Abstract
Background:
The role of the gut microbiota in Anorexia Nervosa (AN) has long been neglected by researchers, although the fact that the former is known to play an important role in health, disease and weight regulation. Cycles of over-weight and underweight due to natural states of starvation and refeeding are normal in many vertebrates in their ecological niches.
Objective:
The aim of this review was to compare the similarities and differences of the gut microbiota in eating disorders with conditions of fasting and refeeding in other vertebrates.
Method:
A systematic literature search was conducted in Pubmed and Web of Science to find all relevant studies examining the gut microbiota in eating disorders and different states of fasting in vertebrates for this narrative review.
Results:
Gut microbiota appears to differ in AN versus normal-weight individuals. Induced fasting conditions in other ver-tebrates resulted in heterogeneous effects on gut microbiota with respect to their richness, diversity and community struc-tures. The findings for hibernating animals were generally consistent. A decrease in microbial richness and diversity was ob-served in the hibernating animal compared to the active animal, and the community structures were linked to these conditions. Some similarities and differences between AN and different states of fasting in other vertebrates were found.
Conclusion:
The complexity of the relationship between fasting and gut microbiota is difficult to interprete. A deeper bio-logical understanding is necessary to identify promising approaches for the modulation of the AN gut microbiota to support established psychotherapies
Keywords: Microbiota, gastrointestinal, hibernation, fasting, starvation, caloric restriction, eating disorder, anorexia nervosa
1. INTRODUCTION
Anorexia Nervosa (AN) is a serious illness associated with a chronic course and high mortality. In addition to psychological and environmental factors, physiological factors could be involved in the aetiology of this disease [1] – the gut microbiota could be one such factor. In 2013 Smith et al. published in Science, that the gut microbiota plays a central role in the cause of kwashiorkor, an acute form of childhood protein-energy malnutrition. Characteristics of kwashiorkor are oedema, irritability, anorexia, ulcerating dermatoses and enlarged livers with fatty infiltrates [2].
The human gut is a complex ecosystem, which harbours a tremendous amount of microbes belonging to the domains of bacteria, archaea and eucaryotes–the gut microbiota. The composition and diversity of microbial species not only vary considerably along the gut compartments, but also differs between individuals and is influenced by age, genetics, health status, diet and other factors [3, 4].
Since the characterization of germ-free animals in comparison to conventional animals in the 1960s and 1970s, it has become evident that the gut microbiota plays a physiological role in weight regulation of the host [5-7]. However, only in the last 15 years, researchers have turned their interest towards the gut microbiota and weight regulation in humans. Microbial activity produces short chain fatty acids (SCFA) such as butyrate, propionate and acetate by fermenting dietary fiber and endogenous substrates. These fatty acids are assumed to contribute to 5 to 10% of the human energy requirements [8]. Moreover, SCFA have been shown to interact with specific G-protein-coupled receptors expressed by enteroendocrine cells in the gut, thereby influencing the release of satiety hormones such as Peptide YY [9]. Additionally, proteins or amino acids, which escape digestion or endogenous mucins, are fermented by gut microbiota to branched chain fatty acids (BCFA) such as isobutyrate and isovalerate and to toxic compounds such as phenols, indols (both co-carcinogens), ammonia (mutagen, cellular poison), amines (neurotransmitter/mutagen precursors), HS- and thiols (both cellular toxins) [10]. Several of these toxic compounds have the potential to negatively impact the host`s gut physiology, motility and psychology, the latter via the brain-gut-axis [11, 12].
The observation that germ-free kept pigs had lower body weight than conventionally raised pigs has been described in 1966 and 1972 [6, 7]. For mice originated from Swiss Webster mice Gordon et al. [5] showed with a large sample size (n=97) the opposite and another study with ICR strain mice demonstrated similar results to the pigs, however the weight differences were lower [13]. Fullfed germ-free Lobund Wistar rats had less body weight when compared to their conventionally held litter mates, however the opposite was shown for these rats with restricted food intake [14]. No differences in body weight were observed in germ-free chickens compared to conventionalized chickens and similar weight loss trends were observed during fasting [15]. Interestingly, around 40 years later, after a thorough morphological characterization of germ-free kept animals, Bäckehed at al. demonstrated for C57BL/6J (B6), that germ free mice had lower body weight and less body fat than conventional animals despite increased chow consumption and decreased energy expenditure [16]. After transferring faecal microbiota of conventional animals into germ free animals, the latter gained body weight and body fat, despite a reduction in chow consumption and an increase in energy expenditure, similar to that of the conventional animals. With respect to the afore-mentioned literature, it appears that the extent to which the gut microbiota influences body weight is dependent on the animal, age and/or specific strain. However, with the publication of Bäckehed at al., the attention of more researchers was drawn towards the gut microbiota and its role in weight regulation. In 2005, Gordon and co-workers reported significantly differing ratios of the phyla Bacteriodetes and Firmicutes in the stools of lean versus leptin deficient obese (ob/ob) mice, with more Firmicutes and fewer Bacteroidetes being present in the obese mice. A similar pattern was found for the diet induced obesity mouse model [17]. More importantly, Turnbaugh et al. demonstrated that the differences in the ratio of the dominant phyla Bacteriodetes and Firmicutes also had functional implications for the host: the gut microbiota was linked to different capacities for energy harvest from the diet [18]. Specifically, the gut microbiota of obese mice produced larger amounts of SCFA and the excreted faeces contained less energy than normal-weight animals. Finally, Ley et al., were the first to show that differences in the Bacterioidetes to Firmicutes ratio existed in normal-weight versus obese humans, and that weight loss in the latter resulted in a shift in this ratio towards one similar to that of normal-weight humans [19]. Since then, many more articles examining obesity and the gut microbiota have been published. For example, considerable alterations of the gut microbiota have been described after bariatric surgery [20] and the role of gut microbiota on functions of the central nervous system (CNS) has recently been debated [21]. However, the contribution of specific microorganisms to the development of obesity remains controversial, with many subsequent studies unable to confirm the Bacteroidetes to Firmicutes differences [22]. Nevertheless, a recent reanalysis of raw data across ten individual obesity studies in humans demonstrated a relationship between the human gut microbial community and obesity status. However, it is important to note that this association was weak [23].
Despite the fact that the composition of the intestinal microbiota plays an important role in gastrointestinal disorders and weight regulation [24] the role of gut microbiota in AN has been neglected by researchers for a long time. However, in previous years a number of studies have been published which show that, similarly to obesity, the gut microbiota in AN is different to normal-weight people, however as with the obesity literature they do not clearly demonstrate causality.
Overweight and underweight in the biological context are both states which are normal in other vertebrates in their ecological niches. Periods of starvation and periods of plenty due to the annual cycles of food availability are typical and drove the evolution of animals with their gut microbiota adapting to this situation. Hibernation of mammals and amphibians, the dietary pattern of reptiles or the moult of penguins are typical and natural states of starvation with subsequent refeeding which give us an opportunity to learn about the adaption processes of the gut microbiota under physiological conditions.
Therefore, it is time to ask the pivotal question: Are changes in gut microbiota during starvation and after refeeding specific to AN? Are there differences in the microbial shifts in AN during starvation and refeeding compared to these conditions in other vertebrates? And if so, are there functional consequences for the host?
We will begin our review by providing an overview of the role of the gut microbiota in health and disease. Aiming to answer our questions, we will then provide an overview of the existing literature regarding the gut microbiota in eating disorders, followed by an overview about the gut microbiota in other vertebrates during fasting. Finally, we will compare our findings and draw conclusion about the specificity of the gut microbiota to eating disorders.
2. METHODS
To assure that all relevant literature on the gut microbiota and eating disorders and fasting was retrieved for this narrative review, the literature search was conducted on the basis of the PRISMA statement [25, 26].
2.1. Literature Information Sources and Search Strategy
We conducted two separate searches. Search 1 was applied to identify relevant studies examining the gut microbiota in eating disorders, and search 2 to identify studies analyzing the gut microbiota in different states of starvation in vertebrates. The databases PubMed and Web of Knowledge were searched for literature on the 2. May 2017. The Pubmed search was updated on the 11. November 2017. The following search terms were used: Search term 1: Pubmed: ((gastrointestinal OR intestinal OR faecal) AND (microbiome OR microflora OR microbiota) AND (“anorexia nervosa” OR “bulimia nervosa” OR “binge eating”)); Web of Science: a) ((gastrointestinal OR intestinal OR faecal) AND (microbiome OR microflora OR microbiota) AND (“anorexia nervosa” OR “bulimia nervosa” OR “binge eating”)); b) TOPIC: (gastrointestinal OR intestinal OR faecal) AND TOPIC: (microbiome OR microflora OR microbiota) AND TOPIC: (“anorexia nervosa” OR “bulimia nervosa” OR “binge eating”) search term 2: Pubmed: ((gastrointestinal OR intestinal OR faecal OR fecal) AND (microbiome OR microflora OR microbiota OR “gut bacteria” OR “intestinal flora”)) AND (kwashiorkor OR marasmus OR hibernation OR fasting OR starvation OR “total parenteral nutrition” OR “TPN”); Web of Science: ((gastrointestinal OR intestinal OR fecal OR faecal) AND (microbiome OR microflora OR microbiota OR “gut bacteria” OR “intestinal flora”)) AND (kwashiorkor OR marasmus OR hibernation OR fasting OR starvation OR “total parenteral nutrition” OR “TPN”).
2.2. Eligibility Criteria
2.2.1. Search 1
We included human and other vertebrate studies, which examined eating disorders and the gut microbiota of the large intestine. Studies with undernutrition in early childhood or undernourished neonatal animals were not included. No restrictions were made concerning ethnicity or sex. To present a complete overview of the current literature, we included randomized and non-randomized, qualitative and quantitative studies with and without comparison groups, pre-post designs and mere observational studies with any sample size. Study settings and outcomes were not required to fulfill any specific criteria, if outcomes regarding the gut microbiota and eating disorders were tested. Overall, no study was excluded due to study design or methodology. We included only peer-reviewed articles written in English and German.
2.2.2. Search 2
We included all human and other vertebrate studies, which examined the gut microbiota of the large intestine and any condition of fasting. We excluded studies with a duration ≤ 72 hours fasting, studies of the oral cavity or small intestine, studies which dealt with overweight or obesity, bariatric surgery, AN, undernutrition in early childhood or undernourished neonatal animals or total parenteral nutrition (although the latter two conditions were included in the literature search term to obtain a broad overview of the topic). The focus of the review lies on studies based on 16S rRNA gut microbiota analyses but other studies including culture-based technologies were also included for this narrative review if appropriate. The remaining criteria were similar to search 1.
2.3. Study Selection and Data Collection
For study selection and data collection, we used a modified PICOS-scheme [27]. For search 1, IM and JD and for search 2, IM and JC conducted the initial literature search on all databases. The duplicates were removed and the titles and abstracts screened to identify appropriate studies. Full-text articles were evaluated regarding their eligibility. Discussions due to uncertainties about study inclusion were held between the respective authors for approximately 5% of the articles. Discrepancies between the respective authors were clarified by including a third person (search 1: JC, search 2: JD).
3. RESULTS
Firstly, we will provide a short overview about the role of the gut microbiota in health and disease, which is not based on a systematic literature review. Next, we present the existing literature regarding the gut microbiota in eating disorders of humans and during fasting in other vertebrates, which is based on a systematic literature search. Finally, we will compare our findings and draw conclusion about the specificity of the gut microbiota to eating disorders.
3.1. The Gastrointestinal Tract and the Intestinal Microbiota in Health
The gut is exposed to numerous potential pathogens and antigens. Therefore, it is of great importance for the host to prevent their uncontrolled penetration into the body. In addition to unspecific immune defenses such as the acidity of the stomach, bactericidal properties of digestive enzymes, peristalsis and mucus secretion, the gut is surrounded by the largest collection of lymphoid tissues in the body, known as the gut-associated lymphoid tissue (GALT). It consists of mesenteric lymph nodes, Peyer’s patches, lymphocytes located in the intestinal lamina propria and large numbers of IgA plasmablasts located in the epithelium [28-30].
Several interactions between the host and the indigenous microorganisms have been described. With regards to health, this symbiosis is beneficial for the host. The resistance of the gastrointestinal tract to colonization by potential pathogens is important. To accomplish this, several mechanisms complement each other. Firstly, the commensal microorganisms occupy ecological niches within the gastrointestinal tract. Thus, other (potential pathogenic) microorganisms not characteristic of the habitat are prevented from colonization. Secondly, the microbiota produce metabolites such as SCFA, lactate and bacteriocins, which are able to influence the pH of their environment or damage other microorgansims, respectively. Thirdly, the microbiota competes for nutrients and growth factors. Besides many other positive impacts the microbiota has on the health of the host, the microbiota also stimulates and influences the immune system of the digestive tract - GALT. Thus, the microbiota is actively involved in maintaining the gut barrier function and overall health of the host [31-33]. Additionally, there is an evidence that the gut microbiota impacts on the function of the CNS by modulating signaling pathways via the microbiota-gut-brain axis [34, 35].
3.2. The Intestinal Microbiota in Disease
Despite its relevance to the human body, the composition and function of the gastrointestinal microbiota is only now beginning to be understood. In the past few years, large collaborative efforts such as META-HIT and the Human Microbiome Project [36] have generated a wealth of data and provided many new insights into the composition of human microbiota, the large interindividual variations and the impact of geography and diet on the human microbiome. Furthermore, one of the most striking findings of META-HIT was the clustering of humans based upon their microbiota composition into 3 distinct enterotypes driven by Prevotella, Bacteroides and Ruminococcus respectively [37]. Despite the considerable scientific debate on the existence of segregated enterotypes rather than a continuum or gradient [38], diet appears to have a strong impact on the balance between Bacteroides and Prevotella [39]. Molecular analyses have shown that the microbiota composition is perturbed in many diseases. Whereas a disturbed microbiota was anticipated and confirmed for intestinal disorders such as inflammatory bowel diseases [40] or colon cancer, its association with atopic diseases (allergies and eczema), diabetes and metabolic syndrome shows that the microbiota has also a systemic impact on human health [3, 24]. Furthermore, there is an evidence that the microbiota influences brain function and the behaviour of the host by communicating with the brain via the gut-brain axis [41, 42]. Altered community structures of gut microorganisms that have been linked with disease states differ between conditions, however for many diseases it is still unclear which species are involved. Nevertheless, it appears that a loss of gut microbial richness and biodiversity is present with most diseases [43]. Similar to other ecosystems such as the rain forests or the water, a loss of species diversity in the gut might be closely linked to a loss of resilience [44].
3.3. Results of the Systematic Literature Search
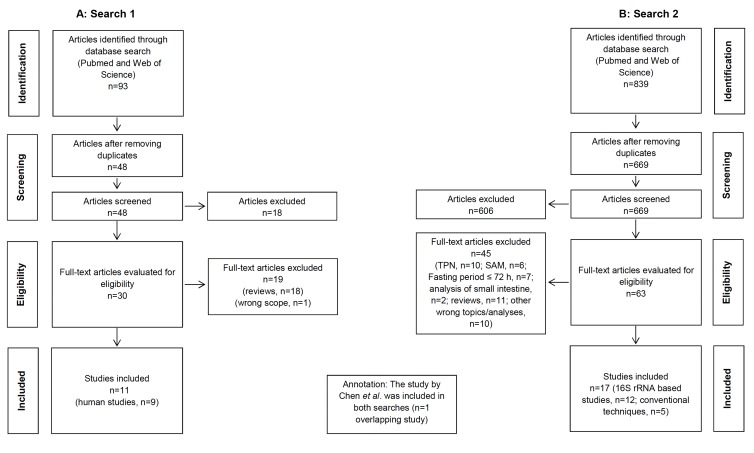
We systematically searched the literature for studies dealing with the gut microbiota and a) eating disorders and b) states of fasting in vertebrates. The detailed study selection process is given in Fig. (1A and 1B), respectively. To give a structured overview we classified the results into 2 groups: group 1: The gut microbiota in eating disorders of humans; group 2: The gut microbiota during food restriction in other vertebrates. The latter group was further structured into the 3 subgroups “fasting”, “hibernation” and “fasting and hibernation”.
3.4. The Gut Microbiota in Eating Disorders of Humans
Eating disorders comprise patients with AN, bulimia nervosa and binge-eating disorder. At present time, we found no studies, which analysed the gut microbiota of patients with bulimia nervosa or binge-eating disorder. However, since patients with a binge-eating disorder are often obese, they may have been included in gut microbiota analyses of obesity studies. Nine studies dealing with AN and the gut microbiota in humans were published between 2009 and 2017 and are summarized in Table 1. One explorative study found 11 new bacterial species in a stool sample of one AN patient [45], whereas another study reported a low diversity of fungal species with 4 microeukaryotes previously not described for the human gut [46]. Armougom et al. [47], Million et al. [48] and Morita et al. [49] performed cross-sectional stool sample analyses for a selected range of gut microorganisms in 9, 15 and 25 AN patients, respectively, using quantitative real-time PCR technologies. Morito et al. additionally applied high-performance liquid chromatography (HPLC) to analyse SCFA. Armougom et al. reported that Methanobrevibacter smithii, a methane producing archaeon, was increased in AN patients compared to normal weight participants [47] which was not confirmed by Million et al., although Methanobrevibacter smithii concentration was lower in obese individuals in comparison to non-obese [48]. Morito et al. found that female AN patients had lower amounts of total bacteria and obligate anaerobes as well as lower levels of acetate and propionate in their faeces when compared to normal-weight, age-matched female participants [49]. Additionally, Borgo et al. performed a cross-sectional stool sample analysis using 16S rRNA gene sequencing in 15 AN-patients and 15 age and sex matched controls [50]. The authors found that that phylogenetic richness and diversity were not different whereas the microbial community structures were distinct between the groups. The phylum Firmicutes and Roseburia spp. along with SCFA (butyrate and propionate) were lower in AN patients in comparison to controls. The archaeon M. smithii was higher in AN patients if detected (found in 30% of AN patients) than in controls. The group found various correlations between microbial species, metabolic parameters, SCFA, BMI and psychometrics. A longitudinal study analyzed the daily changes on the gut microbiota using 16S rRNA gene sequencing in 3 AN patients. The group reported patient-specific changes in microbial composition and diversity in the course of weight gain [51].
Table 1.
Overview of human studies anlayzing the gut microbiota in Anorexia nervosa (AN) patients.
|
Author,
Year |
Title of Study |
Type of
Study |
Species | Further Characteristics | Characteristics of Feces Collection and Storage | Methods |
Main Results
(Focus on Outcomes Regarding AN-Patients in Mixed Studies) |
||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Pfeiderer et al., 2013 |
Culturomics identified 11 new bacterial species from a single anorexia nervosa stool sample. |
Explorative, cross-sectional | Human (homo sapiens sapiens) | Female AN patient (n=1) Age: 21 years BMI: 10.4 kg/m2 Control: no, n.a. for study purpose |
Feces collection at the day of hospitalization, before the introduction of tube feeding. No further information on feces collection and storage provided. |
Large scale of culture conditions. Identification of colonies by MALDI-TOF and 16S rRNA. |
Identification of 11 new bacterial species in feces. | ||||||||||||||||
| Gouba et al., 2014 |
Gut microeukaryotes during anorexia nervosa: a case report. | Explorative, cross-sectional | Human (homo sapiens sapiens) | Female AN patient (n=1) Age: 21 years BMI: 10.4 kg/m2 Control: no, n.a. for study purpose |
Feces collection at the day of hospitalization, before the introduction of tube feeding. No further information on feces collection and storage provided. |
Culture and PCR techniques. | Diversity of fungi low. Identification of 4 new microeukaryote. |
||||||||||||||||
| Armougom et al., 2009 |
Monitoring bacterial community of human gut microbiota reveals an increase in Lactobacillus in obese patients and Methanogens in anorexic patients. |
Explorative, cross-sectional |
Human (homo sapiens sapiens) | AN patients (n=9) Sex: not reported Age: 19-36 years BMI:12.7±1.6 kg/m2 Normal-weight participants (n=20) Sex: not reported Age: 13-68 years BMI:20.7±2.0 kg/m2 Obese patients (n=20) Sex: not reported Age: 17-72 years BMI:47.1±10.7 kg/m2 |
No information on feces collection and storage provided. | 16S rRNA analyses by quantitative Real-time PCR. | M. smithii higher in AN patients in comparison to other participants. | ||||||||||||||||
| Million et al., 2013 |
Correlation between body mass index and gut concentrations of Lactobacillus reuteri, Bifidobacterium animalis, Methanobrevibacter smithii and Escherichia coli. | Explorative, cross-sectional |
Human (homo sapiens sapiens) | AN patients (n=15) Sex: 14 female, 1 male Age: 27.3±10.8 years BMI: 13.5 (11.7–14.6) kg/m2 Normal-weight participants (n=76) Sex: 36 female, 40 male Age: 49.5±18.6 years BMI: 22.4 (20.7–23.7) kg/m2 Overweight participants (n=38) Sex: 6 female, 32 male Age: 54.1±17.8 years BMI: 27.1 (25.9–28.6) kg/m2 Obese participants (n=134) Sex: 69 female, 65 male Age: 51.8±14.7 years BMI: 27.1 40.0 (36.4–46.8) kg/m2 |
No information on feces collection and storage provided. | 16S rRNA analyses by quantitative Real-time PCR. |
M. smithii higher in non-obese participants in comparison to obese patients | ||||||||||||||||
|
Author, Year |
Title of Study |
Type of Study |
Species |
Further Characteristics |
Characteristics of Feces Collection and Storage | Methods |
Main Results (Focus on Outcomes Regarding AN-Patients in Mixed Studies) |
||||||||||||||||
| Morita et al., 2015 |
Gut Dysbiosis in Patients with Anorexia Nervosa. |
Explorative, cross-sectional |
Human (homo sapiens sapiens) | Female AN patients (n=25) Age: 30.0 ± 10.2 years BMI=12.8 ± 1.3 kg/m2 Controls matched for sex and age (n=21) Age: 31.5 ± 7.4 years BMI:20.5 ± 2.1 kg/m2 |
The faecal samples were placed directly into two tubes by the participants or hospital staff members. One tube contained RNAlater and was stored at 4°C and used for the analysis of faecal microbiota. The other tube was empty and was stored at −20°C for the analysis of faecal organic acid concentration and faecal pH. Tubes were stored a indicated above within 30 min of excretion. |
16S and 23S rRNA analysis by quantitiative Real-time PCR (Yakult Intestinal Flora-SCAN); Short chain fatty acids by HPLC. | AN-patients had lower amounts of total bacteria and obligate anaerobes as well as lower levels of acetate and propionate in their feces. | ||||||||||||||||
| Kleiman et al., 2017 |
Daily Changes in Composition and Diversity of the Intestinal Microbiota in Patients with Anorexia Nervosa: A Series of Three Cases. |
Explorative, longitudinal |
Human (homo sapiens sapiens) | Female AN patients (n=3) in the course of weight restoration. T1= before weight gain; T2= after weight gain. Time between T1 and T2: 34, 73, 58 days Age: 16,25,29 BMI at T1: 13.7; 15.6; 17.6 kg/m2 BMI at T2: 15.4; 20.2; 21.1 kg/m2 |
Samples were stored at +4°C and were transferred to the laboratory within 24 h where they were processed and stored at -80°C for future DNA isolation and molecular microbiological analyses. |
16S rRNA analyses by quantitative Real-time PCR and sequencing (MiSeq platform). | In the time course of weight restoration changes of microbial composition and diversity were obeserved and were patient specific. | ||||||||||||||||
| Kleiman et al., 2015 |
The Intestinal Microbiota in Acute Anorexia Nervosa and During Renourishment: Relationship to Depression, Anxiety, and Eating Disorder Psychopathology. |
Explorative, longitudinal |
Human (homo sapiens sapiens) | Female AN patients before weight gain (T1, n=16) and after weight gain (T2, n=10) Time between T1 and T2: not reported Age: 28 ± 11.7 years BMI at T1: 16.2 ± 1.5 kg/m2 BMI at T2: 17.4 ± 6.9 kg/m2 Controls (n=12), matched for sex and age Age: 29.8 ± 11.6 years BMI: 21.5 ± 1.9 kg/m2 |
Procedure unclear: Study refers to another paper with PMID: 22339879. “Subjects unable to provide stool samples at the visit were instructed to collect a specimen at home and return it to study staff at the same morning. Each faecal sample was immediately transferred to the laboratory where it was homogenized, divided into aliquots and stored at -80°C for future DNA isolation ...” | 16S rRNA analysis by sequencing (454 platform). Questionnaires |
AN patients versus controls: Phylogenetic richness↓ Anaerostipes↓ in AN Faecalibacterium↓in AN AN patients after weight gain: Phylogenetic richness↔ Beta diversity: similarity↑ Differences at phylum and genus level (global tests). Ruminococcus spp.↑ Microbial composition and diversity associated with mental health. |
||||||||||||||||
|
Author, Year |
Title of Study |
Type of Study |
Species |
Further Characteristics |
Characteristics of Feces Collection and Storage | Methods |
Main Results (Focus on Outcomes Regarding AN-Patients in Mixed Studies) |
||||||||||||||||
| Mack et al., 2016 |
Weight gain in anorexia nervosa does not ameliorate the faecal microbiota, branched chain fatty acid profiles, and gastrointestinal complaints. | Explorative, longitudinal |
Human (homo sapiens sapiens) | Female AN patients before weight gain (T1, n=55) and after weight gain (T2, n=44) Time between T1 and T2: 14.0 ± 6.8 weeks. Age: 23.8 ± 6.8 years BMI at T1: 15.3 ± 1.4 kg/m2 BMI at T2: 17.7 ± 1.4 kg/m2 Controls (n=55), matched for sex and age Age: 23.7 ± 6.7 years BMI: 21.6 ± 2.0 kg/m2 |
AN patients: Feces was collected as soon as possible after the beginning of their inpatient stay. Patients were provided with a stool sampling kit and instructed to auto-collect their stool. Immediately, upon defaecation, patients delivered their stool samples to the collection point for human specimens at the hospital where one of the receptacles was instantly frozen at −80°C. Controls: Same procedure but after defecation instructed to immediately contact staff from the University Hospital. University staff picked up samples straight away, transported the samples between cool packs (stored ahead at + 4 °C) and subsequently stored the samples at−80°C upon arrival. The median time to freezing was 0:45 [0.15-1:55] hours. |
16S rRNA analyses by quantitative Real-time PCR and sequencing (MiSeq platform); Short chain fatty acids by gas chromatography; Other measurements: Gastro-questionnaire, dietary assessment via 24 h food records, food frequency questionnaires and the multiple source method. |
AN patients versus controls: Phylogenetic richness ↔ Phylogenetic diversity ↔ Beta diversity: similarity↓ in AN Community structure different. Bacterioidetes↑ and Verrucomibrobia↑ in AN patients Actinobacteria↓ in AN patients Mucin-degraders and members of Clostridium clusters I, XI and XVIII↑ in AN patients Butyrate-producing Roseburia spp. branched-chain fatty acid concentrations, being markers for protein fermentation↓ Between AN patients: Community structure different in restrictive versus binge/purging AN-subtypes. AN patients after weight gain: Phylogenetic richness↑ Phylogenetic diversity ↔ Beta diversity: similarity↑. Similarity very high within the subjects. Community structure different Bacteroidetes↓ Firmicutes↑ Verrucomicrobia↓ Perturbations in intestinal microbiota and short chain fatty acid profiles in addition to several gastrointestinal symptoms did not recover. |
||||||||||||||||
| Borgo et al., 2017 |
Microbiota in anorexia nervosa: The triangle between bacterial spcies, metbolites and psychological tests. | Explorative, cross-sectional |
Human (homo sapiens sapiens) | Female AN patients (n=15) Age: not reported BMI:13.9±2.1 Controls (n=15) matched for sex and age Age: not reported BMI:22.1±2.6 |
Feces collected and stored at -80°C. No further information on feces collection provided. |
16S rRNA analyses by quantitative Real-time PCR and sequencing (MiSeq platform); Short chain fatty acids by gas chromato- graphy; Questionnaires: EDI-2, SCL90, STAI-Y, BDI; Blood values. |
AN patients versus controls: Phylogenetic richness↔ Phylogenetic diversity↔ Beta diversity↔ Community structure different. Short chain fatty acids in AN patients↓ Firmicutes↓ in AN patients Proteobacteria↑ in AN patients Roseburia↓ in AN patients Clostridium↓ in AN patients M. smithii↑ (if detected) in AN patients Different correlations between microbial specis, metabolic parameters, short chain fatty acids, BMI and psychometrics. |
||||||||||||||||
↓=decrease, ↑=increase, ↔=no change.
Kleiman et al. [52] and Mack et al. [53] performed longitudinal stool sample analyses using 16S rRNA gene sequencing (Kleiman et al.: Roche 454 Life Sciences Genome Sequencer; Mack et al.: Illumina MiSeq instrument). Kleiman et al. [52] investigated the relationship between mental health and gut microbiota in a small sample of female AN patients before (n=16) and after weight gain (n=10) in comparison to 12 healthy age-matched female controls. The mean age of AN-patients was 28±11.7 years, the mean BMI before weight gain was 16.2±1.5 kg/m2 and after weight gain 17.4±0.9 kg/m2, thus the BMI had increased by mean 1.2 BMI points. The period of treatment was not reported. The study reported that microbial richness was lower in AN patients before and after weight gain in comparison to controls, however no differences were observed in AN patients during the course of weight gain. Beta diversity showed that the samples of AN-patients were more similar after weight gain than before weight gain. Global tests also revealed differences in the phylum and genus levels for AN patients before and after weight gain, whereas univariate tests did not show differences at phylum level and at genus level only Ruminococcus spp. increased with weight gain. In comparison to controls, AN patients before weight gain did not differ at phylum level, but after weight gain there was a trend towards a lower relative abundance of Bacteroidetes and higher abundance of Firmicutes (false discovery rate=0.11). At genus level, the relative abundance of Coribacteriales spp. was lower and Faecalibacterium spp. and Anaerostipes spp. were higher in AN patients before weight gain. After weight gain, the difference in Coribacteriales was still observed and the relative abundance of Ruminococcus spp. was higher in comparison to controls. The authors also reported that the microbial composition and diversity were associated with mental health. It should be considered that due to the small sample size and the accompanying limited statistical power, differences within and between the study groups may have remained undetected.
Mack et al. [53] analysed the stool samples of female AN patients at the beginning (n=55) and at discharge (n=44 paired samples) of an inpatient stay compared to 55 healthy, age-matched female control participants (NW). The mean age of the patients was 23.8±6.8 years, the BMI before weight gain was 15.3±1.4 kg/m2 and after weight gain was 17.7±1.4 kg/m2. Thus, the mean BMI increase was 2.3±1.2 kg/m2. The mean time interval of treatment was 14±6.8 weeks. The authors report that gastrointestinal symptoms were not only present at the beginning of treatment but also remained present at the end of therapy, suggesting that the gastrointestinal tract had not fully recovered after three months inpatient treatment. Before weight gain, AN patients had similar microbial richness and eveness to NW participants. The authors suggested that the observed normal fibre intake in comparison to NW participants, and the normal proportion of energy derived from macronutrients are critical for the inconspicuous alpha diversity observed. Before weight gain AN patients at phylum level had lower levels of Bacteroidetes and higher levels of Actinobacteria and Verrucomicrobia in comparison to NW participants. At genus level, Mack et al. found higher levels of mucin-degrading (e.g. Verrucomicrobia, Bifidobacteria, Anaerotruncus) and protein degrading taxa (e.g. Clostridium cluster I and XI) in AN patients, whereas levels of carbohydrate degraders (e.g. the key butyrate-producing Roseburia spp. and Gemminger spp.) were lower in comparison to NW participants. In addition, gas chromatography revealed higher levels of BCFA (markers for protein fermentation) and lower relative butyrate levels in the faeces of AN patients in comparison to NW participants. The authors concluded that fibre and endogenous host and microbe-derived proteins (e.g. bacterial secreatios, lysis products, mucosal and bacterial cells) and endogenous carbohydrates (e.g. mucins being glycoproteins) were most likely the main substrates nourishing the gut microbiota. Gastrointestinal symptoms, which are linked to slow colonic transit times offered an ecological niche for mucing degraders. In the course of weight gain and exposure to a high-energy, high-fibre diet, symptoms linked to slow colonic transit times also improved. Interestingly, the archaeon Methanobrevibacter smithii was only detected in 20% of the participants, but in those patients where it was detected, the relative abundance was higher before weight gain. In line with the observed taxonomic changes, Mack et al. found that the microbial community structure was related to the disease status (AN patients versus NW participants) and to a lesser extent to age. Moreover, the community structure was also different between AN patients with the restrictive subtype versus those with the binge-purge subtype, a finding which reflects the different feeding behaviour styles of the patients. After weight gain, species richness increased but not when compared to NW participants. The diversity of species (Shannon index) was even higher in AN patients when compared to NW participants. Upon weight gain, the relative abundances of the phyla Bacteroidetes and Verrucomicrobia decreased whereas Firmicutes and Actinobacteria increased. At genus level, no differences in carbohydrate utilizing taxa (Roseburia spp., Gemminger spp.) were observed between AN patients and NW participants. Ruminococcus spp. increased, which may reflect the increased amount of fibre and resistant starch in the diet. Mucin degraders (Akkermansia spp. and Anaerotruncus spp.) decreased. Interestingly, Bifidobacteria were present in the core microbiome of AN patients but not of NW, impying that the vast majority of AN patients had specific bifidobacterial taxa in their microbiome in contast to NW participants. After weight gain, the Bifidobacteria spp. difference compared to NW participants became even larger, suggesting that the already established bifidobacteria had benefitted from the diet-derived carbohydrates. The butyrate proportion normalized after weight gain but the BCFA concentrations were still higher in AN patients compared to NW participants, suggesting that protein fermentation still played an important role.
Overall, few studies on the gut microbiota of AN patients have been published to date, and the studies that have been conducted have applied different technologies and vary in scope. Additionally, the small sample sizes in most of the studies may limit the conclusions drawn from those studies. However, it appears that the microbiota in AN, similarly to obesity, differs in comparison to healthy, NW populations. Regarding microbial richness, Kleiman et al. [52] found differences between AN patients versus controls whereas Mack et al. [53] and Borgo et al. [50] did not. In contrast to Kleiman et al., Mack et al. found an increase of richness and eveness with weight gain. All three studies reported differences regarding microbial taxa at phylum and genus level between controls and AN patients. The finding of Mack et al., that the phyla Firmicutes increased, whereas Bacteriodetes decreased during the course of weight gain, was also observed as trend by Kleiman et al. Additionally, the increased levels of BCFA observed by Mack et al. were observed as a clear trend by Morita et al. [49], thus supporting that protein degradation of gut microbiota may be increased in AN. However, Borgo et al. [50] did not make this observation. Nevertheless, all three authors found that the SCFA profile and/or abundance was different in AN patients compared to controls. Both, Borgo et al. and Mack et al., observed low abundances of Roseburia spp. in AN patients in comparison to controls which they linked to the low butyrate levels observed in these patients. Three studies [47, 50, 53] found higher abundances of M. smithii in AN patients in comparison to controls, with the limitation that two studies [50, 53] reported that only a small subgroup of participants harboured this archaeon. Finally, Ruminococcus spp. benefited from the diet during weight gain in AN as observed by Mack et al. and Kleiman et al.
3.5. The Gut Microbiota During Food Restriction in other Vertebrates
As mentioned previously, AN, which can also be described as a chronic fasting period, is accompanied by gastrointestinal complaints and several symptoms are linked to decreased colonic transit times [53]. A change in the gastrointestinal transit time itself may cause altered microbial community structures in the gut [54]. In addition to stool frequency, stool consistency changes in periods of fasting. For example Sonoyama et al. described that wet weights of cecal contents were higher in torpid hamsters than in active hamsters. Additionally, the caecal contents in fasted hamsters were more fluid compared to fed and torpid hamsters [55].
Starvation of vertebrates can be studied in active and non-active states. Depriving animals of food intake or studying penguins at moult are active states, whereas hibernation is a state of inactivity. The presented studies analyzed either the faeces or the content of gut compartments after sacrificing the animal. If an animal was sacrificed and the caecum was the location of microbial turnover and not the colon (e.g. mice and squirrels), some studies may have analyzed the microbial content of the caecum only. An overview of the studies, which performed 16S rRNA analyses, is provided in Table 2.
Table 2.
Overview of vertebrate studies (excluding humans) anlayzing the gut microbiota in different states of caloric restriction.
| Author, year | Title of study | Type of study, category and species | Further study characteristics |
Characteristics of
specimen collection |
Methods | Results - CR, RF or HN condition | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Findings |
Phylo-genetic
richness |
Phylo-genetic
diversity |
Community
structure |
Firmi-
cutes |
Bacterio-
detes |
Actino-
bacteria |
Proteo-
bacteria |
Verruco-
microbia |
||||||||||||
| Queipo-Ortuno et al., 2013 |
Gut microbiota composition in male rat models under different nutritional status and physical activity and its association with serum leptin and ghrelin levels. |
Explorative, food restriction study. Mammal, rat (Rattus rattus). |
Activity based anorexia (ABA) rat (n=40) 4 groups, n=10 each group: ABA: food rectriction and access to running wheel; Control ABA: food restriction and no access to running wheel; Exercise: fed ad libitum and access to running wheel; Ad libitum: fed ad libitum and no access to running wheel. |
Faecal samples immediately collected and storet at -80°C. |
PCR-denaturating gradient gel electrophoresis and real-time PCR based on 16S rRNA. |
See details to the right. | n.r. | ↓CR | Difference between food-restricted and fed state. | CR↓ | CR↓ | CR↓ | CR↑ | CR↑ | ||||||
| Chen et al., 2016 |
Altered gut microbiota in female mice with persistent low body weights following removal of post-weaning chronic dietary restriction. |
Explorative, food restriction study. Mammal, mouse (Mus musculus). |
BALB/c mice (n=48) 4 groups, n=12 each group: Ad libitum: fed ad libitum; limited fed (LF): food restriction throughout the study to prevent natural weight gain; LF refed: like LF but than refed; Treated LF refed: like LF but treated with insulin growth factor-1 and refed. |
Faecal samples were collected by placing the tubes under the anus at different times before feeting at 10:00. |
16 S rRNA based sequencing (MiSeq platform). | See details to the right. | Increased with age, no group differences reported. | Increased with age, no group differences reported. | Age and diet, but not body weight, were associated with gut microbiota composition. RF of fasted mice changed in comparison to fasted mice; refed mice still differed from the ad libitum fed mice. |
RF↓ | RF↑ | RF↔ | RF↑ | RF↔ | ||||||
| Author, year | Title of study | Type of study, category and species | Further study characteristics |
Characteristics of specimen collection |
Methods | Results - CR, RF or HN condition | ||||||||||||||
| Findings |
Phylo-genetic richness |
Phylo-genetic diversity |
Community structure |
Firmi- cutes |
Bacterio- detes |
Actino- bacteria |
Proteo- bacteria |
Verruco- microbia |
||||||||||||
| Kohl et al., 2014 |
Unique and shared responses of the gut microbiota to prolonged fasting: a comparative study across five classes of vertebrate hosts. |
Explorative, food restriction study. |
Animals were sacrificed in states of being nourished and of being fasted. | Faecal content was collected from the colon and the caecum (from the former only if possible). | 16 S rRNA based sequencing (MiSeq platform). | Phyla distribution across the analyzed species varied considerably. | - | - | Changes to fasting were extremely heterogeneous between the different vertebrates. Most changes occured in the caecum if anatomically present. | - | - | - | - | - | ||||||
| - | - | Fish, nile tilapia (Oreochromis niloticus). |
n=23 | - | - | - | CR: Colon↑ Caecum↓ |
CR: Colon↑ Caecum↓ |
- | CR: Colon↔ Caecum↔ |
CR: Colon↔ Caecum↔ |
CR: Colon↑ Caecum↔ |
CR: Colon↑ Caecum↔ |
CR: Colon↑ Caecum↔ |
||||||
| - | - | Amphibian, southern toad (Anaxyrus terrestris). |
n=23 | - | - | - | CR: Colon↑ Caecum n.a. |
CR: Colon↑ Caecum n.a. |
- | CR: Colon↔ Caecum n.a. |
CR: Colon↑ Caecum n.a. |
CR: Colon↔ Caecum n.a. |
CR: Colon↔ Caecum n.a. |
CR: Colon↔ Caecum n.a. |
||||||
| - | - | Amphibian, leopard gecko (Eublepharis macularius). |
n=25 | - | - | - | CR: Colon↔ Caecum n.a. |
CR: Colon↔ Caecum n.a. |
- | CR: Colon↔ Caecum n.a. |
CR: Colon↔ Caecum n.a. |
CR: Colon↔ Caecum n.a. |
CR: Colon↔ Caecum n.a. |
CR: Colon↔ Caecum n.a. |
||||||
| - | - | Bird, Japanese quail (Coturnix coturnix). |
n=25 | - | - | - | CR: Colon↓ Caecum ↔ |
CR: Colon↓ Caecum ↔ |
- | CR: Colon↔ Caecum↔ |
CR: Colon↔ Caecum↔ |
CR: Colon↔ Caecum↔ |
CR: Colon↔ Caecum↔ |
CR: Colon↔ Caecum↔ |
||||||
| - | - | Mammal, mouse (Mus musculus). |
n=26 | - | - | - | CR: Colon↑ Caecum ↔ |
CR: Colon↑ Caecum ↔ |
- | CR: Colon↔ Caecum↔ |
CR: Colon↓ Caecum ↔ |
CR: Colon↔ Caecum↔ |
CR: Colon↔ Caecum↔ |
CR: Colon↔ Caecum↔ |
||||||
| Xia et al., 2014 |
The intestinal microbiome of fish under CR. |
Explorative, food restriction study. Fish, Asian seabass (Lates calcarifer). |
Fish (n=12) were either fed normally (n=6) or food-restricted (n=6). | Faecal content was collected across the entire intestine. | 16 sRNA based sequencing and real-time PCR. | Most abundant phyla in large intestine different to that of mammals. | CR: ↔ | n.r. | Difference between food-restricted and fed state. |
CR↔ | CR↓ | CR↔ | CR↔ | CR↔ | ||||||
| Author, year | Title of study | Type of study, category and species | Further study characteristics |
Characteristics of specimen collection |
Methods | Results - CR, RF or HN condition | ||||||||||||||
| Findings |
Phylo-genetic richness |
Phylo-genetic diversity |
Community structure |
Firmi- cutes |
Bacterio- detes |
Actino- bacteria |
Proteo- bacteria |
Verruco- microbia |
||||||||||||
| Dhanasiri et al., 2011 |
Changes in the intestinal microbiota of wild Atlantic cod Gadus morhua L. upon captive rearing. |
Explorative, food restriction study. Fish, Atlantic cod (Gadus morhua). |
Free-living cod were caught (n=79). Fresh caught fish, fed fish and starved fish were investigated. Sample size of the groups not clearly reported. | Faecal content and intestinal walls were collected from the intestine. |
PCR-denaturating gradient gel electrophoresis based on 16S rRNA. |
See details to the right. | CR: ↔ | n.r. | No difference between food-restricted and fed state. | n.r. | n.r. | n.r. | n.r. | n.r. | ||||||
| Costello et al., 2010 |
Postprandial remodeling of the gut microbiota in Burmese pythons. |
Explorative, food restriction study. Reptile, Burmese python (Python molurus). |
Snakes were fasted for 30 days before RF (n=32). | Faecal content was collected from the large intestine. |
16 S rRNA based sequencing (454 platform). |
See details to the right. | CR↓ | CR↓ | Difference between food-restricted and fed state. |
CR↓ | CR↑ | CR↔ | CR↔ | CR↑ | ||||||
| Dewar et al., 2014 |
Influence of fasting during moult on the faecal microbiota of penguins. | Explorative, food restriction study. Bird, little penguin (Eudyptula minor) and king penguin (Aptenodytes patagonicus). |
Free-living king penguins (n=12) and little penguins (n=9) during early and late moult were investigated. | A sterile Copan E-swab was inserted into the cloaca. Samples transferred into amine soution for DNA preservation and stored at -20°C in the field and subsequently at -80°C. | 16 S rRNA based sequencing (454 platform). |
See details to the right. | n.r. | n.r. | Difference between early and late mould in both, little and king penguin. | King penguin: CR↔ Little penguin: CR↓ |
King penguin: CR↓ Little penguin: CR↑ |
King and little penguin: CR↔ | King penguin: CR↓ Little penguin: CR↔ |
King and little penguin: CR↔ | ||||||
| Sonoyama et al., 2009 |
Response of gut microbiota to fasting and HN in Syrian hamsters. | Explorative, food restriction and HN study. | Hamsters (n=18) wer fed active nonhibernating (n=6), fasted active nonhibernating (n=6) and hibernating (n=6). | Faecal content was collected from the caecum. | PCR-denaturating gradient gel electrophoresis and real-time PCR based on 16S rRNA. |
Fasting in the active versus the inactive state have large impact on the cecal microbiota. | n.r. | n.r. | n.r. | CR↓ | CR↔ | CR↔ | CR↑ | CR↑ HN↔ |
||||||
| Author, year | Title of study | Type of study, category and species | Further study characteristics |
Characteristics of specimen collection |
Methods | Results - CR, RF or HN condition | ||||||||||||||
| Findings |
Phylo-genetic richness |
Phylo-genetic diversity |
Community structure |
Firmi- cutes |
Bacterio- detes |
Actino- bacteria |
Proteo- bacteria |
Verruco- microbia |
||||||||||||
| Weng et al., 2016 |
Functional analysis for gut microbes of the brown tree frog (Polypedates megacephalus) in artificial HN. |
Explorative, HN study. Amphibian, brown tree frog (Polypedates megacephalus). |
Frogs collected in the wild (n=39) at different seasons: fall (n=12), winter (n=18), spring (n=6). Artificial HN was implemented in 3 frogs. |
Faecal content was collected from the large intestine. |
16 S rRNA based sequencing (454 platform). | See details to the right. | HN↓ | HN↓ | Similar between frogs at different season, difference between HN and other frogs. not specifically reported |
HN↓ | HN↔ | HN↔ | HN↔ | CR↔ | ||||||
| Carey et al., 2013 |
Seasonal restructuring of the ground squirrel gut microbiota over the annual HN cycle. | Explorative, HN study. Mammal, 13-lined ground squirrel (Ictidomys tridecemlineatus) |
Free-living Squirrels were caught (n=6). Pups (n=40) were used for experiments and artificial HN was induced . | Faecal content was collected from the caecum. | 16 S rRNA based sequencing (454 platform). | See details to the right. | HN↓ | HN↓ | Difference between HN and active state. |
HN↓ | HN↑ | HN↔ | HN↑ | HN↑ | ||||||
| Dill-McFarland et al., 2014 |
HN alters the diversity and composition of mucosa-associated bacteria while enhancing antimicrobial defence in the gut of 13-lined ground squirrels. | Explorative, HN study. Mammal, 13-lined ground squirrel (Ictidomys tridecemlineatus). |
Free-living Squirrels were caught (n=5). Pups (n=19) were used for experiments and artificial HN was induced. | Rinsed caecum was frozen and stored at -80°C | 16 S rRNA based sequencing (454 platform). | Mucosal microbiota remained relatively stable across the annual cycle. | HN↓ | HN↓ | Difference between HN and active state. |
HN↓ | HN↑ | HN↔ | HN↔ | HN↑ | ||||||
| Stevenson et al., 2014 |
Effects of season and host physiological state on the diversity, density, and activity of the arctic ground squirrel cecal microbiota. |
Explorative, HN study. Mammal, arctic ground squirrel (Urocitellus parryii) |
Free-living Squirrels were caught across the annual cyle (n=44) | Faecal content was collected from the caecum. | 16s rRNA based sequencing (454 platform). | See details to the right. | HN↓ | HN↓ | Difference between HN and active state. |
HN↓ | HN↑ | HN↔ | HN↑ | HN↑ | ||||||
| Author, year | Title of study | Type of study, category and species | Further study characteristics |
Characteristics of specimen collection |
Methods | Results - CR, RF or HN condition | ||||||||||||||
| Findings |
Phylo-genetic richness |
Phylo-genetic diversity |
Community structure |
Firmi- cutes |
Bacterio- detes |
Actino- bacteria |
Proteo- bacteria |
Verruco- microbia |
||||||||||||
| Sommer et al., 2016 |
The Gut Microbiota Modulates Energy Metabolism in the Hibernating Brown Bear Ursus arctos. |
Explorative, HN study. Mammal, Eurasian brown bear (Ursus arctos) |
Wild bears were tracked with global positioning system collars and anesthesized in winter (n=16) and summer (n=8). N=8 paired samples. |
Faecal samples were collected directly from the rectum and immediately frozen at -80°C. |
16 S rRNA based sequencing (MiSeq platform). | See details to the right. | HN↓ | n.r. | Difference between HN and active state. |
HN↓ | HN↑ | HN↓ | HN↔ | HN↓ | ||||||
CR=caloric restriction, HN=hibernation,RF=refeeding, n.a.=not applicable, n.r.=not reported, ↓=decrease, ↑=increase, ↔=no change.
3.5.1. Fasting
Early studies using microscopy and culture-based techniques described gastrointestinal bacterial shifts upon fasting, for example in vivo and in vitro studies of the flounder, squid and mouse using culture-based and microscopy techniques revealed that fasting led to bacterial shifts to favour bacterial species with survival mechanisms [56].
3.5.1.1. Mammals
To study AN in animals, the activity based anorexia (ABA) rat-model can be used. In this model Spraque Dawley rats are starved by restricting food access to 23 hours per day and are confined to running wheels except during the one hour exposure to food. These animals become more anorexic than food-restricted control animals (no running wheel provided) due to their decreased food-intake and their high activity-level. Using PCR-denaturating gradient gel electrophoresis and quantitative real-time PCR (based on 16S rRNA), Queipo-Ortuno et al. found that food restriction led to higher numbers of Proteobacteria and the archaeon Methanobrevibacter smithii, and lower quantities of Actinobacteria, Firmicutes and Bacteroidetes [57].
Chen et al. analyzed mice subjected to post-weaning chronic dietary restriction and subsequent refeeding [58] and performed 16S rRNA gene sequencing of the faecal microbiota. The group found that age and diet, but not bodyweight itself were associated with faecal microbiota composition. Characteristic for the animals with dietary restriction was a relative immature faecal microbiota, a phenomenon that was abolished after refeeding. The overall microbial community structure was significantly different in the fasted compared to the ad libitum fed mice. Upon refeeding of the fasted mice, the microbial community structure significantly changed compared to mice that were persistently fasted throughout the study period. However, the microbial community structure of refed mice also still differed significantly from the ad libitum fed mice, indicating that the microbiota changed in response to refeeding but did not resume the state of ad libitum fed mice. At phylum level, refeeding was accompanied with increased abundances of Bacteroidetes and Proteobacteria and a decreased abundance of Firmicutes.
The abundances of Ruminoccocus spp., Oscillospria spp., Coprococcus spp., and Adlercreutzia spp. were decreased and Suturella spp. increased in the refed animals.
3.5.1.2. Fish
The intestinal gut-microbiota of eight day long fasted Asian seabass (Lates calcarifer, fish) was analyzed by 16S rRNA gene sequencing and revealed that Proteobacteria, followed by Firmicutes and Bacteroidetes were the most abundant bacterial taxa [59]. Thus, the distribution of the most abundant phyla in the large intestine is different to that of mammals. Starvation led to only minor shifts of microbial richness but at community level large shifts were observed. At phylum level, Bacteroidetes was decreased and Firmicutes increased during fasting.
Another study analyzed the intestinal microbiota in wild Atlantic cod (Gadus morhua) using counting techniques and PCR-denaturating gradient gel electrophoresis (based on 16S rRNA) after five weeks starvation starvation upon captive rearing [60]. They found that the counts of intestinal microbes were similar in the starved versus the non-starved and wild-caught Atlantic cod. Moreover, starvation had not affected the microbial population structure in comparison to the wild-caught Atlantic cod. In contrast, the microbial population differed between the fed-group versus the wild-caught Atlantic cod group, suggesting that the provided fish feed was responsible for this change.
3.5.1.3. Reptile
One animal model used to study starvation and refeeding at physiological conditions are snakes, which belong to the reptiles of the vertebrate class [61]. The gut microbiota was studied in Burmese pythons (Python molurus) under laboratory conditions by Costello et al. using 16S rRNA gene sequencing. Similarly to mammals, Firmicutes and Bacteroidetes were the most abundant phyla. The fasted snakes displayed decreased microbial richness and Faith`s phylogenetic diversity, increased abundances of Bacteroidetes and decreased abundances of Firmicutes in the large intestine. At genus level, Bacteroides spp., Rikenella spp., Synergistes spp. and Akkermansia spp. were increased. Refeeding not only increased the bacterial community diversity and species richness but also changed the ratio of Firmicutes and Bacteroides dramatically.
3.5.1.4. Birds
Dewar et al. analyzed the effect of fasting on the faecal microbiota during moult of penguins [62]. These animals have to survive long periods of starvation during increased metabolic demands for thermoregulation and feather synthesis. The most abundant phyla in little penguins (Eudyptula minor) were Firmicutes, Proteobacteria, Bacteroidetes and Actinobateria whereas in king penguins (Aptenodytes patagonicus) the phyla Proteobacteria, Firmicutes, Fusobacteria and Bacteroidetes dominated. In little penguins, the phylum Bacteroidetes increased during starvation whereas in the king penguin it was the phylum Proteobacteria. Interestingly, the microbial community structure during early and late moult in king penguins differed dramatically, whereas in little penguins only moderate differences were observed.
3.5.1.5. Mammal, Bird, Amphibitate and Fish
Kohl et al. analyzed the influence of 20% to 30% body weight loss under laboratory conditions on the gut microbiota of the nile tilapia (Oreochromis niloticus, fish), southern toad (Anaxyrus terrestris, amphibian), leopard gecko (Eublepharis macularius, amphibian), Japanese quail (Coturnix coturnix, bird) and the mouse (Mus musculus, small mammal) [63]. 16S rRNA gene sequencing revealed that the phyla distribution across the analyzed species varied considerably. Richness and phylogenetic diversity of the colonic microbiota increased with fasting in tilapias, toads and mice, whereas no difference was observed in geckos, and a decrease was observed in quails in comparison to nourished animals. In contrast, in the caecum the microbial richness and Faith`s phylogenetic diversity decreased in tilapias and no differences were observed for mice and quails at late-fasting versus nourished animals. The caecum of the gecko and toad was not analyzed as the caecum is generally either missing or extremely small in amphibians. After fasting the phylum levels in the colon had not changed in the gecko and the quail, but Bacteroidetes had increased in the toad and the mouse, and Tenericutes decreased in mice only. The microbiota of the colon in tilapia changed dramatically upon fasting with increased levels of Proteobacteria, Actinobacteria, Verrucomicrobia and Planctomycetes. Interestingly, in the caecum of tilapia, quail and the mouse no such differences were observed. Overall, the changes to fasting were extremely heterogeneous between the different vertebrates. Shared responses of toads, geckos, quail and mice in the colon were the reduced levels of Coprococcus and Ruminococcus. In the caecum of tilapia, quail and mouse a reduction in Lactobacillus and Prevotella and an increase in Oscillospira was observed upon fasting. This study elegantly highlights the inter-species and inter-compartmental variation in the gut microbiota during the nourished and the energy-restricted state.
3.5.2. Hibernation
Microbial shifts in the intestinal tracts of different hibernating animals such as the leopard frog [64, 65] or the 13-lined ground squirrel [66, 67] have been described using culture-based techniques for many years. In the following, we will only present studies which are based on 16S rRNA gene analyses of the gut microbiota.
3.5.2.1. Amphibian
The effect of hibernation on the large intestinal microbiota of the brown tree frog (Polypedates megacephalus) was analyzed using 16S rRNA gene sequencing under laboratory conditions [68]. In comparison to controls, hibernation was associated with a decreased microbial richness and diversity. At phylum level, the abundance of Firmicutes decreased during hibernation. Interestingly, Citrobacter spp, an opportunistic pathogenic genus increased in this state of starvation.
3.5.2.2. Small and Large Mammals
The cecal microbiota of 13-lined ground squirrel (Ictidomys tridecemlineatus) was analyzed over the annual hibernation cycle under laboratory conditions using 16S rRNA gene sequencing and gas chromatography [69]. The most abundant phyla were Bacteroidetes, Firmicutes and Verrucomicrobia. Microbial richness and phylogenetic diversity were lowest in late winter hibernators and the microbial community structure was clearly related to season. The hibernators had a lower abundance of Firmicutes and higher levels of Bacteroidetes and Verrucomicrobia (for the latter only Akkermansia spp.). Interestingly, Lactobacillus spp. were completely depleted in late winter hibernators. The production of SCFA was dramatically decreased during hibernation. Similar results were reported by another group, which analyzed the structure of the mucosal bacteria community in 13-lined ground squirrels [70]. However, the authors note that although the mucosal microbiota remained relatively stable across the annual cycle, it responded to the changes in substrates.
The influence of the annual cycle (including hibernation) on the caecal microbiota of the arctic ground squirrel (Urocitellus parryii) was also analyzed under laboratory conditions [71]. Hibernation led to a decrease of microbial richness and phylogenetic diversity. The caecal microbial community of squirrels clustered tightly during summer, as indicated by the unweighted Unifrac beta-diversity indice. This demonstrates high inter-individual similarity and low dispersion between animals wheras the dispersion was high at posthibernation. The most abundant phyla were Bacteroidetes and Firmicutes, similar to humans. Hibernation led to a shift towards a decrease in Firmicutes and an increase in Bacteroidetes, Verrucomicrobia and Proteobacteria. SCFA decreased during hibernation. It is important to note that the changes observed in diversity and composition of the caecal microbiota were not reversed immediately after hibernation.
Sommer et al. investigated the faecal microbiota of free-ranging Eurasian brown bears (ursus arctos), which are large mammal hibernator [72]. The most abundant phyla were Proteobacteria, Firmicutes, Bacteroidetes and Actinobacteria. The gut microbial diversity was lower during the hibernation period and the microbial community structure was clearly linked to season (summer/active animal versus winter/hibernating animal). In the hibernating period, the abundances of Actinobacteria and Firmicutes were lower whereas Bacteroidetes was higher in comparison to the active/feeding summer phase. Verrucomicrobia (Akkermansia spp.) were not higher in hibernating animals. Finally, the group performed additional analyses by colonizing germ-free mice with bear microbiota. They found that animals inoculated with summer bear faecal microbiota tended to gain more weight and had increased adipose tissue. Glucose metabolism was similar in the animals and was even improved in the “summer bear microbiota” mice.
3.5.3. Fasting Versus Hibernation
Finally, one study analyzed the differences between fasting and hibernation in the syrian hamster (Mesocricetus auratus). Using flow cytometry, Sonoyama et al. showed that the caecal total bacterial count was lower in fasted animals compared to torpid or fed hamsters [55]. Additionally, the count of viable cells was lowest in fasted animals and highest in torpid animals. SCFA analyzed with high-performance liquid chromatography were decreased in torpid animals when compared to fed animals, and dramatically low levels were observed in fasted hamsters. Using PCR-denaturating gradient gel electrophoresis and quantitative real-time PCR (based on 16S rRNA), they found that the class Clostridia of the phylum Firmicutes was the most abundant taxonomic group. Fasting in the animals led to a shift towards a decrease in Firmicutes, a massive increase of Verrucomicrobia (Akkermansia spp.) and Proteobacteria. This study shows that fasting in the active state versus fasting in the inactive state results in large differences for the gut microbiota.
Overall, fasting in animals resulted in increased, decreased and unchanged gastrointestinal microbial richness and diversity, and depended on the gastrointestinal compartments. If the overall microbial community structure was reported for the different states of feeding, most studies reported clustering according to the feeding state except one study. With respect to the variation in specific microbial taxa, the findings for the relative abundances of the phyla Firmicutes and Bacteroidetes upon starvation and refeeding were inconsistent. However, if a change in the phyla Verrucomicrobia (or the genus Akkermansia spp.) or Proteobacteria was reported, an increase was observed upon starvation. In the toad, gecko, quail and the mouse Ruminococcus spp. was decreased in the colon during starvation, however one study reported for mice a decrease of Ruminococcus spp. upon refeeding. Only one study reported a role of the archaeon Methanobrevibacter smithii and found this taxon to be increased upon starvation.
In contrast to the conflicting results found for the gastrointestinal and faecal microbiota in fasting studies, the findings for hibernating animals were rather consistent. If reported, a decrease of microbial richness and diversity was observed in the hibernating animal in comparison to the active animal. The microbial community structure was linked to the season (hibernation versus active period). Hibernation was also linked to decreased relative abundances of Firmicutes whereas Bacteroidetes and Verrucomicrobia were increased or unchanged with the exception of Sommer et al. where Verrucomicrobia were significantly decreased during winter.
3.6. Similarities and Differences between the Gut Microbiota in Humans with Eating Disorders and other Vertebrates
Keeping in mind that i) the morphology of the gastrointestinal tract and ii) the food and living schemes differ substantially between vertebrates and within vertebrate classes (e.g. mammals) [73], it is clear that the gastrointestinal microbiota has needed to adapt to these different habitats. Thus, it is not surprising that the results of our reviewed studies are rather heterogeneous. The aim to determine similarities and differences between the gut microbiota in humans with AN in comparison to other vertebrates subjected to fasting periods is somewhat difficult since i) only few studies have been published for AN in this field and ii) the results of the fasting studies for other animals were heterogeneous. One explanation for this could be that the gastrointestinal microbiota is extremely species- and even strain- specific (when looking at the characterization of the germ-free animals) and/or that the environmental conditions during the fasting experiments bias the results (e.g. environmental temperature, activity or resting of the animal, dietary intake). An argument against an overall species specificity is that the gut microbiota at hibernation of amphibians and mammals was rather homogeneous. Here the environmental conditions were overall constant and controllable.
The fasting studies show that caloric restriction (except during hibernation) does not necessarily lead to a loss of gut microbial richness and diversity. Turnover of microbes adapting to the specific situations of fasting and/or refeeding is imperative, which explains how generally the microbial community structures were linked to the states of feeding. Thus, conflicting results in microbial richness observed by Kleiman et al. versus Mack et al. and Borgo et al. may simply reflect environmental differences. The directions of change observed for the phyla Firmicutes and Bacteroidetes in AN patients may be specific since the results in the fasting animal studies were heterogeneous, and reported opposite directions in hibernating animals. However, due to the few studies published for AN, this is mere speculation at the present time. Additionally we have to consider that, at least for mice and humans, the Firmicutes to Bacteroidetes ratio should not be compared since it is well known that both species harbor a very different faecal microbial community in terms of composition and function [74]. Regarding the phylum Verrucomicrobia (Akkermansia spp.) Mack et al. found high levels in AN patients before weight gain, a finding which appears to be unspecific to AN itself, since many of the fasting and hibernation studies also reported high abundances for this phylum. Akkermansia spp. have an advantage in fasting periods since they are typical mucin-degraders [75]. The finding of Kleiman et al. and Mack et al. that Ruminococcus spp. increases with weight gain is in line with the fasting studies in other vertebrates, which show a decrease upon fasting, except for one study. Rumminococcus spp. are involved in the degradation of fiber and resistant starch [76, 77].
The role of the gut microbiota and CNS function was not tested in any of the studies, but this was not within the scope of the systematic literature search. However, Kleiman et al., and Borgo et al. found associations with mood and the gut microbiota. There is no clear evidence that allows us to draw the conclusion that the gut microbiota influences the psyche of AN patients. Nevertheless, an important communication link between gut bacteria and the host mucosa (and thus the gut-brain axis) are metabolites and low weight molecular compounds (e.g. SCFA) [78]. Since the amounts and/or proportions of faecal SCFA were consistently different in AN patients compared to controls, it is tempting to speculate that the gut microbiota of AN patients impacts on central nervous functions.
Finally, we asked whether – in the case that the gut microbiota in AN is specific – this would have functional consequences for the host. Since we cannot answer the first question fully, we are far away from answering this key question.
CONCLUSION
We found that the complexity of the relationship between fasting and the gut microbiota in humans with AN and other vertebrates is difficult to interpret. Considering that AN has the highest mortality rate of all mental disorders and only 50% of AN patients fully recover in the long-term [79], alternative therapies in addition to the well established psychotherapies are needed for better outcomes. The finding of decreased abundances of the butyrate producing Roseburia spp. in combination with reduced butyrate levels in AN could provide an interesting target to modulate the gut microbiota. Future opportunities include further investigations of Roseburia spp. as a probiotic, as well as other established probiotics such as Lactobacilli spp. and Bifidobacteria spp., which can increase the abundance of Roseburia spp. via cross-feeding [80]. Modulating the gut microbiota could support therapy by improving the nutritional status and/or common side effects such as gastrointestinal symptoms or psychological disease. A deeper biological understanding will help to find promising approaches for the modulation of the AN gut microbiota.
CONSENT FOR PUBLICATION
Not applicable.
Fig. (1).
PRISMA flow chart for study inclusion.
ACKNOWLEDGEMENTS
We acknowledge the Schweizerische Anorexia Nervosa Stiftung, Switzerland for funding (project number: 32-13). All authors of this paper have read and approved the final version. Isabelle Mack was responsible for the conception of the review, conducted the literature search, interpreted the studies and wrote the paper. John Penders interpreted the studies and wrote the paper. Jaslyn Dugmore, Jessica Cook and Nazar Mazurak performed the literature search and discussed and interpreted it with Isabelle Mack. Paul Enck was responsible for the conception of the review and interpreted the studies.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
REFERENCES
- 1.Kaye W.H., Fudge J.L., Paulus M. New insights into symptoms and neurocircuit function of anorexia nervosa. Nat. Rev. Neurosci. 2009;10(8):573–584. doi: 10.1038/nrn2682. [http://dx.doi.org/10.1038/nrn2682]. [PMID: 19603056]. [DOI] [PubMed] [Google Scholar]
- 2.Smith M.I., Yatsunenko T., Manary M.J., Trehan I., Mkakosya R., Cheng J., Kau A.L., Rich S.S., Concannon P., Mychaleckyj J.C., Liu J., Houpt E., Li J.V., Holmes E., Nicholson J., Knights D., Ursell L.K., Knight R., Gordon J.I. Gut microbiomes of Malawian twin pairs discordant for kwashiorkor. Science. 2013;339(6119):548–554. doi: 10.1126/science.1229000. [http://dx.doi.org/10.1126/science. 1229000]. [PMID: 23363771]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lozupone C.A., Stombaugh J.I., Gordon J.I., Jansson J.K., Knight R. Diversity, stability and resilience of the human gut microbiota. Nature. 2012;489(7415):220–230. doi: 10.1038/nature11550. [http://dx.doi.org/10. 1038/nature11550]. [PMID: 22972295]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Walter J., Ley R. The human gut microbiome: Ecology and recent evolutionary changes. Annu. Rev. Microbiol. 2005;•••:65411–65429. doi: 10.1146/annurev-micro-090110-102830. [PMID: 21682646]. [DOI] [PubMed] [Google Scholar]
- 5.Gordon H.A., Bruckner-Kardoss E., Wostmann B.S. Aging in germ-free mice: life tables and lesions observed at natural death. J. Gerontol. 1966;21(3):380–387. doi: 10.1093/geronj/21.3.380. [http://dx.doi.org/10.1093/geronj/ 21.3.380]. [PMID: 5944800]. [DOI] [PubMed] [Google Scholar]
- 6.Landy J.J., Yerasimides T.G., Growdon J.H., Bausor S.C. Germfree guinea pig delivery by hysterectomy. Surg. Forum. 1960;11:425–426. [PMID: 13758703]. [PubMed] [Google Scholar]
- 7.Waxler G.L., Drees D.T. Comparison of body weights, organ weights and histological features of selected organs of gnotobiotic, conventional and isolator-reared contaminated pigs. Can. J. Comp. Med. 1972;36(3):265–274. [PMID: 4261841]. [PMC free article] [PubMed] [Google Scholar]
- 8.Bergman E.N. Energy contributions of volatile fatty acids from the gastrointestinal tract in various species. Physiol. Rev. 1990;70(2):567–590. doi: 10.1152/physrev.1990.70.2.567. [http://dx.doi.org/10.1152/physrev.1990.70.2.567]. [PMID: 2181501]. [DOI] [PubMed] [Google Scholar]
- 9.Ballantyne G.H. Peptide YY(1-36) and peptide YY(3-36): Part I. Distribution, release and actions. Obes. Surg. 2006;16(5):651–658. doi: 10.1381/096089206776944959. [http://dx.doi.org/10.1381/096089206776944959]. [PMID: 16687037]. [DOI] [PubMed] [Google Scholar]
- 10.Macfarlane G.T., Macfarlane S. Bacteria, colonic fermentation, and gastrointestinal health. J. AOAC Int. 2012;95(1):50–60. doi: 10.5740/jaoacint.sge_macfarlane. [http://dx.doi.org/10.5740/jaoacint.SGE_Macfarlane]. [PMID: 22468341]. [DOI] [PubMed] [Google Scholar]
- 11.Holzer P., Farzi A. Neuropeptides and the microbiota-gut-brain axis. Adv. Exp. Med. Biol. 2014;817:195–219. doi: 10.1007/978-1-4939-0897-4_9. [http://dx.doi.org/ 10.1007/978-1-4939-0897-4_9]. [PMID: 24997035]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Macfarlane G.T. Macfarlane, S. Human colonic microbiota: Ecology, physiology and metabolic potential of intestinal bacteria. Scand. J. Gastroenterol. Suppl. 1997;•••:2223–2229. doi: 10.1080/00365521.1997.11720708. [DOI] [PubMed] [Google Scholar]
- 13.Yamanaka M., Nomura T., Tokioka J., Kametaka M. A comparison of the gastrointestinal tract in germ-free and conventional mice fed an amino acid mixture or purified whole-egg protein. J. Nutr. Sci. Vitaminol. (Tokyo) 1980;26(5):435–447. doi: 10.3177/jnsv.26.435. [http://dx.doi. org/10.3177/jnsv.26.435]. [PMID: 7218047]. [DOI] [PubMed] [Google Scholar]
- 14.Snyder D.L., Wostmann B.S. Growth rate of male germfree Wistar rats fed ad libitum or restricted natural ingredient diet. Lab. Anim. Sci. 1987;37(3):320–325. [PMID: 3613511]. [PubMed] [Google Scholar]
- 15.Yokota H., Mori H., Furuse M. Changes in body composition of germ-free and conventional chickens during starvation. Comp. Biochem. Physiol. Comp. Physiol. 1992;103(3):565–568. [http://dx. doi.org/10.1016/0300-9629(92)90290-7]. [PMID: 1358511]. [PubMed] [Google Scholar]
- 16.Bäckhed F., Ding H., Wang T., Hooper L.V., Koh G.Y., Nagy A., Semenkovich C.F., Gordon J.I. The gut microbiota as an environmental factor that regulates fat storage. Proc. Natl. Acad. Sci. USA. 2004;101(44):15718–15723. doi: 10.1073/pnas.0407076101. [http://dx.doi.org/10.1073/pnas. 0407076101]. [PMID: 15505215]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Turnbaugh P.J., Bäckhed F., Fulton L., Gordon J.I. Diet-induced obesity is linked to marked but reversible alterations in the mouse distal gut microbiome. Cell Host Microbe. 2008;3(4):213–223. doi: 10.1016/j.chom.2008.02.015. [http://dx.doi.org/10.1016/j.chom.2008.02.015]. [PMID: 18407065]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Turnbaugh P.J., Ley R.E., Mahowald M.A., Magrini V., Mardis E.R., Gordon J.I. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature. 2006;444(7122):1027–1031. doi: 10.1038/nature05414. [http://dx.doi.org/10.1038/nature05414]. [PMID: 17183312]. [DOI] [PubMed] [Google Scholar]
- 19.Ley R.E., Turnbaugh P.J., Klein S., Gordon J.I. Microbial ecology: human gut microbes associated with obesity. Nature. 2006;444(7122):1022–1023. doi: 10.1038/4441022a. [http://dx.doi.org/10.1038/4441022a]. [PMID: 17183309]. [DOI] [PubMed] [Google Scholar]
- 20.Guo Y., Huang Z.P., Liu C.Q., Qi L., Sheng Y., Zou D.J. Modulation of the gut microbiome: A systematic review of the effect of bariatric surgery. Eur. J. Endocrinol. 2018;178(1):43–56. doi: 10.1530/EJE-17-0403. [http://dx.doi.org/10.1530/EJE-17-0403]. [PMID: 28916564]. [DOI] [PubMed] [Google Scholar]
- 21.Torres-Fuentes C., Schellekens H., Dinan T.G., Cryan J.F. The microbiota-gut-brain axis in obesity. Lancet Gastroenterol. Hepatol. 2017;2(10):747–756. doi: 10.1016/S2468-1253(17)30147-4. [http://dx.doi.org/10.1016/S2468-1253(17) 30147-4]. [PMID: 28844808]. [DOI] [PubMed] [Google Scholar]
- 22.Harley I.T., Karp C.L. Obesity and the gut microbiome: Striving for causality. Mol. Metab. 2012;1(1-2):21–31. doi: 10.1016/j.molmet.2012.07.002. [http://dx.doi.org/ 10.1016/j.molmet.2012.07.002]. [PMID: 24024115]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sze M.A., Schloss P.D. Looking for a signal in the noise: Revisiting obesity and the microbiome. MBio. 2016;7(4):e01018–e16. doi: 10.1128/mBio.01018-16. [http://dx.doi.org/10.1128/mBio.01018-16]. [PMID: 27555308]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Guinane C.M., Cotter P.D. Role of the gut microbiota in health and chronic gastrointestinal disease: Understanding a hidden metabolic organ. Therap. Adv. Gastroenterol. 2013;6(4):295–308. doi: 10.1177/1756283X13482996. [http://dx.doi.org/10.1177/1756283X13482996]. [PMID: 23814609]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moher D., Liberati A., Tetzlaff J., Altman D.G. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. J. Clin. Epidemiol. 2009;62(10):1006–1012. doi: 10.1016/j.jclinepi.2009.06.005. [http://dx. doi.org/10.1016/j.jclinepi.2009.06.005]. [PMID: 19631508]. [DOI] [PubMed] [Google Scholar]
- 26.Liberati A., Altman D.G., Tetzlaff J., Mulrow C., Gotzsche P.C., Ioannidis J.P., Clarke M., Devereaux P.J., Kleijnen J. Moher, D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339:b2700. doi: 10.1136/bmj.b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.da Costa Santos C.M., de Mattos Pimenta C.A., Nobre M.R. The PICO strategy for the research question construction and evidence search. Rev. Lat. Am. Enfermagem. 2007;15(3):508–511. doi: 10.1590/s0104-11692007000300023. [http:// dx.doi.org/10.1590/S0104-11692007000300023]. [PMID: 17653438]. [DOI] [PubMed] [Google Scholar]
- 28.Macpherson A.J., McCoy K.D., Johansen F.E., Brandtzaeg P. The immune geography of IgA induction and function. Mucosal Immunol. 2008;1(1):11–22. doi: 10.1038/mi.2007.6. [http://dx.doi.org/10.1038/mi.2007.6]. [PMID: 19079156]. [DOI] [PubMed] [Google Scholar]
- 29.Maynard C.L., Elson C.O., Hatton R.D., Weaver C.T. Reciprocal interactions of the intestinal microbiota and immune system. Nature. 2012;489(7415):231–241. doi: 10.1038/nature11551. [http://dx.doi.org/10.1038/ nature11551]. [PMID: 22972296]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pabst O. New concepts in the generation and functions of IgA. Nat. Rev. Immunol. 2012;12(12):821–832. doi: 10.1038/nri3322. [http://dx.doi.org/10. 1038/nri3322]. [PMID: 23103985]. [DOI] [PubMed] [Google Scholar]
- 31.Savage D.C. Microbial ecology of the gastrointestinal tract. Annu. Rev. Microbiol. 1977;31:107–133. doi: 10.1146/annurev.mi.31.100177.000543. [http://dx.doi.org/10.1146/ annurev.mi.31.100177.000543]. [PMID: 334036]. [DOI] [PubMed] [Google Scholar]
- 32.Sommer F., Bäckhed F. The gut microbiota--masters of host development and physiology. Nat. Rev. Microbiol. 2013;11(4):227–238. doi: 10.1038/nrmicro2974. [http://dx.doi.org/10.1038/nrmicro2974]. [PMID: 23435359]. [DOI] [PubMed] [Google Scholar]
- 33.Tremaroli V., Bäckhed F. Functional interactions between the gut microbiota and host metabolism. Nature. 2012;489(7415):242–249. doi: 10.1038/nature11552. [http://dx.doi.org/10.1038/nature11552]. [PMID: 22972297]. [DOI] [PubMed] [Google Scholar]
- 34.Kennedy P.J., Cryan J.F., Dinan T.G., Clarke G. Kynurenine pathway metabolism and the microbiota-gut-brain axis. 2017. http://dx.doi.org/10 [DOI] [PubMed]
- 35.Fernandez-Real J.M., Serino M., Blasco G., Puig J., Daunis-i-Estadella J., Ricart W., Burcelin R., Fernández-Aranda F., Portero-Otin M. Gut microbiota interacts with brain microstructure and function. J. Clin. Endocrinol. Metab. 2015;100(12):4505–4513. doi: 10.1210/jc.2015-3076. [http://dx.doi.org/10.1210/jc.2015-3076]. [PMID: 26445114]. [DOI] [PubMed] [Google Scholar]
- 36.Turnbaugh P.J., Ley R.E., Hamady M., Fraser-Liggett C.M., Knight R., Gordon J.I. The human microbiome project. Nature. 2007;449(7164):804–810. doi: 10.1038/nature06244. [http://dx.doi.org/10.1038/nature06244]. [PMID: 17943116]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Arumugam M., Raes J., Pelletier E., Le Paslier D., Yamada T., Mende D.R., Fernandes G.R., Tap J., Bruls T., Batto J.M., Bertalan M., Borruel N., Casellas F., Fernandez L., Gautier L., Hansen T., Hattori M., Hayashi T., Kleerebezem M., Kurokawa K., Leclerc M., Levenez F., Manichanh C., Nielsen H.B., Nielsen T., Pons N., Poulain J., Qin J., Sicheritz-Ponten T., Tims S., Torrents D., Ugarte E., Zoetendal EG., Wang J., Guarner F., Pedersen O., de Vos W.M., Brunak S., Doré J. ; Ehrlich S.D., Bork P. Enterotypes of the human gut microbiome. Nature. 2011;473(7346):174–180. doi: 10.1038/nature09944. [http://dx.doi.org/10.1038/nature 09944]. [PMID: 21508958]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jeffery I.B., Claesson M.J., O’Toole P.W., Shanahan F. Categorization of the gut microbiota: Enterotypes or gradients? Nat. Rev. Microbiol. 2012;10(9):591–592. doi: 10.1038/nrmicro2859. [http://dx.doi.org/10.1038/ nrmicro2859]. [PMID: 23066529]. [DOI] [PubMed] [Google Scholar]
- 39.Wu G.D., Chen J., Hoffmann C., Bittinger K., Chen Y.Y., Keilbaugh S.A., Bewtra M., Knights D., Walters W.A., Knight R., Sinha R., Gilroy E., Gupta K., Baldassano R., Nessel L., Li H., Bushman F.D., Lewis J.D. Linking long-term dietary patterns with gut microbial enterotypes. Science. 2011;334(6052):105–108. doi: 10.1126/science.1208344. [http://dx.doi.org/10.1126/science.1208344]. [PMID: 21885731]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ohman L., Simrén M. Intestinal microbiota and its role in irritable bowel syndrome (IBS). Curr. Gastroenterol. Rep. 2013;15(5):323. doi: 10.1007/s11894-013-0323-7. [http://dx.doi.org/10.1007/s11894-013-0323-7]. [PMID: 23580243]. [DOI] [PubMed] [Google Scholar]
- 41.Collins S.M., Surette M., Bercik P. The interplay between the intestinal microbiota and the brain. Nat. Rev. Microbiol. 2012;10(11):735–742. doi: 10.1038/nrmicro2876. [http://dx.doi.org/10.1038/nrmicro2876]. [PMID: 23000955]. [DOI] [PubMed] [Google Scholar]
- 42.Foster J.A., McVey Neufeld K.A. Gut-brain axis: How the microbiome influences anxiety and depression. Trends Neurosci. 2013;36(5):305–312. doi: 10.1016/j.tins.2013.01.005. [http://dx.doi.org/10.1016/j.tins.2013.01.005]. [PMID: 23384445]. [DOI] [PubMed] [Google Scholar]
- 43.Lepage P., Leclerc M.C., Joossens M., Mondot S., Blottière H.M., Raes J., Ehrlich D., Doré J. A metagenomic insight into our gut’s microbiome. Gut. 2013;62(1):146–158. doi: 10.1136/gutjnl-2011-301805. [http://dx.doi. org/10.1136/gutjnl-2011-301805]. [PMID: 22525886]. [DOI] [PubMed] [Google Scholar]
- 44.Barnes A.D., Jochum M., Mumme S., Haneda N.F., Farajallah A., Widarto T.H., Brose U. Consequences of tropical land use for multitrophic biodiversity and ecosystem functioning. Nat. Commun. 2014;5:5351. doi: 10.1038/ncomms6351. [http://dx.doi.org/10.1038/ncomms6351]. [PMID: 25350947]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pfleiderer A., Lagier J.C., Armougom F., Robert C., Vialettes B., Raoult D. Culturomics identified 11 new bacterial species from a single anorexia nervosa stool sample. Eur. J. Clin. Microbiol. Infect. Dis. 2013;32(11):1471–1481. doi: 10.1007/s10096-013-1900-2. [http://dx.doi.org/10.1007/ s10096-013-1900-2]. [PMID: 23728738]. [DOI] [PubMed] [Google Scholar]
- 46.Gouba N., Raoult D., Drancourt M. Gut microeukaryotes during anorexia nervosa: A case report. BMC Res. Notes. 2014;7:33. doi: 10.1186/1756-0500-7-33. [http://dx.doi.org/10.1186/1756-0500-7-33]. [PMID: 24418238]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Armougom F., Henry M., Vialettes B., Raccah D., Raoult D. Monitoring bacterial community of human gut microbiota reveals an increase in Lactobacillus in obese patients and Methanogens in anorexic patients. PLoS One. 2009;4(9):e7125. doi: 10.1371/journal.pone.0007125. [http://dx.doi.org/ 10.1371/journal.pone.0007125]. [PMID: 19774074]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Million M., Angelakis E., Maraninchi M., Henry M., Giorgi R., Valero R., Vialettes B., Raoult D. Correlation between body mass index and gut concentrations of Lactobacillus reuteri, Bifidobacterium animalis, Methanobrevibacter smithii and Escherichia coli. Int. J. Obes. 2013;37(11):1460–1466. doi: 10.1038/ijo.2013.20. [http://dx.doi.org/10. 1038/ijo.2013.20]. [PMID: 23459324]. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 49.Morita C., Tsuji H., Hata T., Gondo M., Takakura S., Kawai K., Yoshihara K., Ogata K., Nomoto K., Miyazaki K., Sudo N. Gut dysbiosis in patients with anorexia nervosa. PLoS One. 2015;10(12):e0145274. doi: 10.1371/journal.pone.0145274. [http://dx.doi.org/10.1371/journal.pone.0145274]. [PMID: 26682545]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Borgo F., Riva A., Benetti A., Casiraghi M.C., Bertelli S., Garbossa S., Anselmetti S., Scarone S., Pontiroli A.E., Morace G., Borghi E. Microbiota in anorexia nervosa: The triangle between bacterial species, metabolites and psychological tests. PLoS One. 2017;12(6):e0179739. doi: 10.1371/journal.pone.0179739. [http://dx.doi.org/10.1371/journal.pone. 0179739]. [PMID: 28636668]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kleiman S.C., Glenny E.M., Bulik-Sullivan E.C., Huh E.Y., Tsilimigras M.C.B., Fodor A.A., Bulik C.M., Carroll I.M. Daily changes in composition and diversity of the intestinal microbiota in patients with anorexia nervosa: A series of three cases. Eur. Eat. Disord. Rev. 2017;25(5):423–427. doi: 10.1002/erv.2524. [http://dx.doi.org/10.1002/erv. 2524]. [PMID: 28586130]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kleiman S.C., Watson H.J., Bulik-Sullivan E.C., Huh E.Y., Tarantino L.M., Bulik C.M., Carroll I.M. The intestinal microbiota in acute anorexia nervosa and during renourishment: Relationship to depression, anxiety, and eating disorder psychopathology. Psychosom. Med. 2015;77(9):969–981. doi: 10.1097/PSY.0000000000000247. [http://dx.doi.org/10. 1097/PSY.0000000000000247]. [PMID: 26428446]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mack I., Cuntz U., Grämer C., Niedermaier S., Pohl C., Schwiertz A., Zimmermann K., Zipfel S., Enck P., Penders J. Weight gain in anorexia nervosa does not ameliorate the faecal microbiota, branched chain fatty acid profiles, and gastrointestinal complaints. Sci. Rep. 2016;6:26752. doi: 10.1038/srep26752. [http://dx.doi.org/10.1038/ srep26752]. [PMID: 27229737]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Vandeputte D., Falony G., Vieira-Silva S., Tito R.Y., Joossens M., Raes J. Stool consistency is strongly associated with gut microbiota richness and composition, enterotypes and bacterial growth rates. Gut. 2015 doi: 10.1136/gutjnl-2015-309618. [PMID: 26069274]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sonoyama K., Fujiwara R., Takemura N., Ogasawara T., Watanabe J., Ito H., Morita T. Response of gut microbiota to fasting and hibernation in Syrian hamsters. Appl. Environ. Microbiol. 2009;75(20):6451–6456. doi: 10.1128/AEM.00692-09. [http://dx.doi.org/10.1128/AEM.00692-09]. [PMID: 19700553]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Conway P.L., Maki J., Mitchell R., Kjelleberg S. Starvation of marine flounder, squid and laboratory mice and its effect on the intestinal microbiota. FEMS Microbiol. Ecol. 1986;38(3):187–195. [http://dx.doi.org/10.1111/j.1574-6968.1986.tb01728.x]. [Google Scholar]
- 57.Queipo-Ortuño M.I., Seoane L.M., Murri M., Pardo M., Gomez-Zumaquero J.M., Cardona F., Casanueva F., Tinahones F.J. Gut microbiota composition in male rat models under different nutritional status and physical activity and its association with serum leptin and ghrelin levels. PLoS One. 2013;8(5):e65465. doi: 10.1371/journal.pone.0065465. [http:// dx.doi.org/10.1371/journal.pone.0065465]. [PMID: 23724144]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chen J., Toyomasu Y., Hayashi Y., Linden D.R., Szurszewski J.H., Nelson H., Farrugia G., Kashyap P.C., Chia N., Ordog T. Altered gut microbiota in female mice with persistent low body weights following removal of post-weaning chronic dietary restriction. Genome Med. 2016;8(1):103. doi: 10.1186/s13073-016-0357-1. [http://dx.doi.org/10.1186/ s13073-016-0357-1]. [PMID: 27716401]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Xia J.H., Lin G., Fu G.H., Wan Z.Y., Lee M., Wang L., Liu X.J., Yue G.H. The intestinal microbiome of fish under starvation. BMC Genomics. 2014;15:266. doi: 10.1186/1471-2164-15-266. [http://dx.doi.org/10.1186/1471-2164-15-266]. [PMID: 24708260]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Dhanasiri A.K., Brunvold L., Brinchmann M.F., Korsnes K., Bergh Ø., Kiron V. Changes in the intestinal microbiota of wild Atlantic cod Gadus morhua L. upon captive rearing. Microb. Ecol. 2011;61(1):20–30. doi: 10.1007/s00248-010-9673-y. [http://dx.doi.org/10.1007/s00248-010-9673-y]. [PMID: 20424834]. [DOI] [PubMed] [Google Scholar]
- 61.Costello E.K., Gordon J.I., Secor S.M., Knight R. Postprandial remodeling of the gut microbiota in Burmese pythons. ISME J. 2010;4(11):1375–1385. doi: 10.1038/ismej.2010.71. [http://dx.doi.org/10.1038/ismej.2010.71]. [PMID: 20520652]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Dewar M.L., Arnould J.P., Krause L., Trathan P., Dann P., Smith S.C. Influence of fasting during moult on the faecal microbiota of penguins. PLoS One. 2014;9(6):e99996. doi: 10.1371/journal.pone.0099996. [http://dx.doi. org/10.1371/journal.pone.0099996]. [PMID: 24979619]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kohl K.D., Amaya J., Passement C.A., Dearing M.D., McCue M.D. Unique and shared responses of the gut microbiota to prolonged fasting: A comparative study across five classes of vertebrate hosts. FEMS Microbiol. Ecol. 2014;90(3):883–894. doi: 10.1111/1574-6941.12442. [http://dx.doi.org/10.1111/1574-6941.12442]. [PMID: 25319042]. [DOI] [PubMed] [Google Scholar]
- 64.Banas J.A., Loesche W.J., Nace G.W. Classification and distribution of large intestinal bacteria in nonhibernating and hibernating leopard frogs (Rana pipiens). Appl. Environ. Microbiol. 1988;54(9):2305–2310. doi: 10.1128/aem.54.9.2305-2310.1988. [PMID: 3263838]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gossling J., Loesche W.J., Nace G.W. Response of intestinal flora of laboratory-reared leopard frogs (Rana pipiens) to cold and fasting. Appl. Environ. Microbiol. 1982;44(1):67–71. doi: 10.1128/aem.44.1.67-71.1982. [PMID: 6982026]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Barnes E.M. Effect of hibernation on the intestinal flora. Am. J. Clin. Nutr. 1970;23(11):1519–1524. doi: 10.1093/ajcn/23.11.1519. [http://dx.doi.org/10.1093/ ajcn/23.11.1519]. [PMID: 5475370]. [DOI] [PubMed] [Google Scholar]
- 67.Blossman-Myer B.L., Stangl F.B. Faecal analysis as an indicator of post-hibernation shifts in the gut microflora of the thirteen-lined ground squirrel (Spermophilus tricecemlineatus). Tex. J. Sci. 1999;51(2):159–172. [Google Scholar]
- 68.Weng F.C.H., Yang Y.J., Wang D. Functional analysis for gut microbes of the brown tree frog (Polypedates megacephalus) in artificial hibernation. BMC Genomics. 2016;17(Suppl. 13):1024. doi: 10.1186/s12864-016-3318-6. [http://dx.doi.org/10.1186/s12864-016-3318-6]. [PMID: 28155661]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Carey H.V., Walters W.A., Knight R. Seasonal restructuring of the ground squirrel gut microbiota over the annual hibernation cycle. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2013;304(1):R33–R42. doi: 10.1152/ajpregu.00387.2012. [http://dx.doi.org/10.1152/ajpregu.00387.2012]. [PMID: 23152108]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Dill-McFarland K.A., Neil K.L., Zeng A., Sprenger R.J., Kurtz C.C., Suen G., Carey H.V. Hibernation alters the diversity and composition of mucosa-associated bacteria while enhancing antimicrobial defence in the gut of 13-lined ground squirrels. Mol. Ecol. 2014;23(18):4658–4669. doi: 10.1111/mec.12884. [http://dx.doi.org/10.1111/mec.12884]. [PMID: 25130694]. [DOI] [PubMed] [Google Scholar]
- 71.Stevenson T.J., Duddleston K.N., Buck C.L. Effects of season and host physiological state on the diversity, density, and activity of the arctic ground squirrel cecal microbiota. Appl. Environ. Microbiol. 2014;80(18):5611–5622. doi: 10.1128/AEM.01537-14. [http://dx.doi.org/10.1128/ AEM.01537-14]. [PMID: 25002417]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sommer F., Ståhlman M., Ilkayeva O., Arnemo J.M., Kindberg J., Josefsson J., Newgard C.B., Fröbert O., Bäckhed F. The gut microbiota modulates energy metabolism in the hibernating brown bear ursus arctos. Cell Reports. 2016;14(7):1655–1661. doi: 10.1016/j.celrep.2016.01.026. [http://dx.doi.org/10.1016/j.celrep.2016.01.026]. [PMID: 26854221]. [DOI] [PubMed] [Google Scholar]
- 73.Chivers D.J., Hladik C.M. Morphology of the gastrointestinal tract in primates: comparisons with other mammals in relation to diet. J. Morphol. 1980;166(3):337–386. doi: 10.1002/jmor.1051660306. [http://dx.doi.org/10. 1002/jmor.1051660306]. [PMID: 7441763]. [DOI] [PubMed] [Google Scholar]
- 74.Nguyen T.L., Vieira-Silva S., Liston A., Raes J. How informative is the mouse for human gut microbiota research? Dis. Model. Mech. 2015;8(1):1–16. doi: 10.1242/dmm.017400. [http://dx.doi.org/10.1242/dmm.017400]. [PMID: 25561744]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Marcobal A., Southwick A.M., Earle K.A., Sonnenburg J.L. A refined palate: bacterial consumption of host glycans in the gut. Glycobiology. 2013;23(9):1038–1046. doi: 10.1093/glycob/cwt040. [http://dx.doi.org/10.1093/ glycob/cwt040]. [PMID: 23720460]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Chassard C., Delmas E., Robert C., Lawson P.A., Bernalier-Donadille A. Ruminococcus champanellensis sp. nov., a cellulose-degrading bacterium from human gut microbiota. Int. J. Syst. Evol. Microbiol. 2012;62(Pt 1):138–143. doi: 10.1099/ijs.0.027375-0. [http://dx.doi.org/10.1099/ ijs.0.027375-0]. [PMID: 21357460]. [DOI] [PubMed] [Google Scholar]
- 77.Ze X., Duncan S.H., Louis P., Flint H.J. Ruminococcus bromii is a keystone species for the degradation of resistant starch in the human colon. ISME J. 2012;6(8):1535–1543. doi: 10.1038/ismej.2012.4. [http://dx.doi.org/10. 1038/ismej.2012.4]. [PMID: 22343308]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Mithieux G. Gut microbiota and host metabolism: what relationship? Neuroendocrinology. 2017;106(4):352–356. doi: 10.1159/000484526. [PMID: 29065411]. [DOI] [PubMed] [Google Scholar]
- 79.Zipfel S., Löwe B., Reas D.L., Deter H.C., Herzog W. Long-term prognosis in anorexia nervosa: Lessons from a 21-year follow-up study. Lancet. 2000;355(9205):721–722. doi: 10.1016/S0140-6736(99)05363-5. [http://dx.doi.org/ 10.1016/S0140-6736(99)05363-5]. [PMID: 10703806]. [DOI] [PubMed] [Google Scholar]
- 80.Tamanai-Shacoori Z., Smida I., Bousarghin L., Loreal O., Meuric V., Fong S.B., Bonnaure-Mallet M. Jolivet-Gougeon, A. Roseburia spp.: A marker of health? Future Microbiol. 2017;12:157–170. doi: 10.2217/fmb-2016-0130. [DOI] [PubMed] [Google Scholar]