Abstract
Background
The association between platelet distribution width (PDW) and cancer has been evaluated by a few studies, but the influence of PDW on cancer prognosis is unclear. Therefore, we conducted the present meta-analysis.
Material/Methods
We identified relevant research using identical search strategies. The influence of PDW level on cancer prognosis, as well as clinical characteristics, was analyzed.
Results
A total of 11 studies comprising 2625 cancer patients were included in our meta-analysis. The results suggested that high PDW level was obviously related to poor OS (HR=1.54, 95%CI 1.18–2.00), especially for breast cancer (HR=1.21, 95%CI 1.07–1.36) and pharyngolaryngeal cancer (HR=3.06, 95%CI 1.68–5.57). Furthermore, high PDW was obviously related to poor OS both in older and younger subgroups, with combined HR estimates of 1.58 (95%CI 1.15–2.16) and 1.64 (95%CI 1.19–2.26), respectively. High PDW level was notably related to poor OS in the cut-off value ≥16% subgroup (HR=1.84, 95%CI 1.01–3.40). Moreover, high PDW level was obviously associated with lymph node metastasis (OR=1.43, 95%CI 1.04–1.99).
Conclusions
The findings of this study suggest that PDW is an effective and convenient indicator of cancer prognosis. Furthermore, high PDW level is obviously associated with lymph node metastasis.
MeSH Keywords: Blood Platelets, Meta-Analysis, Prognosis
Background
Cancer is one of the major causes of morbidity and mortality in the world [1]. Although drug treatments and surgical methods develop rapidly, the survival of most tumors is still disappointing [1]. Therefore, it is of great importance to find effective and convenient indicators for predicting prognosis of cancer [2].
Platelets play an important role in cancer progression and metastases [3]. The interactions between platelets and cancer cells lead to tumor growth, abnormal angiogenesis, and tumor metastasis [4,5]. It is speculated that tumor cells can escape from shear-induced damage by binding with platelets, which facilitates tumor colonization [6]. Platelet distribution width (PDW) a platelet index indicating variation in platelet size [7]. In addition, PDW has been reported to be a marker of platelet morphology and activation [7]. The relationship between PDW level and prognosis of cancer is not clear. Recently, several studies have reported that high PDW level was associated with unfavorable prognosis for various types of tumors, including breast cancer, colorectal cancer, and laryngeal cancer [8–10], but other studies found that PDW is not associated with progression of gastric cancer [11].
Therefore, we performed this meta-analysis to investigate the possible association between PDW level and overall survival of cancer patients and determine whether PDW could an effective and convenient indicator of cancer prognosis.
Material and Methods
Literature search strategies
We searched PubMed, Web of Science, and EMBASE databases for relevant articles using the keywords “Platelet distribution width”, “PDW”, „cancer”, „tumor”, and “malignancy” with all combinations. To find additional relevant studies, we also manually searched bibliographies, reviews, and a few related articles. The last update was conducted on June 30, 2018.
Selection criteria
In our meta-analysis, the inclusion criteria for choosing articles were as follows: (1) studies should evaluate the value of PDW in the blood; (2) studies should assess the relationship between PDW and prognostic value or clinical characteristics of cancer patients; (3) studies should provide abundant information for estimating HR of OS, or OR of clinical characteristics; (4) papers should be published in English.
Due to insufficient data needed to properly evaluate review articles, case reports, and conference reports, these were not included in our meta-analysis [12]. In case of duplication, we only included the most complete article when studies included the same patient population.
Data extraction
Two researchers (Wen-Jie Xia and Jiang-Feng Tu) individually checked each related article and gathered relevant information. Disagreements were arbitrated by researcher Wu-Zhen Chen. Basic information was obtained from each article, such as name of first author, publication year, patient population, cancer types, cut-off value of PDW, and HR data [2].
Quality evaluation
The Newcastle-Ottawa quality assessment scale (NOS) was utilized for assessing the quality of each study in our meta-analysis [2,13]. This scale was used to evaluate each study, with 8 items on methodology from 3 dimensions: selection, comparability, and exposure [2]. The studies with scores of 6 or higher were qualified for our meta-analysis [2]. Two investigators each evaluated all studies and scored them, and disagreements were settled by researcher Wu-Zhen Chen.
Statistical analysis
Hazard ratio (HR) with 95% confidence interval (CI) was obtained from each included study for the pooled analysis of survival: when the information on HR was given in the study, it was obtained directly; otherwise, we calculated relevant HR information with the use of data given in the papers by means described by Parmar and Jayne [2,14,15]. The influence of PDW level on cancer prognosis was estimated by the combined HR and 95%CI. The relationship between PDW level and clinical parameters, such as age, sex, differentiation, and lymph node metastasis, was evaluated by combined odds ratio (OR) and its 95% CI. We performed this meta-analysis with the use of STATA v12.0 (Stata Corporation, Collage Station, Texas, USA). Heterogeneity in the analysis was estimated by using the chi-square-based Q statistical test and I2 test [2,16,17]. We used a fixed-effects model when analysis did not show significant heterogeneity (I2 value <50%) [18]; otherwise, we used a random-effects model for pooling data [19]. We used Begg’s test for estimating publication bias [20], which was identified as p value of Begg’s test <0.05 [21].
Results
Study selection and description
Totally 347 published studies were retrieved from databases using the information retrieval strategies described in Figure 1. After duplicates were removed, 166 studies in total were included in preliminary screening. We excluded 138 because they were, for instance, fundamental experimental studies, reviews, not focussed on PDW or cancer; 28 studies were checked with full text, and 17 were excluded due to insufficient data to assess the relationship between PDW and prognostic value or clinicopathologic features of cancer patients.
Figure 1.
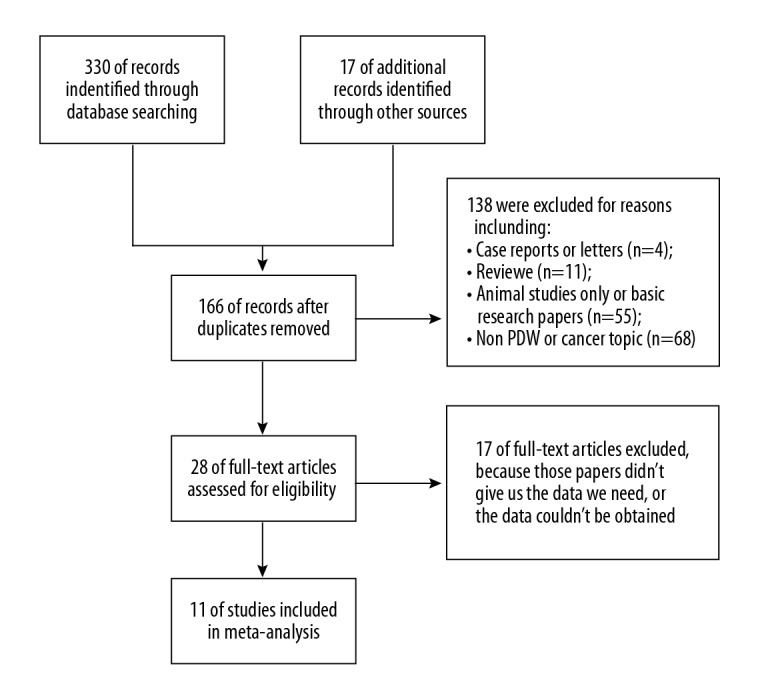
Flow diagram of study selection procedure.
Finally, 11 eligible studies met our requirements and were included in our meta-analysis [8–11,22–28], and their basic characteristics are described in Table 1. In the aggregate, 2625 cancer patients were involved in the meta-analysis. The median population size was 239 (range, 168–379). Eight studies assessed patients from China and 3 studies assessed patients from Western countries. All the included studies passed methodological evaluation and were qualified for analysis.
Table 1.
Basic characteristics of the included studies.
| First author | Year | Country | Patients | Median age (year) | Cancer type | Follow up (months) | Cut-off value | HR estimation | NOS score |
|---|---|---|---|---|---|---|---|---|---|
| Hideya T | 2017 | US | 275 | 64.5 | Breast cancer | 45 | 15.3% | HR for OS | 7 |
| Cheng S | 2017 | China | 227 | NA | Gastric cancer | 61 | 11.5% | HR for OS | 8 |
| Xie X | 2017 | China | 168 | 49 | Nasopharyngeal carcinoma | 65.2 | 16.3% | HR for OS | 7 |
| Zhang H | 2017 | China | 241 | 57.8 | Laryngeal cancer | 60 | 16.70% | HR for OS | 8 |
| Li N | 2017 | China | 220 | 56.3 | Melanoma | 60 | 17.20% | HR for OS | 8 |
| Yun ZY | 2017 | China | 379 | 55.6 | Gastric cancer | NA | NA | NA | 6 |
| Zhang X | 2017 | China | 294 | 56 | Gastric cancer | 60 | 16.80% | HR for OS | 7 |
| Song X | 2017 | China | 206 | 57 | Colorectal cancer | 52 | 17.35% | HR for OS | 7 |
| Gunaldi M | 2016 | Turkey | 269 | 59.4 | Gastric cancer | 14.04 | 16.65% | HR for OS | 7 |
| Wang L | 2015 | China | 168 | 61 | Pancreatic adenocarcinoma | 48 | 14.15% | HR for OS | 7 |
| Okuturlar Y | 2015 | Turkey | 178 | 53.8 | Breast cancer | NA | NA | HR for OS | 6 |
HR – hazard ratio; OS – overall survival; NOS – Newcastle-Ottawa quality assessment scale; NA – not available.
Influence of PDW level on OS of cancer
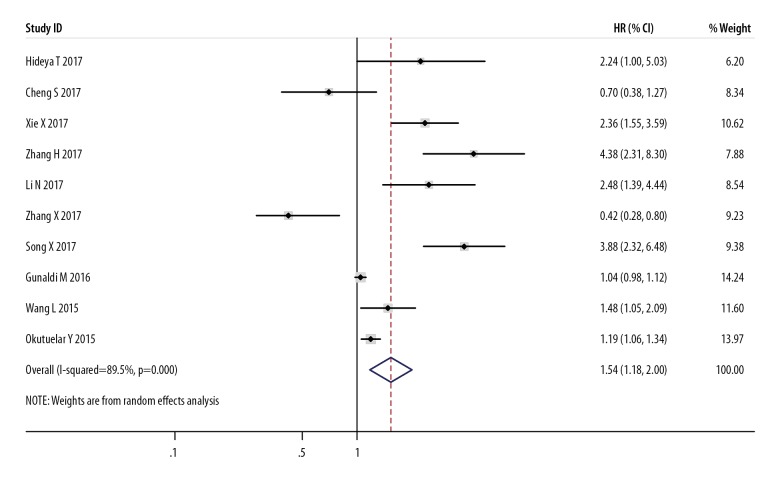
Ten studies estimated the relationship between PDW and OS of cancer using HR data, suggesting that compared with low PDW level, high PDW level substantially raised the mortality risk of cancer patients, with a combined HR estimate of 1.54 (95%CI: 1.18–2.00, I2=89.5%) (Figure 2). Sensitivity analysis was conducted, in which individual studies were sequentially omitted (Figure 3). The result showed no clear variation in the combined HR and the result was robust.
Figure 2.
Forest plot of hazard ratio (HR) and 95%CI for the correlation between PDW level and overall survival (OS). HR >1 indicates worse survival for the group.
Figure 3.
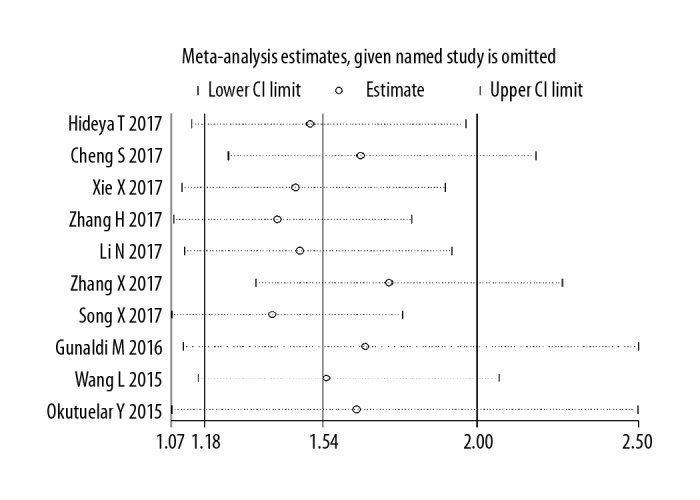
Forest plot of sensitivity analysis for influence of PDW level on OS with HR and 95%CI. HR >1 indicates worse survival for the group.
In addition, subgroup analysis was also performed by cancer type, study location, median age, cut-off value, and length of follow-up (Table 2). Showing significant correlations between high PDW level and poor prognosis for breast cancer (HR=1.21, 95%CI: 1.07–1.36) and pharyngolaryngeal cancer (HR=3.06, 95%CI: 1.68–5.57), but not for gastrointestinal cancer (HR=1.13, 95%CI: 0.68–1.87). High PDW was obviously related to worse OS both in older and younger subgroups, with a combined HR estimate of 1.58 (95%CI: 1.15–2.16) and 1.64 (95%CI: 1.19–2.26), respectively. Furthermore, high PDW level was notably related to worse OS in the shorter follow-up subgroup (HR=1.82, 95%CI: 1.03–3.20) and cut-off value ≥16% subgroup (HR=1.84, 95%CI: 1.01–3.40). Other subgroups did not show significant correlations between PDW and overall survival.
Table 2.
Stratified analysis of pooled hazard ratios for cancer patients with PDW.
| Stratified analysis | Number of studies | Number of patients | Pooled HR (95%CI) |
|---|---|---|---|
| Cancer type | |||
| Breast cancer | 2 | 453 | 1.21 (1.07–1.36) |
| Pharyngolaryngeal cancer | 2 | 409 | 3.06 (1.68–5.57) |
| Gastrointestinal cancer | 5 | 1164 | 1.13 (0.68–1.87) |
| Median age | |||
| ≥60 | 2 | 443 | 1.58 (1.15–2.16) |
| <60 | 7 | 1576 | 1.64 (1.19–2.26) |
| Follow up (months) | |||
| ≥60 | 5 | 1150 | 1.49 (0.65–3.43) |
| <60 | 4 | 918 | 1.82 (1.03–3.20) |
| Study location | |||
| Asian | 7 | 1524 | 1.71 (0.95–3.07) |
| Non-Asian | 3 | 722 | 1.14 (0.97–1.33) |
| Cut-off value | |||
| ≥16% | 6 | 1398 | 1.84 (1.01–3.40) |
| <16% | 3 | 670 | 1.29 (0.72–2.31) |
Relation between PDW level and clinical parameters
Six studies were included that evaluated the relationship between PDW level and clinical parameters of cancer patients. High PDW was strongly associated with lymph node metastasis (OR=1.43, 95%CI: 1.04–1.99, I2=33%) (Table 3), suggesting PDW is an effective and convenient indicator of cancer metastasis. However, PDW was not closely associated with age (OR=0.87, 95%CI 0.67–1.13, I2=10%), sex (OR=1.15, 95%CI: 0.87–1.52, I2=13%), tumor differentiation (OR=1.01, 95%CI: 0.72–1.44, I2=0%), or tumor stage (OR=1.18, 95%CI: 0.75–1.85, I2=65%) (Table 3).
Table 3.
Relationship between PDW level and clinicopathological features.
| Clinical features | Pooled OR | Low value of 95%CI | High value of 95%CI | I square | Model used |
|---|---|---|---|---|---|
| Lymphatic metastasis | 1.43 | 1.04 | 1.99 | 33% | Fixed effect model |
| Differentiation (poor vs. well) | 1.01 | 0.72 | 1.44 | 0% | Fixed effect model |
| Sex (Male vs. Female) | 1.15 | 0.87 | 1.52 | 13% | Fixed effect model |
| Age (older vs. younger) | 0.87 | 0.67 | 1.13 | 10% | Fixed effect model |
| Tumor stage (T3+T4 vs. T1+T2) | 1.18 | 0.75 | 1.85 | 65% | Random effect model |
Publication bias
Begg’s test indicated that after assessing the funnel plot for included studies, there was no noteworthy publication bias in this meta-analysis (Figure 4).
Figure 4.
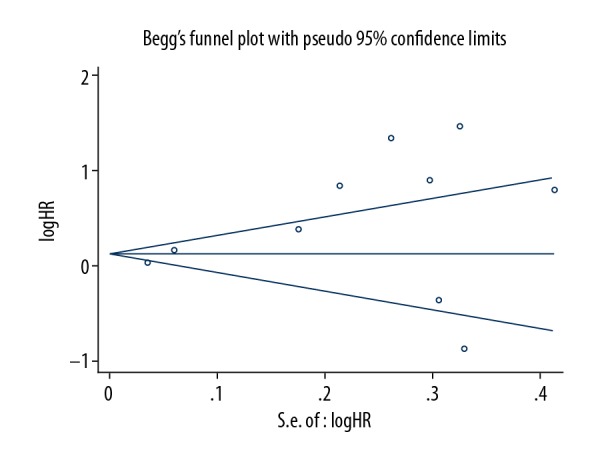
Begg’s funnel plot indicated there was no significant publication bias for studies evaluating the impact of PDW level on OS.
Discussion
Despite rapid development of drug treatments and surgical methods, the survival of most cancers is still very poor [1]. Complicated interactions between platelets and cancer cells lead to tumor growth, abnormal angiogenesis, and metastasis [29]. Platelets are reported to play an important role in the inflammatory response, and can influence the tumor microenvironment and promote tumor growth [30,31]. Tumor cells can secrete many mediators, such as thrombin, which interact with platelet surface receptors via PAR-1, PAR-4, and P2Y12 receptors [32]. Tumor cells can also secrete matrix metalloproteinases (MMPs) and interleukin-6, which activate platelets and promote tumor growth [32–34]. In addition, it was reported that by degranulation, activated platelets can release tumor growth factors, such as vascular endothelial growth factor (VEGF), which promote tumor growth and abnormal angiogenesis [35]. Moreover, platelet-derived growth factor (PDGF) was confirmed to inhibit the cell-killing effects of NK cells [36]. Compared with platelet count, which changes rapidly, PDW was revealed to be a better indicator to reflect the characteristics of activated platelets [37]. A few researches assessed the association between PDW and cancer, but the influence of PDW on cancer prognosis was not clear.
To the best of our knowledge, the present study is the first to show the influence of PDW on cancer prognosis and the association with clinical characteristics.
In this meta-analysis, 10 studies in total estimated the relationship between PDW and OS of cancer, and 6 estimated the relationship between PDW and clinical parameters. Eventually, we found that high PDW level strongly predicts poor OS of cancer patients. The sensitive analysis suggests our results are robust. Thus, PDW appears to be an effective and convenient indicator of cancer prognosis. All the blood samples collected in the included studies were obtained before surgery; thus, the time of collecting blood samples was not a factor affecting the results in our analysis.
Subgroup analysis was conducted by cancer type, study location, median age, cut-off value, and follow-up. High PDW level was obviously related to worse OS for breast cancer and pharyngolaryngeal cancer, suggesting PDW has a better prognostic value in these tumors. Furthermore, high PDW was notably related to poor OS both in older and younger subgroups, indicating PDW could be an efficient predictor of prognosis, regardless of patient age. High PDW seemed to be related to unfavorable OS in the cut-off value ≥16% subgroup, suggesting PDW has a better prognostic value with cut-off values in this range. Moreover, significant correlations were found between PDW level and lymph node metastasis, suggesting PDW is an effective and convenient indicator of cancer metastasis.
There were a few limitations in our study. First of all, the patient populations of the included studies were undersized, and we could not evaluate the prognostic value of PDW in each cancer separately, mainly because some studies only provided average level of PDW, which we could not use, rather than assessing it as high or low. Second, the cut-off values in the included studies were various, which could lead to heterogeneity between studies. Subgroup analysis showed PDW might have a better prognostic value with cut-off value ≥16%.
Conclusions
High PDW level was obviously related to poor OS, especially for breast cancer and pharyngolaryngeal cancer, and high PDW was strongly associated with poor OS in the older and younger subgroups. Furthermore, high PDW was notably related to poor OS in the cut-off value ≥16% subgroup, and high PDW was obviously associated with lymph node metastasis. Therefore, the results of our meta-analysis suggest PDW is an effective and convenient indicator of cancer prognosis and lymph node metastasis.
Abbreviations
- PDW
platelet distribution width
- HR
hazard ratio
- OR
odds ratio
- CI
confidence interval
- PAR
protease activated receptor
- MMP
matrix metalloproteinase
- VEGF
vascular endothelial growth factor
- PDGF
platelet-derived growth factor
- NOS
Newcastle-Ottawa quality assessment scale
Footnotes
Source of support: Departmental sources
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics. Cancer J Clin. 2018;68:7–30. doi: 10.3322/caac.21442. [DOI] [PubMed] [Google Scholar]
- 2.Xia W, Chen W, Zhang Z, et al. Prognostic value, clinicopathologic features and diagnostic accuracy of interleukin-8 in colorectal cancer: A meta-analysis. PLoS One. 2015;10:e0123484. doi: 10.1371/journal.pone.0123484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cui MM, Li N, Liu X, et al. Platelet distribution width correlates with prognosis of non-small cell lung cancer. Sci Rep. 2017;7:3456. doi: 10.1038/s41598-017-03772-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bambace NM, Holmes CE. The platelet contribution to cancer progression. J Thromb Haemost. 2011;9:237–49. doi: 10.1111/j.1538-7836.2010.04131.x. [DOI] [PubMed] [Google Scholar]
- 5.Goubran HA, Stakiw J, Radosevic M, Burnouf T. Platelet-cancer interactions. Semin Thromb Hemost. 2014;40:296–305. doi: 10.1055/s-0034-1370767. [DOI] [PubMed] [Google Scholar]
- 6.Egan K, Cooke N, Kenny D. Living in shear: Platelets protect cancer cells from shear-induced damage. Clin Exp Metastasis. 2014;31:697–704. doi: 10.1007/s10585-014-9660-7. [DOI] [PubMed] [Google Scholar]
- 7.Bath PM, Missouris CG, Buckenham T, MacGregor GA. Increased platelet volume and platelet mass in patients with atherosclerotic renal artery stenosis. Clin Sci (Lond) 1994;87:253–57. doi: 10.1042/cs0870253. [DOI] [PubMed] [Google Scholar]
- 8.Takeuchi H, Abe M, Takumi Y, et al. The prognostic impact of the platelet distribution width-to-platelet count ratio in patients with breast cancer. PLoS One. 2017;12:e0189166. doi: 10.1371/journal.pone.0189166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang H, Liu L, Fu S, et al. Higher platelet distribution width predicts poor prognosis in laryngeal cancer. Oncotarget. 2017;8:48138–44. doi: 10.18632/oncotarget.18306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Song X, Zhu H, Pei Q, et al. Significance of inflammation-based indices in the prognosis of patients with non-metastatic colorectal cancer. Oncotarget. 2017;8:45178–89. doi: 10.18632/oncotarget.16774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cheng S, Han F, Wang Y, et al. The red distribution width and the platelet distribution width as prognostic predictors in gastric cancer. BMC Gastroenterol. 2017;17:163. doi: 10.1186/s12876-017-0685-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen Z, He X, Xia W, et al. Prognostic value and clinicopathological differences of HIFs in colorectal cancer: Evidence from meta-analysis. PLoS One. 2013;8:e80337. doi: 10.1371/journal.pone.0080337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25:603–5. doi: 10.1007/s10654-010-9491-z. [DOI] [PubMed] [Google Scholar]
- 14.Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform meta-analyses of the published literature for survival endpoints. Stat Med. 1998;17:2815–34. doi: 10.1002/(sici)1097-0258(19981230)17:24<2815::aid-sim110>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 15.Tierney JF, Stewart LA, Ghersi D, et al. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials. 2007;8:16. doi: 10.1186/1745-6215-8-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Handoll HH. Systematic reviews on rehabilitation interventions. Arch Phys Med Rehabil. 2006;87:875. doi: 10.1016/j.apmr.2006.04.006. [DOI] [PubMed] [Google Scholar]
- 17.Ioannidis JP, Patsopoulos NA, Evangelou E. Uncertainty in heterogeneity estimates in meta-analyses. BMJ. 2007;335:914–16. doi: 10.1136/bmj.39343.408449.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mantel N, Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst. 1959;22:719–48. [PubMed] [Google Scholar]
- 19.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–60. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50:1088–101. [PubMed] [Google Scholar]
- 21.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–34. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xie X, Zeng X, Cao S, et al. Elevated pretreatment platelet distribution width and platelet count predict poor prognosis in nasopharyngeal carcinoma. Oncotarget. 2017;8:106089–97. doi: 10.18632/oncotarget.22528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li N, Diao Z, Huang X, et al. Increased platelet distribution width predicts poor prognosis in melanoma patients. Sci Rep. 2017;7:2970. doi: 10.1038/s41598-017-03212-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yun ZY, Li N, Zhang X, et al. Mean platelet volume, platelet distribution width and carcinoembryonic antigen to discriminate gastric cancer from gastric ulcer. Oncotarget. 2017;8:62600–5. doi: 10.18632/oncotarget.15898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang X, Cui MM, Fu S, et al. Platelet distribution width correlates with prognosis of gastric cancer. Oncotarget. 2017;8:20213–19. doi: 10.18632/oncotarget.15561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gunaldi M, Erdem D, Goksu S, et al. Platelet distribution width as a predictor of metastasis in gastric cancer patients. J Gastrointest Cancer. 2017;48:341–46. doi: 10.1007/s12029-016-9886-5. [DOI] [PubMed] [Google Scholar]
- 27.Wang L, Sheng L, Liu P. The independent association of platelet parameters with overall survival in pancreatic adenocarcinoma receiving intensity-modulated radiation therapy. Int J Clin Exp Med. 2015;8:21215–21. [PMC free article] [PubMed] [Google Scholar]
- 28.Okuturlar Y, Gunaldi M, Tiken EE, et al. Utility of peripheral blood parameters in predicting breast cancer risk. Asian Pac J Cancer Prev. 2015;16:2409–12. doi: 10.7314/apjcp.2015.16.6.2409. [DOI] [PubMed] [Google Scholar]
- 29.Jurasz P, Alonso-Escolano D, Radomski MW. Platelet – cancer interactions: Mechanisms and pharmacology of tumour cell-induced platelet aggregation. Br J Pharmacol. 2004;143:819–26. doi: 10.1038/sj.bjp.0706013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Desai SJ, Prickril B, Rasooly A. Mechanisms of phytonutrient modulation of Cyclooxygenase-2 (COX-2) and inflammation related to cancer. Nutr Cancer. 2018;70:350–75. doi: 10.1080/01635581.2018.1446091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kamath J. Cancer-related fatigue, inflammation and thyrotropin-releasing hormone. Curr Aging Sci. 2012;5:195–202. doi: 10.2174/1874609811205030005. [DOI] [PubMed] [Google Scholar]
- 32.Lee EC, Cameron SJ. Cancer and thrombotic risk: The platelet paradigm. Front Cardiovasc Med. 2017;4:67. doi: 10.3389/fcvm.2017.00067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Seizer P, May AE. Platelets and matrix metalloproteinases. Thromb Haemost. 2013;110:903–9. doi: 10.1160/TH13-02-0113. [DOI] [PubMed] [Google Scholar]
- 34.Gremmel T, Perkmann T, Seidinger D, et al. Differential impact of inflammation on six laboratory assays measuring residual arachidonic acid-inducible platelet reactivity during dual antiplatelet therapy. J Atheroscler Thromb. 2013;20:630–45. doi: 10.5551/jat.17665. [DOI] [PubMed] [Google Scholar]
- 35.Matowicka-Karna J. Markers of inflammation, activation of blood platelets and coagulation disorders in inflammatory bowel diseases. Postepy Hig Med Dosw (Online) 2016;70:305–12. doi: 10.5604/17322693.1199305. [DOI] [PubMed] [Google Scholar]
- 36.Peterson JE, Zurakowski D, Italiano JE, Jr, et al. VEGF, PF4 and PDGF are elevated in platelets of colorectal cancer patients. Angiogenesis. 2012;15:265–73. doi: 10.1007/s10456-012-9259-z. [DOI] [PubMed] [Google Scholar]
- 37.Leader A, Pereg D, Lishner M. Are platelet volume indices of clinical use? A multidisciplinary review. Ann Med. 2012;44:805–16. doi: 10.3109/07853890.2011.653391. [DOI] [PubMed] [Google Scholar]