Abstract
IN BRIEF This study examined the relationship between patient commitment and A1C. Patients completed the Altarum Consumer Engagement (ACE) measure. Multiple A1C values were extracted from medical records for 273 military beneficiaries. Effects were analyzed with generalized linear models. The ACE Commitment subscale was significantly inversely related to A1C trends. Low-commitment patients were more likely to have a high A1C. High-commitment patients were 16% more likely to have an A1C <7.0%; this likelihood increased to 65% over time. The ACE Commitment domain may be a useful clinical tool. Increasing patients’ commitment to managing diabetes may improve their A1C over time.
Diabetes is a chronic disease that affects 25.8 million Americans, or about 8.3% of the U.S. population (1). About 95% of adults with diabetes are diagnosed with type 2 diabetes, and incidence rates for diabetes have been steadily increasing since the mid‐1990s (1). In addition, more is being asked of patients in health care interactions than ever before. The move toward patient‐centered medical homes is transforming primary care practices and driving patients toward a more active role in health care interactions (2).
Increasingly, patients must be more engaged in managing health information and making complex health care decisions (3). Patient engagement is key within the context of a chronic care model (4). Although being fully engaged in one’s health care can be challenging, the benefits are numerous, yielding safer (5), more effective (6), and less expensive (7) health care. Additionally, patients who actively participate in care decisions report higher satisfaction (5), faster recovery from illness (8), and improved quality of life. Finally, care plans resulting from a shared decision‐making process have been shown to result in more effective use of medication (9) and improved clinical outcomes (10,11).
There are several definitions of what it means for a patient to be fully engaged in health care decisions (12). Patient engagement is often defined within cognitive, emotional, or behavioral components. In biomedical research, patient engagement is distinct in terms of patient attitude; behavioral health research defines patient engagement as clinical alliance; and in nursing research, it is delineated as emotional factors that facilitate healthy behaviors. Public health defines patient engagement as consumer empowerment with a focus on health policy (3). We choose to define patient engagement as personal value in health care, self-efficacy, and use of skills and knowledge to create healthy behaviors within a supportive environment (3,4,13).
Although definitions abound, there has been less work toward developing practical measures of patient engagement. The Diabetes Empowerment Scale has been described as a measure for self-efficacy (14). Although Hibbard’s Patient Activation Measure is widely used (15), it measures only a subset of the full range of the concepts advanced by the Engagement Behavior Framework (16).
Tucked within the notion of patient engagement is the specific topic of patient commitment, which has been an important topic of discussion for patients with chronic disease. Patient commitment is delineated as a personal investment and value toward enacting health-related behaviors (17). Regarding the physician-patient relationship, patient commitment has been cited as positively influencing medical adherence and healthy behaviors (18). Beyond commitment is the notion of patient empowerment from health care providers to promote a psychological state that determines behavior and self-management. This step involves the complexity of supporting patients with autonomy and decision-making and enabling individuals with chronic disease to take charge of their own health (19,20).
Although several scales have captured aspects of patient commitment, in 2013, Altarum Institute, a Michigan‐based nonprofit health care research organization, validated the Altarum Consumer Engagement (ACE) Measure (21) with national and employer respondents of varying levels of health. However, it has not been applied specifically with patients who have chronic diseases such as diabetes. Within the ACE Measure, the Commitment domain focuses on patient commitment to health-promoting behaviors and has been found to be related to both self-rated patient health status and being at least 10 lb overweight (21).
Objective
This study examined the relationship between commitment as measured by the ACE Commitment domain and diabetes management. We hypothesized that higher levels of patient commitment would be associated with more successful diabetes management.
Methods
Design
Wilford Hall Ambulatory Surgical Center institutional review board approval was obtained. The Diabetes Center of Excellence (DCOE), which is a U.S. Air Force diabetes specialty clinic, partnered with Altarum Institute to collect and analyze the data. Potential participants included Department of Defense adult beneficiaries 18–70 years of age. In addition, only patients being treated at the DCOE who were diagnosed with type 2 diabetes for >1 year were eligible.
A DCOE research coordinator identified potentially eligible patients before their scheduled appointment. A red tag with information about the study was placed in patients’ folders. This red tag was a signal to providers to briefly discuss the study with their patients and invite them to participate. After patients checked out with the front office receptionist, interested patients met with the research coordinator to learn more about the study.
After completing the informed consent process, participants were asked questions pertaining to demographics and given the ACE Measure. The instrument was in English; therefore, it excluded participants who could not speak or read English. The next phase of the study included an electronic chart review of the survey respondents. Clinical information was extracted, including retrospective A1C, BMI, and significant health events such heart attacks, strokes, hospitalizations for diabetic complication(s), and emergency room visits.
Measures
The survey included 21 items from the validated ACE Measure (21), which assessed patient engagement in four domains: 1) Commitment, 2) Ownership, 3) Navigation, and 4) Informed Choice. Response choices were as follows: 1 = strongly disagree; 2 = disagree; 3 = neither agree nor disagree; 4 = agree; and 5 = strongly agree. This study focused on the Commitment domain (Table 1), which has been associated with health-related behaviors such as diet, exercise, and medication adherence (21).
TABLE 1.
ACE Measure Commitment Domain
| Patient Instructions: Please read each response. On a scale from 1 to 5, tell us if you agree with the statement. |
|---|
| Commitment |
| C1. I can stick with plans to exercise and eat a healthy diet. |
| C2. Even when life is stressful, I know I can continue to do the things that keep me healthy. |
| C3. When I work to improve my health, I succeed. |
| C4. I handle my health well. |
| C5. I take responsibility for managing my health. |
| C6. I take an active role in my own health care. |
Response choices included 1 = strongly disagree; 2 = disagree; 3 = neither agree nor disagree; 4 = agree; and 5 = strongly agree.
Variables
The ACE Commitment domain variables were scored as described by Duke et al. (21) to create a 0–25 score. This score was then split into three groups to indicate low, moderate, and high levels of commitment. Low-commitment patients scored <16 (26.2%), moderate-commitment patients scored between 16 and 22 (51.3%), and high-commitment patients scored >22 on the 25-point scale (21.8%).
A1C scores for up to three of the most recent readings were categorized as well managed (A1C <7.0%), not well managed (A1C 7.0–8.9%), and poorly managed (A1C ≥9.0%). The three readings occurred approximately 4 months apart on average and were spread over an average of 8 months total (mean 237 days; SD 69 days).
Analysis
Effects were analyzed both descriptively and with generalized linear models using PROC GENMOD in SAS (SAS Institute, Cary, N.C.) to include demographic variables such as patient age, sex, income, education, and self-reported health status. The generalized linear model used a multinomial outcome for three levels of A1C status: <7.0%, 7.0–8.9%, and ≥9.0%. Regardless of whether patients had an A1C value <7.0% or ≥9.0%, the values were treated as two binomial variables. Models across time were analyzed as repeated measures with a generalized estimating equation model, with z tests for individual predictors. Models comparing single time points, such as scores within time 1 or time 3 only, used maximum likelihood parameter estimates with Wald χ2 tests.
Results
Descriptive results including demographics, ACE Commitment level, and health outcomes are shown in Table 2. The sample included 273 participants; 58.2% were male. The overall mean age was 58.46 years, with women (mean age 57.82 years) being slightly younger than men (mean age 58.92 years). Participants were primarily Caucasian (74.8%) or African American (19.5%); one-third were Hispanic. Most participants were retired (61.2%), with 29.3% being employed after retirement from the military. The majority of women (58.7%) and men (50.9%) were employed. More men were college graduates (40.9%) than women (32.4%). However, women were more likely to live in a home with income >$50,000 (54.1%) than men (28.1%). Similar rates of self-reported health status were observed in men and women, with overall self-reported health mostly fair (23.4%), average (26.7%), and good (39.2%). Nearly half of the sample scored in the moderate-commitment category with about one-fourth to one-third in each of the low- and high-commitment groups.
TABLE 2.
Sample Characteristics by Sex
| Overall (n = 273) | Female (n = 114) | Male (n = 159) | |
|---|---|---|---|
| Sex | — | 114 (41.8) | 159 (58.2) |
| Mean age, years | 58.46 | 57.82 | 58.92 |
| Race | |||
| Caucasian | 196 (74.8) | 83 (76.9) | 113 (73.4) |
| African American | 51 (19.5) | 16 (14.8) | 35 (22.7) |
| API | 13 (5.0) | 8 (7.4) | 5 (3.2) |
| AIAN | 2 (0.8) | 1 (0.9) | 1 (0.6) |
| Ethnicity | |||
| Non-Hispanic | 171 (62.6) | 68 (59.6) | 103 (64.8) |
| Hispanic | 100 (36.6) | 45 (39.5) | 55 (34.6) |
| Employment status | |||
| Employed | 67 (24.8) | 53 (46.9) | 14 (8.9) |
| Unemployed | 38 (14.1) | 30 (26.5) | 8 (5.1) |
| Retired military (employed) | 79 (29.3) | 10 (8.8) | 66 (42.0) |
| Retired military (unemployed) | 86 (31.9) | 20 (17.7) | 69 (43.9) |
| Education level | |||
| High school/GED | 45 (16.5) | 27 (23.7) | 18 (11.3) |
| Some college | 119 (43.6) | 45 (39.5) | 74 (46.5) |
| College graduate | 57 (20.9) | 20 (17.5) | 37 (23.3) |
| Graduate degree | 45 (16.5) | 17 (14.9) | 28 (17.6) |
| Income category | |||
| <$15,000 | 38 (13.9) | 32 (28.1) | 6 (3.8) |
| $15,000 to $34,999 | 40 (14.7) | 20 (17.5) | 20 (12.6) |
| $35,000 to $49,999 | 44 (16.1) | 14 (12.3) | 30 (18.9) |
| $50,000 to $64,999 | 38 (13.9) | 13 (11.4) | 25 (15.7) |
| $65,000 to $74,999 | 24 (8.8) | 4 (3.5) | 20 (12.6) |
| $75,000 to $99,999 | 33 (12.1) | 9 (7.9) | 24 (15.1) |
| ≥$100,000 | 23 (8.4) | 6 (5.3) | 17 (10.7) |
| Declined to answer | 33 (12.1) | 16 (14.0) | 17 (10.7) |
| Self-reported health status | |||
| Poor | 18 (6.6) | 6 (5.3) | 12 (7.5) |
| Fair | 64 (23.4) | 26 (22.8) | 38 (23.9) |
| Average | 73 (26.7) | 33 (28.9) | 40 (25.2) |
| Good | 107 (39.2) | 44 (38.6) | 63 (39.6) |
| Excellent | 11 (4.0) | 5 (4.4) | 6 (3.8) |
| ACE Commitment level | |||
| Low | 72 (26.2) | 27 (23.7) | 45 (28.3) |
| Moderate | 141 (51.3) | 61 (53.5) | 80 (50.3) |
| High | 60 (21.8) | 26 (22.8) | 34 (21.4) |
Data are n (%) unless otherwise indicated. Totals may not be 100% because of rounding and missing data. AIAN, American Indian/Alaskan Native; API, Asian/Pacific Islander.
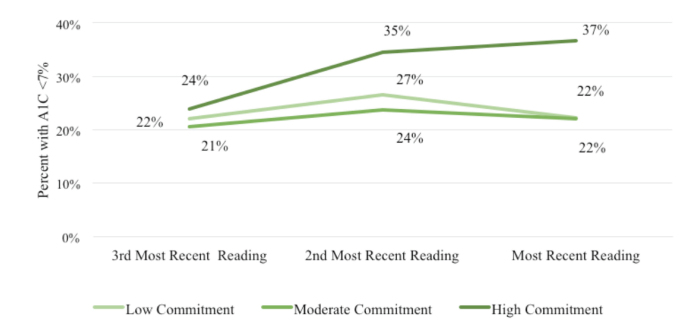
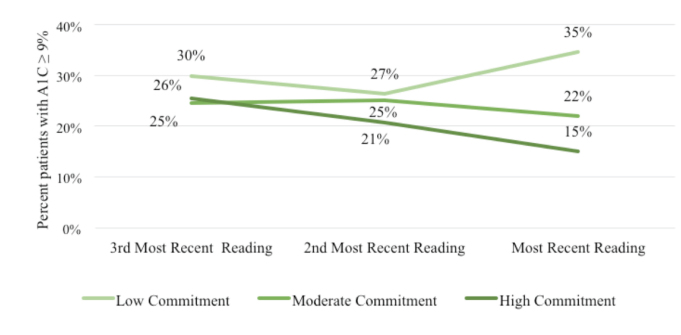
When examining sample characteristics by commitment level, individuals in the high-commitment group were less likely to be obese and more likely to rate their health as “very good” or “excellent” compared to individuals in the low- or moderate-commitment cohorts. The proportion of patients within the extreme A1C groups by commitment level are shown in Figures 1 and 2. DCOE patients with high commitment (37.0%) were more likely to have A1C values <7.0% than patients in the low-commitment (22.0%) or moderate-commitment (22.0%) groups (Figure 1). The converse was also apparent in that DCOE patients with high commitment were less likely to have an A1C ≥9.0% over time than either low-commitment or moderate-commitment patients (Figure 2).
FIGURE 1.
Percentage of people with diabetes with A1C <7% over time by commitment group.
FIGURE 2.
Percentage of people with diabetes with A1C ≥9% over time by commitment group.
To analyze the effect of commitment group on A1C levels over time while controlling for patient characteristics, we conducted a repeated-measures multinomial generalized linear model using PROC GENMOD in SAS. Patient A1C value (three levels: <7.0%, 7.0–8.9%, and ≥9.0%) across three time measurements was the dependent variable (Table 3). Patient ACE Commitment level (low, moderate, or high) was the primary independent variable. Commitment was analyzed as a class variable, with high commitment as the reference class. Additional patient characteristic variables included sex, education, income, age, and self-reported health. These patient characteristics had no significant relationship with A1C over time; parameter estimates for the model are shown in Table 4. The three readings occurred ∼4 months apart on average and were spread over an average of 8 months total.
TABLE 3.
Sample Characteristics by Commitment Level
| Overall (n = 273) | Low Commitment (n = 72) | Moderate Commitment (n = 141) | High Commitment (n = 60) | |
|---|---|---|---|---|
| Third most recent A1C | ||||
| <7.0% | 43 (22.2) | 11 (22.0) | 20 (20.6) | 11 (23.9) |
| 7.0–8.9% | 100 (51.5) | 24 (48.0) | 53 (54.6) | 23 (48.9) |
| ≥9.0% | 51 (26.3) | 15 (30.0) | 24 (24.7) | 12 (25.5) |
| Second most recent A1C | ||||
| <7.0% | 64 (26.8) | 18 (26.5) | 31 (23.7) | 20 (34.5) |
| 7.0–8.9% | 132 (48.6) | 32 (47.1) | 67 (51.1) | 26 (44.8) |
| ≥9.0% | 61 (24.5) | 18 (26.5) | 33 (25.2) | 12 (20.7) |
| Most recent A1C | ||||
| <7.0% | 69 (25.3) | 16 (22.2) | 31 (22.0) | 22 (36.7) |
| 7.0–8.9% | 139 (50.9) | 31 (43.1) | 79 (56.0) | 29 (48.3) |
| ≥9.0% | 65 (23.8) | 25 (34.7) | 31 (22.0) | 9 (15.0) |
| Clinical obesity | ||||
| BMI ≥30 kg/m2 | 197 (73.2) | 54 (76.1) | 106 (76.8) | 37 (61.7) |
| Hospitalization | ||||
| Emergency department use | 91 (33.3) | 21 (29.2) | 51 (36.2) | 19 (31.7) |
| Admitted as inpatient | 54 (19.8) | 22 (30.6) | 21 (14.9) | 11 (18.3) |
| Self-rated health | ||||
| Poor | 18 (6.6) | 11 (15.3) | 6 (4.3) | 1 (1.7) |
| Fair | 64 (23.4) | 20 (27.8) | 35 (24.8) | 9 (15.0) |
| Good | 73 (26.7) | 15 (20.8) | 42 (29.8) | 16 (26.7) |
| Very Good | 107 (39.2) | 25 (34.7) | 53 (37.6) | 29 (48.3) |
| Excellent | 11 (4.0) | 1 (1.4) | 5 (3.5) | 5 (8.3) |
Data are n (%) unless otherwise indicated.
TABLE 4.
Generalized Estimating Equation Parameter Estimates for Repeated-Measures Multinomial Generalized Linear Model of Patient Characteristics and ACE Commitment Level on A1C Over Time
| Parameter | Estimate* | SE | z | P |
|---|---|---|---|---|
| Female | 0.047 | 0.204 | 0.23 | 0.8175 |
| Education | 0.060 | 0.093 | 0.65 | 0.5188 |
| Income | 0.027 | 0.046 | 0.58 | 0.5628 |
| Age | –0.014 | 0.012 | –1.10 | 0.2728 |
| Self-rated health | 0.004 | 0.095 | 0.04 | 0.9697 |
| Time | 0.017 | 0.123 | 0.14 | 0.8893 |
| Low commitment | –1.184 | 0.394 | –3.00 | 0.0027 |
| Moderate commitment | –0.822 | 0.330 | –2.49 | 0.0128 |
| High commitment | 0.000 | 0.000 | Ref. | Ref. |
| Time × low commitment | 0.471 | 0.181 | 2.59 | 0.0095 |
| Time × moderate commitment | 0.392 | 0.153 | 2.56 | 0.0105 |
| Time × high commitment | 0.000 | 0.000 | Ref. | Ref. |
Generalized estimating equation parameter estimate. Ref., reference category.
Model results found that patients with high commitment were significantly more likely to be in a healthier A1C group than low-commitment (z = –3.0, P = 0.003) or moderate-commitment (z = –2.5, P = 0.013) patients. Additionally, the magnitude of this difference in A1C between high- and low-/moderate-commitment groups changed over time, as indicated by two significant interactions between low and high commitment × time (z = 2.6, P = 0.01) and moderate and high commitment × time (z = 2.6, P = 0.01).
To better understand the interaction effects in commitment over time on A1C level, we conducted a number of tests to contrast specific effects, controlling for all demographics in the full model. These effects show that over time, high-commitment patients were more likely to keep their A1C value <7.0% than low-commitment (z = 1.89, P = 0.059) or moderate-commitment (z = 2.08, P = 0.038) patients. Likewise, high-commitment patients were less likely to have an A1C value ≥9.0% over time compared to low-commitment patients (z = 2.74, P = 0.006). This effect was also subject to an interaction where the size of this gap increased over time (z = 1.92, P = 0.055); in other words, as time went on, low-commitment patients were increasingly likely to have a high A1C compared to high-commitment patients. Additionally, high-commitment patients were significantly less likely to be at risk of high A1C at time 3 than at time 1 (z = 2.09, P = 0.037). These results are shown in Table 5.
TABLE 5.
Tests of Specific Contrasts of Commitment Level and A1C Category Over Time
| Comparison | Estimate* | SE | z | P |
|---|---|---|---|---|
| Proportion with A1C <7.0% | ||||
| Low vs. high commitment (main effect, all times) | 0.996 | 0.526 | 1.89 | 0.0585 |
| Moderate vs. high commitment (main effect, all times) | 0.981 | 0.472 | 2.08 | 0.0378 |
| Low vs. high commitment (interaction over time) | –0.306 | 0.268 | –1.14 | 0.2545 |
| Moderate vs. high commitment (interaction over time) | –0.298 | 0.252 | –1.18 | 0.2365 |
| Within low commitment only (time 1 vs. time 3) | 0.049 | 0.167 | 0.29 | 0.7682 |
| Within moderate commitment only (time 1 vs. time 3) | 0.037 | 0.128 | 0.29 | 0.7711 |
| Within high commitment only (time 1 vs. time 3) | 0.308 | 0.195 | 1.58 | 0.1132 |
| Proportion with A1C of ≥9.0% | ||||
| Low vs. high commitment (main effect, all times) | –1.458 | 0.531 | –2.74 | 0.0061 |
| Moderate vs. high commitment (main effect, all times) | –0.624 | 0.496 | –1.26 | 0.2082 |
| Low vs. high commitment (interaction over time) | 0.462 | 0.240 | 1.92 | 0.0545 |
| Moderate vs. high commitment (interaction over time) | 0.302 | 0.221 | 1.37 | 0.1715 |
| Within low commitment only (time 1 vs. time 3) | 0.105 | 0.167 | 0.63 | 0.5288 |
| Within moderate commitment only (time 1 vs. time 3) | –0.015 | 0.192 | –0.36 | 0.7159 |
| Within high commitment only (time 1 vs. time 3) | –0.382 | 0.183 | –2.09 | 0.0369 |
Generalized estimating equation parameter estimate.
Additional tests examined differences between commitment levels at specific time points. At time 1, all three commitment level groups were similar in the proportion of patients with A1C scores <7.0% and ≥9.0%. By time 3, significant differences emerged. High-commitment patients were more likely to have an A1C value <7.0% compared to low-commitment (χ2 = 3.26, P = 0.071) and moderate-commitment (χ2 = 4.42, P = 0.036) patients. Likewise, at time 3, high-commitment patients were less likely to have an A1C of ≥9.0% than low-commitment patients (χ2 = 6.96, P = 0.008), indicating that high-commitment patients were more likely to have healthier A1C at the final reading (Table 6).
TABLE 6.
Tests of A1C by Commitment Level at Specific Time Points
| Comparison | Estimate* | SE | Wald χ2 | P |
|---|---|---|---|---|
| Proportion with A1C <7.0% | ||||
| Time 1 (least recent), low vs. high commitment | 0.189 | 0.520 | 0.13 | 0.7165 |
| Time 1 (least recent), moderate vs. high commitment | 0.208 | 0.448 | 0.21 | 0.6432 |
| Time 3 (most recent), low vs. high commitment | 0.749 | 0.415 | 3.26 | 0.0710 |
| Time 3 (most recent), moderate vs. high commitment | 0.742 | 0.353 | 4.42 | 0.0355 |
| Proportion with A1C of ≥9.0% | ||||
| Time 1 (least recent), low vs. high commitment | –0.163 | 0.495 | 0.11 | 0.7420 |
| Time 1 (least recent), moderate vs. high commitment | 0.230 | 0.441 | 0.27 | 0.6024 |
| Time 3 (most recent), low vs. high commitment | –1.228 | 0.466 | 6.96 | 0.0083 |
| Time 3 (most recent), moderate vs. high commitment | –0.485 | 0.431 | 1.26 | 0.2608 |
Maximum likelihood parameter estimate.
Collectively, these results show that, although early measurements of A1C showed similar outcomes in diabetes management, over an 8-month period, outcomes diverged widely by commitment groups. After 8 months, high-commitment patients were more likely to keep their A1C well managed and <7.0% compared to low- and moderate-commitment patients. Likewise, high-commitment patients were less likely to have poorly managed A1C values ≥9.0% compared to low-commitment patients. This result suggests that the ACE Commitment level may be predictive of diabetes outcomes, specifically blood glucose outcomes.
Discussion
This study demonstrates an associative relationship between patient engagement as measurement by ACE Commitment and glycemic control. Patients with high commitment were more likely to improve their A1C over time, whereas patients with low or moderate commitment did not improve over time. It is not clear whether commitment levels lead directly to better management of A1C, or if they are both influenced by additional variables; the relationship is likely to be complex. However, these findings are consistent with research showing that increased locus of control and self-efficacy have a positive effect on clinical outcomes (22).
The ACE Measure may be a useful tool in clinical encounters. Its Commitment domain could help identify patients who require additional support for self-management. This scale could be used by medical assistants, nursing staff, primary care physicians, and specialists during initial, annual, or follow-up appointments. If the clinic has limited resources, we suggest targeting patients with elevated A1C values. Potential interventions for improving commitment include motivational interviewing, medical health technology, education, and clinician self-management support (23–28).
This study focuses on the relationship between commitment and A1C. Current literature suggests a number of ways commitment may be fostered.
Diabetes Self-Management Education
In current clinical practice, providers may use the ACE Commitment domain to identify patients who would benefit from additional diabetes self-management education (DSME) and support (22). A potential intervention could target strengthening a patient’s locus of control and confidence through DSME and support (29–31). Perceived barriers interfere with patient self-efficacy and have been associated with worse diabetes self-care (30). Certainly, DSME has the potential to decrease self-perceived barriers, increase locus of control, and improve self-efficacy (22). Increasing self-efficacy with use of DSME has increased self-care behaviors and glycemic control in patients with type 2 diabetes in past studies (30,31).
Clinician-Patient Partnerships
Clinician-patient partnerships may be an effective intervention to increase patient commitment (32). These partnership strategies have included clinicians partnering with patients with use of shared decision-making in medical management (33). Key players in clinician-patient partnerships include specialists, primary care providers, diabetes educators, and nursing staff (34). Both knowledge of patients and support of their autonomy are consistent within these partnerships, therefore conveying a respect and spirit of collaboration (18). Clinician partnerships have been noted to use a strategy of intentionally conveying caring and empathy to improve disease self-management (26). Clinician partnerships may also involve enabling patients to contribute to their own electronic medical records or to have full access to their electronic medical record (35). Personalized goal-setting, identifying steps in achieving set goals, and promoting ownership of their diabetes management have been successful strategies (26,36). Thus, a clinician could identify at-risk patients with low commitment through the ACE Commitment scale and develop multidisciplinary appointments to promote a clinician-patient partnership model aimed at improving self-management through increasing commitment.
Online Communication
Technology within health care has enhanced communication, education, and self-management (35,37). A secure online communication tool could share information regarding patients’ personal health, diet and exercise, and appointment reminders to promote self-management among vulnerable patients identified with low commitment (38).
For example, in one study, patients had online access to their clinical notes through OpenNotes, which increased management and confidence. Qualitative feedback suggested that patients who used OpenNotes felt a high level of partnership and engagement (35). This tool also facilitates bidirectional communication and continuing education. Other electronic tools have included registries such as the Swedish Rheumatology Quality Registry. This registry is described as a platform for sharing information electronically between patients and health care providers, providing patient education, and promoting discussion of personal goals and research (39).
Motivational Interviewing
Motivational interviewing could be used as an effective tool for increasing patient commitment. Motivational interviewing interventions have been demonstrated to improve self-management skills and glycemic management (40,41). In clinical practice, the commitment questions could explore barriers or life challenges, confidence, and values. Individual commitment questions could assist in deciding what clinical support strategies to explore. For example, using statement C2, “Even when life is stressful, I know I can continue to do the things that keep me healthy,” may trigger providers to explore life stressors if the Commitment domain score is low. Services provided may include support groups, stress management techniques, or psychology or psychiatry services. Motivational interviewing techniques have the potential to complement clinician-patient partnership strategies, technology, and self-management education.
Limitations
This was a retrospective study that reviewed 1 year of data followed by the ACE Measure. Patients’ Commitment domain scores are only known at one time point close to the final A1C reading. It is unclear what patients’ Commitment domain scores would have been at the first A1C reading or how commitment may have changed over time. It is also unknown whether the relationship between commitment and A1C is causal or only correlational. We suggest the literature on self-efficacy and locus of control makes a case for commitment influencing health outcomes (42). Moreover, patients in the study were all Department of Defense beneficiaries receiving care in the DCOE, a military diabetes specialty clinic; thus, results may not be generalizable to a civilian population or to primary care patients.
Future Research
Additional research to replicate and extend our findings is needed. Future studies could include longitudinal measurement of commitment and how commitment relates to other chronic health conditions, including obesity, prediabetes, hypertension, chronic obstructive pulmonary disease, and chronic heart failure. Furthermore, research studies are needed to translate the commitment scale into clinical practice using specific interventions, including motivational interviewing, use of electronic communication, and clinician partnership models.
In conclusion, type 2 diabetes affects millions of Americans and inundates the U.S. health care system (1). Patients are asked to be more involved in diabetes management, despite limited resources (2,3). Use of the ACE Measure, specifically the Commitment domain, may identify likelihood of successfully managing A1C in diabetes patients, as well as identify patients who need additional support and clinic resources.
Acknowledgments
Duality of Interest
C.D. works for Altarum, Inc., the creator of the survey instrument. No other potential conflicts of interest relevant to this article were reported.
Author Contributions
J.W. and D.B. wrote the manuscript and contributed to the background and discussion. C.D. and T.J.S. reviewed/edited the manuscript and contributed to the discussion. C.D. provided the analysis, created graphs and tables, and wrote the results section. J.W. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Disclaimer
The views expressed are those of the authors and do not reflect the official views of the Department of Defense or its components.
References
- 1.Centers for Disease Control and Prevention National diabetes fact sheet: national estimates and general information on diabetes and prediabetes in the United States, 2014. Atlanta, GA: U.S. Department of Health and Human Services, 2014 [Google Scholar]
- 2.Carney PA, Eiff MP, Saultz JW, et al. . Aspects of the patient-centered medical home currently in place: initial findings from preparing the personal physician for practice. Fam Med 2009;41:632–639 [PubMed] [Google Scholar]
- 3.Barello S, Graffigna G, Vegni E, Bosio AC. The challenges of conceptualizing patient engagement in health care: a lexicographic literature review. J Participat Med 2014:6:e9 [Google Scholar]
- 4.Simmons LA, Wolever RQ, Bechard EM, Snyderman R. Patient engagement as a risk factor in personalized health care: a systematic review of the literature on chronic disease. Genome Med 2014;6:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Weingart SN, Zhu J, Chiappetta L, et al. . Hospitalized patients’ participation and its impact on quality of care and patient safety. Int J Qual Health Care 2011;23:269–277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Remmers C, Hibbard J, Mosen DM, Wagenfield M, Hoye RE, Jones C. Is patient activation associated with future health outcomes and healthcare utilization among patients with diabetes? J Ambul Care Manage 2009;32:320–327 [DOI] [PubMed] [Google Scholar]
- 7.Kennedy AD, Sculpher MJ, Coulter A, et al. . Effects of decision aids for menorrhagia on treatment choices, health outcomes, and costs: a randomized controlled trial. JAMA 2002;288:2701–2708 [DOI] [PubMed] [Google Scholar]
- 8.Clark NM, Janz NK, Dodge JA, et al. . The effect of patient choice of intervention on health outcomes. Contemp Clin Trials 2008;29:679–686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Naik AD, Kallen MA, Walder A, Street RL. Improving hypertension control in diabetes mellitus. Circulation 2008;117:1361–1368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bloomfield HE, Krause A, Greer N, et al. . Meta-analysis: effect of patient self-testing and self-management of long-term anticoagulation on major clinical outcomes. Ann Intern Med 2011:154:472–482 [DOI] [PubMed] [Google Scholar]
- 11.Lorig K, Ritter PL, Laurent DD, et al. . Online diabetes self-management program. Diabetes Care 2010;33:1275–1281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sacks RM, Greene J, Hibbard J, Overton V, Parrotta CD. Does patient activation predict the course of type 2 diabetes? A longitudinal study. Patient Educ Couns 2017:100:1268–1275 [DOI] [PubMed] [Google Scholar]
- 13.Barello S, Graffigna G, Vegni E. Patient engagement as an emerging challenge for healthcare services: mapping the literature. Nurs Res Pract 2012;2012:905934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hernandez-Tejada MA, Campbell JA, Walker RJ, Smalls BL, Davis KS, Egede LE. Diabetes empowerment, medication adherence and self-care behaviors in adults with type 2 diabetes. Diabetes Technol Ther 2012;14:630–634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hibbard JH, Mahoney ER, Stockard J, Tusler M. Development and testing of a short form of the patient activation measure. Health Serv Res 2005;40:1918–1930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gruman J, Rovner MH, French ME, et al. . From patient education to patient engagement: implications for the field of patient education. Patient Educ Couns 2010;78:350–356 [DOI] [PubMed] [Google Scholar]
- 17.Castelnuovo G, Pietrabissa G, Manzoni GM, et al. . Cognitive behavioral therapy to aid weight loss in obese patients: current perspectives. Psychol Res Behav Manag 2017;10:165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Berry LL, Parish JT, Janakiraman R, et al. . Patients’ commitment to their primary physician and why it matters. Ann Fam Med 2008;6:6–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lavalle-González FJ, Chiquete E. Patients’ empowerment, physicians’ perceptions, and achievement of therapeutic goals in patients with type 1 and type 2 diabetes mellitus in Mexico. Patient Prefer Adherence 2016;10:1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Small N, Bower P, Chew-Graham CA, Whalley D, Protheroe J. Patient empowerment in long-term conditions: development and preliminary testing of a new measure. BMC Health Serv Res 2013;13:263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Duke CC, Lynch WD, Smith B, Winstanley J. Validity of a new patient engagement measure: the Altarum Consumer Engagement (ACE) Measure. Patient 2015;8:559–568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marks R, Allegrante JP. A review and synthesis of research evidence for self-efficacy-enhancing interventions for reducing chronic disability: implications for health education practice (part II). Health Promot Pract 2005;6:148–156 [DOI] [PubMed] [Google Scholar]
- 23.Bodenheimer T. Helping patients improve their health-related behaviors: what system changes do we need? Dis Manag 2005;8:319–330 [DOI] [PubMed] [Google Scholar]
- 24.Dellasega C, Añel-Tiangco RM, Gabbay RA. How patients with type 2 diabetes mellitus respond to motivational interviewing. Diabetes Res Clin Pract 2012;95:37–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gimbel R, Shi L, Williams JE, et al. . Enhancing mHealth technology in the patient-centered medical home environment to activate patients with T2DM: a multisite feasibility study protocol. JMIR Res Protoc 2017;6:e38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Greene J, Hibbard JH, Alvarez C, Overton V. Supporting patient behavior change: approaches used by primary care clinicians whose patients have an increase in activation levels. Ann Fam Med 2016;14:148–154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li T, Wu HM, Wang F, et al. . Education programmes for people with diabetic kidney disease. Cochrane Database Syst Rev 2011;CD007374. [DOI] [PubMed] [Google Scholar]
- 28.Linden A, Butterworth SW, Prochaska JO. Motivational interviewing‐based health coaching as a chronic care intervention. J Eval Clin Pract 2010;16:166–174 [DOI] [PubMed] [Google Scholar]
- 29.Khazrai YM, Buzzetti R, Del Prato S, Cahn A, Raz I, Pozzilli P. The addition of E (Empowerment and Economics) to the ABCD algorithm in diabetes care. J Diabetes Complications 2015;29:599–606 [DOI] [PubMed] [Google Scholar]
- 30.Mohebi S, Azadbakht L, Feizi A, Sharifirad G, Kargar M. Review the key role of self-efficacy in diabetes care. J Educ Health Promot 2013;2:36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chrvala CA, Sherr D, Lipman RD. Diabetes self-management education for adults with type 2 diabetes mellitus: a systematic review of the effect on glycemic control. Patient Educ Couns 2016;99:926–943 [DOI] [PubMed] [Google Scholar]
- 32.Alexander JA, Hearld LR, Mittler JN, Harvey J. Patient–physician role relationships and patient activation among individuals with chronic illness. Health Serv Res 2012;47:1201–1223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Baars JE, Markus T, Kuipers EJ, Van Der Woude CJ. Patients’ preferences regarding shared decision-making in the treatment of inflammatory bowel disease: results from a patient-empowerment study. Digestion 2010;81:113–119 [DOI] [PubMed] [Google Scholar]
- 34.Jordan JE, Briggs AM, Brand CA, Osborne RH. Enhancing patient engagement in chronic disease self-management support initiatives in Australia: the need for an integrated approach. Med J Aust 2008;189:S9. [DOI] [PubMed] [Google Scholar]
- 35.Gerard M, Fossa A, Folcarelli PH, Walker J, Bell SK. What patients value about reading visit notes: a qualitative inquiry of patient experiences with their health information. J Med Internet Res 2017;19:e237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lindblad S, Ernestam S, Van Citters AD, Lind C, Morgan TS, Nelson EC. Creating a culture of health: evolving healthcare systems and patient engagement. QJM 2017;110:125–129 [DOI] [PubMed] [Google Scholar]
- 37.Graffigna G, Barello S. Patient engagement in healthcare: pathways for effective medical decision making. Neuropsychol Trends 2015;17:53–65 [Google Scholar]
- 38.Scholten M, Kelders SM, van Gemert-Pijnen JE. How persuasive is a virtual coach in terms of promoting web-based intervention user engagement? Exploring a potential match between user support needs and a virtual coach’s motivational capabilities. Presented at the Fourth International Workshop on Behavior Change Support Systems, BCSS 2016, Salzburg, Austria, 5–7 April 2016. Available from http://ceurl-ws.org/Vol-1573/Paper_6_BCSS2016.pdf. Accessed 13 March 2018 [Google Scholar]
- 39.Lindblad S, Ernestam S, Van Citters AD, Lind C, Morgan TS, Nelson EC. Creating a culture of health: evolving healthcare systems and patient engagement. QJM 2017;110:125–129 [DOI] [PubMed] [Google Scholar]
- 40.Chen SM, Creedy D, Lin HS, Wollin J. Effects of motivational interviewing intervention on self-management, psychological and glycemic outcomes in type 2 diabetes: a randomized controlled trial. Int J Nurs Stud 2012;49:637–644 [DOI] [PubMed] [Google Scholar]
- 41.Dellasega C, Gabbay R, Durdock K, Martinez-King N. Motivational interviewing (MI) to change type 2DM self care behaviors: a nursing intervention. J Diabetes Nurs 2010;14:112. [PMC free article] [PubMed] [Google Scholar]
- 42.Aljasem LI, Peyrot M, Wissow L, Rubin RR. The impact of barriers and self-efficacy on self-care behaviors in type 2 diabetes. Diabetes Educ 2001;27:393–404 [DOI] [PubMed] [Google Scholar]