Abstract
Refractometry is a classic analytical method in analytical chemistry and biosensing. By integrating advanced micro- and nano-optical systems with well-developed microfluidics technology, optofluidics are shown to be a powerful, smart and universal platform for refractive index sensing applications. This paper reviews recent work on optofluidic refractometers based on different sensing mechanisms and structures (e.g., photonic crystal/photonic crystal fibers, waveguides, whisper gallery modes and surface plasmon resonance), and traces the performance enhancement due to the synergistic integration of optics and microfluidics. A brief discussion of future trends in optofluidic refractometers, namely volume sensing and resolution enhancement, are also offered.
Keywords: refractometry, refractive index, microfluidics
1. Introduction
Refractive index (RI), a basic physical substance property, can be used to measure solute concentration and purity in transparent liquor such as Salinity and Brix. RI is highly sensitive and precise, enabling it to monitor extreme variations in tiny particles in solution, which can be used to quantitatively analyze chemical components. For example, 10−9 RIU is equivalent to 1 femto mol/L of salt in water. Compared to other measuring methods, RI measurement does not actually affect the properties of the analyst and offers real-time, convenient analysis of liquid composition (e.g., label-free analysis of various bio-samples, including DNA and protein). Therefore, refractometry with ultra-high sensitivity has great potential in environmental protection [1,2,3], drinking water safety [4,5] and biomedical applications [6].
Microfluidics has achieved great progress recently due to its own excellent performance in fluidic handling [7,8,9], micro-environment control [10,11,12] and signal amplification [13,14]. By integrating advanced micro- and nano-optical systems with well-developed microfluidics technology, optofluidics has ushered in a new era of lab-on-a-chip functionality [15,16,17,18,19,20,21,22], including biochemical sensing with optical measurement [23], optofluidic imaging [24], and light-driven manipulation [25,26]. In the case of RI sensing, many valuable review papers such as Fan’s [27] have found that synergistic integration creates unique characteristics that promote the performance and function of biological/chemical analysis. First, in some well-designed structures, the analyte can be selectively delivered to a location with maximum light-analyte interaction, which can significantly enhance sensors’ sensitivity and resolution. Second, an extremely small analyte volume (i.e., nL) and some related treatments of biological samples, such as cultivating, sorting, trapping, and purification, can be achieved with microfluidics technology. Other analytical methods, including chromatography, electrophoresis, and Raman scattering, can be cascaded with the RI sensing function to carry out complex analysis. Third, issues related to integration, namely alignment and packaging, can be easily solved, and the device’s volume can be reduced substantially. Moerover, device commercialization will facilitate the development of portable, cost-effective, and highly sensitive bio/chemical analysis instruments.
In this paper, recent work pertaining to optofluidic refractometers, based on different sensing mechanisms and structures, is sequentially reviewed. Particularly, this review focuses on the brilliant designs that the optical sensing structures, based on their own various characteristics, synergistically integrated with microfluidics to optimize their sensing performances. These designs enhance the resolutions of the sensors, expand the analysis functions of the sensors or solve issues of sensor applications. The revolution tracks of this integration, showing the elegance of the device design, are also presented. A discussion regarding the field’s ongoing development is also offered with the hope to inspire more new ideas from the readers.
2. RI Sensing and Technologies
2.1. Photonic Crystal Fibers
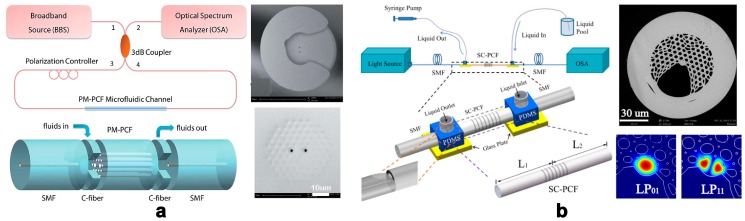
As a classic photonic structure, photonic crystal fibers (PCFs) appear to be an ideal platform for the realization of novel optofluidic sensors [28]. P. Domachuk et al. proposed a compact refractometer utilizing a Fabry–Pérot cavity (FPC) etalon formed between two aligned Bragg grating fibers (one-dimensional PCFs), which located on either side of the microfluidic channel to contain the fluid to be tested [15]. The resolution of the RI sensor based on PCFs can be further improved if the microstructured architecture in the PCFs is filled by analyte [29]. Its waveguide nature ensures strong light-analyte interaction along all PCFs. Some smart structures are designed to feed the analyte into the PCFs’ hollow gap. Many research efforts contribute to design the deliberate structures to achieve the convenient load of liquid sample, and employ special modes to improve the sensitivity of the sensors. For example, C. Wu et al. presented the fabrication and characterization of an in-line photonic crystal fiber microfluidic refractometer outfitted with a C-shaped fiber [30] (Figure 1a). The C-shaped fiber, placed between the PCF and single-mode fiber, achieved two functions simultaneously: in-line optical signal coupling and analyte fluid feeding. Using an arc discharge pre-treatment technique, small air hole voids near the surface were sealed, so only the two central large air hole channels were employed for RI sensing; thus, device sensitivity increased by 70% due to higher power density. Similarly, N. Zhang et al. utilized a side-channel photonic crystal fiber with side-polished single mode fibers to form optofluidic microchannels [31] (Figure 1b). A long-period grating combined with intermodal interference between LP01 and LP11 core modes was used to sense the liquid’s RI in the side channel.
Figure 1.
(a) Scheme and transverse section graph of the SMF-C-PCF-C-SMF microfluidic device. Reprinted with permission from [30]. Copyright (2014) RSC; (b) Scheme of the in-line optofluidic sensing platform and SEM image of the SC-PCF and Simulated intensity distribution of LP01 mode and LP11 mode at the wavelength of 1550 nm. Reprinted with permission from [31]. Copyright (2016) OSA.
2.2. Planar Optical Waveguides
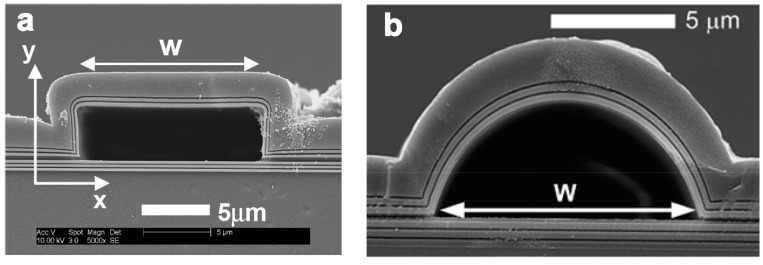
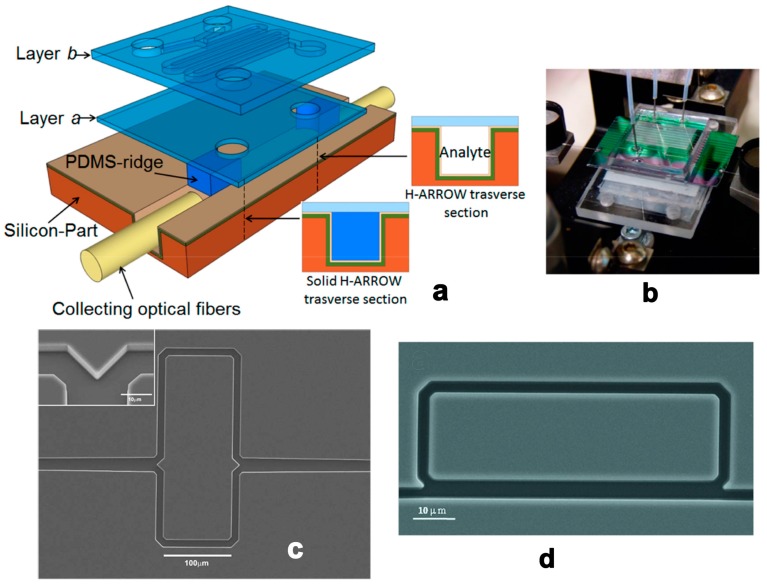
An integrated planar optical waveguide (POW) has been utilized for RI sensing for several decades [32]. In this type of sensor, light propagates along the solid waveguide, around which an evanescent field interacts with the analyte to induce phase shift or intensity variation. The light-analyte interaction is naturally limited by the evanescent field’s range per unit length. Therefore, confining light in the fluidic waveguide is an effective way to achieve strong light-analyte interaction. The liquid core anti-resonant reflecting optical waveguide (ARROW) is a novel photonic structure with tightly integrated optical and fluidic structures [33] (Figure 2). Particularly, Campopiano et al. firstly demonstrated a bulk refractometer based on multimode liquid ARROW structure [34] (Figure 3a,b). And G. Testa et al. offered a comprehensive review of an ARROW-based device’s operation principles and applications [35]. Furthermore, the RI microsensor’s sensitivity can be improved by using the ARROW waveguide as interferometer arms [36] (Figure 3c) and part of the ring resonators [37] (Figure 3d). In addition, the waveguide can act as a carrier, on which 2D materials can be deposited, for novel RI sensors developments. Surface plasmon waves or evanescent wave are tuned by the liquid medium on the surface of Graphene [38] and MoS2 [39] to realize RI sensing.
Figure 2.
Scanning electron microscope (SEM) images of hollow-core anti-resonant reflecting optical waveguides (ARROWs) with (a) rectangular and (b) arch-shaped cross sections fabricated by surface micromachining process. Reprinted with permission from [33]. Copyright (2005) OSA.
Figure 3.
Schematic (a) and fabricated chip (b) of the sensor based on hybrid ARROW optofluidic platform. Reprinted with permission from [34]. Copyright (2014) OSA; (c) SEM picture of liquid-core ARROW. Reprinted with permission from [36]. Copyright (2010) OSA; (d) SEM picture of the integrated silicon optofluidic ring resonator. Reprinted with permission from [37]. Copyright (2010) AIP.
2.3. Whisper Gallery Mode
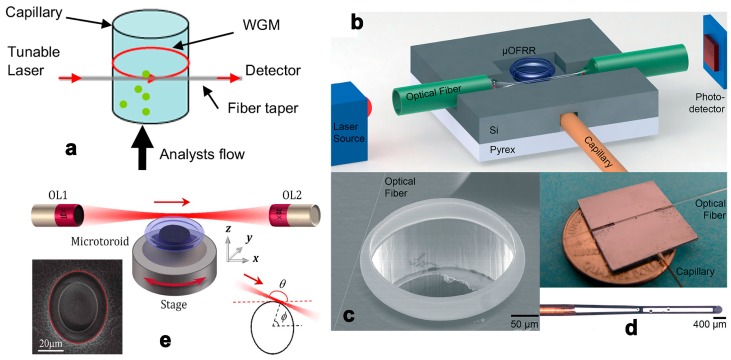
Since F. Vollmer piloted the application of whisper gallery mode (WGM) in protein detection, continued efforts have explored the detection resolution’s potential [40]. A series of well-designed microtoroid resonators were fabricated by L. Yang and Y.-F. Xiao, and related intelligent sensing schemes and noise control methods have also been proposed and optimized [41]. Due to the resonators’ ultra-high Q values, the microsensors have an extremely low detection limit; single nanoparticles have been detected successfully. However, when applying a typical cavity-taper coupling system in an optofluidic system, uncontrollable analyte flow in the microchip may exert a negative effect on cavity-taper coupling, diminishing the Q value to some extent. Thin-wall cylindrical capillaries with spatial analyte-taper separation comprise an alternative scheme that is more convenient for optofluidic integration [42,43] (Figure 4a–d). Since high-Q WGMs in deformed microcavities can be excited by free space coupling [44] (Figure 4e), it is a feasible way to achieve high cavity coupling efficiency in microfluidic chips.
Figure 4.
(a) The optofluidic ring resonator based on thin-walled capillary. Reprinted with permission from [42]. Copyright (2014) Elsevier; Schematic diagram (b), SEM image (c) and photograph (d) of the μOFRR sensor. Reprinted with permission from [43]. Copyright (2014) RSC; (e) Schematic diagram of the experimental setup for free-space coupling between a laser beam and a deformed toroidal microcavity. Reprinted with permission from [44]. Copyright (2016) OSA.
2.4. Surface Plasmon Resonance and Localized Surface Plasmon Resonance
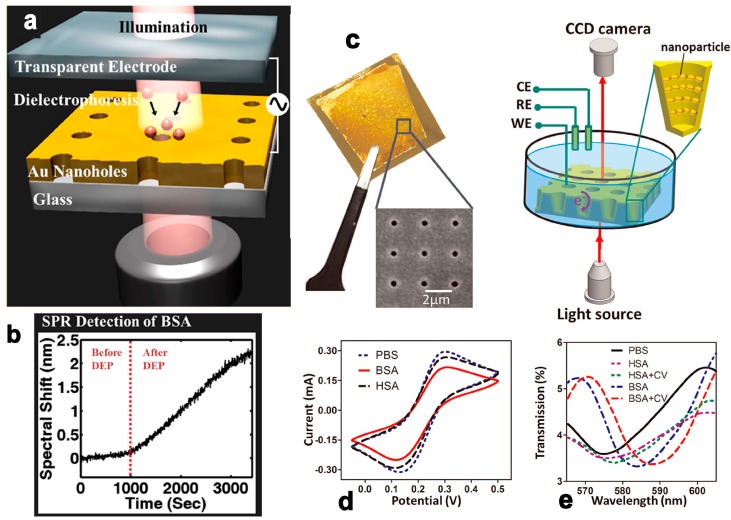
Surface plasmon resonance/localized surface plasmon resonance (SPR/LSPR) refer to the excitation of collective electron charge oscillations on planar metal surfaces or onto the surface of metallic nanoparticles by incident light. The oscillation leads to a wavelength-dependent reduction in the overall reflection, which presents an absorption peak in the reflection/scatter spectrum. As the SPR is very sensitive to the RI near the metal surface (within 300 nm), it is widely used to develop RI sensors [45] and biosensors [46]. Unlike most other types of sensing schemes, metal plane/particle/nanohole arrays represent an indispensable part of the sensing system, which also offers unique opportunities for function appending and upgrading. A. Barik et al. utilized a gold nanohole array-based optofluidic device for label-free detection of analyte molecules, of which the nanohole array also generated gradient dielectrophoretic force to accumulate the measured biological analytes [47] (Figure 5a,b). Real-time detection was over 1000 times faster than the classic diffusion method for 1 pM analyte concentration. S. Kang et al. combined RI sensing with surface-enhanced Raman spectroscopy (SERS) in silver–gold layered bimetallic plasmonic crystals to conduct quantitative and qualitative measurements simultaneously [48]. D. Zhang et al. developed a unique nanoscale cup array (nanoCA) coupling electrochemistry to LSPR spectroscopy measurement, offering a novel method by which to evaluate complex electrochemical reaction processes [49] (Figure 5c–e).
Figure 5.
Schematic of the experimental setup for dielectrophoretic concentration of analyte molecules (a) and spectral shift of BSA’s SPR detection (b). Reprinted with permission from [47]. Copyright (2014) ACS; The nanoCA for electrochemical and LSPR measurement (c) and Transmission spectrum (d) of nanoCA with PBS, 100 μg/mL HSA and HSA plus CV scanning, 100 μg/mL BSA and BSA plus synchronous CV scanning and statistic (e) for shifts in dip wavelength of HSA, HSA plus CV, BSA and BSA plus CV. Reprinted with permission from [49]. Copyright (2015) Elsevier.
3. Discussion and Outlook
3.1. Parameters for Sensor Characterization
Sensitivity, defined as the magnitude in shift of the characteristic wavelength versus the change in a sample’s RI, is a key parameter describing RI sensor performance. However, for measurement response down to the sensor’s detection limit (i.e., resolution), many factors should be considered: the shape of the resonant peak, noise sources, and signal intensity, among others. According to X. D. Fan’s detailed analysis, a sensor with lower sensitivity but a sharper resonance peak (i.e., a higher-quality factor) has higher resolution [50]. Thus, to accurately describe sensor performance, a comprehensive evaluation that includes sensitivity and quality factors is crucial. Several novel parameters, such as figure of merit (FOM) [51] or detectivity [52], have been proposed in some papers. FOM is defined as the ratio of sensitivity to full wave at half maximum (FWHM) as Equation (1), which can take both of the sensitivity and Q value into consideration, thus the performance of the sensor can be characterized accurately by using a single parameter.
| (1) |
Table 1 summarized the critical parameters for qualifying the performances of the RI sensors based on different principles. Although the FOM value (related to resolution/detection limit) is a key parameter for the RI sensors, some other characteristics (detection range, sample volume, cost-effective ratio, portability and capability of integration with other analyzing instruments) are of importance and should be taken into consideration in the specific biochemical applications.
Table 1.
Critical parameters of the refractive index (RI) sensors based on different principles.
| Working Principle | Sensitivity | Q Factor | FOM | Detection Limit | Analyte | Reference |
|---|---|---|---|---|---|---|
| PCF | 8699 nm/RIU | - | - | 4.0 × 10−6 RIU | - | [30] |
| PCF | 1145 nm/RIU | - | - | - | - | [31] |
| POW | 260 nm/RIU | 800 | - | - | - | [37] |
| POW | 1920 nm/RIU | - | - | 5.2 × 10−7 RIU | - | [38] |
| WGM | 1.84 pm/mM | 4 × 105 | - | 0.035 mM | Glucose | [42] |
| WGM | 0.018 pm/mg m−3 | 11,500 | - | 6.9 ppm | Benzene | [43] |
| SPR | - | - | - | 1 pM | BSA | [47] |
| SPR | ~104 nm/RIU | - | - | - | - | [49] |
| FPC | 960 nm/RIU | 600 | 18.79 | 0.01 RIU | - | [53] |
| FPC | 907 nm/RIU | 400 | 9 | 1.7 × 10−5 RIU | - | [54] |
3.2. New Areas for Exploration: Volume Sensing
Currently, most RI sensors are developed based on near-field optics, which uses evanescent waves in the subwavelength region. Dramatically decayed evanescence has marked light-analyte interaction, indicating high sensitivity, but its spatial interaction is intrinsically limited by the attenuation characteristics of evanescent waves. On the other hand, some bio-analytes (e.g., eukaryotic cells) tend to measure 20–30 μm in diameter; in this case, organisms deep inside the cells are outside of the evanescent field and cannot be measured accurately. For RI sensors working on evanescent waves, only the sample in contact with the sensing surface can be measured, which hinders the detection of naturally suspended samples. Furthermore, because the spatial measurement range is expanded from nearly 2D to 3D, volume sensing is a feasible way to increase light-analyte interaction. In volume sensing, the lightwave can completely permeate the targeted sample and detect every particle in the solution—not only the sample attached to the sensing surface. Hence, this method is particularly useful for monolithic biological samples (eukaryotic cells and tissues) and analytes in low-concentration solutions, which is a rising research subject offering clear advantages in a host of applications.
Fabry–Pérot (FP) etalon is considered to be a promising choice for the volume sensing application due to its simple structure and growing performance [16]. Some recent research demonstrated that open-access optical cavities with high Q factor and low mode volume can be achieved by utilizing micro-scale curved-mirrors [55], or even a spherical mirror on a fiber tip-end and an assorted planar mirror [56]. Since RI of living kidney cells [22,57,58], these high performance FP RI sensors are inspired to have bright future in studying cell physiology and pathology.
3.3. Advanced Methods for Performance Enhancement
According to Equation (1), FOM can be optimized from two angles: sensitivity and quality factor. Improving sensitivity depends mainly on enhancing the light-analyte interaction; however, strong light-analyte interaction also indicates intense absorption (i.e., a low Q factor) from solvent, where are often water or phosphate-buffered solution with certain concentration. Therefore, spatially accumulating or attracting interested particles to the area with the strongest light-analyte interaction via external field/force (dielectrophoretic [47], ultrasonic, and magnetic methods [59]) or a microfluidic sorting structure is an effective way to avoid the influence of solvent absorption. Furthermore, cascaded/hybrid structures, such as coupled resonator-induced transparence (CRIT) [60,61,62] and Vernier effect [26], improve the Q factor and sensitivity synchronously and contribute to resolving the interaction-absorption dilemma.
4. Conclusions
This review article has summarized the prominent designs for RI sensing using optofluidic technology. Excellent performance of intelligent designs mentioned in paper indicates a prosperous prospect of optofluidics in bio/chemical analysis. The synergy of photonics and microfluidics offers a great opportunity to achieve the device’s performance improvements and functional extension. And microfluidics technology facilitates devices with portable and cost-effective features, providing steady motivation for their commercialization promotion. In future, more and more subtle and powerful devices will be developed to meet the growing need of measurement in biomedical applications.
Acknowledgments
This study was financially supported by the National Natural Science Foundation of China (No. 61501316), the Shanxi Provincial Foundation for Returned Scholars (2015-047), 863 project (2015AA042601), Fund of State Key Laboratory of Information Photonics and Optical Communications (Beijing University of Posts and Telecommunications).
Author Contributions
Cheng Li wrote the whole manuscript. Gang Bai reviewed the manuscript. Yunxiao Zhang and Min Zhang participated in the discussion. Aoqun Jian supervised the work.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Rotiroti L., De Stefano L., Rendina I., Moretti L., Rossi A.M., Piccolo A. Optical microsensors for pesticides identification based on porous silicon technology. Biosens. Bioelectron. 2005;20:2136–2139. doi: 10.1016/j.bios.2004.09.020. [DOI] [PubMed] [Google Scholar]
- 2.Park J.-H., Byun J.-Y., Yim S.-Y., Kim M.-G. A localized surface plasmon resonance (LSPR)-based, simple, receptor-free and regeneratable Hg2+ detection system. J. Hazard. Mater. 2016;307:137–144. doi: 10.1016/j.jhazmat.2015.12.040. [DOI] [PubMed] [Google Scholar]
- 3.Bao B., Melo L., Davies B., Fadaei H., Sinton D., Wild P. Detecting supercritical CO2 in brine at sequestration pressure with an optical fiber sensor. Environ. Sci. Technol. 2012;47:306–313. doi: 10.1021/es303596a. [DOI] [PubMed] [Google Scholar]
- 4.Yu J., Huang W., Chin L., Lei L., Lin Z., Ser W., Chen H., Ayi T., Yap P., Chen C. Droplet optofluidic imaging for λ-bacteriophage detection via co-culture with host cell escherichia coli. Lab Chip. 2014;14:3519–3524. doi: 10.1039/C4LC00042K. [DOI] [PubMed] [Google Scholar]
- 5.Liu P., Chin L., Ser W., Ayi T., Yap P., Bourouina T., Leprince-Wang Y. An optofluidic imaging system to measure the biophysical signature of single waterborne bacteria. Lab Chip. 2014;14:4237–4243. doi: 10.1039/C4LC00783B. [DOI] [PubMed] [Google Scholar]
- 6.Liu P.Y., Chin L.K., Ser W., Chen H.F., Hsieh C.M., Lee C.H., Sung K.B., Ayi T.C., Yap P.H., Liedberg B., et al. Cell refractive index for cell biology and disease diagnosis: Past, present and future. Lab Chip. 2016;16:634–644. doi: 10.1039/C5LC01445J. [DOI] [PubMed] [Google Scholar]
- 7.Kaler K.V., Prakash R. Droplet microfluidics for chip-based diagnostics. Sensors. 2014;14:23283–23306. doi: 10.3390/s141223283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Strohmeier O., Keller M., Schwemmer F., Zehnle S., Mark D., von Stetten F., Zengerle R., Paust N. Centrifugal microfluidic platforms: Advanced unit operations and applications. Chem. Soc. Rev. 2015;44:6187–6229. doi: 10.1039/C4CS00371C. [DOI] [PubMed] [Google Scholar]
- 9.Fair R.B. Digital microfluidics: Is a true lab-on-a-chip possible? Microfluid. Nanofluid. 2007;3:245–281. doi: 10.1007/s10404-007-0161-8. [DOI] [Google Scholar]
- 10.Yang W., Luo C., Lai L., Ouyang Q. A novel microfluidic platform for studying mammalian cell chemotaxis in different oxygen environments under zero-flow conditions. Biomicrofluidics. 2015;9:044121. doi: 10.1063/1.4929406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen Y.-C., Zhang Z., Fouladdel S., Deol Y., Ingram P.N., McDermott S.P., Azizi E., Wicha M.S., Yoon E. Single cell dual adherent-suspension co-culture micro-environment for studying tumor–stromal interactions with functionally selected cancer stem-like cells. Lab Chip. 2016;16:2935–2945. doi: 10.1039/C6LC00062B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen Y.-C., Cheng Y.-H., Kim H.S., Ingram P.N., Nor J.E., Yoon E. Paired single cell co-culture microenvironments isolated by two-phase flow with continuous nutrient renewal. Lab Chip. 2014;14:2941–2947. doi: 10.1039/C4LC00391H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chang C.M., Chang W.H., Wang C.H., Wang J.H., Mai J.D., Lee G.B. Nucleic acid amplification using microfluidic systems. Lab Chip. 2013;13:1225–1242. doi: 10.1039/c3lc41097h. [DOI] [PubMed] [Google Scholar]
- 14.Lin X., Sun X., Luo S., Liu B., Yang C. Development of DNA-based signal amplification and microfluidic technology for protein assay: A review. TrAC Trends Anal. Chem. 2016;80:132–148. doi: 10.1016/j.trac.2016.02.020. [DOI] [Google Scholar]
- 15.Domachuk P., Littler I.C.M., Cronin-Golomb M., Eggleton B.J. Compact resonant integrated microfluidic refractometer. Appl. Phys. Lett. 2006;88:093513. doi: 10.1063/1.2181204. [DOI] [Google Scholar]
- 16.Bitarafan M.H., DeCorby R.G. On-chip high-finesse fabry-perot microcavities for optical sensing and quantum information. Sensors. 2017;17:1748. doi: 10.3390/s17081748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Trichet A.A., Foster J., Omori N.E., James D., Dolan P.R., Hughes G.M., Vallance C., Smith J.M. Open-access optical microcavities for lab-on-a-chip refractive index sensing. Lab Chip. 2014;14:4244–4249. doi: 10.1039/C4LC00817K. [DOI] [PubMed] [Google Scholar]
- 18.Pang L., Chen H.M., Freeman L.M., Fainman Y. Optofluidic devices and applications in photonics, sensing and imaging. Lab Chip. 2012;12:3543–3551. doi: 10.1039/c2lc40467b. [DOI] [PubMed] [Google Scholar]
- 19.Erickson D., Sinton D., Psaltis D. Optofluidics for energy applications. Nat. Photonics. 2011;5:583–590. doi: 10.1038/nphoton.2011.209. [DOI] [Google Scholar]
- 20.Schmidt H., Hawkins A.R. The photonic integration of non-solid media using optofluidics. Nat. Photonics. 2011;5:598–604. doi: 10.1038/nphoton.2011.163. [DOI] [Google Scholar]
- 21.Monat C., Domachuk P., Eggleton B. Integrated optofluidics: A new river of light. Nat. Photonics. 2007;1:106–114. doi: 10.1038/nphoton.2006.96. [DOI] [Google Scholar]
- 22.Song W., Zhang X., Liu A., Lim C., Yap P., Hosseini H.M.M. Refractive index measurement of single living cells using on-chip fabry-pérot cavity. Appl. Phys. Lett. 2006;89:203901. doi: 10.1063/1.2387965. [DOI] [Google Scholar]
- 23.Bates K.E., Lu H. Optics-integrated microfluidic platforms for biomolecular analyses. Biophys. J. 2016;110:1684–1697. doi: 10.1016/j.bpj.2016.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhao Y., Stratton Z.S., Guo F., Lapsley M.I., Chan C.Y., Lin S.C., Huang T.J. Optofluidic imaging: Now and beyond. Lab Chip. 2013;13:17–24. doi: 10.1039/C2LC90127G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Seow Y.C., Lim S.P., Lee H.P. Optofluidic variable-focus lenses for light manipulation. Lab Chip. 2012;12:3810–3815. doi: 10.1039/c2lc40415j. [DOI] [PubMed] [Google Scholar]
- 26.La Notte M., Troia B., Muciaccia T., Campanella C.E., De Leonardis F., Passaro V.M. Recent advances in gas and chemical detection by vernier effect-based photonic sensors. Sensors. 2014;14:4831–4855. doi: 10.3390/s140304831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fan X., White I.M. Optofluidic microsystems for chemical and biological analysis. Nat. Photonics. 2011;5:591–597. doi: 10.1038/nphoton.2011.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cubillas A.M., Unterkofler S., Euser T.G., Etzold B.J., Jones A.C., Sadler P.J., Wasserscheid P., Russell P.S.J. Photonic crystal fibres for chemical sensing and photochemistry. Chem. Soc. Rev. 2013;42:8629–8648. doi: 10.1039/c3cs60128e. [DOI] [PubMed] [Google Scholar]
- 29.He Z., Tian F., Zhu Y., Lavlinskaia N., Du H. Long-period gratings in photonic crystal fiber as an optofluidic label-free biosensor. Biosens. Bioelectron. 2011;26:4774–4778. doi: 10.1016/j.bios.2011.05.048. [DOI] [PubMed] [Google Scholar]
- 30.Wu C., Tse M.L., Liu Z., Guan B.O., Zhang A.P., Lu C., Tam H.Y. In-line microfluidic integration of photonic crystal fibres as a highly sensitive refractometer. Analyst. 2014;139:5422–5429. doi: 10.1039/C4AN01361A. [DOI] [PubMed] [Google Scholar]
- 31.Zhang N., Humbert G., Wu Z., Li K., Shum P.P., Zhang N.M., Cui Y., Auguste J.L., Dinh X.Q., Wei L. In-line optofluidic refractive index sensing in a side-channel photonic crystal fiber. Opt. Express. 2016;24:27674–27682. doi: 10.1364/OE.24.027674. [DOI] [PubMed] [Google Scholar]
- 32.Kozma P., Kehl F., Ehrentreich-Forster E., Stamm C., Bier F.F. Integrated planar optical waveguide interferometer biosensors: A comparative review. Biosens. Bioelectron. 2014;58:287–307. doi: 10.1016/j.bios.2014.02.049. [DOI] [PubMed] [Google Scholar]
- 33.Yin D., Schmidt H., Barber J.P., Lunt E.J., Hawkins A.R. Optical characterization of arch-shaped arrow waveguides with liquid cores. Opt. Express. 2005;13:10564–10570. doi: 10.1364/OPEX.13.010564. [DOI] [PubMed] [Google Scholar]
- 34.Testa G., Persichetti G., Sarro P.M., Bernini R. A hybrid silicon-pdms optofluidic platform for sensing applications. Biomed. Opt. Express. 2014;5:417–426. doi: 10.1364/BOE.5.000417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Testa G., Persichetti G., Bernini R. Liquid core arrow waveguides: A promising photonic structure for integrated optofluidic microsensors. Micromachines. 2016;7:47. doi: 10.3390/mi7030047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Testa G., Huang Y., Sarro P.M., Zeni L., Bernini R. High-visibility optofluidic mach-zehnder interferometer. Opt. Lett. 2010;35:1584–1586. doi: 10.1364/OL.35.001584. [DOI] [PubMed] [Google Scholar]
- 37.Testa G., Huang Y., Sarro P.M., Zeni L., Bernini R. Integrated silicon optofluidic ring resonator. Appl. Phys. Lett. 2010;97:131110. doi: 10.1063/1.3496027. [DOI] [Google Scholar]
- 38.Dolatabady A., Asgari S., Granpayeh N. Tunable mid-infrared nanoscale graphene-based refractive index sensor. IEEE Sens. J. 2017;18:569–574. doi: 10.1109/JSEN.2017.2778003. [DOI] [Google Scholar]
- 39.Tan Y., He R., Cheng C., Wang D., Chen Y., Chen F. Polarization-dependent optical absorption of MoS2 for refractive index sensing. Sci. Rep. 2014;4:7523. doi: 10.1038/srep07523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vollmer F., Braun D., Libchaber A., Khoshsima M., Teraoka I., Arnold S. Protein detection by optical shift of a resonant microcavity. Appl. Phys. Lett. 2002;80:4057–4059. doi: 10.1063/1.1482797. [DOI] [Google Scholar]
- 41.Zhi Y., Yu X.C., Gong Q., Yang L., Xiao Y.F. Single nanoparticle detection using optical microcavities. Adv. Mater. 2017;29 doi: 10.1002/adma.201604920. [DOI] [PubMed] [Google Scholar]
- 42.Luo Y., Chen X., Xu M., Chen Z., Fan X. Optofluidic glucose detection by capillary-based ring resonators. Opt. Laser Technol. 2014;56:12–14. doi: 10.1016/j.optlastec.2013.07.007. [DOI] [Google Scholar]
- 43.Scholten K., Fan X., Zellers E.T. A microfabricated optofluidic ring resonator for sensitive, high-speed detection of volatile organic compounds. Lab Chip. 2014;14:3873–3880. doi: 10.1039/C4LC00739E. [DOI] [PubMed] [Google Scholar]
- 44.Zhang S.X., Wang L., Li Z.Y., Li Y., Gong Q., Xiao Y.F. Free-space coupling efficiency in a high-q deformed optical microcavity. Opt. Lett. 2016;41:4437–4440. doi: 10.1364/OL.41.004437. [DOI] [PubMed] [Google Scholar]
- 45.Lee B., Roh S., Park J. Current status of micro-and nano-structured optical fiber sensors. Opt. Fiber Technol. 2009;15:209–221. doi: 10.1016/j.yofte.2009.02.006. [DOI] [Google Scholar]
- 46.Homola J. Present and future of surface plasmon resonance biosensors. Anal. Bioanal. Chem. 2003;377:528–539. doi: 10.1007/s00216-003-2101-0. [DOI] [PubMed] [Google Scholar]
- 47.Barik A., Otto L.M., Yoo D., Jose J., Johnson T.W., Oh S.H. Dielectrophoresis-enhanced plasmonic sensing with gold nanohole arrays. Nano Lett. 2014;14:2006–2012. doi: 10.1021/nl500149h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kang S., Lehman S.E., Schulmerich M.V., Le A.P., Lee T.W., Gray S.K., Bhargava R., Nuzzo R.G. Refractive index sensing and surface-enhanced raman spectroscopy using silver-gold layered bimetallic plasmonic crystals. Beilstein J. Nanotechnol. 2017;8:2492–2503. doi: 10.3762/bjnano.8.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhang D., Lu Y., Jiang J., Zhang Q., Yao Y., Wang P., Chen B., Cheng Q., Liu G.L., Liu Q. Nanoplasmonic biosensor: Coupling electrochemistry to localized surface plasmon resonance spectroscopy on nanocup arrays. Biosens. Bioelectron. 2015;67:237–242. doi: 10.1016/j.bios.2014.08.022. [DOI] [PubMed] [Google Scholar]
- 50.White I.M., Fan X. On the performance quantification of resonant refractive index sensors. Opt. Express. 2008;16:1020–1028. doi: 10.1364/OE.16.001020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sherry L.J., Chang S.-H., Schatz G.C., Van Duyne R.P., Wiley B.J., Xia Y. Localized surface plasmon resonance spectroscopy of single silver nanocubes. Nano Lett. 2005;5:2034–2038. doi: 10.1021/nl0515753. [DOI] [PubMed] [Google Scholar]
- 52.Jian A., Zhang X., Zhu W., Yu M. Optofluidic refractometer using resonant optical tunneling effect. Biomicrofluidics. 2010;4:043008. doi: 10.1063/1.3502671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chin L., Liu A., Lim C., Lin C., Ayi T., Yap P. An optofluidic volume refractometer using fabry–pérot resonator with tunable liquid microlenses. Biomicrofluidics. 2010;4:024107. doi: 10.1063/1.3430605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.St-Gelais R., Masson J., Peter Y.-A. All-silicon integrated fabry–pérot cavity for volume refractive index measurement in microfluidic systems. Appl. Phys. Lett. 2009;94:243905. doi: 10.1063/1.3152286. [DOI] [Google Scholar]
- 55.Trichet A.A., Dolan P.R., James D., Hughes G.M., Vallance C., Smith J.M. Nanoparticle trapping and characterization using open microcavities. Nano Lett. 2016;16:6172–6177. doi: 10.1021/acs.nanolett.6b02433. [DOI] [PubMed] [Google Scholar]
- 56.Mader M., Reichel J., Hänsch T.W., Hunger D. A scanning cavity microscope. Nat. Commun. 2015;6:7249. doi: 10.1038/ncomms8249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chin L.K., Liu A.Q., Lim C.S., Zhang X.M., Ng J.H., Hao J.Z., Takahashi S. Differential single living cell refractometry using grating resonant cavity with optical trap. Appl. Phys. Lett. 2007;91:243901. doi: 10.1063/1.2823610. [DOI] [Google Scholar]
- 58.Chin L.K., Liu A.Q., Lim C.S., Lin C.L., Ayi T.C., Yap P.H. An optofluidic volume refractometer using fabry-perot resonator with tunable liquid microlenses. Biomicrofluidics. 2010;4 doi: 10.1063/1.3430605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wang Y., Dostalek J., Knoll W. Magnetic nanoparticle-enhanced biosensor based on grating-coupled surface plasmon resonance. Anal. Chem. 2011;83:6202–6207. doi: 10.1021/ac200751s. [DOI] [PubMed] [Google Scholar]
- 60.Li M., Wu X., Liu L., Fan X., Xu L. Self-referencing optofluidic ring resonator sensor for highly sensitive biomolecular detection. Anal. Chem. 2013;85:9328–9332. doi: 10.1021/ac402174x. [DOI] [PubMed] [Google Scholar]
- 61.Xiao Y.-F., He L., Zhu J., Yang L. Electromagnetically induced transparency-like effect in a single polydimethylsiloxane-coated silica microtoroid. Appl. Phys. Lett. 2009;94:231115. doi: 10.1063/1.3149697. [DOI] [Google Scholar]
- 62.Lu H., Liu X., Mao D., Wang G. Plasmonic nanosensor based on Fano resonance in waveguide-coupled resonators. Opt. Lett. 2012;37:3780–3782. doi: 10.1364/OL.37.003780. [DOI] [PubMed] [Google Scholar]