Abstract
Introduction
Whilst psychological therapies are the main approach to treatment of eating disorders (EDs), advances in aetiological research suggest the need for the development of more targeted, brain-focused treatments. A range of neurostimulation approaches, most prominently repetitive transcranial magnetic stimulation (rTMS), transcranial direct current stimulation (tDCS) and deep brain stimulation (DBS), are rapidly emerging as potential novel interventions. We have previously reviewed these techniques as potential treatments of EDs.
Aim
To provide an update of the literature examining the effects of DBS, rTMS and tDCS on eating behaviours, body weight and associated symptoms in people with EDs and relevant analogue populations.
Methods
Using PRISMA guidelines, we reviewed articles in PubMed, Web of Science, and PsycINFO from 1st January 2013 until 14th August 2017, to update our earlier search. Studies assessing the effects of neurostimulation techniques on eating and weight-related outcomes in people with EDs and relevant analogue populations were included. Data from both searches were combined.
Results
We included a total of 32 studies (526 participants); of these, 18 were newly identified by our update search. Whilst findings are somewhat mixed for bulimia nervosa, neurostimulation techniques have shown potential in the treatment of other EDs, in terms of reduction of ED and associated symptoms. Studies exploring cognitive, neural, and hormonal correlates of these techniques are also beginning to appear.
Conclusions
Neurostimulation approaches show promise as treatments for EDs. As yet, large well-conducted randomised controlled trials are lacking. More information is needed about treatment targets, stimulation parameters and mechanisms of action.
Keywords: Anorexia nervosa, bulimia nervosa, binge eating disorder, repetitive transcranial magnetic stimulation, transcranial direct current stimulation, deep brain stimulation, neurostimulation
1. INTRODUCTION
In recent years, there has been a conceptual shift in psychiatry and clinical psychology, in that there is increasing acceptance that clinical approaches to mental health problems should not remain “brainless”: in this respect, the eating disorders (EDs) are no exception [1]. This paradigm shift has crucially been influenced by developments in basic neuroscience that have increased our understanding of neural pathways and function, such as optogenetics [2], but arguably, the change is mainly due to the increasing clinical and experimental use (and sophistication) of structural and functional neuroimaging, e.g. magnetic resonance imaging (MRI) [3]. These studies have fostered the development of brain-based aetiological models of illness and there is the expectation that these will inform advances in treatment, leading to more targeted and personalised treatments. This applies across psychiatry and again EDs are no exception. In EDs, for many years, the majority of structural and functional neuroimaging studies have focussed on anorexia nervosa (AN) [4, 5-8] and to a lesser extent on bulimia nervosa (BN) [9]. However, there is increasing interest in binge eating disorder (BED) and in obesity [10, 11]. There is also interest in the development of spectral models that can accommodate a transdiagnostic framework for these problems [12, 13].
Many of the neural-based models of EDs are centred around an altered balance between neural mechanisms related to reward and those related to cognitive control/inhibitory systems [14, 15]. Importantly, and as discussed below, they have boosted the development and use of neurostimulation studies in EDs both as illness probes and as potential treatment modalities. They have also provided a rationale for treatment targets, such as the nucleus accumbens for deep brain stimulation (DBS) in AN and the dorsolateral prefrontal cortex (DLPFC) for the application of non-invasive neurostimulation procedures (such as transcranial magnetic stimulation (TMS) or transcranial direct current stimulation (tDCS)). Conversely, as is described below, neurostimulation studies are gradually beginning to inform models of illness and treatment.
Neurostimulation has been defined as ‘any intervention intended to alter nervous system function by using energy fields such as electricity, magnetism, or both’ [16]. Contemporary therapeutic neurostimulation methods use a range of implantable and non-implantable devices to reversibly enhance or suppress brain and neuronal activity for the treatment of disease. The most widely used neurostimulation method in psychiatry remains electro-convulsive therapy (ECT), but - given its side effect and safety profile - ECT is increasingly limited to life-threatening psychiatric disorders, such as severe depression or catatonia. More modern neurostimulation treatments include DBS, repetitive TMS (rTMS) and tDCS. These are the treatments that will be considered here.
Information on these neurostimulation techniques is summarised in Table 1. As can be seen, DBS is a reversible neurosurgical intervention in which electrodes are implanted in a defined brain region, such as the nucleus accumbens, subgenual cingulate cortex, ventral capsule/ventral striatum (VC/VS), or subcallosal cingulate (SCC) and a battery-operated pulse generator (implanted in the chest) is used to alter neural activity. The device is programmed wirelessly, permitting titration of stimulation parameters. With TMS, an electrical current in a coil generates a magnetic field, which induces a secondary electrical current in the targeted brain region. rTMS involves multiple pulses over a short time period with effects that outlast the stimulation period (30–60 min). Low frequency rTMS is thought to suppress neural activity, and high frequency rTMS to enhance activity. rTMS can lead to lasting changes in brain function, i.e. there is some evidence that it leads to long term depression (LTD) and long term potentiation (LTP) in neural systems. tDCS is also non-invasive. It involves application of a low-intensity constant current (1–2mA) to the brain via scalp electrodes. Anodal stimulation generally has cortical excitatory effects, whereas cathodal stimulation inhibits activity. Effects on cortical excitability can last beyond the stimulation period. The currents involved are not considered sufficiently strong to induce action potentials but rather are likely to alter membrane potentials, i.e. the procedure may alter synaptic plasticity by strengthening or weakening synaptic transmission. Candidate targets for non-invasive brain stimulation (NIBS) in EDs include brain regions/circuitry associated with cognitive control, negative and positive valence, and social processing [13]. For pragmatic accessibility reasons, many NIBS studies have targeted the dorsolateral or dorsomedial prefrontal cortex.
Table 1.
Common modern neurostimulation techniques.
| Type | Invasiveness | Mechanism of Action |
|---|---|---|
| Transcranial magnetic stimulation (TMS) | Non-invasive | Electromagnetic induction leads to modulation of underlying cortex and neural activity. |
| Transcranial direct current stimulation (tDCS) | Non-invasive | Weak current alters neuronal excitability. Neural effects depend on the direction of current. |
| Deep brain stimulation (DBS) | Invasive | Electrical pulses delivered to specific brain area/circuitry central to condition. |
It is beyond the scope of this paper to review extensively the evidence relating to potential mechanisms of action underpinning different neurostimulation techniques. The interested reader may wish to consult the following reviews: George and Aston-Jones [17]; Giordano et al. [18]; Lipsman et al. [19]; and Philip et al. [16]. However, there has recently been much interest in the role of learning in the development/maintenance of psychiatric disorders, including EDs, and also in the role of new learning in treatment [20, 21]. Thus, it is appropriate to consider the neural underpinnings of memory as a potential target for neurostimulation and we briefly elaborate on this here. Of particular clinical interest is reconsolidation, the process by which memories can be made labile at the time of their reactivation [22, 23], and is therefore increasingly being used as a treatment target. The rationale is based on the broad assumption that psychological interventions are most effective when the links between pathological stimuli and maladaptive emotional responses/thinking/behaviours are broken [20]. This is, of course, the objective of exposure treatments [24, 25], however, another approach is to update emotional memories by changing their salience during their reconsolidation [26]. This can be done using either psychological [27-29] or pharmacological approaches [30-33]. Importantly, in the present context, there are reports that neurostimulation alters memory reconsolidation, and some investigators have begun to assess the effects of tDCS on reconsolidation [34]. The underlying mechanisms centre around the idea that new memories arise when the balance between excitatory (glutamatergic) and inhibitory (GABA-ergic) (E-I) firing patterns are disrupted [34, 35], as can be promoted by neurostimulation. What is of particular interest here is that very different treatment approaches, such as the ones described above, may share common mechanisms; it is possible that they may all change the balance between E-I systems. On the basis of such studies, our view is that future research on mechanisms related to neurostimulation will need to identify the neurotransmitter systems which are the key targets, e.g. 5-hydroxytryptamine (in relation to affect), dopamine (in relation to reward and habits) and/or glutamate/GABA (E-I) (in relation to memory/synaptic plasticity).
In recent years there has been a surge in interest in neurostimulation techniques and several reasons for this have been identified [16]: Firstly, neurostimulation techniques have the potential for being highly targeted on particular brain regions or networks to alleviate psychiatric symptoms. Secondly, their mechanisms of action differ from those of pharmacotherapy, and thus they offer fresh hope to those who fail to respond to medication. Thirdly, with increasing use of electronic and mobile devices that interface/interact with the human body (e.g. smartphones, watches with sensors and apps that monitor individuals’ vital characteristics and behaviour), use of medical technologies that interact with the central nervous system may also become more acceptable. Fourth, these techniques, especially NIBS, are seen as having a superior side effect profile compared to ECT and medications. Finally, they have been shown in healthy populations to improve cognition and a range of non-specific symptoms (e.g. stress). It is hoped that these kinds of changes will be achieved in psychiatric patients.
We previously systematically reviewed the literature related to DBS, rTMS, tDCS and vagus nerve stimulation (VNS) in human and animal studies [36], focusing on the effects of these techniques on ED symptoms, such as food intake and body weight and related behaviours, in people with clinical EDs, related analogue populations, in those with other psychiatric or neurological disorders and also in animals. Here, we have conducted a more selective search, focusing only on human studies, specifically in people with EDs (and related analogue populations), identifying updates in the literature from 2013 to the present. Although VNS is receiving a resurgence of interest in other disorders, such as depression [37, 38], we decided not to include this in the present review, as to the best of our knowledge, no studies have focused on using VNS in EDs.
2. METHODS
A systematic review was conducted, following the recommendations outlined in the PRISMA guidance [39] and using a similar search strategy to our previous review [36], so as to be able to combine the identified studies.
2.1. Selection Criteria
We included articles in English that investigated the effects of a form of neurostimulation on eating- and weight-related outcomes, for example ED symptoms, food cravings, food intake, and BMI. Studies focusing on clinical EDs and related analogue populations (which in this case refers to individuals who display sub-clinical disordered eating behaviours e.g. people with frequent food cravings, restrained eaters, sub-clinical binge eating disorder) were included. Randomised control trials (RCTs), clinical studies, case series and single case reports were eligible for inclusion.
Studies were excluded if: (i) they did not report on changes to eating-related outcomes as a result of neurostimulation; (ii) the sample did not include participants with clinical EDs or related analogue populations (which in this case refers to samples in which sub-clinical disorder eating behaviours, such as food cravings or restriction, are experimentally elicited in healthy individuals); (iii) the sample comprised of animals; and (iv) they used less common methods of neurostimulation (e.g. VNS). Review articles, meta-analyses, conference proceedings/abstracts, editorials, letters, book chapters, and unpublished theses were also not included.
2.2. Search Strategy
Three electronic databases (PubMed, ISI Web of Science Core Collection, and PsycINFO via Ovid SP) were searched from 1st January 2013 until 14th August 2017 using the following keywords, which were mapped to Medical Subject Headings with the Explode function where possible: eating disorder*, anorexia, anorexi*, bulimia, bulimi*, binge eat*, eating*, food in combination with brain stimulation, DBS, TMS, transcranial magnetic stimulation, tDCS, transcranial direct current stimulation, transcranial stimulation. These searches were supplemented by internet searches and hand searches of reference lists of potentially relevant papers and reviews. Citation tracking in Google Scholar was also performed. The initial search yielded 614 abstracts.
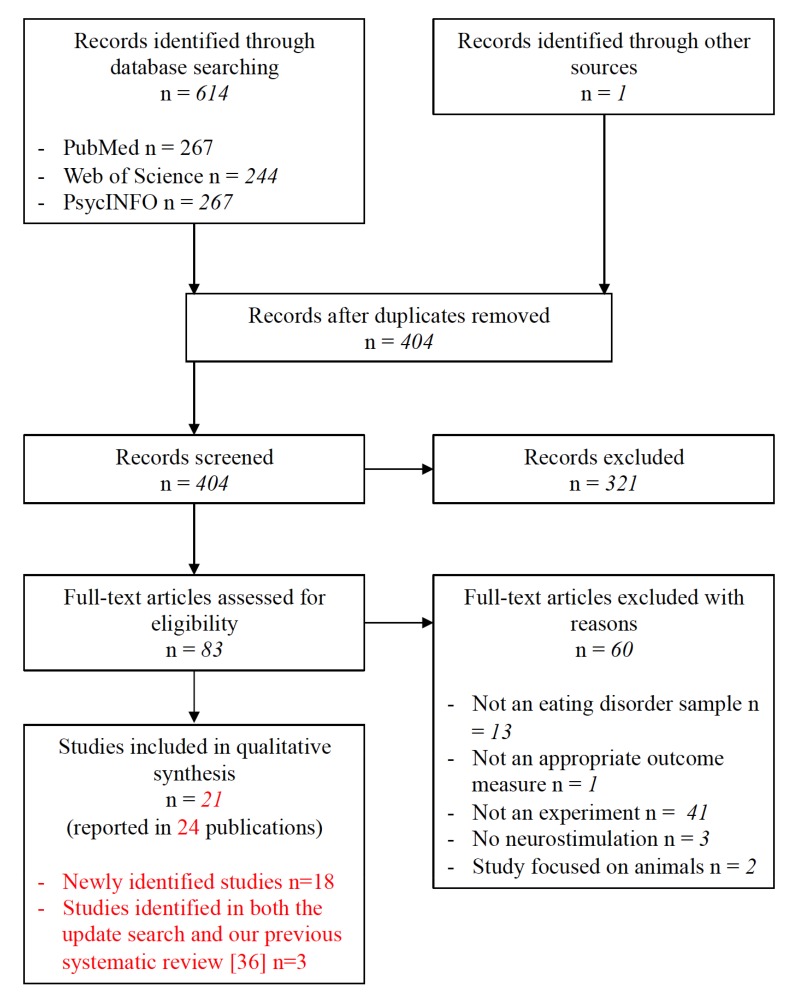
Titles and abstracts of retrieved publications were assessed for relevance, and duplicates were removed. Title and abstracts were screened and based on these, papers that were deemed highly unlikely to be relevant were disregarded. Full-text versions of the remaining articles were then obtained and screened according to the pre-specified eligibility criteria. All papers that did not meet the inclusion criteria were excluded, with the reasons documented (Fig. 1). The entire search process was conducted independently by two reviewers (B.D. and S.B) and disagreements at the final stage were resolved by consensus. The PRISMA flow diagram of the update search is presented in Fig. (1). The paper by McClelland et al. [40] reports an extension and longer term follow-up of an earlier paper by McClelland et al. [41] and the papers by Lipsman et al. [42], Hayes et al. [43], and Lipsman et al. [44] refer to different aspects of the same and increasingly extended sample. In both cases we counted these papers as relating to one study. The findings of the update search were collated together with eligible papers from our earlier review [36]. Of note, four of the eligible studies had already been identified in our earlier review: Lipsman et al. [42]; McLaughlin et al. [45]; Van den Eynde et al. [46]; and Wu et al. [47].
Fig. (1).
PRISMA flow diagram of update search (1st January 2013 until 14th August 2017).
2.3. Data Extraction
The principal reviewer (B.D.) extracted data from all included studies into an electronic summary table, which was then checked by another reviewer (S.B.). Information collected related to the sample characteristics, study design, neurostimulation technique and protocol, target brain area, and relevant findings. A narrative synthesis is presented due to the methodological diversity of the included studies.
3. RESULTS
We identified 18 new studies in the update search (n=368 participants) that met the inclusion criteria for this review, in addition to 14 studies identified in our earlier review [36], yielding a total number of 32 studies to be included in the current review (total n=526 participants). Eight of these studies were conducted in analogue samples of people with food cravings (n=160 participants), 13 in AN patients (n=148 participants), 9 in BN patients (n=187 participants) and 2 in BED patients (n=31 participants). One study included both AN-binge/purge subtype (AN-BP) and BN patients [48], which for the purposes of this review was reported alongside studies in people with BN. The following neurostimulation techniques were investigated: DBS (7 studies), rTMS (17 studies) and tDCS (8 studies). Tables 2-4 show the newly identified studies, together with those that we identified in our earlier review [36].
Table 2.
Research studies assessing the effects of neurostimulation in analogue samples of people with food craving.
| Author | N | Sample | Treatment Type | Design | Area | Protocol | Findings | Comments | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Repetitive transcranial magnetic stimulation | ||||||||||||||||||
| Lowe et al. [50] | 21 | Healthy females with strong and frequent food cravings for experimental foods | cTBS | Double-blind sham-controlled within-subjects crossover Conditions: (i) real cTBS (ii) sham cTBS |
Left DLPFC Located using 10–20 EEG system (F3 for left DLPFC) |
3 stimuli at 50 Hz repeated at 5 Hz for a total of 600 stimuli for 40 seconds, 80% MT 1 session per condition |
After active cTBS, participants reported larger increases in snack food cravings and consumed more snack foods than after sham. | Performance on a Stroop task was more impaired after active cTBS than after sham. The aim of this study was to examine the relationship between DLPFC function and dietary self-control. |
||||||||||
| Barth et al. [55] | 10 | Healthy adults with high food cravings | rTMS | Double-blind sham-controlled within-subjects crossover Conditions: (i) real rTMS (ii) sham rTMS |
Left DLPFC Located using the 5 cm anterior method |
10 Hz, 15 minutes, 100% MT, 3000 pulses 1 session per condition |
No difference between real and sham rTMS in reducing cravings. | - | ||||||||||
| Uher et al. [56] | 28 | Healthy adults with high food cravings | rTMS | RCT; parallel group design Conditions: (i) real rTMS (ii) sham rTMS |
Left DLPFC Located using the 5 cm anterior method |
10 Hz, 20 minutes, 110% MT, 1000 pulses 1 session |
Food cravings during food exposure remained stable after real rTMS and increased after sham rTMS. | - | ||||||||||
| Transcranial direct current stimulation | ||||||||||||||||||
| Ljubisavljevic et al. [49] | 30 | Healthy adults with high food cravings Right handed |
tDCS | RCT Conditions: (i) Active tDCS: anode right/cathode left (ii) Sham tDCS |
Right DLPFC Located using 10–20 EEG system (F4 for right DLPFC) |
2 mA; 20 minutes 5 sessions; 1 per day for 5 days |
Food cravings were significantly reduced by the end of treatment and at 30 days post-treatment in the active, but not the sham, group. | Sham group: Received real stimulation on 1st session. | ||||||||||
| Kekic et al. [53] | 20 | Healthy female adults with high food cravings | tDCS | Double-blind sham-controlled within-subjects crossover Conditions: (i) Active tDCS: anode right/cathode left (ii) Sham tDCS |
Right DLPFC Located using 10–20 EEG system (F4 for right DLPFC, F3 for left DLPFC) |
2mA; 20 minutes 1 session per condition |
Active tDCS did not alter global food craving scores or actual food consumption compared to sham tDCS, although it did lead to a reduction in craving for sweet foods (but not savoury). | The study also investigated the effects of tDCS on temporal discounting (TD, a measure of choice impulsivity). No differences were seen in TD after real vs sham tDCS. Participants who showed more reflective choice behaviour were more susceptible to the anti-craving effects of tDCS than those that displayed more impulsive choice behaviour. |
||||||||||
| Author | N | Sample | Treatment Type | Design | Area | Protocol | Findings | Comments | ||||||||||
| Transcranial direct current stimulation | ||||||||||||||||||
| Lapenta et al. [51] | 9 | Healthy female adults with food cravings | tDCS | Single-blind sham-controlled within-subjects crossover Conditions: (i) Active tDCS: anode right/cathode left (ii) Sham tDCS |
Right DLPFC Located using 10–20 EEG system (F4 for right DLPFC, F3 for left DLPFC) |
2mA; 20 minutes 1 session per condition |
Active tDCS reduced food craving and the amount of calories ingested, compared with sham tDCS. | This study included assessment of evoked potentials in a Go/No-go Task that contained pictures of food and furniture (a control visual stimulus). Active vs sham tDCS, reduced the frontal N2 component and enhanced the P3a component of responses to No-go stimuli, regardless of the stimulus condition (food, furniture). Both N2 and P3a are thought to be markers of inhibitory control. |
||||||||||
| Goldman et al. [54] | 19 | Healthy adults with food cravings | tDCS | RCT, crossover, blinded Conditions: (i) Active tDCS: anode right/cathode left (ii) Sham tDCS |
DLPFC Located using 10–20 EEG system (F3 for left DLPFC; F4 for right DLPFC) |
2mA, 20 minutes 1 session per condition |
Food cravings reduced in both conditions; however, percentage change was significantly greater in active tDCS. Active tDCS reduced food cravings for sweet foods and carbohydrates more than sham. No difference between groups in amount of food ingested. | - | ||||||||||
| Fregni et al. [52] | 23 | Healthy adults with food cravings | tDCS | RCT, crossover, double-blinded Conditions: (i) anode left/cathode right (ii) anode right/cathode left (iii) Sham tDCS |
DLPFC Located using 10–20 EEG system (F3 for left DLPFC; F4 for right DLPFC) |
2mA, 20 minutes 1 session per condition |
Craving of viewed foods decreased with anode right/cathode left, remained stable with anode left/cathode right and increased after sham. Subjects fixated (eye tracking) on food related pictures less after anode right/cathode left. Subjects consumed less food after both types of active stimulation. |
- | ||||||||||
Abbreviations: cTBS - continuous theta burst stimulation, a variant of rTMS that transiently inhibits cortical activity; DLPFC - dorsolateral prefrontal cortex; EEG - electroencephalography; Hz - Hertz; MT - motor threshold; rTMS - repetitive transcranial magnetic stimulation; RCT - randomised controlled trial; tDCS - transcranial direct current stimulation; mA - milliAmpere; TD - temporal discounting.
Table 4.
Research studies assessing the effects of neurostimulation in people with bulimia nervosa.
| Author | N | Sample | Treatment Type | Design | Area | Protocol | Findings | Comments | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Repetitive transcranial magnetic stimulation | ||||||||||||||||||||||||
| Gay et al. [73] | 51 | Females with DSM-IV BN Right handed |
rTMS | RCT Double-blind parallel group Conditions: (i) Real rTMS (ii) Sham rTMS |
Left DLPFC Located using 6 cm anterior method |
20 × 5 s trains/55 s inter-train interval at 10 Hz = 1000 pulses per session; 110% MT 10 sessions |
At post-treatment, no group differences in number of binges in 15 days post-treatment, features of binge episodes, number of days without bingeing, maximal craving before a binge, number of vomiting episodes and mood. | There were no within group differences from pre-to post treatment in either groups in relation to binge or purge episodes. However, within the active rTMS group there was a borderline significant (p=0.05) reduction in depression symptoms over time. | ||||||||||||||||
| Sutoh et al. [66] |
8 | Adults with DSM-IV-TR BN Right handed |
rTMS | Case series | Left DLPFC Located using 5 cm anterior method |
20 × 5 s trains/55 s inter-train interval at 10 Hz = 1000 pulses per session; 110% MT 1 session |
At 4-hours post-rTMS, a significant reduction in the subjective ratings of want to eat, urge to eat, and sense of hunger for high-calorie food stimuli was found. No effect on ED symptoms was identified. | Using near-infrared spectroscopy, haemoglobin concentration changes in the DLPFC was measured during cognitive tasks (rock-paper-scissors and food picture task), measuring self-regulatory control, both at baseline and after a single session of rTMS. A significant decrease in cerebral oxygenation of the left DLPFC was observed after a single session of rTMS. | ||||||||||||||||
| Dunlop et al. [48] |
28 | Adults (n=2 male) with DSM-5 BN (n=17) or AN-BP (n=11) Treatment non-responders |
rTMS | Case series | DMPFC Neuronavigation |
20 × 5 s trains/10 s inter-train interval at 10 Hz = 3000 pulses per session; 120% MT 20 sessions Treatment responders (n=16) received 10 additional sessions |
In whole sample, no change in binge frequency but significant reduction in purge frequency. N=16 achieved >50% reduction in binge and purge frequency (responders). |
Resting state fMRI data were collected before and after rTMS treatment to identify neural predictors and correlates of treatment response. Enhanced frontostriatal connectivity was associated with being an rTMS responder. In non-responders, frontostriatal functional connectivity was high at baseline, and rTMS suppressed functional connectivity in association with symptomatic worsening. |
||||||||||||||||
| Author | N | Sample | Treatment Type | Design | Area | Protocol | Findings | Comments | ||||||||||||||||
| Repetitive transcranial magnetic stimulation | ||||||||||||||||||||||||
| Downar et al. [68] |
1 | Adult with severe refractory BN and depression | rTMS | Case study | Bilateral DMPFC Neuro- navigation |
60 x at 5 s trains/10 s inter-train interval at 10 Hz, 3000 pulses; 120% MT 2 x 20 sessions |
Full remission of binge-purging episodes and depression for more than 2 months post-treatment completion; after a significant stressor some ED symptoms returned. These remitted after a 2nd 20 session course of treatment. |
- | ||||||||||||||||
| Van den Eynde et al. [67] |
7 | Adults with BN Left-handed |
rTMS | Case series | Left DLPFC Located using 5 cm anterior method |
20 × 5 s trains/55 s inter-train interval at 10 Hz = 1000 pulses per session; 110% MT 1 session |
Decrease in reported cravings, but mood deteriorated. | - | ||||||||||||||||
| Van den Eynde et al. [70] |
38 | Adults with BN Right handed |
rTMS | RCT Double blind, parallel groups Conditions: (i) real rTMS (ii) sham rTMS |
Left DLPFC Located using 5 cm anterior method |
20 × 5 s trains/55 s inter-train interval at 10 Hz = 1000 pulses per session; 110% MT 1 session |
Compared with sham, real rTMS was associated with a decrease in self-reported urge to eat and binge eating (24 hours post-treatment). No difference between groups in hunger, tension, mood and urge to binge eat. | An associated study in 22 participants from the same trial [71] found a reduction of salivary cortisol in patients receiving real, compared to those receiving sham rTMS. Another associated study [82] in 33 trial participants found no effect of rTMS on cognition (selective attention, assessed with a Stroop task). | ||||||||||||||||
| Walpoth et al. [72] | 14 | Female adults with DSM-IV BN | rTMS | RCT Double blind, parallel groups Conditions: (i) real rTMS (ii) sham rTMS |
Left DLPFC Neuro- navigation |
10 × 10 s trains/60 s inter-train interval at 20 Hz, = 2000 pulses; 120% MT 15 sessions over 3 weeks |
Improvement in self-reported binge-purge behaviours, depressive and OCD symptoms in both groups. No difference between real and sham groups. | - | ||||||||||||||||
| Hausmann et al. [69] | 1 | Adult with BN and depression | rTMS | Case study | Left DLPFC Neuro- navigation |
10 × 10 s trains/60 s inter-train interval at 20 Hz, = 2000 pulses; 80% MT 10 sessions over 2 weeks |
Remission from binge-purge symptoms and almost 50% decrease in depression score at post-treatment. | - | ||||||||||||||||
| Author | N | Sample | Treatment Type | Design | Area | Protocol | Findings | Comments | ||||||||||||||||
| Transcranial direct current stimulation | ||||||||||||||||||||||||
| Kekic et al. [79] |
39 | Adults with DSM-5 BN Right handed |
tDCS | RCT Double-blind crossover Conditions: (i) Active tDCS: anode left / cathode right (ii) Active tDCS: anode right / cathode left (iii) Sham tDCS |
DLPFC Located using 10–20 EEG system (F3 for left DLPFC and F4 for right DLPFC) |
2 mA; 20 minutes 1 session per condition |
Anode right / cathode left active tDCS led to reductions in eating disorder cognitions and improvement in mood, compared to the other active and sham condition. Both active conditions suppressed the self-reported urge to binge-eat. | The study also assessed temporal discounting (TD), finding that active but not sham tDCS reduced TD behaviour (was associated with more reflective choice behaviour). | ||||||||||||||||
Abbreviations: DSM - Diagnostic and Statistical Manual [74, 75]; BN - bulimia nervosa; rTMS - repetitive transcranial magnetic stimulation; DLPFC - dorsolateral prefrontal cortex; s - seconds; Hz - hertz; MT - motor threshold; ED - eating disorder; AN-BP - anorexia nervosa binge/purge subtype; DMPFC - dorsomedial prefrontal cortex; tDCS - transcranial direct current stimulation; mA - milliAmpere; EEG - electroencephalography; RCT - randomised controlled trial; OCD - obsessive compulsive disorder; TD - temporal discounting.
3.1. Studies in Analogue Samples of Healthy People with Food Craving
We identified 8 small controlled trials; three applied high or low-frequency rTMS (n=59 participants), one of which used continuous theta burst stimulation (cTBS), a variant of rTMS (n=21 participants), and 5 applied tDCS (n=101 participants) to either the left or right DLPFC in people with frequent food cravings (Table 2). Six of these studies used a cross-over design. With the exception of one study, which delivered 5 sessions of tDCS over one week [49], all others only delivered 1 session or 1 session per condition. The cTBS study used this method as an illness probe and as hypothesised found an increase of snack food craving after active cTBS, but not after sham [50]. All other studies found an effect of the active condition on reducing general food-craving, sweet food craving, or on valuation of foods. Out of four studies that reported the effects of neurostimulation on actual food consumption, two found that the active condition seemed to reduce this [51, 52], whereas the others found no difference [53, 54]. The one study that used a multi-session design, found five sessions of active tDCS, but not sham tDCS, to the right DLPFC (anode right/cathode left forehead) to reduce food cravings [49]. This improvement was observed 30 days post-treatment. Compared to sham tDCS, active tDCS also decreased craving for fast food and sweets (but not carbohydrates).
3.2. Studies in People with Anorexia Nervosa
We identified 13 studies investigating the effects of neurostimulation in patients with AN (Table 3).
Table 3.
Research studies assessing the effects of neurostimulation in people with anorexia nervosa.
| Author | N | Sample | Treatment Type | Design | Area | Protocol | Findings | Comments | |||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Deep brain stimulation | |||||||||||||||||||||||||||||||||||
| Blomstedt et al. [60] | 1 | Adult female with chronic AN and severe MDD | DBS | Single case | Bed nucleus of the stria terminalis (BNST) | Bilateral stimulation of 130 Hz, 120 μs pulse width, and 4.3V (at 12 months post-surgery) to the BNST | Food and eating-related anxiety and obsessive thoughts vanished. Virtually stopped vomiting. Food intake more stable and less prone to large variations. No effect on BMI. Profound improvement in depression nine months post-surgery. | Electrodes were initially implanted in the medial forebrain bundle. Due to side effects, stimulation was turned off. Re-operated on for DBS of the BNST two years after first operation. | |||||||||||||||||||||||||||
| Lipsman et al. [44] |
16 | Adults with enduring AN | DBS | Open-label trial | Subcallosal cingulate | Bilateral stimulation of 130 Hz, 90 μs pulse width and 5-6.5 V (at 12 months post-surgery) to the subcallosal cingulate | Mean BMI increased significantly and, anxiety, depression and affective regulation improved over the 12 months post-surgery. | This study is an extension of Lipsman et al. [42] and Hayes et al. [43]. PET imaging identified significant changes in glucose metabolism in several brain structures implicated in AN at 6 and 12 months follow-ups, compared with baseline. |
|||||||||||||||||||||||||||
| Hayes et al. [43] | 8 | Female adults with treatment-refractory DSM-IV AN | DBS | Open-label trial | Subcallosal cingulate | As in Lipsman et al. [44] | Compared to healthy controls widely distributed differences in SCC connectivity were found in AN patients. | These cases are included in the Lipsman et al. [44] series. The study used diffusion magnetic resonance imaging and deterministic multi-tensor tractography to compare anatomical connectivity and microstructure in SCC-associated white matter tracts. |
|||||||||||||||||||||||||||
| Lipsman et al. [42] |
6 | Female adults with chronic or treatment resistant DSM-IV AN | DBS | Open-label trial | Subcallosal cingulate | As in Lipsman et al. [44] | Included in Lipsman et al. [44] | These cases are included in the Lipsman et al. [44] series. | |||||||||||||||||||||||||||
| Wang et al. [57] | 2 | Female adults with AN | DBS | Case series | Nucleus accumbens | Bilateral stimulation of 135-185 Hz, 120-210 μs pulse width, and 2.5-3.8 V to the nucleus accumbens | Pre-operative BMI: Case 1 –13.3 Case 2 – 12.9 BMI at 1 year post-op: Case 1 – 18 Case 2 – 20.8 |
Patient’s illness duration was 2 and 3 years respectively. Depression, anxiety and obsessive compulsive symptoms reduced significantly from pre-op to 1 year post-op. |
|||||||||||||||||||||||||||
| Author | N | Sample | Treatment Type | Design | Area | Protocol | Findings | Comments | |||||||||||||||||||||||||||
| Deep brain stimulation | |||||||||||||||||||||||||||||||||||
| Wu et al. [47] | 4 | Female adolescents with AN with failure to respond to standard psychiatric treatment programme of at least 12 month | DBS | Open-label trial | Nucleus accumbens | Bilateral stimulation of 180 Hz, 90 μs pulse width and >6 V to the nucleus accumbens | Average increase of 65% body weight from baseline to post-surgery follow-up (mean 38 months). Menstruation restored in all participants following surgery. | Patients had short illness duration (13 to 28 months) and BMIs between 10 to 13.3 kgs/m2 at pre-treatment. Improvements in anxiety and obsessive-compulsive symptoms. No definition is given of the standard psychiatric treatment that patients had previously failed to respond to. |
|||||||||||||||||||||||||||
| Zhang et al. [58] | 6 | Adolescent Patients with restricting type AN (age 13 to 17) | DBS | Case series | Nucleus accumbens | DBS protocol not described | Follow-up data at 1 month post-DBS is available for 4 out of 6 patients. All 4 showed improvements in BMI. No longer term follow-up provided. | Patients had a short illness duration (13-42 months) and BMIs between 11.2 and 13.5 at pre-treatment. All had previous unsuccessful behavioural and medication treatments. The main focus of the study was on PET imaging. Compared to healthy controls AN patients showed baseline hypermetabolism in the frontal lobe, hippocampus, and lentiform nucleus. This decreased after DBS. | |||||||||||||||||||||||||||
| McLaughlin et al. [45] | 1 | AN and Obsessive compulsive disorder | DBS | Single case report | VC/VS Bilateral |
Bilateral stimulation of 120 Hz, 120 μs pulse width and 7.5 V to the VC/VS | Food intake, food variety and body weight were increased. BMI maintained between 18.9 and 19.6 postoperatively. | Symptoms worsened when cathode electrode was added. | |||||||||||||||||||||||||||
| Israel et al. [59] | 1 | Adult female with AN and depression | DBS | Single case report | Subgenual cingulate cortex Bilateral |
Right-sided intermittent stimulation (2 minutes on/1 minute off) at 130 Hz, 5 mA and 91 μs pulse width to the subgenual cingulate cortex |
Remission of ED, no relapse and maintained average BMI of 19.1. Remission from ED persisted despite depressive breakthrough. | - | |||||||||||||||||||||||||||
| Author | N | Sample | Treatment Type | Design | Area | Protocol | Findings | Comments | |||||||||||||||||||||||||||
| Repetitive transcranial magnetic stimulation | |||||||||||||||||||||||||||||||||||
| Schmidt [personal communication; for protocol 63] | 34 | Females with chronic treatment-refractory DSM-5 AN Right handed |
rTMS | Feasibility RCT | Left DLPFC Neuronavigation |
20 × 5 s trains/55 s inter-train interval at 10 Hz = 1000 pulses per session; 110% MT ~20 sessions |
At 3-month follow-up between-group differences of medium effect size were noted in measures of depression, anxiety, and obsessional symptoms, favouring active rTMS. Changes in eating disorder symptoms were less pronounced. | Neurocognitive (e.g. temporal discounting) and neural predictors and correlates of rTMS are also being assessed. | |||||||||||||||||||||||||||
| McClelland et al. [41] [40] | 5 | Females with chronic treatment-refractory DSM-5 AN Right handed |
rTMS | Case series | Left DLPFC Neuronavigation |
20 × 5 s trains/55 s inter-train interval at 10 Hz = 1000 pulses per session; 110% MT ~20 sessions |
Compared to baseline, at post-treatment, participants showed significant improvements in ED and affective symptoms. Further improvements were seen at 6 months post-treatment. | - | |||||||||||||||||||||||||||
| McClelland et al. [62] | 60 | Adults with DSM-5 AN Right handed |
rTMS | RCT Double-blind parallel group Conditions: (i) Real rTMS (ii) Sham rTMS |
Left DLPFC Neuronavigation |
20 × 5 s trains/55 s inter-train interval at 10 Hz = 1000 pulses per session; 110% MT 1 session |
In completers (n=49), core AN symptoms were significantly reduced post-rTMS and at 24-hour follow-up in the real, but not sham, rTMS group. | This study also assessed cognitive (temporal discounting; TD) and biomarkers (salivary cortisol) of rTMS. In relation to TD, there was an interaction trend (p = 0.060): real vs sham rTMS resulted in reduced rates of TD (more reflective choice behaviour). Salivary cortisol concentrations were unchanged by stimulation. |
|||||||||||||||||||||||||||
| Van den Eynde et al. [46] | 10 | Adults with DSM-IV-TR AN Right-handed |
rTMS | Pilot study | Left DLPFC Located using 5 cm anterior method |
20 × 5 s trains/55 s inter-train interval at 10 Hz = 1000 pulses per session; 110% MT 1 session |
In completers (n=9), based on VAS scales, sensations of “feeling fat” and “feeling full”, and “anxiety” decreased between pre- and post-rTMS. No change observed in “urge to restrict” or “urge to eat”. No changes in mood following rTMS. | - | |||||||||||||||||||||||||||
| Author | N | Sample | Treatment Type | Design | Area | Protocol | Findings | Comments | |||||||||||||||||||||||||||
| Repetitive transcranial magnetic stimulation | |||||||||||||||||||||||||||||||||||
| Kamolz et al. [61] |
1 | Adult with AN and depression | rTMS | Case report | Left DLPFC Located using 10-20 EEG system (F3) |
100 x 2 s trains/10 s inter-train interval at 10 Hz = 2000 pulses per session, 110% MT 41 sessions |
Improvements in depression and ED symptoms after 10 sessions, after deterioration further rTMS sessions were given including maintenance sessions (2 p/week). This resulted in continuing improvement of depression and ED symptoms. | - | |||||||||||||||||||||||||||
| Transcranial direct current stimulation | |||||||||||||||||||||||||||||||||||
| Khedr et al. [65] | 7 | Adults (n=1 male) with DSM-IV AN | tDCS | Open-label pilot study Active tDCS: anode left / cathode contralateral arm |
Left DLPFC Located using 6 cm anterior method |
2mA; 20 minutes 10 sessions |
Variable response in participants. Significant improvement compared with baseline in the BDI, EDI and EAT at post session and also at one month post-treatment (n=3). | - | |||||||||||||||||||||||||||
Abbreviations: AN - anorexia nervosa; MDD - major depressive disorder; DBS - deep brain stimulation; BNST - bed nucleus of the stria terminalis; Hz - Hertz; μs - micro seconds; V - volts; BMI - body mass index; PET - positron emission tomography; DSM - Diagnostic and Statistical Manual [74, 75]; SCC - subcallosal cingulate; VC/VS - ventral capsule/ventral striatum; ED - eating disorder; rTMS - repetitive transcranial magnetic stimulation; RCT - randomised controlled trial; DLPFC - dorsolateral prefrontal cortex; s - seconds; Hz - Hertz; MT - motor threshold; TD - temporal discounting; VAS - visual analogue scales; tDCS - transcranial direct current stimulation; mA - milliAmpere; EEG - electroencephalography; BDI - Beck Depression Inventory [76]; EDI - Eating Disorder Inventory [77]; EAT - Eating Attitudes Test [78].
3.2.1. DBS
Seven case studies or series were identified that used DBS to treat chronic or treatment-refractory AN. The largest series administered bilateral stimulation to the SCC in 16 patients [44]. This was an extension of an earlier series of 6 patients [42]. DBS treatment increased BMI in the year following surgery. Furthermore, symptoms of depression and anxiety also improved post-surgery. Two patients asked to have their device removed for reasons that were not entirely clear. Whilst 10 out of 16 patients experienced at least one adverse event, only one was a DBS-related surgical site infection, most others were related to the underlying illness. Within this study [44], PET imaging identified significant changes in glucose metabolism in brain structures implicated in AN at 6 and 12 months follow-ups, compared with baseline, suggesting that DBS can directly affect AN-related brain circuitry. An associated study in 8 patients who were part of this DBS series used diffusion MRI and deterministic multi-tensor tractography to compare anatomical connectivity and microstructure in SCC-associated white matter tracts [43]. Compared to healthy controls, AN patients displayed widely distributed heterogeneous differences in SCC connectivity.
Three case series from China (n=12) [47, 57, 58] used nucleus accumbens DBS to treat severe AN. Of note, most of the patients included in these series were adolescents or young adults with short illness duration. Whilst all three series emphasised the benefits of DBS in terms of weight gain, psychological outcomes and longer-term follow-up data were not always reported. Three other single case studies used DBS in enduring treatment-refractory AN with comorbid severe depression or obsessive compulsive disorder, targeting the SCC [59], VC/VS [45] or the bed nucleus of the stria terminalis [60]. In the first two of these cases, ED symptoms remitted and the patient’s BMI returned to normal or near normal [45, 59]. In the latter case, surgery reduced anxious and obsessive thoughts surrounding food and eating, stabilized food intake, reduced self-induced vomiting and improved depression symptoms, but without any improvement in BMI [60].
3.2.2. rTMS
One single case study [61], two case series [40, 41, 46] and two RCTs [62, Schmidt, personal communication; for protocol [63] assessed the effect of rTMS on AN symptoms. Two of these used single session designs [46, 62], the remainder used multi-session designs. All studies targeted the left DLPFC. Van den Eynde et al. [46] conducted a pilot study of a single-session of real rTMS finding that it was generally well-tolerated and reduced some of the core symptoms of AN. No change in urge to restrict food or in mood was found. Building on these findings, we conducted a sham-controlled single session RCT [62]. Patients receiving real rTMS (compared to the sham group) showed an improvement in core AN symptoms (urge to restrict, feeling full, feeling fat) post-rTMS and at a 24-hour follow-up. Real rTMS was also found to encourage more prudent decision-making in a temporal discounting task.
The first report of therapeutic use of rTMS in a case of severe AN and depression was published by Kamolz et al. [61]. A total of 41 sessions of left DLPFC rTMS were delivered. The patient showed improvements in both ED and depressive symptoms after an initial course of 10 rTMS sessions. However, this was followed by deterioration and so, further sessions of rTMS were delivered, with further improvement of depression and ED symptoms. McClelland et al. [40, 41] conducted a case series (n=5) assessing the effect of a treatment course (20 sessions) of real rTMS in patients with severe and enduring AN. At post-treatment significant improvements in ED and affective symptoms were observed. While further improvements were seen at 6-months post-treatment (3/5 participants deemed ‘recovered’ on the Eating Disorders Examination Questionnaire [64]), by 12-months post-treatment, the therapeutic effects had waned and participants had lost some weight compared to baseline. Since then we have completed a sham-controlled feasibility RCT of 20 sessions in 34 patients with severe and enduring AN [Schmidt, personal communication; for protocol see 63]. Cognitive and neural correlates of rTMS treatment are also being examined. Patient retention in the study and treatment completion rates were high. Whilst between group differences at post-treatment were small, at 3 months post-treatment, there were between-group differences of medium effect size in depression, stress and obsessive compulsive symptoms, all favouring the active treatment. Group differences in ED symptoms and BMI were of negligible effect at both post-treatment and follow-up.
Only one study reported on the effect of 10 sessions of tDCS in an open-label pilot study in patients with AN [65]. Variable responses to the treatment were observed: scores on eating and depression questionnaires improved in three patients at post-treatment and follow-up, in two participants improvements were seen at the end of treatment but scores returned to baseline at one-month post-treatment, one participant showed improvements only in depression, and one participant showed no improvements following treatment.
3.3. Studies in People with Bulimia Nervosa
We identified nine studies in patients with BN, eight of these applied rTMS (n=148 participants) and one applied tDCS (n=39 participants) (Table 4).
3.3.1. rTMS
The rTMS studies included 5 single case studies/case series and three RCTs, stimulating either the left DLPFC or the dorsomedial prefrontal cortex (DMPFC). Findings from case studies/series were promising in that they all noted reductions in urge to eat, and in some studies reductions in actual binge and/or purge episodes were reported [48, 66-69]. Of note, one small case series of rTMS to the left DLPFC studied left-handed patients and found that their mood deteriorated after 1 session of rTMS [67], whereas in a comparison group of right-handed BN patients receiving left DLPFC rTMS, their mood improved. A sham-controlled RCT of one session of rTMS found a decrease in self-reported urge to eat and binge eating (24-hours post-treatment) [70]. An associated study [71] in a subgroup of participants found that real rTMS reduced salivary cortisol levels compared to sham.
Multi-session designs (10-20 sessions) were used in one larger case series [48] and two RCTs [72, 15 sessions, 73, 10 sessions]. Dunlop et al. [48] assessed the effect of 20 sessions of DMPFC rTMS on binging and purging in a transdiagnostic sample comprised of patients with BN or AN-BP. Purge frequency, global ED symptom scores and depression scores had significantly reduced at the 4-week post-treatment follow-up. Just over half of the participants were classed as treatment responders with a >50% reduction in binge and purge frequency at follow-up. Whilst in both RCTs [72, 73] there were some improvements in binge-purge symptoms over time, there was no group difference between patients receiving real or sham rTMS.
3.3.2. tDCS
The only study examining the effects of tDCS in patients with BN was a small cross-over study in which two tDCS electrode montages (anode left DLPFC/cathode right DLPFC and in the reverse montage) were compared to sham treatment [79]. The study found that one session of anode right/cathode left active tDCS led to reductions in ED cognitions and improvement in mood, compared to the other active and sham condition. Both active conditions suppressed the self-reported urge to binge eat.
3.4. Studies in People with Binge Eating Disorder
We identified two studies (n=31 participants) (see Table 5).
Table 5.
Research studies assessing the effects of neurostimulation in people with binge eating disorder.
| Author | N | Sample | Treatment Type | Design | Area | Protocol | Findings | Comments |
|---|---|---|---|---|---|---|---|---|
| Repetitive transcranial magnetic stimulation | ||||||||
| Baczynski et al. [80] | 1 | Adult female with refractory BED and comorbid depression | rTMS | Case report | Left DLPFC Located using 10–20 EEG system (F3 for left DLPFC) |
20 × 4 s trains/26 s inter-train interval at 10 Hz = 2400 pulses per session; 120% MT 20 sessions, over 4 weeks and 2 days |
At the end point of rTMS (3 days post-treatment), binge eating episode/week had reduced to 0, clinical global impression score had reduced from 6 pre-treatment to 1 post-treatment. BDI and BES scores also reduced (approx. 40 to 25). | - |
| Transcranial direct current stimulation | ||||||||
| Burgess et al. [81] | 30 | Adults with full or subthreshold (n=11) BED | tDCS | Single-blind sham-controlled crossover Conditions: (i) Active tDCS: anode right / cathode left (ii) Sham tDCS |
DLPFC Located using 10–20 EEG system (F3 for left DLPFC and F4 for right DLPFC) |
2 mA; 20 minutes 1 session per condition |
Active tDCS decreased craving more than sham for desserts, savoury proteins, and the all-foods category. Participants ate less total kcals in the lab after active tDCS compared to following sham tDCS. | Active tDCS reduced desire to binge-eat 5-6 hours post-tDCS, but only in male participants. |
Abbreviations: BED - binge eating disorder; rTMS - repetitive transcranial magnetic stimulation; DLPFC - dorsolateral prefrontal cortex; s - seconds, Hz - hertz; MT - motor threshold; BDI - Beck Depression Inventory [76]; BES - Binge Eating Scale [83]; tDCS - transcranial direct current stimulation; mA - milliAmpere.
3.4.1. rTMS
A case study of a female with refractory BED and comorbid depression found that 20 sessions of high frequency rTMS to the left DLPFC led to a reduction in binge frequency and improvements in the clinical global impression score [80]. Depression and binge eating questionnaire scores also improved.
3.4.2. tDCS
The effects of tDCS were assessed in 30 adults with full or subthreshold BED [81]. Active tDCS (anode right DLPFC/cathode left DLPFC) was found to decrease craving more than sham tDCS for desserts, savoury proteins, and the all-foods category. Interestingly, active tDCS reduced the male participant’s craving more than the female’s craving for desserts and the all-foods category. Participants ate fewer total kilocalories in the lab after active tDCS compared to following sham tDCS. Active tDCS reduced desire to binge eat 5-6 hours post-stimulation, however, this was observed in the male participants only.
4. DISCUSSION
4.1. Overall Findings
Our review shows that there is an expanding literature on the use of neurostimulation procedures for the treatment of EDs and related eating behaviours (food craving). Compared to our earlier systematic review only 4 years ago, the number of available studies and the number of participants in them, has more than doubled, which is encouraging. However, as yet, the majority of these studies are single case studies, case series, or proof-of-concept experimental studies using single session and cross-over designs. These studies provide preliminary evidence that suggests that neurostimulation has potential for altering disordered eating behaviours, food intake and body weight. Therefore, a strong case can be made for continuing to examine and develop neurostimulation protocols that can be used to treat EDs and which can also be used as illness probes [50].
At this point DBS is arguably the treatment with the strongest theoretical underpinnings and clearest rationale for specific treatment targets. It is also the most invasive of these treatments and as such has been mainly advocated for use in severe and enduring AN. DBS has shown promise in different case series, and can give new hope to this group of patients who have often received multiple unsuccessful treatments. However, several small studies from China have applied DBS in adolescent patients with very severe, recent onset of illness (and therefore good prognosis). Whilst these were clearly cases with often alarmingly low BMIs, one cannot help considering whether in a different healthcare system with greater access to specialist ED in-patient treatment programmes and associated family-based treatments, some of these cases would have recovered with the help of less invasive treatments [84, 85].
To date only 3 parallel group RCTs using rTMS as treatment, i.e. applying multi-session protocols, have been reported. All of these used high frequency (excitatory) rTMS and stimulated the left DLPFC. All of these are small trials. Two of these [72, 73], which were focused on BN, did not show an effect of rTMS on ED outcomes at post-treatment assessment. The third is a feasibility trial in patients with severe and enduring AN and this has shown promising results at 3-month follow-up, with group differences of medium effect sizes mainly in relation to improvement of mood [Schmidt, personal communication; for protocol see 63]. Key differences between these studies lie in the number of rTMS sessions offered, the way the stimulation target was determined (with or without MRI guidance) and their assessment schedule. Compared to the AN study, the two BN studies offered fewer sessions (10 or 15) and did not determine the stimulation target with MRI guidance. Moreover, neither of the two BN studies included a follow-up and only immediate post-treatment effects were assessed. In contrast, in the AN study, the largest changes were seen at the 3 month follow-up point. This delay in action is something we had previously observed in a small case series of AN patients treated using the same protocol [40]. It is also known from rTMS trials in depression [86, 87].
Despite the ease of use of tDCS, this procedure/technique has to date been used mainly in analogue populations in relation to food craving (5 studies), with just one small case series in AN and two small cross-over proof-of-concept trials in BN and BED. Given that this is by far the most accessible and easy to use method, this is somewhat surprising.
4.2. Cognitive, Neural and Hormonal Correlates and Predictors of Neurostimulation Treatments
In this review, we have focused predominantly on ED and related clinical outcomes. However, there is a growing literature in healthy and clinical populations on the neuro-cognitive and biological (neural, immunological, electrophysiological, and hormonal) effects of neurostimulation treatments [88]. Only a handful of studies have examined these effects in relation to neurostimulation of EDs. As far as we are aware only two studies assessed the effects of rTMS on salivary cortisol in BN [71] and AN [62] with the former finding an effect, the latter not. Several neuro-cognitive tasks have been studied, such as temporal discounting, assessing choice impulsivity [53, 62; Schmidt, personal communication, 79]; Stroop tasks [50, 82]; the Go-No-go Task [50, 51] and a ‘rock-paper-scissors’ task [66]. All of these are thought to assess elements of self-regulatory or inhibitory control. These various neurocognitive studies do not produce consistent evidence for the effects of neurostimulation on executive function. For example, Kekic et al. [79] and McClelland et al. [62], using temporal discounting, have produced data which are indicative of increased self-regulation following neurostimulation (tDCS in BN and rTMS in AN respectively). Somewhat in agreement with this is the report by Lowe et al. [50], in that decreasing cortical activity using cTBS diminished inhibitory control in food cravers as measured using a Stroop Task. On the other hand, Van den Eynde et al. [82] found no effect of rTMS on Stroop performance in patients with BN. At this point, it is not possible to make definitive statements on how and if executive function is altered by neurostimulation, even when one is stimulating an area known to be involved in self-regulatory and inhibitory control (i.e. DLPFC). Until the effects of neurostimulation of the DLPFC on executive function are more established, it is not possible to determine whether effects on executive function mediate the effect of neurostimulation on clinical change in ED symptoms.
A limited number of studies have included a neuroimaging component and hence the field is in its infancy. Two DBS studies have used PET [44, 58] and one diffusion tensor imaging [43], two rTMS studies [48; Schmidt, personal communication] have used functional MRI and one has used near-infrared spectroscopy (NIRS) [66], identifying stimulation-related changes in brain metabolism and neural connectivity. Both PET-based studies reported significant and quite widespread changes in brain glucose metabolism following DBS [44, 58], but simple conclusions on the mode of action do not appear to be obvious at this point. Findings from Dunlop et al. [48] suggest that treatment responders exhibit an increase in frontostriatal functional connectivity following rTMS treatment; however, in those who do not respond these increases do not occur. Such findings are broadly consistent with “top down-bottom-up” neural models of eating disorders which are centred on frontostriatal circuitry [4, 9].
All in all, as can be seen from the above studies, combining data on cognitive, neuroimaging, other biological markers and neurostimulation data are as yet in their infancy in EDs compared, for example, to depression. In future, such studies in EDs may be able to identify distinct endophenotypes, associated with differential responses to interventions at both the cognitive/biological/neural and the clinical level. In this way, they might also help tailor rTMS parameters to individual patients and they may shed light on illness mechanisms and strengthen the scientific rationale for the use of neurostimulation [48].
4.3. Safety and Acceptability
In general, NIBS seems to be safe and acceptable to patients [89-91]. Potential risks include those that are device-related (e.g. seizure risk (rTMS) or skin irritation/burn (tDCS)), adverse cognitive effects and interference with psychiatric treatment [16]. A systematic review of tDCS studies found similarly low drop-out rates for real and sham tDCS [89]. However, these authors noted that the quality of adverse events reporting was low in most studies. Very limited research on issues of safety and acceptability has been conducted in relation to EDs [92].
4.4. Ethical Considerations
Questions over the ethical implications of these neurostimulation techniques have mainly focused on DBS rather than on NIBS, given the invasiveness of DBS and its use in highly vulnerable patients, such as adolescent patients or physically very frail patients with severe and enduring AN. In both cases the capacity for making health-related decisions may be impaired. Additionally, desperate families may push their loved one towards agreeing to DBS. Ethicists have also raised concerns that DBS or NIBS might be perceived as ‘mind control’, increasing patients’ helplessness and reducing their sense of authenticity [93, 94]. The limited literature exploring ED patients’ views shows that they are able to understand and reflect on issues related to gains and threats to their authenticity [95, 96]. In a small case series of therapeutic rTMS in AN, patients were asked about their experience [40]. They talked about greater cognitive clarity, flexibility & improved mood. Altered authenticity or agency was not mentioned by any of the participants. Recently a neuro-ethics framework for the use of DBS in AN has been published [97].
4.5. Future Directions
Whilst the evidence suggests that neurostimulation treatments have significant potential, both as interventions in the treatment of EDs and as probes of illness mechanisms, much of this potential is still waiting to emerge. There is still a considerable amount that needs to be learnt about patient selection, intervention parameters, treatment targets and how to optimise protocols. For example, protocol optimisation will require a substantial amount of experimental work in order to address issues such as target selection, frequency, intensity and duration, and even the type of neurostimulation. Furthermore, much more needs to be learnt about neurocognitive, neural and other biological predictors and correlates of outcome, as this may help to individualise protocols and deliver personalised treatment. Progress is also being made in relation to developing a rationale for use of neurostimulation treatments, substantially based on evolving neural models of EDs [13], including the role of memory and its reconsolidation in the development and treatment of EDs. These advances together with the rapidly increasing knowledge of neural networks and their interconnectivity will lead to the formulation of new hypotheses on the aetiology and treatment of EDs.
Neurostimulation technologies continue to evolve, and for example, in the case of NIBS, are increasingly allowing more precise targeting of treatment, use of increasingly briefer and more powerful treatment protocols, probing deeper brain areas and stimulating multiple brain targets simultaneously [13]. There is emerging evidence suggesting that these kinds of interventions may work synergistically when applied with different forms of cognitive training, as yet this combination treatment is unexplored in EDs. A framework for combining rTMS with behavioural interventions has been described [98]. Other promising neurotechnologies, such as functional MRI neurofeedback [99] or vagus nerve stimulation, as yet have not been explored in relation to EDs.
At present, the rationale for use of one NIBS procedure over another is unclear. Ultimately this may be mostly influenced by practical considerations such as costs, availability and commercial interests. In this respect, it is noted that portable tDCS devices are available, which can be used at home. However, given the limited research in this field, it is not advised that individuals make use of these neurostimulation technologies without supervision from an experienced therapist.
Finally, we have reported elsewhere [100, 101] that several large scale trials of neurostimulation treatments of EDs are forthcoming, i.e. registered with national and international trial registries. Several of these are transdiagnostic and/or target specific ED symptoms (e.g. binge-eating or body image problems). Thus over the next few years, we are likely to see an explosion of knowledge in this area.
DISCLOSURE OF FUNDING
Bethan Dalton and Savani Bartholdy were supported by a National Institute for Health Research (NIHR) Research for Patient Benefit (RfPB) grant (RB-PG-1013-32049). Iain Campbell, and Ulrike Schmidt receive salary support from the National Institute for Health Research (NIHR) Biomedical Research Centre for Mental Health, South London and Maudsley NHS Foundation Trust and Institute of Psychiatry, Psychology and Neuroscience, King’s College London. Ulrike Schmidt is supported by an NIHR Senior Investigator award. The views expressed herein are not those of NIHR or the NHS.
CONSENT FOR PUBLICATION
Not applicable.
ACKNOWLEDGEMENTS
Declared none.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
REFERENCES
- 1.Schmidt U., Campbell I.C. Treatment of eating disorders can not remain ‘brainless’: The case for brain-directed treatments. Eur. Eat. Disord. Rev. 2013;21(6):425–427. doi: 10.1002/erv.2257. [http://dx.doi.org/10.1002/ erv.2257]. [PMID: 24123463]. [DOI] [PubMed] [Google Scholar]
- 2.Deisseroth K. Optical and chemical discoveries recognized for impact on biology and psychiatry. EMBO Rep. 2017;18(6):859–860. doi: 10.15252/embr.201744405. [http://dx.doi.org/10.15252/embr.201744405]. [PMID: 28566521]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Poldrack R.A., Congdon E., Triplett W., Gorgolewski K.J., Karlsgodt K.H., Mumford J.A., Sabb F.W., Freimer N.B., London E.D., Cannon T.D., Bilder R.M. A phenome-wide examination of neural and cognitive function. Sci. Data. 2016;3:160110. doi: 10.1038/sdata.2016.110. [http://dx.doi.org/10.1038/sdata.2016.110]. [PMID: 27922632]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kaye W.H., Fudge J.L., Paulus M. New insights into symptoms and neurocircuit function of anorexia nervosa. Nat. Rev. Neurosci. 2009;10(8):573–584. doi: 10.1038/nrn2682. [http://dx.doi.org/10.1038/nrn2682]. [PMID: 19603056]. [DOI] [PubMed] [Google Scholar]
- 5.Kaye W.H., Wagner A., Fudge J.L., Paulus M. Neurocircuity of eating disorders. Curr. Top. Behav. Neurosci. 2011;6:37–57. doi: 10.1007/7854_2010_85. [http://dx.doi.org/10.1007/7854_2010_85]. [PMID: 21243469]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhu Y., Hu X., Wang J., Chen J., Guo Q., Li C., Enck P. Processing of food, body and emotional stimuli in anorexia nervosa: a systematic review and meta-analysis of functional magnetic resonance imaging studies. Eur. Eat. Disord. Rev. 2012;20(6):439–450. doi: 10.1002/erv.2197. [http://dx.doi.org/10.1002/erv.2197]. [PMID: 22945872]. [DOI] [PubMed] [Google Scholar]
- 7.Frank G.K. Altered brain reward circuits in eating disorders: chicken or egg? Curr. Psychiatry Rep. 2013;15(10):396. doi: 10.1007/s11920-013-0396-x. [http:// dx.doi.org/10.1007/s11920-013-0396-x]. [PMID: 23963630]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Seitz J., Konrad K., Herpertz-Dahlmann B. Exlend, pathomechanism and clinical consequences of brain volume changes in anorexia nervosa. Curr. Neuropharmacol. 2018;16:1164–1173. doi: 10.2174/1570159X15666171109145651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Berner L.A., Marsh R. Frontostriatal circuits and the development of bulimia nervosa. Front. Behav. Neurosci. 2014;8:395. doi: 10.3389/fnbeh.2014.00395. [http://dx.doi.org/10.3389/fnbeh.2014.00395]. [PMID: 25452718]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Balodis I.M., Grilo C.M., Potenza M.N. Neurobiological features of binge eating disorder. CNS Spectr. 2015;20(6):557–565. doi: 10.1017/S1092852915000814. [http://dx.doi.org/10.1017/S1092852915000814]. [PMID: 26530404]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Val-Laillet D., Aarts E., Weber B., Ferrari M., Quaresima V., Stoeckel L.E., Alonso-Alonso M., Audette M., Malbert C.H., Stice E. Neuroimaging and neuromodulation approaches to study eating behavior and prevent and treat eating disorders and obesity. Neuroimage Clin. 2015;8:1–31. doi: 10.1016/j.nicl.2015.03.016. [http://dx.doi.org/10.1016/j.nicl. 2015.03.016]. [PMID: 26110109]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brooks S.J., Rask-Andersen M., Benedict C., Schiöth H.B. A debate on current eating disorder diagnoses in light of neurobiological findings: is it time for a spectrum model? BMC Psychiatry. 2012;12:76. doi: 10.1186/1471-244X-12-76. [http://dx.doi.org/10.1186/1471-244X-12-76]. [PMID: 22770364]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dunlop K.A., Woodside B., Downar J. Targeting neural endophenotypes of eating disorders with non-invasive brain stimulation. Front. Neurosci. 2016;10:30. doi: 10.3389/fnins.2016.00030. [http://dx.doi.org/10.3389/ fnins.2016.00030]. [PMID: 26909013]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wierenga C.E., Ely A., Bischoff-Grethe A., Bailer U.F. ; Simmons A.N., Kaye W.H. Are extremes of consumption in eating disorders related to an altered balance between reward and inhibition? Front. Behav. Neurosci. 2014;8:410. doi: 10.3389/fnbeh.2014.00410. [http://dx. doi.org/10.3389/fnbeh.2014.00410]. [PMID: 25538579]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.O’Hara C.B., Campbell I.C., Schmidt U. A reward-centred model of anorexia nervosa: A focussed narrative review of the neurological and psychophysiological literature. Neurosci. Biobehav. Rev. 2015;52:131–152. doi: 10.1016/j.neubiorev.2015.02.012. [http://dx.doi.org/10.1016/j.neubiorev.2015.02. 012]. [PMID: 25735957]. [DOI] [PubMed] [Google Scholar]
- 16.Philip N.S., Nelson B.G., Frohlich F., Lim K.O., Widge A.S., Carpenter L.L. Low-intensity transcranial current stimulation in psychiatry. Am. J. Psychiatry. 2017;174(7):628–639. doi: 10.1176/appi.ajp.2017.16090996. [http://dx. doi.org/10.1176/appi.ajp.2017.16090996]. [PMID: 28231716]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.George M.S., Aston-Jones G. Noninvasive techniques for probing neurocircuitry and treating illness: vagus nerve stimulation (VNS), transcranial magnetic stimulation (TMS) and transcranial direct current stimulation (tDCS). Neuropsychopharmacology. 2010;35(1):301–316. doi: 10.1038/npp.2009.87. [http://dx.doi.org/10.1038/npp.2009.87]. [PMID: 19693003]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Giordano J., Bikson M., Kappenman E.S., Clark V.P., Coslett H.B., Hamblin M.R., Hamilton R., Jankord R., Kozumbo W.J., McKinley R.A., Nitsche M.A., Reilly J.P., Richardson J., Wurzman R., Calabrese E. Mechanisms and effects of trans-cranial direct current stimulation. Dose Response. 2017;15(1):1559325816685467. doi: 10.1177/1559325816685467. [http://dx.doi.org/10.1177/1559325816685467]. [PMID: 28210202]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lipsman N., Woodside D.B., Lozano A.M. Neurocircuitry of limbic dysfunction in anorexia nervosa. Cortex. 2015;62:109–118. doi: 10.1016/j.cortex.2014.02.020. [http://dx.doi.org/10.1016/j.cortex.2014.02.020]. [PMID: 24703713]. [DOI] [PubMed] [Google Scholar]
- 20.Lane R.D., Ryan L., Nadel L., Greenberg L. Memory reconsolidation, emotional arousal, and the process of change in psychotherapy: New insights from brain science. Behav. Brain Sci. 2015;38:e1. doi: 10.1017/S0140525X14000041. [http://dx.doi.org/10.1017/S0140525X14000041]. [PMID: 24827452]. [DOI] [PubMed] [Google Scholar]
- 21.Zipfel S., Giel K.E., Bulik C.M., Hay P., Schmidt U. Anorexia nervosa: aetiology, assessment, and treatment. Lancet Psychiatry. 2015;2(12):1099–1111. doi: 10.1016/S2215-0366(15)00356-9. [http://dx.doi.org/10.1016/S2215-0366 (15)00356-9]. [PMID: 26514083]. [DOI] [PubMed] [Google Scholar]
- 22.Milad M.R., Quirk G.J. Fear extinction as a model for translational neuroscience: ten years of progress. Annu. Rev. Psychol. 2012;63:129–151. doi: 10.1146/annurev.psych.121208.131631. [http://dx.doi.org/10.1146/annurev.psych.121208. 131631]. [PMID: 22129456]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Inda M.C., Muravieva E.V., Alberini C.M. Memory retrieval and the passage of time: from reconsolidation and strengthening to extinction. J. Neurosci. 2011;31(5):1635–1643. doi: 10.1523/JNEUROSCI.4736-10.2011. [http://dx.doi.org/ 10.1523/JNEUROSCI.4736-10.2011]. [PMID: 21289172]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Koskina A., Campbell I.C., Schmidt U. Exposure therapy in eating disorders revisited. Neurosci. Biobehav. Rev. 2013;37(2):193–208. doi: 10.1016/j.neubiorev.2012.11.010. [http://dx.doi.org/10.1016/j.neubiorev.2012.11.010]. [PMID: 23201859]. [DOI] [PubMed] [Google Scholar]
- 25.Murray S.B., Treanor M., Liao B., Loeb K.L., Griffiths S., Le Grange D. Extinction theory & anorexia nervosa: Deepening therapeutic mechanisms. Behav. Res. Ther. 2016;87:1–10. doi: 10.1016/j.brat.2016.08.017. [http:// dx.doi.org/10.1016/j.brat.2016.08.017]. [PMID: 27580026]. [DOI] [PubMed] [Google Scholar]
- 26.Lee J.L.C., Nader K., Schiller D. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Das R.K., Lawn W., Kamboj S.K. Rewriting the valuation and salience of alcohol-related stimuli via memory reconsolidation. Transl. Psychiatry. 2015;5:e645. doi: 10.1038/tp.2015.132. [http://dx.doi.org/10.1038/tp. 2015.132]. [PMID: 26393491]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dunsmoor J.E., Campese V.D., Ceceli A.O., LeDoux J.E., Phelps E.A. Novelty-facilitated extinction: providing a novel outcome in place of an expected threat diminishes recovery of defensive responses. Biol. Psychiatry. 2015;78(3):203–209. doi: 10.1016/j.biopsych.2014.12.008. [http:// dx.doi.org/10.1016/j.biopsych.2014.12.008]. [PMID: 25636175]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hon T., Das R.K., Kamboj S.K. The effects of cognitive reappraisal following retrieval-procedures designed to destabilize alcohol memories in high-risk drinkers. Psychopharmacology (Berl.) 2016;233(5):851–861. doi: 10.1007/s00213-015-4164-y. [http://dx.doi.org/10.1007/s00213-015-4164-y]. [PMID: 26667478]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Soeter M., Kindt M. An abrupt transformation of phobic behavior after a post-retrieval amnesic agent. Biol. Psychiatry. 2015;78(12):880–886. doi: 10.1016/j.biopsych.2015.04.006. [http://dx.doi.org/10.1016/j.biopsych.2015.04.006]. [PMID: 25980916]. [DOI] [PubMed] [Google Scholar]
- 31.Makkar S.R., Zhang S.Q., Cranney J. Behavioral and neural analysis of GABA in the acquisition, consolidation, reconsolidation, and extinction of fear memory. Neuropsychopharmacology. 2010;35(8):1625–1652. doi: 10.1038/npp.2010.53. [http://dx.doi.org/10.1038/npp.2010.53]. [PMID: 20410874]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hofmann S.G., Meuret A.E., Smits J.A., Simon N.M., Pollack M.H., Eisenmenger K., Shiekh M., Otto M.W. Augmentation of exposure therapy with D-cycloserine for social anxiety disorder. Arch. Gen. Psychiatry. 2006;63(3):298–304. doi: 10.1001/archpsyc.63.3.298. [http://dx.doi.org/ 10.1001/archpsyc.63.3.298]. [PMID: 16520435]. [DOI] [PubMed] [Google Scholar]
- 33.Levinson C.A., Rodebaugh T.L., Fewell L., Kass A.E., Riley E.N., Stark L., McCallum K., Lenze E.J. D-Cycloserine facilitation of exposure therapy improves weight regain in patients with anorexia nervosa: A pilot randomized controlled trial. J. Clin. Psychiatry. 2015;76(6):e787–e793. doi: 10.4088/JCP.14m09299. [http://dx.doi.org/10.4088/JCP. 14m09299]. [PMID: 26132687]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Barron H.C., Vogels T.P., Emir U.E., Makin T.R., O’Shea J., Clare S., Jbabdi S., Dolan R.J., Behrens T.E. Unmasking latent inhibitory connections in human cortex to reveal dormant cortical memories. Neuron. 2016;90(1):191–203. doi: 10.1016/j.neuron.2016.02.031. [http://dx.doi.org/10. 1016/j.neuron.2016.02.031]. [PMID: 26996082]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yizhar O., Fenno L.E., Prigge M., Schneider F., Davidson T.J., O’Shea D.J., Sohal V.S., Goshen I., Finkelstein J., Paz J.T., Stehfest K., Fudim R., Ramakrishnan C., Huguenard J.R., Hegemann P., Deisseroth K. Neocortical excitation/inhibition balance in information processing and social dysfunction. Nature. 2011;477(7363):171–178. doi: 10.1038/nature10360. [http://dx.doi.org/10.1038/nature10360]. [PMID: 21796121]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McClelland J., Bozhilova N., Campbell I., Schmidt U. A systematic review of the effects of neuromodulation on eating and body weight: evidence from human and animal studies. Eur. Eat. Disord. Rev. 2013;21(6):436–455. doi: 10.1002/erv.2256. [http://dx.doi.org/10.1002/ erv.2256]. [PMID: 24155246]. [DOI] [PubMed] [Google Scholar]
- 37.Aaronson S.T., Sears P., Ruvuna F., Bunker M., Conway C.R., Dougherty D.D., Reimherr F.W., Schwartz T.L., Zajecka J.M.A. 5-Year observational study of patients with treatment-resistant Depression treated with vagus nerve stimulation or treatment as usual: comparison of response, remission, and suicidality. Am. J. Psychiatry. 2017;174(7):640–648. doi: 10.1176/appi.ajp.2017.16010034. [http://dx.doi.org/10.1176/appi.ajp. 2017.16010034]. [PMID: 28359201]. [DOI] [PubMed] [Google Scholar]
- 38.Bremner J.D., Rapaport M.H. Vagus nerve stimulation: Back to the future. Am. J. Psychiatry. 2017;174(7):609–610. doi: 10.1176/appi.ajp.2017.17040422. [http://dx.doi. org/10.1176/appi.ajp.2017.17040422]. [PMID: 28669203]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Moher D., Liberati A., Tetzlaff J., Altman D.G., Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. doi: 10.1371/journal.pmed.1000097. [http:// dx.doi.org/10.1371/journal.pmed.1000097]. [PMID: 19621072]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McClelland J., Kekic M., Campbell I.C., Schmidt U. Repetitive transcranial magnetic stimulation (rTMS) treatment in enduring anorexia nervosa: A case series. Eur. Eat. Disord. Rev. 2016;24(2):157–163. doi: 10.1002/erv.2414. [http://dx.doi.org/10.1002/erv.2414]. [PMID: 26537308]. [DOI] [PubMed] [Google Scholar]
- 41.McClelland J., Bozhilova N., Nestler S., Campbell I.C., Jacob S., Johnson-Sabine E., Schmidt U. Improvements in symptoms following neuronavigated repetitive transcranial magnetic stimulation (rTMS) in severe and enduring anorexia nervosa: findings from two case studies. Eur. Eat. Disord. Rev. 2013;21(6):500–506. doi: 10.1002/erv.2266. [http://dx.doi.org/10.1002/erv.2266]. [PMID: 24155247]. [DOI] [PubMed] [Google Scholar]
- 42.Lipsman N., Woodside D.B., Giacobbe P., Hamani C., Carter J.C., Norwood S.J., Sutandar K., Staab R., Elias G., Lyman C.H., Smith G.S., Lozano A.M. Subcallosal cingulate deep brain stimulation for treatment-refractory anorexia nervosa: a phase 1 pilot trial. Lancet. 2013;381(9875):1361–1370. doi: 10.1016/S0140-6736(12)62188-6. [http://dx.doi.org/ 10.1016/S0140-6736(12)62188-6]. [PMID: 23473846]. [DOI] [PubMed] [Google Scholar]
- 43.Hayes D.J., Lipsman N., Chen D.Q., Woodside D.B., Davis K.D., Lozano A.M., Hodaie M. Subcallosal cingulate connectivity in anorexia nervosa patients differs from healthy controls: A Multi-tensor tractography study. Brain Stimul. 2015;8(4):758–768. doi: 10.1016/j.brs.2015.03.005. [http://dx.doi.org/10.1016/j.brs.2015.03.005]. [PMID: 26073966]. [DOI] [PubMed] [Google Scholar]
- 44.Lipsman N., Lam E., Volpini M., Sutandar K., Twose R., Giacobbe P., Sodums D.J., Smith G.S., Woodside D.B., Lozano A.M. Deep brain stimulation of the subcallosal cingulate for treatment-refractory anorexia nervosa: 1-year follow-up of an open-label trial. Lancet Psychiatry. 2017;4(4):285–294. doi: 10.1016/S2215-0366(17)30076-7. [http://dx.doi. org/10.1016/S2215-0366(17)30076-7]. [PMID: 28238701]. [DOI] [PubMed] [Google Scholar]
- 45.McLaughlin N.C., Didie E.R., Machado A.G., Haber S.N., Eskandar E.N., Greenberg B.D. Improvements in anorexia symptoms after deep brain stimulation for intractable obsessive-compulsive disorder. Biol. Psychiatry. 2013;73(9):e29–e31. doi: 10.1016/j.biopsych.2012.09.015. [http:// dx.doi.org/10.1016/j.biopsych.2012.09.015]. [PMID: 23128051]. [DOI] [PubMed] [Google Scholar]
- 46.Van den Eynde F., Guillaume S., Broadbent H., Campbell I.C., Schmidt U. Repetitive transcranial magnetic stimulation in anorexia nervosa: a pilot study. Eur. Psychiatry. 2013;28(2):98–101. doi: 10.1016/j.eurpsy.2011.06.002. [http://dx.doi.org/10.1016/j.eurpsy.2011.06.002]. [PMID: 21880470]. [DOI] [PubMed] [Google Scholar]
- 47.Wu H., Van Dyck-Lippens P.J., Santegoeds R., van Kuyck K., Gabriels L. Deep-brain stimulation for anorexia nervosa. 2013. [DOI] [PubMed]
- 48.Dunlop K., Woodside B., Lam E., Olmsted M., Colton P., Giacobbe P., Downar J. Increases in frontostriatal connectivity are associated with response to dorsomedial repetitive transcranial magnetic stimulation in refractory binge/purge behaviors. Neuroimage Clin. 2015;8:611–618. doi: 10.1016/j.nicl.2015.06.008. [http://dx.doi.org/10.1016/j.nicl.2015. 06.008]. [PMID: 26199873]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ljubisavljevic M., Maxood K., Bjekic J., Oommen J., Nagelkerke N. Long-Term effects of repeated prefrontal cortex transcranial direct current stimulation (tDCS) on food craving in normal and overweight young Adults. Brain Stimul. 2016;9(6):826–833. doi: 10.1016/j.brs.2016.07.002. [http://dx.doi.org/10.1016/j.brs.2016.07.002]. [PMID: 27498606]. [DOI] [PubMed] [Google Scholar]
- 50.Lowe C.J., Hall P.A., Staines W.R. The effects of continuous theta burst stimulation to the left dorsolateral prefrontal cortex on executive function, food cravings, and snack food consumption. Psychosom. Med. 2014;76(7):503–511. doi: 10.1097/PSY.0000000000000090. [http://dx.doi.org/10.1097/ PSY.0000000000000090]. [PMID: 25215552]. [DOI] [PubMed] [Google Scholar]
- 51.Lapenta O.M., Sierve K.D., de Macedo E.C., Fregni F., Boggio P.S. Transcranial direct current stimulation modulates ERP-indexed inhibitory control and reduces food consumption. Appetite. 2014;83:42–48. doi: 10.1016/j.appet.2014.08.005. [http://dx.doi.org/10.1016/j.appet.2014.08.005]. [PMID: 25128836]. [DOI] [PubMed] [Google Scholar]
- 52.Fregni F., Orsati F., Pedrosa W., Fecteau S., Tome F.A., Nitsche M.A., Mecca T., Macedo E.C., Pascual-Leone A., Boggio P.S. Transcranial direct current stimulation of the prefrontal cortex modulates the desire for specific foods. Appetite. 2008;51(1):34–41. doi: 10.1016/j.appet.2007.09.016. [http://dx.doi.org/10.1016/j.appet.2007.09.016]. [PMID: 18243412]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kekic M., McClelland J., Campbell I., Nestler S., Rubia K., David A.S., Schmidt U. The effects of prefrontal cortex transcranial direct current stimulation (tDCS) on food craving and temporal discounting in women with frequent food cravings. Appetite. 2014;78:55–62. doi: 10.1016/j.appet.2014.03.010. [http://dx.doi.org/10.1016/j.appet.2014.03.010]. [PMID: 24656950]. [DOI] [PubMed] [Google Scholar]
- 54.Goldman R.L., Borckardt J.J., Frohman H.A., O’Neil P.M., Madan A., Campbell L.K., Budak A., George M.S. Prefrontal cortex transcranial direct current stimulation (tDCS) temporarily reduces food cravings and increases the self-reported ability to resist food in adults with frequent food craving. Appetite. 2011;56(3):741–746. doi: 10.1016/j.appet.2011.02.013. [http://dx.doi.org/10.1016/j.appet.2011.02.013]. [PMID: 21352881]. [DOI] [PubMed] [Google Scholar]
- 55.Barth K., Rydin-Gray S., Kose S., Borckardt J., O’Neil P., Shaw D., Madan A., George M. Food cravings and the effects of left prefrontal repetitive transcranial magnetic stimulation using an improved sham condition. Front. Psychol. 2011;2:9. doi: 10.3389/fpsyt.2011.00009. [http://dx.doi. org/10.3389/fpsyt.2011.00009]. [PMID: 21556279]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Uher R., Yoganathan D., Mogg A., Eranti S.V., Treasure J., Campbell I.C., McLoughlin D.M., Schmidt U. Effect of left prefrontal repetitive transcranial magnetic stimulation on food craving. Biol. Psychiatry. 2005;58(10):840–842. doi: 10.1016/j.biopsych.2005.05.043. [http://dx.doi.org/10. 1016/j.biopsych.2005.05.043]. [PMID: 16084855]. [DOI] [PubMed] [Google Scholar]
- 57.Wang J., Chang C., Geng N., Wang X., Gao G. Treatment of intractable anorexia nervosa with inactivation of the nucleus accumbens using stereotactic surgery. Stereotact. Funct. Neurosurg. 2013;91(6):364–372. doi: 10.1159/000348278. [http://dx.doi.org/10.1159/000348278]. [PMID: 24108066]. [DOI] [PubMed] [Google Scholar]
- 58.Zhang H.W., Li D.Y., Zhao J., Guan Y.H., Sun B.M., Zuo C.T. Metabolic imaging of deep brain stimulation in anorexia nervosa: a 18F-FDG PET/CT study. Clin. Nucl. Med. 2013;38(12):943–948. doi: 10.1097/RLU.0000000000000261. [PMID: 24212440]. [DOI] [PubMed] [Google Scholar]
- 59.Israël M., Steiger H., Kolivakis T., McGregor L., Sadikot A.F. Deep brain stimulation in the subgenual cingulate cortex for an intractable eating disorder. Biol. Psychiatry. 2010;67(9):e53–e54. doi: 10.1016/j.biopsych.2009.11.016. [http://dx.doi.org/10.1016/j.biopsych.2009.11.016]. [PMID: 20044072]. [DOI] [PubMed] [Google Scholar]
- 60.Blomstedt P., Naesström M., Bodlund O. Deep brain stimulation in the bed nucleus of the stria terminalis and medial forebrain bundle in a patient with major depressive disorder and anorexia nervosa. Clin. Case Rep. 2017;5(5):679–684. doi: 10.1002/ccr3.856. [http://dx.doi.org/ 10.1002/ccr3.856]. [PMID: 28469875]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kamolz S., Richter M.M., Schmidtke A., Fallgatter A.J. Transcranial magnetic stimulation for comorbid depression in anorexia. Nervenarzt. 2008;79(9):1071–1073. doi: 10.1007/s00115-008-2537-8. [DOI] [PubMed] [Google Scholar]
- 62.McClelland J., Kekic M., Bozhilova N., Nestler S., Dew T., Van den Eynde F., David A.S., Rubia K., Campbell I.C., Schmidt U. A Randomised controlled trial of neuronavigated Repetitive transcranial magnetic stimulation (rTMS) in anorexia nervosa. PLoS One. 2016;11(3):e0148606. doi: 10.1371/journal.pone.0148606. [http://dx.doi.org/10. 1371/journal.pone.0148606]. [PMID: 27008620]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bartholdy S., McClelland J., Kekic M., O’Daly O.G., Campbell I.C., Werthmann J., Rennalls S.J., Rubia K., David A.S., Glennon D., Kern N., Schmidt U. Clinical outcomes and neural correlates of 20 sessions of repetitive transcranial magnetic stimulation in severe and enduring anorexia nervosa (the TIARA study): study protocol for a randomised controlled feasibility trial. Trials. 2015;16:548. doi: 10.1186/s13063-015-1069-3. [http://dx.doi.org/10.1186/s13063-015-1069-3]. [PMID: 26634828]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Fairburn C. Cognitive Behav. Ther. Eating Disorders. New York: Guilford Press; 2008. [Google Scholar]
- 65.Khedr E.M., Elfetoh N.A., Ali A.M., Noamany M. Anodal transcranial direct current stimulation over the dorsolateral prefrontal cortex improves anorexia nervosa: A pilot study. Restor. Neurol. Neurosci. 2014;32(6):789–797. doi: 10.3233/RNN-140392. [PMID: 25189181]. [DOI] [PubMed] [Google Scholar]
- 66.Sutoh C., Koga Y., Kimura H., Kanahara N., Numata N., Hirano Y., Matsuzawa D., Iyo M., Nakazato M., Shimizu E. Repetitive transcranial magnetic stimulation changes cerebral oxygenation on the left dorsolateral prefrontal cortex in bulimia nervosa: A near-infrared spectroscopy pilot study. Eur. Eat. Disord. Rev. 2016;24(1):83–88. doi: 10.1002/erv.2413. [http://dx.doi.org/10.1002/erv.2413]. [PMID: 26481583]. [DOI] [PubMed] [Google Scholar]
- 67.Van den Eynde F., Broadbent H., Guillaume S., Claudino A., Campbell I.C., Schmidt U. Handedness, repetitive transcranial magnetic stimulation and bulimic disorders. Eur. Psychiatry. 2012;27(4):290–293. doi: 10.1016/j.eurpsy.2010.08.015. [http://dx.doi.org/10.1016/j.eurpsy.2010.08.015]. [PMID: 21067901]. [DOI] [PubMed] [Google Scholar]
- 68.Downar J., Sankar A., Giacobbe P., Woodside B., Colton P. Unanticipated rapid remission of refractory bulimia nervosa, during high-dose repetitive transcranial magnetic stimulation of the dorsomedial prefrontal cortex: A case report. Front. Psychiatry. 2012;3:30. doi: 10.3389/fpsyt.2012.00030. [http://dx.doi.org/10.3389/fpsyt.2012.00030]. [PMID: 22529822]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hausmann A., Mangweth B., Walpoth M., Hoertnagel C., Kramer-Reinstadler K., Rupp C.I., Hinterhuber H. Repetitive transcranial magnetic stimulation (rTMS) in the double-blind treatment of a depressed patient suffering from bulimia nervosa: a case report. Int. J. Neuropsychopharmacol. 2004;7(3):371–373. doi: 10.1017/S1461145704004420. [http://dx.doi.org/10.1017/S1461145704004420]. [PMID: 15154975]. [DOI] [PubMed] [Google Scholar]
- 70.Van den Eynde F., Claudino A.M., Mogg A., Horrell L., Stahl D., Ribeiro W., Uher R., Campbell I., Schmidt U. Repetitive transcranial magnetic stimulation reduces cue-induced food craving in bulimic disorders. Biol. Psychiatry. 2010;67(8):793–795. doi: 10.1016/j.biopsych.2009.11.023. [http:// dx.doi.org/10.1016/j.biopsych.2009.11.023]. [PMID: 20060105]. [DOI] [PubMed] [Google Scholar]
- 71.Claudino A.M., Van den Eynde F., Stahl D., Dew T., Andiappan M., Kalthoff J., Schmidt U., Campbell I.C. Repetitive transcranial magnetic stimulation reduces cortisol concentrations in bulimic disorders. Psychol. Med. 2011;41(6):1329–1336. doi: 10.1017/S0033291710001881. [http://dx. doi.org/10.1017/S0033291710001881]. [PMID: 20925970]. [DOI] [PubMed] [Google Scholar]
- 72.Walpoth M., Hoertnagl C., Mangweth-Matzek B., Kemmler G., Hinterhölzl J., Conca A., Hausmann A. Repetitive transcranial magnetic stimulation in bulimia nervosa: preliminary results of a single-centre, randomised, double-blind, sham-controlled trial in female outpatients. Psychother. Psychosom. 2008;77(1):57–60. doi: 10.1159/000110061. [http://dx.doi.org/10.1159/000110061]. [PMID: 18087209]. [DOI] [PubMed] [Google Scholar]
- 73.Gay A., Jaussent I., Sigaud T., Billard S., Attal J., Seneque M., Galusca B., Van Den Eynde F., Massoubre C., Courtet P., Guillaume S. A lack of clinical effect of high-frequency rTMS to dorsolateral prefrontal cortex on bulimic symptoms: A randomised, double-blind trial. Eur. Eat. Disord. Rev. 2016;24(6):474–481. doi: 10.1002/erv.2475. [http://dx.doi.org/10.1002/erv.2475]. [PMID: 27633286]. [DOI] [PubMed] [Google Scholar]
- 74.American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders. 4th ed. Washington, DC: American Psychiatric Association; 2000. [Google Scholar]
- 75.American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Arlington, VA: American Psychiatric Association; 2013. [Google Scholar]
- 76.Beck A.T., Ward C.H., Mendelson M., Mock J., Erbaugh J. An inventory for measuring depression. Arch. Gen. Psychiatry. 1961;4:561–571. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- 77.Garner D.M., Olmstead M.P., Polivy J. Development and validation of a multidimensional eating disorder inventory for anorexia nervosa and bulimia. Int. J. Eat. Disord. 1983;2:15–34. [Google Scholar]
- 78.Garner D.M., Olmsted M.P., Bohr Y., Garfinkel P.E. The Eating Attitudes Test: Psychometric features and clinical correlates. Psych. Med. 1982;12:871–878. doi: 10.1017/s0033291700049163. [PMID: 6961471]. [DOI] [PubMed] [Google Scholar]
- 79.Kekic M., McClelland J., Bartholdy S., Boysen E., Musiat P., Dalton B., Tiza M., David A.S., Campbell I.C., Schmidt U. Single-session transcranial direct current stimulation temporarily improves symptoms, mood, and self-regulatory control in bulimia nervosa: A randomised controlled trial. PLoS One. 2017;12(1):e0167606. doi: 10.1371/journal.pone.0167606. [http://dx.doi.org/10.1371/journal.pone.0167606]. [PMID: 28121991]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Baczynski T.P., de Aquino Chaim C.H., Nazar B.P., Carta M.G., Arias-Carrion O., Silva A.C., Machado S., Nardi A.E. High-frequency rTMS to treat refractory binge eating disorder and comorbid depression: a case report. CNS Neurol. Disord. Drug Targets. 2014;13(5):771–775. doi: 10.2174/1871527313666140307154823. [http://dx.doi.org/10.2174/ 1871527313666140307154823]. [PMID: 24606720]. [DOI] [PubMed] [Google Scholar]
- 81.Burgess E.E., Sylvester M.D., Morse K.E., Amthor F.R., Mrug S., Lokken K.L., Osborn M.K., Soleymani T., Boggiano M.M. Effects of transcranial direct current stimulation (tDCS) on binge eating disorder. Int. J. Eat. Disord. 2016;49(10):930–936. doi: 10.1002/eat.22554. [http://dx.doi.org/10.1002/eat.22554]. [PMID: 27159906]. [DOI] [PubMed] [Google Scholar]
- 82.Van den Eynde F., Claudino A.M., Campbell I.C., Schmidt U. Immediate cognitive effects of repetitive Transcranial Magnetic Stimulation in eating disorders: a pilot study. Eat. Weight Disord. 2011;16(1):e45–e48. doi: 10.1007/BF03327520. [http://dx.doi.org/10.1007/BF03327520]. [PMID: 21727781]. [DOI] [PubMed] [Google Scholar]
- 83.Gormally J., Black S., Daston S., Rardin D. The assessment of binge eating severity among obese persons. Addict. Behav. 1982;7:47–55. doi: 10.1016/0306-4603(82)90024-7. [PMID: 7080884]. [DOI] [PubMed] [Google Scholar]
- 84.Herpertz-Dahlmann B., Schwarte R., Krei M., Egberts K., Warnke A., Wewetzer C., Pfeiffer E., Fleischhaker C., Scherag A., Holtkamp K., Hagenah U., Bühren K., Konrad K., Schmidt U., Schade-Brittinger C., Timmesfeld N., Dempfle A. Day-patient treatment after short inpatient care versus continued inpatient treatment in adolescents with anorexia nervosa (ANDI): a multicentre, randomised, open-label, non-inferiority trial. Lancet. 2014;383(9924):1222–1229. doi: 10.1016/S0140-6736(13)62411-3. [http://dx.doi.org/10.1016/S0140-6736(13)62411-3]. [PMID: 24439238]. [DOI] [PubMed] [Google Scholar]
- 85.Madden S., Miskovic-Wheatley J., Wallis A., Kohn M., Lock J., Le Grange D., Jo B., Clarke S., Rhodes P., Hay P., Touyz S. A randomized controlled trial of in-patient treatment for anorexia nervosa in medically unstable adolescents. Psychol. Med. 2015;45(2):415–427. doi: 10.1017/S0033291714001573. [http://dx.doi.org/10.1017/S0033291714001573]. [PMID: 25017941]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Eche J., Mondino M., Haesebaert F., Saoud M., Poulet E., Brunelin J. Low- vs high-frequency repetitive transcranial magnetic stimulation as an add-on treatment for refractory depression. Front. Psychiatry. 2012;3:13. doi: 10.3389/fpsyt.2012.00013. [http://dx.doi.org/10.3389/ fpsyt.2012.00013]. [PMID: 22408627]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Brunelin J., Jalenques I., Trojak B., Attal J., Szekely D., Gay A., Januel D., Haffen E., Schott-Pethelaz A.M., Brault C., Poulet E. The efficacy and safety of low frequency repetitive transcranial magnetic stimulation for treatment-resistant depression: the results from a large multicenter French RCT. Brain Stimul. 2014;7(6):855–863. doi: 10.1016/j.brs.2014.07.040. [http://dx.doi.org/10.1016/j.brs.2014.07.040]. [PMID: 25192980]. [DOI] [PubMed] [Google Scholar]
- 88.Fidalgo T.M., Morales-Quezada J.L., Muzy G.S., Chiavetta N.M., Mendonca M.E., Santana M.V., Goncalves O.F., Brunoni A.R., Fregni F. Biological markers in noninvasive brain stimulation trials in major depressive disorder: A systematic review. J. ECT. 2014;30(1):47–61. doi: 10.1097/YCT.0b013e31828b34d8. [http://dx.doi.org/10.1097/YCT.0b013 e31828b34d8]. [PMID: 23845938]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Aparício L.V.M., Guarienti F., Razza L.B., Carvalho A.F., Fregni F., Brunoni A.R. A systematic review on the acceptability and tolerability of transcranial direct current stimulation treatment in neuropsychiatry trials. Brain Stimul. 2016;9(5):671–681. doi: 10.1016/j.brs.2016.05.004. [http://dx.doi.org/10.1016/j.brs.2016.05.004]. [PMID: 27261431]. [DOI] [PubMed] [Google Scholar]
- 90.Bikson M., Grossman P., Thomas C., Zannou A.L., Jiang J., Adnan T., Mourdoukoutas A.P., Kronberg G., Truong D., Boggio P., Brunoni A.R., Charvet L., Fregni F., Fritsch B., Gillick B., Hamilton R.H., Hampstead B.M., Jankord R., Kirton A., Knotkova H., Liebetanz D., Liu A., Loo C., Nitsche M.A., Reis J., Richardson J.D., Rotenberg A., Turkeltaub P.E., Woods A.J. Safety of transcranial direct current stimulation: Evidence based update 2016. Brain Stimul. 2016;9(5):641–661. doi: 10.1016/j.brs.2016.06.004. [http://dx.doi. org/10.1016/j.brs.2016.06.004]. [PMID: 27372845]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Rossi S., Hallett M., Rossini P.M., Pascual-Leone A. Safety, ethical considerations, and application guidelines for the use of transcranial magnetic stimulation in clinical practice and research. Clin. Neurophysiol. 2009;120(12):2008–2039. doi: 10.1016/j.clinph.2009.08.016. [http://dx.doi.org/ 10.1016/j.clinph.2009.08.016]. [PMID: 19833552]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Van den Eynde F., Claudino A.M., Campbell I., Horrell L., Andiappan M., Stahl D., Schmidt U. Cardiac safety of repetitive transcranial magnetic stimulation in bulimic eating disorders. Brain Stimul. 2011;4(2):112–114. doi: 10.1016/j.brs.2010.06.003. [http://dx.doi.org/10.1016/j.brs.2010. 06.003]. [PMID: 21511212]. [DOI] [PubMed] [Google Scholar]
- 93.Koivuniemi A., Otto K. When “altering brain function” becomes “mind control”. Front. Syst. Neurosci. 2014;8:202. doi: 10.3389/fnsys.2014.00202. [http://dx. doi.org/10.3389/fnsys.2014.00202]. [PMID: 25352789]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Maslen H., Pugh J., Savulescu J. The ethics of deep brain stimulation for the treatment of anorexia nervosa. Neuroethics. 2015;8(3):215–230. doi: 10.1007/s12152-015-9240-9. [http://dx.doi.org/10.1007/s12152-015-9240-9]. [PMID: 26594256]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Coman A. Emerging technologies in the treatment of anorexia Nervosa and ethics: Sufferers’ accounts of treatment strategies and authenticity. Health Care Anal. 2017;25(3):212–224. doi: 10.1007/s10728-014-0286-3. [http:// dx.doi.org/10.1007/s10728-014-0286-3]. [PMID: 25183320]. [DOI] [PubMed] [Google Scholar]
- 96.Coman A., Skårderud F., Reas D.L., Hofmann B.M. The ethics of neuromodulation for anorexia nervosa: A focus on rTMS. J. Eat. Disord. 2014;2(1):10. doi: 10.1186/2050-2974-2-10. [http://dx.doi.org/10.1186/2050-2974-2-10]. [PMID: 24690315]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Park R.J., Singh I., Pike A.C., Tan J.O. Deep brain stimulation in anorexia nervosa: Hope for the hopeless or exploitation of the vulnerable? The Oxford neuroethics gold standard framework. Front. Psychiatry. 2017;8:44. doi: 10.3389/fpsyt.2017.00044. [http://dx.doi.org/10.3389/fpsyt. 2017.00044]. [PMID: 28373849]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Tsagaris K.Z., Labar D.R., Edwards D.J. A Framework for Combining rTMS with Behavioral Therapy. Front. Syst. Neurosci. 2016;10:82. doi: 10.3389/fnsys.2016.00082. [http://dx.doi.org/10.3389/fnsys.2016.00082]. [PMID: 27895557]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Bartholdy S., Musiat P., Campbell I.C., Schmidt U. The potential of neurofeedback in the treatment of eating disorders: A review of the literature. Eur. Eat. Disord. Rev. 2013;21(6):456–463. doi: 10.1002/erv.2250. [http://dx.doi.org/10.1002/erv.2250]. [PMID: 24115445]. [DOI] [PubMed] [Google Scholar]
- 100.Brockmeyer T., Friederich H-C., Schmidt U. Advances in the treatment of anorexia nervosa: A review of established and emerging interventions. Psychol. Med. 2018;48(8):1228–1256. doi: 10.1017/S0033291717002604. [http:// dx.doi.org/10.1017/S0033291717002604]. [PMID: 28889819]. [DOI] [PubMed] [Google Scholar]
- 101.Dalton B., Campbell I.C., Schmidt U. Neuromodulation and neurofeedback treatments in eating disorders and obesity. Curr. Opin. Psychiatry. 2017;30(6):458–473. doi: 10.1097/YCO.0000000000000361. [http://dx.doi.org/10.1097/ YCO.0000000000000361]. [PMID: 28817418]. [DOI] [PubMed] [Google Scholar]