||||
| Risperidone | + | +++ | +++ | +++ | +++ | + | + | ++ | ++++ | ++ | ++ | +++ | +++ | ++ | ++ | +++ | +++ | +/++ | +/++ | |||||
| (Nor)quetiapine | + | + | + | +++ | ++ | ++ | + | + | ++ | + | + | ++ | + | + | ++ | NET | +++ | +/++ | ++ | |||||
| Paliperidone | + | +++ | +++ | +++ | ++ | + | + | ++ | +++ | ++ | + | +++ | +++ | +++ | +++ | +++ | +++ | +/++ | +/++ | |||||
| Asenapine | +++ | +++ | ++++ | +++ | +++ | +++ | +++ | +++ | ++++ | ++++ | ++++ | ++++ | ++++ | +++ | +++ | ++++ | +++ | ++ | + | + | ||||
| Amisulpride | +++ | +++ | +++ | ++ | ++ | ++ | + | ++(LD) | ||||||||||||||||
| Aripiprazole | +++ | +++ | + | ++ | +++ | + | ++ | ++++ | ++ | + | ++ | ++ | ++ | ++ | ++ | SERT | ++ | + | + | |||||
| Brexpiprazole | + | ++++ | +++ | +++ | ++ | ++++ | ++ | ++++ | +++ | + | ++ | +++ | +++ | ++ | ++ | ++++ | SERT, NET | +(LD) | +(LD) | +(LD) | ||||
| Cariprazine | ++++ | ++++ | ++ | +++ | ++ | ++++ | + | + | + | +(LD) | +(LD) | +(LD) | ||||||||||||
| Haloperidol | + | +++ | +++ | +++ | + | + | + | + | ++ | + | + | + | + | + | + | |||||||||
| Lurasidone | + | +++ | +++ | +++ | + | ++++ | ++ | ++ | +++ | + | + | + | ||||||||||||
| Ziprasidone | + | +++ | +++ | ++ | ++ | +++ | +++ | ++++ | ++ | ++++ | ++ | +++ | ++ | + | ++ | ++ | SERT, NET | + | + | + | ||||
A. Receptor-binding profile. Antagonism and inverse agonism are indicated by blue color whereas partial agonism by yellow. The number of crosses and color intensity are correlated to binding affinity. Quetiapine is demonstrated along with norquetiapine, a metabolite of the drug with distinct binding profile. 100 < Ki < 1000: + weak association. 10< Ki < 100: ++ moderate association. 1< Ki < 10: +++ strong association. 1 > Ki: ++++ very strong association. [Data taken from [30, 43-45, 48-50], PDSP [47] and iPHACE [46] databases]. B. Metabolic risk. The number of crosses are correlated to risk of weight gain (maximum ++++), glucose and lipid abnormalities (maximum ++). (LD): Limited Data, abn: abnormalities. [Data taken from [4, 12, 14-20].
The proposed model of AIMD suggests that antipsychotics disrupt metabolic regulation, by affecting both CNS and peripheral organs [21]. Regarding the CNS, they are suggested to activate hunger centers, inhibit satiety centers and disrupt food reward [22-24]. Additionally, they could impair locomotion and the flow of ANS to the periphery. These effects could mediate weight gain (increased energy intake, decreased energy expenditure) and alterations in peripheral metabolism [21, 23]. Weight gain impairs glucose and lipid metabolism and it is thought to be the core mechanism of AIMD.
In addition to that, in vivo and in vitro studies suggest that antipsychotic drugs could directly dysregulate peripheral metabolism. They may have direct effects on liver (increase lipogenesis, glucose output), adipose tissue (increase adipogenesis, lipogenesis and pro-inflammatory cytokines), skeletal muscles (decrease glucose uptake) [13, 25-27]. They can also derange pancreatic functions, disrupting insulin/glucagon secretion and repressing the compensation of pancreatic islets. These effects may mediate hyperglycemia, diabetes and dyslipidemia [21, 28]. Antipsychotics could increase prolactin and ghrelin, hormones associated with metabolic dysfunctions. They can possibly alter the function of glucagon-like peptide-1 (GLP-1) along with the adipokines, leptin and adiponectin, which have beneficial effects on metabolism [13]. Antipsychotic-induced weight gain increases leptin levels in patients, which may be serving as negative feedback signal [29]. However, in vivo studies suggest that antipsychotics could induce leptin resistance by acting on satiety and reward centers [30, 31].
2.3. Translational Validity Issues of Animal Models of Antipsychotic-induced Metabolic Side Effects
Discrepancy of AIMD preclinical models is a major issue of translational neuropharmacology. Gender-dependent differences in antipsychotic-induced weight gain are evident in both clinical and preclinical setting. Clinical evidence, yet limited, suggest that women may be in higher risk of antipsychotic-induced weight gain than men [32]. However, female rodents seem to be more vulnerable to weight gain, especially olanzapine-induced [33]. This gender gap seems to be more prominent in chronic rather than acute antipsychotic administration [34].
Antipsychotic-induced hyperprolactinemia is more common in rodents than humans, but its contribution to weight gain may be limited [35]. However, prolactin seems to preferentially increase food intake in female rodents. Additionally, hyperprolactinemia is often accompanied by estradiol reduction in females, which could further increase food intake, and decreased testosterone in males, which could decrease lean body mass [36]. Histaminergic, dopaminergic and serotonergic signaling display also gender-dimorphism in rodents [32, 37]. Strain- and gender-dependent behavioral and pharmacokinetic differences could also play important roles. For example, male rats may be more susceptible to stress-induced decreased food intake, whereas females may be exposed to longer drug half-life [34, 38].
Rodent models of clozapine-induced weight gain display also inconsistent results regardless gender. In many models, clozapine may not affect or even decrease weight, partially explained by its sedative effects [34]. Despite the positive association of clozapine administration and weight gain in humans, clozapine may also induce weight loss to some individuals, possibly underpinning genetic factors or variable therapeutic response [39].
As a result, weight cannot solely provide high predictive validity of AIMD animal models. Adjusting experimental designs, such as routes of administration (antipsychotic drugs mixed with chow) and type of diet (high carbohydrate/fat), could diminish gender dimorphism and induce weight gain in olanzapine-treated male rodents [34]. Additionally, both clozapine and olanzapine can increase adiposity measures in rodents, irrespective of gender and weight gain [35, 40]. Examining parameters, such as body composition (fat and lean mass) and impaired satiation, along with carefully selected experimental designs and monitoring of sedation and extrapyramidal symptoms, could enhance the predictive validity of rodent models [34, 36, 41]. On the
contrary, preclinical models of antipsychotic-induced glucose dysregulation seem to be translational valid [42].
3. RECEPTOR MECHANISMS OF ANTIPSYCHOTIC-INDUCED METABOLIC SIDE EFFECTS
Antipsychotic drugs bind to a variety of neurotransmitter receptors [30, 43-50] (see ‘binding affinities’ in Table 1). Clinical and preclinical studies propose that H1, 5-HT2A, 5-HT2c, 5-HT6, D2, α1 and M3 are the key mediators of metabolic side effects [5, 30, 51-54]. Current research in the pathogenesis of AIMD focuses mainly on the central mechanism but recent advances in peripheral neurotransmitter receptors bring new insights [21].
3.1. Dopamine Receptors
Dopamine receptors are G protein-coupled receptors classified into D1-like (D1, D5) that increase cAMP (Gαs) and D2-like (D2, D3, D4) that decrease cAMP (Gαi). All antipsychotic drugs bind to D2 receptors and altered dopaminergic signaling could be a ubiquitous contributor to AIMD (see Table 1). Proper dopaminergic neurotransmission in hypothalamus and reward system regulates feeding behaviors. Palatable food has rewarding properties and it could increase dopaminergic output in regions of the reward system. Presynaptic D2 in midbrain inhibits dopamine release. Mice lacking D2 autoreceptors display disinhibition of dopamine release in the striatum, accompanied by elevated reward-sensitivity and food-seeking behavior [55]. Reward system seems to adapt to chronic overconsumption of palatable food by decreasing striatal D2 signaling. This reward-hyposensitive state could also trigger compensatory and compulsive food intake [9, 56]. Prior to initiation with amisulpride treatment, reduced reward-related activation of the dorsal striatum seems to predispose to weight gain in drug-naïve patients. However, amisulpride, and possibly other antipsychotics, seem to enhance the activation of striatal regions, which may also be related to weight gain [24]. As a result, D2 antagonism could induce overeating, by both promoting reward-sensitive and reward-deficient states. In addition to food reward, striatal dopaminergic signaling seems to interact with insulin signaling and it could also affect glucose tolerance. According to that, dopamine depletion and decreased D2/3 signaling in ventral striatum may be correlated to insulin resistance in healthy individuals [57].
Prefrontal cortex-amygdala network may also play important roles in regulating feeding. Prefrontal D1-expressing neurons may stimulate feeding by activating downstream nuclei of amygdala in mice [58], whereas D2/3 stimulation in amygdala may reduce food intake in rats [59]. Hypothalamic dopaminergic neurotransmission regulates energy balance, glucose homeostasis, circadian rhythms and prolactin secretion [60]. Preclinical research suggests that hypothalamic D1 signaling has site-dependent effects on feeding, whereas D2 could act acts as satiety signal and inhibit food intake [61]. Rodent studies suggest also that D2 mediates anorexic and hypophagic effects, such as by GLP-1 and leptin [31, 59]. Additionally, intracerebroventricular administration of D2 antagonist to mice could induce hyperglycemia, accompanied by the activation of hypothalamic AMPK, which is correlated to AIMD [62]. Furthermore, D2 antagonism in posterior pituitary and adipocytes elevates prolactin secretion [63]. Hyperprolactinemia has been accused for weight gain and insulin resistance. Risperidone, paliperidone, lurasidone, ziprasidone, amisulpride and sertindole increase significantly prolactin levels, however they do not have the same metabolic liabilities [17].
Despite conflicting results, D1 agonism tents to increase food intake, but D2/3 agonism may attenuate it [37, 64, 65]. Consequently, administration of D2 antagonists, haloperidol or sulpiride, seems to be correlated to increased food intake and weight gain in rodents [34], whereas amisulpride could also reduce weight loss in a mouse model of anorexia nervosa [66]. Other preclinical studies demonstrated negligible effects on food intake and weight by administration of aripiprazole or haloperidol in rodents [34, 67], but haloperidol could potentiate weight gain induced by 5-HT2C antagonists in rats [5, 68]. Additionally, administration of D1 or D2 agonists could alleviate clozapine-induced food intake in mice [69]. Accordingly, clinical and preclinical research suggests the beneficial effects of bromocriptine, a D2/3 agonist, on weight and glycemic regulation in obesity or type 2 diabetes [60]. As a result, D2 antagonism seems to be a supportive, rather than a core mechanism, of inducing AIMD.
Antipsychotic drugs can alter basal and stimulus-activated neurotransmitter secretion [42, 70]. Administration of haloperidol to male rats tents to attenuate palatable food-induced dopamine release in nucleus accumbens and food-induced eating, whereas long-term effects on prefrontal cortex seems to be negligible [71]. Conversely, atypical antipsychotics seem to preferentially increase dopaminergic output in prefrontal cortex compared to striatal regions, by acting on 5-HT1A, 5-HT2A and other receptors [72]. Antipsychotics with D1 antagonist properties, such as olanzapine, could partially compensate increased cortical dopaminergic efflux. Clozapine, in comparison with other atypical antipsychotics, may act as D1 partial agonist in prefrontal cortex [73]. Regarding that prefrontal D1 may promote food intake, the different dopaminergic output of atypical antipsychotics could contribute to higher metabolic liabilities.
Dysfunction of the peripheral dopamine system seems to be associated with metabolic disturbances and altered glucose regulation. Human and rodent beta cells seem to express D2/3 receptors, which may inhibit glucose-dependent insulin release and regulate proliferation of beta cells [63, 74]. Accordingly, in vitro administration of haloperidol or sulpiride to human islets seems to increase glucose-dependent insulin secretion [75], which could contribute to nutrient deposition and weight gain [51]. Subsequently, in vivo rodent studies suggest that chronic disinhibition of insulin secretion by D2 antagonism could deplete insulin stores, disrupt pancreatic compensation and finally induce glucose intolerance [76-78].
3.2. 5-Hydroxytryptamine Receptors
The seven 5-HT receptor families are 5-HT1, 5-HT5 (Gαi), 5-HT2, 5-HT4, 5-HT6, 5-HT7 (Gαs/q) and 5-HT3, a ligand-gated ion channel with excitatory properties. Central serotonin produces anorexigenic behaviors, modulates peripheral metabolism and circadian rhythms [79]. Satiety centers express 5-HT2C, whereas hunger centers 5-HT1B and 5-HT6 [10]. Antagonism of 5-HT1B and/or 5-HT2C receptors by many antipsychotic drugs could switch the energy balance setpoint to a more anabolic state, inducing hunger and increasing food intake. In vivo rodent studies suggest that ventral tegmental 5-HT2c stimulation inhibits binge eating by increasing dopaminergic signaling [80], but 5-HT1B and 5-HT2B may also be important [81]. Accordingly, administration of 5-HT2C antagonist in rats induces weight gain, an effect that is potentiated by D2 antagonist, haloperidol, which has no effect on weight alone [5, 68]. In addition, a pharmacodynamic-pharmacoepidemiological study proposes the association of 5-HT2C antagonism and antipsychotic-induced diabetes, a relation that is potentiated by simultaneously H1 antagonism [54]. 5-HT2A antagonism is also associated with AIMD [53, 54]. Binding affinity to neocortical 5-HT2A predicts quetiapine-induced weight gain in drug-naïve patients [82], but the receptor may also have important actions in the periphery. Conversely, antipsychotics with lower risk of weight gain, like ziprasidone, tend to be 5-HT1B partial agonists. Administration of ziprasidone in rats, despite of having dual 5-HT2C and D2 antagonistic effects, increases energy expenditure and induces weight loss [83]. Concerning 5-HT6, a pharmacodynamic analysis of clinical trials suggests that 5-HT6 antagonism may be correlated to antipsychotic-induced weight gain [53].
Antipsychotic drugs can also have indirect effects on serotonin receptors by altering serotonergic efflux in different brain regions. Serotonergic efflux in cortical and subcortical regions seems to remain unchanged (e.g. lurasidone, ziprasidone) or even decrease (e.g. haloperidol, aripiprazole, olanzapine, clozapine). However, risperidone, and to a lesser extent quetiapine, may be the only exceptions. Risperidone could disinhibit serotonin neurotransmission, by 5-HT1A and 5-HT2A antagonism, resulting in increased serotonergic efflux [42, 70]. Additionally, long-term administration of haloperidol to rats seems to attenuate food-related increase of serotonin release in nucleus accumbens [71]. The supplementary and indirectly inhibition of serotonin receptors could further dampen serotonergic regulation of metabolic homeostasis and contribute to AIMD.
Serotonin may have multiple effects on beta islets, which seems to express 5-HT1, 5-HT2 and SERT. In vitro studies suggest that intracellular serotonin increases glucose-dependent insulin secretion whereas extracellular serotonin suppresses it [84]. Extracellular and intracellular serotonin levels can be modulated by serotonin transporters. Clinical data and in vitro studies propose that serotonin transporter inhibitors decrease insulin secretion and could accelerate the progression of diabetes [85, 86], possibly by increasing extracellular serotonin, but other mechanisms cannot be excluded [87]. 5-HT2C antagonism seems to increase directly glucose-dependent insulin secretion in a mouse model of type 2 diabetes, but not in healthy mice [88]. However, 5-HT2C transcripts have not been detected in human islets [89]. In vitro studies of human and rodent islets propose that 5-HT2A/B stimulation increases glucose-dependent insulin secretion, in contrast to 5-HT1A/D stimulation [89, 90]. Additionally, pancreatic 5-HT2B may also promote proliferation and adaption of beta islets during metabolic stress conditions [89]. Administration of 5-HT2A antagonists to rodents suggests that they might directly decrease insulin secretion [52, 62, 77]. Central and peripheral administration of 5-HT1A agonist to rodents suppresses insulin secretion, whereas 5-HT1A antagonism seems to have no effect [52]. However, other 5-HT1 subtypes may be more important in humans. In islets from healthy donors, extracellular serotonin inhibits insulin secretion, probably by 5-HT1D stimulation. This effect is reversed on islets from T2D donors, which display compensatory increased expression of both 5-HT2A and 5-HT1D [90]. A recent study suggests that treatment initiation with aripiprazole or ziprasidone carries risk for diabetes in young antipsychotic-naive patients, despite their estimated lower risk in other groups [91]. These drugs are potent 5-HT1D agonists, which possibly explain this phenomenon in young patients.
3.3. Histamine Receptors
The families of histamine receptors are H1 (Gαq), H2 (Gαs) and the inhibitory H3, H4 (Gαi). CNS histamine is thought to be mainly anorexic reducing hunger and promoting satiety [92]. H1 is expressed in satiety centers and their projections, whereas H3 inhibits hunger centers and neurotransmitter release by acting as an autoreceptor [93]. Binding affinity to H1 is correlated to antipsychotic-induced weight gain and diabetes [53, 54, 94]. Administration of olanzapine or risperidone to rats seems to increase food intake and reduce locomotor activity, accompanied by increased hypothalamic H1R and activated AMPK [23, 95]. The above effects of olanzapine can be reversed by co-administration of H1 agonist [95-97]. Additionally, central administration of H1 antagonists activates hypothalamic AMPK and induces hyperglycemia in mice [62]. Conversely, H1 antagonist could not increase weight in rats, questioning its role in antipsychotic-induced weight gain [68]. H3 signaling may also contribute to AIMD, but its exact role is still not elucidated [98]. Regarding the crosstalk among hypothalamus-brainstem-periphery and the diverge actions of histamine [92, 99], antipsychotic-H1 antagonism may have complex and supplementary contributions to AIMD.
There is evidence, yet controversial [28], that antipsychotic drugs could directly alter hepatic metabolism. A recent in vivo study suggests that olanzapine-induced dyslipidemia is accompanied with reduced activation of hepatic AMPK and increased hepatic H1R expression in rats. Betahistine was able to reverse these actions, probably by acting on hepatic H1 receptors [100]. Administration of clozapine to mice could increase fructose, but not glucose, absorption contributing to weight gain. Probably intestinal H1 interacts with GLUT5 function regulating fructose absorption. This interaction could contribute to weight gain and hepatic insulin resistance induced by antipsychotics [101, 102]. Other possible actions of peripheral histamine should also be noted. A study with healthy volunteers suggests that H1/2 may mediate indirectly glucose uptake by skeletal muscles after exercise and insulin sensitivity [103]. Interestingly, HR1 knockout mice are obese with increased atherosclerosis, whereas HR2 knockout mice display hyperlipidemia, hepatosteatosis but not atherosclerosis [104, 105]. Despite of translational issues, H1 antagonism could be an additive link between metabolic disturbances and cardiovascular risk.
3.4. Acetylcholine Receptors
Acetylcholine receptors are divided into muscarinic and nicotinic. Muscarinic receptors are GPCRs classified into M1, M3, M5 (Gaq) and M2, M4 (Gai) [106]. Clozapine and olanzapine act as partial agonists/allosteric modulators of muscarinic receptors, whereas quetiapine, chloropromazine and zotepine act as antagonists [43, 49]. Binding affinity to M3 may be associated with antipsychotic-induced weight gain and diabetes [53, 54, 107]. Administration of olanzapine to rats increases M3 density in both hypothalamus and DVC, possibly by M3 antagonism. Increased M3 density was associated with increased food intake and decreased insulin secretion [108]. However, central administration of M1 antagonist to mice could not alter glucose metabolism [62]. Additionally, studies of olanzapine-treated rats suggest that presynaptic M2 antagonism could reduce M2 density in DVC, which may also contribute to increased food intake [109].
Pancreatic-M3 deficient mice are prone to glucose intolerance whereas whole body-M3 deficient mice are lean with a good metabolic profile suggesting complex roles of muscarinic signaling [110]. In vitro experiments with rat pancreatic islets suggest that M1 and M3 stimulation promotes basal and glucose-dependent insulin secretion respectively [111]. Accordingly, administration of M3 antagonist to rats acutely inhibits glucose-dependent insulin secretion [77]. Clozapine and olanzapine as muscarinic partial agonists could both increase and decrease insulin secretion. This quite unique dual effect is consistent with in vitro and in vivo studies [28, 112, 113].
Other possible mechanisms cannot be excluded. During the cephalic phase of digestion, clozapine/olanzapine could antagonize acetylcholine at M3 and decrease insulin secretion. The insufficient insulin levels during the cephalic phase increase post-prandial glucose blood levels, stimulating post-prandial insulin secretion. However, clinical evidence suggests that olanzapine increases both cephalic and post-prandial insulin levels, indicating complex and compensatory interactions of central and peripheral muscarinic signaling [51, 114]. Additionally, translational issues between human and rodent models should be furthered clarified. In contrast to rodents, human beta cells seem to express M3 and M5, which increase insulin secretion, whereas delta cells express M1, which stimulates somatostatin secretion, a paracrine hormone that inhibits insulin secretion [115]. As a result, M3 antagonism could decrease whereas M1 antagonism indirectly increases insulin secretion in humans.
3.5. Adrenergic Receptors
Adrenergic receptors are classified into alpha1 (α1A, α1B, α1D), alpha2 (α2A, α2B, α2C) and beta (β1, β2, β3). AIMD-related antipsychotics are potent inhibitors of alpha1 receptors and less or equal of alpha2A, whereas antipsychotics with lower metabolic risk tent to have equal affinities to alpha1/alpha2A (Table 1). Phenylpiperazines, such as aripiprazole, are also beta receptors (β1, β2) antagonists [116].
In vivo rodent studies suggest that hypothalamic adrenergic receptors regulate energy balance, with alpha1 and alpha2 having opposing roles. Noradrenergic transmission to hypothalamus may mediate peripheral anorexic signals [117]. Central alpha1 agonism seems to decrease food intake and weight gain whereas alpha2 agonism has the opposite effects [30]. Additionally, central administration of alpha1 antagonist to mice was able to induce hyperglycemia and activation of hypothalamic AMPK [62]. Αlpha2A inverse agonism, in contrast to alpha1 or alpha2B antagonism, potentiates weight loss by norepinephrine transporter/serotonin transporter inhibitors in rats [118]. The above data demonstrate possible protective roles of central alpha2A antagonism in contrast to alpha1 antagonism.
Peripheral adrenergic receptors mediate the effects of the sympathetic nervous system. Adrenergic and beta receptors seems to have both protective and harmful effects on metabolic regulation [119]. Sympathetic nervous system overreaction may be associated with AIMD [120] and have reciprocal connections with metabolic syndrome and obesity [121]. Central and peripheral D2/alpha2 antagonism could increase catecholamine release and sympathetic activity [122]. Alpha1/2 antagonism by antipsychotics can compensate some of the effects of increased sympathetic tone [4, 43]. Beta receptor stimulation has divergent effects on energy balance and insulin sensitivity [123, 124]. Despite the possible protective effects of beta receptor signaling, sustained sympathetic hyperactivity seems to disrupt beta receptor density and function [121]. As a result, antipsychotics could indirectly influence β adrenergic signaling, given their low affinity to these receptors.
Hepatic insulin resistance and glucose output may be important pathogenetic steps of metabolic syndrome and AIMD [62, 101]. Hypothalamus could affect hepatic glucose and lipid metabolism through the autonomous nervous system. Central administration of olanzapine to mice activates hypothalamic AMPK and increases hepatic glucose production, probably mediated by sympathetic nervous system and beta-adrenergic signaling [125]. Adrenergic receptors may also regulate pancreatic, adipose and skeletal muscle metabolism. Rodent studies suggest that peripheral alpha1 antagonism reduces peripheral vascular resistance, but it can also decrease insulin-independent glucose uptake by adipose tissue and skeletal muscles [119]. Conversely, alpha2 antagonism may promote insulin sensitivity [52] and possibly regulate WAT lipolysis along with insulin and glucagon secretion [119].
3.6. NMDA and GABAA Receptor
Glutamate receptors are possible targets of antipsychotic treatment and may play important roles in metabolic regulation [10, 126]. Clozapine and other antipsychotics, to a lesser degree, could act as weak agonists of NMDA receptors. NMDA receptors are activated when both glutamate and D-serine or D-glycine are binding to different sites of the receptor. Clozapine might activate the receptor by acting like D-serine [49, 127]. D-serine administration seems to suppress unhealthy feeding behaviors in mice [128]. Recently NMDA receptors were detected in mouse and human pancreatic islets. Their blockade could be therapeutic adjuvant for type 2 diabetes, by increasing insulin secretion and beta cell survival [129].
GABAA receptors are structured by a configuration of α, β and γ subunits. Clozapine, olanzapine and maybe other antipsychotics could act as weak antagonists of GABAA receptors [49, 130]. Administration of GABAA antagonists to rats suppresses food intake by acting on energy balance centers [131]. Additionally, preclinical studies suggest that GABAA receptors are also expressed in pancreatic islets, where their stimulation increases glucose-dependent insulin, decreases glucagon secretion and promotes cell survival [132].
3.7. Glucose Transporter Inhibition
Comparisons of central and peripheral administration of olanzapine to rats suggest that hyperglycemia and insulin resistance may be provoked in periphery. They also propose that the primary mediator could be the inhibition of glucose uptake in non-hepatic tissues [26]. Additionally, disrupted glucose-lipid utilization seems to precede insulin resistance in antipsychotic-treated rodents. Inhibition of multiple glucose transporters could be a probable but unlikely mechanism [133, 134]. However, antipsychotic drugs may inhibit directly and/or indirectly glucose transportation in the CNS and periphery, but the exact mechanisms are unknown [13]. A possible mechanism is the interaction of antipsychotic drugs with conserved residues in glucose transporters, mainly the insulin-sensitive GLUT4 [135]. Aerobic exercise and CB1 antagonists seems to ameliorate olanzapine-induced metabolic side effects in rats, accompanied by increasing GLUT4 in skeletal muscles and WAT [136, 137], suggesting the importance of glucose transporters in AIMD.
3.8. Drugs of Abuse, Opioid and Cannabinoid Receptors
Substance abuse is common in patients with schizophrenia, especially nicotine, cannabis, alcohol and psychostimulants [138]. Antipsychotic drugs could also predispose to substance abuse per se. Chronic and continuous administration of antipsychotics, especially typical, could upregulate D2 receptors and increase reward-sensitivity to drugs [139]. Alcohol and drugs of abuse seems to predispose to metabolic syndrome and they are associated with eating disorders, malnutrition, neurotoxicity and hepatotoxicity [140]. As a result, comorbid substance abuse could worsen AIMD. Psychostimulants, such as amphetamine and cocaine, mainly target monoamine systems. They can alter metabolic regulation and they have been used as anorectic agents [64]. However, cocaine seems to increase palatable food intake without increasing weight in men, probably by increasing sympathetic activity and energy expenditure [141].
Neurotransmitter receptors have both protective and harmful effects on metabolism. Secondary metabolic effects, e.g. by ANS disruption, are not mentioned.
Opioid and cannabinoid receptors are widely expressed in energy balance and reward systems. Morphine, a μ opioid agonist, and THC or CB1 agonists can increase food intake [64]. Clinical evidence suggests D2 antagonism could interfere with opioid signaling in order to induce weight gain. According to that, naltrexone, a μ/κ opioid antagonist, was able to attenuate antipsychotic-induced weight gain in overweight women, possibly the portion mediated by D2 antagonism [142]. Additionally, in vivo rodent studies propose the involvement of central and peripheral endocannabinoid signaling in AIMD. Both central and peripheral CB1 antagonism could attenuate olanzapine-induced metabolic alterations in rats. However, CB1 antagonists with low BBB permeability may affect preferentially the periphery and they could counteract AIMD without having the side effects of central CB1 antagonism, such as suicidality and depression [137].
CONCLUSION
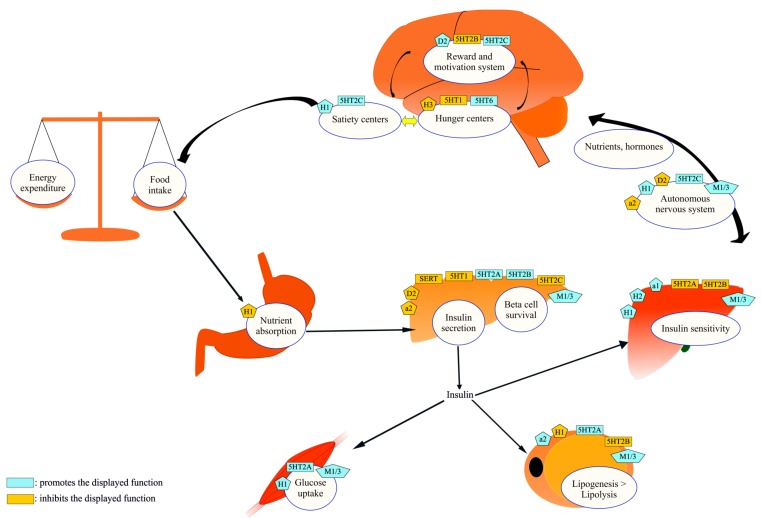
Antipsychotic-induced metabolic side effects are at the forefront of psychopharmacology, but their pathogenesis remains unknown. The current model consists of three interrelated but quite independent pathogenetic pathways: weight gain, insulin resistance and beta cell dysfunction [21]. These pathways are jointly regulated by the CNS, ANS and peripheral organs, where receptors are expressed (Fig. 1). Neurotransmitter receptors and transporters may mediate AIMD (Table 2), but the primary and secondary targets are unclear. Furthermore, there is evidence that antipsychotic drugs have negligible affinities to receptors involved in weight gain [143] and possibly insulin resistance and diabetes.
Fig. (1).
Metabolic regulation and neurotransmitter receptors: the “canvas” of antipsychotic-induced metabolic dysfunctions. CNS and peripheral organs are the two major levels of metabolic regulation. They have bidirectional neuroendocrine connections and express neurotransmitter receptors. Energy balance (energy intake-energy expenditure), adipose tissue function, insulin secretion and insulin sensitivity are important sections of proper metabolic regulation. Antipsychotic drugs, by acting on neurotransmitter receptors, could alter this system and induce metabolic dysfunctions. (The color version of the figure is available in the electronic copy of the article).
Table 2.
Association between pharmacological activity, central and peripheral levels of metabolic regulation.
| Effects | ||
|---|---|---|
| Central | Peripheral | |
| D2/3 antagonism | ↑ Food intake | ↑ Insulin secretion |
| ↑hypohalamic AMPK | ↑ Proliferation and survival of beta cells | |
| ↑EPS | Deplete insulin stores (chronic) | |
| ↑ Catecholamine release | ||
| ↑ Prolactin | ||
| 5-HT1 partial agonism | ↓ Food intake | ↓ Insulin secretion |
| ↓ EPS | ↓ Glucose uptake (adipose tissue) | |
| ↓ Prolactin | ||
| 5-HT2A antagonism | ↑Food intake | ↓ Insulin secretion |
| ↓ EPS | ↑ Hepatic insulin sensitivity | |
| ↓ Prolactin | ↓ Glucose uptake (skeletal muscle) | |
| ↓Adipose tissue lipogenesis | ||
| 5-HT2C antagonism | ↑ Food intake | ↑ Insulin secretion(?) |
| ANS disruption | ||
| H1 antagonism | ↑ Food intake | ↓ Hepatic insulin sensitivity |
| ↑ hypothalamic AMPK | ↓ Glucose uptake (adipose tissue, skeletal muscle) | |
| ↑ Sedation | ↑ Adipose tissue lipogenesis | |
| ANS disruption | ↑ Fructose absorption | |
| ↑ Atherosclerosis | ||
| M3 antagonism | ↑ Food intake | ↓ Insulin secretion |
| ↓ EPS | ↓ Adipose tissue lipogenesis | |
| ANS disruption | ↓ Glucose uptake (skeletal muscle) | |
| alpha1 antagonism | ↑ Food intake | ↓ Hepatic insulin sensitivity |
| ↑ hypothalamic AMPK | ↓ Peripheral vascular resistance | |
| ↑ Sedation | ↓ Glucose uptake (adipose tissue, skeletal muscle) | |
| ANS disruption | ||
| alpha2 antagonism | ↓ Food intake | ↑ Insulin secretion |
| ↓Adipose tissue lipogenesis | ||
| ↑ Catecholamine release | ||
Actions in multiple brain regions, such as the hypothalamus, the reward system and the brainstem and in peripheral organs may disrupt food intake and metabolic regulation. The combination of 5-HT2C/D2/H1 antagonism affects these regions and it is suggested to be the core mechanism of antipsychotic induced weight gain and diabetes [5, 54]. Antagonism of 5-HT2A, 5-HT6, M3 and alpha1 may have additive central and/or peripheral harmful effects, whereas of alpha2A quite protective. Antipsychotics with lower metabolic risk seem to lack 5-HT2C antagonism (amisulpride, haloperidol) or have additional protective mechanisms, such as D2/3 and/or 5-HT1 partial agonism (aripiprazole, lurasidone, and ziprasidone). However, D2/3 or 5-HT1 partial agonism may also disrupt beta cell function and insulin secretion [74, 90]. As a result, “metabolic neutral” drugs may also be capable of inducing metabolic side effects [91].
Antipsychotic drugs may also alter the expression of various receptors, neurotransmitter release along with brain structure and function. These adaptations in central and peripheral systems may mediate important indirect functions of antipsychotic drugs [42, 72]. In addition antipsychotic drugs seem to interfere with the immune system, gut microflora, hormone secretion, function and signaling [13, 144], with yet unclear mechanisms. These indirect mechanisms could help cover the translational gap from binding profiles to metabolic liabilities. As a result, the effects of antipsychotics on metabolism should be considered as the net result of direct receptor mechanisms combined with indirect and compensatory mechanisms, such as changes in receptor expression and neurotransmitter release. Moreover, AIMD exhibits quite distinct time-dependent stages [145], possibly mediated by different receptors and adaptive responses to antipsychotics.
Further preclinical and clinical research is needed in order to elucidate all the pathogenetic mechanisms. Moreover, theoretical and data-driven computational models could help decipher the primary elements of binding profiles and the downstream pathways that mediate AIMD. Consequently, the translation of drug binding profiles to metabolic liability would be of great clinical importance, by guiding therapeutic decision making and intelligent drug design.
Acknowledgements
Declared none.
LIST OF ABBREVIATIONS
- AgRP
Agouti-related peptide
- AIMD
Antipsychotic-induced metabolic dysfunctions
- AMPK
5' adenosine monophosphate-activated protein kinase
- ANS
Autonomous nervous system
- BBB
Blood brain barrier
- CNS
Central nervous system
- DVC
Dorsal vagal complex
- EPS
Extrapyramidal symptoms
- GLP-1
Glucagon-like peptide 1
- GLUT
Glucose transporter
- GPCR
G protein-coupled receptor
- NPY
Neuropeptide Y
- POMC
Pro-opiomelanocortin
- SERT
Serotonin transporter
- T2D
Type 2 diabetes mellitus
- THC
Tetrahydrocannabinol
Consent for Publication
Not applicable.
Conflict of Interest
The authors declare no conflict of interest, financial or otherwise.
References
- 1.Kaidanovich-Beilin O., Cha D.S., McIntyre R.S. Crosstalk between metabolic and neuropsychiatric disorders. F1000 Biol. Rep. 2012;4:14. doi: 10.3410/B4-14. [http://dx.doi.org/10.3410/B4-14]. [PMID: 22802875]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Harris L.W., Guest P.C., Wayland M.T., Umrania Y., Krishnamurthy D., Rahmoune H., Bahn S. Schizophrenia: metabolic aspects of aetiology, diagnosis and future treatment strategies. Psychoneuroendocrinology. 2013;38(6):752–766. doi: 10.1016/j.psyneuen.2012.09.009. [http://dx.doi.org/ 10.1016/j.psyneuen.2012.09.009]. [PMID: 23084727]. [DOI] [PubMed] [Google Scholar]
- 3.Dickerson F.B., Brown C.H., Kreyenbuhl J.A., Fang L., Goldberg R.W., Wohlheiter K., Dixon L.B. Obesity among individuals with serious mental illness. Acta Psychiatr. Scand. 2006;113(4):306–313. doi: 10.1111/j.1600-0447.2005.00637.x. [http://dx.doi.org/10.1111/j.1600-0447.2005.00637.x]. [PMID: 16638075]. [DOI] [PubMed] [Google Scholar]
- 4.De Hert M., Detraux J., van Winkel R., Yu W., Correll C.U. Metabolic and cardiovascular adverse effects associated with antipsychotic drugs. Nat. Rev. Endocrinol. 2011;8(2):114–126. doi: 10.1038/nrendo.2011.156. [http:// dx.doi.org/10.1038/nrendo.2011.156]. [PMID: 22009159]. [DOI] [PubMed] [Google Scholar]
- 5.Correll C.U., Lencz T., Malhotra A.K. Antipsychotic drugs and obesity. Trends Mol. Med. 2011;17(2):97–107. doi: 10.1016/j.molmed.2010.10.010. [http://dx.doi. org/10.1016/j.molmed.2010.10.010]. [PMID: 21185230]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vancampfort D., Stubbs B., Mitchell A.J., De Hert M., Wampers M., Ward P.B., Rosenbaum S., Correll C.U. Risk of metabolic syndrome and its components in people with schizophrenia and related psychotic disorders, bipolar disorder and major depressive disorder: a systematic review and meta-analysis. World Psychiatry. 2015;14(3):339–347. doi: 10.1002/wps.20252. [http://dx.doi.org/10.1002/wps. 20252]. [PMID: 26407790]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Weiden P.J., Mackell J.A., McDonnell D.D. Obesity as a risk factor for antipsychotic noncompliance. Schizophr. Res. 2004;66(1):51–57. doi: 10.1016/s0920-9964(02)00498-x. [http://dx.doi.org/10.1016/S0920-9964(02)00498-X]. [PMID: 14693352]. [DOI] [PubMed] [Google Scholar]
- 8.Koch M., Horvath T.L. Molecular and cellular regulation of hypothalamic melanocortin neurons controlling food intake and energy metabolism. Mol. Psychiatry. 2014;19(7):752–761. doi: 10.1038/mp.2014.30. [http://dx. doi.org/10.1038/mp.2014.30]. [PMID: 24732669]. [DOI] [PubMed] [Google Scholar]
- 9.Volkow N.D., Wang G.J., Baler R.D. 2011. [Google Scholar]
- 10.Sohn J.W., Elmquist J.K., Williams K.W. Neuronal circuits that regulate feeding behavior and metabolism. Trends Neurosci. 2013;36(9):504–512. doi: 10.1016/j.tins.2013.05.003. [http://dx.doi.org/10.1016/j.tins.2013.05.003]. [PMID: 23790727]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zeltser L.M., Seeley R.J., Tschöp M.H. Synaptic plasticity in neuronal circuits regulating energy balance. Nat. Neurosci. 2012;15(10):1336–1342. doi: 10.1038/nn.3219. [http://dx.doi.org/10.1038/nn.3219]. [PMID: 23007188]. [DOI] [PubMed] [Google Scholar]
- 12.Bak M., Fransen A., Janssen J., van Os J., Drukker M. Almost all antipsychotics result in weight gain: a meta-analysis. PLoS One. 2014;9(4):e94112. doi: 10.1371/journal.pone.0094112. [http://dx.doi.org/10.1371/journal.pone.0094112]. [PMID: 24763306]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gonçalves P., Araújo J.R., Martel F. Antipsychotics-induced metabolic alterations: focus on adipose tissue and molecular mechanisms. Eur. Neuropsychopharmacol. 2015;25(1):1–16. doi: 10.1016/j.euroneuro.2014.11.008. [http:// dx.doi.org/10.1016/j.euroneuro.2014.11.008]. [PMID: 25523882]. [DOI] [PubMed] [Google Scholar]
- 14.Rummel-Kluge C., Komossa K., Schwarz S., Hunger H., Schmid F., Lobos C.A., Kissling W., Davis J.M., Leucht S. Head-to-head comparisons of metabolic side effects of second generation antipsychotics in the treatment of schizophrenia: a systematic review and meta-analysis. Schizophr. Res. 2010;123(2-3):225–233. doi: 10.1016/j.schres.2010.07.012. [http://dx.doi.org/10.1016/j.schres.2010.07.012]. [PMID: 20692814]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hert D.E. M.; Correll, C.U.; Bobes, J.; Cetkovich-Bakmas, M.; Cohen, D.; Asai, I.; Detraux, J.; Gautam, S.; Möller, H.J.; Ndetei, D.M.; Newcomer, J.W.; Uwakwe, R.; Leucht, S. Physical illness in patients with severe mental disorders. I. Prevalence, impact of medications and disparities in health care. World Psychiatry. 2011;10(1):52–77. doi: 10.1002/j.2051-5545.2011.tb00014.x. [http://dx.doi.org/10.1002/j.2051-5545.2011.tb00014.x]. [PMID: 21379357]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.De Hert M., Yu W., Detraux J., Sweers K., van Winkel R., Correll C.U. Body weight and metabolic adverse effects of asenapine, iloperidone, lurasidone and paliperidone in the treatment of schizophrenia and bipolar disorder: a systematic review and exploratory meta-analysis. CNS Drugs. 2012;26(9):733–759. doi: 10.2165/11634500-000000000-00000. [http:// dx.doi.org/10.2165/11634500-000000000-00000]. [PMID: 22900950]. [DOI] [PubMed] [Google Scholar]
- 17.Leucht S., Cipriani A., Spineli L., Mavridis D., Orey D., Richter F., Samara M., Barbui C., Engel R.R., Geddes J.R., Kissling W., Stapf M.P., Lässig B., Salanti G., Davis J.M. Comparative efficacy and tolerability of 15 antipsychotic drugs in schizophrenia: a multiple-treatments meta-analysis. Lancet. 2013;382(9896):951–962. doi: 10.1016/S0140-6736(13)60733-3. [http://dx.doi.org/10.1016/S0140-6736(13)60733-3]. [PMID: 23810019]. [DOI] [PubMed] [Google Scholar]
- 18.Correll C.U., Detraux J., De Lepeleire J., De Hert M. Effects of antipsychotics, antidepressants and mood stabilizers on risk for physical diseases in people with schizophrenia, depression and bipolar disorder. World Psychiatry. 2015;14(2):119–136. doi: 10.1002/wps.20204. [http://dx. doi.org/10.1002/wps.20204]. [PMID: 26043321]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lao K.S., He Y., Wong I.C., Besag F.M., Chan E.W. Tolerability and safety profile of cariprazine in treating psychotic disorders, bipolar disorder and major depressive disorder: A systematic review with meta-analysis of randomized controlled trials. CNS Drugs. 2016;30(11):1043–1054. doi: 10.1007/s40263-016-0382-z. [http://dx.doi.org/10.1007/ s40263-016-0382-z]. [PMID: 27550371]. [DOI] [PubMed] [Google Scholar]
- 20.Das S., Barnwal P., Winston A. B.; Mondal, S.; Saha, I. Brexpiprazole: so far so good. Ther. Adv. Psychopharmacol. 2016;6(1):39–54. doi: 10.1177/2045125315614739. [http://dx.doi.org/10.1177/2045125315614739]. [PMID: 26913177]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ballon J.S., Pajvani U., Freyberg Z., Leibel R.L., Lieberman J.A. Molecular pathophysiology of metabolic effects of antipsychotic medications. Trends Endocrinol. Metab. 2014;25(11):593–600. doi: 10.1016/j.tem.2014.07.004. [http://dx.doi.org/10.1016/j.tem.2014.07.004]. [PMID: 25190097]. [DOI] [PubMed] [Google Scholar]
- 22.Weston-Green K., Huang X.F., Deng C. Alterations to melanocortinergic, GABAergic and cannabinoid neurotransmission associated with olanzapine-induced weight gain. PLoS One. 2012;7(3):e33548. doi: 10.1371/journal.pone.0033548. [http://dx.doi.org/10.1371/journal.pone.0033548]. [PMID: 22438946]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lian J., De Santis M., He M., Deng C. Risperidone-induced weight gain and reduced locomotor activity in juvenile female rats: The role of histaminergic and NPY pathways. Pharmacol. Res. 2015;95-96:20–26. doi: 10.1016/j.phrs.2015.03.004. [http://dx.doi.org/10.1016/j.phrs.2015.03.004]. [PMID: 25782398]. [DOI] [PubMed] [Google Scholar]
- 24.Nielsen M.O., Rostrup E., Wulff S., Glenthøj B., Ebdrup B.H. Striatal reward activity and antipsychotic-associated weight change in patients with schizophrenia undergoing initial treatment. JAMA Psychiatry. 2016;73(2):121–128. doi: 10.1001/jamapsychiatry.2015.2582. [http://dx.doi.org/10.1001/ jamapsychiatry.2015.2582]. [PMID: 26747088]. [DOI] [PubMed] [Google Scholar]
- 25.Vestri H.S., Maianu L., Moellering D.R., Garvey W.T. Atypical antipsychotic drugs directly impair insulin action in adipocytes: effects on glucose transport, lipogenesis, and antilipolysis. Neuropsychopharmacology. 2007;32(4):765–772. doi: 10.1038/sj.npp.1301142. [http://dx.doi.org/10. 1038/sj.npp.1301142]. [PMID: 16823387]. [DOI] [PubMed] [Google Scholar]
- 26.Girault E.M., Alkemade A., Foppen E., Ackermans M.T., Fliers E., Kalsbeek A. Acute peripheral but not central administration of olanzapine induces hyperglycemia associated with hepatic and extra-hepatic insulin resistance. PLoS One. 2012;7(8):e43244. doi: 10.1371/journal.pone.0043244. [http:// dx.doi.org/10.1371/journal.pone.0043244]. [PMID: 22905238]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sárvári A.K., Veréb Z., Uray I.P., Fésüs L., Balajthy Z. Atypical antipsychotics induce both proinflammatory and adipogenic gene expression in human adipocytes in vitro. Biochem. Biophys. Res. Commun. 2014;450(4):1383–1389. doi: 10.1016/j.bbrc.2014.07.005. [http://dx.doi.org/10. 1016/j.bbrc.2014.07.005]. [PMID: 25019983]. [DOI] [PubMed] [Google Scholar]
- 28.Smith G.C., Zhang Z.Y., Mulvey T., Petersen N., Lach S., Xiu P., Phillips A., Han W., Wang M.W., Shepherd P.R. Clozapine directly increases insulin and glucagon secretion from islets: implications for impairment of glucose tolerance. Schizophr. Res. 2014;157(1-3):128–133. doi: 10.1016/j.schres.2014.05.003. [http://dx.doi.org/10.1016/j.schres.2014.05.003]. [PMID: 24906220]. [DOI] [PubMed] [Google Scholar]
- 29.Potvin S., Zhornitsky S., Stip E. Antipsychotic-induced changes in blood levels of leptin in schizophrenia: a meta-analysis. Can. J. Psychiatry. 2015;60(3) Suppl. 2:S26–S34. [PMID: 25886677]. [PMC free article] [PubMed] [Google Scholar]
- 30.Reynolds G.P., Kirk S.L. Metabolic side effects of antipsychotic drug treatment--pharmacological mechanisms. Pharmacol. Ther. 2010;125(1):169–179. doi: 10.1016/j.pharmthera.2009.10.010. [http://dx.doi.org/10.1016/j.pharmthera. 2009.10.010]. [PMID: 19931306]. [DOI] [PubMed] [Google Scholar]
- 31.Billes S.K., Simonds S.E., Cowley M.A. Leptin reduces food intake via a dopamine D2 receptor-dependent mechanism. Mol. Metab. 2012;1(1-2):86–93. doi: 10.1016/j.molmet.2012.07.003. [http://dx.doi.org/10.1016/j.molmet. 2012.07.003]. [PMID: 24024122]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Seeman M.V. Secondary effects of antipsychotics: women at greater risk than men. Schizophr. Bull. 2009;35(5):937–948. doi: 10.1093/schbul/sbn023. [http://dx.doi.org/10.1093/schbul/sbn023]. [PMID: 18400811]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Panariello F., De Luca V., de Bartolomeis A. Weight Gain, Schizophrenia and Antipsychotics: New Findings from Animal Model and Pharmacogenomic Studies. Schizophr. Res. Treatment. 2011;2011:16. doi: 10.1155/2011/459284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Benarroch L., Kowalchuk C., Wilson V., Teo C., Guenette M., Chintoh A., Nesarajah Y., Taylor V., Selby P., Fletcher P., Remington G.J., Hahn M.K. A typical antipsychotics and effects on feeding: from mice to men. Psychopharmacology (Berl.) 2016;233:2629–2653. doi: 10.1007/s00213-016-4324-8. [http://dx.doi.org/10.1007/s00213-016-4324-8]. [DOI] [PubMed] [Google Scholar]
- 35.Cooper G.D. A parametric analysis of olanzapine-induced weight gain in female rats. 2005. [DOI] [PubMed]
- 36.van der Zwaal E.M., Janhunen S.K. 2013.
- 37.Meguid M.M., Fetissov S.O., Varma M., Sato T., Zhang L., Laviano A., Rossi-Fanelli F. Hypothalamic dopamine and serotonin in the regulation of food intake. Nutrition. 2000;16(10):843–857. doi: 10.1016/s0899-9007(00)00449-4. [DOI] [PubMed] [Google Scholar]
- 38.Faraday M.M. Rat sex and strain differences in responses to stress. Physiol. Behav. 2002;75(4):507–522. doi: 10.1016/s0031-9384(02)00645-5. [http://dx.doi.org/10.1016/ S0031-9384(02)00645-5]. [DOI] [PubMed] [Google Scholar]
- 39.Tungaraza T.E. Significant weight loss following clozapine use, how is it possible? A case report and review of published cases and literature relevant to the subject. Ther. Adv. Psychopharmacol. 2016;6(5):335–342. doi: 10.1177/2045125316649534. [http://dx.doi.org/10.1177/2045125316649534]. [PMID: 27721972]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cooper G.D., Pickavance L.C., Wilding J.P., Harrold J.A. ; Halford J.C., Goudie A.J. Effects of olanzapine in male rats: enhanced adiposity in the absence of hyperphagia, weight gain or metabolic abnormalities. J. Psychopharmacol. 2007;21(4):405–413. doi: 10.1177/0269881106069637. [DOI] [PubMed] [Google Scholar]
- 41.Cope M.B., Nagy T.R., Fernandez J.R., Geary N., Casey D.E., Allison D.B. Antipsychotic drug-induced weight gain: development of an animal model. Int. J. Obes. 2005;29(6):607–614. doi: 10.1038/sj.ijo.0802928. [DOI] [PubMed] [Google Scholar]
- 42.Amato D., Beasley C.L., Hahn M.K., Vernon A.C. Neuroadaptations to antipsychotic drugs: Insights from pre-clinical and human post-mortem studies. Neurosci. Biobehav. Rev. 2016 doi: 10.1016/j.neubiorev.2016.10.004. [PMID: 27756689]. [DOI] [PubMed] [Google Scholar]
- 43.Nasrallah H.A. A typical antipsychotic-induced metabolic side effects: insights from receptor-binding profiles. Mol. Psychiatry. 2008;13(1):27–35. doi: 10.1038/sj.mp.4002066. [http://dx.doi.org/10.1038/sj.mp.4002066]. [PMID: 17848919]. [DOI] [PubMed] [Google Scholar]
- 44.Correll C.U. From receptor pharmacology to improved outcomes: individualising the selection, dosing, and switching of antipsychotics. Eur. Psychiatry. 2010;25(Suppl. 2):S12–S21. doi: 10.1016/S0924-9338(10)71701-6. [http://dx.doi.org/ 10.1016/S0924-9338(10)71701-6]. [PMID: 20620881]. [DOI] [PubMed] [Google Scholar]
- 45.Ishibashi T., Horisawa T., Tokuda K., Ishiyama T., Ogasa M., Tagashira R., Matsumoto K., Nishikawa H., Ueda Y., Toma S., Oki H., Tanno N., Saji I., Ito A., Ohno Y., Nakamura M. Pharmacological profile of lurasidone, a novel antipsychotic agent with potent 5-hydroxytryptamine 7 (5-HT7) and 5-HT1A receptor activity. J. Pharmacol. Exp. Ther. 2010;334(1):171–181. doi: 10.1124/jpet.110.167346. [http://dx.doi.org/10.1124/jpet.110.167346]. [PMID: 20404009]. [DOI] [PubMed] [Google Scholar]
- 46.Garcia-Serna R., Ursu O., Oprea T.I., Mestres J. iPHACE: integrative navigation in pharmacological space. Bioinformatics. 2010;26(7):985–986. doi: 10.1093/bioinformatics/btq061. [http://dx.doi.org/10.1093/bioinformatics/btq061]. [PMID: 20156991]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Besnard J., Ruda G.F., Setola V., Abecassis K., Rodriguiz R.M., Huang X.P., Norval S., Sassano M.F., Shin A.I., Webster L.A., Simeons F.R., Stojanovski L., Prat A., Seidah N.G., Constam D.B., Bickerton G.R., Read K.D., Wetsel W.C., Gilbert I.H., Roth B.L., Hopkins A.L. Automated design of ligands to polypharmacological profiles. Nature. 2012;492(7428):215–220. doi: 10.1038/nature11691. [http://dx.doi.org/10.1038/nature11691]. [PMID: 23235874]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Maeda K., Sugino H., Akazawa H., Amada N., Shimada J., Futamura T., Yamashita H., Ito N., McQuade R.D., Mørk A., Pehrson A.L., Hentzer M., Nielsen V., Bundgaard C., Arnt J., Stensbøl T.B., Kikuchi T. Brexpiprazole I: in vitro and in vivo characterization of a novel serotonin-dopamine activity modulator. J. Pharmacol. Exp. Ther. 2014;350(3):589–604. doi: 10.1124/jpet.114.213793. [http://dx.doi. org/10.1124/jpet.114.213793]. [PMID: 24947465]. [DOI] [PubMed] [Google Scholar]
- 49.O’Connor W.T., O’Shea S.D. Clozapine and GABA transmission in schizophrenia disease models: establishing principles to guide treatments. Pharmacol. Ther. 2015;150:47–80. doi: 10.1016/j.pharmthera.2015.01.005. [http://dx.doi. org/10.1016/j.pharmthera.2015.01.005]. [PMID: 25585121]. [DOI] [PubMed] [Google Scholar]
- 50.Fountoulakis K.N., Gazouli M., Kelsoe J., Akiskal H. The pharmacodynamic properties of lurasidone and their role in its antidepressant efficacy in bipolar disorder. Eur. Neuropsychopharmacol. 2015;25(3):335–342. doi: 10.1016/j.euroneuro.2014.11.010. [http://dx.doi.org/10.1016/j.euroneuro.2014. 11.010]. [PMID: 25596883]. [DOI] [PubMed] [Google Scholar]
- 51.Teff K.L., Kim S.F. Atypical antipsychotics and the neural regulation of food intake and peripheral metabolism. Physiol. Behav. 2011;104(4):590–598. doi: 10.1016/j.physbeh.2011.05.033. [http://dx.doi.org/10.1016/j.physbeh.2011. 05.033]. [PMID: 21664918]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Guenette M.D., Giacca A., Hahn M., Teo C., Lam L., Chintoh A., Arenovich T., Remington G. Atypical antipsychotics and effects of adrenergic and serotonergic receptor binding on insulin secretion in-vivo: an animal model. Schizophr. Res. 2013;146(1-3):162–169. doi: 10.1016/j.schres.2013.02.023. [http://dx.doi.org/10.1016/j.schres.2013.02.023]. [PMID: 23499243]. [DOI] [PubMed] [Google Scholar]
- 53.Michl J., Scharinger C., Zauner M., Kasper S., Freissmuth M., Sitte H.H., Ecker G.F., Pezawas L. A multivariate approach linking reported side effects of clinical antidepressant and antipsychotic trials to in vitro binding affinities. Eur. Neuropsychopharmacol. 2014;24(9):1463–1474. doi: 10.1016/j.euroneuro.2014.06.013. [http://dx.doi.org/10.1016/j.euroneuro. 2014.06.013]. [PMID: 25044049]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Montastruc F., Palmaro A., Bagheri H., Schmitt L., Montastruc J.L., Lapeyre-Mestre M. Role of serotonin 5-HT2C and histamine H1 receptors in antipsychotic-induced diabetes: A pharmacoepidemiological-pharmacodynamic study in VigiBase. Eur. Neuropsychopharmacol. 2015;25(10):1556–1565. doi: 10.1016/j.euroneuro.2015.07.010. [http://dx.doi.org/10. 1016/j.euroneuro.2015.07.010]. [PMID: 26256010]. [DOI] [PubMed] [Google Scholar]
- 55.Bello E.P., Mateo Y., Gelman D.M., Noaín D., Shin J.H., Low M.J., Alvarez V.A., Lovinger D.M., Rubinstein M. Cocaine supersensitivity and enhanced motivation for reward in mice lacking dopamine D2 autoreceptors. Nat. Neurosci. 2011;14(8):1033–1038. doi: 10.1038/nn.2862. [http://dx.doi.org/10.1038/nn.2862]. [PMID: 21743470]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Johnson P.M., Kenny P.J. Dopamine D2 receptors in addiction-like reward dysfunction and compulsive eating in obese rats. Nat. Neurosci. 2010;13(5):635–641. doi: 10.1038/nn.2519. [http://dx.doi.org/10.1038/nn. 2519]. [PMID: 20348917]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Caravaggio F., Borlido C., Hahn M., Feng Z., Fervaha G. ; Gerretsen P., Nakajima S., Plitman E., Chung J.K., Iwata Y., Wilson A., Remington G., Graff-Guerrero A. Reduced insulin sensitivity is related to less endogenous dopamine at D2/3 receptors in the ventral striatum of healthy nonobese humans. Int. J. Neuropsychopharmacol. 2015;18(7):pyv014. doi: 10.1093/ijnp/pyv014. [http://dx.doi.org/10.1093/ ijnp/pyv014]. [PMID: 25716779]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Land B.B., Narayanan N.S., Liu R.J., Gianessi C.A., Brayton C.E., Grimaldi D.M., Sarhan M., Guarnieri D.J., Deisseroth K., Aghajanian G.K., DiLeone R.J. Medial prefrontal D1 dopamine neurons control food intake. Nat. Neurosci. 2014;17(2):248–253. doi: 10.1038/nn.3625. [http://dx.doi.org/10.1038/nn.3625]. [PMID: 24441680]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Anderberg R.H., Anefors C., Bergquist F., Nissbrandt H., Skibicka K.P. Dopamine signaling in the amygdala, increased by food ingestion and GLP-1, regulates feeding behavior. Physiol. Behav. 2014;136:135–144. doi: 10.1016/j.physbeh.2014.02.026. [http://dx.doi.org/10.1016/j.physbeh. 2014.02.026]. [PMID: 24560840]. [DOI] [PubMed] [Google Scholar]
- 60.Lopez Vicchi F., Luque G.M., Brie B., Nogueira J.P., Garcia Tornadu I., Becu-Villalobos D. Dopaminergic drugs in type 2 diabetes and glucose homeostasis. Pharmacol. Res. 2016;109:74–80. doi: 10.1016/j.phrs.2015.12.029. [http://dx.doi.org/10.1016/j.phrs.2015.12.029]. [PMID: 26748034]. [DOI] [PubMed] [Google Scholar]
- 61.Fetissov S.O., Meguid M.M., Sato T., Zhang L.H. Expression of dopaminergic receptors in the hypothalamus of lean and obese Zucker rats and food intake. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2002;283(4):R905–R910. doi: 10.1152/ajpregu.00092.2002. [http://dx.doi.org/10.1152/ ajpregu.00092.2002]. [PMID: 12228060]. [DOI] [PubMed] [Google Scholar]
- 62.Ikegami M., Ikeda H., Ohashi T., Kai M., Osada M., Kamei A., Kamei J. Olanzapine-induced hyperglycemia: possible involvement of histaminergic, dopaminergic and adrenergic functions in the central nervous system. Neuroendocrinology. 2013;98(3):224–232. doi: 10.1159/000356119. [http://dx.doi.org/10.1159/000356119]. [PMID: 24135197]. [DOI] [PubMed] [Google Scholar]
- 63.Rubí B., Maechler P. Minireview: new roles for peripheral dopamine on metabolic control and tumor growth: let’s seek the balance. Endocrinology. 2010;151(12):5570–5581. doi: 10.1210/en.2010-0745. [http://dx.doi.org/ 10.1210/en.2010-0745]. [PMID: 21047943]. [DOI] [PubMed] [Google Scholar]
- 64.Adan R.A. Mechanisms underlying current and future anti-obesity drugs. Trends Neurosci. 2013;36(2):133–140. doi: 10.1016/j.tins.2012.12.001. [http://dx.doi.org/ 10.1016/j.tins.2012.12.001]. [PMID: 23312373]. [DOI] [PubMed] [Google Scholar]
- 65.Cooper S.J., Al-Naser H.A. Dopaminergic control of food choice: contrasting effects of SKF 38393 and quinpirole on high-palatability food preference in the rat. Neuropharmacology. 2006;50(8):953–963. doi: 10.1016/j.neuropharm.2006.01.006. [http://dx.doi.org/10.1016/j.neuropharm.2006.01. 006]. [PMID: 16549074]. [DOI] [PubMed] [Google Scholar]
- 66.Klenotich S.J., Ho E.V., McMurray M.S., Server C.H., Dulawa S.C. Dopamine D2/3 receptor antagonism reduces activity-based anorexia. Transl. Psychiatry. 2015;5:e613. doi: 10.1038/tp.2015.109. [http://dx.doi.org/ 10.1038/tp.2015.109]. [PMID: 26241351]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.De Santis M., Pan B., Lian J., Huang X.F., Deng C. Different effects of bifeprunox, aripiprazole, and haloperidol on body weight gain, food and water intake, and locomotor activity in rats. Pharmacol. Biochem. Behav. 2014;124:167–173. doi: 10.1016/j.pbb.2014.06.004. [http://dx.doi.org/ 10.1016/j.pbb.2014.06.004]. [PMID: 24933333]. [DOI] [PubMed] [Google Scholar]
- 68.Kirk S.L., Glazebrook J., Grayson B., Neill J.C., Reynolds G.P. Olanzapine-induced weight gain in the rat: role of 5-HT2C and histamine H1 receptors. Psychopharmacology (Berl.) 2009;207(1):119–125. doi: 10.1007/s00213-009-1639-8. [http://dx.doi.org/10.1007/s00213-009-1639-8]. [PMID: 19688201]. [DOI] [PubMed] [Google Scholar]
- 69.Kaur G., Kulkarni S.K. Studies on modulation of feeding behavior by atypical antipsychotics in female mice. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2002;26(2):277–285. doi: 10.1016/s0278-5846(01)00266-4. [http://dx.doi. org/10.1016/S0278-5846(01)00266-4]. [PMID: 11817504]. [DOI] [PubMed] [Google Scholar]
- 70.Amato D. Serotonin in antipsychotic drugs action. Behav. Brain Res. 2015;277:125–135. doi: 10.1016/j.bbr.2014.07.025. [http://dx.doi.org/10.1016/j.bbr.2014. 07.025]. [PMID: 25078293]. [DOI] [PubMed] [Google Scholar]
- 71.Amato D., Natesan S., Yavich L., Kapur S., Müller C.P. Dynamic regulation of dopamine and serotonin responses to salient stimuli during chronic haloperidol treatment. Int. J. Neuropsychopharmacol. 2011;14(10):1327–1339. doi: 10.1017/S1461145711000010. [http://dx.doi.org/10.1017/ S1461145711000010]. [PMID: 21281560]. [DOI] [PubMed] [Google Scholar]
- 72.Lieberman J.A., Bymaster F.P., Meltzer H.Y., Deutch A.Y., Duncan G.E., Marx C.E., Aprille J.R., Dwyer D.S., Li X-M., Mahadik S.P., Duman R.S., Porter J.H., Modica-Napolitano J.S., Newton S.S., Csernansky J.G. Antipsychotic drugs: comparison in animal models of efficacy, neurotransmitter regulation, and neuroprotection. Pharmacol. Rev. 2008;60(3):358–403. doi: 10.1124/pr.107.00107. [http://dx. doi.org/10.1124/pr.107.00107]. [PMID: 18922967]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Oerther S., Ahlenius S. Atypical antipsychotics and dopamine D(1) receptor agonism: an in vivo experimental study using core temperature measurements in the rat. J. Pharmacol. Exp. Ther. 2000;292(2):731–736. [PMID: 10640312]. [PubMed] [Google Scholar]
- 74.Ustione A., Piston D.W., Harris P.E. Minireview: Dopaminergic regulation of insulin secretion from the pancreatic islet. Mol. Endocrinol. 2013;27(8):1198–1207. doi: 10.1210/me.2013-1083. [http://dx.doi.org/10.1210/me. 2013-1083]. [PMID: 23744894]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Simpson N., Maffei A., Freeby M., Burroughs S., Freyberg Z., Javitch J., Leibel R.L., Harris P.E. Dopamine-mediated autocrine inhibitory circuit regulating human insulin secretion in vitro. Mol. Endocrinol. 2012;26(10):1757–1772. doi: 10.1210/me.2012-1101. [http://dx.doi.org/10.1210/ me.2012-1101]. [PMID: 22915827]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.García-Tornadú I., Ornstein A.M., Chamson-Reig A., Wheeler M.B., Hill D.J., Arany E., Rubinstein M., Becu-Villalobos D. Disruption of the dopamine d2 receptor impairs insulin secretion and causes glucose intolerance. Endocrinology. 2010;151(4):1441–1450. doi: 10.1210/en.2009-0996. [http://dx.doi.org/10.1210/en.2009-0996]. [PMID: 20147524]. [DOI] [PubMed] [Google Scholar]
- 77.Hahn M., Chintoh A., Giacca A., Xu L., Lam L., Mann S., Fletcher P., Guenette M., Cohn T., Wolever T., Arenovich T., Remington G. Atypical antipsychotics and effects of muscarinic, serotonergic, dopaminergic and histaminergic receptor binding on insulin secretion in vivo: an animal model. Schizophr. Res. 2011;131(1-3):90–95. doi: 10.1016/j.schres.2011.06.004. [http://dx.doi.org/10.1016/j.schres.2011.06.004]. [PMID: 21696923]. [DOI] [PubMed] [Google Scholar]
- 78.de Leeuw van Weenen J.E., Auvinen H.E., Parlevliet E.T., Coomans C.P., Schröder-van der Elst J.P., Meijer O.C., Pijl H. Blocking dopamine D2 receptors by haloperidol curtails the beneficial impact of calorie restriction on the metabolic phenotype of high-fat diet induced obese mice. J. Neuroendocrinol. 2011;23(2):158–167. doi: 10.1111/j.1365-2826.2010.02092.x. [http://dx.doi.org/10.1111/j.1365-2826.2010.02092.x]. [PMID: 21062378]. [DOI] [PubMed] [Google Scholar]
- 79.Versteeg R.I., Serlie M.J., Kalsbeek A., la Fleur S.E. Serotonin, a possible intermediate between disturbed circadian rhythms and metabolic disease. Neuroscience. 2015;301:155–167. doi: 10.1016/j.neuroscience.2015.05.067. [http://dx. doi.org/10.1016/j.neuroscience.2015.05.067]. [PMID: 26047725]. [DOI] [PubMed] [Google Scholar]
- 80.Xu P., He Y., Cao X., Valencia-Torres L., Yan X., Saito K., Wang C., Yang Y., Hinton A., Jr, Zhu L., Shu G., Myers M.G., Jr, Wu Q., Tong Q., Heisler L.K., Xu Y. Activation of serotonin 2C receptors in dopamine neurons inhibits binge-like eating in mice. Biol. Psychiatry. 2016 doi: 10.1016/j.biopsych.2016.06.005. [PMID: 27516377]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Pratt W.E., Clissold K.A., Lin P., Cain A.E., Ciesinski A.F., Hopkins T.R., Ilesanmi A.O., Kelly E.A., Pierce-Messick Z., Powell D.S., Rosner I.A. 2016.
- 82.Rasmussen H., Ebdrup B.H., Oranje B., Pinborg L.H., Knudsen G.M., Glenthøj B. Neocortical serotonin2A receptor binding predicts quetiapine associated weight gain in antipsychotic-naive first-episode schizophrenia patients. Int. J. Neuropsychopharmacol. 2014;17(11):1729–1736. doi: 10.1017/S1461145714000777. [http://dx.doi.org/10.1017/S1461145714000777]. [PMID: 24830305]. [DOI] [PubMed] [Google Scholar]
- 83.Park S., Kim M.S., Namkoong C., Park M.H., Hong J.P. The effect of ziprasidone on body weight and energy expenditure in female rats. Metabolism. 2012;61(6):787–793. doi: 10.1016/j.metabol.2011.10.011. [http://dx.doi. org/10.1016/j.metabol.2011.10.011]. [PMID: 22209671]. [DOI] [PubMed] [Google Scholar]
- 84.Paulmann N., Grohmann M., Voigt J.P., Bert B., Vowinckel J., Bader M., Skelin M., Jevsek M., Fink H., Rupnik M., Walther D.J. Intracellular serotonin modulates insulin secretion from pancreatic beta-cells by protein serotonylation. PLoS Biol. 2009;7(10):e1000229. doi: 10.1371/journal.pbio.1000229. [http://dx.doi.org/10.1371/journal.pbio.1000229]. [PMID: 19859528]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Isaac R., Boura-Halfon S., Gurevitch D., Shainskaya A., Levkovitz Y., Zick Y. Selective serotonin reuptake inhibitors (SSRIs) inhibit insulin secretion and action in pancreatic β cells. J. Biol. Chem. 2013;288(8):5682–5693. doi: 10.1074/jbc.M112.408641. [http://dx.doi.org/10.1074/jbc. M112.408641]. [PMID: 23275337]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Noordam R., Aarts N., Peeters R.P., Hofman A., Stricker B.H., Visser L.E. Selective serotonin reuptake inhibitors decrease pancreatic insulin secretion in older adults and increase the risk of insulin dependence in type 2 diabetes patients. J. Clin. Psychiatry. 2016;77(9):e1124–e1129. doi: 10.4088/JCP.15m10048. [http://dx.doi.org/10.4088/JCP.15m10048]. [PMID: 27487004]. [DOI] [PubMed] [Google Scholar]
- 87.Cataldo L.R., Cortés V.A., Mizgier M.L., Aranda E., Mezzano D., Olmos P., Galgani J.E., Suazo J., Santos J.L. Fluoxetine impairs insulin secretion without modifying extracellular serotonin levels in MIN6 β-cells. Exp. Clin. Endocrinol. Diabetes. 2015;123(8):473–478. doi: 10.1055/s-0035-1549964. [http://dx.doi.org/10.1055/s-0035-1549964]. [PMID: 26011169]. [DOI] [PubMed] [Google Scholar]
- 88.Zhang Q., Zhu Y., Zhou W., Gao L., Yuan L., Han X. Serotonin receptor 2C and insulin secretion. PLoS One. 2013;8(1):e54250. doi: 10.1371/journal.pone.0054250. [http://dx.doi.org/10.1371/journal.pone.0054250]. [PMID: 23349838]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Bennet H., Mollet I.G., Balhuizen A., Medina A., Nagorny C., Bagge A., Fadista J., Ottosson-Laakso E., Vikman P., Dekker-Nitert M., Eliasson L., Wierup N., Artner I., Fex M. Serotonin (5-HT) receptor 2b activation augments glucose-stimulated insulin secretion in human and mouse islets of Langerhans. Diabetologia. 2016;59(4):744–754. doi: 10.1007/s00125-015-3847-6. [http://dx.doi.org/10.1007/s00125-015-3847-6]. [PMID: 26733006]. [DOI] [PubMed] [Google Scholar]
- 90.Bennet H., Balhuizen A., Medina A., Dekker Nitert M., Ottosson Laakso E., Essén S., Spégel P., Storm P., Krus U., Wierup N., Fex M. Altered serotonin (5-HT) 1D and 2A receptor expression may contribute to defective insulin and glucagon secretion in human type 2 diabetes. Peptides. 2015;71:113–120. doi: 10.1016/j.peptides.2015.07.008. [http://dx. doi.org/10.1016/j.peptides.2015.07.008]. [PMID: 26206285]. [DOI] [PubMed] [Google Scholar]
- 91.Rubin D.M., Kreider A.R., Matone M., Huang Y.S., Feudtner C., Ross M.E., Localio A.R. Risk for incident diabetes mellitus following initiation of second-generation antipsychotics among Medicaid-enrolled youths. JAMA Pediatr. 2015;169(4):e150285. doi: 10.1001/jamapediatrics.2015.0285. [http://dx.doi.org/10.1001/jamapediatrics.2015.0285]. [PMID: 25844991]. [DOI] [PubMed] [Google Scholar]
- 92.Tabarean I.V. Histamine receptor signaling in energy homeostasis. Neuropharmacology. 2015;106:13–19. doi: 10.1016/j.neuropharm.2015.04.011. [PMID: 26107117]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Provensi G., Blandina P., Passani M.B. The histaminergic system as a target for the prevention of obesity and metabolic syndrome. Neuropharmacology. 2015;106:3–12. doi: 10.1016/j.neuropharm.2015.07.002. [PMID: 26164344]. [DOI] [PubMed] [Google Scholar]
- 94.Kroeze W.K., Hufeisen S.J., Popadak B.A., Renock S.M., Steinberg S., Ernsberger P., Jayathilake K., Meltzer H.Y., Roth B.L. H1-histamine receptor affinity predicts short-term weight gain for typical and atypical antipsychotic drugs. Neuropsychopharmacology. 2003;28(3):519–526. doi: 10.1038/sj.npp.1300027. [http://dx.doi.org/10.1038/sj.npp.1300027]. [PMID: 12629531]. [DOI] [PubMed] [Google Scholar]
- 95.He M., Zhang Q., Deng C., Wang H., Huang X.F. Olanzapine-activated AMPK signaling in the dorsal vagal complex is attenuated by histamine H1 receptor agonist in female rats. Endocrinology. 2014;155(12):4895–4904. doi: 10.1210/en.2014-1326. [http://dx.doi.org/10.1210/en.2014-1326]. [PMID: 25264935]. [DOI] [PubMed] [Google Scholar]
- 96.Lian J., Huang X.F., Pai N., Deng C. Betahistine ameliorates olanzapine-induced weight gain through modulation of histaminergic, NPY and AMPK pathways. Psychoneuroendocrinology. 2014;48:77–86. doi: 10.1016/j.psyneuen.2014.06.010. [http://dx.doi.org/10.1016/j.psyneuen.2014.06.010]. [PMID: 24992721]. [DOI] [PubMed] [Google Scholar]
- 97.Lian J., Huang X.F., Pai N., Deng C. Preventing olanzapine-induced weight gain using betahistine: a study in a rat model with chronic olanzapine treatment. PLoS One. 2014;9(8):e104160. doi: 10.1371/journal.pone.0104160. [http:// dx.doi.org/10.1371/journal.pone.0104160]. [PMID: 25084453]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Deng C., Weston-Green K., Huang X.F. The role of histaminergic H1 and H3 receptors in food intake: a mechanism for atypical antipsychotic-induced weight gain? Prog. Neuropsychopharmacol. Biol. Psychiatry. 2010;34(1):1–4. doi: 10.1016/j.pnpbp.2009.11.009. [http://dx.doi.org/10.1016/j. pnpbp.2009.11.009]. [PMID: 19922755]. [DOI] [PubMed] [Google Scholar]
- 99.Kim Y.S., Kim Y.B., Kim W.B., Yoon B.E., Shen F.Y., Lee S.W., Soong T.W., Han H.C., Colwell C.S., Lee C.J., Kim Y.I. Histamine resets the circadian clock in the suprachiasmatic nucleus through the H1R-CaV 1.3-RyR pathway in the mouse. Eur. J. Neurosci. 2015;42(7):2467–2477. doi: 10.1111/ejn.13030. [http://dx.doi.org/10.1111/ejn. 13030]. [PMID: 26215659]. [DOI] [PubMed] [Google Scholar]
- 100.Liu X., Lian J., Hu C.H., Deng C. Betahistine co-treatment ameliorates dyslipidemia induced by chronic olanzapine treatment in rats through modulation of hepatic AMPKα-SREBP-1 and PPARα-dependent pathways. Pharmacol. Res. 2015;100:36–46. doi: 10.1016/j.phrs.2015.07.023. [http:// dx.doi.org/10.1016/j.phrs.2015.07.023]. [PMID: 26218603]. [DOI] [PubMed] [Google Scholar]
- 101.Bremer A.A., Mietus-Snyder M., Lustig R.H. Toward a unifying hypothesis of metabolic syndrome. Pediatrics. 2012;129(3):557–570. doi: 10.1542/peds.2011-2912. [http://dx.doi.org/10.1542/peds.2011-2912]. [PMID: 22351884]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Palavicino-Maggio C.B., Kuzhikandathil E.V. Dietary fructose and GLUT5 transporter activity contribute to antipsychotic-induced weight gain. Schizophr. Bull. 2016;42(5):1270–1279. doi: 10.1093/schbul/sbw037. [http://dx. doi.org/10.1093/schbul/sbw037]. [PMID: 27056716]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Pellinger T.K., Dumke B.R., Halliwill J.R. Effect of H1- and H2-histamine receptor blockade on postexercise insulin sensitivity. Physiol. Rep. 2013;1(2):e00033. doi: 10.1002/phy2.33. [http://dx.doi.org/10.1002/phy2.33]. [PMID: 24303118]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Wang K.Y., Tanimoto A., Yamada S., Guo X., Ding Y., Watanabe T., Watanabe T., Kohno K., Hirano K., Tsukada H., Sasaguri Y. Histamine regulation in glucose and lipid metabolism via histamine receptors: model for nonalcoholic steatohepatitis in mice. Am. J. Pathol. 2010;177(2):713–723. doi: 10.2353/ajpath.2010.091198. [http://dx.doi.org/10.2353/ ajpath.2010.091198]. [PMID: 20566747]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Yamada S., Wang K.Y., Tanimoto A., Sasaguri Y. Novel function of histamine signaling in hyperlipidemia-induced atherosclerosis: Histamine H1 receptors protect and H2 receptors accelerate atherosclerosis. Pathol. Int. 2015;65(2):67–80. doi: 10.1111/pin.12246. [http://dx.doi. org/10.1111/pin.12246]. [PMID: 25581821]. [DOI] [PubMed] [Google Scholar]
- 106.Eglen R.M. Muscarinic receptor subtypes in neuronal and non-neuronal cholinergic function. Auton. Autacoid Pharmacol. 2006;26(3):219–233. doi: 10.1111/j.1474-8673.2006.00368.x. [http://dx.doi.org/10.1111/j.1474-8673.2006.00368.x]. [PMID: 16879488]. [DOI] [PubMed] [Google Scholar]
- 107.Silvestre J.S., Prous J. Research on adverse drug events. I. Muscarinic M3 receptor binding affinity could predict the risk of antipsychotics to induce type 2 diabetes. Methods Find. Exp. Clin. Pharmacol. 2005;27(5):289–304. doi: 10.1358/mf.2005.27.5.908643. [http://dx.doi.org/10.1358/mf. 2005.27.5.908643]. [PMID: 16082416]. [DOI] [PubMed] [Google Scholar]
- 108.Weston-Green K., Huang X.F., Lian J., Deng C. Effects of olanzapine on muscarinic M3 receptor binding density in the brain relates to weight gain, plasma insulin and metabolic hormone levels. Eur. Neuropsychopharmacol. 2012;22(5):364–373. doi: 10.1016/j.euroneuro.2011.09.003. [http://dx.doi. org/10.1016/j.euroneuro.2011.09.003]. [PMID: 21982116]. [DOI] [PubMed] [Google Scholar]
- 109.Deng C., Weston-Green K.L., Han M., Huang X.F. Olanzapine treatment decreases the density of muscarinic M2 receptors in the dorsal vagal complex of rats. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2007;31(4):915–920. doi: 10.1016/j.pnpbp.2007.02.009. [http://dx.doi.org/10.1016/j. pnpbp.2007.02.009]. [PMID: 17368684]. [DOI] [PubMed] [Google Scholar]
- 110.Gautam D., Jeon J., Li J.H., Han S.J., Hamdan F.F., Cui Y., Lu H., Deng C., Gavrilova O., Wess J. Metabolic roles of the M3 muscarinic acetylcholine receptor studied with M3 receptor mutant mice: a review. J. Recept. Signal Transduct. Res. 2008;28(1-2):93–108. doi: 10.1080/10799890801942002. [http://dx.doi.org/10.1080/10799890801942002]. [PMID: 18437633]. [DOI] [PubMed] [Google Scholar]
- 111.Renuka T.R., Robinson R., Paulose C.S. Increased insulin secretion by muscarinic M1 and M3 receptor function from rat pancreatic islets in vitro. Neurochem. Res. 2006;31(3):313–320. doi: 10.1007/s11064-005-9022-6. [http:// dx.doi.org/10.1007/s11064-005-9022-6]. [PMID: 16733808]. [DOI] [PubMed] [Google Scholar]
- 112.Johnson D.E., Yamazaki H., Ward K.M., Schmidt A.W., Lebel W.S., Treadway J.L., Gibbs E.M., Zawalich W.S., Rollema H. Inhibitory effects of antipsychotics on carbachol-enhanced insulin secretion from perifused rat islets: role of muscarinic antagonism in antipsychotic-induced diabetes and hyperglycemia. Diabetes. 2005;54(5):1552–1558. doi: 10.2337/diabetes.54.5.1552. [http://dx.doi.org/10.2337/diabetes.54.5.1552]. [PMID: 15855345]. [DOI] [PubMed] [Google Scholar]
- 113.Melkersson K., Jansson E. Effects of the atypical antipsychotic clozapine on insulin release in vitro. Neuroendocrinol. Lett. 2007;28(6):854–860. [PMID: 18063937]. [PubMed] [Google Scholar]
- 114.Teff K.L., Rickels M.R., Grudziak J., Fuller C., Nguyen H.L., Rickels K. Antipsychotic-induced insulin resistance and postprandial hormonal dysregulation independent of weight gain or psychiatric disease. Diabetes. 2013;62(9):3232–3240. doi: 10.2337/db13-0430. [http://dx. doi.org/10.2337/db13-0430]. [PMID: 23835329]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Molina J., Rodriguez-Diaz R., Fachado A., Jacques-Silva M.C., Berggren P-O., Caicedo A. Control of insulin secretion by cholinergic signaling in the human pancreatic islet. Diabetes. 2014;63(8):2714–2726. doi: 10.2337/db13-1371. [http://dx.doi.org/10.2337/db13-1371]. [PMID: 24658304]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Shapiro D.A., Renock S., Arrington E., Chiodo L.A., Liu L.X., Sibley D.R., Roth B.L., Mailman R. Aripiprazole, a novel atypical antipsychotic drug with a unique and robust pharmacology. Neuropsychopharmacology. 2003;28(8):1400–1411. doi: 10.1038/sj.npp.1300203. [http://dx.doi. org/10.1038/sj.npp.1300203]. [PMID: 12784105]. [DOI] [PubMed] [Google Scholar]
- 117.Romano A., Potes C.S., Tempesta B., Cassano T., Cuomo V., Lutz T., Gaetani S. Hindbrain noradrenergic input to the hypothalamic PVN mediates the activation of oxytocinergic neurons induced by the satiety factor oleoylethanolamide. Am. J. Physiol. Endocrinol. Metab. 2013;305(10):E1266–E1273. doi: 10.1152/ajpendo.00411.2013. [http://dx.doi. org/10.1152/ajpendo.00411.2013]. [PMID: 24064338]. [DOI] [PubMed] [Google Scholar]
- 118.Janhunen S.K., van der Zwaal E.M., la Fleur S.E., Adan R.A. Inverse agonism at α2A adrenoceptors augments the hypophagic effect of sibutramine in rats. Obesity (Silver Spring) 2011;19(10):1979–1986. doi: 10.1038/oby.2011.51. [http://dx.doi.org/10.1038/oby.2011.51]. [PMID: 21475142]. [DOI] [PubMed] [Google Scholar]
- 119.Boyda H.N., Procyshyn R.M., Pang C.C., Barr A.M. Peripheral adrenoceptors: the impetus behind glucose dysregulation and insulin resistance. J. Neuroendocrinol. 2013;25(3):217–228. doi: 10.1111/jne.12002. [http:// dx.doi.org/10.1111/jne.12002]. [PMID: 23140239]. [DOI] [PubMed] [Google Scholar]
- 120.Savoy Y.E., Ashton M.A., Miller M.W., Nedza F.M., Spracklin D.K., Hawthorn M.H., Rollema H., Matos F.F., Hajos-Korcsok E. Differential effects of various typical and atypical antipsychotics on plasma glucose and insulin levels in the mouse: evidence for the involvement of sympathetic regulation. Schizophr. Bull. 2010;36(2):410–418. doi: 10.1093/schbul/sbn104. [http://dx.doi.org/10.1093/schbul/sbn104]. [PMID: 18703666]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Thorp A.A., Schlaich M.P. Relevance of sympathetic nervous system activation in obesity and metabolic syndrome. J. Diabetes Res. 2015;2015:341583. doi: 10.1155/2015/341583. [http://dx.doi.org/10.1155/2015/341583]. [PMID: 26064978]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Scigliano G., Ronchetti G. Antipsychotic-induced metabolic and cardiovascular side effects in schizophrenia: a novel mechanistic hypothesis. CNS Drugs. 2013;27(4):249–257. doi: 10.1007/s40263-013-0054-1. [http://dx.doi.org/ 10.1007/s40263-013-0054-1]. [PMID: 23533011]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Ziegler M.G., Elayan H., Milic M., Sun P., Gharaibeh M. Epinephrine and the metabolic syndrome. Curr. Hypertens. Rep. 2012;14(1):1–7. doi: 10.1007/s11906-011-0243-6. [http://dx.doi.org/10.1007/s11906-011-0243-6]. [PMID: 22124970]. [DOI] [PubMed] [Google Scholar]
- 124.Cypess A.M., Weiner L.S., Roberts-Toler C., Franquet Elía E., Kessler S.H., Kahn P.A., English J., Chatman K., Trauger S.A., Doria A., Kolodny G.M. Activation of human brown adipose tissue by a β3-adrenergic receptor agonist. Cell Metab. 2015;21(1):33–38. doi: 10.1016/j.cmet.2014.12.009. [http://dx.doi.org/10.1016/j.cmet.2014.12.009]. [PMID: 25565203]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Ikegami M., Ikeda H., Ohashi T., Ohsawa M., Ishikawa Y., Kai M., Kamei A., Kamei J. Olanzapine increases hepatic glucose production through the activation of hypothalamic adenosine 5′-monophosphate-activated protein kinase. Diabetes Obes. Metab. 2013;15(12):1128–1135. doi: 10.1111/dom.12148. [http://dx.doi.org/10.1111/dom.12148]. [PMID: 23782571]. [DOI] [PubMed] [Google Scholar]
- 126.Papanastasiou E., Stone J.M., Shergill S. When the drugs don’t work: the potential of glutamatergic antipsychotics in schizophrenia. Br. J. Psychiatry. 2013;202:91–93. doi: 10.1192/bjp.bp.112.110999. [http://dx.doi.org/10.1192/ bjp.bp.112.110999]. [PMID: 23377207]. [DOI] [PubMed] [Google Scholar]
- 127.Heresco-Levy U. N-Methyl-D-aspartate (NMDA) receptor-based treatment approaches in schizophrenia: the first decade. Int. J. Neuropsychopharmacol. 2000;3(3):243–258. doi: 10.1017/S1461145700001978. [http://dx.doi.org/ 10.1017/S1461145700001978]. [PMID: 11343602]. [DOI] [PubMed] [Google Scholar]
- 128.Sasaki T., Kinoshita Y., Matsui S., Kakuta S., Yokota-Hashimoto H., Kinoshita K., Iwasaki Y., Kinoshita T., Yada T., Amano N., Kitamura T. N-methyl-d-aspartate receptor coagonist d-serine suppresses intake of high-preference food. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2015;309(5):R561–R575. doi: 10.1152/ajpregu.00083.2015. [http:// dx.doi.org/10.1152/ajpregu.00083.2015]. [PMID: 26157056]. [DOI] [PubMed] [Google Scholar]
- 129.Marquard J., Otter S., Welters A., Stirban A., Fischer A., Eglinger J., Herebian D., Kletke O., Klemen M.S., Stožer A., Wnendt S., Piemonti L., Köhler M., Ferrer J., Thorens B., Schliess F., Rupnik M.S., Heise T., Berggren P.O., Klöcker N., Meissner T., Mayatepek E., Eberhard D., Kragl M., Lammert E. Characterization of pancreatic NMDA receptors as possible drug targets for diabetes treatment. Nat. Med. 2015;21(4):363–372. doi: 10.1038/nm.3822. [http://dx.doi.org/10.1038/nm.3822]. [PMID: 25774850]. [DOI] [PubMed] [Google Scholar]
- 130.Squires R.F., Saederup E. Clozapine and several other antipsychotic/antidepressant drugs preferentially block the same ‘core’ fraction of GABA(A) receptors. Neurochem. Res. 1998;23(10):1283–1290. doi: 10.1023/a:1020796200769. [http://dx.doi.org/10.1023/A:1020796200769]. [PMID: 9804284]. [DOI] [PubMed] [Google Scholar]
- 131.Kamatchi G.L., Rathanaswami P. Inhibition of deprivation-induced food intake by GABA(A) antagonists: roles of the hypothalamic, endocrine and alimentary mechanisms. J. Clin. Biochem. Nutr. 2012;51(1):19–26. doi: 10.3164/jcbn.11-85. [http://dx.doi.org/10.3164/jcbn.11-85]. [PMID: 22798708]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Wan Y., Wang Q., Prud’homme G.J. GABAergic system in the endocrine pancreas: a new target for diabetes treatment. Diabetes Metab. Syndr. Obes. 2015;8:79–87. doi: 10.2147/DMSO.S50642. [PMID: 25678807]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Albaugh V.L., Vary T.C., Ilkayeva O., Wenner B.R., Maresca K.P., Joyal J.L., Breazeale S., Elich T.D., Lang C.H., Lynch C.J. Atypical antipsychotics rapidly and inappropriately switch peripheral fuel utilization to lipids, impairing metabolic flexibility in rodents. Schizophr. Bull. 2012;38(1):153–166. doi: 10.1093/schbul/sbq053. [http://dx.doi. org/10.1093/schbul/sbq053]. [PMID: 20494946]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Klingerman C.M., Stipanovic M.E., Bader M., Lynch C.J. Second-generation antipsychotics cause a rapid switch to fat oxidation that is required for survival in C57BL/6J mice. Schizophr. Bull. 2014;40(2):327–340. doi: 10.1093/schbul/sbs196. [http://dx.doi.org/10.1093/schbul/sbs196]. [PMID: 23328157]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Babkin P., George Thompson A.M., Iancu C.V., Walters D.E., Choe J.Y. Antipsychotics inhibit glucose transport: Determination of olanzapine binding site in Staphylococcus epidermidis glucose/ H(+) symporter. FEBS Open Bio. 2015;5:335–340. doi: 10.1016/j.fob.2015.04.006. [http://dx. doi.org/10.1016/j.fob.2015.04.006]. [PMID: 25941630]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Boyda H.N., Ramos-Miguel A., Procyshyn R.M., Töpfer E., Lant N., Choy H.H., Wong R., Li L., Pang C.C., Honer W.G., Barr A.M. Routine exercise ameliorates the metabolic side-effects of treatment with the atypical antipsychotic drug olanzapine in rats. Int. J. Neuropsychopharmacol. 2014;17(1):77–90. doi: 10.1017/S1461145713000795. [http://dx.doi. org/10.1017/S1461145713000795]. [PMID: 23953063]. [DOI] [PubMed] [Google Scholar]
- 137.Lazzari P., Serra V., Marcello S., Pira M., Mastinu A. Metabolic side effects induced by olanzapine treatment are neutralized by CB1 receptor antagonist compounds co-administration in female rats. Eur. Neuropsychopharmacol. 2017;27(7):667–678. doi: 10.1016/j.euroneuro.2017.03.010. [http:// dx.doi.org/10.1016/j.euroneuro.2017.03.010]. [PMID: 28377074]. [DOI] [PubMed] [Google Scholar]
- 138.Volkow N.D. Substance use disorders in schizophrenia--clinical implications of comorbidity. Schizophr. Bull. 2009;35(3):469–472. doi: 10.1093/schbul/sbp016. [http://dx.doi.org/10.1093/schbul/sbp016]. [PMID: 19325163]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Samaha A.N. Can antipsychotic treatment contribute to drug addiction in schizophrenia? Prog. Neuropsychopharmacol. Biol. Psychiatry. 2014;52:9–16. doi: 10.1016/j.pnpbp.2013.06.008. [http://dx.doi.org/10.1016/j.pnpbp.2013. 06.008]. [PMID: 23793001]. [DOI] [PubMed] [Google Scholar]
- 140.Virmani A., Binienda Z.K., Ali S.F., Gaetani F. Metabolic syndrome in drug abuse. Ann. N. Y. Acad. Sci. 2007;1122:50–68. doi: 10.1196/annals.1403.004. [http://dx.doi.org/10.1196/annals.1403.004]. [PMID: 18077564]. [DOI] [PubMed] [Google Scholar]
- 141.Ersche K.D., Stochl J., Woodward J.M., Fletcher P.C. The skinny on cocaine: insights into eating behavior and body weight in cocaine-dependent men. Appetite. 2013;71:75–80. doi: 10.1016/j.appet.2013.07.011. [http://dx. doi.org/10.1016/j.appet.2013.07.011]. [PMID: 23920064]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Tek C., Ratliff J., Reutenauer E., Ganguli R., O’Malley S.S.A. A randomized, double-blind, placebo-controlled pilot study of naltrexone to counteract antipsychotic-associated weight gain: proof of concept. J. Clin. Psychopharmacol. 2014;34(5):608–612. doi: 10.1097/JCP.0000000000000192. [http:// dx.doi.org/10.1097/JCP.0000000000000192]. [PMID: 25102328]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Theisen F.M., Haberhausen M., Firnges M.A., Gregory P., Reinders J.H., Remschmidt H., Hebebrand J., Antel J. No evidence for binding of clozapine, olanzapine and/or haloperidol to selected receptors involved in body weight regulation. Pharmacogenomics J. 2007;7(4):275–281. doi: 10.1038/sj.tpj.6500418. [http://dx.doi.org/10.1038/sj.tpj.6500418]. [PMID: 16983399]. [DOI] [PubMed] [Google Scholar]
- 144.Bahr S.M., Tyler B.C., Wooldridge N., Butcher B.D., Burns T.L., Teesch L.M., Oltman C.L., Azcarate-Peril M.A., Kirby J.R., Calarge C.A. Use of the second-generation antipsychotic, risperidone, and secondary weight gain are associated with an altered gut microbiota in children. Transl. Psychiatry. 2015;5:e652. doi: 10.1038/tp.2015.135. [http://dx.doi.org/10.1038/tp.2015.135]. [PMID: 26440540]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Deng C. Effects of antipsychotic medications on appetite, weight, and insulin resistance. Endocrinol. Metab. Clin. North Am. 2013;42(3):545–563. doi: 10.1016/j.ecl.2013.05.006. [http://dx.doi.org/10.1016/j.ecl.2013.05.006]. [PMID: 24011886]. [DOI] [PubMed] [Google Scholar]