Abstract
Introduction:
Brain volume deficits of grey matter (GM) and white matter (WM) are often found in patients with anorexia nervosa (AN). However, until recently, little was known about the influencing factors of these brain volume alterations, nor their exact quantification and rehabilitation.
Methods:
This review addresses these open questions and further explores what is now known about the underlying patho-biology and the clinical consequences including human studies as well as animal studies mimicking anorexia nervosa in ro-dents.
Results:
GM was reduced by 3.7% in adults and 7.6% in adolescents with AN. WM was reduced on average 2.2% in adult patients and 3.2% in adolescents. Most volume deficits in adults are reversible after long-term recovery; for adolescents, data are less clear. The main influencing factors for GM were absolute lowest weight at admission and illness duration. Cerebellar and WM reductions at admission predicted clinical outcome at one year follow-up. New studies found GABA receptor changes in GM and astrocyte loss in both GM and WM, as well as a possible role for oestrogen deficit. All three could part-ly explain clinical symptoms of anxiety, rigidity and learning impairments in patients with AN.
Conclusion:
Brain volume deficits in AN seem to play a causal role in the course and the prognosis of AN. A better under-standing of these brain changes could lead to more targeted therapies for patients with AN, including astrocyte-directed ap-proaches
Keywords: Anorexia nervosa, grey matter, white matter, volume reduction, prognosis, neuropsychologic deficits, astrocytes
1. INTRODUCTION
1.1. Anorexia Nervosa
Anorexia Nervosa (AN) is the third most common chronic illness in adolescence [1] with a lifetime prevalence of 0.5-1%, and it has the highest mortality of all psychiatric illnesses. Patients with AN show a significant weight loss or insufficient growth and development-adapted weight gain, feel overweight despite being underweight and suffer from weight phobia. There is a strong female-to-male ratio imbalance (10-20 females vs 1 male) [2]. The average illness duration is 6 years [3]; the incidence seems to be on the rise with a decreasing age of onset [4, 5]. The illness often runs a chronic course with severe suffering for patients and caregivers alike. Somatic consequences of semi-starvation lead to amenorrhea, a delay of pubertal development and growth as well as osteopenia and later osteoporosis [2]. In all of these processes, oestrogen deficiency seems to play a major role. Chronically, ill patients tend to show neuroprogressive changes, including neuropsychological deficits such as inflexibility, impaired learning, impaired memory, altered visuospatial processing skills and traits of autism [6, 7]. The role of brain changes in AN, potentially underlying these symptoms, however, remains poorly understood.
1.2. Brain Volume Loss
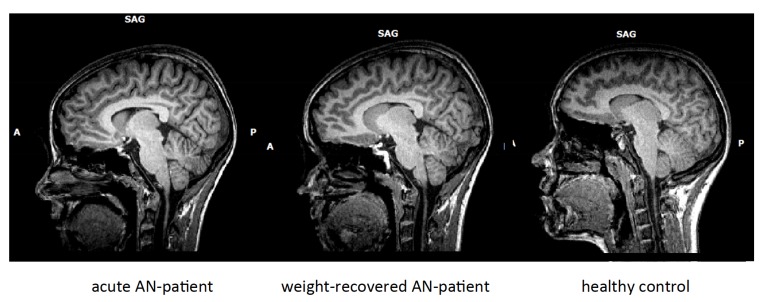
Semi-starvation in AN leads to a so called “pseudoatrophia cerebri”, a potentially reversible brain volume loss. Several earlier studies documented a significant loss of grey and potentially white matter volume during acute AN. These volume reductions are so significant that they can often be identified with the bare eye upon studying MRT-images of the brain: Ventricle volumes are increased and more cerebro-spinal fluid (CSF) fills the gaps between retracted gyri (see Fig. 1). Most studies, but not all, suggest reversibility upon weight rehabilitation. The exact amount of brain volume loss and its course upon refeeding, its underlying pathophysiology, and its importance for illness symptoms and prognosis are largely unclear. Moreover, it is not clear if it is a local or global phenomenon in the brain. This review gives an overview on the current knowledge of brain volume loss and about its course and reversibility, influencing factors, functional consequences and underlying pathophysiology including microstructural white matter changes, loss of astrocytes in the brain and the role of oestrogen. A better understanding of this brain volume loss in AN could spur new, more focused research on the underlying pathophysiology of AN to open new avenues for more targeted therapeutic interventions.
Fig. (1).
From left to right: T1-weighted sagittal MRI pictures of a female patient with acute anorexia nervosa (AN), the same patient short-term weight-recovered and a healthy control subject. Note that the external and internal cerebro-spinal fluid cavities are enlarged, most prominently in the acutely ill patient with AN.
2. METHODS
To this end, we performed a systematic literature review updating our meta-analysis from early 2016 [8] that quantifies brain volume loss in AN in human neuroimaging studies with the additional literature from October 2015 - October 2017. In a qualitative review we cover possible underlying mechanisms of brain volume changes as derived from animal studies that mimic starvation in rodents. For the systematic review we searched PubCentral and Medline databases for the keywords: “Anorexia nervosa” or “eating disorder” in conjunction with “grey/gray matter”, “white matter”, “volume” or “cortical thickness” starting 1997. All English original MRI studies with AN patients that contained brain volume information were selected and subdivided in studies of acute AN, shortly weight-recovered and long-term weight recovered (longer than 1.5 years). GM, WM, CSF and total brain volume were extracted and RevMan 5.3 (Cochrane Library, http://ims.cochrane.org/revman) was used as a statistical tool calculating random effects model. Additionally, we estimated the mean volume loss at each time point by weighting the average volume loss with the number of patients included is each study. Subgroup analysis separated adult from adolescent patients with AN.
For the qualitative review we focussed on articles pertaining to functional correlates, prognostic value and underlying pathomechanisms of brain volume changes in AN.
3. RESULTS
3.1. Systematic Review: Quantification and Reversibility of Brain Volume Loss
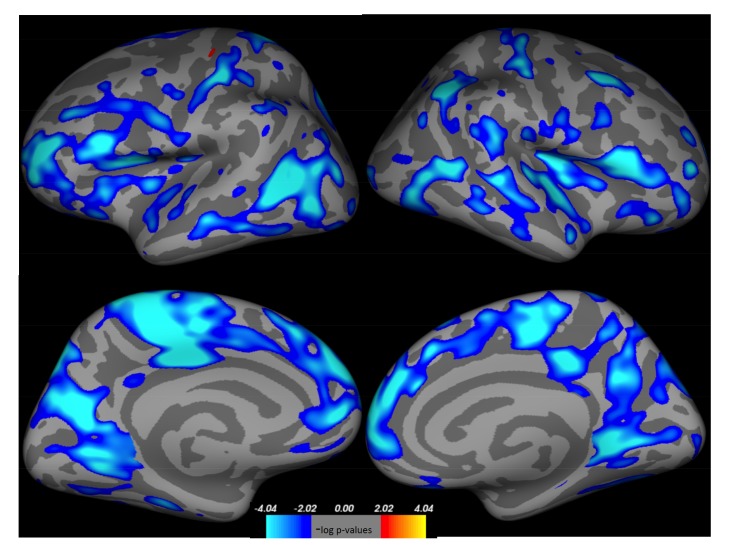
Our systematic review identified 34 eligible studies combining 532 acutely ill patients, 8 short-term recovery studies with 121 patients and 14 studies with 323 long-term recovered patients (Supplementary Fig. 1). Acutely ill patients showed a highly significant grey matter (GM) volume reduction of 3.7% in adults and of even 7.6% in adolescent patients (Table 1). White matter (WM) was also significantly reduced in adult and adolescent patients on average 2.2% and 3.2%, respectively. CSF was conversely increased by 15%. After short-term recovery on average 3.6% of GM remained reduced, while a 0.9% remaining reduction of WM volume did not reach significance any more. Long-term recovered patients analysis showed -0.5% GM and -1% WM which did not reach significance. These findings are well in line with the previous two reviews [9, 10] and two meta-analyses [8, 11] and extend our last metanalysis by another 160 patients, further adding to the validity of the findings. Both GM and WM volume loss seem to represent global phenomena, affecting virtually all regions in the brain [12, 13] (Fig. 2). Regional decreases were most pronounced in the anterior cingulate gyrus (ACC) [14, 15], the hippocampus [16, 17] and supplementary motor regions [18, 19], the potential significance of which will be discussed in the following section.
Table 1.
Average brain volume changes in acute, short-term weight recovered and long-recovered AN compared to healthy controls.
| Acute AN | Short-term Weight Recovered AN | Long-term (>1.5y) Recovered AN | |||||
|---|---|---|---|---|---|---|---|
| all | adults | adol. | all | adults | adol. | all | |
| N | 532 | 352 | 180 | 121 | 45 | 76 | 323 |
| Gray Matter | |||||||
| %Vol | -5.1%*** | -3.7%*** | -7.6%*** | -3.6%* | -3.5% | -3.7% | -0.5% |
| White Matter | |||||||
| %Vol | -2.6%*** | -2.2%* | -3.2%** | -0.9% | -1.4% | -0.6% | -1.0% |
| Cerebro-spinal Fluid# | |||||||
| %Vol | 15.0%*** | 12.8%*** | 22.8%* | 9.3%*** | 1.0% | ||
Adol.: adolescents; %Vol: % volume change compared to healthy controls; #: only SPM-VBM Data; *p<0.05, **p<0.01, ***p<0.001.
Fig. (2).
Whole brain surface based comparison of cortical grey matter volume between 50 healthy controls (HC) and 56 AN patients at admission. Red: AN > HC, blue: AN < HC. FDR corrected for multiple comparison. (The color version of the figure is available in the electronic copy of the article).
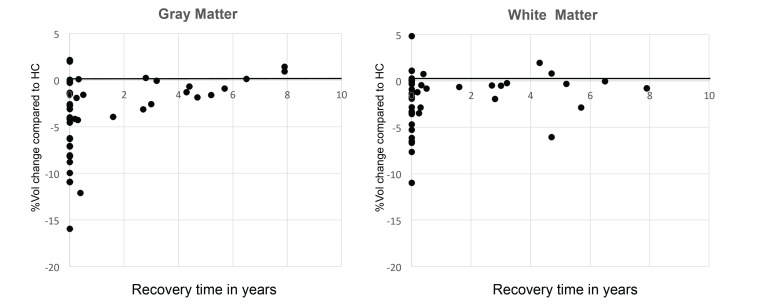
GM showed a protracted reversibility with approximately 50% of the reductions being reversed upon short-term weight rehabilitation while WM seemed to recover more quickly in those patients who reached a “healthy weight” (Fig. 3). One and a half to eight years after weight rehabilitation, adult patients still showed a mild reduction of brain volume, however, this difference was no longer significant. Thus, it is unclear whether small “scars” remain in some of the brains of recovered patients [8]. Unfortunately, there are not enough longitudinal studies of adolescent patients, so no clear conclusion can be drawn about the long-term consequences of brain volume loss in younger patients. Those 20-40% chronically ill patients who stay underweight, however, remain to have reduced brain volume for the duration of their illness, potentially inhibiting normal growth of these often still developing brains and potentially responsible for the neuroprogressive changes and neuropsychological deficits mentioned above that are seen in chronic patients with AN.
Fig. (3).
From left to right: time course of recovery for grey matter and white matter with time of recovery in years on the X-axis and volume changes in percent compared to healthy controls on the Y-axis.
4. QUALITATIVE REVIEW
4.1. Influencing Factors
A systematic analysis of potential factors influencing brain volume loss in 56 adolescent AN patients encompassed age of onset, illness duration, amount of weight loss, weight loss velocity and absolute lowest weight during the course of illness. The results showed that especially the absolute lowest weight in terms of body mass index (BMI) was relevant to explain GM volume reduction [13]. This finding was underscored by earlier studies linking the severity of starvation (i.e., BMI) with GM and WM changes [15, 18, 20]. Two more studies with more chronically ill adult patients also revealed an effect of illness duration on GM volume reductions [21, 22] that potentially was not as relevant in the rather short-term ill adolescents. As GM loss was largely reversible upon weight restoration, GM volume reductions could thus be seen as a state marker for the degree of semi-starvation.
Theories linking brain volume loss in AN to dehydration could not be validated to date. Several authors analysed potential dehydration parameters in blood serum and urine but could not find any association with brain volume changes, so this alternative cause does not seem likely [12, 23].
The fact that GM and WM reductions were much more pronounced in adolescents than adults could be attributed to at least two different causes. For one, this could be an acute versus chronic effect differing in rather short-term ill adolescents versus generally longer term ill adult patients due to a different acute versus chronic pathophysiologic mechanism. However, in patients turning from acute to chronic, larger brain volume deficits would then have to consolidate over time into the smaller brain volume differences shown above in (chronically ill) adults. Alternatively, and potentially more probable, the still developing brain could prove more susceptible to volume reduction due to semi-starvation than the adult brain. In the adolescent brain, there are several normal developmental processes going on, such as increased synapse formation followed by pruning of unused neurons and synapses. This normally causes GM volume to peak at region-specific ages between childhood and adolescence [24] and then to slowly and continuously decline in volume. WM volume, on the contrary, continues to increase almost linearly with age until the beginning of the third decade of life, reflecting ongoing myelination, especially of long-distance fibres [25]. Both of these dynamic processes could be affected by AN in adolescents, especially if the illness turns chronic. In fact, newly developed WM fibres and still developing fibres have proven to be more susceptible to insults and exterior influences than already existing fibres [25]. This also fits to findings from both human and animal neuroimaging studies that demonstrated particularly strong adverse effects of cannabis or alcohol use during adolescence (relative to adulthood), showing high vulnerability of the brain during development [26, 27]. Also internalizing and externalizing symptoms in childhood have been shown to be followed by brain volume reduction compared to healthy controls [28] (but symptoms did not “follow” brain volume), hinting again at a susceptibility of the developing brain to stressors.
There are indications that these brain volume changes might be mediated by hormonal changes in AN. Increased cortisol levels and/or decreased thyroid hormone have been associated with GM volume loss [29-31]. In addition, brain-derived neurotrophic growth factor and leptin are lacking in AN [32] and could affect brain volume changes. Nogal et al [31] also found associations between the lack of gonadal hormones and increased sulcal width, an indirect measure of brain volume reduction. Interestingly, Mainz et al. also found an increase in follicle stimulation hormone (FSH) to be associated with GM volume increase upon weight recovery [17], further hinting to a role of gonadal hormones for GM volume changes in AN. This was underscored by the study of Chui et al. [30] that found persisting amenorrhea to be associated with decreased cognitive functioning in otherwise similarly recovered patients with AN.
4.2. Functional Consequences of Brain Volume Changes
The marked changes in global and regional brain volume loss in AN are in sharp contrast to relatively mild neuropsychological impairments typically observed in this patient group across many cognitive domains, potentially pointing to functional compensatory brain processes that need further investigations. However, there is preliminary evidence for global or regional GM loss in acute AN that is associated with neuropsychological performance and psychopathological traits. Global GM reduction was associated with reduced visuospatial functioning [29] while smaller dorsal ACC was associated with reduced perceptual organization and reasoning skills [14]. Reduced inferior parietal cortex GM correlated with increased drive for thinness [33], dorsolateral prefrontal cortex GM correlated with dietary restraint and BMI [16] and medial orbitofrontal cortex correlated with sucrose pleasantness ratings [34]. Finally, GM of the extrastriatal body area was reduced and showed decreased functional connectivity with related brain areas during a body-image distortion task using functional MRI [35]. After psychotherapy focusing on improving body-image distortion, the connectivity was no longer reduced [36].
4.3. Clinical Prognosis
McCormick et al. [14] were the first to show an influence of brain volume normalization on one-year outcome in AN. They found GM volume reductions in the ACC in acutely ill patients with AN to normalize after weight rehabilitation at the end of the treatment in most patients. However, those who did not normalize had significantly more relapses at one year follow-up. In our own study, we found cerebellar and WM volume loss at admission to significantly predict the outcome of adolescent patients with AN at one year follow-up [13]. This finding was independent of BMI at admission and accounted for an additional 20% of explained variance of BMI at follow-up. Cerebellar and WM volume loss was less dependent on the state of starvation than GM volume, pointing to potentially different underlying mechanisms causing these volume reductions and cerebellar and WM volume changes to be more structurally ingrained and thus more imposing as a trait marker. The prognostic value of reduced cerebellar volumes for worse clinical outcome fits well with previous findings of smaller cerebellar volumes in chronically ill patients with AN [18, 21] and its correlation with illness duration [22], as patients with smaller cerebellar volumes would suffer a greater risk of turning chronic and be overrepresented in the group of chronically ill patients over time. Functionally, the cerebellum plays a role in habit formation and ritualistic behaviour [37] which fits well with obsessive-compulsive traits and increased perseverance often noted in patients with AN [38]. The pathophysiology of WM changes is expanded below.
4.4. Potential Pathomechanisms
Two differing pathomechanisms could explain the predictive value of small brain volumes at admission for prognosis. On one hand, small volumes at the beginning of the illness could be indicators of a predisposition for a chronic course of AN. Then, over time, more and more patients with small cerebellums and WM would “aggregate” in the relapsing and chronic AN group. On the other hand, the cerebellum and WM could be tissues that are more susceptible to semi-starvation in some patients, especially in adolescence. Then, patients with more strongly reduced volumes would be more affected and take a more dire course than those without. This “scarring” hypothesis would fit well with developmental findings showing that the cerebellum and WM take longer than other brain tissues to mature and that they are influenced by learning and environmental factors [39-41]. As has been explained above, impairing WM maturation during adolescence and early adulthood has been shown to affect mostly newly forming long-distance connections in the brain at this age [25]. Altered development of these WM fibres (“scars”) could be associated with increased body-image distortion [42] and life-long risks of increased anxiety and depression, even in weight restored patients with AN [2]. However, longitudinal studies are lacking in humans, so this conclusion currently remains putative. Animal studies in mice, however, seem to point towards smaller WM-volumes and WM lipid content following malnutrition early in life (following birth or after 30 days), persisting even after (60 days) long-term follow-up with normal feeding [43].
4.5. WM Microstructural Changes
A shown above, cortical and cerebellar WM predicted the clinical outcome independent of weight at admission. These findings are underscored by diffusion-weighted MRI studies. These studies found evidence of decreased integrity of WM fibres mainly in frontal and parietal tracks in adults with AN [44]. One study found reduced WM integrity to be associated with lowest life-time BMI, a measure of clinical severity [45], while other studies showed associations with current BMI [44-46]. Permanent scarring of white matter could be responsible for reduced WM integrity found in recovered adult patients with AN [47], although opposite changes and more structured WM were found in another study [45]. Findings in adolescents, who are less often chronically ill, are more divergent. Frank et al. [48] and Travis et al. [49] found evidence for decreased integrity but also increased structure of WM (measured by fractional anisotropy, FA). Our group found mainly increased FA in frontal and parietal fibre tracts in acute adolescent AN that partly declined after weight recovery [23]. Increased FA could stem from more structured or larger fibres or a tighter packing of fibres. It could be interpreted as pre-existing changes in brain architecture of patients predisposed for AN or, more likely, as loss of surrounding cells and tissue around the WM fibres during semi-starvation (see below). The latter hypothesis would also nicely explain the WM volume reductions found in AN patients that are even more pronounced in adolescents with AN.
4.6. Cellular Changes
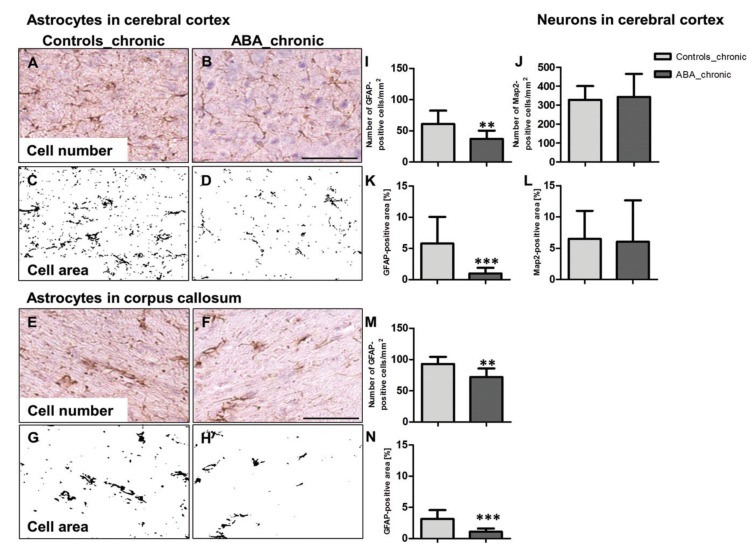
Until recently, cellular changes in AN have rarely been investigated, which might be due, in part, to the lack of post-mortem analyses. Only two papers reported on a total of three human post-mortem analyses showing signs of cellular degeneration, altered dendritic ramification patterns and an altered dendritic spine morphology of neurons in the GM [50, 51]. More recently, several groups have used animal models of AN to start to unravel these cellular processes and thus gain more insight into the underlying pathophysiology of AN brain volume reduction. The most commonly used animal model for AN is the activity based anorexia (ABA) model in rodents [52, 53]. Here, reduced food intake is coupled with the availability of a running wheel. Counterintuitively, susceptible mice or rats start to run more instead of less upon semi-starvation, furthering their weight loss. This is typically interpreted as food seeking behaviour [54], potentially an evolutionarily preserved mechanism aimed at increasing survival. Symptoms of ABA animals that are similar to AN include weight loss, hyperactivity [52], hypothermia [55], hypoleptinemia [56] and amenorrhea [57]. Aoki et al. [58] showed increased GABA receptors in spines of hippocampal neurons, potentially linked to increased anxiety and tonic inhibition, exacerbating hyperactivity and weight loss. Recent findings including our own showed that also glia could be affected by semi-starvation. Barbarich-Marsteller [59] found reduced glia cell proliferation in the dentate gyrus, the surrounding dorsal hippocampus and corpus callosum but not in regions with known neurogenesis such as the subgranular zone of the dentate gyrus. Reyes-Haro et al. [60, 61] found a slightly reduced number of astrocytes in the corpus callosum and hippocampus in a slightly different (dehydration) model for AN. Our own group could expand these findings showing widespread astrocyte loss and reduced astrocyte size in the GM and WM of adolescent ABA rats that were starved for at least three weeks [53, 62] (Fig. 4). This was underscored by a more than halved mRNA production of glial fibrillary acidic protein (GFAP), typically found in astrocytes, a finding also confirmed by Reyes-Haro et al. [60]. Our group also confirmed brain volume reductions of the same extent (6-9%) in the rat model as shown in the human patients above in the very same animals, effectively linking astrocyte reduction with brain volume loss. Interestingly, acute starvation for only 1 week was not sufficient to incur astrocyte loss [62], emphasizing again the role of chronic starvation and illness duration. Neuron number and size were not altered during these trials and neither were oligodendrocytes, showing the specificity of astrocyte loss in the brain of ABA animals.
Fig. (4).
Cell number and cell area of glial fibrillary acidic protein (GFAP)-stained astrocytes in the (A-D) cerebral cortex and the (E-H) corpus callosum of activity-based anorexia (ABA) and control rats. Astrocyte cell number and cell areas were significantly reduced in both brain regions (I, K, M, N). The neuron cell number (J) and neuron cell area (L) were quantified with Map2, which showed no significant alteration in ABA rats compared to controls. **: P<0.01, ***: P<0.001, two-sided Student’s t-test. (From Frintrop et al., 2017, with permission). (The color version of the figure is available in the electronic copy of the article).
4.7. Potential Role of Astrocytes During Semi-starvation
Reduced astrocyte count has also been found in fronto-limbic areas of the brain in patients with depression, which is very often comorbid in AN patients [63, 64]. Its causal role was underscored by animal studies showing depressive symptoms after the destruction of frontocortical astrocytes [65]. In major depressive disorder, a reduction of GFAP has also been shown and linked to impaired vesicle transport in astrocytes leading to memory deficits, which has also been described in AN [66, 67]. Furthermore, other psychiatric illnesses, such as anxiety disorder and symptoms following chronic stress have been linked to astrocyte alterations and reduced GFAP [67]. Astrocytes might thus play a much more important role in psychiatric psychopathology than previously envisioned [68]. Astrocyte functions include supporting structure (brain matrix surrounding neurons, blood-brain barrier), metabolism (neurons have very low energy depots themselves), endocrine functions (neurotransmitter reuptake, “gliotransmitters”) have direct and indirect influences on synapse formation and learning [69-73]. Thus, fewer astrocytes could lead to a “leaky” blood brain barrier, impaired neuron metabolism and aberrant neuronal and synaptic functions leading to deficits in learning and memory [70]. This would potentially help explain the findings of mildly reduced cognitive abilities in patients with chronic AN mentioned above [30, 74, 75]. Our own studies also showed reduced learning and memory in chronically starved ABA animals [57]. Astrocyte loss could thus also be partly responsible for the slow therapeutic progress made in the psychotherapy of severely affected patients with AN (and those with depression), especially during the acute stage of AN when starvation effects are particularly pronounced. Identifying reduced and altered astrocytes as an important feature of AN pathophysiology opens up important new lines of research for the future. Astrocytes could become a new research target for the study of AN.
4.8. The Effects of Oestrogen
The lack of oestrogen seems to play a major role in the somatic symptoms of AN such as amenorrhea, growth retardation, halted sexual maturation and osteopenia. It is a trophic hormone, and oestrogen receptors are found in a wide array of tissues far beyond primary sexual functionality. It is not surprising that the rise of oestrogen during puberty has been linked to progressive brain volume changes especially in parahippocampal areas in typically developing girls [76]. As mentioned above, several studies could link gonadal hormones to brain volume changes in AN [17, 31], underscoring their potential importance for this disease. Furthermore, the rise of oestrogen levels was associated with recovering learning and memory function [66] and failure to reach menstruation resulted in impaired cognitive functioning [30]. The effect of oestrogen on certain forms of learning and memory during normal oestrous cycle, menopause and following ovariectomy has been well researched in animals and humans [77, 78]. In our ABA experiments, we could also demonstrate oestrogen deficiency and amenorrhea [57], a finding supported by previous studies [79]. We also found impaired learning and memory in the novel object recognition task [57] and could show that oestrogen reduction upon semi-starvation was positively associated with the degree of this learning and memory impairment. To counter this deficiency in oestrogen, replacement therapy in patients with AN would be the logical next step to try. Cognitive improvements have been shown following oestrogen replacement in normal weight patients after menopause [77]. To counter osteopenia and osteoporosis in AN, oestrogen replacement therapy has previously been attempted orally many times unsuccessfully but were recently successfully used transdermally (Misra 2011, 2016). Misra et al. could show a significant increase in bone density in both adults and adolescents with AN upon using oestrogen patches; however, more research is needed before oestrogen can be clinically recommended to be administered to patients on a larger scale.
CONCLUSION
Brain volume reductions of GM and WM in AN seem to be significant and global. GM changes can be seen as a state marker mostly influenced by semi-starvation and illness duration, while cerebellar and WM changes seem to be more of a trait-marker important for clinical prognosis. Astrocyte loss seems to be a relevant factor for volume reduction and potentially for functional impairments associated with brain volume decrease; they could prove to be a new promising research target for future studies. Oestrogen replacement therapy, already proven to be effective against osteopenia, could also be found to be an effective treatment against brain volume reduction in the future. Further, understanding the underlying causes and effects of brain volume reductions in AN seems to be an important future direction in AN research to gain even more insights into AN pathophysiology and to more successfully target long-term consequences on the brain level in AN patients. This might be of high clinical relevance in order to improve long-term outcome and the development of additional psychopathology in the course of the disorder.
CONSENT FOR PUBLICATION
Not applicable.
ACKNOWLEDGEMENTS
Declared none.
SUPPLEMENTARY MATERIAL
Supplementary material is available on the publisher’s web site along with the published article.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
REFERENCES
- 1.Gonzalez A., Kohn M.R., Clarke S.D. Eating disorders in adolescents. Aust. Fam. Physician. 2007;36(8):614–619. [PMID: 17676184]. [PubMed] [Google Scholar]
- 2.Herpertz-Dahlmann B. Adolescent eating disorders: Update on definitions, symptomatology, epidemiology, and comorbidity. Child Adolesc. Psychiatr. Clin. N. Am. 2015;24(1):177–196. doi: 10.1016/j.chc.2014.08.003. [http://dx.doi.org/10.1016/j.chc.2014.08.003]. [PMID: 25455581]. [DOI] [PubMed] [Google Scholar]
- 3.Darby A., Hay P., Mond J., Quirk F., Buttner P., Kennedy L. The rising prevalence of comorbid obesity and eating disorder behaviors from 1995 to 2005. Int. J. Eat. Disord. 2009;42(2):104–108. doi: 10.1002/eat.20601. [http://dx.doi.org/10.1002/eat.20601]. [PMID: 18949767]. [DOI] [PubMed] [Google Scholar]
- 4.Smink F.R., van Hoeken D., Hoek H.W. Epidemiology of eating disorders: incidence, prevalence and mortality rates. Curr. Psychiatry Rep. 2012;14(4):406–414. doi: 10.1007/s11920-012-0282-y. [http://dx.doi.org/10.1007/s11920-012-0282-y]. [PMID: 22644309]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Steinhausen H-C., Jensen C.M. Time trends in lifetime incidence rates of first-time diagnosed anorexia nervosa and bulimia nervosa across 16 years in a Danish nationwide psychiatric registry study. Int. J. Eat. Disord. 2015;48(7):845–850. doi: 10.1002/eat.22402. [http://dx.doi.org/10. 1002/eat.22402]. [PMID: 25809026]. [DOI] [PubMed] [Google Scholar]
- 6.Treasure J., Stein D., Maguire S. Has the time come for a staging model to map the course of eating disorders from high risk to severe enduring illness? An examination of the evidence. Early Interv. Psychiatry. 2015;9(3):173–184. doi: 10.1111/eip.12170. [http://dx.doi.org/10. 1111/eip.12170]. [PMID: 25263388]. [DOI] [PubMed] [Google Scholar]
- 7.Wentz E., Gillberg I.C., Anckarsäter H., Gillberg C., Råstam M. 2009.
- 8.Seitz J., Herpertz-Dahlmann B., Konrad K. Brain morphological changes in adolescent and adult patients with anorexia nervosa. J. Neural Transm. (Vienna) 2016;123(8):949–959. doi: 10.1007/s00702-016-1567-9. [http://dx.doi. org/10.1007/s00702-016-1567-9]. [PMID: 27188331]. [DOI] [PubMed] [Google Scholar]
- 9.Titova O.E., Hjorth O.C., Schiöth H.B., Brooks S.J. Anorexia nervosa is linked to reduced brain structure in reward and somatosensory regions: a meta-analysis of VBM studies. BMC Psychiatry. 2013;13:110. doi: 10.1186/1471-244X-13-110. [http://dx.doi.org/10.1186/1471-244X-13-110]. [PMID: 23570420]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Van den Eynde F., Suda M., Broadbent H., Guillaume S., Van den Eynde M., Steiger H., Israel M., Berlim M., Giampietro V., Simmons A., Treasure J., Campbell I., Schmidt U. Structural magnetic resonance imaging in eating disorders: A systematic review of voxel-based morphometry studies. Eur. Eat. Disord. Rev. 2012;20(2):94–105. doi: 10.1002/erv.1163. [http://dx.doi.org/10.1002/erv.1163]. [PMID: 22052722]. [DOI] [PubMed] [Google Scholar]
- 11.Seitz J., Bühren K., von Polier G.G., Heussen N., Herpertz-Dahlmann B., Konrad K. 2014. [DOI] [PubMed]
- 12.King J.A., Geisler D., Ritschel F., Boehm I., Seidel M., Roschinski B., Soltwedel L., Zwipp J., Pfuhl G., Marxen M., Roessner V., Ehrlich S. Global cortical thinning in acute anorexia nervosa normalizes following long-term weight restoration. Biol. Psychiatry. 2015;77(7):624–632. doi: 10.1016/j.biopsych.2014.09.005. [http://dx.doi.org/10.1016/ j.biopsych.2014.09.005]. [PMID: 25433902]. [DOI] [PubMed] [Google Scholar]
- 13.Seitz J., Walter M., Mainz V., Herpertz-Dahlmann B., Konrad K., von Polier G. Brain volume reduction predicts weight development in adolescent patients with anorexia nervosa. J. Psychiatr. Res. 2015;68:228–237. doi: 10.1016/j.jpsychires.2015.06.019. [http://dx.doi.org/10.1016/j.jpsychires. 2015.06.019]. [PMID: 26228424]. [DOI] [PubMed] [Google Scholar]
- 14.McCormick L.M., Keel P.K., Brumm M.C., Bowers W., Swayze V., Andersen A., Andreasen N. Implications of starvation-induced change in right dorsal anterior cingulate volume in anorexia nervosa. Int. J. Eat. Disord. 2008;41(7):602–610. doi: 10.1002/eat.20549. [http://dx.doi.org/10.1002/eat.20549]. [PMID: 18473337]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mühlau M., Gaser C., Ilg R., Conrad B., Leibl C., Cebulla M.H., Backmund H., Gerlinghoff M., Lommer P., Schnebel A., Wohlschläger A.M., Zimmer C., Nunnemann S. Gray matter decrease of the anterior cingulate cortex in anorexia nervosa. Am. J. Psychiatry. 2007;164(12):1850–1857. doi: 10.1176/appi.ajp.2007.06111861. [http://dx.doi.org/10.1176/ appi.ajp.2007.06111861]. [PMID: 18056240]. [DOI] [PubMed] [Google Scholar]
- 16.Brooks S.J., Barker G.J., O’Daly O.G., Brammer M., Williams S.C., Benedict C., Schiöth H.B., Treasure J., Campbell I.C. Restraint of appetite and reduced regional brain volumes in anorexia nervosa: A voxel-based morphometric study. BMC Psychiatry. 2011;11:179. doi: 10.1186/1471-244X-11-179. [http://dx.doi.org/10.1186/1471-244X-11-179]. [PMID: 22093442]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mainz V., Schulte-Rüther M., Fink G.R., Herpertz-Dahlmann B., Konrad K. Structural brain abnormalities in adolescent anorexia nervosa before and after weight recovery and associated hormonal changes. 2012. [DOI] [PubMed]
- 18.Amianto F., Caroppo P., D’Agata F., Spalatro A., Lavagnino L., Caglio M., Righi D., Bergui M., Abbate-Daga G., Rigardetto R., Mortara P., Fassino S. Brain volumetric abnormalities in patients with anorexia and bulimia nervosa: A voxel-based morphometry study. 2013. [DOI] [PubMed]
- 19.Bär K-J., de la Cruz F., Berger S., Schultz C.C., Wagner G. Structural and functional differences in the cingulate cortex relate to disease severity in anorexia nervosa. J. Psychiatry Neurosci. 2015;40(4):269–279. doi: 10.1503/jpn.140193. [http://dx.doi.org/10.1503/jpn.140193]. [PMID: 25825813]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Katzman D.K., Lambe E.K., Mikulis D.J., Ridgley J.N., Goldbloom D.S., Zipursky R.B. Cerebral gray matter and white matter volume deficits in adolescent girls with anorexia nervosa. J. Pediatr. 1996;129(6):794–803. doi: 10.1016/s0022-3476(96)70021-5. [http://dx.doi.org/10.1016/S0022-3476(96)70021-5]. [PMID: 8969719]. [DOI] [PubMed] [Google Scholar]
- 21.Boghi A., Sterpone S., Sales S., D’Agata F., Bradac G.B., Zullo G., Munno D. In vivo evidence of global and focal brain alterations in anorexia nervosa. Psychiatry Res. 2011;192(3):154–159. doi: 10.1016/j.pscychresns.2010.12.008. [http://dx.doi.org/10.1016/j.pscychresns.2010.12.008]. [PMID: 21546219]. [DOI] [PubMed] [Google Scholar]
- 22.Fonville L., Giampietro V., Williams S.C.R., Simmons A., Tchanturia K. Alterations in brain structure in adults with anorexia nervosa and the impact of illness duration. Psychol. Med. 2014;44(9):1965–1975. doi: 10.1017/S0033291713002389. [http://dx.doi.org/10.1017/S0033291713002389]. [PMID: 24074139]. [DOI] [PubMed] [Google Scholar]
- 23.Vogel K., Timmers I., Kumar V., Nickl-Jockschat T., Bastiani M., Roebroek A., Herpertz-Dahlmann B., Konrad K., Goebel R., Seitz J. White matter microstructural changes in adolescent anorexia nervosa including an exploratory longitudinal study. Neuroimage Clin. 2016;11:614–621. doi: 10.1016/j.nicl.2016.04.002. [http://dx.doi.org/10.1016/j.nicl. 2016.04.002]. [PMID: 27182488]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shaw P., Kabani N.J., Lerch J.P., Eckstrand K., Lenroot R., Gogtay N., Greenstein D., Clasen L., Evans A., Rapoport J.L., Giedd J.N., Wise S.P. Neurodevelopmental trajectories of the human cerebral cortex. J. Neurosci. 2008;28(14):3586–3594. doi: 10.1523/JNEUROSCI.5309-07.2008. [http:// dx.doi.org/10.1523/JNEUROSCI.5309-07.2008]. [PMID: 18385317]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gibson K.R., Petersen A.C. 1991.
- 26.Camchong J., Lim K.O., Kumra S. Adverse effects of cannabis on adolescent brain development: A longitudinal study. Cereb. Cortex. 2017;27:1922–1930. doi: 10.1093/cercor/bhw015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Meruelo A.D., Castro N., Cota C.I., Tapert S.F. Cannabis and alcohol use, and the developing brain. 2017. [DOI] [PMC free article] [PubMed]
- 28.Muetzel R.L., Blanken L.M.E., van der Ende J., El Marroun H., Shaw P., Sudre G., van der Lugt A., Jaddoe V.W.V., Verhulst F.C., Tiemeier H., White T. Tracking brain development and dimensional psychiatric symptoms in children: A longitudinal population-based neuroimaging study. 2018. [DOI] [PubMed]
- 29.Castro-Fornieles J., Bargalló N., Lázaro L., Andrés S., Falcon C., Plana M.T., Junqué C. A cross-sectional and follow-up voxel-based morphometric MRI study in adolescent anorexia nervosa. J. Psychiatr. Res. 2009;43(3):331–340. doi: 10.1016/j.jpsychires.2008.03.013. [http://dx.doi.org/10.1016/ j.jpsychires.2008.03.013]. [PMID: 18486147]. [DOI] [PubMed] [Google Scholar]
- 30.Chui H.T., Christensen B.K., Zipursky R.B., Richards B.A., Hanratty M.K., Kabani N.J., Mikulis D.J., Katzman D.K. Cognitive function and brain structure in females with a history of adolescent-onset anorexia nervosa. Pediatrics. 2008;122(2):e426–e437. doi: 10.1542/peds.2008-0170. [http://dx.doi.org/10.1542/peds.2008-0170]. [PMID: 18676530]. [DOI] [PubMed] [Google Scholar]
- 31.Nogal P., Pniewska-Siark B., Lewinski A. Relation of trophic changes in the central nervous system, measured by the width of cordical sulci, to the clinical course of anorexia nervosa (II). Neuroendocrinol. Lett. 2008;29(6):879–883. [PMID: 19112395]. [PubMed] [Google Scholar]
- 32.Holtkamp K., Herpertz-Dahlmann B., Hebebrand K., Mika C., Kratzsch J., Hebebrand J. Physical activity and restlessness correlate with leptin levels in patients with adolescent anorexia nervosa. Biol. Psychiatry. 2006;60(3):311–313. doi: 10.1016/j.biopsych.2005.11.001. [http://dx.doi.org/10.1016/j. biopsych.2005.11.001]. [PMID: 16376860]. [DOI] [PubMed] [Google Scholar]
- 33.Joos A., Klöppel S., Hartmann A., Glauche V., Tüscher O., Perlov E., Saum B., Freyer T., Zeeck A., Tebartz van Elst L. Voxel-based morphometry in eating disorders: correlation of psychopathology with grey matter volume. Psychiatry Res. 2010;182(2):146–151. doi: 10.1016/j.pscychresns.2010.02.004. [http://dx.doi.org/10.1016/j.pscychresns.2010.02. 004]. [PMID: 20400273]. [DOI] [PubMed] [Google Scholar]
- 34.Frank G.K. Alterations in brain structures related to taste reward circuitry in Ill and recovered anorexia nervosa and in bu-limia nervosa. Am. J. Psychiatry. 2013;170:1152–1160. doi: 10.1176/appi.ajp.2013.12101294. [http://dx. doi.org/10.1176/appi.ajp.2013.12101294]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vocks S., Busch M., Grönemeyer D., Schulte D., Herpertz S., Suchan B. Neural correlates of viewing photographs of one’s own body and another woman’s body in anorexia and bulimia nervosa: an fMRI study. J. Psychiatry Neurosci. 2010;35(3):163–176. doi: 10.1503/jpn.090048. [http://dx.doi.org/10.1503/jpn.090048]. [PMID: 20420767]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vocks S., Busch M., Schulte D., Grönermeyer D., Herpertz S., Suchan B. Effects of body image therapy on the activation of the extrastriate body area in anorexia nervosa: an fMRI study. Psychiatry Res. 2010;183:114–118. doi: 10.1016/j.pscychresns.2010.05.011. [http://dx.doi.org/10.1016/j.pscychresns. 2010.05.011]. [DOI] [PubMed] [Google Scholar]
- 37.Schmahmann J.D., Weilburg J.B., Sherman J.C. The neuropsychiatry of the cerebellum - insights from the clinic. Cerebellum. 2007;6(3):254–267. doi: 10.1080/14734220701490995. [http://dx.doi.org/10.1080/14734220701490995]. [PMID: 17786822]. [DOI] [PubMed] [Google Scholar]
- 38.Friederich H-C., Herzog W. Cognitive-behavioral flexibility in anorexia nervosa. Curr. Top. Behav. Neurosci. 2011;6:111–123. doi: 10.1007/7854_2010_83. [http://dx.doi.org/10.1007/7854_2010_83]. [PMID: 21243473]. [DOI] [PubMed] [Google Scholar]
- 39.Draganski B., May A. Training-induced structural changes in the adult human brain. Behav. Brain Res. 2008;192(1):137–142. doi: 10.1016/j.bbr.2008.02.015. [http://dx.doi.org/10.1016/j.bbr.2008.02.015]. [PMID: 18378330]. [DOI] [PubMed] [Google Scholar]
- 40.Koziol L.F., Budding D., Andreasen N., D’Arrigo S., Bulgheroni S., Imamizu H., Ito M., Manto M., Marvel C., Parker K., Pezzulo G., Ramnani N., Riva D., Schmahmann J., Vandervert L., Yamazaki T. Consensus paper: the cerebellum’s role in movement and cognition. Cerebellum. 2014;13(1):151–177. doi: 10.1007/s12311-013-0511-x. [http://dx.doi.org/10.1007/s12311-013-0511-x]. [PMID: 23996631]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tang Y-Y., Lu Q., Fan M., Yang Y., Posner M.I. Mechanisms of white matter changes induced by meditation. Proc. Natl. Acad. Sci. USA. 2012;109(26):10570–10574. doi: 10.1073/pnas.1207817109. [http://dx.doi.org/10.1073/ pnas.1207817109]. [PMID: 22689998]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vocks S., Schulte D., Busch M., Grönemeyer D., Herpertz S., Suchan B. Changes in neuronal correlates of body image processing by means of cognitive-behavioural body image therapy for eating disorders: a randomized controlled fMRI study. Psychol. Med. 2011;41(8):1651–1663. doi: 10.1017/S0033291710002382. [http://dx.doi.org/10.1017/S0033291710002382]. [PMID: 21205361]. [DOI] [PubMed] [Google Scholar]
- 43.Yusuf H.K., Haque Z., Mozaffar Z. Effect of malnutrition and subsequent rehabilitation on the development of mouse brain myelin. J. Neurochem. 1981;36(3):924–930. doi: 10.1111/j.1471-4159.1981.tb01683.x. [http://dx.doi.org/ 10.1111/j.1471-4159.1981.tb01683.x]. [PMID: 7205282]. [DOI] [PubMed] [Google Scholar]
- 44.Kazlouski D., Rollin M.D.H., Tregellas J., Shott M.E., Jappe L.M., Hagman J.O., Pryor T., Yang T.T., Frank G.K.W. Altered fimbria-fornix white matter integrity in anorexia nervosa predicts harm avoidance. Psychiatry Res. 2011;192(2):109–116. doi: 10.1016/j.pscychresns.2010.12.006. [http://dx. doi.org/10.1016/j.pscychresns.2010.12.006]. [PMID: 21498054]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yau W-Y.W., Bischoff-Grethe A., Theilmann R.J., Torres L., Wagner A., Kaye W.H., Fennema-Notestine C. Alterations in white matter microstructure in women recovered from anorexia nervosa. Int. J. Eat. Disord. 2013;46:701–708. doi: 10.1002/eat.22154. [http://dx.doi.org/ 10.1002/eat.22154]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nagahara Y., Nakamae T., Nishizawa S., Mizuhara Y., Moritoki Y., Wada Y., Sakai Y., Yamashita T., Narumoto J., Miyata J., Yamada K., Fukui K. A tract-based spatial statistics study in anorexia nervosa: abnormality in the fornix and the cerebellum. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2014;51:72–77. doi: 10.1016/j.pnpbp.2014.01.009. [http://dx.doi.org/10.1016/j.pnpbp.2014.01.009]. [PMID: 24462618]. [DOI] [PubMed] [Google Scholar]
- 47.Shott M.E., Pryor T.L., Yang T.T., Frank G.K.W. Greater insula white matter fiber connectivity in women recovered from anorexia nervosa. Off. Publ. Am. Coll. Neuropsychopharmacology. 2016;41(2):498–507. doi: 10.1038/npp.2015.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Frank G.K., Shott M.E., Hagman J.O., Yang T.T. Localized brain volume and white matter integrity alterations in adolescent anorexia nervosa. 2013. [DOI] [PMC free article] [PubMed]
- 49.Travis K.E., Golden N.H., Feldman H.M., Solomon M., Nguyen J., Mezer A., Yeatman J.D., Dougherty R.F. Abnormal white matter properties in adolescent girls with anorexia nervosa. Neuroimage Clin. 2015;9:648–659. doi: 10.1016/j.nicl.2015.10.008. [http://dx.doi.org/10.1016/j.nicl. 2015.10.008]. [PMID: 26740918]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Martin F. Pathology of neurological & psychiatric aspects of various deficiency manifestations with digestive & neuro-endocrine disorders: study of the changes of the central nervous system in 2 cases of anorexia in young girls (so-called mental anorexia). Acta Neurol. Psychiatr. Belg. 1958;58(9):816–830. [PMID: 13605672]. [PubMed] [Google Scholar]
- 51.Neumärker K.J., Dudeck U., Meyer U., Neumärker U., Schulz E., Schönheit B. Anorexia nervosa and sudden death in childhood: clinical data and results obtained from quantitative neurohistological investigations of cortical neurons. Eur. Arch. Psychiatry Clin. Neurosci. 1997;247(1):16–22. doi: 10.1007/BF02916248. [http://dx.doi.org/10.1007/ BF02916248]. [PMID: 9088801]. [DOI] [PubMed] [Google Scholar]
- 52.Routtenberg A., Kuznesof A.W. Self-starvation of rats living in activity wheels on a restricted feeding schedule. J. Comp. Physiol. Psychol. 1967;64(3):414–421. doi: 10.1037/h0025205. [http://dx.doi.org/10.1037/ h0025205]. [PMID: 6082873]. [DOI] [PubMed] [Google Scholar]
- 53.Frintrop L., Trinh S., Liesbrock J., Paulukat L., Kas M.J., Tolba R., Konrad K., Herpertz-Dahlmann B., Beyer C., Seitz J. Establishment of a chronic activity-based anorexia rat model. J. Neurosci. Methods. 2017 doi: 10.1016/j.jneumeth.2017.09.018. [http://dx.doi.org/10.1016/j.jneumeth. 2017.09.018]. [PMID: 28970163]. [DOI] [PubMed] [Google Scholar]
- 54.Kas M.J.H., Adan R.A.H. Animal models of eating disorder traits. Curr. Top. Behav. Neurosci. 2011;6:209–227. doi: 10.1007/7854_2010_84. [http://dx.doi.org/ 10.1007/7854_2010_84]. [PMID: 21243478]. [DOI] [PubMed] [Google Scholar]
- 55.Hillebrand J.J., van Elburg A.A., Kas M.J., van Engeland H., Adan R.A. Olanzapine reduces physical activity in rats exposed to activity-based anorexia: possible implications for treatment of anorexia nervosa? Biol. Psychiatry. 2005;58(8):651–657. doi: 10.1016/j.biopsych.2005.04.008. [http://dx. doi.org/10.1016/j.biopsych.2005.04.008]. [PMID: 16018979]. [DOI] [PubMed] [Google Scholar]
- 56.Exner C., Hebebrand J., Remschmidt H., Wewetzer C., Ziegler A., Herpertz S., Schweiger U., Blum W.F., Preibisch G., Heldmaier G., Klingenspor M. Leptin suppresses semi-starvation induced hyperactivity in rats: implications for anorexia nervosa. Mol. Psychiatry. 2000;5(5):476–481. doi: 10.1038/sj.mp.4000771. [http://dx.doi.org/10.1038/sj.mp. 4000771]. [PMID: 11032380]. [DOI] [PubMed] [Google Scholar]
- 57.Paulukat L., Frintrop L., Liesbrock J., Heussen N., Johann S., Exner C., Kas M.J., Tolba R., Neulen J., Konrad K., Herpertz-Dahlmann B., Beyer C., Seitz J. Memory impairment is associated with the loss of regular oestrous cycle and plasma oestradiol levels in an activity-based anorexia animal model. World J. Biol. Psychiatry. 2016;17(4):274–284. doi: 10.3109/15622975.2016.1173725. [http://dx.doi.org/10.3109/ 15622975.2016.1173725]. [PMID: 27160428]. [DOI] [PubMed] [Google Scholar]
- 58.Aoki C., Wable G., Chowdhury T.G., Sabaliauskas N.A., Laurino K., Barbarich-Marsteller N.C. α4-containing GABA receptors at the hippocampal CA1 spines is a biomarker for resilience to food restriction-evoked excessive exercise and weight loss of adolescent female rats. Neuroscience. 2014;265:108–123. doi: 10.1016/j.neuroscience.2014.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Barbarich-Marsteller N.C., Fornal C.A., Takase L.F., Bocarsly M.E., Arner C., Walsh B.T., Hoebel B.G., Jacobs B.L. Activity-based anorexia is associated with reduced hippocampal cell proliferation in adolescent female rats. Behav. Brain Res. 2013;236(1):251–257. doi: 10.1016/j.bbr.2012.08.047. [http://dx.doi.org/10.1016/j.bbr.2012.08.047]. [PMID: 22981561]. [DOI] [PubMed] [Google Scholar]
- 60.Reyes-Haro D., Labrada-Moncada F.E., Varman D.R., Krüger J., Morales T., Miledi R., Martínez-Torres A. Anorexia reduces GFAP+ cell density in the rat hippocampus. Neural Plast. 2016;2016:2426413. doi: 10.1155/2016/2426413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Reyes-Haro D., Labrada-Moncada F.E., Miledi R., Martínez-Torres A. Dehydration-induced anorexia reduces astrocyte density in the rat corpus callosum. Neural Plast. 2015;2015:474917. doi: 10.1155/2015/474917. [http://dx.doi.org/10.1155/2015/474917]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Frintrop L., Liesbrock J., Paulukat L., Johann S., Kas M.J.H., Tolba R., Heussen N., Neulen J., Konrad K., Herpertz-Dahlmann B., Beyer C., Seitz J. Reduced astrocyte density underlying brain volume reduction in female activity-based anorexia rats. World J. Biol. Psychiatry. 2017;19(3):225–235. doi: 10.1080/15622975.2016.1273552. [DOI] [PubMed] [Google Scholar]
- 63.Banasr M., Dwyer J.M., Duman R.S. Cell atrophy and loss in depression: Reversal by antidepressant treatment. Curr. Opin. Cell Biol. 2011;23(6):730–737. doi: 10.1016/j.ceb.2011.09.002. [http://dx.doi.org/10.1016/j.ceb. 2011.09.002]. [PMID: 21996102]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Verkhratsky A., Steardo L., Parpura V., Montana V. Translational potential of astrocytes in brain disorders. Prog. Neurobiol. 2016;144:188–205. doi: 10.1016/j.pneurobio.2015.09.003. [http://dx.doi.org/10.1016/j.pneurobio.2015. 09.003]. [PMID: 26386136]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rial D., Lemos C., Pinheiro H., Duarte J.M., Gonçalves F.Q., Real J.I., Prediger R.D., Gonçalves N., Gomes C.A., Canas P.M., Agostinho P., Cunha R.A. Depression as a glial-based synaptic dysfunction. Front. Cell. Neurosci. 2016;9:521. doi: 10.3389/fncel.2015.00521. [http:// dx.doi.org/10.3389/fncel.2015.00521]. [PMID: 26834566]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Buehren K., Konrad K., Schaefer K., Kratzsch J., Kahraman-Lanzerath B., Lente C., Herpertz-Dahlmann B. Association between neuroendocrinological parameters and learning and memory functions in adolescent anorexia nervosa before and after weight recovery. J. Neural Transm. (Vienna) 2011;118(6):963–968. doi: 10.1007/s00702-010-0567-4. [http://dx.doi.org/10.1007/s00702-010-0567-4]. [PMID: 21207075]. [DOI] [PubMed] [Google Scholar]
- 67.Elsayed M., Magistretti P.J. A new outlook on mental illnesses: glial involvement beyond the glue. Front. Cell. Neurosci. 2015;9:468. doi: 10.3389/fncel.2015.00468. [http://dx.doi.org/10.3389/fncel.2015.00468]. [PMID: 26733803]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Stevens H.E. In this issue/abstract thinking: Glial contributions to childhood psychiatric disorders, here and there, September 2009. J. Am. Acad. Child Adolesc. Psychiatry. 2009;48(9):871–872. doi: 10.1097/CHI.0b013e3181ae0a1b. [http:// dx.doi.org/10.1097/CHI.0b013e3181ae0a1b]. [PMID: 19692851]. [DOI] [PubMed] [Google Scholar]
- 69.Bélanger M., Allaman I., Magistretti P.J. Brain energy metabolism: focus on astrocyte-neuron metabolic cooperation. Cell Metab. 2011;14(6):724–738. doi: 10.1016/j.cmet.2011.08.016. [http://dx.doi.org/10.1016/j.cmet.2011. 08.016]. [PMID: 22152301]. [DOI] [PubMed] [Google Scholar]
- 70.Henneberger C., Papouin T., Oliet S.H.R., Rusakov D.A. Long-term potentiation depends on release of D-serine from astrocytes. Nature. 2010;463(7278):232–236. doi: 10.1038/nature08673. [http://dx.doi.org/10.1038/ nature08673]. [PMID: 20075918]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Molofsky A.V., Krencik R., Ullian E.M., Tsai H.H., Deneen B., Richardson W.D., Barres B.A., Rowitch D.H., Barres B.A., Rowitch D.H. Astrocytes and disease: A neurodevelopmental perspective. Genes Dev. 2012;26(9):891–907. doi: 10.1101/gad.188326.112. [http://dx.doi.org/ 10.1101/gad.188326.112]. [PMID: 22549954]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Paixão S., Klein R. Neuron-astrocyte communication and synaptic plasticity. Curr. Opin. Neurobiol. 2010;20:466–473. doi: 10.1016/j.conb.2010.04.008. [http://dx. doi.org/10.1016/j.conb.2010.04.008]. [DOI] [PubMed] [Google Scholar]
- 73.Rose C.R., Karus C. Two sides of the same coin: sodium homeostasis and signaling in astrocytes under physiological and pathophysiological conditions. Glia. 2013;61(8):1191–1205. doi: 10.1002/glia.22492. [http://dx. doi.org/10.1002/glia.22492]. [PMID: 23553639]. [DOI] [PubMed] [Google Scholar]
- 74.Reville M-C., O’Connor L., Frampton I. Literature review of cognitive neuroscience and anorexia nervosa. Curr. Psychiatry Rep. 2016;18(2):18. doi: 10.1007/s11920-015-0651-4. [http://dx.doi.org/10.1007/s11920-015-0651-4]. [PMID: 26797860]. [DOI] [PubMed] [Google Scholar]
- 75.Terhoeven V., Kallen U., Ingenerf K., Aschenbrenner S., Weisbrod M., Herzog W., Brockmeyer T., Friederich H-C., Nikendei C. Meaningful memory in acute anorexia nervosa patients-comparing recall, learning, and recognition of semantically related and semantically unrelated word stimuli. Eur. Eat. Disord. Rev. 2017;25(2):89–97. doi: 10.1002/erv.2496. [http://dx.doi.org/10.1002/erv.2496]. [PMID: 28032373]. [DOI] [PubMed] [Google Scholar]
- 76.Neufang S., Specht K., Hausmann M., Güntürkün O., Herpertz-Dahlmann B., Fink G.R., Konrad K. Sex differences and the impact of steroid hormones on the developing human brain. Cereb. Cortex. 2009;19(2):464–473. doi: 10.1093/cercor/bhn100. [http://dx.doi.org/10.1093/cercor/ bhn100]. [PMID: 18550597]. [DOI] [PubMed] [Google Scholar]
- 77.Koebele S.V., Bimonte-Nelson H.A. The endocrine-brain-aging triad where many paths meet: female reproductive hormone changes at midlife and their influence on circuits important for learning and memory. Exp. Gerontol. 2017;94:14–23. doi: 10.1016/j.exger.2016.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Luine V. Recognition memory tasks in neuroendocrine research. Behav. Brain Res. 2014 doi: 10.1016/j.bbr.2014.04.032. [http://dx.doi.org/10.1016/j.bbr.2014. 04.032]. [PMID: 24837746]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Dos Santos Z.A., Da Silva R.J., Bacurau R.F.P., Tirapegui J., Ribeiro S.M.L. Effect of food restriction and intense physical training on estrous cyclicity and plasma leptin concentrations in rats. J. Nutr. Sci. Vitaminol. (Tokyo) 2011;57(1):1–8. doi: 10.3177/jnsv.57.1. [http://dx. doi.org/10.3177/jnsv.57.1]. [PMID: 21512284]. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material is available on the publisher’s web site along with the published article.