Abstract
Objective
The National Comprehensive Cancer Network (NCCN) guidelines recommend local excision and observation as standard treatment for selected patients with clinical T1N0M0 rectal cancer. In patients with pathological T1 (pT1) rectal cancer who received local excision, the local recurrence rate is at least 10%. We studied oncological outcomes in patients with pT1 rectal cancer who received chemoradiotherapy (CRT) after local excision.
Methods
Local excision was performed in 65 patients with clinical T1N0M0 rectal cancer (≤8 cm from the anal verge, tumor size < 30 mm, well or moderately differentiated adenocarcinoma). The patients received CRT (40 or 45 Gy in 1.8–2.0 fractions with concurrent oral UFT [tegafur/uracil] or S-1 [tegafur/gimeracil/oteracil]) after confirmation of pT1 and negative margins.
Results
Patients who had pT2 cancer or who did not provide informed consent were excluded. The remaining 50 patients additionally received CRT. The CRT was completed in 48 patients (96%). The median follow-up period was 71 months. Local recurrence occurred in 1 patient (2%). Distant metastases occurred in 3 patients (6%). The 5-year disease-free survival rate was 86%, and the 5-year overall survival rate was 92%.
Conclusions
Our study suggested that multidisciplinary treatment with local excision plus CRT can be used as a treatment option in selected patients with clinical T1N0M0 rectal cancer.
Keywords: Early rectal cancer, Local excision, Chemoradiotherapy
Introduction
The standard treatments for rectal cancer invading the submucosa with no lymph node metastasis (clinical T1N0) are local excision and radical surgery [1]. Radical surgery is well known to be associated with a relatively high morbidity and can cause postoperative dysfunction of the rectum and the bladder and sexual dysfunction, negatively affecting patients' quality of life [2, 3, 4, 5]. Therefore, local excision has been performed in some patients with clinical T1 rectal cancer. The National Comprehensive Cancer Network (NCCN) guidelines recommend the transanal local excision for patients with tumors of < 30% circumference of the bowel, < 3 cm in size, with a negative margin, and located ≤8 cm from the anal verge. Follow-up observation can be performed in patients who do not have a histologically positive margin, lymphovascular invasion, poorly differentiated adenocarcinoma, or invasion of the lower third of the submucosa (sm3) [1].
The rate of local recurrence has been reported to be relatively high after local excision alone [6, 7, 8, 9, 10, 11, 12, 13, 14, 15]. However, local recurrence is almost completely inhibited by additionally administering chemoradiotherapy (CRT) after local excision [11, 16, 17, 18]. A meta-analysis reported that the rate of local recurrence was 5% in patients who received radiotherapy or CRT after local excision, which was similar to that (4%) in those who underwent total mesorectal excision (TME) [19]. The relations between image analysis of cancer invasion and lymph node metastasis have been studied in T1 colorectal cancer. The rate of lymph node metastasis was reported to increase in parallel to the depth of tumor invasion [20]. The width of T1 invasion [21, 22], cross-sectional area of T1 invasion [22], lymphatic invasion [20, 22], venous invasion [22, 23], and histologic type [20, 21, 22] have been reported to be risk factors for local recurrence. However, accurate predictive factors remain to be established.
We performed transanal full-thickness local excision in patients with cT1N0 rectal cancer in whom transanal tumor resection was feasible. We then confirmed pathological T1 stage with negative margins and the absence of poorly differentiated adenocarcinoma. All patients subsequently received CRT. We report the treatment outcomes of such patients.
Patients and Methods
From June 2000 through October 2017, local excision was performed in 65 patients with clinical T1N0M0 rectal cancer in whom the tumor was located ≤8 cm from the anal verge and the tumor diameter was < 30 mm and who had biopsy-proven well-differentiated or moderately differentiated adenocarcinoma. Clinical stage was diagnosed on the basis of digital rectal examination, barium enema examination, colonoscopy, endorectal ultrasonography, computed tomography of the chest, abdomen, and pelvis, and pelvic magnetic resonance imaging. After local excision, patients who had pathological T2 (pT2) cancer or included components of poorly differentiated adenocarcinoma or mucinous carcinoma additionally underwent TME surgery. Patients who had well-differentiated or moderately differentiated adenocarcinoma with negative resection margins and provided informed consent additionally received postoperative CRT.
Surgery
Transanal full-thickness local resection was performed while securing a horizontal margin of 1 cm from the tumor.
Adjuvant CRT
Postoperative radiotherapy was performed with 15 MV X-rays delivered by a linear accelerator (Clinac 21EX, Varian Medical Systems, Inc., Palo Alto, CA, USA) using a 4-field technique. Irradiation was carried out once (1.8 or 2 Gy) daily to a total dose of 40–45 Gy.
Concomitant chemotherapy with oral UFT (tegafur/uracil; 400 mg/m2) or oral S-1 (tegafur/gimeracil/oteracil; 80 mg/m2) was started at the same time as radiotherapy. Oral UFT was simultaneously given with radiotherapy on 5 weekdays, followed by a 2-day rest on the weekends. This cycle was repeated during irradiation. Oral S-1 (80 mg/m2) was simultaneously begun with radiotherapy. S-1 was given for 2 consecutive weeks, followed by a 1-week rest, and was then given for another 2 weeks [24, 25].
Surveillance
After local excision, surveillance was performed on an outpatient basis for 5 years. Examinations were performed every 3–4 months during the first 2 years and every 6 months thereafter until 5 years. Examinations included physical examination, digital rectal examination, blood tests, including serum carcinoembryonic antigen, and abdominal ultrasonography. Computed tomography of the chest, abdomen, and pelvis was performed every 6 months. Colonoscopy was performed 6 months and 1, 3, and 5 years after surgery.
Statistical Analysis
Statistical analysis was performed using SPSS for Windows, version 24 (IBM Japan, Ltd., Tokyo, Japan). Actuarial survival curves were calculated according to the Kaplan-Meier method. The rates of disease-free survival (DFS) and overall survival (OS) were determined by log-rank analysis.
This study was approved by the institutional review board of our university (17R-366), and all patients provided written informed consent.
Results
Patient and Tumor Characteristics
Local excision was performed in 65 patients with cT1N0M0 rectal cancer. Four patients found to have pT2 disease on histopathological examination and 11 patients who refused CRT were excluded from the analysis. The remaining 50 patients additionally received postoperative CRT. The patient characteristics are shown in Table 1. The median tumor diameter was 19 mm (range, 7–29 mm). The median distance from the anal verge was 4.0 cm (range, 1–8 cm).
Table 1.
Patient characteristics
| Gender, n (%) | |
| Male | 31 (62) |
| Female | 19 (38) |
| Median age (range), years | 64 (42–91) |
| Median tumor size (range), cm | 1.9 (0.7–2.9) |
| Tumor location, n (%) | |
| Middle rectuma | 9 (18) |
| Lower rectumb | 41 (82) |
| Histologic type, n (%) | |
| Well differentiatedc | 39 (78) |
| Moderately differentiatedd | 11 (22) |
| Radiation dose, n (%) | |
| 40 Gy | 27 (54) |
| 45 Gy | 23 (46) |
| Concurrent chemotherapy, n (%) | |
| UFT | 47 (94) |
| S-1 | 3 (6) |
| Lymphatic invasion, n (%) | |
| (–) | 29 (58) |
| (+) | 21 (42) |
| Venous invasion, n (%) | |
| (–) | 41 (82) |
| (+) | 9 (18) |
| Lymphovascular invasion, n (%) | |
| (–) | 25 (50) |
| (+) | 25 (50) |
UFT, tegafur/uracil; S-1, tegafur/gimeracil/oteracil.
Middle third of the rectum.
Lower third of the rectum.
Well differentiated adenocarcinoma.
Moderately differentiated adenocarcinoma.
CRT-Related Adverse Events
Two patients had grade 2 or higher adverse events. One patient discontinued concurrent chemotherapy because of grade 2 leukopenia. The other patient had grade 3 diarrhea, leukopenia, and neutropenia and discontinued CRT at a dose of 40 Gy. The scheduled course of CRT was completed in 48 patients (96%) (Table 2).
Table 2.
Chemoradiotherapy intervention
| Chemotherapy completed | |
| Yes | 48 (96) |
| No | 2 (4) |
| Radiotherapy completed | |
| Yes | 49 (98) |
| No | 1 (2) |
| Chemoradiotherapy completed | |
| Yes | 48 (96) |
| No | 2 (4) |
Values are n (%).
Survival Analysis
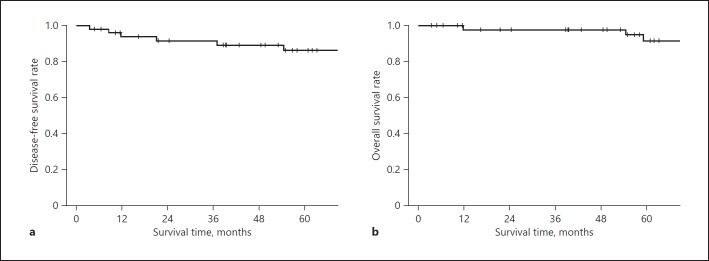
The median follow-up period was 71 months (range, 4–211 months). The 5-year DFS rate was 86%, the 5-year local recurrence-free survival rate was 86%, and the 5-year OS rate was 92% (Fig. 1). Recurrence occurred in 4 patients (8%). Recurrence was local in 1 patient (2%), involved the liver in 1 patient (2%), and involved the lung in 2 patients (4%) (Table 3). Local recurrence developed in the mesorectal lymph nodes 37 months after surgery, and abdominoperineal resection was performed. As of 179 months after surgery, the patient is still alive, with no recurrence.
Fig. 1.
Disease-free survival rate (a) and overall survival rate (b).
Table 3.
Treatment failures
| Death from original disease | 2 (4) |
| Death unrelated to disease | 3 (6) |
| Recurrence | 4 (8) |
| Local failure | 1 (2) |
| Distant | 3 (6) |
| Liver | 1 (2) |
| Lung | 2 (4) |
Values are n (%).
Overall, 5 patients died. Two of the 3 patients with distant recurrence died of recurrent cancer. The other 3 patients died of other causes.
Discussion
Lymph node metastases have been reported to occur in 9–17% of patients with pT1 rectal cancer [20, 21, 22, 23]. The rate of local recurrence after local excision ranges from 11 to 18% [6, 7, 8, 10, 12, 13, 14, 26, 27]. In rectal cancer, 27% of metastatic lymph nodes were reported to be 3 mm or less in diameter. Lymph node metastasis is, thus, difficult to accurately diagnose preoperatively on the basis of lymph node size [28].
Nascimbeni et al. [20] studied the relation between infiltration patterns and lymph node metastasis in patients with T1 colorectal cancer. The depth of invasion was divided into 3 levels. The rate of lymph node metastasis was 3% in patients whose tumors invaded the upper third of the submucosa (sm1), 8% in patients whose tumors invaded the middle third of the submucosa (sm2), and 23% in patients whose tumors invaded the lower third of the submucosa (sm3). The rate of lymph node metastasis, thus, increased in parallel to the depth of invasion. We previously reported that the depth of invasion was unrelated to the rate of lymph node metastasis in patients with T1 rectal cancer. Lymph node metastasis was not found in patients with a width of T1 invasion of < 5 mm and occurred in 30% of patients with a width of T1 invasion of ≥5 mm. The width of tumor invasion was, thus, closely related to the rate of lymph node metastasis in patients with T1 rectal cancer (p = 0.005) [21]. Toh et al. [22] reported no relation between the incidence of lymph node metastasis and the depth of invasion and found that the incidence of lymph node metastasis was closely related to the width and cross-sectional area of invasion in patients with T1 rectal cancer (p = 0.001, p < 0.001). Diagnostic methods for lymph node metastasis on the basis of image analysis of tumor invasion remain to be established.
Min et al. [26] designated lymphovascular invasion, margin involvement, and sm2 or deeper invasion of the submucosa as risk factors for recurrence in patients with T1 rectal cancer and found local recurrence after local excision with no risk factors in 3 (12%) of 26 patients. Blumberg et al. [23] reported that lymphovascular invasion and poorly differentiated adenocarcinoma are risk factors for recurrence in patients with T1 rectal cancer and found local recurrence in 3 (7%) of 42 patients who underwent local excision for T1 rectal cancer with no risk factors. The rate of local recurrence was reported to be high after local excision alone even in patients who had T1 rectal cancer with no risk factors. We, therefore, administered CRT in all patients with pT1 rectal cancer postoperatively. Some studies have found no difference between local excision and TME surgery in local recurrence rates or outcomes of patients with T1 rectal cancer [7, 14, 29]. However, Stornes et al. [6] studied 543 patients with T1 rectal cancer and reported that the local recurrence rate was 14.5% in patients who underwent transanal endoscopic microsurgery, which was significantly higher than the recurrence rate (1.4%) in patients who underwent TME (p < 0.001). The 5-year OS rate was significantly lower in the transanal endoscopic microsurgery group (65.3%) than in the TME group (81.5%, p = 0.012). You et al. [8] used the National Cancer Data Base (NCDB) and found that the local recurrence rate was significantly higher after local excision (12.5%) than after standard TME surgery (6.9%, p = 0.003) in patients with pT1 rectal cancer. The 5-year OS rate was significantly lower in the local excision (78%) than the standard surgery (86%, p = 0.009) [30]. Consequently, an increasing number of patients with T1 rectal cancer are additionally receiving CRT after local excision. Borstlap et al. [19] performed a meta-analysis of 14 studies and found that the local recurrence rate in patients with T1 rectal cancer who additionally received CRT or radiotherapy after local excision (5%) was similar to that in patients who underwent TME (4%).
In our study of patients with pT1 cancer who additionally received radiotherapy after local resection, only 1 patient (2%) had local recurrence, the 5-year OS rate was 91.4%, and the 5-year DFS rate was 85.8%, indicating good outcomes.
Conclusions
The results of our study suggested that multidisciplinary treatment combining local resection with postoperative CRT can be a treatment option in selected patients with pT1N0M0 rectal cancer without risk factors.
Disclosure Statement
The authors have no potential conflicts of interest.
default
References
- 1.NCCN (National Comprehensive Cancer Network) NCCN Clinical Practice Guidelines in Oncology, Rectal Cancer. 2018;v.4 doi: 10.6004/jnccn.2009.0057. https://www.nccn.org/professionals/physician_gls/pdf/rectal.pdf. [DOI] [PubMed] [Google Scholar]
- 2.Pucciarelli S, Giandomenico F, De Paoli A, Gavaruzzi T, Lotto L, Mantello G, Barba C, Zotti P, Flora S, Del Bianco P. Bowel function and quality of life after local excision or total mesorectal excision following chemoradiotherapy for rectal cancer. Br J Surg. 2017;104:138–147. doi: 10.1002/bjs.10318. [DOI] [PubMed] [Google Scholar]
- 3.Breukink SO, van der Zaag-Loonen HJ, Bouma EM, Pierie JP, Hoff C, Wiggers T, Meijerink WJ. Prospective evaluation of quality of life and sexual functioning after laparoscopic total mesorectal excision. Dis Colon Rectum. 2007;50:147–155. doi: 10.1007/s10350-006-0791-z. [DOI] [PubMed] [Google Scholar]
- 4.Camilleri-Brennan J, Steele RJ. Quality of life after treatment for rectal cancer. Br J Surg. 1998;85:1036–1043. doi: 10.1046/j.1365-2168.1998.00808.x. [DOI] [PubMed] [Google Scholar]
- 5.Gavaruzzi T, Lotto L, Giandomenico F, Perin A, Pucciarelli S. Patient-reported outcomes after neoadjuvant therapy for rectal cancer: a systematic review. Expert Rev Anticancer Ther. 2014;14:901–918. doi: 10.1586/14737140.2014.911090. [DOI] [PubMed] [Google Scholar]
- 6.Stornes T, Wibe A, Nesbakken A, Myklebust TA, Endreseth BH. National early rectal cancer treatment revisited. Dis Colon Rectum. 2016;59:623–629. doi: 10.1097/DCR.0000000000000591. [DOI] [PubMed] [Google Scholar]
- 7.Patel SA, Chen YH, Hornick JL, Catalano P, Nowak JA, Zukerberg LR, Bleday R, Shellito PC, Hong TS, Mamon HJ. Early-stage rectal cancer: clinical and pathologic prognostic markers of time to local recurrence and overall survival after resection. Dis Colon Rectum. 2014;57:449–459. doi: 10.1097/DCR.0b013e3182a70709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.You YN, Baxter NN, Stewart A, Nelson H. Is the increasing rate of local excision for stage I rectal cancer in the United States justified? A nationwide cohort study from the National Cancer Database. Ann Surg. 2007;245:726–733. doi: 10.1097/01.sla.0000252590.95116.4f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Paty PB, Nash GM, Baron P, Zakowski M, Minsky BD, Blumberg D, Nathanson DR, Guillem JG, Enker WE, Cohen AM, Wong WD. Long-term results of local excision for rectal cancer. Ann Surg. 2002;236:522–529. doi: 10.1097/00000658-200210000-00015. discussion 529–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mellgren A, Sirivongs P, Rothenberger DA, Madoff RD, Garcia-Aguilar J. Is local excision adequate therapy for early rectal cancer? Dis Colon Rectum. 2000;43:1064–1071. doi: 10.1007/BF02236551. discussion 1071–1064. [DOI] [PubMed] [Google Scholar]
- 11.Chakravarti A, Compton CC, Shellito PC, Wood WC, Landry J, Machuta SR, Kaufman D, Ancukiewicz M, Willett CG. Long-term follow-up of patients with rectal cancer managed by local excision with and without adjuvant irradiation. Ann Surg. 1999;230:49–54. doi: 10.1097/00000658-199907000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Garcia-Aguilar J, Mellgren A, Sirivongs P, Buie D, Madoff RD, Rothenberger DA. Local excision of rectal cancer without adjuvant therapy: a word of caution. Ann Surg. 2000;231:345–351. doi: 10.1097/00000658-200003000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Greenberg JA, Shibata D, Herndon JE, 2nd, Steele GD, Jr, Mayer R, Bleday R. Local excision of distal rectal cancer: an update of cancer and leukemia group B 8984. Dis Colon Rectum. 2008;51:1185–1191. doi: 10.1007/s10350-008-9231-6. discussion 1191–1184. [DOI] [PubMed] [Google Scholar]
- 14.Nascimbeni R, Nivatvongs S, Larson DR, Burgart LJ. Long-term survival after local excision for T1 carcinoma of the rectum. Dis Colon Rectum. 2004;47:1773–1779. doi: 10.1007/s10350-004-0706-9. [DOI] [PubMed] [Google Scholar]
- 15.Ikematsu H, Yoda Y, Matsuda T, Yamaguchi Y, Hotta K, Kobayashi N, Fujii T, Oono Y, Sakamoto T, Nakajima T, Takao M, Shinohara T, Murakami Y, Fujimori T, Kaneko K, Saito Y. Long-term outcomes after resection for submucosal invasive colorectal cancers. Gastroenterology. 2013;144:551–559. doi: 10.1053/j.gastro.2012.12.003. quiz e14. [DOI] [PubMed] [Google Scholar]
- 16.Sasaki T, Ito Y, Ohue M, Kanemitsu Y, Kobatake T, Ito M, Moriya Y, Saito N. Postoperative chemoradiotherapy after local resection for high-risk T1 to T2 low rectal cancer: results of a single-arm, multi-institutional, phase II clinical trial. Dis Colon Rectum. 2017;60:914–921. doi: 10.1097/DCR.0000000000000870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gonzalez QH, Heslin MJ, Shore G, Vickers SM, Urist MM, Bland KI. Results of long-term follow-up for transanal excision for rectal cancer. Am Surg. 2003;69:675–678. discussion 678. [PubMed] [Google Scholar]
- 18.Lamont JP, McCarty TM, Digan RD, Jacobson R, Tulanon P, Lichliter WE. Should locally excised T1 rectal cancer receive adjuvant chemoradiation? Am J Surg. 2000;180:402–405. doi: 10.1016/s0002-9610(00)00493-1. discussion 405–406. [DOI] [PubMed] [Google Scholar]
- 19.Borstlap WA, Coeymans TJ, Tanis PJ, Marijnen CA, Cunningham C, Bemelman WA, Tuynman JB. Meta-analysis of oncological outcomes after local excision of pT1–2 rectal cancer requiring adjuvant (chemo)radiotherapy or completion surgery. Br J Surg. 2016;103:1105–1116. doi: 10.1002/bjs.10163. [DOI] [PubMed] [Google Scholar]
- 20.Nascimbeni R, Burgart LJ, Nivatvongs S, Larson DR. Risk of lymph node metastasis in T1 carcinoma of the colon and rectum. Dis Colon Rectum. 2002;45:200–206. doi: 10.1007/s10350-004-6147-7. [DOI] [PubMed] [Google Scholar]
- 21.Suzuki T, Sadahiro S, Mukoyama S, Ishikawa K, Yasuda S, Tajima T, Makuuchi H, Murayama C. Risk of lymph node and distant metastases in patients with early invasive colorectal cancer classified as Haggitt's level 4 invasion: image analysis of submucosal layer invasion. Dis Colon Rectum. 2003;46:203–208. doi: 10.1007/s10350-004-6525-1. [DOI] [PubMed] [Google Scholar]
- 22.Toh EW, Brown P, Morris E, Botterill I, Quirke P. Area of submucosal invasion and width of invasion predicts lymph node metastasis in pT1 colorectal cancers. Dis Colon Rectum. 2015;58:393–400. doi: 10.1097/DCR.0000000000000315. [DOI] [PubMed] [Google Scholar]
- 23.Blumberg D, Paty PB, Guillem JG, Picon AI, Minsky BD, Wong WD, Cohen AM. All patients with small intramural rectal cancers are at risk for lymph node metastasis. Dis Colon Rectum. 1999;42:881–885. doi: 10.1007/BF02237095. [DOI] [PubMed] [Google Scholar]
- 24.Sadahiro S, Suzuki T, Tanaka A, Okada K, Kamijo A, Murayama C, Akiba T, Nakayama Y. Phase I/II study of preoperative concurrent chemoradiotherapy with S-1 for locally advanced, resectable rectal adenocarcinoma. Oncology. 2011;81:306–311. doi: 10.1159/000334580. [DOI] [PubMed] [Google Scholar]
- 25.Suzuki T, Sadahiro S, Tanaka A, Okada K, Kamata H, Kamijo A, Murayama C, Akiba T, Kawada S. Biopsy specimens obtained 7 days after starting chemoradiotherapy (CRT) provide reliable predictors of response to CRT for rectal cancer. Int J Radiat Oncol Biol Phys. 2013;85:1232–1238. doi: 10.1016/j.ijrobp.2012.09.031. [DOI] [PubMed] [Google Scholar]
- 26.Min BS, Kim NK, Ko YT, Lee KY, Baek SH, Cho CH, Sohn SK. Long-term oncologic results of patients with distal rectal cancer treated by local excision with or without adjuvant treatment. Int J Colorectal Dis. 2007;22:1325–1330. doi: 10.1007/s00384-007-0339-2. [DOI] [PubMed] [Google Scholar]
- 27.Bentrem DJ, Okabe S, Wong WD, Guillem JG, Weiser MR, Temple LK, Ben-Porat LS, Minsky BD, Cohen AM, Paty PB. T1 adenocarcinoma of the rectum: transanal excision or radical surgery? Ann Surg. 2005;242:472–477. doi: 10.1097/01.sla.0000183355.94322.db. discussion 477–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Langman G, Patel A, Bowley DM. Size and distribution of lymph nodes in rectal cancer resection specimens. Dis Colon Rectum. 2015;58:406–414. doi: 10.1097/DCR.0000000000000321. [DOI] [PubMed] [Google Scholar]
- 29.Bhangu A, Brown G, Nicholls RJ, Wong J, Darzi A, Tekkis P. Survival outcome of local excision versus radical resection of colon or rectal carcinoma: a Surveillance, Epidemiology, and End Results (SEER) population-based study. Ann Surg. 2013;258:563–569. doi: 10.1097/SLA.0b013e3182a4e85a. discussion 569–571. [DOI] [PubMed] [Google Scholar]
- 30.Gabriel E, Thirunavukarasu P, Al-Sukhni E, Attwood K, Nurkin SJ. National disparities in surgical approach to T1 rectal cancer and impact on outcomes. Am Surg. 2016;82:1080–1091. [PubMed] [Google Scholar]