Abstract
Background:
Functional magnetic resonance imaging (fMRI) has provided insight on how neural abnormalities are related to the symptomatology of the eating disorders (EDs): anorexia nervosa (AN), bulimia nervosa (BN), and binge eating disorder (BED). More specifically, an increasingly growing number of brain imaging studies has shed light on how func-tionally connected brain networks contribute not only to disturbed eating behavior, but also to transdiagnostic alterations in body/interoceptive perception, reward processing and executive functioning.
Methods:
This narrative review aims to summarize recent advances in fMRI studies of patients with EDs by highlighting studies investigating network alterations that are shared across EDs.
Results and Conclusion:
Findings on reward processing in both AN and BN patients point to the presence of altered sensi-tivity to salient food stimuli in striatal regions and to the possibility of hypothalamic inputs being overridden by top-down emotional-cognitive control regions. Additionally, innovative new lines of research suggest that increased activations in fron-to-striatal circuits are strongly associated with the maintenance of restrictive eating habits in AN patients. Although signifi-cantly fewer studies have been carried out in patients with BN and BED, aberrant neural responses to both food cues and an-ticipated food receipt appear to occur in these populations. These altered responses, coupled with diminished recruitment of prefrontal cognitive control circuitry, are believed to contribute to the binge eating of palatable foods. Results from functional network connectivity studies are diverse, but findings tend to converge on indicating disrupted resting-state connectivity in executive networks, the default-mode network and the salience network across EDs.
Keywords: Eating disorders, fMRI, neuroimaging, anorexia, bulimia, binge eating disorder
1. INTRODUCTION
Brain imaging techniques have enabled researchers to reframe our understanding of the pathophysiology of eating disorders (ED) [1, 2]. The majority of neuroimaging studies thus far have concentrated on anorexia nervosa (AN), a psychiatric disorder characterized by the restriction of food intake, an extreme fear of gaining weight, significantly low body weight, and disturbances in the perception of one’s body figure [3, 4]. To a considerably lesser degree, neuroimaging studies have also been carried out on patients with bulimia nervosa (BN) and binge eating disorder (BED). Binge eating is defined by the overconsumption of food in a relatively short period of time and accompanying feelings of loss of control. Apart from bingeing episodes, patients with BN also present compensatory purging: any behaviors used to offset weight gain, such as self-induced vomiting or the use of laxatives or diuretics [5, 6].
Functional magnetic resonance imaging (fMRI) is an inferential neuroimaging technique used to assess brain activity associated with temporal changes in regional cerebral blood flow. Thanks to its low level of invasiveness, the widespread availability of MRI machines, and its high level of spatial resolution, fMRI has become the gold-standard approach for assessing neural function in both currently ill and healthy individuals. fMRI can be performed in conjunction with paradigms that are designed to pinpoint regions of the brain that are associated with ED pathophysiology (e.g. food-related decision-making or body-image perception) and to elucidate their neural mechanisms [7]. The most common fMRI paradigms to determine changes in neural response use either block-related designs or event-related designs. In block-related designs, different conditions are presented and alternated in order to contrast differences in neural activations between conditions. By contrast, event-related designs are not presented in a set sequence and are randomized, with varying times in-between stimuli. This technique allows for the modeling of changes in fMRI signal in response to neural events associated with behavioral trials [8].
fMRI can also be used to investigate synchronous brain activity to determine functional connectivity between brain regions [9]. Functional connectivity is measured either during the execution a task, or via a stimulus-free fMRI approach, defined as resting-state fMRI [10]. Functional connectivity studies have led to the identification of distinct, large-scale brain networks that are understood to drive behavior [11].
In this narrative review, we will seek to provide an overview of fMRI studies on patients with EDs published within the past five years. As opposed to compiling recent findings of neuroimaging studies on this population, here, we will attempt to delineate shared alterations in neural networks across disorders, with special emphasis being given to fMRI studies examining reward processing, cognitive control, and self-monitoring networks in currently ill ED patients. This review will also cover a finite number of noteworthy studies focusing on neural activity in recovered ED patients. Such studies are useful in that they control for the obscuring effects of malnutrition and help to disentangle the “state” and “trait” characteristics of a given disorder [12]. It is our hope that a dimensional approach may prove to be valuable in pinpointing neurobiological targets that underlie ED symptomatology. Due to space limitations, not all fMRI studies involving patients with EDs can be included in this review; instead our aim is to evaluate a limited selection of recent studies that highlight common neural alterations in EDs. In addition, the article will solely focus on patients with AN, BN, or BED, since fMRI studies of patients with other specified feeding or eating disorders (OSFED) are scarce. Likewise, studies exclusively examining patients with obesity and/or excess weight will be overlooked as they are beyond the scope of this review. Lastly, this review will conclude by highlighting potential future directions for neuroimaging ED research.
2. REWARD PROCESSING NETWORKS
In order to decide whether individuals approach or avoid rewarding food stimuli, the brain reward system must integrate metabolic hunger and satiety signals with higher-order processing of taste and cognitive motivational factors [13]. The role of dopamine in such “wanting” processes is well established, and multiple studies have shown that the striatum, cortical regions, including the orbital frontal cortex (OFC), and the amygdala, are involved in driving the acquisition of both primary (e.g. palatable foods) and secondary (e.g. money) rewards [14, 15]. On the other hand, “liking”, or the hedonic experience generated from rewarding stimuli, is associated with increased opioid system activity. In the case of food stimuli, these systems are modulated by the primary taste cortex in the insula, which serves as a gatekeeper between dopaminergic basal ganglia and top-down prefrontal control regions [16]. Researchers have raised the possibility that intrinsic disturbances of reward processing could be a potential mechanistic explanation for the extremes of food intake seen in EDs [17]. A table summarizing the fMRI reward processing studies mentioned in this section can be found in Supplementary Information Table 1.
2.1. Reward Processing in Anorexia Nervosa
2.1.1. Response to Reward
The food restriction typical of AN has been found to sensitize reward circuits and several reward-centered models of the pathophysiology of AN have been proposed [18-20]. These models overlap in supporting that AN is maintained by a reward-based learned behavior in which central reward systems are altered by abnormal eating- and weight-related cognitions. Additionally, cues that are compatible with this aberrant mode of thinking become rewarding for the individual and thus promote anorectic behaviors.
As of late, more fMRI studies have focused on testing neurotransmitter-based hypotheses, with midbrain dopaminergic neurons being understood to mediate reward learning [21]. fMRI prediction error paradigms that use a computational model for reward receipt and omission have been especially helpful in shedding light on alterations in neural dopamine responsiveness in AN [22]. Numerous studies in AN patients have found prediction error response to be heightened in the dorsal striatum, using both taste [23] and monetary stimuli [24]. DeGuzman et al., even found that greater caudate prediction error response was associated with lower weight recovery during treatment, suggesting that elevated prediction error neural activity could potentially be a neurobiological marker of AN [24]. It should be noted however that the aforementioned paradigms did not require any behavioral responses by the participants and that the clinical relevance of prediction error in EDs remains speculative. In this same vein, other researchers have found AN participants exhibited an exaggerated response to losses compared to wins during a monetary reward task in both executive, and striatal regions [25]. These findings imply that altered responses in the circuitry responsible for coding the affective content of stimuli might be partly responsible for the exaggerated negative bias observed in AN. Murao et al., also observed heightened activations in the right posterior insula and the cingulate during the anticipation of losses (i.e. punishment) in AN patients with binge-eating/purging subtypes compared to patients with restricting subtype and healthy controls [26]. This hypersensitivity to punishment has been further demonstrated in the context of social behavior, with one study identifying ventral striatum activation during rejection on a social-judgment task to be positively correlated with AN severity scores [27]. Being that attentional bias modification strategies have been utilized in treating AN and other psychiatric disorders [28, 29], it might be particularly beneficial to develop interventions aimed at rectifying dysfunctional reward-related response biases to negative stimuli.
2.1.2. Habit Formation
New evidence supports that the neural circuits engaged by individuals with AN during food choice differ significantly from healthy controls, and that these circuits are associated with habit formation [30]. In an elegantly designed study by Foerde et al., participants with AN were asked to make a series of choices between self-rated “Neutral” food items and other food items during fMRI scanning [31]. In the AN group, less-caloric food choices were strongly associated with dorsal striatum activity, a pattern that was not found in the control group. Anatomical and interference studies uphold that the dorsal lateral striatum is critical for control of habitual action and learned automatic behaviors [32], thereby suggesting that the persistent, maladaptive food intake behaviors seen in AN are underpinned by fronto-striatal networks crucial for forming habits [18]. Moreover, the authors found that the AN patients actual food consumption in a meal the day following fMRI scanning was also robustly associated with dorsolateral prefrontal cortex (dlPFC) and dorsal striatum connectivity. By observing that individuals with AN engaged in neural circuits associated with habit formation during food choice to a greater extent than healthy controls, one can postulate that the deeply entrenched and stereotyped dietary practices of AN are ultimately mediated by these neural circuits.
2.1.3. Taste Processing
Taste is another known driver of food intake and therefore could be implicated in the pathophysiology of restricted eating in AN [33]. The insula has been shown to respond to taste stimuli and it plays a central role in transmitting salience information to both ventral striatal and OFC reward pathways [34]. A recent study by Frank et al., using Multivariate Bayesian pattern analysis found that AN was associated with reduced taste pattern classification accuracy in the insula when contrasting caloric sucrose against a control solution during a neural taste discrimination paradigm [35]. As normal taste/smell discrimination is altered in patients AN [36], it is feasible that disruptions in afferents from the insula to basal ganglia reward centers and to higher-order taste processing influence their drive to eat [33]. Moreover, the hypothesis that the under-consumption of energy-dense foods in AN is partly due to the over-activation of top-down control regions when viewing energy-dense foods has been corroborated by a recent study showing a differential pattern of activation in the lateral frontal pole [37]. In this study, AN patients displayed increased activations in supervisory attentional control centers following the presentation of high-calorie stimuli and decreased activations during the presentation of low-calorie food pictures, the opposite of what occurred in control participants. Likewise, Monteleone et al., identified a similar paradoxical brain response to basic sweet taste stimuli over bitter taste stimuli in several taste-reward pathway areas in AN subjects, with the opposite pattern of activation appearing in healthy subjects [38]. This finding suggests that pleasant taste stimuli is intrinsically more salient for individuals with AN, being that sweet flavors are attributed to foods with a higher calorie content, and hence, perceived as more aversive than bitter taste stimuli. What is more, a recent study examining sucrose taste processing found that the hypothalamus drove ventral striatal activity in healthy subjects, but, in both AN and BN, patients’ effective connectivity was directed from the anterior cingulate via the ventral striatum to the hypothalamus [39]. Such findings give further support to the notion that cognitive-emotional top-down control affects food reward processing, possibly by overriding hypothalamic inputs to the ventral striatum.
2.2. Reward Processing in Bulimia Nervosa and Binge Eating Disorder
2.2.1. Addictive-like Eating?
As opposed to AN, the overeating behavior observed in BN and BED patients is hypothesized to be driven by an unstable reward system which overrides homeostatic and cognitive regulation systems [17, 40]. Animal research, for example, has shown that sugar binging is associated with the sensitization of dopamine-related reward systems [41]. One study using the temporal difference model to examine dopamine-related learning identified reduced brain responses in BN patients when learning associations between arbitrary visual stimuli and taste rewards compared to controls [42]. These attenuated responses were found in the insula, ventral putamen, amygdala, and OFC, and were also linked to the frequency of binge/purge episodes. The episodic excessive food intake seen in BN and BED could lead to excessive episodic dopamine release and bring about the subsequent desensitization of dopamine circuits via the downregulation of D2 receptors. These alterations in the dopamine taste reward system coincide with the addictive-like model of craving for excessive food stimulation [15]. Another longitudinal study found that diminished recruitment of the ventral striatum and the inferior frontal gyrus during the anticipatory phase of reward processing, and reduced activity in the medial prefrontal cortex (PFC) during the outcome phase of reward processing was linked to poor response to treatment in BED patients [43]. As similarly posited in the obesity literature, hypo-responsivity in reward regions in BN and BED patients is hypothesized to be a result of a history of repeatedly overeating highly palatable foods, which thereby leads to higher levels of food consumption in order to compensate for this deficit [44].
However, some divergent findings have been observed which put into question the addiction-like neurobiological model of heightened anticipatory reward processing and blunted response to reward receipt as a driver of overeating [45]. Namely, Simon et al., observed the opposite pattern in a large sample of BN and BED patients during the performance of a food incentive delay task [46]. Compared to controls, patients exhibited reduced brain activation in the posterior cingulate cortex (PCC) during the expectation of food and increased activity in the medial OFC cortex, anterior medial PFC and posterior cingulate cortex during the receipt of food reward. Likewise, another study using a chocolate milkshake vs. tasteless solution task found that women with BN displayed hypoactivation in the right anterior insula in response to the anticipation of taste receipt compared to controls [44]. BN patients also showed hypoactivations in the left middle frontal gyrus, right posterior insula, right precentral gyrus, and right dorsal insula in response to consumption. Reduced activations during the expectation of reward may be indicative of reduced involvement of self-control brain regions, whereas the sensitization of brain regions involved in determining the hedonic valuation of food may be the result of persistent attempts at dieting and recurring binge eating episodes.
2.2.2. Drivers of Overeating
Numerous studies suggest that BN patients are less responsive to both internal and external reward cues, which is believed to contribute to their tendency to overeat during binge episodes [45]. One study found, in concordance with models of binge eating that highlight the role of negative reinforcement in driving overeating [18, 47], a positive association between negative affect and the responsivity of reward regions (the putamen, caudate, and pallidum) to the anticipated intake of palatable food in BN patients [48]. This result implies that negative affect may positively modulate the reward value of food and become a conditioned cue due to a history of binge eating when in a negative mood. This coincides with other evidence suggesting that unpleasant acute stressors contribute to diminished hippocampal activation responsiveness in relation to external food cues and increased food consumption in BED-symptomatic women [49]. Other researchers have postulated that impaired hippocampal activity can lead to interference in the coding of conditional cues evoked by food and potentially distort interoceptive satiety [50]. Lastly, using a novel approach, Weygandt et al., applied an ensemble classifier to predict the clinical status of subjects (BED, BN, overweight controls, and normal-weight controls) based on food-related brain response patterns [51]. The authors found that patterns in the right insular cortex provided a maximum diagnostic accuracy for the separation of BED and BN patients from normal-weight controls, thereby highlighting the importance of the insula in processing food stimuli [51]. Taking these results into account and the reduced sensitivity of the primary gustatory cortex to aversive tastes found in Monteleone et al., [38], it is possible that dysfunctional insula cortex taste processing may correspond to a neurobiological correlate of the propensity of BED and BN patients to ingest both highly palatable and non-pleasurable foods during binging episodes.
2.3. Reward Processing in Recovered Eating Disorder Patients
Increasingly, researchers have opted to recruit recovered ED patients for fMRI studies in order to avoid the confounding effects of altered nutritional states [52]. It has been argued that this approach allows for the isolation of core temperament and personality traits that persist after recovery and that are similar to the symptoms described premorbidly in ED patients [53]. Results from such studies have largely been consistent with studies in ill ED patients [54] and it can be argued that alterations in recovered patients may also partly be the result of the “scarring” effect of a prolonged semi-starvation state [55]. For example, Wegner et al., found that both recovered AN and BN patients presented disturbed sensitization patterns in response to sweet taste stimuli, with subjects recovered from AN specifically displaying decreased sensitization to sucrose, whereas subjects recovered from BN displayed increased sensitization [56]. Interestingly, the sensitization and habituation effects primarily occurred in appraisal circuits in the medial frontal cortex, but did not appear in the insula, as has been observed in currently ill ED patients [38]. Wierenga et al., inventively used a delay discounting monetary decision task during hunger and satiated states to examine whether diminished response to reward could underlie food restriction in women remitted from AN [57]. These researchers found that, whereas in the control group hunger significantly increased activation in reward salience circuitry (ventral striatum, dorsal caudate, anterior cingulate cortex) during processing of immediate reward, brain response in reward and cognitive neurocircuitry did not differ during hunger and satiety in the recovered AN group. These findings uphold the notion that decreased sensitivity to the motivational drive of hunger could be a crucial maintenance factor for individuals with AN [57]. Another study examined food-cue processing in both chronically ill and long-term recovered women with AN and indentified increased activations in top-down control regions (i.e. medial and lateral PFC, and anterior cingulate) in both patient groups when compared to healthy controls [58]. This suggests that a stronger aversion to food cues still remains after recovery and that attentional biases may represent a trait marker of the disorder. Likewise, it may be that recovered AN patients require a greater allocation of top-down cognitive neural resources to process subjectively aversive stimuli, whereas control subjects process such stimuli through more intuitive mechanisms. Other reward processing alterations, such as heightened sensitivity to punishment [54], also persist after recovery, whereas taste discrimination deficits seem to normalize in AN [35].
3. COGNITIVE CONTROL NETWORKS
Cognitive control refers to the processes related to the mindful deployment of attentional and cognitive resources for the flexible response to shifting contingencies [59]. The synergy of cognitive processes, spanning across domains such as working memory, set shifting, and performance monitoring, are integral to the deployment of adaptive decision making [60] and emotion regulation [61, 62]. A frontoparietal network containing the dlPFC (dlPFC) and posterior parietal cortices, and a cingulo-opercular network containing the dorsal anterior cingulate cortex (dACC), anterior insula, and the anterior PFC have been identified as crucial for the adaptive functioning of cognitive control [63, 64]. No fMRI studies to date have been published using cognitive reappraisal emotion regulation paradigms in ED patients, though aberrant insula activity during the generation and down-regulation of negative emotions has been linked to excess weight [65]. Maladaptive cognitive functioning has been proposed to be prevalent across eating disorders (i.e., transdiagnostically), with AN patients presenting increased self-control, cognitive rigidity and impairments in set-shifting [66], and patients with BN and BED symptoms displaying increased food-related impulsivity [67, 68]. A table summarizing the cognitive control fMRI studies mentioned in this section can be found in Supplementary Information Table 2.
3.1. Cognitive Control in Anorexia Nervosa
Excessive cognitive control characteristics such as perfectionism, dichotomous thinking, and obsessive–compulsive personality traits are predisposing factors for AN that very often persist after clinical recovery [69]. More specifically, patients with AN often display weak central coherence [70] (i.e. a tendency to focus on local detail at the expense of global processing), which is thought to hinder the appropriate adjustment of cognitive strategies during treatment [71]. Although the neural substrates of central coherence impairments in AN are still relatively unknown, Fonville et al. found that AN patients showed diminished activation in the precuneus compared to controls when completing an embedded figures test [72]. This region is known to be implicated in visuospatial imagery processing and in shifting attention between targets [73]. Furthermore, the AN group showed greater activation in the right fusiform gyrus, a region associated with object perception, suggesting that AN patients use alternative cognitive mechanisms to controls to carry out the task. This pattern of elevated cognitive rigidity and performance monitoring is supported by the findings of Geisler et al., who opted to use a probabilistic reversal learning task to investigate the neural correlates of flexibility in response to changing reward contingencies in acute AN patients [66]. This study found that increased neural response in the dACC on trials with negative feedback was followed by behavioral adaptation in AN patients. Furthermore, the authors found group differences in feedback-dependent changes in functional connectivity between the dACC and right amygdala, which is consistent with the notion of over-responsiveness to conflict and negative feedback in AN. Abnormally elevated performance monitoring and cognitive control manifested as hyperfunctioning of the dACC could be reflective of the additional allocation of cognitive resources. These resources could reinforce the fixation on goals commonly seen in AN (e.g. skinny body and food restriction). However, it is worth noting that other studies have found evidence of both reduced and increased PFC activations in AN patients during error processing [74] and inhibitory control [75], and that some cognitive domains, such as verbal working memory, do not appear to be affected in restrictive-type AN patients [76].
Another commonly reported risk factor of AN is impaired set shifting [77] (i.e. the ability to alter a behavior in response to changing contingencies), with the ventrolateral PFC (vlPFC) being a key area for successful execution of set shifting tasks [78]. One study found that AN patients showed decreased activity in the right vlPFC and bilateral parahippocampal cortex during an fMRI-adapted version of the Wisconsin Card Sorting Test compared to controls [79]. Taken further, Garrett et al., found that improvements in set-shifting performance following AN treatment were predicted by lower vlPFC activation and higher anterior middle frontal activation [80]. These findings imply that impairments in cognitive flexibility found in AN patients are associated with alterations in the recruitment of PFC resources and that the vlPFC specifically is imperative for shifting between context-appropriate responses. Relatedly, Sulston et al., identified that reduced right dACC activation was correlated with perseverative errors on a set-shifting task in a sample of currently ill AN patients, suggesting that these patients require greater neural resource allocation in order to properly perform such tasks [81]. The hypothesis that the altered efficiency of neural resource allocation might underlie the increased levels of self-control in AN was also supported by the findings of King et al., [82]. In their study, currently ill adolescent AN patients presented decreased activations in lateral prefrontal and posterior parietal regions during decision making on a delay discounting task. Being that choices were also consistently made faster by AN patients, the authors suggest that their results might reflect a sustained high-level of anticipatory cognitive control in AN patients. It is possible that this ingrained and proactive mechanism might compromise control mechanisms that are necessary to adapt to shifting cognitive demands.
3.2. Cognitive Control Networks in Bulimia Nervosa and Binge Eating Disorder
In contrast to AN, individuals with bulimic-type EDs display diminished self-regulatory capacities in numerous facets not limited to overeating, but also other impulsive-compulsive behaviors (e.g. substance abuse, behavioral addictions), which is suggestive of more extensive cognitive dysregulation [83, 84]. Abnormalities in frontostriatal circuits likely contribute to diminished self-regulatory capacity, which subsequently become manifest as an inability to sufficiently curb eating behavior in individuals with BN and BED [68]. For example, Hege et al., found that increased rash-spontaneous behavior in BED subjects was related to decreased response inhibition performance during a go/no-go task, as well as reduced activity in the prefrontal control network [85]. Correspondingly, Skunde et al., observed diminished sensorimotor and the dorsal striatum activity during a go/no-go task in patients with BN with high symptom severity compared to controls [86]. Curiously, these differences were specific to the general go/no-go paradigm but not to the food-specific go/no-go paradigm, which is suggestive of a more generalized impairment of behavioral inhibition rather than a disorder-specific impairment. This coincides with a recent study by Dreyfuss et al., which found that patients with BN did not show enhanced activity in the prefrontal cortex as observed in controls during a negative-emotional-arousal cognitive-control task [87]. Furthermore, in contrast to controls, patients with BN did not display an age-dependent improvement in performance, indicating that the emergence and maintenance of BN in late adolescence could be linked to the altered recruitment of prefrontal control circuitry. Another study using Stroop-Match-to-Sample task found that both BED and BN subjects exhibited stronger activation of striatal regions compared to controls, whereas only BN subjects presented heightened premotor cortex activation [88]. This result partially dovetails with the findings of Balodis et al., who found that individuals with BED had diminished activity in the vmPFC, the inferior frontal gyrus, and the insula during Stroop performance, when compared to obese and normal-weight controls [89]. These observed differences in the neural correlates of inhibitory processing are demonstrative of a diminished ability to recruit impulse-control-related brain regions in individuals with BN [17] and BED [47].
Patients suffering from BN and BED are also characterized by a tendency to make disadvantageous food decisions and by a failure to adapt their behavior in the face of the negative consequences of overeating [90]. Burgeoning evidence on decision-making impairments in these disorders has led to the emergence of a limited number of fMRI studies examining the neural substrates of these alterations [91]. Reiter et al., for example, used computational modeling of choice behavior to identify specific signatures of altered decision-making in BED patients. The authors found BED patients to be more likely to switch between choices, indicating a bias towards exploratory decisions during behavioral adaptation in a dynamic environment. Parallel to this behavioral observation, BED patients showed reduced anterior insula and vlPFC activation during exploratory decisions than healthy controls [92]. This study stands out as it provides a mechanistic account of the maintenance of maladaptive behaviors despite negative consequences that is a hallmark of binge-spectrum disorders. By identifying the specific impairments in reward-guided decision-making in BED, these results advance our understanding of the neurocognitive phenotype of BED. Marsh et al., also identified abnormal patterns of activation in frontostriatal circuits in adolescent BN patients during a conflict resolution task [93]. More specifically, during correct responses in conflict trials, the right inferolateral and dorsolateral prefrontal cortices, and putamen failed to activate to the same degree in adolescents with BN as in healthy comparison subjects. Instead, deactivation was seen in the left inferior frontal gyrus, as well as a neural system encompassing the posterior cingulate cortex and superior frontal gyrus [93]. These findings coincide with another study which found that BN patients showed hypoactivation during reorienting and executive attention in anterior cingulate regions, the temporo-parietal junction and parahippocampus compared with controls [94].
Novel approaches using ecological momentary assessment (EMA) have also sought to clarify the association between individual differences in neural response to food cues under stress and natural environment binge eating episodes. Using EMA, Fischer et al., found that changes in activation in the ACC, precuneus, and dlPFC prefrontal cortex (dlPFC) moderated the relationship of stress to binge eating, to the extent that women with BN who exhibited decreased response reported increasing stress prior to binges [95]. The authors postulate that it is possible that increases in stress may bring about reduced sensitivity to the negatively reinforcing effects of food and thereby increase the vulnerability to binge eating.
3.3. Cognitive Control Networks in Recovered Eating Disorder Patients
The extent to which alterations in cognitive control and perception persist after recovery from EDs remains unclear and the vast majority of the literature has thus far focused on examining recovered AN patients. Decker et al., used a delay discounting task to explore choice behavior in a large sample of controls and AN patients at pre- and post-treatment [96]. However, instead of showing increased neural activity in regions associated with executive control, underweight AN patients displayed relatively less activity in the dACC and striatum. Interestingly, the authors found that the tendency to prefer larger, delayed rewards in the acutely ill state normalized as health improved. Being that neural activity in the cingulostriatal and frontoparietal circuits was linked to behavioral changes on the task after weight restoration, the authors suggest that the tendency to prefer larger, delayed rewards in the acutely ill state of AN may reflect a state-specific shift in decision making. Whether this normalization occurs in the context of the anticipation and receipt of reward remains uncertain. Another study in a sample of recovered AN patients and matched controls found no group differences behaviorally or in neural responses in the mesocorticolimbic system during the anticipation of- or in response to monetary rewards [97]. However, during both anticipation and response phases, recovered AN patients presented increased recruitment of the dlPFC, a brain region broadly implicated in top–down executive control. The study authors assert that patients with elevated cognitive control may in turn be able to adhere to dietary restriction more meticulously than others, which could render them vulnerable for an onset or relapse of AN. This suggests that an imbalance between brain systems subserving bottom–up and top–down processes may be a trait marker of AN.
4. SELF-MONITORING NETWORKS
4.1. Body-image Distortions
In AN, body image distortions are commonly focused on areas of the body that are considered by the patient to be too fat, and are often accompanied by the patient checking their own body by pinching skin folds, or measuring specific areas of the body (hips, thighs, upper arms, etc) [98, 99]. These compulsive behaviors and alterations in self perception in AN patients are understood to be driven by cognitive distortions regarding the individual’s own weight and shape, and their neurobiological underpinnings have received increased interest by neuroimaging researchers in recent years [100]. Regions that have commonly been found to display either hyper- or hypoactivations in response to images of body shape include the fusiform gyrus, the precuneus, the insula, and regions in the PFC [101]. The right precuneus in particular appears to hold a central role in developing and maintaining body representation [102]. In a skillfully designed paradigm, Nico et al., had participants predict whether a stimulus would hit or miss their body if it continued its linear motion [103]. The researchers found that healthy volunteers and stroke patients with focal left parietal damage estimated body boundaries very accurately. Conversely, AN patients and stroke patients with right parietal lesions underestimated the boundaries of their body, supporting that this region is implicated in the deviations of body schema found in AN. The findings of Via et al., during a task in which AN patients viewed video clips of their own body and another's body also identified the precuneus as a key component of a network supporting self-other-evaluative processes implicated in body distortion [104].
However, some fMRI studies have not obtained differences in right parietal lobe activations when comparing AN patients to controls. For example, Suda et al., found that patients with AN had less activation in the medial PFC and right fusiform gyrus compared to controls in response to body checking compared to neutral action images [105]. Likewise, another study identified increased activity in the dlPFC in AN patients in response to the presentation of oversized body pictures [106]. Hyperactivation in the dlPFC was also significantly correlated with shape concern in these patients. This direct association may represent an increased need for top-down cognitive control in AN when confronting emotionally salient cues, such as body images, which otherwise would be experienced as overly aversive. In order to test whether dysfunctional ventral–striatal signaling contributes to the maintenance of AN, Fladung et al., used a body weight estimation and self-referential task in a sample of adolescent patients with AN [107]. Their results showing that underweight stimuli were associated with greater activity of the ventral striatum support that reduced food intake in AN may serve as conditioned response that evolves over time by associating underweight body stimuli and starvation to motivational value. Body size processing biases have also been found to be present in BN [108]. Given that there is evidence in longitudinal studies that body size overestimation is a predictor for the development of ED, the exploration of the neurobiological underpinnings of body image distortion and interception alterations in these patients is warranted [108].
4.1.1. Interoception and Perception
An individual’s sensitivity to bodily signals, such as heartbeats and hunger, is strongly linked to the perception and regulation of emotions [109], with the insula playing a key role in integrating interoceptive signals [16]. Furthermore, it has recently been proposed that reduced levels of interoceptive awareness may predispose individuals for greater body-image dissatisfaction [100,110]. A recent review of neuroimaging studies developed a speculative model of body image distortion by dividing these alterations into three neurobiologically based components: (1) a perceptive component mainly related to alterations of the precuneus and the inferior parietal lobe; (2) an affective component mainly related to alterations of the PFC, the insula and the amygdala; (3) a yet to be fully defined cognitive component focusing on beliefs concerning body shape and appearance [101].
As previously mentioned, aberrant visceral interoceptive processing within the insula has been hypothesized to be an important mechanism in the pathophysiology of EDs due to its links to interoception, homeostatic signals that drive food consumption, emotion regulation, and body-image dissatisfaction [100, 111]. Using a well-validated interoceptive attention task in which participants focus on the sensations in specific parts of their bodies, Kerr et al., found that recovered AN patients presented decreased activity in the dorsal mid-insula during gastric interoception and that these patients also presented heightened activity in this region during anxious rumination [112]. In addition to abnormal insula activity, individuals with AN exhibited decreased activity in the precuneus during interoception. This finding reinforces the role of the insula and precuneus in self-monitoring and is in agreement with other research showing that individuals with AN demonstrate alterations in the precuneus when responding to statements relating to self-knowledge [113].
McAdams et al., 2016 opted to use a Faces task to compare viewing oneself to a stranger [114]. AN participants displayed elevated activity in the bilateral fusiform gyri for self-images, unlike the weight-recovered and healthy women, leading the authors to suggest cognitive distortions about physical appearance are a state rather than trait feature in AN [114]. However, one recent study identified persistent hyperactivation in the medial PFC during attentional bias to angry faces in recovered AN patients [115], possibly reflecting the existence of compensatory mechanisms. Lastly, abnormal spatiotemporal activation also appear to persists after recovery in AN, with one study identifying alterations in configural/holistic information for appearance- and non-appearance-related stimuli processing in patients with body dysmorphic disorder (BDD) and weight-restored AN patients [116]. This study found that both AN and BDD groups demonstrated similar hypoactivity in early secondary visual processing regions and the dorsal visual stream when viewing low spatial frequency faces, indicating a common phenotype of abnormal early visual system functioning. The above-mentioned differential patterns of response imply that alterations emerging both during the processing of the self and others, as well as while perceiving emotions [117], may be partly due to a hypersensitive inward attentional system response to self-image, and difficulties in processing and interpreting information from others during self processes.
A table summarizing the self-monitoring fMRI studies mentioned in this section can be found in Supplementary Information Table 3.
5. RESTING-STATE FUNCTIONAL CONNECTIVITY
In contrast to having subjects perform a particular task, resting-state fMRI studies allow for the examination of temporal correlations between spontaneous fluctuations in blood-oxygen-level dependent (BOLD) activations in distinct brain regions [118, 119]. Resting-state fMRI analysis generally either uses a seed-based or a network-based approach [11]. A seed-based approach preselects a region-of-interest and creates a functional connectivity map to assess the temporal correlations between this seed and other regions of the brain.
Network-based approaches, on the other hand, investigate putatively intrinsic neural networks from BOLD signal. One commonly utilized network-based approach is independent component analysis, which isolates sets of regions showing the strongest levels of temporal synchronicity [120]. The default-mode network (DMN), for example, is the most studied network in resting-state research and is recruited during internally focused, non-goal oriented activity [121]. The DMN, which is deactivated when cognitive resources are needed in order to carry out a task, includes areas such as the precuneus-posterior cingulate, the inferior parietal cortex, the medial PFC, and the hippocampus. Other networks implied in ED pathology include the superior parietal/PFC-centered executive control network, and the salience network (a circuit linking the anterior cingulate, the frontal, and the anterior insular cortices), which is understood to mediate shifts between the DMN to goal-directed networks [10, 122]. Other analytical approaches less commonly found in the ED literature include graph analysis, which consists of dividing the brain into a network of nodes in order to assess degree centrality (i.e. the number of links a given node has) [10, 123].
A table summarizing the resting-state fMRI studies mentioned in this section can be found in Supplementary Information Table 4.
5.1. Resting-state Functional Connectivity in Eating Disorder Patients
Although the methodological approaches employed to examine resting-state functional connectivity vary greatly across studies, results have fairly consistently overlapped in identifying cortico-limbic network abnormalities in individuals with EDs [119]. Of particular interest are findings from seed-based studies examining regions involved in cognitive control. For example, Lee et al., chose to explore the functional connectivity of the dACC in a sample of AN and BN patients and matched healthy controls [124]. The authors found that the AN group exhibited stronger synchronous activity between the dACC and retrosplenial cortex, whereas the BN group presented heightened synchronous activity between the dACC and the OFC. Furthermore, both groups demonstrated greater synchronous activity between the dACC and precuneus, which correlated with higher levels of body shape concerns. These findings suggest that altered dACC-precuneus connectivity could conceivably underlie the disorder-specific weight and body shape concerns found in ED patients. Biezonski et al., singled out the thalamus, a key mediator of information flow through frontal-basal ganglia circuit loops, as their region of interest of resting-state functional connectivity in a sample of patients with AN and matched controls [125]. Relative to controls, AN patients displayed heightened functional connectivity between the central-medial thalamus and the bilateral dlPFC, along with lower connectivity between the anterior thalamus and the left anterior PFC. Furthermore, alterations in thalamo-frontal connectivity were associated with deficits in performance on cognitive control and working memory tasks. These findings coincide with those of Boehm et al., which revealed increased functional connectivity between the angular gyrus and the other regions of the fronto-parietal network in patients with AN in comparison to controls [126]. Heightened functional connectivity within the fronto-parietal network might contribute to the high levels of anxiety and rumination commonly found in AN by making it more difficult for patients with AN to disengage from an internally oriented mental states.
Instead of using a seed-based approach, Favaro et al., analyzed the spontaneous organization of visuospatial and somatosensory networks in currently ill and recovered AN patients through resting-state functional connectivity [127]. Their findings point to AN being associated with a double disruption of brain connectivity, one associated with visuospatial difficulties and the other with abnormalities in processing somatosensory perceptual information. In their sample, both AN groups showed decreased connectivity in the occipitotemporal junction, a network involved in the “what?” pathway of visual perception, whereas only the currently-ill AN group displayed increased coactivation in the left parietal cortex, a region within the somatosensory cortex and implicated in integrating visual and interoceptive representations. Within-network connectivity of the somatosensory network has also been found to be reduced in BN, though the authors did not observe any significant between-group differences in the average within-network connectivity of the DMN, the executive network, and the salience network
[128]. Interestingly, functional connectivity of the right middle occipital gyrus, a region implicated in body processing, also correlated inversely with the bulimia severity and interoceptive awareness levels. The study authors suggested that this increased dependence on interoceptive awareness may result from dysfunctional multisensory integration and a reduced reliance on visual inputs.
Results on alterations in the DMN in patients with AN have proven to be inconsistent, with some studies showing altered DMN activity in both currently ill [104, 126] and recovered AN patients [129], whereas other studies have not identified significant differences in the DMN between AN patients and controls [130, 131]. These discordant results are in all likelihood due to differences in methodological approach between studies (e.g. seed-based studies vs. network-based approaches) and sample heterogeneity (e.g. ED duration, pharmacological treatment, prandial state). Contrastingly, the few studies using graph analysis in AN patients have been fairly consistent in identifying brain regions with functional connectivity alterations. These data-driven methods tend to show reduced functional connectivity in the insula and the thalamus, which is suggestive of impaired integration of visuospataial and homeostatic signaling [132-134]. Kullmann et al., also found reduced functional connectivity in the bilateral inferior frontal gyrus in AN patients compared to controls, which the authors postulate might contribute to the alterations in salience processing and hyperactivity found in AN [135].
To the best of our knowledge, only one study to date has examined resting-state functional connectivity in BED patients, though it should be noted that BED patients were analyzed alongside individuals with obesity not presenting bingeing symptomatology [136]. Consistent with habit formation theories, the authors found evidence of disruption in global network properties and motor cortico-striatal networks in their sample with obesity.
6. IMPLICATIONS AND FUTURE DIRECTIONS
Our global understanding of the neurobiology of the brain and its role in psychiatric disorders is still not adequately advanced for techniques such as fMRI to be reliably used to diagnose or determine the severity of EDs. Even though the identification of neural targets for effective brain-based treatments is ultimately one of the goals of neuroimaging research, at present, more research is needed to first develop comprehensive models of the behaviors that underpin EDs [137].
As highlighted in the present narrative review, the range and diversity of methodological approaches applied in fMRI studies for EDs, and the overall lack of reproducibility studies in the literature greatly hinders researchers’ ability to grasp the generalizability of any recent findings. Nonetheless, this body of research indicates that commonalities in specific neural network alterations are present across EDs and endorse the potential advantages of using a dimensional approach to elucidate the neurobiology of specific behavioral constructs [38, 39]. Transdiagnostic studies are increasingly more frequent in research of other psychiatric conditions, such as mood disorders [62], and similar approaches could prove to be beneficial to the field of EDs.
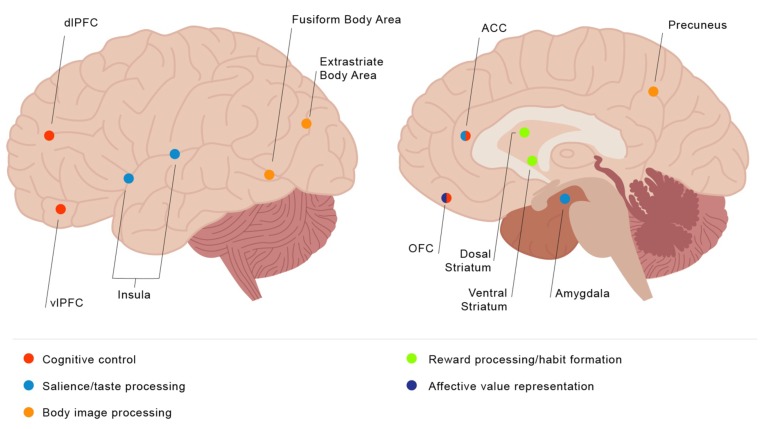
The fMRI studies presented in this review have repeatedly demonstrated that altered activation in fronto-striato and limbic regions play a significant role in the pathophysiology of EDs. In the case of AN, the evidence presented suggests that divergent responses in bottom-up regions such as the striatum during food reward processing mediated by increased activation in top-down information processing regions may contribute to the formation of restrictive eating habits (see Fig. 1). Though the number of studies in this population is much more limited, abnormalities in top-down control regions have also been identified in BN and BED, though differing results regarding hypo- or hyper-activation in comparison to controls during cognitive tasks could be due to the choice of task or compensatory activations for these groups. Likewise, resting-state functional connectivity studies indicate that executive network, DMN, and salience network alterations are implicated in the pathophysiology of EDs.
Although we strived to be parsimonious with our selection of the publications featured in this review, the highly blurred and distorted lens of neuroimaging techniques like fMRI often requires researchers to reason backwards from differential activation patterns to infer the existence of alterations in specific mental processes. The use of reverse inference to provide an interpretation of neuroimaging results has rightly come under fire due to the multiple drawbacks this line of reasoning entails [138]. Likewise, many studies fail to detect behavioral differences between patient groups and controls during fMRI scanning, despite significant differences in neural activations being present. Interpreting results based solely on differences in physiological response runs on the risk of further reifying informal reverse inferences (e.g. mining the literature to find past publications linking activations in the anterior insula to interoceptive processes in order to reason to the best explanation for a given finding). This is one of the many motives for which researchers have increasingly opted to formally test the ability to infer mental states from neuroimaging data using tools from the field of machine learning.
There are numerous approaches used in the research of other psychiatric disorders that could be applicable to ED research. Multimodal imaging techniques which utilize a combination of resting state fMRI, task-based fMRI, magnetic resonance spectroscopy (MRS), and diffusion tensor imaging (DTI) have proven to be especially useful in highlighting distinct levels of neuropathology in multiple inter-related brain systems [139, 140]. Likewise, findings from such studies could potentially be used to identify targets for non-invasive neuromodulation strategies such as transcranial direct-current stimulation (tDCS) and real-time fMRI [141]. Lastly, moving beyond the limitations of categorical, symptom-based psychiatric diagnoses and adopting dimensional frameworks such as those found in The Research Domain Criteria (RDoC) when it comes to designing future studies and testing hypotheses would be a symbol of advancement in the field [142].
Fig. (1).
Visual guide of brain regions implicated in eating disorder symptomatology. (The color version of the figure is available in the electronic copy of the article).
ACKNOWLEDGEMENTS
The authors would like to thank Beatriz Ramirez-Vaquero for her help in preparing the figure and Maria Picó-Pérez for her aid in reviewing the article content.
This work was supported by the Ministerio de Economía y Competitividad (PSI2015-68701-R); AGAUR (2009SGR1554) and Instituto de Salud Carlos III (FIS14/00290, PI13/01958 and PI16/00889). CIBER Fisiopatología de la Obesidad y Nutrición (CIBERobn) and CIBER Salud Mental (CIBERsam) are supported by ISCIII. C.S-M. is funded by a ‘Miguel Servet’ contract from the Carlos III Health Institute (CPII16/00048).
SUPPLEMENTARY MATERIAL
Supplementary material is available on the publisher’s web site along with the published article.
CONSENT FOR PUBLICATION
Not applicable.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
REFERENCES
- 1.Frank G.K.W. Advances from neuroimaging studies in eating disorders. CNS Spectr. 2015;20(4):391–400. doi: 10.1017/S1092852915000012. [http://dx.doi.org/10.1017/S1092852915000012]. [PMID: 25902917]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kaye W.H., Wagner A., Fudge J.L., Paulus M. Neurocircuity of eating disorders. Curr. Top. Behav. Neurosci. 2011;6:37–57. doi: 10.1007/7854_2010_85. [http://dx.doi.org/10.1007/7854_2010_85]. [PMID: 21243469]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zipfel S., Giel K.E., Bulik C.M., Hay P., Schmidt U. Anorexia nervosa: Aetiology, assessment, and treatment. Lancet Psychiatry. 2015;2(12):1099–1111. doi: 10.1016/S2215-0366(15)00356-9. [http://dx.doi.org/10.1016/S2215-0366 (15)00356-9]. [PMID: 26514083]. [DOI] [PubMed] [Google Scholar]
- 4.Treasure J., Zipfel S., Micali N., Wade T., Stice E., Claudino A., Schmidt U., Frank G.K., Bulik C.M., Wentz E. Anorexia nervosa. Nat. Rev. Dis. Primers. 2015;1:15074. doi: 10.1038/nrdp.2015.74. [http://dx.doi.org/10.1038/nrdp.2015.74]. [PMID: 27189821]. [DOI] [PubMed] [Google Scholar]
- 5.Treasure J., Claudino A.M., Zucker N. Eating disorders. Lancet. 2010;375(9714):583–593. doi: 10.1016/S0140-6736(09)61748-7. [http://dx.doi.org/10.1016/S0140-6736 (09)61748-7]. [PMID: 19931176]. [DOI] [PubMed] [Google Scholar]
- 6.Heaner M.K., Walsh B.T. A history of the identification of the characteristic eating disturbances of Bulimia Nervosa, Binge Eating Disorder and Anorexia Nervosa. Appetite. 2013;65:185–188. doi: 10.1016/j.appet.2013.01.005. [http://dx.doi.org/10.1016/j.appet.2013.01.005]. [PMID: 23348361]. [DOI] [PubMed] [Google Scholar]
- 7.Chow M.S.M., Wu S.L., Webb S.E., Gluskin K., Yew D.T. Functional magnetic resonance imaging and the brain: A brief review. World J. Radiol. 2017;9(1):5–9. doi: 10.4329/wjr.v9.i1.5. [http://dx.doi.org/10.4329/wjr.v9.i1.5]. [PMID: 28144401]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Clark V.P. A history of randomized task designs in fMRI. Neuroimage. 2012;62(2):1190–1194. doi: 10.1016/j.neuroimage.2012.01.010. [http://dx.doi.org/10.1016/j.neuroimage. 2012.01.010]. [PMID: 22245352]. [DOI] [PubMed] [Google Scholar]
- 9.Ugurbil K. What is feasible with imaging human brain function and connectivity using functional magnetic resonance imaging. Philos Trans. R. Soc. B. Biol. Sci. 2016;371:20150361. doi: 10.1098/rstb.2015.0361. [http://dx. doi.org/10.1098/rstb.2015.0361]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barkhof F., Haller S., Rombouts S.A.R.B. Resting-state functional MR imaging: a new window to the brain. Radiology. 2014;272(1):29–49. doi: 10.1148/radiol.14132388. [http://dx.doi.org/10.1148/radiol.14132388]. [PMID: 24956047]. [DOI] [PubMed] [Google Scholar]
- 11.Salim A., Sofia I.K., Antonios M., Emma C., Robinson D.R., Sarah P. Human brain mapping: A systematic comparison of parcellation methods for the human cerebral cortex. Neuroimage. 2018;170:5–30. doi: 10.1016/j.neuroimage.2017.04.014. [http://dx.doi.org/10.1016/j.neuroimage.2017.04. 014]. [PMID: 28412442]. [DOI] [PubMed] [Google Scholar]
- 12.Kaye W.H., Wierenga C.E., Bailer U.F., Simmons A.N., Wagner A., Bischoff-Grethe A. Does a shared neurobiology for foods and drugs of abuse contribute to extremes of food ingestion in anorexia and bulimia nervosa? Biol. Psychiatry. 2013;73(9):836–842. doi: 10.1016/j.biopsych.2013.01.002. [http://dx.doi.org/10.1016/j.biopsych.2013.01.002]. [PMID: 23380716]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Edmund T.R. Taste, olfactory, and food reward value processing in the brain. Prog. Neurobiol. 2015;127-128:64–90. doi: 10.1016/j.pneurobio.2015.03.002. [http://dx.doi. org/10.1016/j.pneurobio.2015.03.002]. [DOI] [PubMed] [Google Scholar]
- 14.Nicola S.M. Taste, olfactory, and food reward value processing in the brain. Prog. Neurobiol. 2015;127-128:64–90. doi: 10.1016/j.pneurobio.2015.03.002. [http://dx.doi. org/10.1152/ajpregu.00234.2016]. [DOI] [PubMed] [Google Scholar]
- 15.Robinson M.J.F., Fischer A.M., Ahuja A., Lesser E.N., Maniates H. Roles of “wanting” and “liking” in motivating behavior: gambling, food, and drug addictions. Curr. Top. Behav. Neurosci. 2016;27:105–136. doi: 10.1007/7854_2015_387. [http://dx.doi.org/10.1007/7854_2015_387]. [PMID: 26407959]. [DOI] [PubMed] [Google Scholar]
- 16.Craig A.D. How do you feel? Interoception: The sense of the physiological condition of the body. Nat. Rev. Neurosci. 2002;3(8):655–666. doi: 10.1038/nrn894. [http://dx.doi.org/10.1038/nrn894]. [PMID: 12154366]. [DOI] [PubMed] [Google Scholar]
- 17.Friederich H-C., Wu M., Simon J.J., Herzog W. Neurocircuit function in eating disorders. Int. J. Eat. Disord. 2013;46(5):425–432. doi: 10.1002/eat.22099. [http://dx.doi.org/10.1002/eat.22099]. [PMID: 23658085]. [DOI] [PubMed] [Google Scholar]
- 18.Steinglass J.E., Walsh B.T. Neurobiological model of the persistence of anorexia nervosa. J. Eat. Disord. 2016;4:19. doi: 10.1186/s40337-016-0106-2. [http://dx. doi.org/10.1186/s40337-016-0106-2]. [PMID: 27195123]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.O’Hara C.B., Campbell I.C., Schmidt U. A reward-centred model of anorexia nervosa: A focussed narrative review of the neurological and psychophysiological literature. Neurosci. Biobehav. Rev. 2015;52:131–152. doi: 10.1016/j.neubiorev.2015.02.012. [http://dx.doi.org/10.1016/j.neubiorev.2015.02. 012]. [PMID: 25735957]. [DOI] [PubMed] [Google Scholar]
- 20.Alessio M.M., Giovanni C., Umberto V., Valdo R., Lorenzo L., Palmiero M., Mario M. Neuroendocrinology and brain imaging of reward in eating disorders: A possible key to the treatment of anorexia nervosa and bulimia nervosa. 2018 doi: 10.1016/j.pnpbp.2017.02.020. https://doi.org/10 [DOI] [PubMed]
- 21.Schultz W., Dayan P., Montague P.R. A neural substrate of prediction and reward. Science. 1997;275(5306):1593–1599. doi: 10.1126/science.275.5306.1593. [http://dx.doi.org/10.1126/science.275.5306.1593]. [PMID: 9054347]. [DOI] [PubMed] [Google Scholar]
- 22.Garrison J., Erdeniz B., Done J. Prediction error in reinforcement learning: A meta-analysis of neuroimaging studies. Neurosci. Biobehav. Rev. 2013;37:1297–1310. doi: 10.1016/j.neubiorev.2013.03.023. [http://dx.doi.org/10.1016/j. neubiorev.2013.03.023]. [DOI] [PubMed] [Google Scholar]
- 23.Frank G.K.W., Reynolds J.R., Shott M.E., Jappe L., Yang T.T., Tregellas J.R., O’Reilly R.C. Anorexia nervosa and obesity are associated with opposite brain reward response. Neuropsychopharmacology. 2012;37(9):2031–2046. doi: 10.1038/npp.2012.51. [http://dx.doi.org/10.1038/npp.2012.51]. [PMID: 22549118]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Deguzman M., Shott M.E., Yang T.T., Ph D., Riederer J., Frank G.K.W.
- 25.Bischoff-Grethe A, McCurdy D, Grenesko-Stevens E. 2013.
- 26.Murao E., Sugihara G., Isobe M., Noda T., Kawabata M., Matsukawa N., Takahashi H., Murai T., Noma S. Differences in neural responses to reward and punishment processing between anorexia nervosa subtypes: An fMRI study. Psychiatry Clin. Neurosci. 2017;71(9):647–658. doi: 10.1111/pcn.12537. [http://dx.doi.org/10.1111/pcn.12537]. [PMID: 28459134]. [DOI] [PubMed] [Google Scholar]
- 27.Via E., Soriano-Mas C., Sánchez I., Forcano L., Harrison B.J., Davey C.G., Pujol J., Martínez-Zalacaín I., Menchón J.M., Fernández-Aranda F., Cardoner N. Abnormal social reward responses in anorexia nervosa: an fMRI study. PLoS One. 2015;10(7):e0133539. doi: 10.1371/journal.pone.0133539. [http://dx.doi.org/10.1371/journal.pone.0133539]. [PMID: 26197051]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tchanturia K., Giombini L., Leppanen J., Kinnaird E. Evidence for cognitive remediation therapy in young people with anorexia nervosa: systematic review and meta-analysis of the literature. Eur. Eat. Disord. Rev. 2017;25(4):227–236. doi: 10.1002/erv.2522. [http://dx.doi.org/10.1002/erv.2522]. [PMID: 28573705]. [DOI] [PubMed] [Google Scholar]
- 29.Cella M., Stahl D., Morris S., Keefe R., Bell M., Wykes T. Effects of cognitive remediation on negative symptoms dimensions: Exploring the role of working memory. Psychol. Med. 2017;47(15):2593–2601. doi: 10.1017/S0033291717000757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Compan V., Walsh B.T., Kaye W., Geliebter A. How does the brain implement adaptive decision making to eat? J. Neurosci. 2015;35(41):13868–13878. doi: 10.1523/JNEUROSCI.2602-15.2015. [http://dx.doi.org/10.1523/JNEUROSCI. 2602-15.2015]. [PMID: 26468187]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Foerde K., Steinglass J.E., Shohamy D., Walsh B.T. Neural mechanisms supporting maladaptive food choices in anorexia nervosa. Nat. Neurosci. 2015;18(11):1571–1573. doi: 10.1038/nn.4136. [http://dx.doi.org/10.1038/nn.4136]. [PMID: 26457555]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Burton A.C., Nakamura K., Roesch M.R. From ventral-medial to dorsal-lateral striatum: Neural correlates of reward-guided decision-making. Neurobiol. Learn. Mem. 2015;117:51–59. doi: 10.1016/j.nlm.2014.05.003. [http://dx.doi.org/10.1016/j.nlm.2014.05.003]. [PMID: 24858182]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Keating C., Tilbrook A.J., Rossell S.L., Enticott P.G., Fitzgerald P.B. Reward processing in anorexia nervosa. Neuropsychologia. 2012;50(5):567–575. doi: 10.1016/j.neuropsychologia.2012.01.036. [http://dx.doi.org/10.1016/j.neuropsychologia. 2012.01.036]. [PMID: 22349445]. [DOI] [PubMed] [Google Scholar]
- 34.Small D.M. Flavor is in the brain. Physiol. Behav. 2012;107(4):540–552. doi: 10.1016/j.physbeh.2012.04.011. [http://dx.doi.org/10.1016/j.physbeh.2012.04.011]. [PMID: 22542991]. [DOI] [PubMed] [Google Scholar]
- 35.Frank G.K.W., Shott M.E., Keffler C., Cornier M.A. Extremes of eating are associated with reduced neural taste discrimination. Int. J. Eat. Disord. 2016;49(6):603–612. doi: 10.1002/eat.22538. [http://dx.doi.org/10. 1002/eat.22538]. [PMID: 27083785]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fernández-Aranda F., Agüera Z., Fernández-García J.C., Garrido-Sanchez L., Alcaide-Torres J., Tinahones F.J., Giner-Bartolomé C., Baños R.M., Botella C., Cebolla A., de la Torre R., Fernández-Real J.M., Ortega F.J., Frühbeck G., Gómez-Ambrosi J., Granero R., Islam M.A., Jiménez-Murcia S., Tárrega S., Menchón J.M., Fagundo A.B., Sancho C., Estivill X., Treasure J., Casanueva F.F. Smell-taste dysfunctions in extreme weight/eating conditions: Analysis of hormonal and psychological interactions. Endocrine. 2016;51(2):256–267. doi: 10.1007/s12020-015-0684-9. [http://dx.doi.org/10.1007/s12020-015-0684-9]. [PMID: 26198367]. [DOI] [PubMed] [Google Scholar]
- 37.Scaife J.C., Godier L.R., Reinecke A., Harmer C.J., Park R.J. Differential activation of the frontal pole to high vs low calorie foods: The neural basis of food preference in Anorexia Nervosa? Psychiatry Res. Neuroimaging. 2016;258:44–53. doi: 10.1016/j.pscychresns.2016.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Monteleone A.M., Monteleone P., Esposito F., Prinster A., Volpe U., Cantone E., Pellegrino F., Canna A., Milano W., Aiello M., Di Salle F., Maj M. Altered processing of rewarding and aversive basic taste stimuli in symptomatic women with anorexia nervosa and bulimia nervosa: An fMRI study. J. Psychiatr. Res. 2017;90:94–101. doi: 10.1016/j.jpsychires.2017.02.013. [http://dx.doi.org/10.1016/j.jpsychires. 2017.02.013]. [PMID: 28249187]. [DOI] [PubMed] [Google Scholar]
- 39.Frank G.K.W., Shott M.E., Riederer J., Pryor T.L. Altered structural and effective connectivity in anorexia and bulimia nervosa in circuits that regulate energy and reward homeostasis. Transl. Psychiatry. 2016;6(11):e932. doi: 10.1038/tp.2016.199. [http://dx.doi.org/10.1038/tp.2016.199]. [PMID: 27801897]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Smith D.G., Robbins T.W. The neurobiological underpinnings of obesity and binge eating: A rationale for adopting the food addiction model. Biol. Psychiatry. 2013;73(9):804–810. doi: 10.1016/j.biopsych.2012.08.026. [http://dx.doi. org/10.1016/j.biopsych.2012.08.026]. [PMID: 23098895]. [DOI] [PubMed] [Google Scholar]
- 41.Murray S.M., Tulloch A.J., Chen E.Y., Avena N.M. Insights revealed by rodent models of sugar binge eating. CNS Spectr. 2015;20(6):530–536. doi: 10.1017/S1092852915000656. [http://dx.doi.org/10.1017/S1092852915000656]. [PMID: 26510689]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Frank G.K.W., Reynolds J.R., Shott M.E., O’Reilly R.C. Altered temporal difference learning in bulimia nervosa. Biol. Psychiatry. 2011;70(8):728–735. doi: 10.1016/j.biopsych.2011.05.011. [http://dx.doi.org/10.1016/j.biopsych.2011. 05.011]. [PMID: 21718969]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Balodis I.M., Grilo C.M., Kober H., Worhunsky P.D., White M.A., Stevens M.C., Pearlson G.D., Potenza M.N. A pilot study linking reduced fronto-Striatal recruitment during reward processing to persistent bingeing following treatment for binge-eating disorder. Int. J. Eat. Disord. 2014;47(4):376–384. doi: 10.1002/eat.22204. [http://dx. doi.org/10.1002/eat.22204]. [PMID: 24729034]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bohon C., Stice E. Reward abnormalities among women with full and subthreshold bulimia nervosa: A functional magnetic resonance imaging study. Int. J. Eat. Disord. 2011;44(7):585–595. doi: 10.1002/eat.20869. [http://dx.doi.org/10.1002/eat.20869]. [PMID: 21997421]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Balodis I.M., Kober H., Worhunsky P.D., White M.A., Stevens M.C., Pearlson G.D., Sinha R., Grilo C.M., Potenza M.N. Monetary reward processing in obese individuals with and without binge eating disorder. Biol. Psychiatry. 2013;73(9):877–886. doi: 10.1016/j.biopsych.2013.01.014. [http://dx. doi.org/10.1016/j.biopsych.2013.01.014]. [PMID: 23462319]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Simon J.J., Skunde M., Walther S., Bendszus M., Herzog W., Friederich H.C. Neural signature of food reward processing in bulimic-type eating disorders. Soc. Cogn. Affect. Neurosci. 2016;11(9):1393–1401. doi: 10.1093/scan/nsw049. [http://dx.doi.org/10.1093/scan/nsw049]. [PMID: 27056455]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kessler R.M., Hutson P.H., Herman B.K., Potenza M.N. The neurobiological basis of binge-eating disorder. Neurosci. Biobehav. Rev. 2016;63:223–238. doi: 10.1016/j.neubiorev.2016.01.013. [http://dx.doi.org/10.1016/j.neubiorev. 2016.01.013]. [PMID: 26850211]. [DOI] [PubMed] [Google Scholar]
- 48.Bohon C., Stice E. Negative affect and neural response to palatable food intake in bulimia nervosa. Appetite. 2012;58(3):964–970. doi: 10.1016/j.appet.2012.02.051. [http://dx.doi.org/10.1016/j.appet.2012.02.051]. [PMID: 22387716]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lyu Z., Jackson T. Acute stressors reduce neural inhibition to food cues and increase eating among binge eating disorder symptomatic women. Front. Behav. Neurosci. 2016;10:188. doi: 10.3389/fnbeh.2016.00188. [http://dx. doi.org/10.3389/fnbeh.2016.00188]. [PMID: 27790097]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Davidson T.L., Chan K., Jarrard L.E., Kanoski S.E., Clegg D.J., Benoit S.C. Contributions of the hippocampus and medial prefrontal cortex to energy and body weight regulation. Hippocampus. 2009;19(3):235–252. doi: 10.1002/hipo.20499. [http://dx.doi.org/10.1002/hipo.20499]. [PMID: 18831000]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Weygandt M., Schaefer A., Schienle A., Haynes J.D. Diagnosing different binge-eating disorders based on reward-related brain activation patterns. Hum. Brain Mapp. 2012;33(9):2135–2146. doi: 10.1002/hbm.21345. [http://dx.doi.org/10.1002/hbm.21345]. [PMID: 22887826]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kaye W.H., Wierenga C.E., Bailer U.F., Simmons A.N., Wagner A., Bischoff-Grethe A. Does a shared neurobiology for foods and drugs of abuse contribute to extremes of food ingestion in anorexia and bulimia nervosa? Biol. Psychiatry. 2013;73(9):836–842. doi: 10.1016/j.biopsych.2013.01.002. [http://dx.doi.org/10.1016/j.biopsych.2013.01.002]. [PMID: 23380716]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wagner A., Barbarich-Marsteller N.C., Frank G.K., Bailer U.F., Wonderlich S.A., Crosby R.D., Henry S.E., Vogel V., Plotnicov K., McConaha C., Kaye W.H. Personality traits after recovery from eating disorders: Do subtypes differ? Int. J. Eat. Disord. 2006;39(4):276–284. doi: 10.1002/eat.20251. [http://dx.doi.org/10.1002/eat.20251]. [PMID: 16528697]. [DOI] [PubMed] [Google Scholar]
- 54.Frank G.K.W., Collier S., Shott M.E., O’Reilly R.C. Prediction error and somatosensory insula activation in women recovered from anorexia nervosa. J. Psychiatry Neurosci. 2016;41(5):304–311. doi: 10.1503/jpn.150103. [http://dx.doi.org/10.1503/jpn.150103]. [PMID: 26836623]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Frank G.K.W. Altered brain reward circuits in eating disorders: chicken or egg? Curr. Psychiatry Rep. 2013;15(10):396. doi: 10.1007/s11920-013-0396-x. [http://dx.doi.org/10.1007/s11920-013-0396-x]. [PMID: 23963630]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wagner A., Simmons A.N., Oberndorfer T.A., Frank G.K.W., McCurdy-McKinnon D., Fudge J.L., Yang T.T., Paulus M.P., Kaye W.H. Altered sensitization patterns to sweet food stimuli in patients recovered from anorexia and bulimia nervosa. Psychiatry Res. 2015;234(3):305–313. doi: 10.1016/j.pscychresns.2015.10.010. [http://dx.doi.org/10.1016/j.pscychresns. 2015.10.010]. [PMID: 26596520]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wierenga C.E., Bischoff-Grethe A., Melrose A.J., Irvine Z., Torres L., Bailer U.F., Simmons A., Fudge J.L., McClure S.M., Ely A., Kaye W.H. Hunger does not motivate reward in women remitted from anorexia nervosa. Biol. Psychiatry. 2015;77(7):642–652. doi: 10.1016/j.biopsych.2014.09.024. [http://dx.doi.org/10.1016/j.biopsych.2014.09.024]. [PMID: 25481622]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sanders N., Smeets P.A., van Elburg A.A., Danner U.N., van Meer F., Hoek H.W., Adan R.A. Altered food-cue processing in chronically ill and recovered women with anorexia nervosa. Front. Behav. Neurosci. 2015;9:46. doi: 10.3389/fnbeh.2015.00046. [http://dx.doi.org/10.3389/fnbeh. 2015.00046]. [PMID: 25774128]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.McTeague L.M., Goodkind M.S., Etkin A. Transdiagnostic impairment of cognitive control in mental illness. J. Psychiatr. Res. 2016;83:37–46. doi: 10.1016/j.jpsychires.2016.08.001. [http://dx.doi.org/10.1016/j.jpsychires.2016.08. 001]. [PMID: 27552532]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Guillaume S., Gorwood P., Jollant F., Van den Eynde F., Courtet P., Richard-Devantoy S. Impaired decision-making in symptomatic anorexia and bulimia nervosa patients: A meta-analysis. Psychol. Med. 2015;45(16):3377–3391. doi: 10.1017/S003329171500152X. [http://dx.doi.org/10. 1017/S003329171500152X]. [PMID: 26497047]. [DOI] [PubMed] [Google Scholar]
- 61.Donofry S.D., Roecklein K.A., Wildes J.E., Miller M.A. ; Erickson K.I. Alterations in emotion generation and regulation neurocircuitry in depression and eating disorders: A comparative review of structural and functional neuroimaging studies. Neurosci. Biobehav. Rev. 2016;68:911–927. doi: 10.1016/j.neubiorev.2016.07.011. [http://dx.doi.org/10.1016/j. neubiorev.2016.07.011]. [PMID: 27422451]. [DOI] [PubMed] [Google Scholar]
- 62.Picó-Pérez M., Radua J., Steward T., Menchón J.M., Soriano-Mas C. Emotion regulation in mood and anxiety disorders: A meta-analysis of fMRI cognitive reappraisal studies. 2017. [DOI] [PubMed]
- 63.Kohn N., Eickhoff S.B., Scheller M., Laird A.R., Fox P.T., Habel U. Neural network of cognitive emotion regulation--an ALE meta-analysis and MACM analysis. Neuroimage. 2014;87:345–355. doi: 10.1016/j.neuroimage.2013.11.001. [http://dx.doi.org/10.1016/j.neuroimage.2013.11.001]. [PMID: 24220041]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lückmann H.C., Jacobs H.I.L., Sack A.T. The cross-functional role of frontoparietal regions in cognition: internal attention as the overarching mechanism. Prog. Neurobiol. 2014;116:66–86. doi: 10.1016/j.pneurobio.2014.02.002. [http://dx.doi.org/10.1016/j.pneurobio.2014.02.002]. [PMID: 24530293]. [DOI] [PubMed] [Google Scholar]
- 65.Steward T., Picó-Pérez M., Mata F., Martínez-Zalacaín I., Cano M., Contreras-Rodríguez O., Fernández-Aranda F., Yucel M., Soriano-Mas C., Verdejo-García A. Emotion regulation and excess weight: Impaired Affective processing characterized by dysfunctional insula activation and connectivity. PLoS One. 2016;11(3):e0152150. doi: 10.1371/journal.pone.0152150. [http://dx.doi.org/10.1371/journal.pone.0152150]. [PMID: 27003840]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Geisler D., Ritschel F., King J.A., Bernardoni F., Seidel M., Boehm I., Runge F., Goschke T., Roessner V., Smolka M.N., Ehrlich S. Increased anterior cingulate cortex response precedes behavioural adaptation in anorexia nervosa. Sci. Rep. 2017;7:42066. doi: 10.1038/srep42066. [http://dx.doi.org/10.1038/srep42066]. [PMID: 28198813]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Schag K., Schönleber J., Teufel M., Zipfel S., Giel K.E. Food-related impulsivity in obesity and binge eating disorder--a systematic review. Obes. Rev. 2013;14(6):477–495. doi: 10.1111/obr.12017. [http://dx.doi.org/10.1111/obr.12017]. [PMID: 23331770]. [DOI] [PubMed] [Google Scholar]
- 68.Berner L.A., Marsh R. Frontostriatal circuits and the development of bulimia nervosa. Front. Behav. Neurosci. 2014;8:395. doi: 10.3389/fnbeh.2014.00395. [http://dx.doi.org/10.3389/fnbeh.2014.00395]. [PMID: 25452718]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Johnson J.G., Cohen P., Kasen S., Brook J.S. Personality disorder traits evident by early adulthood and risk for eating and weight problems during middle adulthood. Int. J. Eat. Disord. 2006;39(3):184–192. doi: 10.1002/eat.20223. [http://dx.doi.org/10.1002/eat.20223]. [PMID: 16498587]. [DOI] [PubMed] [Google Scholar]
- 70.Lang K., Lopez C., Stahl D., Tchanturia K., Treasure J. Central coherence in eating disorders: an updated systematic review and meta-analysis. World J. Biol. Psychiatry. 2014;15(8):586–598. doi: 10.3109/15622975.2014.909606. [http://dx.doi.org/10.3109/15622975.2014.909606]. [PMID: 24882144]. [DOI] [PubMed] [Google Scholar]
- 71.Tchanturia K., Lock J. 2010.
- 72.Fonville L., Lao-Kaim N.P., Giampietro V., van den Eynde F., Davies H., Lounes N. Evaluation of enhanced attention to local detail in anorexia nervosa using the embedded figures test; an fMRI study. PLoS One. 2013;8:1–7. doi: 10.1371/journal.pone.0063964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Cavanna A.E., Trimble M.R. The precuneus: A review of its functional anatomy and behavioural correlates. Brain. 2006;129(Pt 3):564–583. doi: 10.1093/brain/awl004. [http://dx.doi.org/10.1093/brain/awl004]. [PMID: 16399806]. [DOI] [PubMed] [Google Scholar]
- 74.Wierenga C., Bischoff-Grethe A., Melrose A.J., Grenesko-Stevens E., Irvine Z., Wagner A., Simmons A., Matthews S., Yau W.Y., Fennema-Notestine C., Kaye W.H. Altered BOLD response during inhibitory and error processing in adolescents with anorexia nervosa. PLoS One. 2014;9(3):e92017. doi: 10.1371/journal.pone.0092017. [http://dx.doi. org/10.1371/journal.pone.0092017]. [PMID: 24651705]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kullmann S., Giel K.E., Hu X., Bischoff S.C., Teufel M., Thiel A., Zipfel S., Preissl H. Impaired inhibitory control in anorexia nervosa elicited by physical activity stimuli. Soc. Cogn. Affect. Neurosci. 2014;9(7):917–923. doi: 10.1093/scan/nst070. [http://dx.doi.org/10.1093/scan/nst070]. [PMID: 23677490]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lao-Kaim N.P., Giampietro V.P., Williams S.C.R., Simmons A., Tchanturia K. Functional MRI investigation of verbal working memory in adults with anorexia nervosa. Eur. Psychiatry. 2014;29(4):211–218. doi: 10.1016/j.eurpsy.2013.05.003. [http://dx.doi.org/10.1016/j.eurpsy.2013.05.003]. [PMID: 23849992]. [DOI] [PubMed] [Google Scholar]
- 77.Reville M-C., O’Connor L., Frampton I. Literature review of cognitive neuroscience and anorexia nervosa. Curr. Psychiatry Rep. 2016;18(2):18. doi: 10.1007/s11920-015-0651-4. [http://dx.doi.org/10.1007/s11920-015-0651-4]. [PMID: 26797860]. [DOI] [PubMed] [Google Scholar]
- 78.Shafritz K.M., Kartheiser P., Belger A. Dissociation of neural systems mediating shifts in behavioral response and cognitive set. Neuroimage. 2005;25:600–606. doi: 10.1016/j.neuroimage.2004.12.054. [http://dx.doi.org/10.1016/j. neuroimage.2004.12.054]. [DOI] [PubMed] [Google Scholar]
- 79.Sato Y, Saito N, Utsumi A, Aizawa E, Shoji T, Izumiyama M. 2013. [DOI] [PMC free article] [PubMed]
- 80.Garrett A.S., Lock J., Datta N., Beenhaker J., Kesler S.R., Reiss A.L. Dissociation of neural systems mediating shifts in behavioral response and cognitive set. Neuroimage. 2005;25:600–606. doi: 10.1016/j.neuroimage.2004.12.054. [DOI] [PubMed] [Google Scholar]
- 81.Sultson H., van Meer F., Sanders N., van Elburg A.A., Danner U.N., Hoek H.W., Adan R.A., Smeets P.A. Associations between neural correlates of visual stimulus processing and set-shifting in ill and recovered women with anorexia nervosa. Psychiatry Res. Neuroimaging. 2016;255:35–42. doi: 10.1016/j.pscychresns.2016.07.004. [http://dx.doi.org/10.1016/j. pscychresns.2016.07.004]. [PMID: 27518327]. [DOI] [PubMed] [Google Scholar]
- 82.King J.A., Geisler D., Bernardoni F., Ritschel F., Böhm I., Seidel M., Mennigen E., Ripke S., Smolka M.N., Roessner V., Ehrlich S. Altered neural efficiency of decision making during temporal reward discounting in anorexia nervosa. J. Am. Acad. Child Adolesc. Psychiatry. 2016;55(11):972–979. doi: 10.1016/j.jaac.2016.08.005. [http://dx.doi. org/10.1016/j.jaac.2016.08.005]. [PMID: 27806865]. [DOI] [PubMed] [Google Scholar]
- 83.Fernández-Aranda F., Steward T., Jimenez-Murcia S. Substance-related disorders in eating disorders. Encycl. Feed. Eat. Disord. Springer Singapore; 2016. pp. 1–5. [Google Scholar]
- 84.Van den Eynde F., Guillaume S., Broadbent H., Stahl D., Campbell I.C., Schmidt U., Tchanturia K. Neurocognition in bulimic eating disorders: a systematic review. Acta Psychiatr. Scand. 2011;124:120–140. doi: 10.1111/j.1600-0447.2011.01701.x. [DOI] [PubMed] [Google Scholar]
- 85.2014.
- 86.Skunde M., Walther S., Simon J.J., Wu M., Bendszus M., Herzog W, Friederich H.C. Neural signature of behavioural inhibition in women with bulimia nervosa. 2016. [DOI] [PMC free article] [PubMed]
- 87.Dreyfuss M.F.W., Riegel M.L., Pedersen G.A., Cohen A.O., Silverman M.R., Dyke J.P., Mayer L.E.S., Walsh B.T., Casey B.J., Broft A.I. Patients with bulimia nervosa do not show typical neurodevelopment of cognitive control under emotional influences. 2017. [DOI] [PubMed]
- 88.Lee J.E., Namkoong K., Jung Y-C. Impaired prefrontal cognitive control over interference by food images in binge-eating disorder and bulimia nervosa. 2017. [DOI] [PubMed]
- 89.Balodis I.M., Molina N.D., Kober H., Worhunsky P.D., White M.A., Sinha R., Grilo C.M., Potenza M.N. Divergent neural substrates of inhibitory control in binge eating disorder relative to other manifestations of obesity. 2013. [DOI] [PMC free article] [PubMed]
- 90.Voon V. Cognitive biases in binge eating disorder: The hijacking of decision making. CNS Spectr. 2015;20:566–573. doi: 10.1017/S1092852915000681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wum M., Brockmeyer T., Hartmann M., Skunde M., Herzog W., Friederich H-C. Reward-related decision making in eating and weight disorders: A systematic review and meta-analysis of the evidence from neuropsychological studies. Neurosci. Biobehav. Rev. 2016;61:177–196. doi: 10.1016/j.neubiorev.2015.11.017. [DOI] [PubMed] [Google Scholar]
- 92.Reiter A.M., Heinze H-J., Schlagenhauf F., Deserno L. 2016.
- 93.Marsh R., Horga G., Wang Z., Wang P., Klahr K.W., Berner LA, Walsh B.T., Peterson B. S. An fMRI study of self-regulatory control and conflict resolution in adolescents with bulimia nervosa. 2011. [DOI] [PMC free article] [PubMed]
- 94.Seitz J., Hueck M., Dahmen B., Schulte-Ruther M., Legenbauer T., Herpertz-Dahlmann B., Konrad K. 1. Attention network dysfunction in bulimia nervosa - An fMRI study. PLoS One. 2016;11:1–18. doi: 10.1371/journal.pone.0161329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Fischer S., Breithaupt L., Wonderlich J., Westwater M.L., Crosby R.D., Engel S.G., Thompson J., Lavender J., Wonderlich S. Impact of the neural correlates of stress and cue reactivity on stress related binge eating in the natural environment. J. Psychiatr. Res. 2017;92:15–23. doi: 10.1016/j.jpsychires.2017.03.017. [DOI] [PubMed] [Google Scholar]
- 96.Decker J.H., Figner B., Steinglass J.E. 2015.
- 97.Ehrlich S., Geisler D., Ritschel F., King J.A., Seidel M., Boehm I., Breier M., Clas S., Weiss J., Marxen M., Smolka M.N., Roessner V., Kroemer N.B. Elevated cognitive control over reward processing in recovered female patients with anorexia nervosa. J. Psychiatry Neurosci. 2015;40:307–315. doi: 10.1503/jpn.140249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Gadsby S. Distorted body representations in anorexia nervosa. 2017. [DOI] [PubMed]
- 99.Gaudio S., Brooks S.J., Riva G. Nonvisual multisensory impairment of body perception in anorexia nervosa: A systematic review of neuropsychological studies. PLoS One. 2014;9 doi: 10.1371/journal.pone.0110087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Esposito R., Cieri F., di Giannantonio M., Tartaro A. 2016.
- 101.Gaudio S., Quattrocchi C.C. Neural basis of a multidimensional model of body image distortion in anorexia nervosa. 2012. [DOI] [PubMed]
- 102.Naito E., Morita T., Amemiya K. Body representations in the human brain revealed by kinesthetic illusions and their essential contributions to motor control and corporeal awareness. Neurosci. Res. 2016;104:16–30. doi: 10.1016/j.neures.2015.10.013. [DOI] [PubMed] [Google Scholar]
- 103.Nico D., Daprati E., Nighoghossian N., Carrier E., Duhamel J-R., Sirigu A. The role of the right parietal lobe in anorexia nervosa. Psychol. Med. 2010;40:1531–1539. doi: 10.1017/S0033291709991851. [DOI] [PubMed] [Google Scholar]
- 104.Via E., Goldberg X., Sánchez I., Forcano L., Harrison B.J., Davey C.G., et al. Self and other body perception in anorexia nervosa: The role of posterior DMN nodes. World J. Biol. Psychiatry. 2016;2975:1–15. doi: 10.1080/15622975.2016.1249951. [DOI] [PubMed] [Google Scholar]
- 105.Suda M., Brooks S.J., Giampietro V., Friederich H.C., Uher R., Brammer M.J., Williams S.C., Campbell I.C., Treasure J. Functional neuroanatomy of body checking in people with anorexia nervosa. Int. J. Eat. Disord. 2013;46:653–662. doi: 10.1002/eat.22150. [DOI] [PubMed] [Google Scholar]
- 106.Castellini G., Polito C., Bolognesi E., D’Argenio A., Ginestroni A., Mascalchi M., Pellicanò G., Mazzoni L.N., Rotella F., Faravelli C., Pupi A., Ricca V. Looking at my body. similarities and differences between anorexia nervosa patients and controls in body image visual processing. Eur. Psychiatry. 2013;28:427–435. doi: 10.1016/j.eurpsy.2012.06.006. [DOI] [PubMed] [Google Scholar]
- 107.Fladung A-K., Schulze U.M.E., Schöll F., Bauer K., Grön G. Role of the ventral striatum in developing anorexia nervosa. Transl. Psychiatry. 2013;3:e315. doi: 10.1038/tp.2013.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Mohr H.M., Röder C., Zimmermann J., Hummel D., Negele A., Grabhorn R. 2011. [DOI] [PubMed]
- 109.Damasio A.R. Descartes’ error: Emotion, rationality and the human brain. New York Putnam; 1994. p. 352. [Google Scholar]
- 110.Badoud D., Tsakiris M. From the body’s viscera to the body’s image: Is there a link between interoception and body image concerns? Neurosci. Biobehav. Rev. 2017;77:237–246. doi: 10.1016/j.neubiorev.2017.03.017. [DOI] [PubMed] [Google Scholar]
- 111.Munro C., Randell L., Lawrie S.M. An Integrative Bio-Psycho-Social Theory of Anorexia Nervosa. Clin. Psychol. Psychother. 2017;24:1–21. doi: 10.1002/cpp.2047. [DOI] [PubMed] [Google Scholar]
- 112.Kerr K.L., Moseman S.E., Avery J.A., Bodurka J., Zucker N.L., Simmons W.K. Altered Insula Activity during Visceral Interoception in Weight-Restored Patients with Anorexia Nervosa. Neuropsychopharmacology. 2015;41:521–528. doi: 10.1038/npp.2015.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.McAdams C.J., Krawczyk D.C. Who am I? How do I look? Neural differences in self-identity in anorexia nervosa. Soc. Cogn. Affect. Neurosci. 2014;9:12–21. doi: 10.1093/scan/nss093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.McAdams C.J., Jeon-Slaughter H., Evans S., Lohrenz T., Montague P.R., Krawczyk D.C. Neural differences in self-perception during illness and after weight-recovery in anorexia nervosa. 2016. [DOI] [PMC free article] [PubMed]
- 115.Bang L., Rø Ø., Endestad T. Threat-detection and attentional Bias to threat in women recovered from anorexia nervosa: neural Alterations in extrastriate and medial prefrontal cortices. Eur. Eat. Disord. Rev. 2017;25:80–88. doi: 10.1002/erv.2494. [DOI] [PubMed] [Google Scholar]
- 116.Li W., Lai T.M., Bohon C., Loo S.K., McCurdy D., Strober M., Bookheimer S., Feusner J. Anorexia nervosa and body dysmorphic disorder are associated with abnormalities in processing visual information. Psychol. Med. 2015:1–12. doi: 10.1017/S0033291715000045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Mcadams C.J., Lohrenz T., Montague P.R. Neural responses to kindness and malevolence differ in illness and recovery in women with anorexia nervosa. Hum. Brain Mapp. 2015;36:5207–5219. doi: 10.1002/hbm.23005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Fox M.D., Raichle M.E. Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat. Rev. Neurosci. 2007;8:700–711. doi: 10.1038/nrn2201. [DOI] [PubMed] [Google Scholar]
- 119.Gaudio S., Wiemerslage L., Brooks S.J., Schioth H.B. 2016.
- 120.Smith S.M., Fox P.T., Miller K.L., Glahn D.C., Fox P.M., Mackay C.E., Filippini N., Watkins K.E., Toro R., Laird A.R., Beckmann C.F. Correspondence of the brain’s functional architecture during activation and rest. Proc. Natl. Acad. Sci. USA. 2009;106:13040–13045. doi: 10.1073/pnas.0905267106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Mohan A., Roberto A.J., Mohan A., Lorenzo A., Jones K., Carney M.J., et al. The Significance of the Default Mode Network (DMN) in Neurological and Neuropsychiatric Disorders: A Review. Yale J. Biol. Med. 2016;89:49–57. [PMC free article] [PubMed] [Google Scholar]
- 122.Peters S.K., Dunlop K., Downar J. Cortico-Striatal-Thalamic Loop Circuits of the Salience Network: A Central Pathway in Psychiatric Disease and Treatment. Front. Syst. Neurosci. 2016;10:104. doi: 10.3389/fnsys.2016.00104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Mears D., Pollard H.B. Network science and the human brain: Using graph theory to understand the brain and one of its hubs, the amygdala, in health and disease. J. Neurosci. Res. 2016;94:590–605. doi: 10.1002/jnr.23705. [DOI] [PubMed] [Google Scholar]
- 124.Lee S., Ran K.K., Ku J., Lee J.H., Namkoong K., Jung Y.C. Resting-state synchrony between anterior cingulate cortex and precuneus relates to body shape concern in anorexia nervosa and bulimia nervosa. Psychiatry Res. Neuroimaging. 2014;221:43–48. doi: 10.1016/j.pscychresns.2013.11.004. [DOI] [PubMed] [Google Scholar]
- 125.Biezonski D., Cha J., Steinglass J., Posner J. Evidence for thalamocortical circuit abnormalities and associated cognitive dysfunctions in underweight individuals with anorexia nervosa. 2015. [DOI] [PMC free article] [PubMed]
- 126.Boehm I., Geisler D., King J.A., Ritschel F., Seidel M., Deza A. Y., Petermann J. 2014.
- 127.Favaro A., Santonastaso P., Manara R., Bosello R., Bommarito G., Tenconi E., Di Salle F. Disruption of visuospatial and somatosensory functional connectivity in anorexia nervosa. Biol. Psychiatry. 2012;72:864–870. doi: 10.1016/j.biopsych.2012.04.025. [DOI] [PubMed] [Google Scholar]
- 128.Lavagnino L., Amianto F., D’Agata F., Huang Z., Mortara P., Abbate-Daga G., Marzola E., Spalatro A., Fassino S., Northoff G. Reduced resting-state functional connectivity of the somatosensory cortex predicts psychopathological symptoms in women with bulimia nervosa. 2014. [DOI] [PMC free article] [PubMed]
- 129.Cowdrey F.A., Filippini N., Park R.J., Smith S.M., Mccabe C. Increased resting state functional connectivity in the default mode network in recovered anorexia nervosa. Hum. Brain Mapp. 2014;35:483–491. doi: 10.1002/hbm.22202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Boehm I., Geisler D., Tam F., King J.A., Ritschel F., Seidel M., Bernardoni F., Murr J., Goschke T., Calhoun V.D., Roessner V., Ehrlich S. Partially restored resting-state functional connectivity in women recovered from anorexia nervosa. J. Psychiatry Neurosci. 2016;41:377–385. doi: 10.1503/jpn.150259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Phillipou A., Abel L.A., Castle D.J., Hughes M.E., Nibbs R.G., Gurvich C., Rossell S.L. Resting state functional connectivity in anorexia nervosa. 2016. [DOI] [PubMed]
- 132.Ehrlich S., Lord A.R., Geisler D., Borchardt V., Boehm I., Seidel M., Ritschel F., Schulze A., King J.A., Weidner K., Roessner V., Walter M. Reduced functional connectivity in the thalamo-insular subnetwork in patients with acute anorexia nervosa. Hum. Brain Mapp. 2015;36:1772–1781. doi: 10.1002/hbm.22736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Lord A., Ehrlich S., Borchardt V., Geisler D., Seidel M., Huber S., Murr J., Walter M. Brain parcellation choice affects disease-related topology differences increasingly from global to local network levels. Psychiatry Res. Neuroimaging. 2016;249:12–19. doi: 10.1016/j.pscychresns.2016.02.001. [DOI] [PubMed] [Google Scholar]
- 134.Geisler D., Borchardt V., Lord A.R., Boehm I., Ritschel F., Zwipp J., Clas S., King J.A., Wolff-Stephan S., Roessner V., Walter M., Ehrlich S. Abnormal functional global and local brain connectivity in female patients with anorexia nervosa. J. Psychiatry Neurosci. 2015;40:140310. doi: 10.1503/jpn.140310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Kullmann S., Giel K.E., Teufel M., Thiel A., Zipfel S., Preissl H. Aberrant network integrity of the inferior frontal cortex in women with anorexia nervosa. Neuroimage Clin. 2014;4:615–622. doi: 10.1016/j.nicl.2014.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Baek K., Morris L.S., Kundu P., Voon V. Disrupted resting-state brain network properties in obesity: decreased global and putaminal cortico-striatal network efficiency. Psychol. Med. 2016:1–12. doi: 10.1017/S0033291716002646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Frank G.K.W. Recent advances in neuroimaging to model eating disorder neurobiology. Curr. Psychiatry Rep. 2015;17:559. doi: 10.1007/s11920-015-0559-z. [DOI] [PubMed] [Google Scholar]
- 138.Poldrack R.A. Inferring mental states from neuroimaging data: From reverse inference to large-scale decoding. Neuron. 2011;72:692–697. doi: 10.1016/j.neuron.2011.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Duncan N.W., Hayes D.J., Wiebking C., Tiret B., Pietruska K., Chen D.Q., Rainville P., Marjańska M., Ayad O., Doyon J., Hodaie M., Northoff G. Negative childhood experiences alter a prefrontal-insular-motor cortical network in healthy adults: A preliminary multimodal rsfMRI-fMRI-MRS-dMRI study. Hum. Brain Mapp. 2015;36:4622–4637. doi: 10.1002/hbm.22941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Vasic N., Wolf N.D., Grön G., Sosic-Vasic Z., Connemann B.J., Sambataro F., von Strombeck A., Lang D., Otte S., Dudek M., Wolf R.C. Baseline brain perfusion and brain structure in patients with major depression: A multimodal magnetic resonance imaging study. J. Psychiatry Neurosci. 2015;40:412–421. doi: 10.1503/jpn.140246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Val-Laillet D., Aarts E., Weber B., Ferrari M., Quaresima V., Stoeckel L.E. Neuroimaging and neuromodulation approaches to study eating behavior and prevent and treat eating disorders and obesity. 2015. [DOI] [PMC free article] [PubMed]
- 142.Wildes J.E., Marcus M.D. Application of the research domain criteria (RDoC) framework to eating disorders: Emerging concepts and research. Curr. Psychiatry Rep. 2015;17:30. doi: 10.1007/s11920-015-0572-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material is available on the publisher’s web site along with the published article.