Abstract
Background:
Neuropathic pain (NP) is an important public health problem and despite recent progress in the un-derstanding, diagnosis, pathophysiological mechanisms and the treatment of NP, many patients remain refractory to pharma-cotherapy.
Objective:
Currently used drugs have limited efficacy and dose-limiting adverse effects, and thus there is a substantial need for further development of novel medications for its treatment. Alternatively, drugs approved for use in diseases other than NP can be applied as experimental for NP conditions. This paper covers advances in the field of NP treatment.
Results:
The prime focus of this paper is on drugs with well-established pharmacological activity whose current therapeutic applications are distinct from NP. These drugs could be a potential novel treatment of NP. Data from preclinical studies and clinical trials on these experimental drugs are presented. The development of advanced methods of genomics enabled to pro-pose new targets for drugs which could be effective in the NP treatment.
Conclusion:
Experimental drugs for NP can be a treatment option which should be tailor-made for each individual on the basis of pain features, previous therapies, associated clinical conditions, recurrence of pain, adverse effects, contraindications and patients’ preferences. At present, there are only some agents which may have potential as novel treatments. Increasing knowledge about mechanisms underlying NP, mechanisms of drug action, as well as available data from preclinical and clin-ical studies make botulinum toxin A, minocycline, ambroxol, statins and PPAR agonists (ATx086001) promising potential future treatment options.
Keywords: Neuropathic pain, adrenergic β2 receptors agonists, histamine H3 receptors, TRPA1 channels, T-type calcium channels, p38 mitogen-activated protein kinase, 3-hydroxy-3-methyl glutaryl coenzyme A reductase, reactive oxygen species
1. INTRODUCTION
As defined by the International Association for the Study of Pain (IASP), neuropathic pain (NP) is the pain caused by a lesion or disease affecting the somatosensory nervous system [1]. This pain type is a debilitating condition which is a significant burden to individuals and society worldwide [2], being a substantial cause for patients’ disability, decreased quality of life and reduced productivity [3, 4]. The estimated prevalence of NP in general population ranges between 1.5% and 6.9%, and is mainly associated with middle age (50-64 years), manual professions, and living in rural areas [5].
Available data indicate that 40-50% of patients with NP do not receive appropriate treatment, which is due to low diagnostic efficacy, ineffectiveness of available analgesics and insufficient knowledge about the pathophysiology of NP [3-5]. Bearing that in mind, not only new recommendations for treatment protocols [3], but also exploration of novel drug targets for agents relieving NP are a strong medical demand [6, 7].
Impaired sensation which is typical for NP reflects and is a consequence of pathological phenomena that occur within the nervous system – both in the periphery (nerves, plexus, roots and sensitive lymph nodes) and at the central nervous system (CNS: spinal cord and brain) level. As such, NP can be triggered by a variety of pathological situations which are able to alter physiological somatosensory functions of the nervous system. These pathologies are responsible for tissue-specific symptoms of NP and they comprise: viral infections (Herpes simplex, Varicella zoster, HIV), metabolic impairments (e.g. diabetes) accompanied by mitochondrial dysfunctions, stroke, mechanical injuries to the CNS, or peripheral nerves [8-10] and adverse (toxic) effects of drugs, in particular some anti-cancer drugs (e.g. oxaliplatin, vincristine, bortezomib, taxanes) [10-12].
Recently published results from preclinical research have provided insights into NP mechanisms and they offered detailed knowledge about the molecular basis of this pain type [13, 14]. These mechanisms are strictly correlated with maladaptive alterations within the central and peripheral nervous system (Fig. 1) which comprise: (i) nociceptor sensitization and hyperexcitability caused by the release of pro-nociceptive neurotransmitters within afferent nerve fibers, (ii) increased ectopic excitability/firing of affected neurons, (iii) facilitation of pronociceptive stimuli at the dorsal horn of the spinal cord level, (iv) nociceptive disinhibition of the spinal inhibitory network, and (v) central sensitization of pain defined as increased responsiveness of nociceptive neurons in the CNS to normal or subthreshold afferent inputs which results from repeated and persistent painful stimulation [9, 15, 16].
Fig. (1).
Maladaptive alterations in NP within the central and peripheral nervous system. (The color version of the figure is available in the electronic copy of the article).
These abovementioned phenomena are thought to be strongly associated with molecular plastic changes in the somatosensory nervous system – in NP overexpression of voltage-gated ion channels, algogen-sensitive receptors and altered synthesis of selected neurotransmitters are observed. In NP, nociceptor activation is one of the most relevant peripheral mechanisms underlying the development of pain sensitization and subsequent fiber density changes, neuronal hyperexcitability, hyperalgesia and allodynia. These receptors are located at free nerve endings of unmyelinated C fibers and myelinated Aδ fibers and they can be activated or sensitized by metabolic damage, various mechanical, thermal and chemical stimuli, being sensitive to a variety of endogenous substances, such as: substance P (SP), bradykinin, serotonin, calcitonin gene-related peptide (CGRP), prostaglandins, excitatory amino acids (glutamate), histamine, growth factors and proinflammatory cytokines [13, 14, 16]. In addition, a pivotal role of abnormal ectopic excitability of afferent neurons has been demonstrated in NP. This phenomenon is mainly responsible for the development of positive symptoms in NP - paraesthesias and dysesthesias, and is thought to be mediated by voltage-gated sodium channels (mainly Nav1.7, 1.8 and 1.9) and voltage-gated calcium channels (Cav) overexpressed on the sensory nerves (C, Aδ, Aβ fibers) and overactivated in NP conditions [17].
Sensory C and Aδ fibers which are strongly implicated in NP pathophysiology terminate at two distinct types of spinal dorsal column neurons, i.e. spinal projection neurons which innervate higher neuronal structures, and interneurons which modify synaptic transmission at the spinal cord level. Here, pronociceptive facilitation is mainly mediated by glutamate – a main excitatory neurotransmitter within the CNS and pain pathways [14], and spinal glial cell activation [18]. Glutamate is an endogenous ligand of three types of glutamate receptors that are implicated in the transmission of pain signals from the periphery to the brain: ionotropic AMPA and NMDA receptors and metabotropic (mGluR) receptors. These proteins are responsible for basic response to painful stimuli (AMPA receptors), amplification and prolongation of painful input signals in the spinal dorsal horn (NMDA receptors), as well as enhancement of synaptic transmission and neuronal discharge via the activation of intracellular signaling cascades, thus contributing to synaptic plasticity and long-term potentiation [16]. Taken together, these mechanisms result in the increased excitability of CNS neurons and phenotypic and functional switch of Aβ fibers (‘Aβ sprout’). The latter phenomenon is due to extended receptive fields of peripheral sensory fibers, which alters the function of Aβ fibers from previously mediating innocuous stimuli to fibers transmitting mechanical stimuli and participating in the development of allodynia and hyperalgesia [8, 16].
The activity of the dorsal horn neurons projecting towards the brain plays an important role in pain modulation and pain perception. This activity is strongly influenced by the descending inhibitory noradrenergic and serotonergic, as well as an opioid, GABAergic and cannabinoid pathways that stem from the periaqueductal gray, raphe nuclei, locus coeruleus and rostral ventral medulla [19]. Both serotonergic and noradrenergic projection neurons from these regions can modulate (i.e. inhibit or facilitate) pain, whereas spinal dorsal horn GABA and glycine neurotransmitting systems are responsible for inhibitory effects. This inhibitory synaptic transmission of GABA and glycine is reduced or completely lost in NP [16] and its restoration might provide analgesia [20, 21], while the disinhibition of nociceptive input at the spinal level contributes to pain signal facilitation and increased pain sensitivity [22].
Taken together, a better understanding of the contribution of the abovementioned mechanisms and elucidation of their role in NP remains an important scientific challenge as it may provide clues to more efficacious treatments for pharmacoresistant NP. So far, several novel drug targets have been discovered for antiallodynic and antihyperalgesic agents. This review is focused on several investigational drugs that have been recently tested in preclinical models of NP and in clinical trials in patients suffering from NP. The prime focus of this paper is on drugs approved for use in diseases other than NP for which they are still considered experimental.
2. DRUG TARGETS FOR EXPERIMENTAL ANTIALLODYNIC AND ANTIHYPERALGESIC AGENTS
Many pathologies involved in the etiology of NP make this pain type complex and resistant to available pharmacotherapy. On the other hand, these mechanisms involved in NP allow for a therapeutic interference in various neurotransmitter, ion channel and receptor systems to alleviate NP symptoms. These interventions involve interdisciplinary multimodal strategies to obtain pain relief, including pharmacotherapy, complementary alternative medicine, cognitive-behavioral therapy and surgical procedures [7, 16]. Despite this, a significant proportion of patients is not treated optimally and there is an unmet clinical need to search for novel efficacious and safe antiallodynic and antihyperalgesic agents. In the recent years, potential new targets for experimental analgesics have been explored. These include:
Adrenergic system: β2 adrenoreceptors
Angiotensin AT2 receptors
Histaminergic system: H3 receptors
Cholinergic system
Transient Receptor Potential A1 channels (TRPA1)
T-type voltage-gated calcium channels
Voltage-gated sodium channels
Endocrine system
Peroxisome proliferator-activated receptors and cytokine/chemokine receptors
Reactive oxygen species
p38 mitogen-activated protein kinase
3-hydroxy-3-methyl glutaryl coenzyme A reductase
2.1. Adrenergic β2 Adrenoreceptors
Apart from their wide distribution in the cardiovascular and respiratory systems, β2 adrenoreceptors are expressed within pain pathways. Although these receptors do not participate in the regulation of mechanical nociceptive threshold in physiological conditions, recently it has been shown that they are important for the antiallodynic action of antidepressant drugs (tricyclic antidepressants, serotonin-noradrenaline and selective noradrenaline re-uptake inhibitors) used in NP [23-25]. It was suggested that noradrenaline increased by the action of antidepressant drugs on reuptake transporters acts through β2 adrenoceptors. Potential mechanism of the antiallodynic action of β2 receptor stimulation seems to be related to their expression by non-neuronal satellite cells within the dorsal horn of the spinal cord. This location of β2 receptors in pain pathways can be a potential molecular and neuroanatomical target for antidepressants in NP.
Antidepressants (nortriptyline, venlafaxine) and β2 adrenoceptor agonist (terbutaline) were effective in a mouse model of neuropathic pain induced by cuffing the sciatic nerve. This antiallodynic effect involved a peripheral nervous system and was due to β2 adrenoceptor stimulation which resulted in the inhibition of neuropathy-induced tumor necrosis factor α (TNFα) production. TNFα is a pro-inflammatory cytokine that participates in the development of allodynia in NP by influencing nociceptor sensitivity via intercellular signaling [26].
Noteworthy, the action of tricyclic antidepressants in NP is faster as compared to their effect on mood [27]. Chronic but not acute treatment with the antidepressant drug - nortriptyline and the β2 adrenoceptor agonist – terbutaline, alleviated tactile allodynia in obese leptin-deficient mice (ob/ob) which are regarded a genetic model of human type 2 diabetes useful for studies on diabetes and diabetic polyneuropathy [28]. It has been demonstrated that β2 adrenoceptors and δ opioid receptors seem important to these drugs’ antiallodynic action and a possible use of β2 adrenoceptor agonists to reduce NP in diabetic patients without contraindication for glycemic control was suggested. At present, the mechanism of the antiallodynic effect is not fully understood, as yet there is no evidence that both β2 and δ receptors may be co-expressed by the same cells in the pain pathways, or such oligomeric complexes of β2 and δ receptors may be formed by native receptors, so this issue requires further studies.
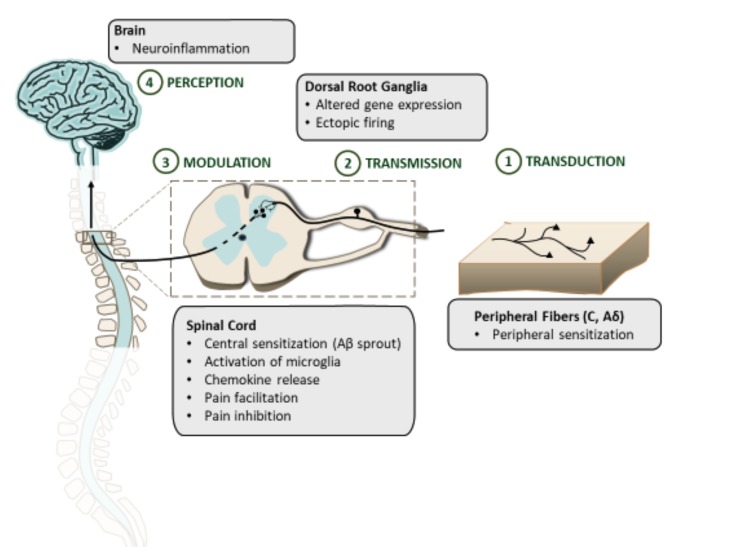
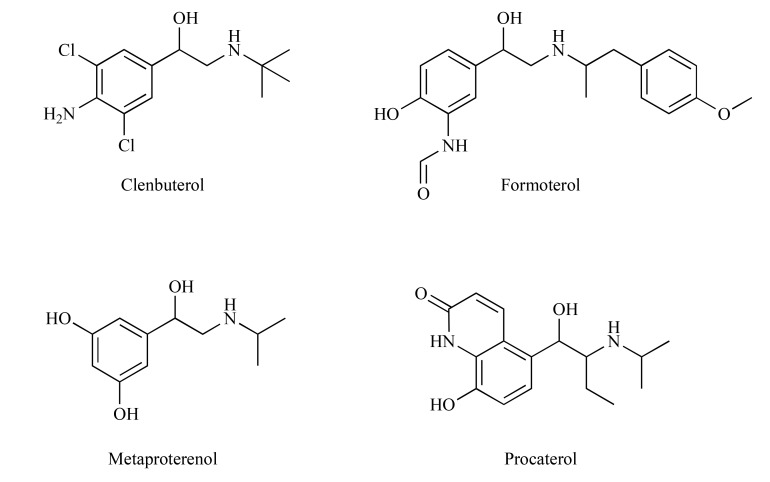
Chronic treatment with β2-agonists (clenbuterol, formoterol, metaproterenol and procaterol) (Fig. 2) suppressed allodynia in neuropathic mice with the therapeutic onset similar to that reported for antidepressant drugs [23, 25]. Similar results were obtained by Choucair-Jaafar et al. [29] for terbutaline (Fig. 3) used chronically at clinically effective doses against asthma and no serious change in cardiac parameters was observed at antiallodynic doses. Taken together these results indicate that β2-agonists may offer an alternative to currently available drugs for the treatment of NP [29]. This potential application of β2-agonists has been recently supported by a case study with salbutamol (Fig. 3) used for 1 month in 6 patients with pharmacoresistant NP but these results require further confirmation in randomized, controlled and blind studies [23]. A clinical trial evaluating the analgesic effect of the β2-agonist, i.e. terbutaline sustained release 5 mg in post-thoracoscopy NP (Betapain), has already started but the results are not available, yet [30]. β2-agonists were also effective in patients with NP secondary to thoracotomy. In addition, this study revealed a pain-type specificity of β2 receptors showing that non-NP syndromes, such as for exapmle musculoskeletal pain can be alleviated by low doses of non-selective β receptor antagonists [24]. A positive effect of β2-agonists on post-thoracotomy NP seems to be related to their inhibitory effect on the glial production of TNFα shown previously [26].
Fig. (2).
Structures of clenbuterol, formoterol, metaproterenol and procaterol.
Fig. (3).
Structures of terbutaline and salbutamol.
2.2. Angiotensin AT2 Receptors
Angiotensin II (Ang II) is an octapeptide that regulates blood pressure and fluid balance via two G protein-coupled receptors, namely AT1 and AT2. Contraction of smooth muscles leading to vasoconstriction and increase in blood pressure, increase in water and sodium intake, renal sodium retention and secretion of vasopressin and aldosterone are mediated by the stimulation of AT1 receptors, while the role of AT2 receptors is not so well understood. It is suggested that the stimulation of AT2 receptors may oppose the biological effects of AT1 receptors – in particular their effects on cell growth, blood pressure and fluid intake.
Apart from the role within the circulatory renin-angiotensin system (RAS), Ang II is a neurohormone and a neuromodulator [31] in the local RAS in various body tissues, including the brain [31, 32] and the spinal cord [32, 33]. Recent evidence showing the co-localization of Ang II with substance P, nerve growth factor and CGRP in neurons of the dorsal root ganglion points out for its role in nociception [34, 35].
Preclinical studies using cultured human and rat dorsal root ganglia cells demonstrated the expression of AT2 receptors in sensory neurons. In addition, abundant distribution of AT2 receptors was demonstrated in the brain and viscera of adult mice and on small- to medium-sized dorsal root ganglia neurons in adult rats and humans. In humans, AT2 receptors are expressed on peripheral nerve fibers, in the skin, urinary bladder and bowel [36]. In vitro studies showed that Ang II and AT2 receptors regulate neuronal membrane excitability and induce neurite outgrowth. This latter effect was caused by a persistent activation of p44/p42 mitogen-activated protein kinase (MAPK). The stimulation of MAPK activates multiple receptors and ion channels that participate in the development of NP [37].
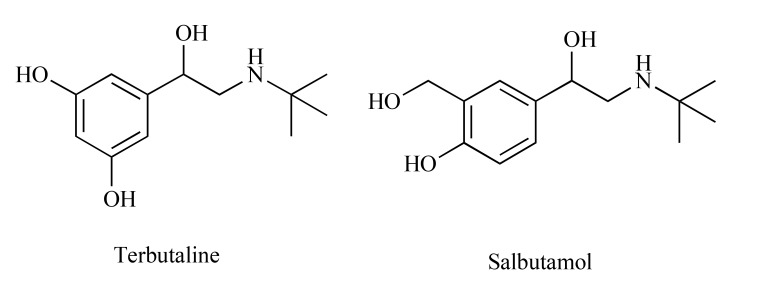
Available literature indicates that AT2 receptor antagonists are efficacious in rodent models of NP [38]. Several AT2 receptor antagonists with >1,000-fold selectivity over AT1 receptor have been developed as potential novel analgesics for alleviation of NP: EMA200 (also referred to as PD 123319), EMA300 (PD 121981), EMA400 (PD 126055) and EMA401 ([S]-enantiomer of EMA400) [38, 39] (Fig. 4). Their analgesic efficacy was proven in several preclinical models of NP [31, 35, 38] and inflammatory pain [18, 31]. The high analgesic potency without significant CNS distribution or serious CNS-related adverse effects in rats selected EMA401 as a lead compound for further clinical investigations on its antinociceptive efficacy in NP [40]. Several mechanisms underlying this antiallodynic efficacy of EMA401 have been hypothesized, including the blockade of Ang II/AT2 receptor signaling pathway in the peripheral somatosensory system and EMA401-induced decrease in p38 MAPK and ERK activation in human dorsal root ganglia sensory neurons [37]. AT2 receptor antagonists including EMA401 may thus act on paracrine/autocrine mechanisms at peripheral nerve terminals, or intracrine mechanisms, to reduce neuropathic pain signalling in Ang II/NGF/TRPV1- convergent pathways.
Fig. (4).
Structures of EMA200, EMA300, EMA400 and EMA401.
EMA401 has been tested in a phase II programme in humans as a novel analgesic drug for the treatment of NP-related to postherpetic neuralgia (PHN) and diabetic NP [38-40], but these studies have been withdrawn, recently [41].
2.3. Histamine H3 Receptors
G protein-coupled histamine H3 receptors are involved in numerous biological functions such as sleep-wake rhythms. They have been identified as mainly presynaptic autoreceptors in the histaminergic neurons of the CNS which regulate the release of histamine, as well as heteroreceptors on non-histaminergic neurons regulating the release of other neurotransmitters. H3 receptors are thought to play a role in several CNS disorders, i.e., attention-deficient hyperactivity disorder, epilepsy, cognitive deficits and obesity. Being localized in many regions of the CNS which are involved in nociception (e.g. dorsal horn of the spinal cord, locus coeruleus), recently H3 receptors have been linked to pain [42].
H3 receptors are involved in central sensitization of pain. H3 receptor blockade reduces spontaneous neuronal firing and decreases responses of spinal nociceptive neurons to mechanical stimulation. Previously, it was reported that this effect is mainly mediated via supraspinal sites, including locus coeruleus whose activity modulates the functions of spinal neurons [43].
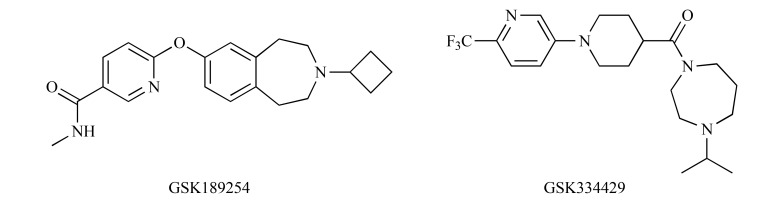
The potential role of H3 receptors in nociception has been studied in preclinical models of NP using various H3 receptor agonists and antagonists [43, 44]. The available results suggest that although these agents reduce allodynia and hyperalgesia [43], their efficacy strongly depends on the site of administration. Hence, the clinical role of H3 antagonists [44] and inverse agonists [43] is questionable and conflicting. On the other hand, inverse agonists of H3 receptors: GSK189254 and GSK334429 (Fig. 5) showed efficacy in animal models of surgically- and virally-induced NP [43-45] pointing out a possible application for these short-acting and poorly brain-penetrating ligands.
Fig. (5).
Structures of GSK189254 and GSK334429.
In a study by Hsieh et al. [42], intraperitoneally administered GSK189254 attenuated central sensitization of pain and reduced tactile allodynia in the rat spinal nerve ligation model of NP. This effect was strongly associated with the activation of the noradrenergic system at the spinal cord level. Safety and efficacy of GSK189254 were investigated and compared to duloxetine in a phase I study to demonstrate that the electrical hyperalgesia model can be extrapolated from animals to patients. This trial has been completed but the results are not available, yet [46].
2.4. Cholinergic System
The idea to use cholinergic drugs to achieve pain relief is not novel and is supported by numerous preclinical and clinical studies which demonstrated that acetylcholinesterase inhibitors (AChEI) administered intrathecally provided analgesia in response to noxious cold in healthy volunteers and in postoperative pain. Moreover, several cholinesterase inhibitors were efficacious in animal models of NP [47].
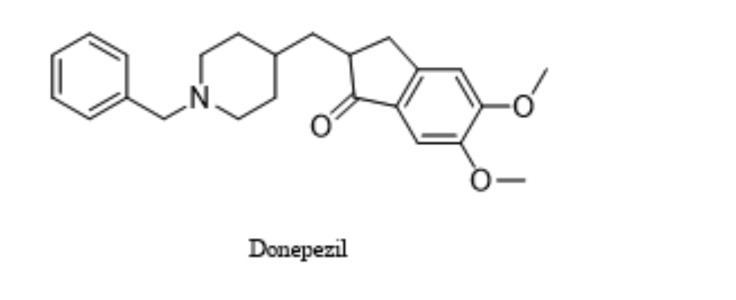
Donepezil is an orally available AChEI with improved tolerability and safety profile as compared to older AChEI [47]. Recently, it has been investigated as adjunctive therapy with gabapentin in the management of NP. The results obtained suggest that donepezil may provide benefits when added to patients with NP who do not receive sufficient pain relief from gabapentin used as monotherapy. Of note, this combination may provide additional benefits for patients who experience troublesome adverse effects from gabapentin treatment, enabling gabapentin dose reduction [47]. At present, donepezil (Fig. 6) 5 mg once daily for 6 weeks is being investigated in a randomized phase 4 clinical trial for its efficacy in NP (diabetic neuropathy, failed back syndrome with neuropathic symptoms). The estimated completion date for this trial was December 2016 [48].
Fig. (6).
Structure of donepezil.
Botulinum toxin A is a potent neurotoxin derived from the anaerobic bacterium Clostridium botulinum. There are several subtypes of botulinum toxin, but the most commonly used is the subtype A. It acts primarily as a muscle paralytic agent by inhibiting the presynaptic release of acetylcholine from motor neurons at the neuromuscular junction. Botulinum toxin also has effects on the afferent nerves by decreasing sensory response of the afferent C-fibers [49].
This potent neurotoxin cleaves the 25-kDa synaptosomal-associated protein (SNAP-25), which is anchored to the cell membrane. This cleavage results in the formation of a non-functional soluble N-ethylmaleimide-sensitive factor attachment receptor (SNARE) complex which blocks synaptic transmission and inhibits neurotransmitter and neuropeptide release. A retrograde transport and transcytosis of botulinum toxin A partially responsible for the analgesic effect of this agent has been also suggested. The latter mechanism enables the action of botulinum toxin A not only at the injection site but also at distant areas that project to the infused region [50].
Analgesic properties of botulinum toxin A both in animal models and humans are well known. The administration of botulinum toxin A attenuated NP in chronic constriction injury (CCI)-exposed rats and restored the neuroimmune balance. It reduced the levels of upregulated after injury IL1β and IL-18 in the spinal cord and dorsal root ganglia and increased the levels of the antinociceptive cytokines, such as IL-10 and IL-1RA. The changes observed at the dorsal root ganglia level suppressed microglial activation within the spinal cord. Therefore, it has been suggested that botulinum toxin A, similarly to minocycline, can influence microglia and monocytes. Intraplantar injection of botulinum toxin A diminished the CCI-mediated increase in the levels of IL-18 in dorsal root ganglia and IL-1β in both the spinal cord and dorsal root ganglia. Additionally, botulinum toxin A injection simultaneously increased the levels of the antinociceptive interleukins IL-1RA and IL-10 in the dorsal root ganglia of CCI-exposed rats compared to control animals [50].
Mechanisms underlying a beneficial effect of botulinum toxin A in NP are still not fully explored. It has been suggested that this neurotoxin: (i) cleaves neuronal SNARE, (ii) inhibits the release of pain mediators (CGRP) from the peripheral nerve terminals, dorsal root ganglia, and spinal cord neurons, (iii) reduces TRPV1 protein expression, (iv) reduces inflammation by decreasing the release of peripheral neurotransmitters and pro-inflammatory mediators, (v) deactivates sodium channels [51]. Botulinum toxin A showed efficacy in pain related to nociceptor sensitization, ectopic firing, and central sensitization and these mechanisms might be important for botulinum toxin A -mediated analgesia in NP [52].
Clinical applications of botulinum toxin A is still increasing. There is level A evidence for established efficacy of botulinum toxin A injection in PHN; level B evidence for probable efficacy of botulinum toxin A injection in trigeminal neuralgia and post-traumatic neuralgia; level C evidence for possible efficacy of botulinum toxin A injection in diabetic polyneuropathy; and level U (insufficient) evidence for botulinum toxin A injection in complex regional pain syndrome (CRPS), phantom limb and stump pain, and occipital neuralgia. There is also some concern regarding unwanted neuromuscular adverse effects, especially weakness [53, 54].
The impact of botulinum toxin A on trigeminal neuralgia has been reviewed thoroughly in [51]. It has been also suggested that botulinum toxin A might be a promising alternative treatment option for refractory cases of trigeminal neuralgia [55].
The efficacy of 50 U botulinum toxin A (intramuscular injection) in trigeminal neuralgia accompanied by severe, episodic pain in the lower left gingival area was also confirmed. Interestingly, a good therapeutic effect lasted for about 5 months [56]. Botulinum toxin A reduced pain severity and attack frequency in trigeminal neuralgia when injected to the maxillary and mandibular roots [57]. In trigeminal neuralgia, it was efficacious and well-tolerated. It should be however noted that the mechanism underlying its analgesic effect in this type of NP may not be strictly related to the cholinergic system [58], but it rather involves the inhibition of the release of pronociceptive neurotransmitters (e.g. SP, CGRP, glutamate) [59].
In PHN botulinum toxin A-treated group exhibited a significant reduction of pain intensity scores and improved sleep quality compared with control groups [51].
In post-surgical neuralgia, 20–190 U of botulinum toxin A was injected intradermally. A significant improvement in postoperative pain, and a reduction of postoperative analgesic use was observed [51].
Two randomized, double-blind, placebo-controlled studies used botulinum toxin A for pain control in diabetic neuropathy. In this type of NP, botulinum toxin A provided pain relief [51].
Botulinum toxin A (50 U) was also tested in the occipital neuralgia. Although pain scores were significantly reduced, it was concluded that these studies were insufficiently reliable to prove its full effectiveness in this pain type [51].
There is also a small clinical report regarding two patients with CRPS who experienced a reduction of pain after intramuscular administration of botulinum toxin A. However, it should be emphasized that the effect of botulinum toxin A in CRPS patients has not been reliably proven [51].
Patients with phantom limb pain were also treated with botulinum toxin A. At present, the effect of botulinum toxin A on phantom limb pain cannot be verified as the results were inconclusive and therefore not reliable [51].
In patients with the spinal cord injury-induced NP, the use of botulinum toxin A provided promising results [51]. In these subjects, a single dose of subcutaneous botulinum toxin A provided analgesia. Two administrations of botulinum toxin A 12 weeks apart significantly reduced pain intensity over 24 weeks compared with placebo [52]. It was suggested that botulinum toxin A may reduce intractable chronic NP in patients with the spinal cord injury [60]. Subcutaneous injection of type A botulinum toxin was effective and relatively well-tolerated in NP related to traumatic spinal cord injury [60]. Botulinum toxin A may be also effective in patients with central post-stroke pain [51].
2.5. TRPA1 Channels
In the mammalian organisms, TRPA1 are strongly involved in chemonociception, being sensitive to numerous chemical (e.g. reactive oxygen species, formalin, bacterial products, allicin, isothyocyanates) and physical (UV light) stimulators [61]. Since TRPA1 channels are widely expressed in nociceptors and in Schwann cells, they are regarded as important sensors of various pain types, including NP. Recent studies on the role of TRPA1 in NP have shown that TRPA1 silencing in Schwann cells reduces both allodynia and neuroinflammation in neuropathic mice [62].
One of the main functions of TRPA1 within pain pathways is thought to be related to its contribution to mechanotransduction and noxious cold sensation [61]. These activities of TRPA1 make this channel particularly important in NP-related to chemotherapy-induced peripheral neuropathy (CIPN) [11, 12] and diabetes [63, 64]. In diabetic animals, it has been demonstrated that the blockade of TRPA1 channels attenuates the development of mechanical hypersensitivity and in the advanced phase of this disease TRPA1 might mediate the loss of SP-expressing fibers causing alterations in tactile sensitivity, and TRPA1 inhibitors could restore their function [63]. As mentioned, TRPA1 is strongly implicated in CIPN caused by oxaliplatin, paclitaxel, or vincristine [63, 64]. Recently, it has been demonstrated that oxaliplatin-induced neuropathies are related to altered expression of TRPA1 channels which serve as sensors of reactive oxygen species generated during oxaliplatin administration. An increased expression of TRPA1 and other members of TRP channel family has been shown in dorsal root ganglia neurons at an early stage following oxaliplatin administration, and it has been suggested that these changes may contribute to the development of oxaliplatin-induced NP [65, 66]. This increased expression of TRPA1 via the activation of p38 MAPK in small-diameter dorsal root ganglia neurons might at least in part contribute to the development of cold hyperalgesia and cold allodynia induced by oxaliplatin. Hence, TRPA1 antagonists have been widely tested as potential lead compounds and drug candidates for the treatment of CIPN [67].
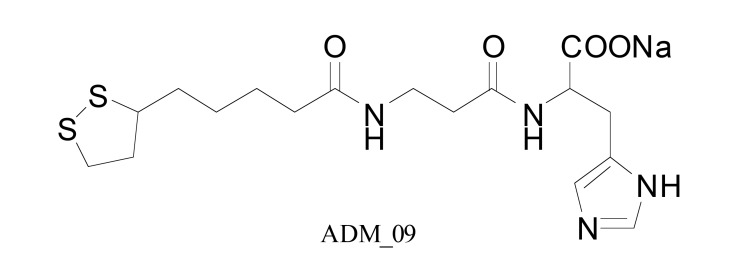
A novel TRPA1 antagonist and a derivative of lipoic acid, ADM_09 (Fig. 7), reverted oxaliplatin-induced NP in rats without causing adverse effects. Of note, ADM_09 had lower antioxidant capacities than the parent compound [68]. In preclinical research that involved paclitaxel-induced peripheral neuropathy HC-030031, a TRPA1 channel antagonist, attenuated tactile allodynia in the von Frey test in mice [64].
Fig. (7).
Structure of ADM_09.
2.6. T-type Calcium Channels
Impaired function of T-type calcium channels is linked to epilepsy but recently, these channels have also emerged as a potential novel target for the treatment of NP [69, 70]. It has been demonstrated that their activation contributes to nociceptive signaling as it facilitates action potential bursting and modulates membrane potentials of hyperexcitable neurons. The role of T-type Ca2+ channels in chronic pain is also proven by gene knockdown experiments showing that their decreased expression attenuates neuropathic pain in the CCI model in rats [71].
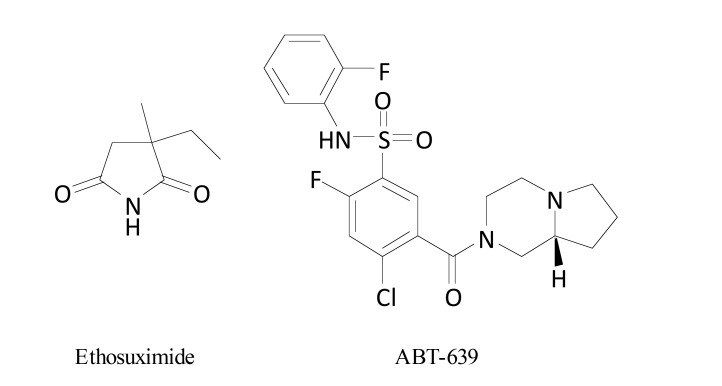
Currently, an antiepileptic drug, a T-type calcium channel blocker, ethosuximide (Fig. 8), is assessed in phase 2 clinical trial for its efficacy in traumatic NP and the estimated primary completion date of this trial is April 2018 [72].
Fig. (8).
Structures of ethosuximide and ABT-639.
ABT-639 (Fig. 8) is an orally-active peripherally-acting and highly selective T-type calcium channel blocker with efficacy in numerous preclinical NP models (spinal nerve ligation, CCI, vincristine-induced NP) [17]. In healthy adults, it demonstrated acceptable safety profile and was investigated in a phase 2 proof-of-concept study in patients with NP-related to diabetes. Since significant improvements in pain intensity score were not observed, ABT-639 is no longer in development [17, 69, 70].
2.7. Sodium Channels
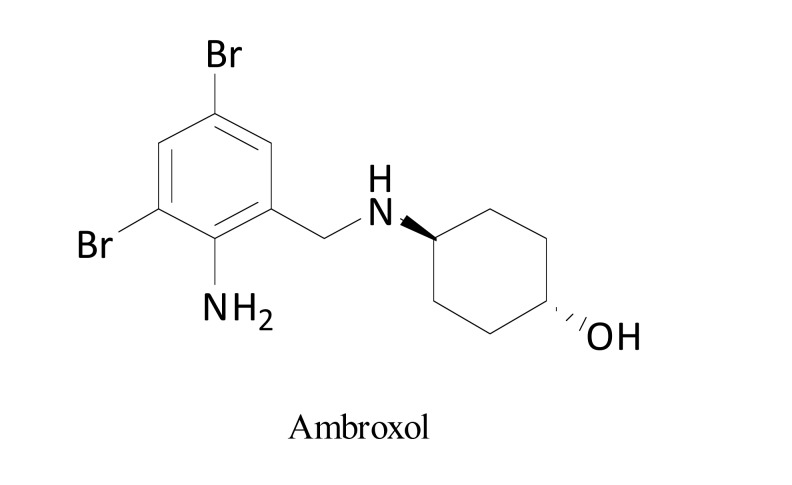
Voltage-gated sodium channels (Nav) are widely distributed within primary afferent nociceptors, mediating persistent hyperexcitability in dorsal root ganglia neurons. Orally administered mucolytic drug, ambroxol (Fig. 9), blocks sodium channels [73] with preference towards Nav1.8 [74, 75]. It acts as a strong local anesthetic agent and demonstrates antinociceptive properties in rat models of NP without impairing motor coordination [73], or sedation [74]. These results indicate its potential use in the clinic. Topical ambroxol in a form of 20% cream alleviated NP in patients with PHN, postoperative neuralgia, mononeuropathy multiplex, deafferentation pain, phantom pain and foot neuropathy of unknown origin without causing severe adverse effects [75].
Fig. (9).
Structure of ambroxol.
2.8. Endocrine System
Apart from its key role in the regulation of circadian rhythms, sleep and mood [76], melatonin (Fig. 10), a hormone synthesized in the pineal gland, modulates pain. This effect results from the activation of melatonin MT2 receptors, inhibition of 5-lipooxygenase and cyclooxygenase expression, indirect activation of opioid receptors by opening potassium channels and antioxidant activity [77]. Melatonin attenuated NP in animal models [76-78] by reducing thermal hyperalgesia and this effect involved nitric oxide synthase, as well as µ and δ opioid receptors [77].
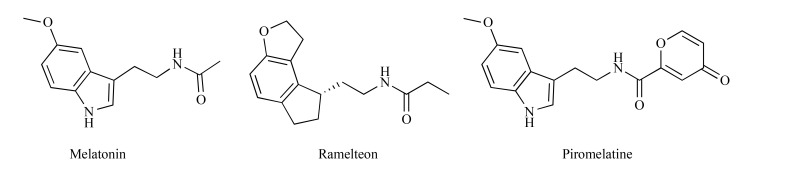
Fig. (10).
Structures of melatonin, ramelteon and piromelatine.
Melatonin showed neuroprotective properties in NP induced by oxaliplatin administration. In vitro, in oxaliplatin-exposed neuro-2a cells, it prevented the loss of mitochondrial membrane potential and it stimulated neuritogenesis without affecting cytotoxic activity of oxaliplatin in human colon cancer HT-29 cell line. Melatonin significantly attenuated oxaliplatin-induced pain behavior and it maintained physiological density of epidermal nerve fibers in oxaliplatin-treated neuropathic rats. It reduced nitro-oxidative stress caused by oxaliplatin and prevented nitrosylation of proteins. Apart from this, melatonin significantly improved functions of the mitochondrial electron transport chain and maintained optimal ATP levels in cells. These protective effects of melatonin resulted from its autophagy-inducing and mitoprotective properties within peripheral nerves and dorsal root ganglia [79].
Data from human studies indicate that melatonin is effective in patients with fibromyalgia [77]. In patients receiving melatonin during chemotherapy treatment with taxanes and cisplatin a decreased incidence of neuropathy was noted. This effect was attributed to its anti-inflammatory and antioxidant properties [78].
Recently, clinical trials with a non-selective MT1/MT2 receptor agonist, ramelteon (8 mg) in patients with NP have been terminated because recruitment did not accrue as expected [80]. Piromelatine, a novel melatonin MT1/MT2 and serotonin 5-HT1A/1D receptor agonist, with pain-related P2X3, TRPV1 and Nav1.7 channel-inhibition properties, exerted antinociceptive effects in the partial sciatic nerve ligation model in mice via melatonin receptors, opioid and 5-HT1A receptors. It also improved sleep and was devoid of motor-impairing properties. It was concluded that piromelatine may serve as an effective analgesic drug able to improve sleep disturbances in patients with NP accompanied by insomnia [81].
Endocrinopathies could be present in patients treated for NP using various drugs (e.g., opioids, antidepressants and antiepileptics), thus leading to serious impairments of their quality of life. In view of this, the restoration of the physiological endocrine balance seems to play a key role. The status of hypothalamic-pituitary-gonado-adrenal axis with a potential collapse in hormone concentration in patients with NP is now assessed in clinical trials. Additionally, a study evaluating analgesic efficacy of intrathecal oxytocin (100 micrograms) in patients with NP is currently recruiting patients [82].
2.9. Peroxisome Proliferator-activated Receptors and Cytokines/chemokine Receptors
Cytokines and chemokines have immunomodulatory properties, being released in response to tissue injury, or inflammation [78]. In both humans and experimental animals, it has been demonstrated that NP might be associated with an increased level of inflammatory state-related proteins [83]. Analgesic therapies focused on the reduction of these molecules have been explored and pharmacological manipulations lowering levels of inflammatory cytokines have been demonstrated to reduce NP in both animal models and clinical studies. Several pro-inflammatory cytokines, including TNFα, IL-1, IL-6, and IL-17 were elevated in animal models of NP and tissues (e.g. blood, cerebrospinal fluid) of patients suffering from NP. A better understanding of the interaction between inflammation and neuropathic pain led to the development of potential novel therapies for NP conditions [52].
There is also evidence for a crucial role of various chemokines in the development and maintenance of NP [84], in particular CIPN [78] and diabetic NP [85]. Chemokines are co-expressed in neurons along with pronociceptive neurotransmitters and neuromodulators, and chemokine receptors are expressed in dorsal root ganglia, which also suggests their role in pain pathways [84]. Chemokines are divided into three different subfamilies – XC (XCL1), CC (CCL2 and CCL5) and CXC (CXCL1, CXCL5, CXCL9, and CXCL12). Recently, it has been shown that some chemokines play a key role in diabetic neuropathy. Zychowska et al. [86] studied the involvement of the chemokine CC motif ligand 1 (CCL1)-chemokine and CC motif receptor 8 (CCR8) interaction and the role of CCL1/CCR8 neuronal signaling in the development of diabetic neuropathy. Previously, it has been reported that intrathecal injection of recombinant CCL1 in naïve mice induced hypersensitivity and resulted in the activation of microglia to release pronociceptive cytokines, such as IL-1β and IL-6. Recent studies have also implicated CCL1/CCR8 in the development of remifentanil-induced hyperalgesia. It has been also postulated that chemokines such as CCL3, CCL2, CCL4, and CCL5 are also important for opioid analgesia. Hence, it was concluded that the spinal CCL1/CCR8 axis might be not only a potential target for the treatment of NP but also a novel therapeutic target for the development of drugs used in the treatment of opioid-induced hyperalgesia [86].
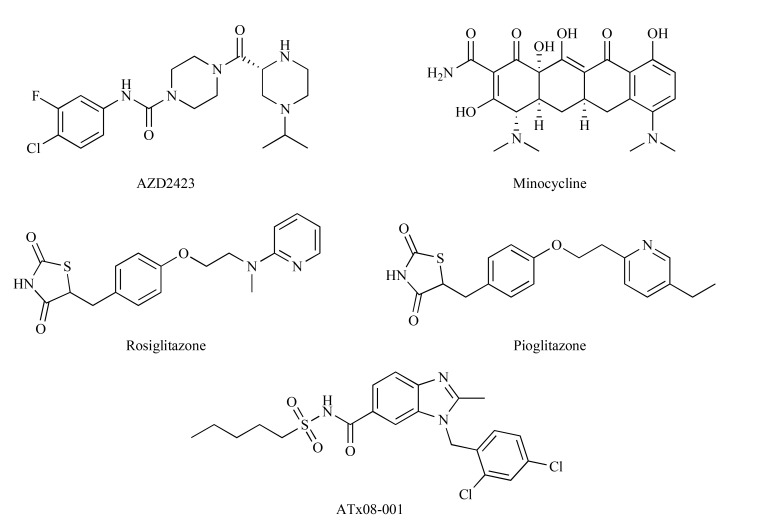
A number of cytokine and chemokine receptor antagonists have been tested for their potential use in NP [84]. AZD2423 (Fig. 11) is a potent and selective inhibitor of chemokine CCR2 receptors which are overexpressed in the spinal cord after nerve injury. This compound was efficacious in a rat model of NP. It reversed hyperalgesia due to CCI but two phase 2a clinical trials with AZD2423 (20 mg or 150 mg once daily for 28 days) in patients with post-traumatic or diabetic NP failed to show its efficacy against primary endpoints [84].
Fig. (11).
Structures of AZD2423, minocycline, rosiglitazone, pioglitazone and ATx08-001.
Minocycline (Fig. 11), the inhibitor of matrix metalloproteinase 3, an enzyme controlling the integrity of blood-brain barrier, myelin protein turnover and phenotypic remodeling of glia and neurons, is currently being investigated for prevention and treatment of CIPN and pain caused by nerve damage [50]. Minocycline prevented paclitaxel-evoked allodynia and was well-tolerated. Hence, it might be a potential agent for preventing paclitaxel-induced NP and improving patients' outcome [81].
The repeated administration of minocycline – a microglial inhibitor and a chemokine CCR2 receptor antagonist (RS504393) attenuated pain symptoms in neuropathic rats subjected to CCI of the sciatic nerve. This activity was associated with a decreased spinal microglia activation and the protein level of CCL2 and CCR2 [87].
In the spinal nerve-injured rats at chronic stages of NP, a role of not only spinal but also brain microglia/macrophages in the anti-inflammatory and analgesic actions of minocycline was demonstrated [88]. In the same model of NP in mice, it has been shown that minocycline alleviated NP through actin binding and this effect was due to its modulatory effect on long non-coding RNAs expression in the spinal cord of these neuropathic animals [89].
In microglia primary cell cultures minocycline was able to downregulate lipopolysaccharide-stimulated expression of both CCL2 and CCR2 proteins. In astroglia, primary cell cultures minocycline, reduced the expression of CCL2. These results showed that modulation of CCL2/CCR2 pathway by minocycline might be an efficacious approach to attenuate the development of NP [87].
The role of activated microglia in NP is well-established and relatively well documented by the beneficial effects of minocycline which attenuated allodynia, hyperalgesia, prevented the up-regulation of pro-inflammatory proteins and increased the levels of anti-inflammatory molecules in diabetic NP. Zychowska et al. [85] investigated the role of chemokine-C-motif ligand (XCL) subfamily in diabetic neuropathy. They demonstrated that the XCL1/XCR1 signaling pathway plays an important role in diabetic neuropathy and the prevention of microglial activation by the use of minocycline strongly affects XCL1/XCR1 levels. These authors showed that minocycline not only caused analgesia in a mouse model of diabetic NP but it also prevented microglial activation and influenced immune factors. Minocycline inhibited the up-regulation of XCL1 and XCR1. Its administration in diabetic NP models increased the levels of antinociceptive interleukins (IL-1α, -2, and -10) and diminished the up-regulation of TNFα, IL-1β, and inducible nitric oxide synthase (iNOS) [85, 90]. These data are in line with clinical findings showing that minocycline is efficacious in diabetic neuropathy, being safe and well tolerated. It has high analgesic potential and seems to be an efficient analgesic agent for the treatment of diabetic NP [85, 91, 92] and possibly for its prevention [90].
In CCI-exposed rats minocycline was also able to potentiate antihyperalgesic and antiallodynic effects of ceftriaxone against thermal: heat [93], cold and mechanical stimuli [94]. Both drugs diminished the levels of TNFα, IL-1β [94].
Available literature suggests that there is a link between chemokines in NP and the activation of peroxisome proliferator-activated receptors (PPARs). PPARs are a family of nuclear receptors which act as lipid-activated transcription factors. This family consists of three different isoforms: PPARα, PPARβ/δ, and PPARγ [84]. It has been demonstrated that ligands that bind PPARs inhibit the expression of inflammatory genes in a process called transrepression which is mediated by targeting specific transcription factors, including NF-κB, AP-1 and STAT [84]. Interestingly, experimental evidence suggests a connection between pain-relieving effects of PPAR agonists and suppression of inflammatory gene expression. Among genes that undergo PPAR-induced transrepression there are those of chemokines linked to NP (MCP-1, RANTES, MIP-1α, fractalkine, SDF-1) and their receptors [84]. In cultured monocytes, PPARγ agonists were also able to decrease levels of pro-inflammatory cytokines, such as: TNFα, IL-6, and IL-1β [95].
The PPARɣ subtype is expressed both in neurons and glial cells and its stimulation is correlated to the protection of neuronal tissue under oxidative stress conditions. Rosiglitazone – a PPARɣ agonist improved catalase activity in the nervous tissue of oxaliplatin-treated rats and prevented oxidative stress in the spinal cord. The restoration of proper redox balance contributed to analgesia in oxaliplatin-treated rats tested in the cold plate test. This effect was observed at a low dose (3 mg/kg) but not at a high dose (10 mg kg), so it was concluded that a mild PPARɣ stimulation is necessary for the attenuation of NP [96].
Results of clinical trials suggest that PPAR agonists can effectively alleviate NP, even if it is resistant to other therapies. The mechanisms underlying this activity are complex. Data from animal studies suggest that the analgesic effect of PPARα agonists (fibrates) and PPARγ agonists (rosiglitazone, pioglitazone) (Fig. 11) results from their action at targets in the pain pathways via PPAR-dependent mechanisms, their influence on gene transcription and alteration in the expression of pro-inflammatory mediators, including chemokines and their receptors [84]. Recently, positive results for a novel PPAR agonist, ATx08-001 (Fig. 11), have been shown in patients with PHN. ATx08-001 is an orally available, selective PPARγ modulator with a good safety profile. ATx08-001 is being developed as a first-in-class treatment for NP, in particular, pain associated with PHN. ATx08-001 is chemically and functionally distinct from other PPARγ agonists, such as thiazolidinediones, displaying a distinct target gene activation profile and a higher selectivity for PPARγ as compared to rosiglitazone and pioglitazone [97].
2.10. Reactive Oxygen Species
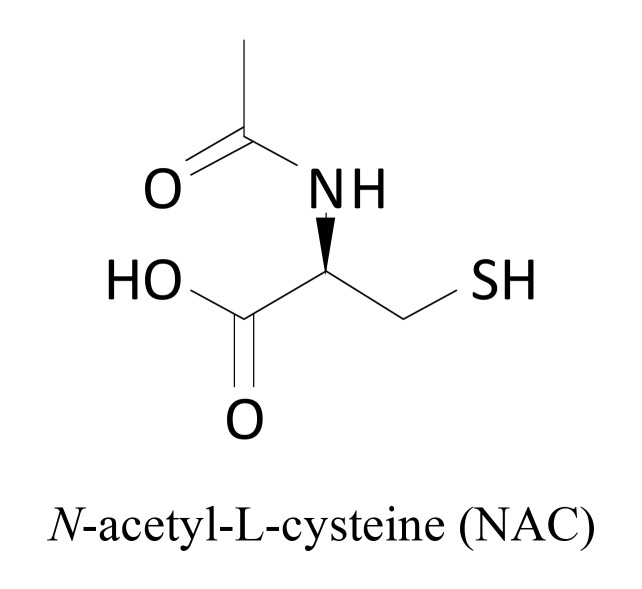
Oxidative stress has been implicated in the pathophysiology of NP and there is considerable evidence that superoxide and peroxynitrite play a key role in the development of chronic pain, central sensitization, the transition of acute to chronic pain, opiate-induced hyperalgesia and antinociceptive tolerance [98, 99]. The increased level of superoxide in the dorsal horn neurons was observed in NP conditions [100], whereas heat hyperalgesia in neuropathic rats was reduced by systemic administration of antioxidants [98]. N-acetyl-L-cysteine (NAC) (Fig. 12) is a mucolytic agent used in the diseases of the respiratory system and as a treatment for paracetamol poisoning where it acts to maintain glutathione levels in the liver. In CCI rats, NAC reduced mechanical and heat hyperalgesia, as well as cold allodynia [98]. Recent preclinical studies revealed that its antihyperalgesic properties in CCI rats were due to its inhibitory effect on iNOS gene expression, which significantly reduced nitric oxide level. Moreover, NAC suppressed c-Jun N-terminal kinase and p38 proteins which are strongly involved in the central sensitization of pain [101]. A single systemic injection of this drug causes analgesia in NP conditions. This effect is due to the reinforcement of mGlu2 receptor activation [102].
Fig. (12).
Structure of N-acetylcysteine.
2.11. p38 Mitogen-activated Protein Kinase
p38 MAPK phosphorylates intracellular proteins, including signal transduction molecules and transcription factors that are involved in the regulation of synthesis of pro-inflammatory cytokines (IL-1, TNFα) [103]. p38 MAPK has two major isoforms, i.e., α and β, which are expressed in the adult spinal cord, each of them having a distinct role in pain processing [104]. Results from in vivo and in vitro studies show that p38 MAPK inhibitors decrease cytokine production thereby leading to the attenuation of inflammatory pain and NP [103, 104]. p38 MAPK inhibitors reduce heat hyperalgesia and mechanical allodynia in CCI and diabetic rats [103].
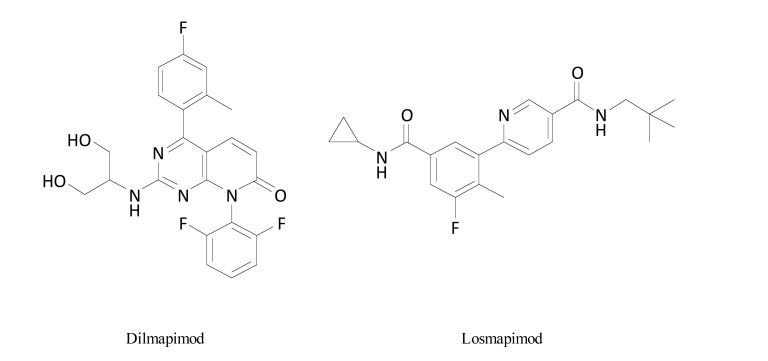
Dilmapimod (SB-681323) (Fig. 13) is a potent, selective, reversible inhibitor of p38α MAPK which inhibits cytokine production. Results from a placebo-controlled clinical trial aiming to assess its efficacy after oral 14-day administration in NP showed that dilmapimod significantly reduced average daily pain intensity and was well tolerated in patients with NP following nerve injury with a maximum permissible clinical dosing period of 28 days, which finally precluded further development of this compound in chronic pain conditions [103-105].
Fig. (13).
Structures of dilmapimod and losmapimod.
Despite its previously established efficacy equal to gabapentin in the attenuation of mechanical allodynia after oral dosing in CCI rats, losmapimod (GW856553) (Fig. 13), a p38αβ MAPK inhibitor (given 7.5 mg twice a day, 28 days) was tested in a double-blind, placebo-controlled study in patients with NP following traumatic peripheral nerve injury. In these patients, it failed to show clinically relevant analgesic properties [104].
Cetuximab, the inhibitor of epidermal growth factor receptor and MAPK-signaling inhibitor was shown to alleviate NP in cancer patients. There is also some evidence from
2.12. 3-Hydroxy-3-methyl Glutaryl Coenzyme A Reductase
Statins, the inhibitors of 3-hydroxy-3-methyl glutaryl coenzyme A reductase, are widely used for the treatment of hypercholesterolemia. Because of their cholesterol-independent pleiotropic effects, such as anti-inflammatory, antioxidant, immunomodulatory and neuroprotective activities, they have also been tested in non-cardiovascular disorders, including NP. Data from preclinical studies indicate that atorvastatin reversed the decrease in mechanical and thermal nociceptive threshold in CCI model in rats and this effect was due to its antioxidant [15, 107] and anti-inflammatory [108] properties. It inhibited cytokines, matrix metalloproteases and nerve growth factor in the sciatic nerve and in the spinal cord, which altogether suggested that it could be a treatment option for NP [108].
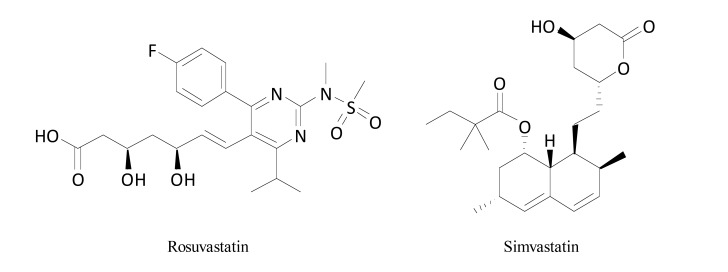
Rosuvastatin and simvastatin (Fig. 14) attenuated symptoms of painful diabetic neuropathy in streptozotocin-treated diabetic animals and ischemia-reperfusion-induced nerve injury models [15]. In the cold plate test in oxaliplatin-treated neuropathic mice cold allodynia caused by oxaliplatin was not influenced by a single dose of simvastatin, but in contrast to this, a 7-day treatment with this drug partially reversed allodynia induced by cold. It indicated that the repeated administration of simvastatin elevated pain threshold in animals with CIPN symptoms [109].
Fig. (14).
Structures of rosuvastatin and simvastatin.
Similarly, data from clinical studies indicate that statins may protect against neuropathic stump pain, phantom limb pain [107] and peripheral sensory neuropathy in type 2 diabetes [15]. This effect is not related to lipid-lowering action of statins and it may comprise distinct mechanisms, such as: inhibition of iNOS and increase in eNOS expression, decreased translocation of RhoA (a small molecular GTPase) from the cytoplasm to plasma membrane, reduced microglial and astrocyte activation and a marked reduction in IL-1β secretion in human peripheral blood mononuclear cells [108].
CONCLUSION
Most analgesic drugs used in NP not only offer limited efficacy but they also cause dose-limiting side effects, which negatively influences patients’ quality of life. Hence, it is evident that the identification of novel drug targets for analgesic drugs used in NP treatment, as well as using reliable animal models for the prediction of the efficacy of analgesic compounds in humans is of special relevance (Table 1). To achieve this, at the preclinical stage of analgesic drug development process, rodents are widely used. However, as in other areas of experimental pharmacology, one may face translation problems from animals to humans. This issue has been shown by numerous studies in which animal models of NP were poorly predictive of clinical efficacy of analgesic drugs in neuropathic patients (Table 1). Because of this, many promising drug candidates for NP selected in the preclinical phase failed to be effective in clinical trials. It should be however emphasized that this did not preclude their use in the drug discovery process as these agents could remain valuable tools for experimental studies on the role of certain biomolecules (e.g., ion channels, receptors or enzymes) in NP etiology.
Table 1.
Experimental drugs for the treatment of neuropathic pain - a summary.
|
Pharmacological Class
(Example) |
Mechanism of Action in NP | Preclinical Efficacy (Animal Models) | Clinical Efficacy |
|---|---|---|---|
| β2-adrenomimetics (terbutaline, salbutamol) |
↓TNFα | Traumatic nerve injury-related NP; diabetic NP |
Post-thoracotomy NP (salbutamol); post-thoracoscopy NP (terbutaline) |
| Angiotensin AT2 receptor antagonists (EMA-401) | ↓ p38 MAPK; ↓ ERK | Surgically-induced NP | PHN; diabetic NP (withdrawn) |
| H3 receptor inverse agonists (GSK189254, GSK334429) |
↓ central sensitization; ↓ spontaneous neuronal firing |
Surgically- and virally-induced NP models; Spinal nerve ligation NP model |
No data available |
| Acetylcholinesterase inhibitors (donepezil) |
↑ acetylcholine – M2 receptor stimulation |
CIPN; spared nerve injury model |
Diabetic NP; patients with chronic neuropathic pain who are currently taking gabapentin or pregabalin (phase 4) |
| Neurotoxins (botulinum toxin A) |
SNAP-25 degradation; ↓ glutamate; ↓CGRP; ↓ TRPV1 expression; ↓ IL-1β, ↓ IL-18; ↑ IL-10, ↑ IL-1RA; ↓central sensitization sodium channel inactivation |
Diabetic NP; Surgically-induced NP |
Established efficacy in PHN; probable efficacy in trigeminal neuralgia and post-traumatic neuralgia; possible efficacy in diabetic polyneuropathy; insufficient evidence in CRPS, phantom limb and stump pain, and occipital neuralgia |
| Microglial activation inhibitors (minocycline) |
Microglia inhibition; matrix metalloproteinase 3 inhibitor; ↓ chemokines; ↓ TNFα; ↓ IL-1β; ↓iNOS; ↑ IL-1α; ↑ IL-2; ↑ IL-10 |
CIPN; diabetic NP; traumatic nerve injury-related NP |
Prevention of postoperative intercostal neuralgia |
| PPARγ agonists (rosiglitazone, ATx08-001) |
↓ IL-1β, ↓ IL-6; ↓ TNFα; ↓chemokines |
CIPN | PHN |
| Kinase inhibitors (dilmapimod, losmapimod) |
↓ p38 MAPK | NP following traumatic peripheral nerve injury | No longer in development due to low efficacy |
| T-type calcium channel antagonists (ethosuximide, ABT-639) |
T-type calcium channel antagonism | NP following traumatic peripheral nerve injury (ethosuximide, ABT-639); spinal nerve ligation NP model; CIPN (ABT-639) |
Diabetic NP – ABT-639 (no longer in development) |
| Sodium channel antagonists (ambroxol) |
Nav1.8 sodium channel antagonism | CIPN; Spinal cord injury-related NP |
PHN; Post-operative neuralgia; phantom pain; foot neuropathy; deafferentation pain |
| Melatonin MT1/MT2 receptor agonists (ramelteon, melatonin, piromelatine) |
Neuritogenesis stimulation; neuroprotection; ↓ nitro-oxidative stress; induction of autophagy; mitochondrial protection |
CIPN | Ramelteon: terminated (recruitment did not accrue as expected) |
| TRPA1 antagonists (ADM_09; HC-030031 |
↓ oxidative stress; ↓ p38 MAPK |
CIPN; diabetic NP |
No data available |
| 3-Hydroxy-3-methyl glutaryl coenzyme A reductase inhibitors = statins (simvastatin, rosuvastatin) |
↓ oxidative stress; iNOS inhibition; decreased translocation of RhoA; reduced microglial and astrocyte activation; ↓IL-1β secretion; immunomodulation; neuroprotection |
CIPN; CRPS; surgically-induced NP |
Diabetic NP; phantom limb pain |
clinical trials that it prevents the development of oxaliplatin-induced neuropathy, but further detailed studies are required to confirm these preliminary observations [106].
Patients’ needs for a more effective pain relief without unwanted side effects are currently being developed by the introduction of new targets for analgesic drugs, or by the search for new analgesic agents with a known mechanism of action. Also, the application of genetic approaches and biochemical assays leading to deeper understanding of molecular mechanisms of NP enables the progress in NP treatment.
In this context, novel experimental drugs can be considered a treatment option which should be tailor-made for each individual on the basis of pain features, previous therapies, associated clinical conditions, recurrence of pain, adverse effects, contraindications and patients’ preferences. Furthermore, physicians wishing to treat patients with these drugs in an off-label fashion should have experience using them for other conditions. Noteworthy, one of the undoubted benefits of using well-known drugs as experimental drugs for new therapeutic indications (here in the treatment of NP) is the fact that these drugs have been thoroughly studied in humans, in terms of their tolerability, drug-drug interactions and potential toxic effects.
Importantly, it should be emphasized that there is a strong medical demand to discover drugs that act causally, i.e., by the elimination of mechanisms that trigger NP. As shown in Table 1 experimental therapies for NP have the ability to interfere with pathomechanisms responsible for the development of NP. Such an approach would provide not only pain relief, but it could also cease the progress of neuropathy.
Moreover, the use of experimental drugs for NP treatment potentially allows multidirectional approach to neuropathic patients using a single drug to treat symptoms of NP and other co-morbidities. This is of particular importance in the case of many lifestyle diseases, such as asthma, diabetes, cardiovascular diseases.
At present, among numerous drug classes tested as experimental therapies for NP, there are only some which may have potential as novel treatments. Increasing knowledge about mechanisms underlying NP, mechanisms of drug action, as well as available efficacy and safety data from preclinical and clinical studies, make some of the abovementioned agents promising potential future treatment options. In that context, botulinum toxin A, minocycline, ambroxol, statins and PPAR agonists (ATx086001) are particularly interesting. Of note, NAC, statins and β2-agonists may also offer an alternative to currently available analgesic drugs for the treatment of NP, as they may be used in patients suffering not only from chronic pain but also other serious comorbidities. Moreover, their safety profile is well established, which might be of great importance for some groups of patients.
ACKNOWLEDGEMENTS
Declared none.
LIST OF ABBREVIATIONS
- AChEI
Acetylcholinesterase inhibitors
- Ang II
Angiotensin II
- Cav
Voltage-gated calcium channels
- CCI
Chronic constriction injury
- CGRP
Calcitonin gene-related peptide
- CIPN
Chemotherapy-induced peripheral neuropathy
- CRPS
Complex regional pain syndrome
- IL
Interleukin
- MAPK
Mitogen-activated protein kinase
- NAC
N-acetyl-L-cysteine
- Nav
Voltage-gated sodium channels
- eNOS
Endothelial (constitutive) nitric oxide synthase
- iNOS
Inducible nitric oxide synthase
- NP
Neuropathic pain
- PHN
Postherpetic neuralgia
- PPARs
Peroxisome proliferator-activated receptors
- RAS
Renin-angiotensin system
- SNAP-25
Synaptosomal-associated protein 25
- SNARE
Soluble N-ethylmaleimide-sensitive factor attachment protein receptor
- SP
Substance P
- TNFα
Tumor necrosis factor α
- TRPA1
Transient receptor potential ankyrin 1
FUNDING SOURCE
The study was supported by the National Science Centre, Poland grants No. 2014/15/B/NZ7/00930 and UMO-2015/17/B/NZ7/02937.
CONSENT FOR PUBLICATION
Not applicable.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
REFERENCES
- 1.Treede R-D., Jensen T.S., Campbell J.N., Cruccu G., Dostrovsky J.O., Griffin J.W., Hansson P., Hughes R., Nurmikko T., Serra J. Neuropathic pain: Redefinition and a grading system for clinical and research purposes. Neurology. 2008;70(18):1630–1635. doi: 10.1212/01.wnl.0000282763.29778.59. [http://dx.doi.org/10.1212/01.wnl.0000282763.29778.59]. [PMID: 18003941]. [DOI] [PubMed] [Google Scholar]
- 2.Finnerup N.B., Attal N., Haroutounian S., McNicol E., Baron R., Dworkin R.H., Gilron I., Haanpää M., Hansson P., Jensen T.S., Kamerman P.R., Lund K., Moore A., Raja S.N., Rice A.S., Rowbotham M., Sena E., Siddall P., Smith B.H., Wallace M. Pharmacotherapy for neuropathic pain in adults: A systematic review and meta-analysis. Lancet Neurol. 2015;14(2):162–173. doi: 10.1016/S1474-4422(14)70251-0. [http://dx.doi.org/10.1016/S1474-4422(14)70251-0]. [PMID: 25575710]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Percie du Sert N., Rice A.S.C. Improving the translation of analgesic drugs to the clinic: animal models of neuropathic pain. Br. J. Pharmacol. 2014;171(12):2951–2963. doi: 10.1111/bph.12645. [http://dx.doi.org/10.1111/ bph.12645]. [PMID: 24527763]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brookes M.E., Eldabe S., Batterham A. Ziconotide monotherapy: A systematic review of randomised controlled trials. Curr. Neuropharmacol. 2017;15(2):217–231. doi: 10.2174/1570159X14666160210142056. [http://dx.doi.org/10. 2174/1570159X14666160210142056]. [PMID: 26861472]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kerstman E., Ahn S., Battu S., Tariq S., Grabois M. Neuropathic pain. Handb. Clin. Neurol. 2013;110:175–187. doi: 10.1016/B978-0-444-52901-5.00015-0. [http://dx. doi.org/10.1016/B978-0-444-52901-5.00015-0]. [PMID: 23312640]. [DOI] [PubMed] [Google Scholar]
- 6.Gilron I., Coderre T.J. Emerging drugs in neuropathic pain. Expert Opin. Emerg. Drugs. 2007;12(1):113–126. doi: 10.1517/14728214.12.1.113. [http://dx.doi.org/ 10.1517/14728214.12.1.113]. [PMID: 17355217]. [DOI] [PubMed] [Google Scholar]
- 7.Gilron I., Dickenson A.H. Emerging drugs for neuropathic pain. Expert Opin. Emerg. Drugs. 2014;19(3):329–341. doi: 10.1517/14728214.2014.915025. [http://dx.doi. org/10.1517/14728214.2014.915025]. [PMID: 24793304]. [DOI] [PubMed] [Google Scholar]
- 8.Jensen T.S., Finnerup N.B. Allodynia and hyperalgesia in neuropathic pain: clinical manifestations and mechanisms. Lancet Neurol. 2014;13(9):924–935. doi: 10.1016/S1474-4422(14)70102-4. [http://dx.doi.org/10.1016/S1474-4422(14)70102-4]. [PMID: 25142459]. [DOI] [PubMed] [Google Scholar]
- 9.Taneja A., Di Iorio V.L., Danhof M., Della Pasqua O. Translation of drug effects from experimental models of neuropathic pain and analgesia to humans. Drug Discov. Today. 2012;17(15-16):837–849. doi: 10.1016/j.drudis.2012.02.010. [http://dx.doi.org/10.1016/j.drudis.2012.02.010]. [PMID: 22445930]. [DOI] [PubMed] [Google Scholar]
- 10.Areti A., Yerra V.G., Komirishetty P., Kumar A. Potential Therapeutic Benefits of Maintaining Mitochondrial Health in Peripheral Neuropathies. Curr. Neuropharmacol. 2016;14(6):593–609. doi: 10.2174/1570159X14666151126215358. [http://dx.doi.org/10.2174/1570159X14666151126215358]. [PMID: 26818748]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ewertz M., Qvortrup C., Eckhoff L. Chemotherapy-induced peripheral neuropathy in patients treated with taxanes and platinum derivatives. Acta Oncol. 2015;54(5):587–591. doi: 10.3109/0284186X.2014.995775. [http://dx.doi.org/ 10.3109/0284186X.2014.995775]. [PMID: 25751757]. [DOI] [PubMed] [Google Scholar]
- 12.Staff N.P., Grisold A., Grisold W., Windebank A.J. Chemotherapy-induced peripheral neuropathy: A current review. Ann. Neurol. 2017;81(6):772–781. doi: 10.1002/ana.24951. [http://dx.doi.org/10.1002/ana.24951]. [PMID: 28486769]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Meacham K., Shepherd A., Mohapatra D.P., Haroutounian S. Neuropathic pain: Central vs. peripheral mechanisms. Curr. Pain Headache Rep. 2017;21(6):28. doi: 10.1007/s11916-017-0629-5. [http://dx.doi.org/10.1007/s11916-017-0629-5]. [PMID: 28432601]. [DOI] [PubMed] [Google Scholar]
- 14.Nickel F.T., Seifert F., Lanz S., Maihöfner C. Mechanisms of neuropathic pain. Eur. Neuropsychopharmacol. 2012;22(2):81–91. doi: 10.1016/j.euroneuro.2011.05.005. [http://dx.doi.org/10.1016/j.euroneuro.2011.05.005]. [PMID: 21672666]. [DOI] [PubMed] [Google Scholar]
- 15.Bhalla S., Singh N., Jaggi A.S. Statins: do they aggravate or ameliorate neuropathic pain? J. Pain. 2014;15(11):1069–1080. doi: 10.1016/j.jpain.2014.06.012. [http://dx.doi.org/10.1016/j.jpain.2014.06.012]. [PMID: 25086324]. [DOI] [PubMed] [Google Scholar]
- 16.Yan Y.Y., Li C.Y., Zhou L., Ao L.Y., Fang W.R., Li Y.M. Research progress of mechanisms and drug therapy for neuropathic pain. Life Sci. 2017;190:68–77. doi: 10.1016/j.lfs.2017.09.033. [http://dx.doi.org/10.1016/j.lfs. 2017.09.033]. [PMID: 28964813]. [DOI] [PubMed] [Google Scholar]
- 17.Tibbs G.R., Posson D.J., Goldstein P.A. Voltage-Gated ion channels in the PNS: Novel therapies for neuropathic pain? Trends Pharmacol. Sci. 2016;37(7):522–542. doi: 10.1016/j.tips.2016.05.002. [http://dx.doi.org/10.1016/ j.tips.2016.05.002]. [PMID: 27233519]. [DOI] [PubMed] [Google Scholar]
- 18.Tsuda M., Inoue K., Salter M.W. Neuropathic pain and spinal microglia: a big problem from molecules in “small” glia. Trends Neurosci. 2005;28(2):101–107. doi: 10.1016/j.tins.2004.12.002. [http://dx.doi.org/10.1016/j.tins. 2004.12.002]. [PMID: 15667933]. [DOI] [PubMed] [Google Scholar]
- 19.Starowicz K., Finn D.P. Cannabinoids and Pain: Sites and Mechanisms of Action. Adv. Pharmacol. 2017;80:437–475. doi: 10.1016/bs.apha.2017.05.003. [http:// dx.doi.org/10.1016/bs.apha.2017.05.003]. [PMID: 28826543]. [DOI] [PubMed] [Google Scholar]
- 20.Fijałkowski Ł., Sałat K., Podkowa A., Zaręba P., Nowaczyk A. Potential role of selected antiepileptics used in neuropathic pain as human GABA transporter isoform 1 (GAT1) inhibitors-Molecular docking and pharmacodynamic studies. Eur. J. Pharm. Sci. 2017;96:362–372. doi: 10.1016/j.ejps.2016.10.004. [http://dx.doi.org/10.1016/j.ejps.2016.10.004]. [PMID: 27721044]. [DOI] [PubMed] [Google Scholar]
- 21.Yadav R., Yan X., Maixner D.W., Gao M., Weng H-R. Blocking the GABA transporter GAT-1 ameliorates spinal GABAergic disinhibition and neuropathic pain induced by paclitaxel. J. Neurochem. 2015;133(6):857–869. doi: 10.1111/jnc.13103. [http://dx.doi.org/10.1111/jnc.13103]. [PMID: 25827582]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Prescott S.A. Synaptic inhibition and disinhibition in the spinal dorsal horn. Prog. Mol. Biol. Transl. Sci. 2015;131:359–383. doi: 10.1016/bs.pmbts.2014.11.008. [http://dx.doi.org/10.1016/bs.pmbts.2014.11.008]. [PMID: 25744679]. [DOI] [PubMed] [Google Scholar]
- 23.Cok O.Y., Eker H.E., Yalcin I., Barrot M., Aribogan A. Is there a place for β-mimetics in clinical management of neuropathic pain? Salbutamol therapy in six cases. Anesthesiology. 2010;112(5):1276–1279. doi: 10.1097/ALN.0b013e3181d40399. [http://dx.doi.org/10.1097/ALN.0b013e3181d40399]. [PMID: 20395826]. [DOI] [PubMed] [Google Scholar]
- 24.Salvat E., Schweitzer B., Massard G., Meyer N., de Blay F., Muller A., Barrot M. Effects of β2 agonists on post-thoracotomy pain incidence. Eur. J. Pain. 2015;19(10):1428–1436. doi: 10.1002/ejp.673. [http://dx. doi.org/10.1002/ejp.673]. [PMID: 25766791]. [DOI] [PubMed] [Google Scholar]
- 25.Barrot M., Yalcin I., Choucair-Jaafar N., Benbouzid M., Freund-Mercier M-J. From antidepressant drugs to beta-mimetics: preclinical insights on potential new treatments for neuropathic pain. Recent Patents CNS Drug Discov. 2009;4(3):182–189. doi: 10.2174/157488909789104794. [http://dx. doi.org/10.2174/157488909789104794]. [PMID: 19538157]. [DOI] [PubMed] [Google Scholar]
- 26.Bohren Y., Tessier L-H., Megat S., Petitjean H., Hugel S., Daniel D., Kremer M., Fournel S., Hein L., Schlichter R., Freund-Mercier M.J., Yalcin I., Barrot M. Antidepressants suppress neuropathic pain by a peripheral β2-adrenoceptor mediated anti-TNFα mechanism. Neurobiol. Dis. 2013;60:39–50. doi: 10.1016/j.nbd.2013.08.012. [http://dx.doi.org/ 10.1016/j.nbd.2013.08.012]. [PMID: 23978467]. [DOI] [PubMed] [Google Scholar]
- 27.Yalcin I., Choucair-Jaafar N., Benbouzid M., Tessier L-H., Muller A., Hein L., Freund-Mercier M-J., Barrot M. β(2)-adrenoceptors are critical for antidepressant treatment of neuropathic pain. Ann. Neurol. 2009;65(2):218–225. doi: 10.1002/ana.21542. [http://dx.doi.org/ 10.1002/ana.21542]. [PMID: 19259968]. [DOI] [PubMed] [Google Scholar]
- 28.Choucair-Jaafar N., Salvat E., Freund-Mercier M-J., Barrot M. The antiallodynic action of nortriptyline and terbutaline is mediated by β(2) adrenoceptors and δ opioid receptors in the ob/ob model of diabetic polyneuropathy. Brain Res. 2014;1546:18–26. doi: 10.1016/j.brainres.2013.12.016. [http:// dx.doi.org/10.1016/j.brainres.2013.12.016]. [PMID: 24361988]. [DOI] [PubMed] [Google Scholar]
- 29.Choucair-Jaafar N., Beetz N., Gilsbach R., Yalcin I., Waltisperger E., Freund-Mercier M-J., Monassier L., Hein L., Barrot M. Cardiovascular effects of chronic treatment with a β2-adrenoceptor agonist relieving neuropathic pain in mice. Neuropharmacology. 2011;61(1-2):51–60. doi: 10.1016/j.neuropharm.2011.02.015. [http://dx.doi.org/10.1016/ j.neuropharm.2011.02.015]. [PMID: 21352833]. [DOI] [PubMed] [Google Scholar]
- 30.University Hospital 2012 https://clinicaltrials. gov/ct2/show/NCT01582646?term=Betapain&rank=1%20NLM
- 31.Campbell J.N., Meyer R.A. Mechanisms of neuropathic pain. Neuron. 2006;52(1):77–92. doi: 10.1016/j.neuron.2006.09.021. [http://dx.doi.org/10.1016/j.neuron. 2006.09.021]. [PMID: 17015228]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lötsch J., Geisslinger G. Pharmacogenetics of new analgesics. Br. J. Pharmacol. 2011;163(3):447–460. doi: 10.1111/j.1476-5381.2010.01074.x. [http://dx.doi.org/10.1111/ j.1476-5381.2010.01074.x]. [PMID: 20942817]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Haven P.I.S.N. ClinicalTrials.gov. Bethesda, MD: National Library of Medicine (US); 2015. United States, Evaluation of the efficacy and safety of single doses of PF-05089771 in patients with primary inherited erythromelalgia IEM.https://clinicaltrials. gov/ct2/show/NCT01769274?term=NCT01769274&rank=1 Internet. [cited 2016 Mar 13] [Google Scholar]
- 34.Goldberg Y.P., Price N., Namdari R., Cohen C.J., Lamers M.H., Winters C., Price J., Young C.E., Verschoof H., Sherrington R., Pimstone S.N., Hayden M.R. Treatment of Na(v)1.7-mediated pain in inherited erythromelalgia using a novel sodium channel blocker. Pain. 2012;153(1):80–85. doi: 10.1016/j.pain.2011.09.008. [http://dx.doi.org/10.1016/ j.pain.2011.09.008]. [PMID: 22035805]. [DOI] [PubMed] [Google Scholar]
- 35.Corrigan H.N. Calcium channel bloker treats Chronic Pain. Drug Discov. Dev; 2012. [Google Scholar]
- 36.Smith M.T., Wyse B.D., Edwards S.R. Small molecule angiotensin II type 2 receptor (AT2R) antagonists as novel analgesics for neuropathic pain: comparative pharmacokinetics, radioligand binding, and efficacy in rats. Pain Med. 2013;14(5):692–705. doi: 10.1111/pme.12063. [http:// dx.doi.org/10.1111/pme.12063]. [PMID: 23489258]. [DOI] [PubMed] [Google Scholar]
- 37.Smith M.T., Anand P., Rice A.S.C. Selective small molecule angiotensin II type 2 receptor antagonists for neuropathic pain: preclinical and clinical studies. Pain. 2016;157(Suppl. 1):S33–S41. doi: 10.1097/j.pain.0000000000000369. [http://dx.doi.org/10.1097/j.pain.0000000000000369]. [PMID: 26785154]. [DOI] [PubMed] [Google Scholar]
- 38.Sałat K., Kowalczyk P., Gryzło B., Jakubowska A., Kulig K. New investigational drugs for the treatment of neuropathic pain. Expert Opin. Investig. Drugs. 2014;23(8):1093–1104. doi: 10.1517/13543784.2014.916688. [http:// dx.doi.org/10.1517/13543784.2014.916688]. [PMID: 24896842]. [DOI] [PubMed] [Google Scholar]
- 39.Nemoto W., Nakagawasai O., Yaoita F., Kanno S., Yomogida S., Ishikawa M., Tadano T., Tan-No K., Angiotensin I.I. Angiotensin II produces nociceptive behavior through spinal AT1 receptor-mediated p38 mitogen-activated protein kinase activation in mice. Mol. Pain. 2013;9:38. doi: 10.1186/1744-8069-9-38. [http://dx.doi.org/10.1186/1744-8069-9-38]. [PMID: 23898828]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pavel J., Tang H., Brimijoin S., Moughamian A., Nishioku T., Benicky J., Saavedra J.M. 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Keppel Hesselink J.M., Schatman M.E. EMA401: an old antagonist of the AT2R for a new indication in neuropathic pain. J. Pain Res. 2017;10:439–443. doi: 10.2147/JPR.S128520. [http://dx.doi.org/10.2147/JPR.S128520]. [PMID: 28255254]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hsieh G.C., Honore P., Pai M., Wensink E.J., Chandran P., Salyers A.K., Wetter J.M., Zhao C., Liu H., Decker M.W., Esbenshade T.A., Cowart M.D., Brioni J.D. Antinociceptive effects of histamine H3 receptor antagonist in the preclinical models of pain in rats and the involvement of central noradrenergic systems. Brain Res. 2010;1354:74–84. doi: 10.1016/j.brainres.2010.07.083. [http://dx.doi.org/10.1016/j. brainres.2010.07.083]. [PMID: 20682302]. [DOI] [PubMed] [Google Scholar]
- 43.McGaraughty S., Chu K.L., Cowart M.D., Brioni J.D. Antagonism of supraspinal histamine H3 receptors modulates spinal neuronal activity in neuropathic rats. J. Pharmacol. Exp. Ther. 2012;343(1):13–20. doi: 10.1124/jpet.112.194761. [http://dx.doi.org/10.1124/jpet.112.194761]. [PMID: 22729221]. [DOI] [PubMed] [Google Scholar]
- 44.Zhang D.D., Sisignano M., Schuh C.D., Sander K., Stark H., Scholich K. Overdose of the histamine H3 inverse agonist pitolisant increases thermal pain thresholds. Inflamm. Res. 2012;61(11):1283–1291. doi: 10.1007/s00011-012-0528-5. [http://dx.doi.org/10.1007/s00011-012-0528-5]. [PMID: 22820944]. [DOI] [PubMed] [Google Scholar]
- 45.Medhurst S.J., Collins S.D., Billinton A., Bingham S., Dalziel R.G., Brass A., Roberts J.C., Medhurst A.D., Chessell I.P. Novel histamine H3 receptor antagonists GSK189254 and GSK334429 are efficacious in surgically-induced and virally-induced rat models of neuropathic pain. Pain. 2008;138(1):61–69. doi: 10.1016/j.pain.2007.11.006. [http://dx.doi.org/ 10.1016/j.pain.2007.11.006]. [PMID: 18164820]. [DOI] [PubMed] [Google Scholar]
- 46.Sander K., Kottke T., Stark H. Histamine H3 receptor antagonists go to clinics. Biol. Pharm. Bull. 2008;31(12):2163–2181. doi: 10.1248/bpb.31.2163. [http:// dx.doi.org/10.1248/bpb.31.2163]. [PMID: 19043195]. [DOI] [PubMed] [Google Scholar]
- 47.Basnet A., Butler S., Honoré P.H., Butler M., Gordh T.E., Kristensen K., Bjerrum O.J. Donepezil provides positive effects to patients treated with gabapentin for neuropathic pain: an exploratory study. Acta Anaesthesiol. Scand. 2014;58(1):61–73. doi: 10.1111/aas.12218. [http://dx.doi. org/10.1111/aas.12218]. [PMID: 24261316]. [DOI] [PubMed] [Google Scholar]
- 48.Wake Forest School of Medicine. National Institute of Neurological Disorders and Stroke (NINDS) Donepezil Compared to Placebo in Patients With Chronic Neuropathic Pain. 2012 https://clinicaltrials.gov/ct2/ show/NCT01743976?term=diabetic+neuropathy+donepezil&rank=2%20NLM
- 49.Committee opinion: Onabotulinumtoxin A and the bladder. Female Pelvic Med. Reconstr. Surg. 2014;20(5):245–247. doi: 10.1097/SPV.0000000000001047. [http://dx. doi.org/10.1097/SPV.0000000000000112]. [PMID: 25181372]. [DOI] [PubMed] [Google Scholar]
- 50.Zychowska M., Rojewska E., Makuch W., Luvisetto S., Pavone F., Marinelli S., Przewlocka B., Mika J. Participation of pro- and anti-nociceptive interleukins in botulinum toxin A-induced analgesia in a rat model of neuropathic pain. Eur. J. Pharmacol. 2016;791:377–388. doi: 10.1016/j.ejphar.2016.09.019. [http://dx.doi.org/10.1016/j.ejphar.2016.09.019]. [PMID: 27619001]. [DOI] [PubMed] [Google Scholar]
- 51.Park J., Park H.J. Botulinum toxin for the treatment of neuropathic pain. Toxins (Basel) 2017;9(9):260. doi: 10.3390/toxins9090260. [http://dx.doi.org/ 10.3390/toxins9090260]. [PMID: 28837075]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hung A.L., Lim M., Doshi T.L. Targeting cytokines for treatment of neuropathic pain. Scand. J. Pain. 2017;17(1):287–293. doi: 10.1016/j.sjpain.2017.08.002. [http://dx.doi.org/10.1016/j.sjpain.2017.08.002]. [PMID: 29229214]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Brown E.A., Schütz S.G., Simpson D.M. Botulinum toxin for neuropathic pain and spasticity: An overview. Pain Manag. 2014;4(2):129–151. doi: 10.2217/pmt.13.75. [http://dx.doi.org/10.2217/pmt.13.75]. [PMID: 24641437]. [DOI] [PubMed] [Google Scholar]
- 54.Zhang H., Lian Y., Ma Y., Chen Y., He C., Xie N., Wu C. Two doses of botulinum toxin type A for the treatment of trigeminal neuralgia: Observation of therapeutic effect from a randomized, double-blind, placebo-controlled trial. J. Headache Pain. 2014;15(1):65. doi: 10.1186/1129-2377-15-65. [http://dx.doi.org/10.1186/1129-2377-15-65]. [PMID: 25263254]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Morra M.E., Elgebaly A., Elmaraezy A., Khalil A.M., Altibi A.M.A., Vu T.L-H., Mostafa M.R., Huy N.T., Hirayama K. Therapeutic efficacy and safety of botulinum toxin a therapy in trigeminal neuralgia: A systematic review and meta-analysis of randomized controlled trials. J. Headache Pain. 2016;17(1):63. doi: 10.1186/s10194-016-0651-8. [http://dx.doi.org/10.1186/s10194-016-0651-8]. [PMID: 27377706]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wu C., Xie N., Liu H., Zhang H., Zhang L., Lian Y. A new target for the treatment of trigeminal neuralgia with botulinum toxin type A. Neurol. Sci. 2017 doi: 10.1007/s10072-017-3171-7. [PMID: 29086125]. [DOI] [PubMed] [Google Scholar]
- 57.Türk B. Ü.; Duman, A.; Bölük, C.; Coşkun Duman, S.; Taşdemir, M. Botulinum toxin in the treatment of trigeminal neuralgia: 6-Month follow-up. Medicine (Baltimore) 2017;96(39):e8133. doi: 10.1097/MD.0000000000008133. [http://dx.doi.org/10.1097/MD.0000000000008133]. [PMID: 28953646]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kowacs P.A., Utiumi M.A.T., Nascimento F.A., Piovesan E.J., Teive H.A.G. OnabotulinumtoxinA for trigeminal neuralgia: a review of the available data. Arq. Neuropsiquiatr. 2015;73(10):877–884. doi: 10.1590/0004-282X20150109. [http://dx.doi.org/10.1590/0004-282X20150109]. [PMID: 26291995]. [DOI] [PubMed] [Google Scholar]
- 59.Merz Pharmaceuticals L.L.C. 2014 https://clinicaltrials.gov/ct2/show/NCT02088632?term=trigeminal+
- 60.Han Z-A., Song D.H., Oh H-M., Chung M.E. Botulinum toxin type A for neuropathic pain in patients with spinal cord injury. Ann. Neurol. 2016;79(4):569–578. doi: 10.1002/ana.24605. [http://dx.doi.org/10.1002/ana.24605]. [PMID: 26814620]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sałat K., Moniczewski A., Librowski T. Transient receptor potential channels-emerging novel drug targets for the treatment of pain. Curr. Med. Chem. 2013;20(11):1409–1436. doi: 10.2174/09298673113209990107. [http://dx.doi. org/10.2174/09298673113209990107]. [PMID: 23409716]. [DOI] [PubMed] [Google Scholar]
- 62.De Logu F., Nassini R., Materazzi S., Carvalho Gonçalves M., Nosi D. Rossi Degl’Innocenti, D.; Marone, I.M.; Ferreira, J.; Li Puma, S.; Benemei, S.; Trevisan, G.; Souza Monteiro de Araújo, D.; Patacchini, R.; Bunnett, N.W.; Geppetti, P. Schwann cell TRPA1 mediates neuroinflammation that sustains macrophage-dependent neuropathic pain in mice. Nat. Commun. 2017;8(1):1887. doi: 10.1038/s41467-017-01739-2. [http://dx.doi.org/10.1038/s41467-017-01739-2]. [PMID: 29192190]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Basso L., Altier C. Transient receptor potential channels in neuropathic pain. Curr. Opin. Pharmacol. 2017;32:9–15. doi: 10.1016/j.coph.2016.10.002. [http://dx.doi. org/10.1016/j.coph.2016.10.002]. [PMID: 27835802]. [DOI] [PubMed] [Google Scholar]
- 64.Sałat K., Filipek B. Antinociceptive activity of transient receptor potential channel TRPV1, TRPA1, and TRPM8 antagonists in neurogenic and neuropathic pain models in mice. J. Zhejiang Univ. Sci. B. 2015;16(3):167–178. doi: 10.1631/jzus.B1400189. [http://dx.doi.org/10.1631/jzus.B1400189]. [PMID: 25743118]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chukyo A., Chiba T., Kambe T., Yamamoto K., Kawakami K., Taguchi K., Abe K. Oxaliplatin-induced changes in expression of transient receptor potential channels in the dorsal root ganglion as a neuropathic mechanism for cold hypersensitivity. Neuropeptides. 2018;67:95–101. doi: 10.1016/j.npep.2017.12.002. [http://dx.doi.org/10.1016/j.npep.2017.12.002]. [PMID: 29274843]. [DOI] [PubMed] [Google Scholar]
- 66.Yamamoto K., Chiba N., Chiba T., Kambe T., Abe K., Kawakami K., Utsunomiya I., Taguchi K. Transient receptor potential ankyrin 1 that is induced in dorsal root ganglion neurons contributes to acute cold hypersensitivity after oxaliplatin administration. Mol. Pain. 2015;11:69. doi: 10.1186/s12990-015-0072-8. [http://dx.doi.org/10.1186/s12990-015-0072-8]. [PMID: 26567040]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Taguchi K. Role of Transient receptor potential channels in paclitaxel- and oxaliplatin-induced peripheral neuropathy. Yakugaku Zasshi. 2016;136(2):287–296. doi: 10.1248/yakushi.15-00214. [http://dx.doi.org/10.1248/yakushi. 15-00214]. [PMID: 26831807]. [DOI] [PubMed] [Google Scholar]
- 68.Nativi C., Gualdani R., Dragoni E., Di Cesare Mannelli L., Sostegni S., Norcini M., Gabrielli G., la Marca G., Richichi B., Francesconi O., Moncelli M.R., Ghelardini C., Roelens S.A. TRPA1 antagonist reverts oxaliplatin-induced neuropathic pain. Sci. Rep. 2013;3:2005. doi: 10.1038/srep02005. [http://dx.doi.org/10.1038/srep02005]. [PMID: 23774285]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Serra J., Duan W.R., Locke C., Solà R., Liu W., Nothaft W. Effects of a T-type calcium channel blocker, ABT-639, on spontaneous activity in C-nociceptors in patients with painful diabetic neuropathy: a randomized controlled trial. Pain. 2015;156(11):2175–2183. doi: 10.1097/j.pain.0000000000000249. [http://dx.doi.org/10.1097/j.pain.0000000000000249]. [PMID: 26035253]. [DOI] [PubMed] [Google Scholar]
- 70.Ziegler D., Duan W.R., An G., Thomas J.W., Nothaft W. A randomized double-blind, placebo-, and active-controlled study of T-type calcium channel blocker ABT-639 in patients with diabetic peripheral neuropathic pain. Pain. 2015;156(10):2013–2020. doi: 10.1097/j.pain.0000000000000263. [http:// dx.doi.org/10.1097/j.pain.0000000000000263]. [PMID: 26067585]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Jarvis M.F., Scott V.E., McGaraughty S., Chu K.L., Xu J., Niforatos W., Milicic I., Joshi S., Zhang Q., Xia Z. A peripherally acting, selective T-type calcium channel blocker, ABT-639, effectively reduces nociceptive and neuropathic pain in rats. Biochem. Pharmacol. 2014;89(4):536–544. doi: 10.1016/j.bcp.2014.03.015. [http://dx.doi.org/10.1016/ j.bcp.2014.03.015]. [PMID: 24726441]. [DOI] [PubMed] [Google Scholar]
- 72.Clermont-Ferrand . Fondation Apicil. 2014. [Google Scholar]
- 73.Hama A.T., Plum A.W., Sagen J. Antinociceptive effect of ambroxol in rats with neuropathic spinal cord injury pain. Pharmacol. Biochem. Behav. 2010;97(2):249–255. doi: 10.1016/j.pbb.2010.08.006. [http://dx.doi.org/10.1016/ j.pbb.2010.08.006]. [PMID: 20732348]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gaida W., Klinder K., Arndt K., Weiser T. Ambroxol, a Nav1.8-preferring Na(+) channel blocker, effectively suppresses pain symptoms in animal models of chronic, neuropathic and inflammatory pain. Neuropharmacology. 2005;49(8):1220–1227. doi: 10.1016/j.neuropharm.2005.08.004. [http://dx.doi. org/10.1016/j.neuropharm.2005.08.004]. [PMID: 16182323]. [DOI] [PubMed] [Google Scholar]
- 75.Kern K-U., Weiser T. Topical ambroxol for the treatment of neuropathic pain. An initial clinical observation. Schmerz. 2015;29(Suppl. 3):S89–S96. doi: 10.1007/s00482-015-0060-y. [http://dx.doi.org/10.1007/s00482-015-0060-y]. [PMID: 26589711]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Srinivasan V., Pandi-Perumal S.R., Spence D.W., Moscovitch A., Trakht I., Brown G.M., Cardinali D.P. Potential use of melatonergic drugs in analgesia: Mechanisms of action. Brain Res. Bull. 2010;81(4-5):362–371. doi: 10.1016/j.brainresbull.2009.12.001. [http://dx.doi.org/10.1016/j.brainresbull. 2009.12.001]. [PMID: 20005925]. [DOI] [PubMed] [Google Scholar]
- 77.Ambriz-Tututi M., Rocha-González H.I., Cruz S.L., Granados-Soto V. Melatonin: A hormone that modulates pain. Life Sci. 2009;84:489–498. doi: 10.1016/j.lfs.2009.01.024. [http://dx.doi.org/10.1016/j.lfs.2009.01.024]. [DOI] [PubMed] [Google Scholar]
- 78.Wang X-M., Lehky T.J., Brell J.M., Dorsey S.G. Discovering cytokines as targets for chemotherapy-induced painful peripheral neuropathy. Cytokine. 2012;59(1):3–9. doi: 10.1016/j.cyto.2012.03.027. [http://dx.doi.org/10.1016/ j.cyto.2012.03.027]. [PMID: 22537849]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Areti A., Komirishetty P., Akuthota M., Malik R.A., Kumar A. Melatonin prevents mitochondrial dysfunction and promotes neuroprotection by inducing autophagy during oxaliplatin-evoked peripheral neuropathy. J. Pineal Res. 2017;62(3):e12393. doi: 10.1111/jpi.12393. [http:// dx.doi.org/10.1111/jpi.12393]. [PMID: 28118492]. [DOI] [PubMed] [Google Scholar]
- 80.2014 https:// clinicaltrials.gov/ct2/show/
- 81.Liu Y-Y., Yin D., Chen L., Qu W-M., Chen C-R., Laudon M., Cheng N-N., Urade Y., Huang Z-L. Piromelatine exerts antinociceptive effect via melatonin, opioid, and 5HT1A receptors and hypnotic effect via melatonin receptors in a mouse model of neuropathic pain. Psychopharmacology (Berl.) 2014;231(20):3973–3985. doi: 10.1007/s00213-014-3530-5. [http://dx.doi.org/10.1007/s00213-014-3530-5]. [PMID: 24700387]. [DOI] [PubMed] [Google Scholar]
- 82.James C. Efficacy of Intrathecal Oxytocin in Patients With Neurophatic Pain. 2014 https://clinicaltrials.gov/ct2/ show/NCT02100956?term=
- 83.Sałat K., Gdula-Argasińska J., Malikowska N., Podkowa A., Lipkowska A., Librowski T. Effect of pregabalin on contextual memory deficits and inflammatory state-related protein expression in streptozotocin-induced diabetic mice. Naunyn Schmiedebergs Arch. Pharmacol. 2016;389(6):613–623. doi: 10.1007/s00210-016-1230-x. [http://dx.doi.org/10. 1007/s00210-016-1230-x]. [PMID: 26984821]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Freitag C.M., Miller R.J. Peroxisome proliferator-activated receptor agonists modulate neuropathic pain: A link to chemokines? Front. Cell. Neurosci. 2014;8:238. doi: 10.3389/fncel.2014.00238. [http://dx.doi.org/10.3389/ fncel.2014.00238]. [PMID: 25191225]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zychowska M., Rojewska E., Piotrowska A., Kreiner G., Mika J. Microglial inhibition influences XCL1/XCR1 expression and causes analgesic effects in a mouse model of diabetic neuropathy. Anesthesiology. 2016;125(3):573–589. doi: 10.1097/ALN.0000000000001219. [http://dx.doi.org/10.1097/ ALN.0000000000001219]. [PMID: 27387353]. [DOI] [PubMed] [Google Scholar]
- 86.Zychowska M., Rojewska E., Piotrowska A., Kreiner G., Nalepa I., Mika J. Spinal CCL1/CCR8 signaling interplay as a potential therapeutic target - Evidence from a mouse diabetic neuropathy model. Int. Immunopharmacol. 2017;52:261–271. doi: 10.1016/j.intimp.2017.09.021. [http://dx.doi. org/10.1016/j.intimp.2017.09.021]. [PMID: 28961489]. [DOI] [PubMed] [Google Scholar]
- 87.Piotrowska A., Kwiatkowski K., Rojewska E., Slusarczyk J., Makuch W., Basta-Kaim A., Przewlocka B., Mika J. Direct and indirect pharmacological modulation of CCL2/CCR2 pathway results in attenuation of neuropathic pain - In vivo and in vitro evidence. J. Neuroimmunol. 2016;297:9–19. doi: 10.1016/j.jneuroim.2016.04.017. [http://dx.doi.org/ 10.1016/j.jneuroim.2016.04.017]. [PMID: 27397071]. [DOI] [PubMed] [Google Scholar]
- 88.Li Z., Wei H., Piirainen S., Chen Z., Kalso E., Pertovaara A., Tian L. Spinal versus brain microglial and macrophage activation traits determine the differential neuroinflammatory responses and analgesic effect of minocycline in chronic neuropathic pain. Brain Behav. Immun. 2016;58:107–117. doi: 10.1016/j.bbi.2016.05.021. [http://dx.doi.org/10.1016/ j.bbi.2016.05.021]. [PMID: 27262531]. [DOI] [PubMed] [Google Scholar]
- 89.Liu Z., Liang Y., Wang H., Lu Z., Chen J., Huang Q., Sheng L., Ma Y., Du H., Gong Q. LncRNA expression in the spinal cord modulated by minocycline in a mouse model of spared nerve injury. J. Pain Res. 2017;10:2503–2514. doi: 10.2147/JPR.S147055. [http://dx.doi.org/10. 2147/JPR.S147055]. [PMID: 29123421]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Pabreja K., Dua K., Sharma S., Padi S.S.V., Kulkarni S.K. Minocycline attenuates the development of diabetic neuropathic pain: possible anti-inflammatory and anti-oxidant mechanisms. Eur. J. Pharmacol. 2011;661(1-3):15–21. doi: 10.1016/j.ejphar.2011.04.014. [http://dx.doi.org/10. 1016/j.ejphar.2011.04.014]. [PMID: 21536024]. [DOI] [PubMed] [Google Scholar]
- 91.Amorim D., Puga S., Bragança R., Braga A., Pertovaara A., Almeida A., Pinto-Ribeiro F. Minocycline reduces mechanical allodynia and depressive-like behaviour in type-1 diabetes mellitus in the rat. Behav. Brain Res. 2017;327:1–10. doi: 10.1016/j.bbr.2017.03.003. [http://dx.doi.org/10. 1016/j.bbr.2017.03.003]. [PMID: 28286285]. [DOI] [PubMed] [Google Scholar]
- 92.Sun J.S., Yang Y.J., Zhang Y.Z., Huang W., Li Z.S., Zhang Y. Minocycline attenuates pain by inhibiting spinal microglia activation in diabetic rats. Mol. Med. Rep. 2015;12(2):2677–2682. doi: 10.3892/mmr.2015.3735. [http://dx.doi.org/10.3892/mmr.2015.3735]. [PMID: 25955348]. [DOI] [PubMed] [Google Scholar]
- 93.Amin B., Hajhashemi V., Hosseinzadeh H. Minocycline potentiates the anti-hyperalgesic effect of ceftriaxone in CCI-induced neuropathic pain in rats. Res. Pharm. Sci. 2015;10(1):34–42. [PMID: 26430455]. [PMC free article] [PubMed] [Google Scholar]
- 94.Amin B., Hajhashemi V., Hosseinzadeh H., Abnous Kh. Antinociceptive evaluation of ceftriaxone and minocycline alone and in combination in a neuropathic pain model in rat. Neuroscience. 2012;224:15–25. doi: 10.1016/j.neuroscience.2012.07.058. [http://dx.doi.org/10.1016/j.neuroscience.2012. 07.058]. [PMID: 22871519]. [DOI] [PubMed] [Google Scholar]
- 95.Jiang C., Ting A.T., Seed B. PPAR-γ agonists inhibit production of monocyte inflammatory cytokines. Nature. 1998;391(6662):82–86. doi: 10.1038/34184. [http://dx.doi.org/10.1038/34184]. [PMID: 9422509]. [DOI] [PubMed] [Google Scholar]
- 96.Zanardelli M., Micheli L., Cinci L., Failli P., Ghelardini C., Di Cesare Mannelli L. Oxaliplatin neurotoxicity involves peroxisome alterations. PPARγ agonism as preventive pharmacological approach. PLoS One. 2014;9(7):e102758. doi: 10.1371/journal.pone.0102758. [http://dx.doi.org/10.1371/ journal.pone.0102758]. [PMID: 25036594]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Zanardelli M., Micheli L., Cinci L., Failli P., Ghelardini C., Di Cesare Mannelli L. Oxaliplatin neurotoxicity involves peroxisome alterations. PPARγ agonism as preventive pharmacological approach. PLoS One. 2014;9(7):e102758. doi: 10.1371/journal.pone.0102758. [http://dx.doi.org/10. 1371/journal.pone.0102758]. [PMID: 25036594]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Naik A.K., Tandan S.K., Dudhgaonkar S.P., Jadhav S.H., Kataria M., Prakash V.R., Kumar D. Role of oxidative stress in pathophysiology of peripheral neuropathy and modulation by N-acetyl-L-cysteine in rats. Eur. J. Pain. 2006;10(7):573–579. doi: 10.1016/j.ejpain.2005.08.006. [http://dx.doi.org/10.1016/j.ejpain.2005.08.006]. [PMID: 16214382]. [DOI] [PubMed] [Google Scholar]
- 99.Nishio N., Taniguchi W., Sugimura Y.K., Takiguchi N., Yamanaka M., Kiyoyuki Y., Yamada H., Miyazaki N., Yoshida M., Nakatsuka T. Reactive oxygen species enhance excitatory synaptic transmission in rat spinal dorsal horn neurons by activating TRPA1 and TRPV1 channels. Neuroscience. 2013;247:201–212. doi: 10.1016/j.neuroscience.2013.05.023. [http:// dx.doi.org/10.1016/j.neuroscience.2013.05.023]. [PMID: 23707800]. [DOI] [PubMed] [Google Scholar]
- 100.Salvemini D., Little J.W., Doyle T., Neumann W.L. Roles of reactive oxygen and nitrogen species in pain. Free Radic. Biol. Med. 2011;51(5):951–966. doi: 10.1016/j.freeradbiomed.2011.01.026. [http://dx.doi.org/10.1016/j.freeradbiomed. 2011.01.026]. [PMID: 21277369]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Horst A., Kolberg C., Moraes M.S., Riffel A.P.K., Finamor I.A., Belló-Klein A., Pavanato M.A., Partata W.A. Effect of N-acetylcysteine on the spinal-cord glutathione system and nitric-oxide metabolites in rats with neuropathic pain. Neurosci. Lett. 2014;569:163–168. doi: 10.1016/j.neulet.2014.03.063. [http://dx.doi.org/10.1016/j.neulet.2014.03.063]. [PMID: 24704379]. [DOI] [PubMed] [Google Scholar]
- 102.Bernabucci M., Notartomaso S., Zappulla C., Fazio F., Cannella M., Motolese M., Battaglia G., Bruno V., Gradini R., Nicoletti F. N-Acetyl-cysteine causes analgesia by reinforcing the endogenous activation of type-2 metabotropic glutamate receptors. Mol. Pain. 2012;8:77. doi: 10.1186/1744-8069-8-77. [http://dx.doi.org/10.1186/1744-8069-8-77]. [PMID: 23088864]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Anand P., Shenoy R., Palmer J.E., Baines A.J., Lai R.Y.K., Robertson J., Bird N., Ostenfeld T., Chizh B.A. Clinical trial of the p38 MAP kinase inhibitor dilmapimod in neuropathic pain following nerve injury. Eur. J. Pain. 2011;15(10):1040–1048. doi: 10.1016/j.ejpain.2011.04.005. [http://dx.doi.org/10.1016/j.ejpain.2011.04.005]. [PMID: 21576029]. [DOI] [PubMed] [Google Scholar]
- 104.Ostenfeld T., Krishen A., Lai R.Y., Bullman J., Baines A.J., Green J., Anand P., Kelly M. Analgesic efficacy and safety of the novel p38 MAP kinase inhibitor, losmapimod, in patients with neuropathic pain following peripheral nerve injury: a double-blind, placebo-controlled study. Eur. J. Pain. 2013;17(6):844–857. doi: 10.1002/j.1532-2149.2012.00256.x. [http:// dx.doi.org/10.1002/j.1532-2149.2012.00256.x]. [PMID: 23239139]. [DOI] [PubMed] [Google Scholar]
- 105.2006 https://clinicaltrials. gov/ct2/show/NCT00390845?term=SB-681323+neuropathic+pain&
- 106.Kersten C., Cameron M.G. Cetuximab alleviates neuropathic pain despite tumour progression. BMJ Case Rep. 2012;2012:bcr1220115374. doi: 10.1136/bcr.12.2011.5374. [http://dx.doi.org/10.1136/bcr.12.2011.5374]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Gillon J.T., Smith S.E., Lowden M.R. Atorvastatin as novel treatment for neuropathic pain: A case report. Clin. J. Pain. 2013;29(12):e46–e48. doi: 10.1097/AJP.0b013e3182a03c2e. [http://dx.doi.org/10.1097/AJP.0b013e3182a03c2e]. [PMID: 23887344]. [DOI] [PubMed] [Google Scholar]
- 108.Pathak N.N., Balaganur V., Lingaraju M.C., More A.S., Kant V., Kumar D., Kumar D., Tandan S.K. Antihyperalgesic and anti-inflammatory effects of atorvastatin in chronic constriction injury-induced neuropathic pain in rats. Inflammation. 2013;36(6):1468–1478. doi: 10.1007/s10753-013-9688-x. [http://dx.doi.org/10.1007/s10753-013-9688-x]. [PMID: 23872719]. [DOI] [PubMed] [Google Scholar]
- 109.Sałat K., Furgała A., Sałat R. Evaluation of cebranopadol, a dually acting nociceptin/orphanin FQ and opioid receptor agonist in mouse models of acute, tonic, and chemotherapy-induced neuropathic pain. Inflammopharmacology. 2018;26(2):361–374. doi: 10.1007/s10787-017-0405-5. [PMID: 29071457]. [DOI] [PMC free article] [PubMed] [Google Scholar]