Abstract
Introduction:
Whole-slide imaging (WSI) technology can be used for primary diagnosis and consultation, including intraoperative (IO) frozen section (FS). We aimed to implement and validate a digital system for the FS evaluation of cancer and transplant specimens following recommendations of the College of American Pathologists.
Materials and Methods:
FS cases were routinely scanned at ×20 employing the “Navigo” scanner system. IO diagnoses using glass versus digital slides after a 3-week washout period were recorded. Intraobserver concordance was evaluated using accuracy rate and kappa statistics. Feasibility of WSI diagnoses was assessed by the way of sensitivity, specificity, as well as positive and negative predictive values. Participants also completed a survey denoting scan time, time spent viewing cases, preference for glass versus WSI, image quality, interface experience, and any problems encountered.
Results:
Of the 125 cases submitted, 121 (436 slides) were successfully scanned including 93 oncological and 28 donor-organ FS biopsies. Four cases were excluded because of failed digitalization due to scanning problems or sample preparation artifacts. Full agreement between glass and digital-slide diagnosis was obtained in 90 of 93 (97%, κ = 0.96) oncology and in 24 of 28 (86%, κ = 0.91) transplant cases. There were two major and one minor discrepancy for cancer cases (sensitivity 100%, specificity 96%) and two major and two minor disagreements for transplant cases (sensitivity 96%, specificity 75%). Average scan and viewing/reporting time were 12 and 3 min for cancer cases, compared to 18 and 5 min for transplant cases. A high diagnostic comfort level among pathologists emerged from the survey.
Conclusions:
These data demonstrate that the “Navigo” digital WSI system can reliably support an IO FS service involving complicated cancer and transplant cases.
Keywords: Digital pathology, frozen section, transplant, validation, whole-slide imaging
INTRODUCTION
Digital pathology (DP), including whole-slide imaging (WSI), holds great promise for supporting many of the functions required in general surgical pathology service.[1] WSI comprises two components: image acquisition to create digital slides that replicate glass slides and viewing these whole-slide images in a manner that simulates (virtual) microscopy.[2] The key components of a DP system include hardware (scanner, workstation with display, server), software (image management system, image viewer, and image analysis), and network connectivity.[3] Primary diagnosis by WSI is defined as establishing a final diagnosis solely by review of digital images without relying on manually examining glass slides using a conventional light microscope.[4] Using WSI for primary diagnosis has now been approved in many countries including Canada, the European Union, and most recently in the United States by the Food and Drug Administration.[5] Apart from primary diagnosis, WSI has also been utilized for remote frozen section (FS) interpretation, teleconsultation for a second opinion, image analysis, education, and proficiency testing.[6]
For teleconsultation, glass slides at the client site can be scanned, then uploaded, and hosted or transmitted to a networked server along with relevant metadata (e.g., case accession number, clinical information) and made available to a single or multiple distant consultant pathologists almost instantly.[7] Leveraging such telepathology can allow surgical pathology support to be remotely offered to pathologists located at distant sites, including during intraoperative (IO) procedures.[8] In the field of transplant pathology, the introduction of high-quality WSI scanners within facilities such as organ retrieval centers enables the establishment of regional, national, and/or international networks of on-call expert pathologists who are subspecialized in all organ systems.[9] Such an on-call service could reduce wastage of organs and thereby expand the donor pool for transplantation.
Before using WSI for diagnostic work, it is imperative that a DP system be validated for its intended clinical use.[10] The College of American Pathologists (CAP) guidelines published in 2013 provided recommendations to help pathology laboratories undertake such a validation study.[11] The CAP guidelines included specific recommendations such as utilizing at least 60 cases per application, training of participants beforehand, employing a washout period of at least 2 weeks between viewing slide sets with different modalities, and preferentially recording intraobserver variability.[3]
To date, most validation studies using the CAP guidelines have been conducted using WSI for primary diagnosis. Up to now, numerous studies have investigated various digital technologies (static and robotic telepathology, live streaming, DP) for IO FS and cytological diagnoses,[8,12,13,14,15,16,17,18,19,20,21,22,23] but only few validation studies have been published regarding the use of WSI for the assessment of FS biopsies.[17,24] The aim of this study was to investigate the validity and accuracy of using WSI for IO FS diagnoses specific to cancer and transplant surgery.
MATERIALS AND METHODS
Ethics statement
This study was approved by the Institutional Review Board of the University of Verona in accordance with the Seventh Revision (2013) of the Declaration of Helsinki 1975.
Equipment
The “Navigo” digital system (Visia Imaging) was installed in the laboratory affiliated with the surgical operating rooms [Figure 1]. This system was connected via a 100 Mbps internal hospital network to the Central Pathology Laboratory which is located in a different building. The system includes a bright field scanner for image acquisition with a 2-slide capacity for standard microscope slides, customized profiles for hematoxylin and eosin (H&E), immunohistochemistry, and cytology slides and two scanning modalities, fast and accurate; a microscope with different objectives (×4, ×10, ×20, ×40) which is part of the scanner; a 10.1-inch color touchscreen for navigation and analysis; local archive 30 TB of capacity for storing digital slides; and web-based software for image management (e.g., transfer of digital images). The file format output is JPEG 2000 and the scanner resolution is of 0.5 μm/pixel at ×20 and 0.25 μm/pixel at ×40. This system can be used as a local digital microscope or for remote live viewing and sharing of digitized slides (e.g., telepathology). As stated by the manufacturers in the fast modality with a number of focal points reduced approximately by a half compared to the accurate modality, the average scan speed per a tissue section of 5 cm2 is <10 s at ×4, <12 min at ×20, <30 min at ×40 while in the accurate modality is of <1 min at ×4, <20 min at ×20, and <60 min at ×40.
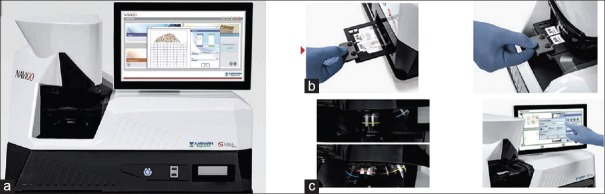
Figure 1.
The Navigo System. (a) Scanner case and integrated 15-inch touchscreen monitor with onboard computer processor. (b) Slide loading mechanism. (c) High-resolution objectives
The Navigo scanning system allows users to manage each component of the imaging process in a single work environment, thereby optimizing workflow tailored to the needs of each pathologist.
After specimen identification, the scanning device determines the optimal set of focus points by placing focal points on adjacent regions (spatial locality) with fine coverage on the borders and a diamond grid placed on the inside of the field to be scanned [Figure 2a]. After rapid preview, the user can select scanning areas, the objective for scanning, and the scan protocol. The scanning mode is suited both for relatively planar samples (e.g., tissue sections) and for thick samples (e.g., cytology specimens with three-dimensional cell clusters). Different focus points are used to extract a best fitting predictive focus plane that guides scanning along the slide, following the predictive Z-position [Figure 2b and c]. For thick samples, a vertical drive along the Z-axis is used for each tile scanned. The image viewer permits users to not only change magnification and zoom into images but also incorporate a focus slider to view different scanned layers in Z-stacked images [Figure 3a]. The viewer includes several other mark-ups (e.g., measurements) and image management (e.g., sharing) tools. Digital slides can be viewed locally on the Navigo monitor or remotely through a web browser with integrated web viewer (Naviweb) [Figure 3b and c]. Once the acquired images are uploaded to the web server, they can be accessed at any time from any web-device after obtaining permissions. The Naviweb digital microscope allows real-time live sample viewing and enables intranet and internet users to securely access live slides and to navigate them as with conventional microscope. The following actions may be carried out: multi-using sharing of digitized slides, simultaneous examination of images, synchronized navigation, fast image streaming, and instant notification to remote users when a case for teleconsultation was assigned for review.
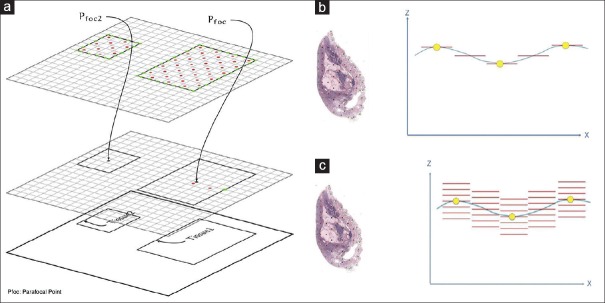
Figure 2.
Scanning focus technology. (a) Focus point placement. (b) Horizontal drive along the Z-axis for planar samples. (c) Vertical drive along the Z-axis for thick samples
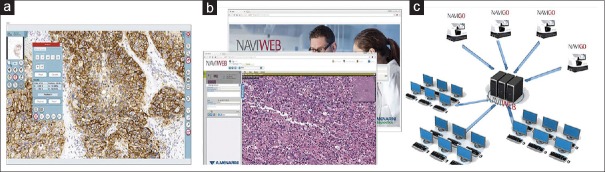
Figure 3.
Image viewing and management. (a) Advanced image viewer. (b) Naviweb web-based application. (c) Web server and multi-user sharing functionality
Samples
Archival slides, all stained by H&E, which were used for IO consecutive examinations performed in 2017 were collected from the Anatomic Pathology Unit of the University Hospital of Verona by all the pathologists routinely involved in the IO examinations. This incorporated a variety of benign, atypical, and neoplastic diagnoses rendered during oncology and transplant IO examinations. The first group of cases (Group 1, n = 96) included those FS cases requested to assess surgical margins, tumor biological behavior, and lymph node status for cancer staging, while the second group (Group 2, n = 29) were cases submitted for FS to assess organ quality of donors during transplantation. All specimens were frozen using the Bech freezer “PrestoCHILL” (Milestone Medical). PrestoCHILL allows ultrafast freezing (60 s) at −40°C for all types of tissue improving the quality of FSs by eliminating freezing, compression, and retraction artifacts and consequently giving better WSIs. During routine processing, a technician recorded the time between arrival of the specimen for FS and communication of the final diagnosis.
Digitization
This validation study started with a 3-month educational phase during which three pathologists (A.E., G.R., and L.C.) and one laboratory technician (A.S.) excluded from the collection of cases, were familiarized with the use of the digital imaging system. Thereafter, glass slides of each FS were scanned at ×20 using the scanner's accurate quality setting. Most slides were scanned using automated tissue detection and focus point assignments. Some slides with limited tissue required manual adjustment. Two pathologists (A.E. and G.R.) managed the scanner by conducting the digitization of the glass slides and their storage. They also performed quality control through a detail view of each digital image and accurate comparison with the corresponding glass slides.
For incomplete or blurry digital slides, scanning was repeated; if the problem occurred again, the case was excluded. Finally, they confirmed, for each case, the original IO diagnosis. The number of slides scanned, repeat scans, and time of scanning for each case were recorded on a digital platform developed by Arsenàl, Veneto's Research Center for eHealth Innovation, and accessible, after obtaining permissions, from each computer connected to the central server of the hospital.
Assessment of glass slides
FS glass slides were anonymized and made available to a third pathologist (L.C.). The laboratory technician (A.S.) provided a case history for each case including patient age, gender, clinical history, and gross description. A “virtual” IO histological report was provided with the following parameters and reported on the digital platform: name and age of the patient, date of report, anatomical site, type of FS, diagnosis, and time needed to render a diagnosis for each case.
Assessment of digital slides
Following a 3-week washout period, all slides were re-evaluated in digital format by L.C. on a widescreen flat panel monitor (HP LP2465): viewable image area (diagonal), 60.96 cm; screen opening, 52 cm × 32.6 cm; pixel pitch, 0.270; color depth support, 16.7 million colors; 1920 × 1200 at 60 Hz. The monitor was integrated into the “Navigo” digital system and equipped with a sensible wireless mouse (Logitech M705) able to ease the navigation inside the digital images. A “virtual” IO histological report was provided, with the same parameters listed previously, and reported on the digital platform.
The decision to evaluate all the glass slides on first and all the digital slides on second was made arbitrarily.
Morphological evaluation
The oncology IO cases were classified as negative (e.g., for benign neoplasm, negative margin, or negative for metastases) or positive (e.g., malignancy, positive margin, or positive for metastases).
The transplant IO donor biopsies were considered negative when the histopathology diagnosis allowed the transplant surgery to proceed and positive when the histopathology interpretation blocked transplant. Kidney biopsies were evaluated using the Remuzzi score assessing four parameters: glomerular sclerosis (GS: score 0–3), tubular atrophy (TA: 0–3), interstitial fibrosis (IF: 0–3), and arterial narrowing (AR: 0–3). The final score ranged from a minimum of 0 to a maximum of 12. Kidneys with a Remuzzi score of 4 or lower were used as single transplants. Those with a score of 5 or 6 were used as dual transplants. Kidneys with a score ≥7 were discarded.[25] Liver biopsies were graded 0–3 based on the percentage of hepatocytes with macrosteatosis: 0 (<5% hepatocytes involved); 1, mild macrosteatosis (5–33% hepatocytes involved); 2, moderate macrosteatosis (34–66% hepatocytes involved); 3, severe macrosteatosis (>66% hepatocytes involved).[26] Liver with a score of 0 or 1 should be used while those with a score of 2 or 3 should be discarded.
Assessment of quality
Quality indicators related to reporting were score relying on subjective perception. Variables taken into consideration in the digital survey and scored were reliance on the best aspects of digital reporting, problems encountered with WSI, preferred reporting methods (i.e., digital vs. glass slides), and the need for more technical tools. The overall perception of reading digital FS slides with a computer monitor has been classified as poor, sufficient, good, very good, and excellent through the comparison, for every single case, with the optical microscope.
Statistical analysis
For each case, the glass-slide diagnosis was compared to that given following digital-slide review and evaluated as concordant or discordant. Discordances were categorized as major (change in patient management) or minor (no change in patient management). Accuracy rate and weighted Cohen κ test were used to determine the validity of agreement between diagnoses by glass and digital microscopy (intraobserver concordance). Sensitivity, specificity, positive predictive value, and negative predictive value were used to determine the validity of digital assessments. Statistical values were obtained with calculations made using SPSS version 22.0 (IBM, Armonk, NY, USA). The level of agreement for κ coefficients is generally accepted as follows: 0.0–0.2, slight; 0.21–0.4, fair; 0.41–0.6, moderate; 0.61–0.8, substantial; and 0.81–1.0, almost perfect.
RESULTS
Clinicopathological results
There were 125 IO examinations, corresponding to 176 FSs and 445 slides. A total of 96 “oncology IO examination” cases were collected that comprised cases submitted to evaluate tumor biological behavior (55%), resection margins (30%), suspicious lesions (10%), lymph nodes for cancer staging (4%), and other miscellaneous reasons (1%). There were 19 “oncology IO examination” cases with multiple tissue blocks per case (range 2–12 FSs, total 126 FSs). The 29 “transplant IO examination” cases included 16 kidney donors and 13 liver donors evaluated by FS before harvesting. All the kidney donors were biopsied bilaterally (32 FSs) while five out of 13 liver donors underwent two biopsies (18 FSs). Specimens underwent fast freezing (60 s) and cutting of tissue section at 5 μm thick. Sections were stained using H&E and examined at three levels. The distribution of IO cases according to organ type is shown in Table 1 and discordances summarized in Table 2.
Table 1.
Summary of intraoperative consultation cases
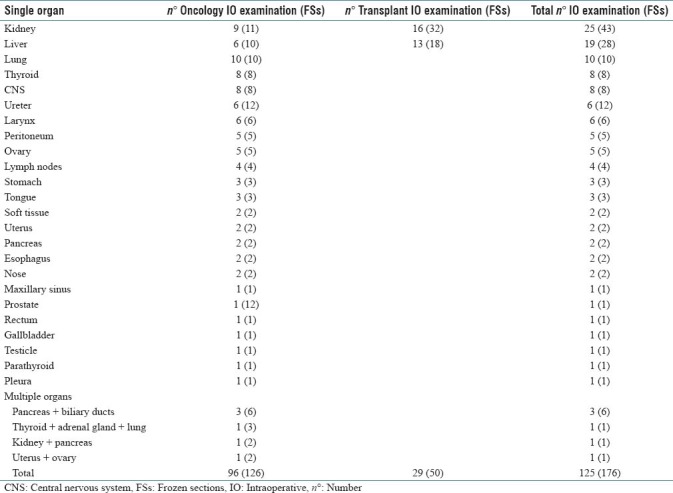
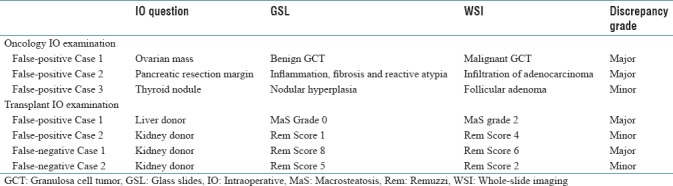
Table 2.
Discordant cases between glass slides and whole-slide imaging showing intraobserver assessment
Digitization findings
Of the 125 submitted cases, 110 (88%) were successfully scanned on the first attempt and 11 (9%) needed a second scan. Four cases (3%) were excluded because of failed digitization 2 times for scanning reasons. The first case was an omental biopsy for surgical staging of uterine endometrioid adenocarcinoma, the FS had high-fat content, and the digital image resulted partially blurry. The second case was a poorly differentiated squamous cell carcinoma of the lung with massive comedo-type necrosis that resulted in an incomplete scanning. The latter two cases failed digitization for sample preparation artifacts and were represented by a pleural biopsy-diagnosed with mesothelioma and a transplant liver biopsy with a great amount of fibrosis. Scan times for each case averaged 12 min (range: 4–30) for the “oncology IO” group and 18 min (range: 3–40) for the “transplant IO” group. The average digital slide file size is 420 MB (range: 260–620) for the “oncology IO” group and 290 MB (range: 210–430) for the “transplant IO” group.
Diagnostic accuracy and concordance
In 90 of 93 “oncology IO examinations,” there was concordance between glass and digital-slide assessment with a 97% accuracy rate (κ =0.96, confidence interval [CI], 0.941–0.985). There were two major and one minor discrepancy; all considered to represent false-positive cases [Table 2, Figure 4a and b]. Sensitivity was 100% and specificity was 96% [Table 3]. Reporting time averaged 6 min for glass slides and 3 min for digital slides.
Figure 4.
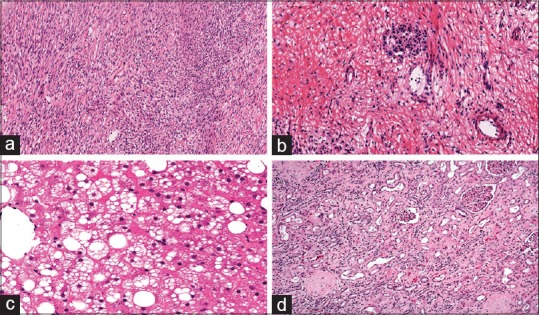
Clinically significant errors with whole-slide imaging. (a) Malignant granulosa cell tumor misinterpreted to be benign in an ovarian frozen section (H&E, ×10). (b) Positive resection margin instead of negative in a pancreatic FS (H&E, ×20). (c) Macrosteatosis grade 2 instead of grade 0 in a liver donor FS (H&E, ×20). (d) Remuzzi score 8 instead of score 6 in a kidney donor FS (H&E, ×10)
Table 3.
Validity of whole-slide imaging diagnoses for intraoperative examinations
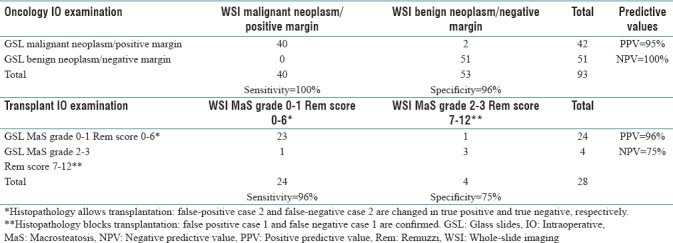
In 24 of 28 “transplant IO examinations,” there was concordance between glass and digital-slide assessment with an 86% accuracy rate (κ = 0.91, CI, 0.877–0.958). There were two major and two minor discrepancies, with 2 false-positive and 2 false-negative cases [Table 2, Figure 4c and d]. Sensitivity was 96% and specificity was 75% [Table 3]. Reporting time for each case averaged 9 min for glass slides and 5 min for digital slides. The average time for tissue sampling, FS case scanning, and digital slide interpretation averaged 26 min per case for “oncology IO examinations” and 31 min per case for “transplant IO examinations.”
Assessment of quality
The overall experience of digital reporting was considered favorable. A high diagnostic comfort level was reported in the majority of cases with 25% classified as excellent and 37% as very good [Figure 5]. The reading pathologist stated as favorite reporting method the glass slides only in 5% of cases [Figure 5], including those cases with major discrepancies on WSI. The survey reported the high quality of digital images and ease of navigating the WSI system as the best features of the digital workstation experience. The reading pathologist also suggested additional technical tools able to quantify, in a precise manner, the grade of macrosteatosis in the transplant liver biopsies and the Remuzzi score in the transplant kidney biopsies.
Figure 5.
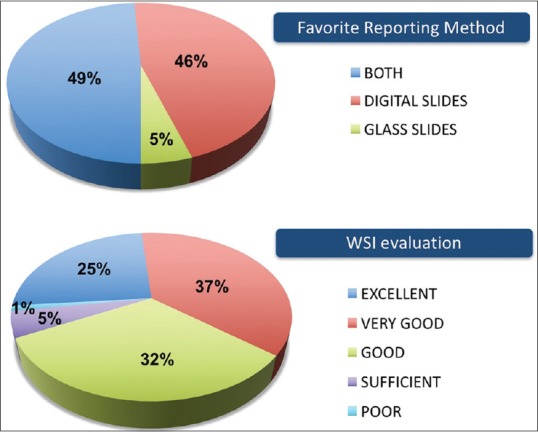
User perception of whole-slide imaging experience. Favorite reporting method and overall evaluation of whole-slide imaging experience
DISCUSSION
The Anatomic Pathology Unit at the University Hospital of Verona recently implemented a WSI system to provide quick consultations for their IO service, particularly during transplant surgeries that often take place outside of conventional working hours. The Navigo system was successfully validated for this intended clinical use [Figure 6] by performing an intraobserver concordance study in accordance with the CAP clinical validation recommendations for WSI. Both oncology and transplant FS cases were diagnosed on glass slides and WSI by the same pathologist after a washout period of 3 weeks. These data show an almost perfect intraobserver agreement (κ > 0.80) between glass and digital assessments for both groups.
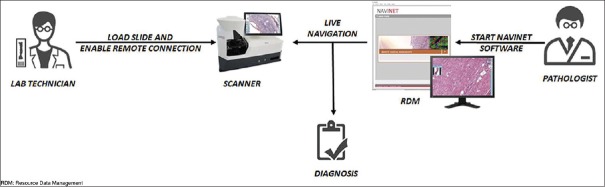
Figure 6.
Digital intraoperative teleconsultation workflow with the Navigo system
The use of telepathology for remotely rendering FS diagnoses has steadily grown as the field of pathology has moved into an age of increasing subspecialization and centralization of pathology services.[17] Before 2000, the technology to support telepathology was limited, especially for handling urgent IO cases, with an ensuing concern for potential diagnostic errors with medicolegal implications. This changed with the development of WSI that offered color digital images of entire glass slides that could be remotely reviewed at high resolution, comparable to a light microscope.[27] WSI systems can facilitate reporting of FS diagnoses from remote locations (e.g., outside the laboratory) at any time (e.g., during night call and on weekends).[10] This capability, in turn, can help reduce unnecessary two-stage surgery. Providing support for lone pathologists working in provincial hospitals facilitates the recruitment and retention of both surgeons and pathologists.[28] This is particularly germane for transplant surgery, where limited exposure to solid-organ transplantation often results in the sense of uneasiness for many practicing pathologists when evaluating transplant pathology specimens.[29] Furthermore, the majority of donor-retrieval operations tend to occur outside normal working hours that necessitates the use of an on-call pathology service which may not be available in all hospitals but might be replaced by a WSI system of remote consultation.[30]
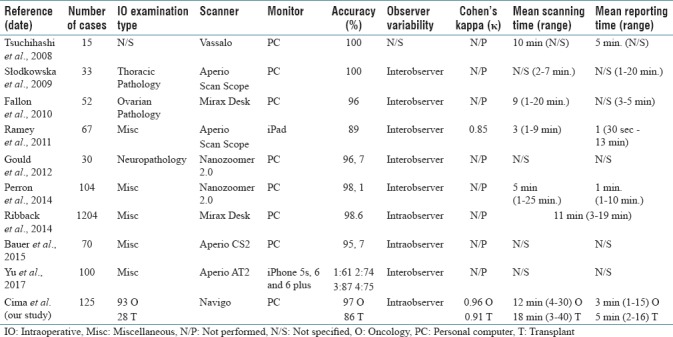
To date, several studies have compared the diagnostic concordance of WSI and conventional microscopy in the IO setting [Table 4].[17,18,19,20,24,31,32,33,34,35] In 2008, Tsuchihashi et al. reported one of the first experiences using remote FS evaluation with WSI technology in a pilot study comprising 15 test cases. The diagnoses made in this historic study with a virtual-slide system were all correct and required around 10 min to scan and transmit each digital image and another 5 min to read these images with a remote viewer. Based on these early, limited results, the authors concluded WSI to be superior to conventional telepathology.[31] Subsequent similar studies reported accuracy rates of over 90% for WSI systems for FS of various organs and tumors.[17,18,32] Ramey et al. were the first to report performing FS assessments using WSI on a mobile device (iPad) in which they obtained a diagnostic accuracy rate of 89%.[33] In 2014, Ribback et al. reported the largest series of FS diagnoses made with the use of digital imaging. They included 1204 FS cases scanned and showed a diagnostic accuracy rate of 98.6% using digital slides.[20] Bauer et al. in their intraobserver study of 70 FS cases reported the longest washout time (just over 1 year) and diagnostic accuracy of 95.7% for interpreting scanned FS slides.[24]
Table 4.
Summary of publications analyzing frozen section diagnosis by whole-slide imaging
The diagnostic accuracy for the “oncology IO examination” group (97%, κ = 0.96) in our study is comparable with that reported in the literature. The major discrepancies in this group were related to pathologist interpretation errors and not directly related to technology issues. Such errors, however, are not totally unexpected. For example, although a granulosa cell tumor of the ovary in this study was misinterpreted as mesenchymal malignant neoplasm, the literature on the accuracy of ovarian FS diagnoses reports that this particular neoplasm is often misinterpreted.[36] In the case where a pancreatic resection margin was erroneously considered to be positive with WSI, it is well known that the presence of prominent inflammation in the region can lead to reactive atypia, even severe, which can be misinterpreted as a malignant process.[37] The diagnostic accuracy for the “transplant IO examination” of this study (86%, κ = 0.91) cannot be compared, given the lack of concordance studies between the conventional microscope and WSI system for liver and kidney FS transplant biopsies. Of note, the major discrepancies observed in this study were due to overestimation of macrosteatosis in a liver biopsy and underestimation of interstitial fibrosis in a kidney biopsy.
Our results showed that the time required by a pathologist for WSI interpretation was shorter than that for conventional light microscopy as has been highlighted in the studies by Ribback et al.[20] However, one of the limiting factors to turnaround time (TAT) using WSI telepathology is scan time as also demonstrated in our study. The CAP TAT for single block FS measured from the time that pathologists receive FS specimens to the time that pathologists return FS diagnoses to the surgeon is 20 min;[38] in our study, we obtained a total average TATs of 26 and 31 min for oncology and transplant cases, respectively. The nonconformity to the CAP accreditation requirements for FS must be compared with the benefits in providing quick consultations for difficult cases, in particular during transplant surgeries.
The average scan time required in our study was longer than the range published in other similar studies (viz., 3–11 min), while the average reporting time was within the range presented in the literature (viz., 1–5 min). The longer scan time noted for the “transplant IO examination” patient group can be explained by the presence of multiple tissue fragments in the same FS, while the longer average reporting time can be attributed to the difficulty often experienced with the interpretation of transplant IO donor biopsies. Notably, average digital reporting time is lower than the average microscope reporting time. The reason for this can perhaps be linked to the ease of navigating high-resolution digital slides on a high-resolution monitor equipped with a sensible wireless mouse. The total average time for digital reporting of “oncology IO examination” cases was quicker than those reported by Ribback et al. (i.e., 35 min.) but longer than those reported by Perron et al. (i.e., 20 min).[20,34]
The limitations of our study include involving only a single reading pathologist which precluded intra–interobserver comparisons and the absence of an analysis of the potential impact of preimaging factors (e.g., sampling, cutting and freezing of the specimens, artifacts). The reason for not including more pathologists in the reading component is due to the need to fill out the IO timesheets correctly. The strengths include strict adherence to the CAP recommendation for validating WSI for clinical use, the complete exclusion of the personnel involved in the study from the IO timesheets preventing the potential bias of having seen some of the study cases at the time they were originally reported and the inclusion of challenging IO transplant cases. In 2001, Minervini et al. reported one of the first digital experiences with IO transplant cases. In their study, both real-time interactive and store-and-forward static telepathology were used to evaluate 49 transplant pathology cases, including six IO donor liver biopsy specimens. Full diagnostic agreement was obtained for all of their IO cases.[29] We successfully included 28 IO donor kidney and liver biopsies and employed a WSI approach and demonstrated almost perfect intraobserver agreement. To the best of our knowledge, this work is the first to investigate the use of WSI technology for transplant FS. WSI technology not only enables easy sharing of problematic cases but can also be leveraged for quality assurance and image analytics in transplant pathology.[39]
CONCLUSIONS
Our study demonstrates that the diagnostic accuracy of FS diagnosis by WSI is comparable with that of light microscopy even though may not follow CAP accreditation requirements for FS. This study is the first, to the best of our knowledge, of consecutive transplant FS cases including routine donor organ liver and kidney biopsies. The ultrafast Navigo scanner and user-friendly software make this DP system particularly suitable for quick teleconsultations as is often requested for FS evaluation in cancer and transplant surgeries.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Footnotes
Available FREE in open access from: http://www.jpathinformatics.org/text.asp?2018/9/1/34/242915
REFERENCES
- 1.Campbell WS, Lele SM, West WW, Lazenby AJ, Smith LM, Hinrichs SH, et al. Concordance between whole-slide imaging and light microscopy for routine surgical pathology. Hum Pathol. 2012;43:1739–44. doi: 10.1016/j.humpath.2011.12.023. [DOI] [PubMed] [Google Scholar]
- 2.Weinstein RS, Graham AR, Richter LC, Barker GP, Krupinski EA, Lopez AM, et al. Overview of telepathology, virtual microscopy, and whole slide imaging: Prospects for the future. Hum Pathol. 2009;40:1057–69. doi: 10.1016/j.humpath.2009.04.006. [DOI] [PubMed] [Google Scholar]
- 3.Pantanowitz L, Sinard JH, Henricks WH, Fatheree LA, Carter AB, Contis L, et al. Validating whole slide imaging for diagnostic purposes in pathology: Guideline from the College of American Pathologists Pathology and Laboratory quality center. Arch Pathol Lab Med. 2013;137:1710–22. doi: 10.5858/arpa.2013-0093-CP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hanna MG, Pantanowitz L, Evans AJ. Overview of contemporary guidelines in digital pathology: What is available in 2015 and what still needs to be addressed? J Clin Pathol. 2015;68:499–505. doi: 10.1136/jclinpath-2015-202914. [DOI] [PubMed] [Google Scholar]
- 5.Abels E, Pantanowitz L. Current state of the regulatory trajectory for whole slide imaging devices in the USA. J Pathol Inform. 2017;8:23. doi: 10.4103/jpi.jpi_11_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Taylor CR. Issues in using whole slide imaging for diagnostic pathology: “Routine” stains, immunohistochemistry and predictive markers. Biotech Histochem. 2014;89:419–23. doi: 10.3109/10520295.2013.861512. [DOI] [PubMed] [Google Scholar]
- 7.Bauer TW, Slaw RJ. Validating whole-slide imaging for consultation diagnoses in surgical pathology. Arch Pathol Lab Med. 2014;138:1459–65. doi: 10.5858/arpa.2013-0541-OA. [DOI] [PubMed] [Google Scholar]
- 8.Kaplan KJ, Burgess JR, Sandberg GD, Myers CP, Bigott TR, Greenspan RB, et al. Use of robotic telepathology for frozen-section diagnosis: A retrospective trial of a telepathology system for intraoperative consultation. Mod Pathol. 2002;15:1197–204. doi: 10.1097/01.MP.0000033928.11585.42. [DOI] [PubMed] [Google Scholar]
- 9.Neil DA, Demetris AJ. Digital pathology services in acute surgical situations. Br J Surg. 2014;101:1185–6. doi: 10.1002/bjs.9576. [DOI] [PubMed] [Google Scholar]
- 10.Boyce BF. Whole slide imaging: Uses and limitations for surgical pathology and teaching. Biotech Histochem. 2015;90:321–30. doi: 10.3109/10520295.2015.1033463. [DOI] [PubMed] [Google Scholar]
- 11.Mukhopadhyay S, Feldman MD, Abels E, Ashfaq R, Beltaifa S, Cacciabeve NG, et al. Whole slide imaging versus microscopy for primary diagnosis in surgical pathology: A Multicenter blinded randomized noninferiority study of 1992 cases (Pivotal study) Am J Surg Pathol. 2018;42:39–52. doi: 10.1097/PAS.0000000000000948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nordrum I, Engum B, Rinde E, Finseth A, Ericsson H, Kearney M, et al. Remote frozen section service: A telepathology project in northern Norway. Hum Pathol. 1991;22:514–8. doi: 10.1016/0046-8177(91)90226-f. [DOI] [PubMed] [Google Scholar]
- 13.Adachi H, Inoue J, Nozu T, Aoki H, Ito H. Frozen-section services by telepathology: Experience of 100 cases in the San-in district, Japan. Pathol Int. 1996;46:436–41. doi: 10.1111/j.1440-1827.1996.tb03634.x. [DOI] [PubMed] [Google Scholar]
- 14.Baak JP, van Diest PJ, Meijer GA. Experience with a dynamic inexpensive video-conferencing system for frozen section telepathology. Anal Cell Pathol. 2000;21:169–75. doi: 10.1155/2000/908426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hufnagl P, Bayer G, Oberbamscheidt P, Wehrstedt K, Guski H, Hauptmann S, et al. Comparison of different telepathology solutions for primary frozen section diagnostic. Anal Cell Pathol. 2000;21:161–7. doi: 10.1155/2000/123057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Frierson HF, Jr, Galgano MT. Frozen-section diagnosis by wireless telepathology and ultra portable computer: Use in pathology resident/faculty consultation. Hum Pathol. 2007;38:1330–4. doi: 10.1016/j.humpath.2007.02.006. [DOI] [PubMed] [Google Scholar]
- 17.Evans AJ, Chetty R, Clarke BA, Croul S, Ghazarian DM, Kiehl TR, et al. Primary frozen section diagnosis by robotic microscopy and virtual slide telepathology: The university health network experience. Hum Pathol. 2009;40:1070–81. doi: 10.1016/j.humpath.2009.04.012. [DOI] [PubMed] [Google Scholar]
- 18.Fallon MA, Wilbur DC, Prasad M. Ovarian frozen section diagnosis: Use of whole-slide imaging shows excellent correlation between virtual slide and original interpretations in a large series of cases. Arch Pathol Lab Med. 2010;134:1020–3. doi: 10.5858/2009-0320-OA.1. [DOI] [PubMed] [Google Scholar]
- 19.Gould PV, Saikali S. A comparison of digitized frozen section and smear preparations for intraoperative neurotelepathology. Anal Cell Pathol (Amst) 2012;35:85–91. doi: 10.3233/ACP-2011-0026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ribback S, Flessa S, Gromoll-Bergmann K, Evert M, Dombrowski F. Virtual slide telepathology with scanner systems for intraoperative frozen-section consultation. Pathol Res Pract. 2014;210:377–82. doi: 10.1016/j.prp.2014.02.007. [DOI] [PubMed] [Google Scholar]
- 21.Vitkovski T, Bhuiya T, Esposito M. Utility of telepathology as a consultation tool between an off-site surgical pathology suite and affiliated hospitals in the frozen section diagnosis of lung neoplasms. J Pathol Inform. 2015;6:55. doi: 10.4103/2153-3539.168515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sirintrapun SJ, Rudomina D, Mazzella A, Feratovic R, Alago W, Siegelbaum R, et al. Robotic telecytology for remote cytologic evaluation without an on-site cytotechnologist or cytopathologist: A tale of implementation and review of constraints. J Pathol Inform. 2017;8:32. doi: 10.4103/jpi.jpi_26_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sirintrapun SJ, Rudomina D, Mazzella A, Feratovic R, Lin O. Successful secure high-definition streaming telecytology for remote cytologic evaluation. J Pathol Inform. 2017;8:33. doi: 10.4103/jpi.jpi_18_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bauer TW, Slaw RJ, McKenney JK, Patil DT. Validation of whole slide imaging for frozen section diagnosis in surgical pathology. J Pathol Inform. 2015;6:49. doi: 10.4103/2153-3539.163988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Remuzzi G, Grinyò J, Ruggenenti P, Beatini M, Cole EH, Milford EL, et al. Early experience with dual kidney transplantation in adults using expanded donor criteria. Double kidney transplant group (DKG) J Am Soc Nephrol. 1999;10:2591–8. doi: 10.1681/ASN.V10122591. [DOI] [PubMed] [Google Scholar]
- 26.Brunt EM, Janney CG, Di Bisceglie AM, Neuschwander-Tetri BA, Bacon BR. Nonalcoholic steatohepatitis: A proposal for grading and staging the histological lesions. Am J Gastroenterol. 1999;94:2467–74. doi: 10.1111/j.1572-0241.1999.01377.x. [DOI] [PubMed] [Google Scholar]
- 27.Goacher E, Randell R, Williams B, Treanor D. The diagnostic concordance of whole slide imaging and light microscopy: A systematic review. Arch Pathol Lab Med. 2017;141:151–61. doi: 10.5858/arpa.2016-0025-RA. [DOI] [PubMed] [Google Scholar]
- 28.Griffin J, Treanor D. Digital pathology in clinical use: Where are we now and what is holding us back? Histopathology. 2017;70:134–45. doi: 10.1111/his.12993. [DOI] [PubMed] [Google Scholar]
- 29.Minervini MI, Yagi Y, Marino IR, Lawson A, Nalesnik M, Randhawa P, et al. Development and experience with an integrated system for transplantation telepathology. Hum Pathol. 2001;32:1334–43. doi: 10.1053/hupa.2001.29655. [DOI] [PubMed] [Google Scholar]
- 30.Neil DA, Roberts IS, Bellamy CO, Wigmore SJ, Neuberger JM. Improved access to histopathology using a digital system could increase the organ donor pool and improve allocation. Transpl Int. 2014;27:759–64. doi: 10.1111/tri.12320. [DOI] [PubMed] [Google Scholar]
- 31.Tsuchihashi Y, Takamatsu T, Hashimoto Y, Takashima T, Nakano K, Fujita S, et al. Use of virtual slide system for quick frozen intra-operative telepathology diagnosis in Kyoto, Japan. Diagn Pathol. 2008;3(Suppl 1):S6. doi: 10.1186/1746-1596-3-S1-S6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Słodkowska J, Pankowski J, Siemiatkowska K, Chyczewski L. Use of the virtual slide and the dynamic real-time telepathology systems for a consultation and the frozen section intra-operative diagnosis in thoracic/pulmonary pathology. Folia Histochem Cytobiol. 2009;47:679–84. doi: 10.2478/v10042-010-0009-z. [DOI] [PubMed] [Google Scholar]
- 33.Ramey J, Fung KM, Hassell LA. Use of mobile high-resolution device for remote frozen section evaluation of whole slide images. J Pathol Inform. 2011;2:41. doi: 10.4103/2153-3539.84276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Perron E, Louahlia S, Nadeau L, Boilard F, Ing M, Orain M, et al. Telepathology for intraoperative consultations and expert opinions: The experience of the Eastern Québec Telepathology Network. Arch Pathol Lab Med. 2014;138:1223–8. doi: 10.5858/arpa.2013-0466-OA. [DOI] [PubMed] [Google Scholar]
- 35.Yu H, Gao F, Jiang L, Ma S. Development of a whole slide imaging system on smartphones and evaluation with frozen section samples. JMIR Mhealth Uhealth. 2017;5:e132. doi: 10.2196/mhealth.8242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pinto PB, Andrade LA, Derchain SF. Accuracy of intraoperative frozen section diagnosis of ovarian tumors. Gynecol Oncol. 2001;81:230–2. doi: 10.1006/gyno.2001.6133. [DOI] [PubMed] [Google Scholar]
- 37.Khalifa MA. Intraoperative assessment of the Whipple resection specimen. J Clin Pathol. 2007;60:975–80. doi: 10.1136/jcp.2006.044834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Novis DA, Zarbo RJ. Interinstitutional comparison of frozen section turnaround time. A College of American Pathologists Q-probes study of 32868 frozen sections in 700 hospitals. Arch Pathol Lab Med. 1997;121:559–67. [PubMed] [Google Scholar]
- 39.Isse K, Lesniak A, Grama K, Roysam B, Minervini MI, Demetris AJ, et al. Digital transplantation pathology: Combining whole slide imaging, multiplex staining and automated image analysis. Am J Transplant. 2012;12:27–37. doi: 10.1111/j.1600-6143.2011.03797.x. [DOI] [PMC free article] [PubMed] [Google Scholar]