Abstract
Background. Corporate interests have the potential to influence public debate and policymaking by influencing the research agenda, namely the initial step in conducting research, in which the purpose of the study is defined and the questions are framed.
Objectives. We conducted a scoping review to identify and synthesize studies that explored the influence of industry sponsorship on research agendas across different fields.
Search Methods. We searched MEDLINE, Scopus, and Embase (from inception to September 2017) for all original research and systematic reviews addressing corporate influence on the research agenda. We hand searched the reference lists of included studies and contacted experts in the field to identify additional studies.
Selection Criteria. We included empirical articles and systematic reviews that explored industry sponsorship of research and its influence on research agendas in any field. There were no restrictions on study design, language, or outcomes measured. We excluded editorials, letters, and commentaries as well as articles that exclusively focused on the influence of industry sponsorship on other phases of research such as methods, results, and conclusions or if industry sponsorship was not reported separately from other funding sources.
Data Collection and Analysis. At least 2 authors independently screened and then extracted any quantitative or qualitative data from each study. We grouped studies thematically for descriptive analysis by design and outcome reported. We developed the themes inductively until all studies were accounted for. Two investigators independently rated the level of evidence of the included studies using the Oxford Centre for Evidence-Based Medicine ratings.
Main Results. We included 36 articles. Nineteen cross-sectional studies quantitatively analyzed patterns in research topics by sponsorship and showed that industry tends to prioritize lines of inquiry that focus on products, processes, or activities that can be commercialized. Seven studies analyzed internal industry documents and provided insight on the strategies the industry used to reshape entire fields of research through the prioritization of topics that supported its policy and legal positions. Ten studies used surveys and interviews to explore the researchers’ experiences and perceptions of the influence of industry funding on research agendas, showing that they were generally aware of the risk that sponsorship could influence the choice of research priorities.
Conclusions. Corporate interests can drive research agendas away from questions that are the most relevant for public health. Strategies to counteract corporate influence on the research agenda are needed, including heightened disclosure of funding sources and conflicts of interest in published articles to allow an assessment of commercial biases. We also recommend policy actions beyond disclosure such as increasing funding for independent research and strict guidelines to regulate the interaction of research institutes with commercial entities.
Public Health Implications. The influence on the research agenda has given the industry the potential to affect policymaking by influencing the type of evidence that is available and the kinds of public health solutions considered. The results of our scoping review support the need to develop strategies to counteract corporate influence on the research agenda.
PLAIN-LANGUAGE SUMMARY
Industry-sponsored studies tend to be biased in favor of the sponsor’s products. Several studies have explored this issue, documenting how the funding source can influence the design, conduct, and publication of research. Although more difficult to define, sponsorship can also influence the research agenda, namely the initial step in conducting research, during which the research questions are chosen and framed. In our scoping review, we aimed to synthesize studies that have explored the influence of industry sponsorship on research agendas across different fields of research. We identified 36 studies. Corporations adopted similar techniques across different industry sectors and fields of research. Industry tended to prioritize lines of inquiry that focused on products or activities that can be commercialized (e.g., drugs or devices). The included studies also suggest that industry reshaped fields of research through the prioritization of topics that supported its policy and legal positions, while distracting from research that could be unfavorable. Our findings suggest that corporate interests can influence research agendas. Bias in the research agenda can produce results that support only certain policy responses to tackle pressing public health problems, which in turn affect the population’s health.
Biases in research are systematic errors that restrict the validity of a study and can threaten the credibility of the evidence on which decisions are based. Bias is commonly conceptualized as a methodological problem, resulting from features of study design that affect a study’s internal validity.1 However, multiple phases of the research process are susceptible to bias, conceptualized as a “cycle of bias.”2 Bias, intentional or unintentional, may be introduced into research by the kinds of research questions that are asked, the way a study is designed and conducted, the nature of results and conclusions that are drawn, and the way they are disseminated.2
Industry sponsorship is a key source of bias that can affect research at multiple stages. Data from several fields have shown biases in the design, conduct, and publication of research that are related to industry funding sources.3–5 For example, analyses of food and pharmaceutical industry–sponsored research have demonstrated that industry-sponsored studies are more likely to have results and conclusions that favor the sponsor’s product than are those that are not industry sponsored.4–6
Although less studied and more difficult to define, sponsorship may also influence the research agenda, namely the initial step in conducting research, during which the purpose of the research is defined and the research questions are chosen and framed. A bias in the research agenda can affect all the subsequent stages of the research process and has the potential to affect policymaking by limiting the type of evidence that is available. Although it is self-evident that corporations would focus research on their products, industry influence on research agendas may be broader in scope. Findings from investigative journalism have shown, for example, that Coca-Cola shifted the focus of the obesity research agenda by funding research that focused on physical activity, thereby downplaying the role of sugar-sweetened beverages in obesity.7 Moreover, both Coca-Cola and the recipient of its funding (the nonprofit group known as the Global Energy Balance Network) hid the true extent of industry involvement in those research projects.7,8 This recent case shows not only how corporations can shape the research agenda in ways that advance their interests but also how the lack of transparency does not allow understanding of the magnitude of the problem.
Although several reviews have explored how sponsorship can bias research methods and outcomes,5,9 a synthesis of studies examining whether industry sponsorship influences the research agenda across different fields of research had not been conducted to our knowledge. Our aim in performing this scoping review was to identify and synthesize studies that have explored the influence of industry sponsorship on research agendas across different fields of research. The research questions we addressed were (1) Are there patterns in research agendas related to type of sponsorship? (2) What strategies does industry use to influence research agendas, and what is industry’s motivation in shaping research agendas? (3) What is the impact of bias in research agendas on the research enterprise? (4) How do researchers perceive and experience the influence of industry sponsorship on research agendas?
METHODS
We chose a scoping review approach to answer these questions. Unlike systematic reviews, a scoping study tends to address complex and exploratory research questions. Through a systematic search, selection, and synthesis of the literature, a scoping review is performed to map “key concepts, types of evidence, and gaps in research related to a defined area or field.”10(p1294)
Study Selection Criteria
We searched for all original research and systematic reviews that addressed industry sponsorship of research and its influence on research agendas in any field. There were no restrictions on study design, language, or outcomes measured. We excluded nonempirical articles, including editorials, letters, and commentaries. We included a study if it included any data (qualitative or quantitative) related to industry influence on research agenda, regardless of whether this was the main aim of the study. We excluded articles if they exclusively focused on the influence of industry sponsorship on other phases of research, such as methods, results, and conclusions, or if industry sponsorship was not reported separately from other funding sources.
Data Sources and Searches
We searched MEDLINE, Scopus, and Embase (from inception to September 2017) for all original research and systematic reviews addressing the relationship between industry sponsorship and the research agenda. We created a search strategy in consultation with a medical librarian on the basis of previous reviews of sponsorship bias in research5 using appropriate subject headings and keywords for each respective database. We combined 2 domains of keywords: (1) industry sponsorship of research and (2) research agenda (see the Appendix, available as a supplement to the online version of this article at http://www.ajph.org) After identifying relevant studies, we hand searched the reference lists for any additional articles.
Study Selection
A. L. conducted the search. Two authors (Q. G., A. F., or A. L.) independently screened for relevant titles and abstracts and assessed the full texts of 143 articles for inclusion, with a third author reviewing any discrepancies.
Data Collection and Synthesis
After retrieving the full texts, the following information was extracted from each study:
Authors,
Year of publication,
Journal,
Study design,
Study funding source (taken verbatim from the article),
Author conflicts of interest (taken verbatim from the article),
Field of research,
Type of industry,
Data sources, and
Sample size.
We extracted any quantitative or qualitative data related to industry sponsorship and influence on research agendas. We grouped studies thematically for descriptive analysis by design and outcome reported. We did not identify these groups a priori; instead we developed them inductively until all studies were accounted for.11 We first identified the range of study designs and grouped studies correspondingly until all were accounted for: observational studies of published literature, content analyses of industry documents, surveys, and qualitative interview studies. Then, we identified all outcomes measured and synthesized findings within the study design groupings accordingly, until all data related to the research question were included. All authors participated in identifying and refining these groups and themes. We then analyzed findings descriptively and reported them using tables and a narrative approach.
Methodological Rigor
Included studies were largely descriptive and of heterogeneous design, including observational, survey, and qualitative studies. Thus established tools for assessing risk of bias or methodological quality were not available, nor could we compare risk of bias across studies. Therefore, 2 investigators (Q. G. and A. F.) independently rated the level of evidence of the included studies using the Oxford Centre for Evidence-Based Medicine ratings.12 We report study design, level of evidence, data sources, and sample size in Table 1 and Table A (available as a supplement to the online version of this article at http://www.ajph.org) to aid the reader in assessing the methodological rigor of included studies.
TABLE 1—
Characteristics of Studies That Explored the Influence of Industry Sponsorship on Research Agendas: 1986–2017
| Characteristic | No. (%) |
| Total | 36 |
| Type of study | |
| Cross-sectional | 19 (52.8) |
| Content analysis | 7 (19.4) |
| Survey | 6 (16.7) |
| Qualitative | 3 (8.3) |
| Systematic review | 1 (2.8) |
| Level of evidencea | |
| 1 | 4 (11.1) |
| 2 | 1 (2.8) |
| 3 | 2 (5.6) |
| 4 | 29 (80.5) |
| 5 | 0 (0.0) |
| Industry type | |
| Medically related | 19 (52.8) |
| Tobacco | 4 (11.1) |
| Food | 3 (8.3) |
| Plant or animal biotechnology | 3 (8.3) |
| Chemical | 1 (2.8) |
| Alcohol | 1 (2.8) |
| Mining | 1 (2.8) |
| Not specified | 4 (11.1) |
| Funding source of the study | |
| Not for profit | 21 (58.3) |
| Mixed | 1 (2.8) |
| None | 4 (11.1) |
| No statement | 10 (27.8) |
1 indicates the highest level of evidence and 5 the lowest; assessed using “The Oxford 2011 Levels of Evidence” from the Oxford Centre for Evidence-Based Medicine.12
RESULTS
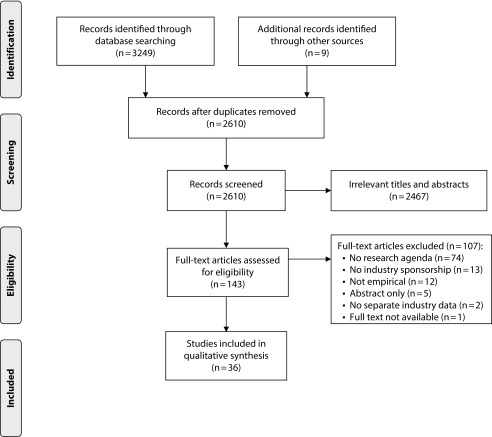
As shown in Figure 1, we initially identified 2610 studies through searching electronic databases and hand searching references, after removing duplicates. On the basis of our inclusion and exclusion criteria, we included 36 articles (Figure 1).
FIGURE 1—
PRISMA Flowchart of Study Selection
Note. PRISMA = Preferred Reporting Items for Systematic Reviews and Meta-Analyses.
Characteristics of the Included Studies
The 36 articles were published between 1986 and 2017. As Table 1 shows, more than half of the articles (n = 19; 52.8%) focused on the medically related industry (e.g., pharmaceutical, medical device, diagnostic test), 4 on tobacco companies, 3 on the food industry, 3 on plant or animal biotechnology, 1 on chemical, 1 on alcohol, and 1 on the mining industry; 4 did not specify the industry sectors covered. Additional information on the characteristics of included studies are available in Table A.
We grouped our findings into 3 domains on the basis of study design and outcomes measured: (1) patterns in research topics by sponsorship, (2) industry strategies to influence research agendas, and (3) experiences and perceptions of industry influence on research agenda.
Patterns in Research Topics by Sponsorship
As Table B (available as a supplement to the online version of this article at http://www.ajph.org) shows, 19 cross-sectional studies quantitatively explored patterns in research topics according to sponsorship by analyzing published articles, conference abstracts, registries of clinical trials, research data from funding agencies, or projects submitted to ethics committees. Two studies focused on the food industry, whereas 17 focused on medically related industry and analyzed patterns in research topics by sponsorship in a wide range of clinical fields (e.g., oncology [n = 4], infectious diseases [n = 2], surgery [n = 2]). The proportion of articles or projects in the included studies that were industry sponsored ranged from 7.4% to 75.7% of the sample.
Generally, the studies found that industry funding focused on research topics with commercial applications. For example, research funded by medically related industry tended to study interventions involving drugs or devices13–16 by contrast to public health or behavioral interventions.17–21 An analysis of 231 comparative effectiveness studies registered in ClinicalTrials.gov showed that 69.2% (n = 45/65) of industry-funded studies involved the study of a drug, 26.2% (n = 17/65) a device, 3.1% (n = 2/65) a procedure, and 1.5% (n = 1/65) behavioral change. Conversely, studies with government or nonprofit funding were less likely to study a drug or device intervention and were more likely to study behavioral change (P < .001).17 Similarly, an analysis of the abstracts presented during the annual meetings of the European Association for the Study of Diabetes between 2010 and 2013 revealed that approximately 80% of research on insulin and orally administered therapies and more than 50% of research on blood glucose measurement was industry sponsored. Conversely, noncommercial research primarily focused on pathophysiology, transplantation, nonpharmaceutical therapy, complications, and treatment care structures.13
The focus on product-related interventions in industry-sponsored studies was confirmed when considering different industry sectors, such as food.22,23 An analysis of randomized controlled trials included in Cochrane Reviews of nutrition interventions to address obesity and overweight, showed that 66.7% (n = 16/24) of the food industry–sponsored studies involved the manipulation of specific nutrients, whereas only 25% (n = 6/24) examined broader levels of dietary composition, such as whole foods. Manufactured foods with specific nutrient profiles, compared with whole foods, are potentially more profitable for food companies. Food industry–sponsored trials were less likely to address dietary behaviors than were those that were non–industry-funded (33.3% vs 66.9%; χ2 test: P = .002).23
The included studies also showed that industry tended to fund research on areas that guaranteed a large market share. This is why, for example, pharmaceutical companies are more likely to sponsor studies on diseases that disproportionally affect high- versus low-income countries.19,24,25 An analysis of whether biomedical research funding in the United States was aligned with projected disease burden estimates showed that industry funding in 2005 was more aligned with projected disease burden in high-income countries than with disease burden globally, whereas the opposite happened when considering funding from the National Institutes of Health.25
Industry Strategies to Influence Research Agendas
Seven studies examined historic documents (e.g., policy statements, briefing articles, reports, research projects, internal correspondence) from industry, trade organizations, and research bodies to assess the strategies industry used to influence research agendas. The studies focused on tactics used by the tobacco industry (n = 4), the alcohol industry (n = 1), the sugar industry (cane and beet, n = 1), and the mining industry (lead and zinc, n = 1).
The included studies documented a wide array of strategies. One of these was to establish priority research agendas that were favorable to industry’s legal and policy positions. For example, an analysis of the Legacy Tobacco Documents illustrated how, with the advent of behavioral genetics, the tobacco industry saw an opportunity to advance the “constitutional hypothesis,” suggesting that there was a genetic influence on nicotine addiction that could be protective. This allowed the industry to counter the claim that smoking caused cancer and to argue that the cause of smoking addiction lay in the individual’s genetic makeup.26 Similar tactics were documented in other sectors as well. Concerned about the scientific evidence showing the link between sugar intake and caries, in the 1950s, the Sugar Research Foundation, an industry trade organization, decided to use industry research as a strategy to divert attention from sugar restriction. In its 1950 annual report, the foundation set a priority research agenda that stated, “The ultimate aim of the Foundation in dental research has been to discover effective means of controlling tooth decay by methods other than restricting carbohydrate intake.”27(p6)
A second strategy was to strategically fund research along priority research agendas in a way that appeared scientifically credible. An analysis of the research projects of the tobacco industry–funded Center for Indoor Air Research showed that although the center stated that its research projects were funded through a peer review process, documents showed that some received a “special review.” These projects were chosen on the basis of recommendations by industry executives and lawyers and were “goal oriented”: they were more likely to have principal investigators from the private sector and to support the tobacco industry position once published.28 Scientific credibility was gained by producing scholarly publications as well. For example, building on the experience of an industry consultant and former World Health Organization staff, the International Center for Alcohol Policies (an alcohol industry–funded organization), commissioned, created, and distributed a plethora of scientific publications, including edited collections, reports, reviews, and journal articles (103 publications in total).29
A third strategy was to disseminate the industry’s research agenda by enrolling nonindustry stakeholders through conferences, committees, and other joint initiatives. For example, in 1972, Philip Morris, a tobacco company, initiated a conference titled the “Motivational Mechanisms of Cigarette Smoking,” which was held in the French Antilles. It was attended by all 6 major tobacco companies and by well-known behavioral and social scientists, who were both industry and government funded—lending scientific stature and credibility to the conference. Internal documents indicated that the goal of the conference was to “provide authoritative statements in support of smoking,” which could serve as the basis for a procigarette public relations campaign.30(p413)
The mining industry used similar strategies. In 1935, closely timed with a congressional hearing into the deaths from silicosis of hundreds of tunnel workers, mining industry executives met in Pittsburgh, Pennsylvania. The discussion centered on the necessity of creating an organization representing industry that had “a broad outlook, a sympathetic understanding of the problem, and wide contacts with all cooperating agencies.”31
The outcome of the meeting was a 5-point program that established a research agenda for silicosis research and action nationally, a working committee populated with prominent actors in silicosis research and engineering communities, and research links with important institutions such as Harvard University. These ties provided industry access to the National Silicosis Conference held by the Department of Labor, during which a research agenda was established that would define silicosis issues for decades.32
The outcomes of these strategies industry used were distinct patterns in the research agendas in these fields, all of which were favorable to the industry’s policy and legal positions; these also had the potential to reshape an entire field of research. For example, the previously mentioned special-reviewed projects of the tobacco industry–funded Center for Indoor Air Research were significantly more likely to focus on secondhand smoke than were peer-reviewed projects: 63% (n = 12/19) special-reviewed versus 30% (n = 12/40) peer-reviewed projects (P = .02). The majority of special-reviewed projects on secondhand smoke focused on exposure (67%; n = 8/12) rather than health effects (11%, n = 2/19). This pattern likely reflected an effort to divert attention from research that showed secondhand smoke was a health hazard. Moreover, the results of these studies were presented in legal and policy settings to support the tobacco industry position.28
Experiences and Perceptions
Ten studies explored the experiences and perceptions of the influence of industry funding on research agendas among university- and industry-based scientists and administrators (Table C, available as a supplement to the online version of this article at http://www.ajph.org). Six studies were surveys, 3 were qualitative interview-based studies, and 1 was a systematic review (which included 3 of the surveys). All surveyed individuals worked in the life sciences, including the subfields of biotechnology, agriculture, and clinical research.
Experiences with industry influence on research agendas.
Among survey respondents, receipt of industry funding was associated with a tendency to shift research agendas toward more applied research with commercial application.33–36 In a 1985 survey of university-based scientists in biotechnology, those with industry funding were significantly more likely to report that their choice of research topics had been affected by the potential for commercial application,33 a finding replicated more than 10 years later.34,37 In the subfield of weed science, researchers with industry funding were significantly more likely to work on field crop research or herbicide efficacy; those who were publicly funded focused on ecological relationships, nonchemical weed management, or rangeland and natural areas research.35 Modeling similar survey results confirmed that scientists’ percentage of funding from industry was negatively correlated with the percentage of their research program focused on basic research (B = −0.247; P < .001)38 and that a 10% increase in private funding reduced the proportion of a research program dedicated to basic research by 1.2%.36 The second association was also significant in the opposite direction: for every 1% increase in the proportion of a research program dedicated to applied research, $900 of private funding was attracted.36
Perceptions and values.
The majority of scientists working in academia and industry tended to agree that industry funding of academic research posed the risk of shifting agendas toward applied research with commercial application, favoring the sponsor.33,37,39,40 For example, among biotechnology faculty in a 1985 survey, 78% of those without industry support and 70% of those with industry support said that to some extent or to a great extent, industry relationships posed the risk of shifting too much emphasis to applied research.33 In the subfield of pediatrics, stakeholders believed that industry would fund research where there were greater financial rewards rather than for “humanitarian reasons,” resulting in greater focus on research in adult diseases and a paucity of funding for pediatric trials.41
There was greater variability among scientists in terms of whether they judged industry influence to be a benefit or to pose a risk to scientific integrity, which confirms the findings of a previous systematic review.39 A survey of university scientists focused on agriculturally related bioscience showed that scientists valued theoretical contributions and scientific curiosity more highly than they valued patenting, believing that publicly supported scientists should focus on research with public benefit.36 Receipt of industry funding seemed to influence whether respondents believed that industry influence over research agendas posed a benefit or a risk. For example, a greater proportion of non–industry-funded (50%) than industry-funded (38.5%) scientists in Australia agreed that industry funding leads to an emphasis on quick-fix solutions rather than long-term basic research.37 Conversely, industry-funded scientists were more likely than were non–industry-funded scientists (70% vs 48%) to agree that the benefit of industry funding was in opening new and promising avenues of research.37
Similarly, scientists had mixed views on the role industry should play in shaping research agendas. In 2 separate surveys of scientists working in agricultural biotechnology, most respondents believed that scientists should determine university research agendas, whereas fewer thought industry should have significant influence.36,38 An in-depth ethnography of a long-term collaboration in the context of an exclusively industry-funded, university-based biotechnology laboratory found that both university and company scientists emphasized that the research agenda was determined collaboratively rather than by company interests.42 Because of the long-term, exclusive nature of the collaboration, the agenda was a blend of basic and applied research, and individual projects were vetted by company executives and the academic research director under the broad funding agreement. From the company’s perspective, the university-based lab served as a “discovery group” with a focus on basic and strategic research questions; the company set up a group of scientist employees to work alongside on commercial applications. Thus, relationships between the academic director and company allies—and the impact of company staff turnover—were key factors in determining the research agenda.42
DISCUSSION
The studies that quantitatively analyzed patterns in research topics by sponsorship showed that industry tends to prioritize lines of inquiry that focus on products, processes, or activities that can be commercialized and marketed. In the case of the medically related industry, this included funding research on interventions involving products (e.g., drugs and devices),13–21 with a focus on high-income markets.19,24,25 With regard to the food industry, industry-sponsored research tended to focus on single nutrients or constituents instead of dietary patterns, which allows the marketing of manufactured products containing certain nutrients as beneficial to health.43
Qualitative and quantitative studies included in our review suggest that industry also used research funding as a strategy to reshape fields of research through the prioritization of topics that supported its policy and legal positions, while distracting from research that could be unfavorable. Analysis of internal industry documents provides insight into how and why industry influenced research agendas. It is particularly interesting to note how corporations adopted similar techniques across different industry sectors (i.e., tobacco, alcohol, sugar, and mining) and fields of research. The strategies included establishing research agendas within the industry that were favorable to its positions,26,27,29,44 strategically funding research along these lines in a way that appeared scientifically credible,26,29,30,32,44,45 and disseminating these research agendas by creating collaborations with prominent institutions and researchers.27,30,32
Finally, surveys and interviews with researchers working in academia and industry suggested that they were generally aware of the risk that sponsorship could influence the choice of research priorities, particularly shifting toward lines of inquiry with potential for commercial application.33–36 The researchers’ views on the role industry should play in shaping research agendas tended to be aligned with the acceptance or nonacceptance of industry funding.
Our findings suggest that corporate interests can influence research agendas, thus affecting the type of evidence that is available to inform decisions. Although it is self-evident that companies will sponsor research that is aligned with their commercial interests, this can be misaligned with the values of evidence-based public health and may circumscribe the available evidence to tackle pressing public health problems. Bias in the research agenda can produce results that support specific policy responses, which in turn affect public health. For example, in the 1980s the findings of the tobacco industry–funded projects on general indoor air quality were presented in legislative settings to support the industry position and influence the development of smoking policies.45
Therefore, commercial biases in the research agenda need to be routinely appraised when assessing the available evidence on a topic. To allow that assessment, requirements for disclosing funding sources and investigators’ financial conflicts of interest should be enforced. Analyses of the activities of tobacco46 and, more recently, food companies7,47 have shown how funding agreements can be hidden from public scrutiny. Disclosure policies should be strengthened, as transparency is essential to understanding the magnitude of the problem. In the long term, strategies to counteract corporate influence on the research agenda need to be explored. Increasing funding for independent research would clearly be the most effective strategy, but some intermediate steps can also be taken. Research institutes should develop strict guidelines to regulate their interaction with all commercial entities and implement sound mechanisms for reviewing external funding. Some research institutions have, for example, banned research funds from tobacco companies.
Before engaging with commercial entities, scientific institutions should also consider conducting a risk–benefit analysis. Useful frameworks that could guide this assessment are already available and are on the basis of domains such as the alignment of the sponsor with the recipient’s mission, the level of harm generated by the product of the sponsor,48 and the reputational risk to the recipient institution.49 Individual researchers could also learn to recognize when genuine commitments to advance research are being hijacked by industry agendas and avoid participating in such initiatives.50
Limitations
Because of the range and heterogeneity in search terms, our search strategy was likely more sensitive than specific. We hand searched references lists and asked experts to recommend any studies we had missed; nevertheless, it is possible that relevant studies were not included. Furthermore, few studies focused on the influence of industry sponsorship on research agenda as their principal aim, thus we might have missed relevant data in studies in which this was a secondary aim. Studies covered a broad period, and attitudes about research and collaboration generally may have changed over time.
Moreover, our study presents the limitations of scoping reviews. Although our search was systematic, we sought to answer exploratory research questions, and thus some relevant studies may have been missed. Because of the heterogeneity of the included studies, we could not compare risk of bias across designs, nor perform a meta-analysis, and we could provide only a narrative synthesis of the available literature. Finally, although other research sponsors, such as private foundations, might also influence research agendas,51 we focused on industry sponsorship because it is prevalent across a wide variety of public health topics and there is substantial evidence that industry sponsorship influences the design and publication of research.
Conclusions
Although several studies have explored how industry sponsorship can bias research outcomes, this is the first review, to our knowledge, to examine the influence of industry sponsorship on research agendas across different fields of research. Our findings suggest that corporate funding of research with commercial implications drives the research agenda away from public health priorities.
ACKNOWLEDGMENTS
Q. G. is supported by a postdoctoral fellowship from the Canadian Institutes of Health Research.
HUMAN PARTICIPANT PROTECTION
No protocol approval was necessary because no human participants were involved in this study.
Footnotes
REFERENCES
- 1.Cochrane Collaboration. Cochrane handbook for systematic reviews of interventions. 2011. Available at: https://training.cochrane.org/handbook. Accessed June 30, 2018.
- 2.Odierna DH, Forsyth SR, White J, Bero LA. The cycle of bias in health research: a framework and toolbox for critical appraisal training. Account Res. 2013;20(2):127–141. doi: 10.1080/08989621.2013.768931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barnes DE, Bero LA. Why review articles on the health effects of passive smoking reach different conclusions. JAMA. 1998;279(19):1566–1570. doi: 10.1001/jama.279.19.1566. [DOI] [PubMed] [Google Scholar]
- 4.Bero L, Oostvogel F, Bacchetti P, Lee K. Factors associated with findings of published trials of drug–drug comparisons: why some statins appear more efficacious than others. PLoS Med. 2007;4(6):e184. doi: 10.1371/journal.pmed.0040184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lundh A, Lexchin J, Mintzes B, Schroll JB, Bero L. Industry sponsorship and research outcome. Cochrane Database Syst Rev. 2017;2(MR000033):1–140. doi: 10.1002/14651858.MR000033.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lesser LI, Ebbeling CB, Goozner M, Wypij D, Ludwig DS. Relationship between funding source and conclusion among nutrition-related scientific articles. PLoS Med. 2007;4(1):e5. doi: 10.1371/journal.pmed.0040005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.O’Connor A. Coca-Cola funds scientists who shift blame for obesity away from bad diets. 2015. Available at: https://well.blogs.nytimes.com/2015/08/09/coca-cola-funds-scientists-who-shift-blame-for-obesity-away-from-bad-diets. Accessed September 11, 2018.
- 8.Huehnergarth NF. Emails reveal how Coca-Cola shaped the anti-obesity global energy balance network. 2015. Available at: https://www.forbes.com/sites/nancyhuehnergarth/2015/11/24/emails-reveal-how-coca-cola-shaped-the-anti-obesity-global-energy-balance-network/#7971eb7979a7. Accessed September 2018. [Google Scholar]
- 9.Chartres N, Fabbri A, Bero LA. Association of industry sponsorship with outcomes of nutrition studies: a systematic review and meta-analysis. JAMA Intern Med. 2016;176(12):1769–1777. doi: 10.1001/jamainternmed.2016.6721. [DOI] [PubMed] [Google Scholar]
- 10.Colquhoun HL, Levac D, O’Brien KK et al. Scoping reviews: time for clarity in definition, methods, and reporting. J Clin Epidemiol. 2014;67(12):1291–1294. doi: 10.1016/j.jclinepi.2014.03.013. [DOI] [PubMed] [Google Scholar]
- 11.Arksey H, O’Malley L. Scoping studies: towards a methodological framework. Int J Soc Res Methodol. 2005;8(1):19–32. [Google Scholar]
- 12.Oxford Centre for Evidence-Based Medicine; OCEBM Levels of Evidence Working Group. The Oxford 2011 levels of evidence. Available at: https://www.cebm.net/wp-content/uploads/2014/06/CEBM-Levels-of-Evidence-2.1.pdf. Accessed June 30, 2018.
- 13.Arnolds S, Heckermann S, Heise T, Sawicki PT. Spectrum of diabetes research does not reflect patients’ scientific preferences: a longitudinal evaluation of diabetes research areas 2010–2013 vs. a cross-sectional survey in patients with diabetes. Exp Clin Endocrinol Diabetes. 2015;123(5):299–302. doi: 10.1055/s-0034-1398591. [DOI] [PubMed] [Google Scholar]
- 14.Haidich AB, Pilalas D, Contopoulos-Ioannidis DG, Ioannidis JP. Most meta-analyses of drug interventions have narrow scopes and many focus on specific agents. J Clin Epidemiol. 2013;66(4):371–378. doi: 10.1016/j.jclinepi.2012.10.014. [DOI] [PubMed] [Google Scholar]
- 15.Momeni A, Becker A, Bannasch H, Antes G, Blümle A, Stark GB. Association between research sponsorship and study outcome in plastic surgery literature. Ann Plast Surg. 2009;63(6):661–664. doi: 10.1097/SAP.0b013e3181951917. [DOI] [PubMed] [Google Scholar]
- 16.Sun GH, Houlton JJ, Moloci NM et al. Prospective head and neck cancer research: a four-decade bibliometric perspective. Oncologist. 2013;18(5):584–591. doi: 10.1634/theoncologist.2012-0415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bourgeois FT, Murthy S, Mandl KD. Comparative effectiveness research: an empirical study of trials registered in ClinicalTrials.gov. PLoS One. 2012;7(1):e28820. doi: 10.1371/journal.pone.0028820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jagsi R, Sheets N, Jankovic A, Motomura AR, Amarnath S, Ubel PA. Frequency, nature, effects, and correlates of conflicts of interest in published clinical cancer research. Cancer. 2009;115(12):2783–2791. doi: 10.1002/cncr.24315. [DOI] [PubMed] [Google Scholar]
- 19.Goswami ND, Pfeiffer CD, Horton JR, Chiswell K, Tasneem A, Tsalik EL. The state of infectious diseases clinical trials: a systematic review of ClinicalTrials.gov. PLoS One. 2013;8(10):e77086. doi: 10.1371/journal.pone.0077086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hartmann M, Knoth H, Schulz D, Knoth S. Industry-sponsored economic studies in oncology vs studies sponsored by nonprofit organisations. Br J Cancer. 2003;89(8):1405–1408. doi: 10.1038/sj.bjc.6601308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hemminki E, Virtanen J, Veerus P, Regushevskaya E. Clinical research in Finland in 2002 and 2007: quantity and type. Health Res Policy Syst. 2013;11:17. doi: 10.1186/1478-4505-11-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fabbri A, Chartres N, Bero LA. Study sponsorship and the nutrition research agenda: analysis of cohort studies examining the association between nutrition and obesity. Public Health Nutr. 2017;20(17):3193–3199. doi: 10.1017/S1368980017002178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fabbri A, Chartres N, Scrinis G, Bero LA. Study sponsorship and the nutrition research agenda: analysis of randomized controlled trials included in systematic reviews of nutrition interventions to address obesity. Public Health Nutr. 2017;20(7):1306–1313. doi: 10.1017/S1368980016003128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bourgeois FT, Murthy S, Pinto C, Olson KL, Ioannidis JPA, Mandl KD. Pediatric versus adult drug trials for conditions with high pediatric disease burden. Pediatrics. 2012;130(2):285–292. doi: 10.1542/peds.2012-0139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dorsey ER, Thompson JP, Carrasco M et al. Financing of U.S. biomedical research and new drug approvals across therapeutic areas. PLoS One. 2009;4(9):e7015. doi: 10.1371/journal.pone.0007015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gundle KR, Dingel MJ, Koenig BA. “To prove this is the industry’s best hope”: big tobacco’s support of research on the genetics of nicotine addiction. Addiction. 2010;105(6):974–983. doi: 10.1111/j.1360-0443.2010.02940.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kearns CE, Glantz SA, Schmidt LA. Sugar industry influence on the scientific agenda of the National Institute of Dental Research’s 1971 National Caries Program: a historical analysis of internal documents. PLoS Med. 2015;12(3):e1001798. doi: 10.1371/journal.pmed.1001798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Barnes DE, Bero LA. Industry-funded research and conflict of interest: an analysis of research sponsored by the tobacco industry through the Center for Indoor Air Research. J Health Polit Policy Law. 1996;21(3):515–542. doi: 10.1215/03616878-21-3-515. [DOI] [PubMed] [Google Scholar]
- 29.Jernigan DH. Global alcohol producers, science, and policy: the case of the International Center for Alcohol Policies. Am J Public Health. 2012;102(1):80–89. doi: 10.2105/AJPH.2011.300269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Petticrew MP, Lee K. The “father of stress” meets “big tobacco”: Hans Selye and the tobacco industry. Am J Public Health. 2011;101(3):411–418. doi: 10.2105/AJPH.2009.177634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Weidlein ER. Plan for study of dust problems. In: WG Hazard to Dr LR Thompson, National Archives, Record Group 90. Pittsburgh, PA: State Boards of Health; March 1935.
- 32.Rosner D, Markowitz G. Workers, industry, and the control of information: silicosis and the Industrial Hygiene Foundation. J Public Health Policy. 1995;16(1):29–58. [PubMed] [Google Scholar]
- 33.Blumenthal D, Gluck M, Louis KS, Stoto MA, Wise D. University–industry research relationships in biotechnology: implications for the university. Science. 1986;232(4756):1361–1366. doi: 10.1126/science.3715452. [DOI] [PubMed] [Google Scholar]
- 34.Blumenthal D, Campbell EG, Causino N, Louis KS. Participation of life science faculty in research relationships with industry. N Engl J Med. 1996;335(23):1734–1739. doi: 10.1056/NEJM199612053352305. [DOI] [PubMed] [Google Scholar]
- 35.Davis AS, Hall JC, Jasieniuk M et al. Weed science research and funding: a call to action. Weed Sci. 2009;57(4):442–448. [Google Scholar]
- 36.Buccola S, Ervin D, Yang H. Research choice and finance in university bioscience. South Econ J. 2009;75(4):1238–1255. [Google Scholar]
- 37.Harman G. Australian science and technology academics and university–industry research links. High Educ. 1999;38(1):83–103. [Google Scholar]
- 38.Glenna LL, Welsh R, Ervin D, Lacy WB, Biscotti D. Commercial science, scientists’ values, and university biotechnology research agendas. Res Policy. 2011;40(7):957–968. [Google Scholar]
- 39.Glaser BE, Bero LA. Attitudes of academic and clinical researchers toward financial ties in research: a systematic review. Sci Eng Ethics. 2005;11(4):553–573. doi: 10.1007/s11948-005-0026-z. [DOI] [PubMed] [Google Scholar]
- 40.Glenna LL, Welsh R, Lacy WB, Biscotti D. Industry perceptions of university–industry relationships related to agricultural biotechnology research. Rural Sociol. 2007;72(4):608–631. [Google Scholar]
- 41.Joseph PD, Craig JC, Tong A, Caldwell PH. Researchers’, regulators’, and sponsors’ views on pediatric clinical trials: a multinational study. Pediatrics. 2016;138(4):e20161171. doi: 10.1542/peds.2016-1171. [DOI] [PubMed] [Google Scholar]
- 42.Webster A. University–corporate ties and the construction of research agendas. Sociology. 1994;28(1):123–142. [Google Scholar]
- 43.Nestle M. Food Politics. How the Food Industry Influences Nutrition and Health. Berkeley, CA: University of California Press; 2013. [Google Scholar]
- 44.Petticrew MP, Lee K, McKee M. Type A behavior pattern and coronary heart disease: Philip Morris’s “crown jewel. Am J Public Health. 2012;102(11):2018–2025. doi: 10.2105/AJPH.2012.300816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Barnes DE, Bero LA. Industry-funded research and conflict of interest: an analysis of research sponsored by the tobacco industry through the Center for Indoor Air Research. J Health Polit Policy Law. 1996;21(3):515–542. doi: 10.1215/03616878-21-3-515. [DOI] [PubMed] [Google Scholar]
- 46.Schick SF, Glantz SA. Old ways, new means: tobacco industry funding of academic and private sector scientists since the Master Settlement Agreement. Tob Control. 2007;16(3):157–164. doi: 10.1136/tc.2006.017186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Serôdio PM, McKee M, Stuckler D. Coca-Cola—a model of transparency in research partnerships? A network analysis of Coca-Cola’s research funding (2008–2016) Public Health Nutr. 2018;21(9):1594–1607. doi: 10.1017/S136898001700307X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Adams PJ. Assessing whether to receive funding support from tobacco, alcohol, gambling and other dangerous consumption industries. Addiction. 2007;102(7):1027–1033. doi: 10.1111/j.1360-0443.2007.01829.x. [DOI] [PubMed] [Google Scholar]
- 49.Charles Perkins Centre. Engagement with industry guidelines. 2016. Available at: https://sydney.edu.au/perkins/documents/cpc_engagement_with_industry_guidelines_2016.pdf. Accessed June 30, 2018.
- 50.Bero L. Tob Control. 2018. Ten tips for spotting industry involvement in science policy. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 51.Global Health Watch 2: An Alternative World Health Report. London, UK: ZED Books; 2008. Peoples Health Movement. [Google Scholar]